Abstract
Introduction
The production of excessive amounts of nitric oxide (NO) through inducible nitric oxide synthase (iNOS) contributes to organ injury, inflammation, and mortality after shock. Resveratrol (RSV) is a natural polyphenol that decreases shock-induced hepatic injury and inflammation. We hypothesized that RSV would mediate these effects by reducing hepatocyte iNOS production.
Methods
Rat hepatocytes were isolated, cultured with varying concentrations of RSV, and then stimulated to induce iNOS with interleukin-1 and interferon. Induction of iNOS protein was measured by western blot, iNOS mRNA by polymerase chain reaction, and NO production was measured by culture supernatant nitrite. Activation of intracellular signaling pathways involving Akt, JNK, and NF-κB were measured by western blot using isoform-specific antibodies.
Results
RSV significantly reduced the expression of iNOS mRNA, protein, and supernatant nitrite in a dose-dependent manner. Our prior work has demonstrated that Akt and JNK both inhibit hepatic iNOS production, while NF-κB increases iNOS expression. Analysis of signaling pathways in this study demonstrated that RSV increased JNK phosphorylation but decreased Akt phosphorylation and increased NF-κB activation.
Conclusion
RSV decreases cytokine-induced hepatocyte iNOS expression, possibly through upregulation of the JNK signaling pathway. RSV merits further investigation to determine its mechanism as a compound that can decrease inflammation following shock.
INTRODUCTION
Hemorrhagic shock and traumatic injury induce a profound inflammatory response that results in systemic inflammation, organ and tissue injury, and the multiple organ failure syndrome (1). In addition to its systemic effects, shock-induced inflammation complicates recovery by prolonging hospitalization, increasing complications, and contributing to the dysfunction of other organ systems (2). However, current therapies to treat trauma-induced inflammation and organ dysfunction are limited.
Resveratrol (RSV) is a polyphenolic phytoestrogen that is a naturally occurring compound found in berries, nuts, grapes, and red wine (3). A popular nutritional supplement, RSV has antioxidant, anti-proliferative, and anti-inflammatory properties that are thought to be partially responsible for the health benefits of these foods (4). Additionally, RSV has been shown to reduce the deleterious effects of inflammation and hemorrhagic shock (5–8). These salutary effects extend to the liver, where RSV decreases hepatic injury after hemorrhagic shock (5, 6), decreases hepatic hypoxic injury (7), and attenuates hepatic pro-inflammatory signaling (5, 6, 8). While several anti-inflammatory effects of RSV have been identified, the mechanism for the beneficial effects of RSV during shock and inflammation remains unknown.
Hepatic dysfunction contributes significantly to the morbidity and mortality of trauma and hemorrhagic shock (9). Increased production of nitric oxide (NO) exacerbates hepatic inflammation and injury after hemorrhage (10). In particular, NO production from upregulation of the inducible nitric oxide synthase (iNOS) is associated with increased shock-induced hepatic injury (11). Reducing the excessive amounts of NO released after hemorrhagic shock decreases hepatic injury and inflammation (10–12), suggesting that methods to diminish excess NO may reduce organ injury and tissue damage after trauma. Since the upregulation of iNOS is a major source of deleterious NO production, the inhibition of iNOS activation is a promising strategy to modulate the inflammatory response to shock. We know that several intracellular signaling pathways regulate hepatocyte iNOS expression, including nuclear factor κB (NF-κB), Protein kinase B/Akt, and c-Jun N-terminal kinase (JNK) (13–16). RSV has been shown to activate several of these intracellular signaling pathways, including Akt (6, 8, 17). Since RSV activates Akt, and we know that Akt downregulates iNOS expression and activation (14, 15), we hypothesized that RSV would decrease inflammation-induced hepatocyte iNOS activation.
