Abstract
Objective:
We investigated the associations of morning and evening salivary cortisol levels with regional brain volumes and cognitive functioning in community-dwelling older persons without dementia.
Method:
From the Age, Gene/Environment Susceptibility (AGES)–Reykjavik Study, we included 4,244 persons without dementia (age 76 ± 5 years, 58% women) who had 1.5T brain MRI, assessment of cognitive functioning, and saliva collected at home 45 minutes after awakening and at night. Linear regression analysis was used to estimate the cross-sectional relationship among cortisol levels, brain volumes, and cognitive functioning, adjusting for covariates.
Results:
Higher evening cortisol was associated with smaller total brain volume (highest vs lowest tertile −16.0 mL; 95% confidence interval −19.7 to −12.2 mL, adjusted for age, sex, education, intracranial volume, smoking, steroid use, white matter lesions, and brain infarcts on MRI). The smaller volumes were observed in all brain regions, but were significantly smaller in gray matter than in white matter regions. Poorer cognitive functioning across all domains was also associated with higher evening cortisol. Higher levels of morning cortisol were associated with slightly greater normal white matter volume and better processing speed and executive functioning, but not with gray matter volume or with memory performance.
Conclusions:
In older persons, evening and morning cortisol levels may be differentially associated with tissue volume in gray and white matter structures and cognitive function. Understanding these differential associations may aid in developing strategies to reduce the effects of hypothalamic-pituitary-adrenal axis dysfunction on late-life cognitive impairment.
With increasing age, the prevalence of dementia, including Alzheimer disease (AD), increases exponentially.1 At older age, depressive symptoms are also common,2 often persist over years,3 and are frequently comorbid with cognitive impairment and dementia.4,5 Prospective studies indicate that depression may also increase the risk of future dementia.6,7 Still, the neurobiologic substrate of the relation between depression and AD remains poorly understood. Several studies have found structural brain abnormalities to be associated with late-life depression, including hippocampal volume reduction.8,9 Also, it has often been hypothesized that persistently high cortisol levels, often observed in depression,10 have neurotoxic effects on the hippocampus.6 Because the hypothalamic-pituitary-adrenal (HPA) axis is inhibited by the hippocampus,11 a cascade would occur in which age-associated hippocampal atrophy would lead to diminished ability to inhibit cortisol and, in turn, further atrophy of the hippocampus,12 eventually leading to development of clinical dementia. Functional changes of the HPA axis have been observed in late-life depression.13 However, relatively few studies have examined the association between HPA axis activity and hippocampal volumes at older age and findings have been inconsistent,14–17 possibly because different measures of cortisol (e.g., saliva, urine) and different time points (e.g., morning, evening) were used. Also, because the hippocampus is involved in regulating the HPA axis11 and the high expression of corticosteroid receptors in limbic areas,18 the majority of studies have only focused on the hippocampus and not on other brain regions. However, corticosteroid receptors are expressed throughout the brain18 and other brain regions may thus be affected. Further, morning and night levels may be differentially associated with brain volumes.17
We aimed to determine the association between morning and evening salivary cortisol levels and regional brain volumes in a large cohort of older persons without dementia. We also investigated the relationship with cognitive functioning to determine the potential clinical relevance of observed differences in brain volume.
METHODS
Participants.
Participants came from the Age, Gene/Environment Susceptibility (AGES)–Reykjavik Study, a single-center prospective population-based cohort study originating from the Reykjavik Study, as has been described elsewhere.19 The Reykjavik Study started in 1967 and included men and women born between 1907 and 1935 living in Reykjavik. From 2002 through 2006, 5,764 persons, randomly chosen from survivors of the Reykjavik Study, were included in the AGES-Reykjavik Study, aimed to investigate the contribution of genetic and environmental factors to (sub)clinical disease in older people. Assessments were performed at the Reykjavik research center and included questionnaires, clinical examinations, venipuncture, cognitive testing, and 1.5T MRI of the brain.
Standard protocol approvals, registrations, and patient consents.
The AGES-Reykjavik Study was approved by the Icelandic National Bioethics Committee (VSN: 00-063), the Icelandic Data Protection Authority, and the Institutional Review Board for the National Institute on Aging, NIH, USA. Written informed consent was obtained from all participants.
MRI protocol.
