Abstract
Objective:
To characterize the natural history and neuropathologic basis of unawareness of memory loss in late-life dementia.
Methods:
Analyses are based on 2,092 older persons from 3 longitudinal clinical-pathologic cohort studies who had no memory or cognitive impairment at baseline. Annual evaluations included clinical classification of dementia plus self-rating and performance testing of memory. At death, there was a uniform neuropathologic examination to quantify 7 dementia-related pathologies.
Results:
In the full group, memory ratings were modestly correlated with memory performance (intercepts r = 0.26, p < 0.001; slopes r = 0.23, p < 001) and so we regressed each person's memory performance on their memory ratings, and the residuals provided longitudinal indicators of memory awareness. In a subset of 239 persons who developed dementia, episodic memory awareness was stable until a mean of 2.6 years before dementia onset (95% credible interval −2.7, –1.6); thereafter, memory awareness declined rapidly (mean annual change −0.32, 95% credible interval –0.37, –0.28). Older age at baseline was associated with later onset of memory unawareness. In a subset of 385 persons who died and underwent neuropathologic examination, transactive response DNA-binding protein 43 (TDP-43) pathology, tau tangles, and gross cerebral infarcts were related to decline in memory awareness. In the absence of these pathologies, no decline in memory awareness was evident. Results were similar in subgroups with and without dementia.
Conclusions:
Awareness of memory impairment typically begins to decline about 2–3 years before dementia onset and is associated with postmortem evidence of TDP-43 pathology, tangles, and gross cerebral infarcts.
Progressive loss of memory is a central feature of dementia, but affected persons are not always aware of this impairment, and despite much research it remains uncertain how common unawareness of memory impairment is in persons with dementia, when it develops, or why some are apparently affected more than others. Unawareness has been associated with dementia severity in some cross-sectional studies1–4 but not others.5–7 Longitudinal studies have also been inconsistent, with some suggesting that unawareness increases over time,8–10 but other studies reporting mixed results1,11 or no change.12–17 This inconsistency may reflect several factors. First, because this research is based on prevalent dementia with relatively brief observation periods, it covers only a fraction of the symptomatic phase of dementia. Second, in most studies, objective determination of memory impairment has been based not on performance testing but on ratings by a knowledgeable informant or clinician, which are subject to bias, particularly when short observation periods limit the actual amount of change that is occurring.
The present analyses are based on data from 3 longitudinal clinical-pathologic studies of older persons without cognitive impairment at enrollment. They had annual clinical evaluations that included global self-ratings of memory and a battery of memory performance tests from which we derived measures of residual deviation in memory performance not explained by memory rating. In those who subsequently developed dementia, we characterized change in these residual measures of memory unawareness in relation to clinical milestones. In those who subsequently died and underwent a postmortem neuropathologic examination, we tested the hypothesis that unawareness of memory impairment is a manifestation of dementia-related pathology.
METHODS
Participants.
Those included in analyses are from 3 ongoing longitudinal clinical-pathologic cohort studies. The Religious Orders Study began in 1994 and its participants are older Catholic nuns, priests, and brothers from groups across the United States.18 The Rush Memory and Aging Project began in 1997 and includes older individuals from the metropolitan Chicago area.19 The Minority Aging Research Study began in 2004 and its participants are older black persons in the metropolitan Chicago area recruited from the community and the clinical core of the Rush Alzheimer's Disease Core Center.20 At the time of enrollment, participants were at least 50 years old and had not been diagnosed with dementia. In addition, everyone in the Religious Orders Study and the Rush Memory and Aging Project and a subset of those in the Minority Aging Research Study agreed to brain autopsy at death.
At the time of these analyses, 3,298 persons had completed the baseline evaluation and signed an Anatomical Gift Act agreeing to brain donation. Of these, 107 died before the first follow-up and 217 had been enrolled less than 1 year. From the remaining 2,974 individuals, we excluded 859 with cognitive impairment at baseline (153 with dementia and 706 with mild cognitive impairment). This left 2,115 eligible for follow-up, and follow-up data were available in 2,092 (98.9%). As shown in table 1, persons in this full group had a mean baseline age of 76.1 years and a mean of 7.7 years of follow-up.
Table 1.
Descriptive data on participantsa
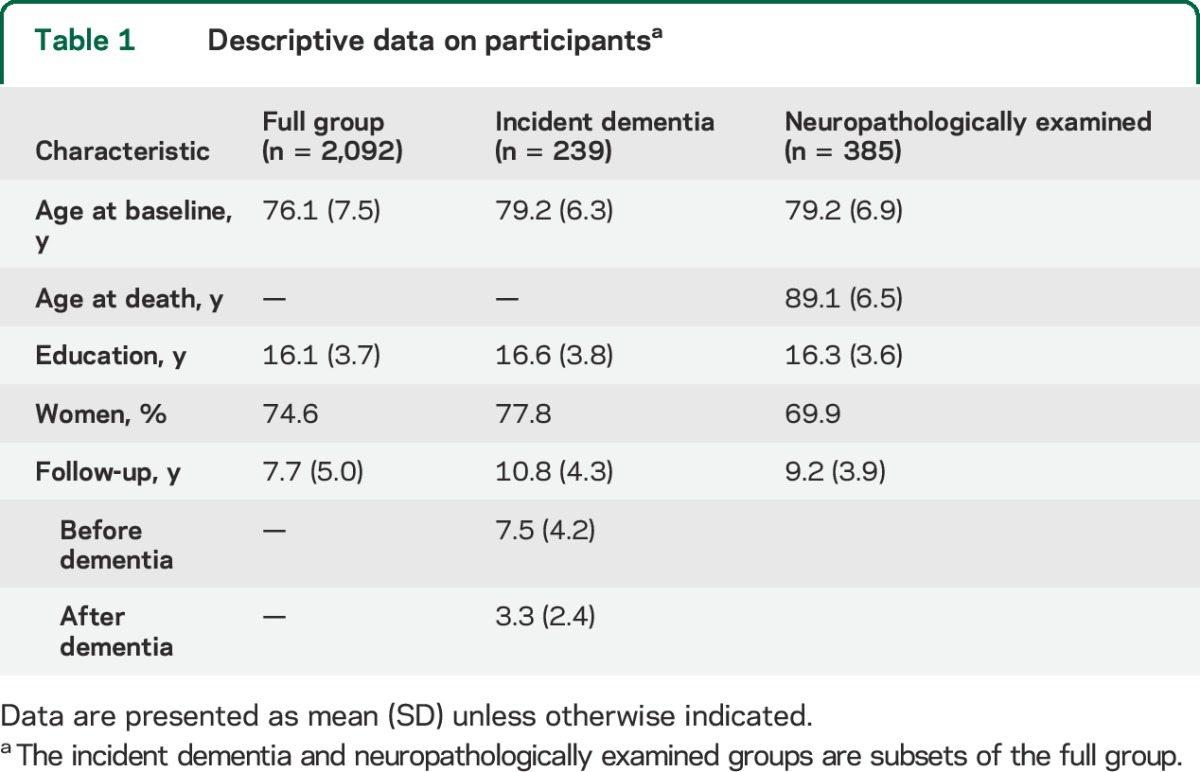