METHODS
Hepatocyte Isolation and Culture
Primary rat hepatocytes were isolated and cultured from male Sprague Dawley rats (250–300 g) (Harlan Sprague Dawley, Madison, WI) using the Seglen technique as previously described (18). All experiments were performed in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by the University of Louisville’s Institutional Animal Care and Use Committee. In brief, the liver was perfused with collagenase, homogenized, and hepatocytes separated from nonparenchymal cells by differential centrifugation. Viability was assessed by Trypan blue exclusion and was consistently greater than 92%. Hepatocytes were plated onto collagen-coated dishes using standard media containing 5% calf serum as described (18), incubated in 5% CO2 at 37° for 4 hours, and then washed to remove nonadherent cells with fresh media added. After overnight incubation, the hepatocytes were washed again with Phosphate Buffered Saline (PBS) and the culture conditions established. Williams medium E, Interferon-gamma (IFN), and Interleukin-1β (IL-1β) were from Invitrogen Life Science Inc. (Carlsbad, CA). Unless otherwise noted, Resveratrol and all other materials were purchased from Sigma (St. Louis, MO).
Western Blot Analysis
Proteins from total cellular lysates were collected at the indicated time point by scraping cells into ice-cold PBS in 500 ml lysis buffer containing protease inhibitors and separated on 8% polyacrylamide gels. The proteins were transferred onto nitrocellulose membranes and nonspecific binding was blocked with Tris buffered saline (50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 0.1% Tween 20) and 5% nonfat dry milk for 1 hour. The membranes were incubated with primary antibodies, washed, incubated with secondary antibodies, and then the bands were visualized with chemiluminescence. Antibodies to iNOS were from BD Bioscience (Billerica MA) and antibodies to phosphorylated and total Akt, IκB, and actin were from Cell Signaling Technologies (Danvers MA). Antibodies to phosphorylated and total JNK were from Santa Cruz Biotechnology Inc (Dallas, TX). Band densities were assessed by NIH ImageJ imaging software (ImageJ, National Institutes of Health, Bethesda MD) and normalized to actin as described (18).
Polymerase Chain Reaction
We measured iNOS mRNA expression using the Applied Biosystems StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA). Total RNA was isolated from cultured hepatocytes at the indicated time point using TRIzol Reagent (Life Technologies, Grand Island, NY) according to the manufacturer’s protocol. Complementary DNA was generated using Taqman Reverse Transcription Reagents, and Taqman Gene Expression Assays (Applied Biosystems) were used as probe and primer sets. Samples were tested in triplicate and 18S rRNA was used as an internal control. The comparative Cycle Threshold method was used to quantify gene expression using StepOne software (Applied Biosystems) according to the manufacturer’s instructions.
Nitrite Measurement and Hepatocyte Viability Assay
Supernatant nitrite (NO2−) accumulation was used as an index of NO production and was measured using the Griess reaction as described (18). Hepatocyte viability was assessed using the 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (14).
Statistical Analysis