The MRI protocol has been described elsewhere.20 All participants without contraindications were eligible for brain MRI scan on a study-dedicated 1.5T Signa Twinspeed system (General Electric Medical Systems, Waukesha, WI). The image protocol included an axial T1-weighted 3D spoiled gradient echo sequence (time to echo [TE] 8 ms; repetition time [TR] 21 ms; flip angle [FA] 30°; field of view [FOV] 240 mm; matrix 256 × 256; slice thickness 1.5 mm); a fluid-attenuated inversion recovery (FLAIR) sequence (TE 100 ms; TR 8,000 ms; inversion time 2,000 ms; FA 90°; FOV 220 mm; matrix 256 × 256); a proton density/T2-weighted fast spin echo sequence (TE1 22 ms; TE2 90 ms; TR 3,220 ms; echo train length 8; FA 90°; FOV 220 mm; matrix 256 × 256); and a T2*-weighted gradient echo type echoplanar sequence (TE 50 ms; TR 3,050 ms; FA 90°; FOV 220 mm; matrix 256 × 256). The FLAIR, proton density/T2, and T2* sequences were acquired with 3-mm-thick interleaved slices. All images were acquired to give full brain coverage and slices were angled parallel to the anterior commissure–posterior commissure line.
Brain volumes, white matter lesions, and infarcts.
Regional brain volumes and CSF were segmented fully automatically with a modified algorithm as described previously.20 The pipeline is based on a multispectral tissue segmentation method that estimated volumes for 4 tissue classes: gray and white matter regions, white matter lesions (WML), and CSF. Gray matter, normal white matter, and WML volumes were summed to obtain total brain volume, and total brain volume and total CSF volume were summed to obtain total intracranial volume (ICV). Total brain volume was divided by ICV to obtain brain parenchymal fraction. Regional brain volumes were based on an anatomical and a regional probabilistic atlas, and were created from 314 participants of the AGES-Reykjavik Study.20 Infarct-like lesions in subcortical regions were defined as parenchymal defects not spreading into the cortex surrounded by an area of high signal intensity on FLAIR images and a diameter of ≥3 mm. Infarct-like lesions in the cortex or cerebellum did not have size criteria.21 For the analyses, we calculated Z scores of brain region volumes.
Cortisol levels.
Saliva samples were collected with the Salivette device (Sarstedt, Rommelsdorf, Germany). Participants were instructed to take the first sample the evening before the clinic visit, prior to going to sleep. The second sample was to be taken in the morning 45 minutes after awaking. Participants were instructed not to brush their teeth before saliva sampling, to allow at least 30 minutes to pass after eating or drinking before taking the evening sample, and to refrain from eating and drinking until after the morning sample was taken. Salivary cortisol was analyzed with a time-resolved immunoassay with fluorescence detection (Delfia; PerkinElmer, Waltham, MA) as described previously.22 Intra-assay and interassay variability were <10% and 12%, respectively. The lower detection limit was 0.43 nmol/L for a 50-μL salivary sample. Morning or evening cortisol values >100 nmol/L were considered unreliable23 and excluded (0.7% of morning samples and 0.5% of evening samples).
Assessment of dementia.
Dementia ascertainment was a 3-step protocol and has been described elsewhere.19,21 In brief, all participants underwent the Mini-Mental State Examination (MMSE) and the Digit Symbol Substitution test (DSST). Participants with MMSE <24 or DSST <18 had a diagnostic battery of neuropsychological tests and those with positive screen results on these tests were examined by a neurologist. Also, a proxy interview was administered regarding medical history, social, cognitive, and daily functioning changes of the participant. A consensus diagnosis according to international guidelines was made by a multidisciplinary panel including a neurologist, geriatrician, neuroradiologist, and neuropsychologist.
Cognition.
A battery of 6 different cognitive tests was administered to all participants24 and 3 cognitive domain scores were calculated: (1) memory composite score, which included immediate and delayed recall of a modified version of the California Verbal Learning Test; (2) processing speed composite score, which included the Stroop 1 and 2, the DSST, and the Figure Comparison Test; (3) executive function composite score, which included the Cambridge Neuropsychological Test Automated Battery Spatial Working Memory test (short version), the Digits Backward test, and Stroop 3. We computed composite measures by averaging the standardized Z scores across the tests in each domain.
Covariates.
Covariates assessed via questionnaires included age, sex, educational level (categorized into primary, secondary, and college/university education) being currently employed, having sleeping problems (early awakening or difficulty falling asleep), smoking history (current vs nonsmoker), alcohol intake (g/wk), physical activity (moderate/high vs never/rarely/occasionally in the past 12 months), and body mass index (BMI) (in kg/m2). Systolic and diastolic blood pressure was measured with a standard mercury sphygmomanometer. Diabetes mellitus was defined as use of blood glucose–lowering drugs or fasting blood glucose level ≥7.0 mmol/L or self-reported history of diabetes. Steroid use included glucocorticoids, inhaled steroids, and oral steroids as determined from medication bottles brought to the clinic. Depressive symptoms were assessed with the 15-item Geriatric Depression Scale25 and the Mini International Neuropsychiatric Interview was used to assess lifetime history of major depressive disorder (current and past).9,26
Study sample.