Additional analyses were conducted on 2 subsets. To track the temporal course of memory awareness in dementia, the first subset consisted of 239 persons who completed at least 4 annual evaluations (to allow for nonlinear change) and developed incident dementia before their last evaluation. They had a mean baseline age of 79.2 years and a mean follow-up of 10.8 years, including 7.5 years before dementia onset and 3.3 years after dementia onset (table 1). To investigate the pathologic basis of unawareness of memory impairment, the second subset consisted of neuropathologically examined individuals. Of the 772 deaths in the full group, 662 (85.8%) underwent a brain autopsy and neuropathologic examination, the results of which were available on the first consecutive 627 persons. Because transactive response DNA-binding protein 43 (TDP-43) pathology is associated with memory loss21 and preliminary analyses in the 627 were similar to results in the 385 with TDP-43 data, we analyzed the association of memory awareness with pathology in this subset. They died at a mean age of 89.1 years with a mean of 9.2 years of follow-up (table 1).
Standard protocol approvals, registrations, and patient consents.
Following detailed discussion with staff, persons in each study signed informed consent forms. The institutional review board of Rush University Medical Center approved each study.
Clinical evaluation.
Participants had an annual clinical assessment that included a medical history, neurologic examination, and tests of memory and cognition. After each evaluation, a clinician, who was unaware of previously collected data, diagnosed dementia with widely used criteria22 that call for a history of cognitive decline and impairment in 2 or more cognitive domains.
Self-assessment of memory.
At each annual evaluation, participants were asked 2 questions about their memory. The first was “How often do you have trouble remembering things?” and the response options were very often (1), often (2), sometimes (3), rarely (4), never (5). The second was “Compared to 10 years ago, would you say that your memory is much worse (1), a little worse (2), the same (3), a little better (4), or much better (5)?” Because the items were correlated and preliminary analyses with each item yielded similar results, we added the 2 item scores to yield a subjective memory measure.23 Scores ranged from 2 to 10 with higher values indicating better subjective memory function.
Performance testing of memory.
Nineteen cognitive tests were administered annually to support clinical classification of dementia and measure change in cognitive function. The battery included 7 episodic memory tests (immediate and delayed recall of the East Boston Story and Story A from Logical Memory, Word List Memory, Word List Recall, Word List Recognition); 3 semantic memory tests (15-item Boston Naming Test, Verbal Fluency, 15-item word reading test); and 3 working memory tests (Digit Span Forward, Digit Span Backward, Digit Ordering). Supported by factor analyses in these24–26 and other27 cohorts, composite measures of episodic memory, semantic memory, and working memory were constructed by converting raw scores on component tests to z scores, using the baseline mean and SD of all persons in the parent studies, and then averaging the component z scores to yield the composite score. The composite episodic memory measure was used as the primary measure of memory performance and the mean of the composites of episodic, semantic, and working memory served as a more broadly defined memory performance measure in secondary analyses.
Neuropathologic examination.
Brain removal took place a mean of 8.2 hours after death (SD 5.1), which was a mean of 9.7 months after the last clinical evaluation. The neuropathologic examination followed a standard protocol.28 The age, volume, and location of gross cerebral infarcts were recorded. Hematoxylin & eosin staining was used to identify microinfarcts in 9 regions of one hemisphere. In analyses, gross and microscopic infarcts were each treated as present or absent. Hippocampal sclerosis was defined as severe neuronal loss and gliosis in the pyramidal cell layer of any cornu ammonis (CA) subfield or the subiculum, based on a hemisection of the midhippocampus at the level of the lateral geniculate body.29
We used computer-assisted sampling and immunohistochemistry to quantify β-amyloid-immunoreactive plaques, with an N-terminus-directed monoclonal antibody (1:1,000, 10D5; Elan Pharmaceuticals, Dublin, Ireland), and tau-immunoreactive tangles, with an anti-paired helical filament-tau antibody clone AT8 (1:2,000; Thermo Scientific, Rockford, IL) in 8 brain regions (entorhinal cortex, CA1/subiculum, anterior cingulate cortex, primary visual cortex, inferior parietal cortex, inferior temporal cortex, superior frontal cortex, dorsolateral prefrontal cortex). Regional scores were averaged to make composite measures of each pathology.30 TDP-43 was assessed in 6 brain regions (amygdala, entorhinal cortex, CA1/subculum, dentate gyrus, middle temporal cortex, midfrontal cortex) with a monoclonal antibody to phosphorylated TDP-43 (pS409/410; 1:100).31 In each region, neuronal and glial TDP-43 cytoplasmic inclusions were rated on a 6-point scale from none to severe, and regional ratings were averaged to yield a total score.21 Lewy bodies were investigated in 6 brain regions (inferior parietal cortex, superior or middle temporal cortex, midfrontal cortex, entorhinal cortex, anterior cingulate cortex, substantia nigra) with a monoclonal antibody to α-synuclein (Zymed LB 509; 1:50).28 Lewy bodies were treated as present or absent.