Culture conditions were performed in duplicate or triplicate and each experiment was repeated using separate rat hepatocyte isolations to ensure reproducibility. Data are presented as the mean ± SEM and statistical significance was determined using Analysis of Variance and Fisher’s test as indicated. Statistical significance was set at p<0.05.
RESULTS
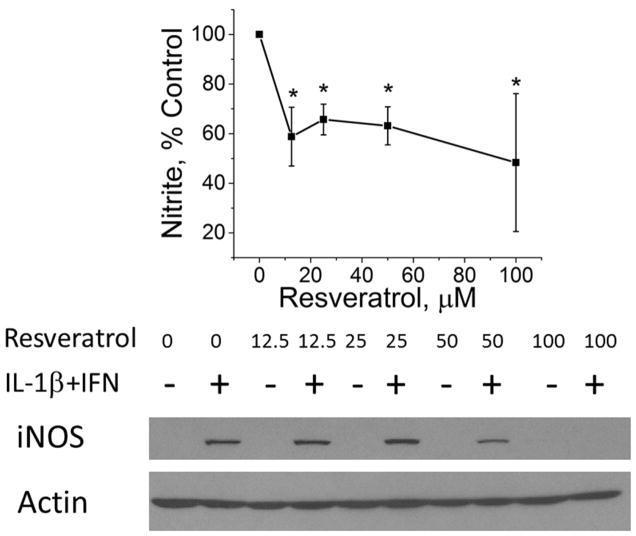
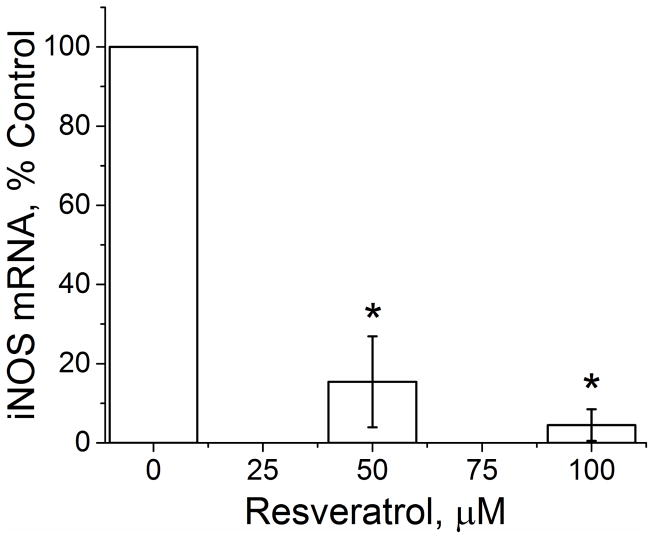
Interleukin-1β (IL-1β) combined with IFN is the most effective cytokine combination for inducing hepatocyte iNOS expression (19, 20). Therefore, to evaluate the effect of resveratrol on iNOS expression, cultured hepatocytes were stimulated with IL-1β/IFN in the presence and absence of different concentrations of RSV. We then assessed iNOS activation at the level of NO production, iNOS protein expression, and iNOS gene expression. NO production was quantified with supernatant nitrite concentrations, and RSV produced a significant decrease in cytokine-stimulated NO production (Figure 1A). The decrease in iNOS activation was also reflected by dose-dependent decreases in hepatocyte iNOS protein expression as measured by Western blot (Figure 1A). iNOS gene expression as measured by PCR was decreased by both 50 μM and 100 μM RSV (Figure 1B). These data suggest that RSV regulates iNOS activation through proximal events involving iNOS gene and protein expression. Hepatocyte viability after 24 hours of RSV treatment was measured using the MTT assay. MTT absorbance was slightly reduced at 100 μM RSV in both media-treated and cytokine-stimulated hepatocytes but was similar to control hepatocytes at all other doses tested (Figure 2).
Figure 1.
Effect of RSV on iNOS production in both control media and IL-1β+IFN-stimulated hepatocytes. A) Cell culture supernatants were collected and nitrite levels measured using the Greiss reaction (top). Western blot analysis of total cellular protein isolates were probed for iNOS (bottom). The blot shown is representative of 4 separate experiments. B) iNOS mRNA expression as measured by PCR. *indicates p<0.05 compared to control.
Figure 2.
Effect of Resveratrol on Hepatocyte MTT absorbance. Hepatocytes were cultured for 24 hours in media supplemented with the indicated concentration of resveratrol in the presence (filled columns) or absence (open columns) of IL-1β+IFN. MTT absorbance was measured as described. *p<0.05 vs 0 μM RSV without IL-1β+IFN, #p<0.05 vs 0 μM RSV with IL-1β+IFN.
RSV regulates a number of pathways that modulate cell surface receptor-mediated intracellular signaling, including PI3K/Akt (6), 5′ AMP-activated protein kinase (AMPK) (4), and MAPK (8, 21). We have previously shown that increased Akt activation downregulates iNOS expression in hepatocytes (14–16, 20), and other investigators have suggested that changes in Akt activation mediate the effects of RSV on organ function after hemorrhagic shock (6). We therefore examined the hypothesis that RSV decreased iNOS expression by increasing Akt activation by measuring Akt phosphorylation using Western blot. RSV had no significant effect on total Akt in hepatocytes cultured with or without cytokines (Figure 3B). In hepatocytes cultured with media alone, there was a low level of Akt phosphorylation that was decreased further by RSV (Figure 3). In hepatocytes cultured with cytokines to induce iNOS, RSV decreased IL-1β+IFN-induced Akt phosphorylation (Figure 3), making it unlikely that RSV-induced changes in Akt activation are responsible for the effect of RSV on hepatocyte NO production.
Figure 3.