We had 4,244 persons for analysis. Of the initial 5,764 persons, 4,811 had brain MRI; 953 had missing MRI scans because of claustrophobia or refusal (n = 227), contraindications (n = 290), machine maintenance (n = 54), home visits (n = 382), and postprocessing failures (n = 59). Of the remaining 4,614 persons, those with dementia were excluded (n = 260) and 110 persons had missing morning and evening cortisol level.
Data analysis.
We used multiple imputation (AregImpute) (10 datasets) to deal with missing values (missingness of variables used in the analyses ranged from 0% to 9.3%) in the 4,244 persons (R version 2.13.1). We analyzed data with PASW version 20.0 (Chicago, IL) and SAS version 9.3 (SAS Institute, Cary, NC).
We used linear regression analyses and analysis of covariance to estimate associations of evening and morning cortisol (in tertiles) with brain tissue volumes and cognitive domain scores, respectively. We adjusted the analyses with evening cortisol for age, sex, education, smoking, steroid use, and when brain volume was the dependent variable for ICV, white matter lesion volume (natural log transformed), and brain infarcts (model 1). Analyses for morning cortisol were also adjusted for being currently employed and sleeping problems (model 1). In model 2, we also adjusted for systolic and diastolic blood pressure, BMI, diabetes mellitus, physical activity, alcohol intake, and depressive symptoms. We repeated the analyses after excluding persons who used steroids (n = 294), with mild cognitive impairment (n = 430), and with a lifetime diagnosis of major depressive disorder (n = 187), adjusting for covariates in model 1.
To examine whether specific brain tissue regions were significantly more affected than others, we identified, from visual inspection of graphs and tables, brain tissue regions that were potentially differentially affected by cortisol levels. These regions were entered into multilevel (mixed) regression models as correlated outcomes, adjusting for covariates used in model 1.
RESULTS
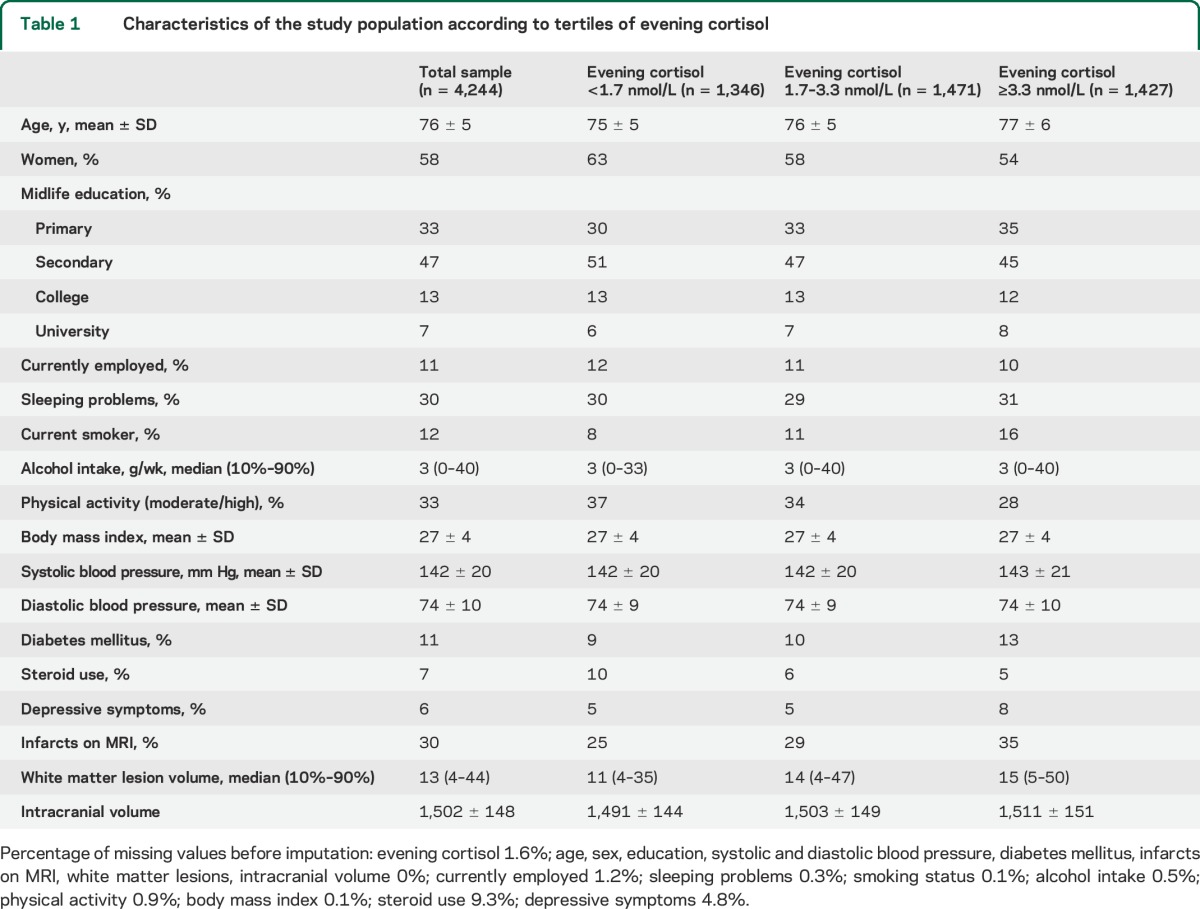
In the 4,244 participants, the mean morning cortisol level was 20 nmol/L (SD 13.5) and the median (10th–90th percentile) evening cortisol level was 2.3 nmol/L (0.9–6.9). Table 1 presents the baseline characteristics across tertiles of evening cortisol (for morning cortisol, see table e-1 on the Neurology® Web site at Neurology.org).