Statistical analysis.
We analyzed change in memory performances and memory ratings in the full group with a simultaneous mixed model that yielded estimates of the correlation between their initial levels and rates of change. To assess each person's memory awareness, a composite measure of memory performance was regressed on memory rating, and the residuals capture the deviation of observed memory performance from that predicted by the memory rating. For example, a negative residual means that memory performance is worse than perceived, suggesting a lack of awareness. Therefore, these residuals were used to characterize person-specific change in memory awareness over time.
In the incident dementia subgroup, we analyzed change in memory awareness with mixed-effects change point models using a Bayesian Monte Carlo Markov Chain approach32 with Open BUGS software,33 with time expressed as years before and after dementia onset. In the neuropathologically examined subgroup, we assessed change in memory awareness in mixed-effects models, with time expressed as years before death (because only a subset had developed dementia). The initial model included terms for time, age, sex, education, and the interactions of time with each demographic variable. Terms for each neuropathologic marker and its interaction with time were added to a second model. The second model was repeated separately in those with dementia and those without dementia.
RESULTS
Developing a measure of memory awareness.
At baseline in the full group (n = 2,092), participants' subjective memory rating (mean 5.05, SD 1.32, skewness −0.10) and the composite measure of episodic memory performance (mean 0.342, SD 0.484, skewness −0.14) had approximately normal distributions, with higher scores on each measure indicating better memory function. We constructed a simultaneous mixed model to estimate the relation between the measures over time. There was decline in both objective memory performances (estimate −0.065, SE 0.003, p < 0.001) and subjective memory ratings (estimate −0.022, SE 0.002, p < 0.001), their intercepts (r = 0.26, p < 0.001) and slopes (r = 0.23, p < 0.001) were correlated, and higher subjective memory at baseline predicted less episodic memory decline (r = 0.29, p < 0.001).
To assess memory awareness in each individual, we regressed their annual episodic memory performances on their annual memory ratings. The residuals capture the deviation of the observed episodic memory performance from the performance predicted by memory ratings and therefore provide a measure of episodic memory awareness, with a residual of zero indicating agreement between rating and performance, positive scores indicating underestimation of memory ability, and negative scores indicating overestimation of memory ability. At baseline in the full group (n = 2,092), this measure had a mean of 0.016 (SD 0.378, skewness 0.97), suggesting no systematic tendency to overestimate or underestimate memory ability at that time.
Memory awareness during dementia development.
There were 239 individuals with no memory or cognitive impairment at baseline, at least 4 annual assessments with valid memory ratings and performance, and a diagnosis of dementia before their last evaluation. To characterize change over time in this residual episodic memory awareness measure, we constructed a mixed-effects model. To determine when the problem began to develop, we allowed decline in memory awareness to accelerate at some variable time after baseline. To provide a clinical context, we scaled time in relation to dementia onset. The crude paths of change for each individual (colored lines in the figure) suggest loss of memory awareness in nearly all affected persons but variability in its onset and rate of progression. In the analysis (model A in table 2, black lines in the figure), the initial memory awareness score was close to zero, consistent with a lack of bias in the ratings, and there was no decline before the change point. However, starting a mean of 2.6 years before dementia onset, episodic memory awareness began to sharply decline at a mean rate of 0.260 unit per year, which is nearly half of the baseline SD (0.545). Older age was associated with later onset of the change point (model A, table 2). The figure shows that memory awareness began to decline 2–3 years earlier in younger persons (solid black line, baseline age 70.2, 10th percentile) compared to older persons (dotted black line, baseline age 87.3, 90th percentile). Neither sex nor education was related to change in memory awareness.
Figure. Declining awareness of memory impairment.
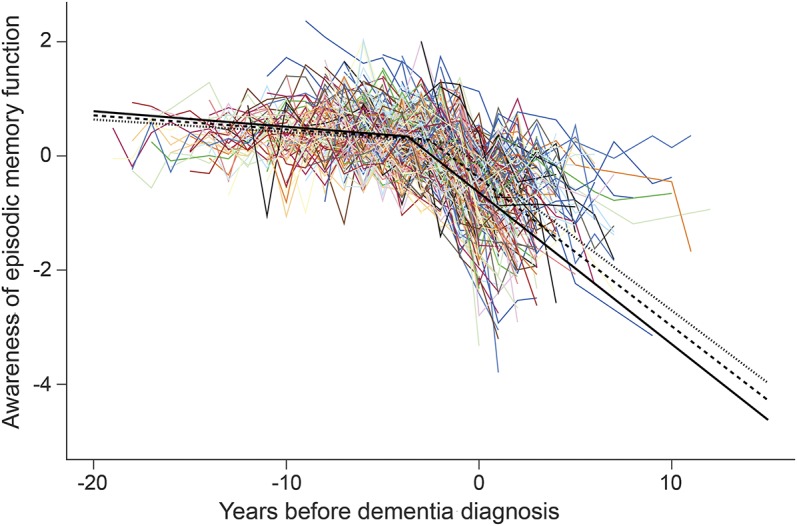
Change in awareness of episodic memory impairment during the course of dementia, including crude trajectories (colored lines) and predicted trajectories for persons beginning the study at ages 70.2 years (10th percentile, solid black line), 79.2 years (mean, dashed black line), or 87.3 years (90th percentile, dotted black line), from a mixed-effects change point model adjusted for sex and education.
Table 2.