Resveratrol decreases Akt phosphorylation. A) Densitometry results of Western blot analysis for phosphorylated Akt (pAkt) with varying concentrations of RSV in control media (open bars) and cytokine-stimulated (closed bars) hepatocytes. Proteins were collected after 60 minutes of culture and probed for pAkt. B) A representative Western blot demonstrating total and phosphorylated Akt. *indicates p<0.05 compared to IL-1β+IFN-treated hepatocytes
NF-κB is another important signaling pathway in iNOS expression and NF-κB activation induces iNOS expression and NO production (13, 19). RSV decreases NF-κB in some models of inflammation (22), and decreased NF-κB activation would be expected with the RSV-induced decrease in iNOS expression seen earlier. To assess NF-κB activation, we performed Western blot with antibodies against phosphorylated and total forms of the inhibitory IκB protein that binds the p50/p65 NF-κB subunits in the cytosol (26). In hepatocytes cultured with media alone, IL-1β+IFN increased phosphorylated IκB and decreased total IκB, consistent with increased NF-κB activation. RSV had little effect on either phosphorylated IκB (Figure 4A) or total IκB (Figure 4B) in the absence of cytokines. In hepatocytes stimulated to produce iNOS with IL-1β+IFN, RSV caused a small but statistically insignificant increase in phosphorylated IκB after 1 hour of culture compared to cells cultured with IL-1β+IFN alone (Figure 4). However, RSV decreased total IκB levels in cytokine-stimulated hepatocytes, consistent with increased activation of the NF-κB pathway. Since NF-κB is a positive regulator of iNOS expression, these data suggest that RSV-induced changes in NF-κB activation do not mediate the inhibitory effect of resveratrol on hepatocyte iNOS expression.
Figure 4.
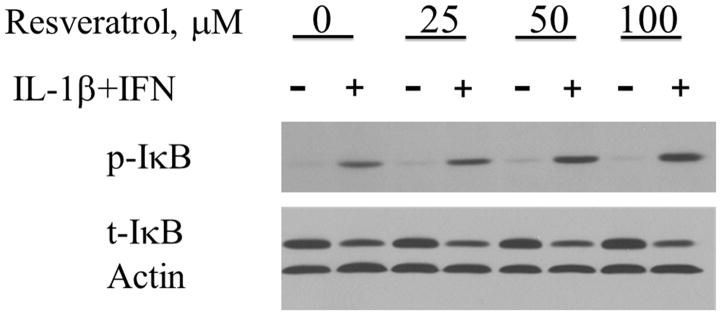
Resveratrol increases NF-κB activation in cultured hepatocytes. Densitometry analysis of Western blots of cellular proteins collected after 60 minutes of culture after stimulation with IL-1β+IFN and probed for phosphorylated IκB (pIκB) (A) and total IκB (tIκB) (B). A representative Western blot is shown (C). *indicates p<0.05 compared to control media hepatocytes. #indicates p<0.05 compared to IL-1β+IFN-treated hepatocytes.
We have previously shown that JNK activation downregulates hepatocyte iNOS expression (16). JNK is a member of the MAPK family of signaling proteins, and RSV regulates MAPK activation (8). We therefore evaluated the effect of RSV on JNK signaling. RSV had little effect on total JNK in hepatocytes cultured either with or without cytokines (Figure 5B). At baseline and with low doses of RSV (0 and 25 μM), cells induced to produce iNOS with IL-1β+IFN demonstrated a slight, non-significant increase in JNK phosphorylation compared to non-induced cells. However, with higher doses of RSV (50 or 100 μM), a statistically significant increase in JNK phosphorylation was observed (Figure 5). The increase in JNK phosphorylation corresponded to the decrease in iNOS mRNA that was also seen at the same RSV doses (Figure 1). These data suggest that RSV-mediated JNK activation contributes to the suppression of cytokine-stimulated hepatocyte iNOS expression. To test this hypothesis further, we cultured hepatocytes with the JNK inhibitor SP 600125 in the presence and absence of cytokines and RSV. There was no effect of SP 600125 on supernatant nitrite in hepatocytes cultured without cytokines (data not shown). In the presence of IL-1β+IFN, SP 600125 increased cytokine-stimulated nitrite accumulation, consistent with our previous work (Figure 6) (16). SP600125 also reversed the inhibition of nitrite accumulation produced by RSV in iNOS-producing hepatocytes (Figure 6).
Figure 5.

Resveratrol increases JNK activation. A) Densitometry analysis of Western blots probed for phosphorylated JNK from control media (open bars) and IL-1β+IFN-treated hepatocytes (filled bars). A representative Western blot is shown (B). *indicates p<0.05 compared to IL-1β+IFN-treated hepatocytes.