Table 1.
Characteristics of the study population according to tertiles of evening cortisol
Evening cortisol.
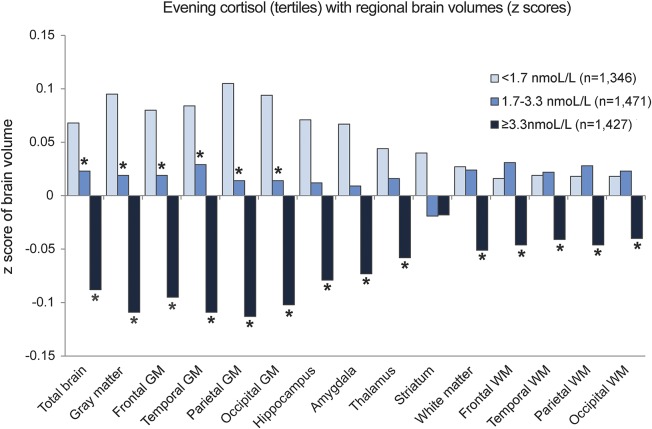
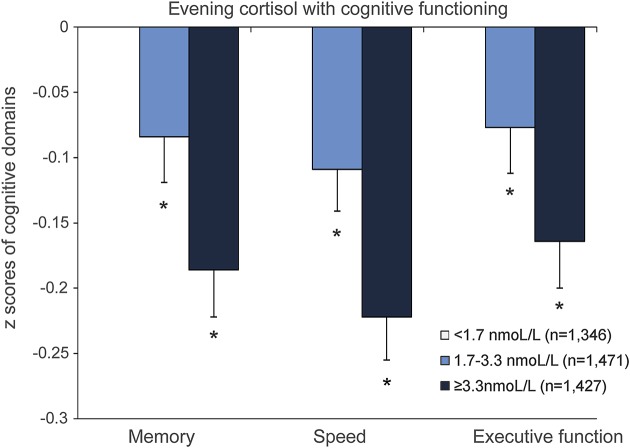
Higher evening cortisol levels were significantly associated with smaller total and regional brain volumes (figure 1; table e-2). The mean adjusted difference in total brain volume between the middle vs lowest tertile of evening cortisol was −4.6 mL (95% confidence interval [CI] −8.2 to −0.9 mL) and for the highest vs lowest tertile −16.0 mL (95% CI −19.7 to −12.2 mL) (model 1). After additional adjustment for BMI, systolic and diastolic blood pressure, diabetes mellitus, physical activity, alcohol intake, and depressive symptom score, the associations attenuated but remained statistically significant (middle vs lowest tertile: −3.7 mL; 95% CI −7.3 to −0.11 mL; highest vs lower tertile: −13.8 mL; 95% CI −17.5 to −10.1 mL). All regional brain volumes, except for the striatum, were significantly smaller in the highest tertile of evening cortisol compared to the lowest tertile (figure 1, table e-2). Further, results from the multilevel models showed that gray matter was significantly (p < 0.001) more affected by higher evening cortisol levels than white matter. Results of the regression analyses with cognitive domains were consistent with those for brain volumes: with increasing levels of cortisol, cognitive functioning was poorer in all domains (figure 2; for estimates and 95% CI, see figure legend).
Figure 1. Adjusted mean total and regional tissue volumes (z scores) according to tertiles of evening cortisol.