Change in awareness of memory function during the development of dementiaa
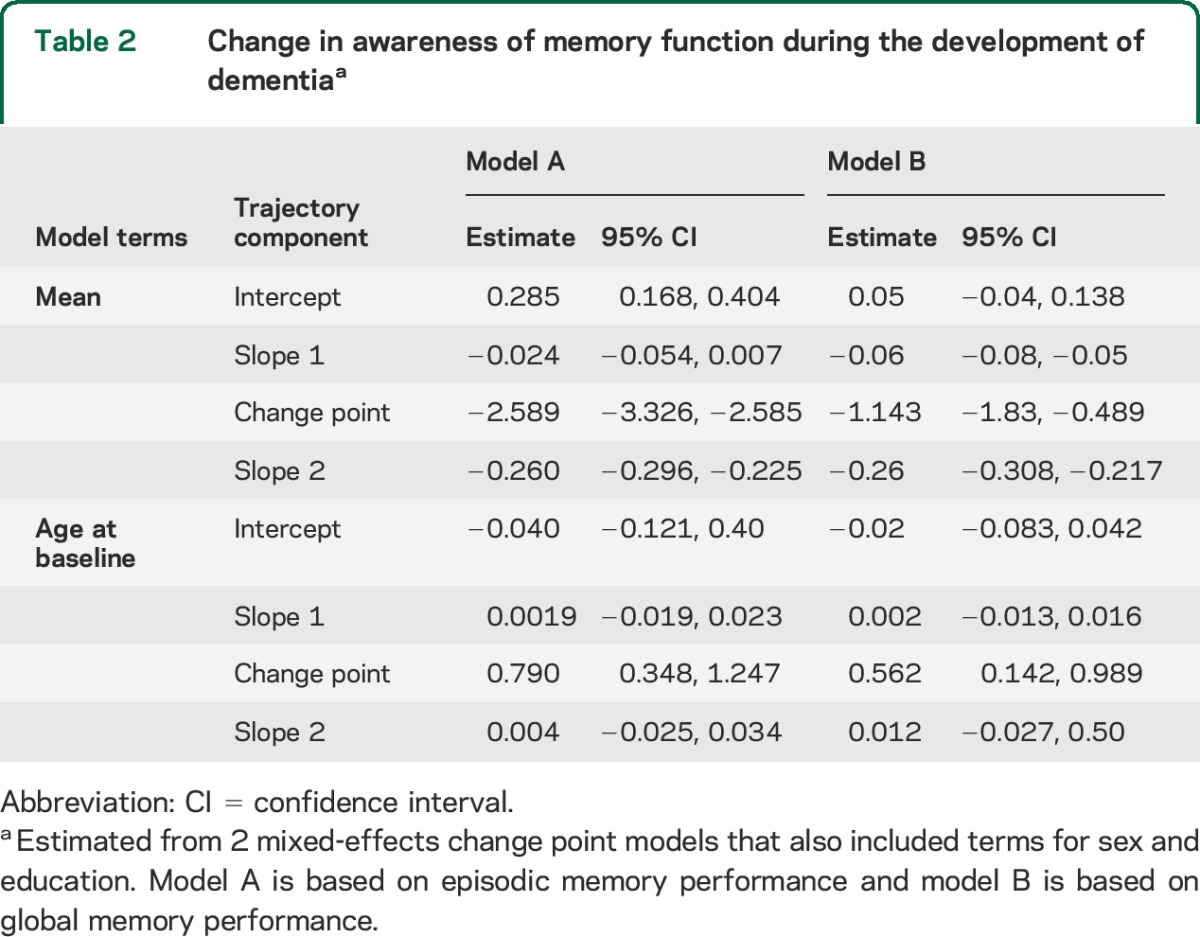
We defined memory performance with episodic memory tests, but participants' ratings may have taken other kinds of memory into consideration. Therefore, we created a global measure of memory performance by averaging measures of episodic memory, semantic memory, and working memory (baseline mean 0.129, SD 0.452, skewness −0.22). We regressed the global measure of memory performance on the composite memory rating and modeled the residuals. As shown in model B of table 2, results were similar to those obtained with episodic memory performance except that some decline in global memory awareness occurred prior to the change point.
Pathologic basis of unawareness of memory impairment.
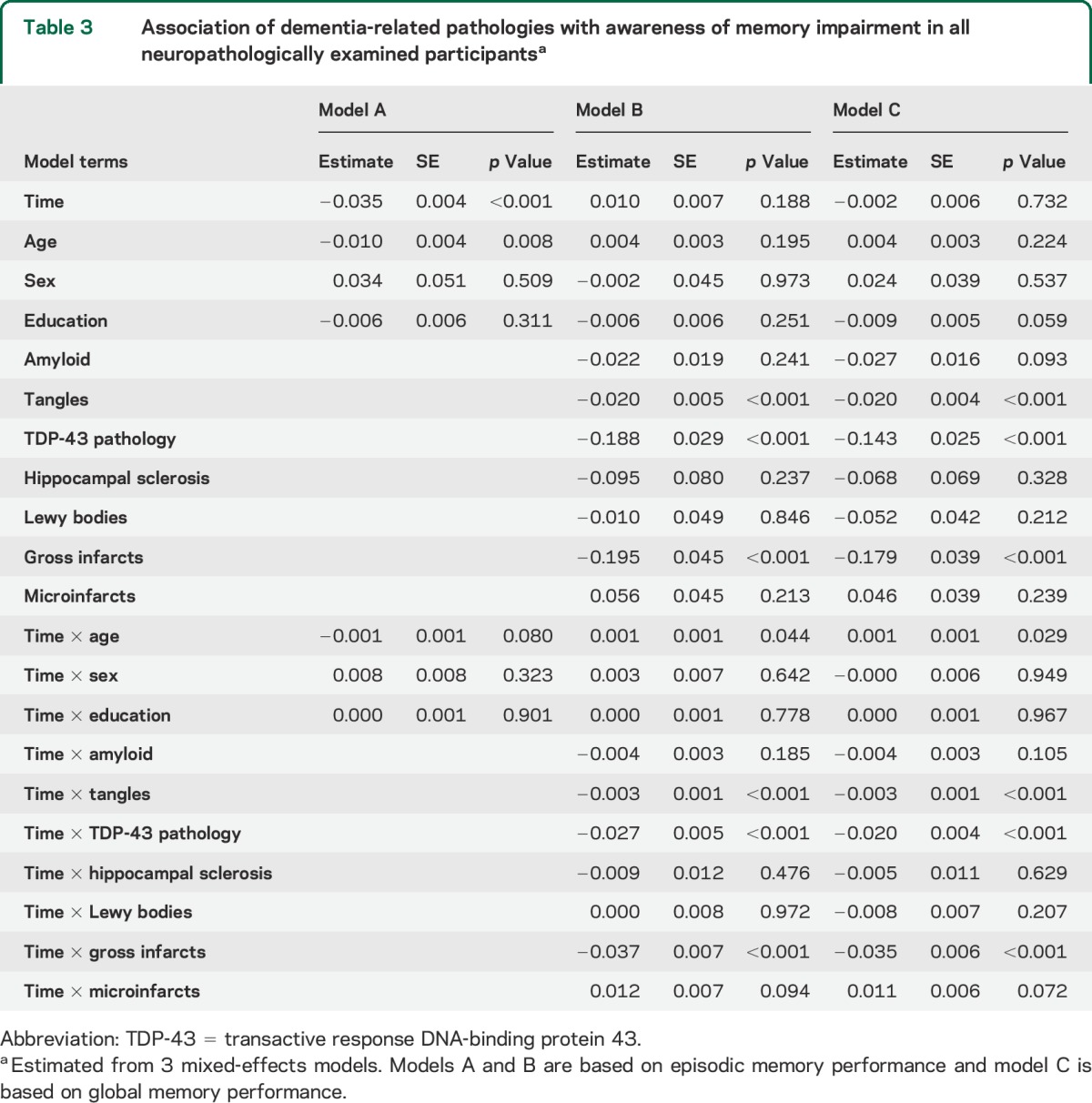
There were 385 individuals with no memory or cognitive impairment at baseline, at least 2 assessments of residual episodic memory awareness, and postmortem data on the 7 neuropathologic markers of interest. In a mixed-effects model adjusted for age at death, sex, and education, the