Figure 6.
Inhibition of JNK blocks the effect of Resveratrol. Hepatocytes were cultured for 24 hours in standard media or media supplemented with Resveratrol (100 μM) in the presence of plain media (open column), IL-1β+IFN (filled column), or IL-1β+IFN plus the JNK inhibitor SP 600125 (10 μM). Supernatant nitrite was analyzed by the Griess reaction as described. *represents p<0.05 compared to media-treated cells with IL-1β+IFN. # represents p<0.05 vs Resveratrol-treated hepatocytes stimulated with IL-1β+IFN.
DISCUSSION
Excessive NO production from hepatic iNOS contributes to the profound systemic inflammatory response and the morbidity/mortality that follows traumatic injury and hemorrhagic shock (10, 12). Inhibition of iNOS therefore represents a potential strategy to mitigate hepatic inflammation following injury. In this study, we evaluated the effect of RSV on hepatocyte expression of iNOS and NO production with the hypothesis that RSV mediates its anti-inflammatory effects on the liver through decreased expression of iNOS. Our results demonstrate that RSV decreased NO production, iNOS mRNA levels, and iNOS protein expression in cytokine-induced hepatocytes in vitro. Hepatocyte NO production is primarily regulated at the level of gene expression (23). While further elaboration of the mechanism of action for RSV is necessary, the reduction of iNOS mRNA and protein in this study suggests that RSV likely controls NO production by decreasing iNOS mRNA transcription.
Initial exploration of the pathways through which RSV modulates iNOS expression indicates a possible role for JNK, a member of the mitogen-activated protein kinase (MAPK) superfamily. While we have previously identified Akt and NF-κB as important pathways in the transcriptional control of iNOS expression (13–15), these pathways did not appear to be responsible for iNOS regulation by RSV in this study. On the other hand, RSV regulates MAPK activation (8), and we have previously reported that JNK signaling plays an important role in the inhibition of iNOS expression by cyclic AMP (16). The results from the current study are consistent with these prior findings and demonstrate that activation of JNK correlates with decreased iNOS expression and NO production. RSV activates a number of intracellular pathways in addition to those studied above and has additional effects on cell metabolism that can potentially be important in our results. RSV activates the 5′ AMP-activated protein kinase (AMPK) (4, 27), activates the deacetylase SIRT1 (28), phosphorylates GSK3β (29), and has general antioxidant properties (4, 5). Any of these effects of RSV could contribute to the findings seen in the current study. Additional work will be required to further clarify the mechanisms mediating the relationship between RSV and iNOS expression.
RSV is a polyphenol and is structurally similar to estrogen and estrogen-like plant derivatives (21). RSV can bind to estrogen receptors and may protect tissues against damage following hemorrhagic shock through an estrogen receptor-related pathway (5). We have previously shown that 17-β estradiol (E2) decreased cytokine-stimulated NO production and iNOS expression although the exact signaling mechanism is unclear (18). Estrogen activates MAPK signaling cascades that contribute to a cellular protective response following shock including increased activation of JNK (24). RSV may activate JNK through E2 receptors to decrease iNOS expression but this hypothesis will need to be validated through further experiments.
RSV is a well-tolerated compound that has been tested in multiple clinical trials with several studying cancer or modification of cardiovascular risk factors. It has a satisfactory safety profile when administered at fairly high doses (30). A recent study reviewing 30 clinical trials included no examples of RSV use to reduce inflammation following injury in human subjects, even though its ability to decrease inflammation in animal models of hemorrhage is well established (5–7, 25). This represents an area of future investigation that has not been examined previously.
This study is not without limitations. As with any in vitro study, extrapolation of these findings to an in vivo model must be done with caution. RSV decreased nitrite production at the 12.5 μM dose but its most substantial effects were at 100 μM. This higher dose was associated with decreased MTT absorbance, suggesting potential toxicity. However, hepatocytes can proliferate in vitro when properly induced and RSV decreases cell proliferation (31). Determining whether the decreased MTT absorbance seen in Figure 2 is due to increased hepatocyte injury/death or due to decreased proliferation will require further study. RSV is highly absorbed and rapidly metabolized and therefore has rather low bioavailability (32). Whether in vivo levels of RSV can be achieved that correspond to the levels used in this study could be debated. However, several RSV metabolites are biologically active and cumulative levels of RSV and its metabolites can reach 50 μM in the peripheral circulation of healthy human volunteers (30). Levels achieved in the portal circulation and prior to hepatic metabolization have never been measured. The doses used in this study approximate those achieved in vivo and therefore appear to be clinically relevant.