Volumes are adjusted for intracranial volume, age, sex, educational level, current smoking status, any steroid use, white matter lesion volume (natural log transformed), and infarcts on MRI. *Significantly (p < 0.05) different from lowest tertile of evening cortisol. GM = gray matter; WM = white matter.
Figure 2. Adjusted mean cognitive domain z scores according to tertiles of evening cortisol.
Cognitive scores are adjusted for age, sex, educational level, current smoking status, alcohol intake, any steroid use, depressive symptoms, body mass index, systolic and diastolic blood pressure, diabetes mellitus, and physical activity. *Significantly (p < 0.05) different from lowest tertile of evening cortisol (reference value = 0, not visible in figure). Adjusted mean z score differences for memory: middle vs lowest tertile −0.084 (95% confidence interval [CI] −0.152 to −0.015); highest vs lowest tertile −0.186 (95% CI −0.256 to −0.116); for speed: middle vs lowest tertile −0.109 (95% CI −0.172 to −0.045); highest vs lowest tertile −0.222 (95% CI −0.288 to −0.157); and for executive functioning: middle vs lowest tertile −0.077 (95% CI −0.145 to −0.009); highest vs lowest tertile −0.164 (95% CI −0.235 to −0.094).
Morning cortisol.
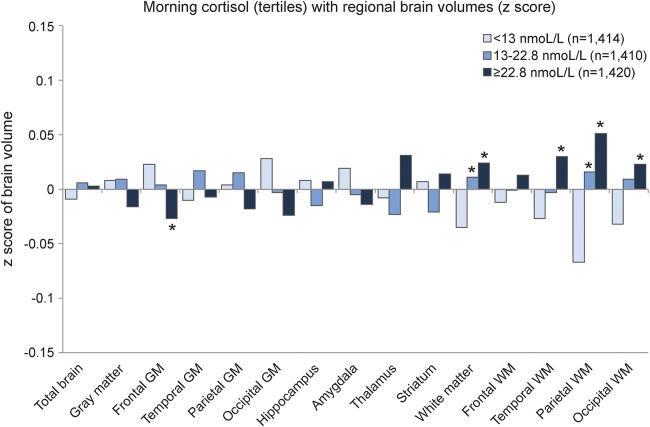
Morning cortisol was not associated with total brain volume. We observed a small but statistically significant difference between lower and higher morning cortisol levels in normal white matter volume (total white matter and white matter in the temporal, parietal, and occipital lobe), indicating that higher morning cortisol levels were associated with slightly less white matter volume reduction (figure 3; table e-3). The gray matter regions were not associated with morning cortisol, except for frontal gray matter, where the highest tertile of morning cortisol was associated with slightly smaller frontal gray matter (figure 3; table e-3). The results of the multilevel models indicated that white matter was significantly (p = 0.008) more affected by morning cortisol than gray matter. Results of the regression analyses with cognitive domains were in the same direction as the brain volumes: no significant association was found with memory, while higher morning cortisol levels were associated with better speed and executive functioning (figure 4; for estimates and 95% CI, see figure legend).
Figure 3. Adjusted mean total and regional tissue volumes (z scores) according to tertiles of morning cortisol.
Volumes are adjusted for intracranial volume, age, sex, educational level, current smoking status, any steroid use, sleeping problems, being currently employed, white matter lesion volume (natural log transformed), and infarcts on MRI. *Significantly (p < 0.05) different from lowest tertile of morning cortisol. GM = gray matter; WM = white matter.
Figure 4. Adjusted mean cognitive domain z scores according to tertiles of morning cortisol.