measure of episodic memory awareness declined a mean of 0.035 unit per year (table 3, model A). When terms for the 7 pathologic measures were added (table 3, model B), 3 were associated with more rapid decline in episodic memory awareness: TDP-43 pathology, tangle density, and gross cerebral infarcts. With these associations accounted for, there was no change in episodic memory awareness, as shown by the term for time. Results were similar for the measure of global memory awareness (table 3, model C).
Table 3.
Association of dementia-related pathologies with awareness of memory impairment in all neuropathologically examined participantsa
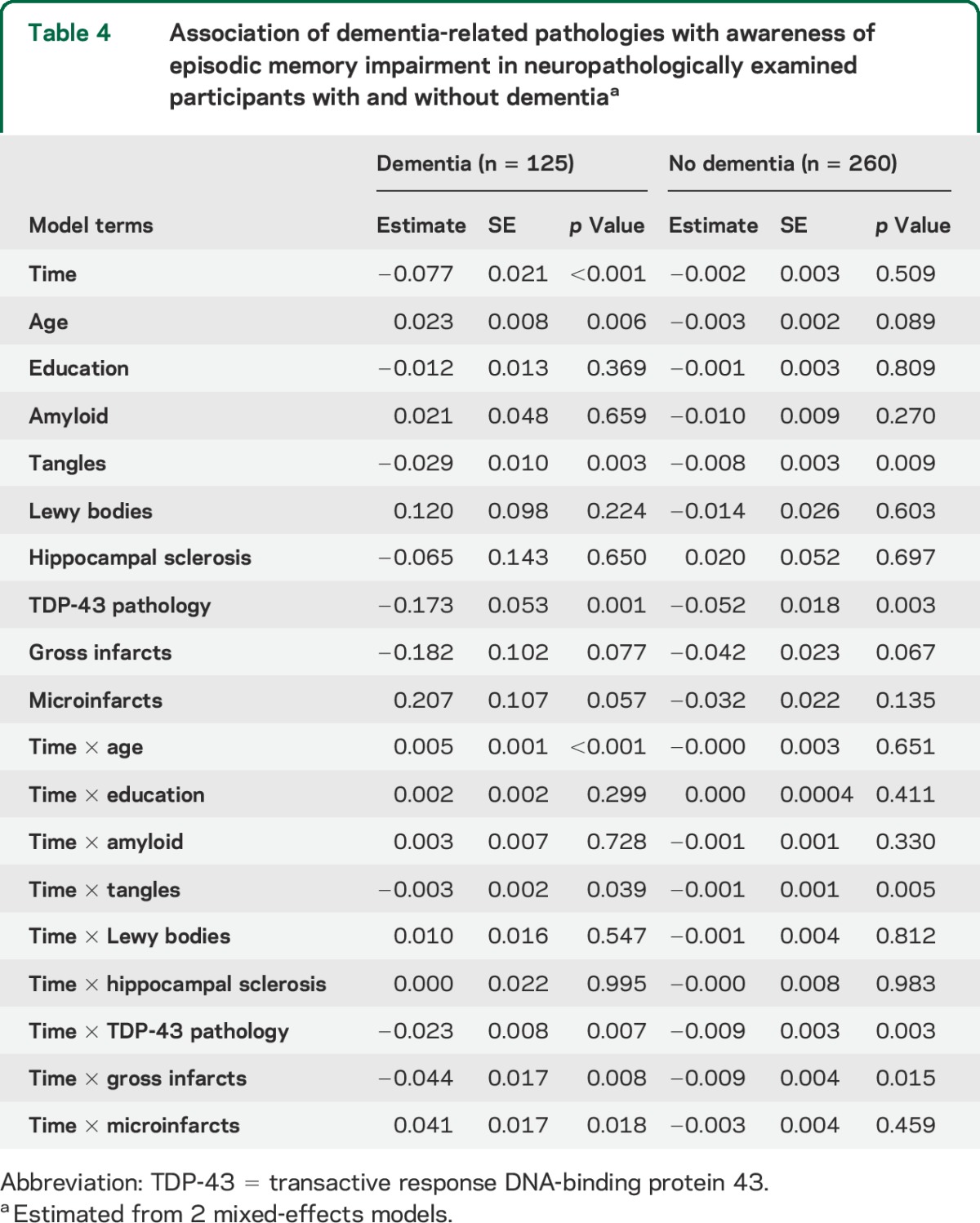
To determine whether the association of the pathologic markers with episodic memory unawareness was confined to persons with dementia, we separately analyzed those who died with (n = 125) and without (n = 260) dementia (table 4). TDP-43 pathology, tangle density, and gross infarcts were each associated with declining awareness of episodic memory impairment in each subgroup, though the associations were stronger in the dementia subgroup.