CONCLUSION
Sustained hepatic inflammation and injury following trauma and hemorrhagic shock is mediated in part by excessive NO production from iNOS. Resveratrol decreased cytokine-induced hepatocyte iNOS expression and activation, possibly through upregulation of the JNK signaling pathway. Resveratrol merits further investigation to define its mechanisms of action in regulating metabolism and hepatic function associated with shock and traumatic injury.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-55664 (BGH) and T35 DK07293: Summer Endocrine Research Training Program (MW, AG)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Minei JP, Cuschieri J, Sperry J, Moore EE, West MA, Harbrecht BG, et al. The changing pattern and implications of multiple organ failure after blunt injury with hemorrhagic shock. Critical care medicine. 2012;40(4):1129–35. doi: 10.1097/CCM.0b013e3182376e9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zenati MS, Billiar TR, Townsend RN, Peitzman AB, Harbrecht BG. A brief episode of hypotension increases mortality in critically ill trauma patients. The Journal of trauma. 2002;53(2):232–6. doi: 10.1097/00005373-200208000-00007. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 3.Hiroto Y, Tadokoro K, Tsuda T, Nakazono E, Ohnaka K, Takayanagi R, et al. Resveratrol, a phytoestrogen found in red wine, down-regulates protein S expression in HepG2 cells. Thrombosis research. 2011;127(1):e1–7. doi: 10.1016/j.thromres.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Chung JH, Manganiello V, Dyck JR. Resveratrol as a calorie restriction mimetic: therapeutic implications. Trends in cell biology. 2012;22(10):546–54. doi: 10.1016/j.tcb.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu HP, Hsu JC, Hwang TL, Yen CH, Lau YT. Resveratrol attenuates hepatic injury after trauma-hemorrhage via estrogen receptor-related pathway. Shock (Augusta, Ga) 2008;30(3):324–8. doi: 10.1097/SHK.0b013e318164f013. [DOI] [PubMed] [Google Scholar]
- 6.Yu HP, Yang SC, Lau YT, Hwang TL. Role of Akt-dependent up-regulation of hemeoxygenase-1 in resveratrol-mediated attenuation of hepatic injury after trauma hemorrhage. Surgery. 2010;148(1):103–9. doi: 10.1016/j.surg.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Powell RD, Swet JH, Kennedy KL, Huynh TT, McKillop IH, Evans SL. Resveratrol attenuates hypoxic injury in a primary hepatocyte model of hemorrhagic shock and resuscitation. The journal of trauma and acute care surgery. 2014;76(2):409–17. doi: 10.1097/TA.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 8.Zong Y, Sun L, Liu B, Deng YS, Zhan D, Chen YL, et al. Resveratrol inhibits LPS-induced MAPKs activation via activation of the phosphatidylinositol 3-kinase pathway in murine RAW 264.7 macrophage cells. PloS one. 2012;7(8):e44107. doi: 10.1371/journal.pone.0044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harbrecht BG, Zenati MS, Doyle HR, McMichael J, Townsend RN, Clancy KD, et al. Hepatic dysfunction increases length of stay and risk of death after injury. The Journal of trauma. 2002;53(3):517–23. doi: 10.1097/00005373-200209000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Hierholzer C, Harbrecht B, Menezes JM, Kane J, MacMicking J, Nathan CF, et al. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. The Journal of experimental medicine. 1998;187(6):917–28. doi: 10.1084/jem.187.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menezes JM, Hierholzer C, Watkins SC, Billiar TR, Peitzman AB, Harbrecht BG. The modulation of hepatic injury and heat shock expression by inhibition of inducible nitric oxide synthase after hemorrhagic shock. Shock (Augusta, Ga) 2002;17(1):13–8. doi: 10.1097/00024382-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Menezes J, Hierholzer C, Watkins SC, Lyons V, Peitzman AB, Billiar TR, et al. A novel nitric oxide scavenger decreases liver injury and improves survival after hemorrhagic shock. The American journal of physiology. 1999;277(1 Pt 1):G144–51. doi: 10.1152/ajpgi.1999.277.1.G144. [DOI] [PubMed] [Google Scholar]