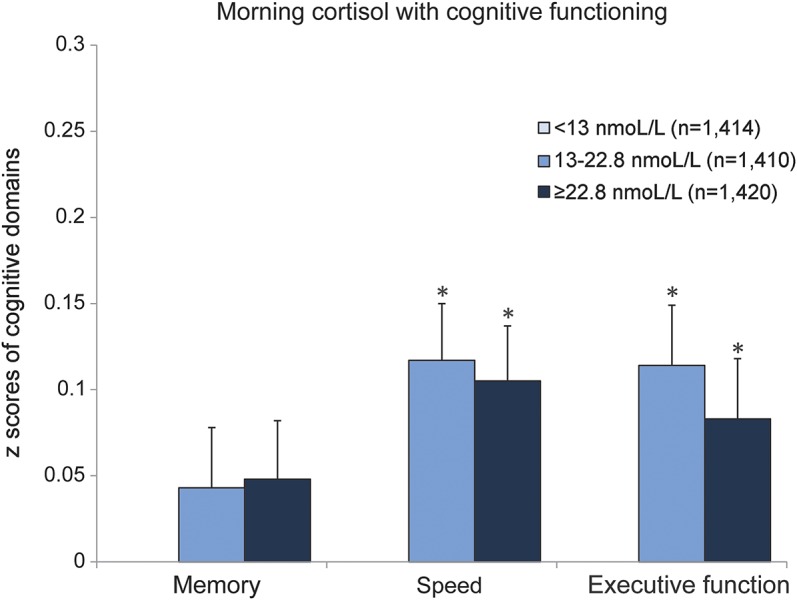
Cognitive scores are adjusted for age, sex, educational level, current smoking status, alcohol intake, any steroid use, sleeping problems, being currently employed, depressive symptoms, body mass index, systolic and diastolic blood pressure, diabetes mellitus, and physical activity. *Significantly (p < 0.05) different from lowest tertile of morning cortisol (reference value = 0, not visible in figure). Adjusted mean z score differences for memory: Middle vs lowest tertile 0.043 (95% confidence interval [CI] −0.026 to 0.112); highest vs lowest tertile 0.048 (95% CI −0.019 to 0.115); for speed: middle vs lowest tertile 0.117 (95% CI 0.054 to 0.181); highest vs lowest tertile 0.105 (95% CI 0.042 to 0.168); and for executive functioning: middle vs lowest tertile 0.114 (95% CI 0.046 to 0.181); highest vs lowest tertile 0.083 (95% CI 0.015 to 0.152).
Results did not materially change when persons who used steroids, with mild cognitive impairment, or with a lifetime diagnosis of major depressive disorder were excluded (data not shown).
DISCUSSION
We found that higher levels of salivary evening cortisol were associated with smaller volume in multiple brain regions, significantly more in gray than in white matter, as well as with poorer cognitive performance in multiple domains. Morning cortisol levels were not associated with gray matter volume in the majority of regions studied or with memory performance, but they were associated with slightly larger volumes in several white matter regions as well as with better processing speed and executive functioning.
To date, most of the studies that investigated the relation between hippocampal volume and HPA axis activity were performed in (younger) adults. Some studies reported more hippocampal atrophy with higher cortisol,27–29 while others reported no association.14,15,30 The largest study in older persons published to date reported that increased evening cortisol levels and increased awakening levels after dexamethasone were associated with reduced volume of the hippocampus, while the cortisol awakening response was not associated with hippocampal volume.17 Although we did not have data on awakening levels after dexamethasone, our findings are consistent with that study but also extend those findings, because we report more widespread gray matter involvement. Moreover, we observed differential gray and normal white matter involvement, where higher evening cortisol levels were more strongly associated with gray than white matter volume reduction, and higher morning cortisol levels were associated with larger white but not gray matter volumes. Interpretation of this differential association is not clear-cut, one reason being that no other study reported on gray–white matter differences.
Interestingly, the results for cognitive functioning were in the same direction as for the brain volumes: higher evening cortisol levels were associated with poorer cognitive performance, while higher morning cortisol levels were associated with better cognitive performance. Furthermore, evening cortisol levels were associated with poorer performance across all cognitive domains, consistent with the diffuse and widespread volume reductions in gray and white matter, while morning cortisol was more selectively associated with speed and executive functioning.
Since this was a cross-sectional study, we do not know what came first: HPA axis dysregulation or brain volume reduction. Possibly, as has often been hypothesized, our findings reflect a cascade in which age-associated brain volume reductions lead to diminished ability to inhibit cortisol and, in turn, further brain atrophy.12 Biological evidence exists that corticosteroid receptors in the brain inhibit the HPA axis and that depletion of these receptors results in hypersecretion of glucocorticoids.31 With aging, brain volume reduction will thus lead to reduction in corticosteroid receptors. Although it has been suggested that the hippocampal glucocorticoid receptors are most vulnerable to this downregulation,31 glucocorticoid receptors are expressed not only in the hippocampus but throughout the brain18 and our data suggest that this phenomenon is not specific to the hippocampus but is more diffuse throughout the gray matter. The stronger relationship with gray matter than white matter may be consistent with this because gray matter volume loss starts at an earlier age and progresses more rapidly than white matter volume loss.32 Alternatively, HPA axis dysregulation may have come first. Indirect evidence for this originates from studies that found that cumulative physiologic wear and tear or allostatic load increases risk of cognitive decline.33,34 It is also possible that HPA axis dysregulation resulted from repeated depressive episodes earlier in life10 which, in turn, led to brain volume reduction and poorer cognitive performance. However, our results were similar after excluding participants with a lifetime diagnosis of major depressive disorder, making this explanation less likely. With respect to the morning cortisol levels, severe life events are associated with relative hypersecretion if the stress occurred recently, whereas relative hyposecretion of morning cortisol has been observed with longer passing of the stressor.35 If our findings were explained by accumulation of chronic stress before the brain atrophy progressed, one would expect to find a relationship between low cortisol levels in the morning and brain atrophy. Although we did find that lower levels of morning cortisol were significantly associated with more white matter reduction, the effect estimates were very small, and the relevance of this finding is uncertain. Clearly, to determine the direction of association, prospective studies are needed that integrate measures of chronic stress, HPA axis activity, and brain volumes.