Table 4.
Association of dementia-related pathologies with awareness of episodic memory impairment in neuropathologically examined participants with and without dementiaa
DISCUSSION
The current analyses are based on older people with no memory or cognitive impairment at study onset who annually rated their memory ability and completed a battery of memory performance tests. In those who developed incident dementia, awareness of memory impairment began to decline a mean of about 2–3 years before dementia onset. In those who died and underwent a brain autopsy, multiple dementia-related pathologies were associated with decline in memory awareness and after adjustment for the pathologic markers, there was no decline in memory awareness. The results suggest that declining awareness of memory impairment is an essentially inevitable manifestation of late-life dementia.
Knowledge about unawareness of memory impairment in dementia is mainly based on cross-sectional or brief longitudinal studies of prevalent dementia. This research has suggested that unawareness of memory impairment is a variable feature of the dementia syndrome, perhaps explained partly by dementia severity1–4 but also associated with dementia type,34,35 brain regions affected,36,37 and psychosocial factors.38,39 In contrast, we followed individuals who were initially cognitively healthy and subsequently developed dementia during a mean of more than a decade of observation, allowing us to track awareness of memory function as memory abilities deteriorated. Although individual differences were apparent in the onset and progression of unawareness of memory impairment, virtually all affected persons manifested unawareness at some point during the disease course. Among persons who died and underwent a neuropathologic examination, decline in memory awareness was associated with multiple dementia-related pathologies, and no change in memory awareness was observed after controlling for these pathologies. These observations suggest that unawareness of amnestic dysfunction is part of the natural history of late-life dementia and is driven by accumulation of dementia-related pathologies.
Because most previous longitudinal research has been based on prevalent dementia, it has been uncertain when unawareness of memory impairment develops during the temporal course of dementia. We found that memory awareness began to decline a mean of 2.6 years before dementia was diagnosed. This is after several years of memory decline and suggests that clinically manifest unawareness of memory impairment typically becomes apparent around the time that dementia is diagnosed. This impairment in awareness is why in clinical classification of dementia, a history of cognitive decline must sometimes be based on expert judgment or a knowledgeable informant rather than self-report.
An unexpected finding was that decline in awareness of memory impairment in dementia began earlier in younger than older persons. The reason for this is uncertain, but it likely reflects a general awareness that memory loss is more normative at older ages rather than an age-related difference in awareness of one's own memory loss.
Unawareness of memory impairment was associated with some dementia-related pathologies but not others. TDP-43 pathology has previously been shown to have a selective association with episodic memory impairment.21 Tangles21,40 and gross cerebral infarcts40 have been associated with impairment in multiple cognitive domains, including episodic memory. The associations of hippocampal sclerosis and microinfarcts with memory awareness may have been diminished by their associations with TDP-43 pathology and gross infarcts, respectively. Much of the contribution of Lewy bodies to cognitive impairment may occur after awareness of memory impairment is diminished.40
Strengths and limitations of this study should be noted. Participants were followed at regular intervals for several years with high rates of participation in follow-up and brain autopsy, minimizing the likelihood that selective attrition affected results. By focusing on persons who were initially cognitively healthy and later developed dementia, we were able to link the development of memory awareness to the natural history of dementia. The availability of postmortem data from a uniform neuropathologic examination allowed us to show that unawareness of memory impairment is linked to the accumulation of dementia-related pathologies. The primary limitation is that participants were selected, underscoring the importance of replicating these results in other cohorts. The extent to which these findings apply to awareness of other dementia signs is also uncertain.
GLOSSARY
- CA
cornu ammonis
- TDP-43
transactive response DNA-binding protein 43
AUTHOR CONTRIBUTIONS
Drafting/revising manuscript for content: Dr. Wilson, Dr. Boyle, Dr. Yu, Dr. Barnes, J. Sytsma, Dr. Buchman, Dr. Bennett, Dr. Schneider. Study concept or design: Dr. Wilson, Dr. Boyle, Dr. Yu, Dr. Barnes, J. Sytsma, Dr. Buchman, Dr. Bennett, Dr. Schneider. Analyses or interpretation of the data: Dr. Wilson, Dr. Boyle, Dr. Yu, Dr. Barnes, J. Sytsma, Dr. Buchman, Dr. Bennett, Dr. Schneider. Acquisition of data: Dr. Barnes, Dr. Bennett, Dr. Schneider. Statistical analysis: Dr. Yu. Study supervision or coordination: Dr. Boyle, Dr. Barnes, Dr. Bennett, Dr. Schneider. Obtaining funding: Dr. Boyle, Dr. Barnes, Dr. Bennett, Dr. Schneider.