A second limitation is that cortisol was assessed during one day. To reduce measurement error, it has been recommended to sample saliva over several days,36 but increasing the sample size, as we did in our cohort, will also improve reliability, and may thus have balanced this. Third, because we had only 1 morning sample, the awakening response could not be determined. The mean levels were, however, remarkably similar to those reported in other studies,17,37,38 and the single morning measurement itself is thus likely to be reliable.
Strengths of this study are the population-based design, the large sample size, the exclusion of participants with dementia, and the adjustment for potential confounders. Second, the measures of several brain regions and the saliva sampling in the morning as well as in the evening allowed us to examine differential associations between HPA axis activity and brain volumes. Third, the inclusion of measures of cognitive functioning allowed us to examine whether the associations with MRI measures were also reflected in functioning. The combination of these factors makes this study unique.
In older persons, evening and morning cortisol levels may be differentially associated with volume reductions in gray and white matter and cognitive functioning. Understanding these differential associations may aid in developing strategies to reduce the effects of HPA axis dysfunction on late-life cognitive impairment.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the employees of the Icelandic Heart Preventive Clinic (Hjartavernd) for their contribution to the data collection; the participants for their participation; and Dr. B. Yu for his statistical expertise with respect to the multilevel models.
GLOSSARY
- AD
Alzheimer disease
- AGES–Reykjavik Study
Age, Gene/Environment Susceptibility–Reykjavik Study
- BMI
body mass index
- CI
confidence interval
- DSST
Digit Symbol Substitution test
- FA
flip angle
- FLAIR
fluid-attenuated inversion recovery
- FOV
field of view
- HPA
hypothalamic-pituitary-adrenal
- ICV
intracranial volume
- MMSE
Mini-Mental State Examination
- TE
time to echo
- TR
repetition time
- WML
white matter lesions
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. M.I. Geerlings was involved in the analysis and interpretation of data and writing of the manuscript. S. Sigurdsson was involved in the collection of data and critical review of the final manuscript. M.E. Garcia and G. Eiriksdottir were involved in the study coordination and critical review of the final manuscript. Dr. Harris and Dr. Gudnason were involved in the study design and critical review of the final manuscript. Dr. L.J. Launer was involved in the study design, analysis and interpretation of data, and writing of the final manuscript.
STUDY FUNDING
Supported by NIH (N01-AG-12100), NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and Althingi (the Icelandic Parliament). Dr. M.I. Geerlings was supported by a grant from the Netherlands Organization for Scientific Research (NWO: project no. 917-66-311) and a grant (Internationalisation program) from the University Medical Center Utrecht, the Netherlands.
DISCLOSURE
M.I. Geerlings was supported by a grant from the Netherlands Organization for Scientific Research (917-66-311), the Netherlands, and a grant (Internationalisation program) from the University Medical Center Utrecht, the Netherlands. S. Sigurdsson, G. Eiriksdottir, M. Garcia, T. Harris, V. Gudnason, and L. Launer report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013;80:1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beekman AT, Deeg DJ, van Tilburg T, Smit JH, Hooijer C, Van Tilburg W. Major and minor depression in later life: a study of prevalence and risk factors. J Affect Disord 1995;36:65–75. [DOI] [PubMed] [Google Scholar]
- 3.Beekman AT, Geerlings SW, Deeg DJ, et al. The natural history of late-life depression: a 6-year prospective study in the community. Arch Gen Psychiatry 2002;59:605–611. [DOI] [PubMed] [Google Scholar]
- 4.Steffens DC, Potter GG. Geriatric depression and cognitive impairment. Psychol Med 2008;38:163–175. [DOI] [PubMed] [Google Scholar]
- 5.Starkstein SE, Jorge R, Mizrahi R, Robinson RG. The construct of minor and major depression in Alzheimer's disease. Am J Psychiatry 2005;162:2086–2093. [DOI] [PubMed] [Google Scholar]
- 6.Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol 2011;7:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF., III Late-life depression and risk of vascular dementia and Alzheimer's disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry 2013;202:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sexton CE, Mackay CE, Ebmeier KP. A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. Am J Geriatr Psychiatry 2013;21:184–195. [DOI] [PubMed] [Google Scholar]