STUDY FUNDING
Supported by the National Institute on Aging (R01AG17917, P30AG10161, R01AG15819, R01AG34374, R01AG42210) and the Illinois Department of Public Health. The funding organizations had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
DISCLOSURE
R. Wilson serves as a Consulting Editor for Aging, Neuropsychology, and Cognition, Psychology and Aging, and Neuropsychology; has served as a consultant for Pain Therapeutics, Inc.; and receives research support from the NIH (P30AG010161 [coinvestigator], RF1AG015819 [coinvestigator], R01AG017917 [coinvestigator], R01AG034374 [coinvestigator], R01AG039478 [coinvestigator], R01AG036042 [coinvestigator], R01AG036836 [coinvestigator], R01AG041797 [coinvestigator], R01AG042210 [coinvestigator], and R01NR013151 [coinvestigator]), the Alzheimer's Association (NIRGD-11-205469), and Zinfandel Pharmaceuticals. P. Boyle receives research support from the NIH (R01AG034374 [principal investigator], R01AG034119 [coinvestigator], R01AG033678 [principal investigator], and R01AG040039 [coinvestigator]). L. Yu receives research support from the NIH (R01AG038651 [biostatistician], R01AG017917 [biostatistician], U18NS082140 [biostatistician], RF1AG015819 [biostatistician], R01AG036042 [biostatistician], U01AG046152 [biostatistician], R01AG033678 [biostatistician], U01AG032984 [biostatistician], and R01DK099269 [biostatistician]) and the Shapiro Foundation. L. Barnes receives research support from the NIH (R01AG022018 [principal investigator], P20MD006886 [principal investigator], and P30AG010161 [co-core leader]) and Zinfandel Pharmaceuticals. J. Sytsma receives research support from the NIH (P30AG010161 [research assistant], R01AG017917 [research assistant], R01NS078009 [research assistant], and K23AG040625-S1 [research assistant]). A. Buchman receives research support from the NIH (R01NS078009 [principal investigator], R01AG043379 [principal investigator], R01AG017917 [coinvestigator], P30AG010161 [coinvestigator], R01AG022018 [coinvestigator], R01AG040039 [coinvestigator], and P20MD006886 [coinvestigator]), the Canadian Institutes of Health Research (MOP-12593), and the Shapiro Foundation. D. Bennett serves on the editorial board of Neurology®; has served as a consultant to Schering-Plough Corp., Mediation, Inc., and the Gerson Lehrman Group; and receives research support from Danone Inc., Zinfandel Pharmaceuticals, the NIH (R01AG017917 [principal investigator], RF1AG015819 [principal investigator], R01AG036042 [principal investigator], U01AG032984 [co–principal investigator, leader of epidemiological cohort studies], R01HL096944 [coinvestigator], R01AG033678 [coinvestigator], R01AG034374 [coinvestigator], R01AG022018 [coinvestigator], R01AG034119 [coinvestigator], P30AG010161 [principal investigator–administrative core leader, Religious Orders Study core leader], U01AG046152 [coinvestigator], R01AG040039 [coinvestigator], R01AG039478 [coinvestigator], R01AG038651 [coinvestigator], R01AG036836 [coinvestigator], R01AG041797 [coinvestigator], R01AG042210 [coinvestigator], P20MD006886 [coinvestigator], R01NS078009 [coinvestigator], R01AG043617 [coinvestigator], R01AG043975 [coinvestigator], U18NS082140 [coinvestigator], R01NS082416 [coinvestigator], R01NS084965 [coinvestigator], R01NS086736 [coinvestigator], R01DK099269 [coinvestigator], R01AG046174 [coinvestigator], R01AG048015 [coinvestigator], P01AG014449 [coinvestigator]), and the Illinois Department of Public Health. J. Schneider has served as a consultant to Navidea Biopharmaceuticals and Genentech USA and receives research support from NIH (R01AG042210 [principal investigator], P30AG010161 [core leader], R01HL096944 [neuropathologist], R01AG039478 [neuropathologist], R01AG017917 [neuropathologist], RF1AG015819 [neuropathologist], R01AG022018 [neuropathologist], R01AG036042 [neuropathologist], R01AG040039 [neuropathologist], R01AG036836 [neuropathologist], R01AG034374 [neuropathologist], U01AG046152 [neuropathologist], R01AG043379 [neuropathologist], R01NS078009 [neuropathologist], R01AG043975 [neuropathologist], R01AG033678 [neuropathologist], R21ES021290 [neuropathologist] R01NS084965 [neuropathologist], R01AG043617 [neuropathologist], R56NS08967 [neuropathologist], R01AG038651 [neuropathologist], R01DK099269 [neuropathologist], and P01AG014449 [neuropathologist]). Go to Neurology.org for full disclosures.
REFERENCES
- 1.McDaniel KD, Edland SD, Heyman A. Relationship between level of insight and severity of dementia in Alzheimer disease. Alzheimer Dis Assoc Disord 1995;9:101–104. [DOI] [PubMed] [Google Scholar]
- 2.McGlynn SM, Kaszniak AW. When metacognition fails: impaired awareness of deficit in Alzheimer's disease. J Cogn Neurosci 1991;3:183–187. [DOI] [PubMed] [Google Scholar]
- 3.Zanetti O, Vallotti B, Frisoni GB, et al. Insight in dementia: when does it occur? Evidence for a nonlinear relationship between insight and cognitive status. J Gerontol B Psychol Sci Soc Sci 1999;54:100–106. [DOI] [PubMed] [Google Scholar]
- 4.Sevush S, Leve N. Denial of memory deficit in Alzheimer's disease. Am J Psychiatry 1993;150:748–751. [DOI] [PubMed] [Google Scholar]
- 5.Reed BR, Jagust WJ, Coulter L. Anosognosia in Alzheimer's disease: relationships to depression, cognitive function, and cerebral perfusion. J Clin Exp Neuropsychol 1993;15:231–244. [DOI] [PubMed] [Google Scholar]
- 6.Starkstein SE, Vazquez S, Migliorelli R, Teson A, Sabe L, Leiguarda R. A single-photon emission computed tomographic study of anosognosia in Alzheimer's disease. Arch Neurol 1995;52:415–420. [DOI] [PubMed] [Google Scholar]
- 7.Farias ST, Mungas D, Jagust W. Degree of discrepancy between self and other-reported everyday functioning by cognitive status: dementia, mild cognitive impairment, and health elders. Int J Geriatr Psychiatry 2005;20:827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasterling JJ, Seltzer B, Watrous WE. Longitudinal assessment of deficit unawareness in Alzheimer's disease. Neuropsychiatry Neuropsychol Behav Neurol 1997;10:197–202. [PubMed] [Google Scholar]
- 9.Starkstein SE, Chemerinski E, Sabe L, et al. Prospective longitudinal study of depression and anosognosia in Alzheimer's disease. Br J Psychiatry 1997;171:47–52. [DOI] [PubMed] [Google Scholar]
- 10.Derouesne C, Thibault S, Lagha-Pierucci S, Baudouin-Madec V, Ancri D, Lacomblez L. Decreased awareness of cognitive deficits in patients with mild dementia of the Alzheimer type. Int J Geriatr Psychiatry 1999;14:1019–1030. [PubMed] [Google Scholar]
- 11.Akai T, Hanyu H, Sakurai H, Sato T, Iwamoto T. Longitudinal patterns of unawareness of memory deficits in mild Alzheimer's disease. Geriatr Gerontol Int 2009;9:16–20. [DOI] [PubMed] [Google Scholar]
- 12.Kiyak HA, Teri L, Borson S. Physical and functional health assessment in normal aging and in Alzheimer's disease: self-reports vs family reports. Gerontologist 1994;34:324–330. [DOI] [PubMed] [Google Scholar]
- 13.Sevush S. Relationship between denial of memory deficit and dementia severity in Alzheimer disease. Neuropsychiatry Neuropsychol Behav Neurol 1999;12:88–94. [PubMed] [Google Scholar]
- 14.Clare L, Wilson BA. Longitudinal assessment of awareness in early-stage Alzheimer's disease using comparable questionnaire-based and performance-based measures: a prospective one-year follow-up study. Aging Ment Health 2006;10:156–165. [DOI] [PubMed] [Google Scholar]
- 15.Clare L, Nelis SM, Martyr A, et al. Longitudinal trajectories of awareness in early-stage dementia. Alzheimer Dis Assoc Disord 2012;26:140–147. [DOI] [PubMed] [Google Scholar]
- 16.Sousa MF, Santos RL, Nogueira ML, et al. Awareness of disease is different for cognitive and functional aspects in mild Alzheimer's disease: a one-year observation study. J Alzheimers Dis 2015;43:905–913. [DOI] [PubMed] [Google Scholar]
- 17.Vogel A, Waldorff FB, Waldemar G. Longitudinal changes in awareness over 36 months in patients with mild Alzheimer's disease. Int Psychogeriatr 2015;27:95–102. [DOI] [PubMed] [Google Scholar]
- 18.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Curr Alzheimer Res 2012;9:628–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res 2012;9:646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. The Minority Aging Research Study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res 2012;9:734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson RS, Yu L, Trojanowski JQ, et al. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol 2013;70:1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 1984:34:939–944. [DOI] [PubMed] [Google Scholar]
- 23.Barnes LL, Schneider JA, Boyle PA, Bienias JL, Bennett DA. Memory complaints are related to Alzheimer disease pathology in older persons. Neurology 2006;67:1581–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging 2002;17:179–193. [PubMed] [Google Scholar]
- 25.Wilson RS, Barnes LL, Bennett DA. Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Neuropsychol 2003;25:634–642. [DOI] [PubMed] [Google Scholar]
- 26.Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc 2005;11;400–407. [PubMed] [Google Scholar]
- 27.Wilson RS, Aggarwal NT, Barnes LL, Bienias JL, Mendes de Leon CF, Evans DA. Biracial population study of mortality in mild cognitive impairment and Alzheimer disease. Arch Neurol 2009;66:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider JA, Li JL, Li Y, Wilson RS, Kordower JH, Bennett DA. Substantia nigra tangles are related to gait impairment in older persons. Ann Neurol 2006;59:166–173. [DOI] [PubMed] [Google Scholar]
- 29.Wilson RS, Capuano AW, Boyle PA, et al. Clinical-pathologic study of depressive symptoms and cognitive decline in old age. Neurology 2014;83:702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol 2004;61:378–384. [DOI] [PubMed] [Google Scholar]
- 31.Neumann M, Kwong LK, Lee EB, et al. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol 2009;117:137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gelman A, Carli JB, Stern HS, Rubin DB. Bayesian Data Analysis. New York: Chapman and Hall; 2004. [Google Scholar]
- 33.Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: evolution, critique and future directions. Stat Med 2009;28:3049–3067. [DOI] [PubMed] [Google Scholar]
- 34.Williamson C, Alcantar O, Rothlind J, Cahn-Weiner D, Miller BL, Rosen HJ. Standardised measurement of self-awareness deficits in FTD and AD. J Neurol Neurosurg Psychiatry 2010;81:140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosen HJ, Alcantar O, Zakrzewski J, Shimamura AP, Neuhaus J, Miller BL. Metacognition in the behavioral variant of frontotemporal dementia and Alzheimer's disease. Neuropsychology 2014;28:436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ries ML, McLaren DG, Bendlin BB, et al. Medial prefrontal functional connectivity: relation to memory self-appraisal accuracy in older adults with and without memory disorders. Neuropsychologia 2012;50:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shany-Ur T, Lin N, Rosen HJ, Sollberger M, Miller BL, Rankin KP. Self-awareness in neurodegenerative disease relies on neural structures mediating reward-driven attention. Brain 2014;137:2368–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogel A, Hasselbalch SG, Gade A, Ziebell M, Waldemar G. Cognitive and functional neuroimaging correlate for anosognosia in mild cognitive impairment and Alzheimer's disease. Int J Geriatr Psychiatry 2005;20:238–246. [DOI] [PubMed] [Google Scholar]
- 39.Clare L, Nelis SM, Martyr A, et al. The influence of psychological, social and contextual factors on the expression and measurement of awareness in early-stage dementia: testing a biopsychosocial model. Int J Geriatr Psychiatry 2012:27:167–177. [DOI] [PubMed] [Google Scholar]
- 40.Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology 2010;75:1070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]