- 9.Geerlings MI, Sigurdsson S, Eiriksdottir G, et al. Associations of current and remitted major depressive disorder with brain atrophy: the AGES-Reykjavik Study. Psychol Med 2013;43:317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 2011;73:114–126. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev 1991;12:118–134. [DOI] [PubMed] [Google Scholar]
- 12.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 2000;57:925–935. [DOI] [PubMed] [Google Scholar]
- 13.Belvederi Murri M, Pariante C, Mondelli V, et al. HPA axis and aging in depression: systematic review and meta-analysis. Psychoneuroendocrinology 2014;41:46–62. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry 2004;161:2081–2090. [DOI] [PubMed] [Google Scholar]
- 15.MacLullich AM, Deary IJ, Starr JM, Ferguson KJ, Wardlaw JM, Seckl JR. Plasma cortisol levels, brain volumes and cognition in healthy elderly men. Psychoneuroendocrinology 2005;30:505–515. [DOI] [PubMed] [Google Scholar]
- 16.Bruehl H, Wolf OT, Convit A. A blunted cortisol awakening response and hippocampal atrophy in type 2 diabetes mellitus. Psychoneuroendocrinology 2009;34:815–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knoops AJ, Gerritsen L, van der Graaf Y, Mali WP, Geerlings MI. Basal hypothalamic pituitary adrenal axis activity and hippocampal volumes: the SMART-Medea study. Biol Psychiatry 2010;67:1191–1198. [DOI] [PubMed] [Google Scholar]
- 18.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci 2005;6:463–475. [DOI] [PubMed] [Google Scholar]
- 19.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility–Reykjavik study: multidisciplinary applied phenomics. Am J Epidemiol 2007;165:1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sigurdsson S, Aspelund T, Forsberg L, et al. Brain tissue volumes in the general population of the elderly: the AGES-Reykjavik Study. Neuroimage 2012;59:3862–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saczynski JS, Sigurdsson S, Jonsdottir MK, et al. Cerebral infarcts and cognitive performance: importance of location and number of infarcts. Stroke 2009;40:677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dressendorfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol 1992;43:683–692. [DOI] [PubMed] [Google Scholar]
- 23.Gerritsen L, Geerlings MI, Beekman AT, Deeg DJ, Penninx BW, Comijs HC. Early and late life events and salivary cortisol in older persons. Psychol Med 2010;40:1569–1578. [DOI] [PubMed] [Google Scholar]
- 24.Vidal JS, Sigurdsson S, Jonsdottir MK, et al. Coronary artery calcium, brain function and structure: the AGES-Reykjavik Study. Stroke 2010;41:891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982;17:37–49. [DOI] [PubMed] [Google Scholar]
- 26.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- 27.Lupien SJ, de Leon M, de Santi S, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci 1998;1:69–73. [DOI] [PubMed] [Google Scholar]
- 28.Wolf OT, Convit A, de Leon MJ, Caraos C, Qadri SF. Basal hypothalamo-pituitary-adrenal axis activity and corticotropin feedback in young and older men: relationships to magnetic resonance imaging-derived hippocampus and cingulate gyrus volumes. Neuroendocrinology 2002;75:241–249. [DOI] [PubMed] [Google Scholar]
- 29.Vythilingam M, Vermetten E, Anderson GM, et al. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol Psychiatry 2004;56:101112. [DOI] [PubMed] [Google Scholar]
- 30.Colla M, Kronenberg G, Deuschle M, et al. Hippocampal volume reduction and HPA system activity in major depression. J Psychiatr Res 2007;41:553–560. [DOI] [PubMed] [Google Scholar]
- 31.Sapolsky RM, Plotsky PM. Hypercortisolism and its possible neural bases. Biol Psychiatry 1990;27:937–952. [DOI] [PubMed] [Google Scholar]
- 32.Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology 2005;64:1032–1039. [DOI] [PubMed] [Google Scholar]
- 33.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci USA 2001;98:4770–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE. Allostatic load as a predictor of functional decline: MacArthur studies of successful aging. J Clin Epidemiol 2002;55:696–710. [DOI] [PubMed] [Google Scholar]
- 35.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull 2007;133:25–45. [DOI] [PubMed] [Google Scholar]
- 36.Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state- and trait components. Psychoneuroendocrinology 2007;32:80–86. [DOI] [PubMed] [Google Scholar]
- 37.Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress 2004;7:29–37. [DOI] [PubMed] [Google Scholar]
- 38.Vreeburg SA, Kruijtzer BP, van Pelt J, et al. Associations between sociodemographic, sampling and health factors and various salivary cortisol indicators in a large sample without psychopathology. Psychoneuroendocrinology 2009;34:1109–1120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.