- 13.Hong G, Zhang B, Harbrecht BG. Cyclic AMP inhibits IL-1beta plus IFNgamma-induced NF-kappaB translocation in hepatocytes by a PKA independent mechanism. The Journal of surgical research. 2010;159(1):565–71. doi: 10.1016/j.jss.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harbrecht BG, Nweze I, Smith JW, Zhang B. Insulin inhibits hepatocyte iNOS expression induced by cytokines by an Akt-dependent mechanism. American journal of physiology Gastrointestinal and liver physiology. 2012;302(1):G116–22. doi: 10.1152/ajpgi.00114.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang B, Li S, Harbrecht BG. Akt-mediated signaling is induced by cytokines and cyclic adenosine monophosphate and suppresses hepatocyte inducible nitric oxide synthase expression independent of MAPK P44/42. Biochimica et biophysica acta. 2011;1813(1):73–9. doi: 10.1016/j.bbamcr.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang B, Perpetua M, Fulmer M, Harbrecht BG. JNK signaling involved in the effects of cyclic AMP on IL-1beta plus IFNgamma-induced inducible nitric oxide synthase expression in hepatocytes. Cellular signalling. 2004;16(7):837–46. doi: 10.1016/j.cellsig.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Tsai YF, Liu FC, Lau YT, Yu HP. Role of Akt-dependent pathway in resveratrol-mediated cardioprotection after trauma-hemorrhage. The Journal of surgical research. 2012;176(1):171–7. doi: 10.1016/j.jss.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 18.Nweze IC, Smith JW, Zhang B, Klinge CM, Lakshmanan J, Harbrecht BG. 17beta-Estradiol attenuates cytokine-induced nitric oxide production in rat hepatocyte. The journal of trauma and acute care surgery. 2012;73(2):408–12. doi: 10.1097/TA.0b013e31825a789b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geller DA, de Vera ME, Russell DA, Shapiro RA, Nussler AK, Simmons RL, et al. A central role for IL-1 beta in the in vitro and in vivo regulation of hepatic inducible nitric oxide synthase. IL-1 beta induces hepatic nitric oxide synthesis. Journal of immunology (Baltimore, Md: 1950) 1995;155(10):4890–8. [PubMed] [Google Scholar]
- 20.Zhang B, Nweze I, Lakshmanan J, Harbrecht BG. Activation of a cyclic amp-guanine exchange factor in hepatocytes decreases nitric oxide synthase expression. Shock (Augusta, Ga) 2013;39(1):70–6. doi: 10.1097/SHK.0b013e3182760530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klinge CM, Blankenship KA, Risinger KE, Bhatnagar S, Noisin EL, Sumanasekera WK, et al. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. The Journal of biological chemistry. 2005;280(9):7460–8. doi: 10.1074/jbc.M411565200. [DOI] [PubMed] [Google Scholar]
- 22.Rieder SA, Nagarkatti P, Nagarkatti M. Multiple anti-inflammatory pathways triggered by resveratrol lead to amelioration of staphylococcal enterotoxin B-induced lung injury. British journal of pharmacology. 2012;167(6):1244–58. doi: 10.1111/j.1476-5381.2012.02063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobbs AJ, Higgs A, Moncada S. Inhibition of nitric oxide synthase as a potential therapeutic target. Annual review of pharmacology and toxicology. 1999;39:191–220. doi: 10.1146/annurev.pharmtox.39.1.191. [DOI] [PubMed] [Google Scholar]
- 24.Stice JP, Mbai FN, Chen L, Knowlton AA. Rapid activation of nuclear factor kappaB by 17beta-estradiol and selective estrogen receptor modulators: pathways mediating cellular protection. Shock (Augusta, Ga) 2012;38(2):128–36. doi: 10.1097/SHK.0b013e31825da754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tome-Carneiro J, Larrosa M, Gonzalez-Sarrias A, Tomas-Barberan FA, Garcia-Conesa MT, Espin JC. Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Current pharmaceutical design. 2013;19(34):6064–93. doi: 10.2174/13816128113199990407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Z, Andersson R. NF-kB activation and inhibition: a review. Shock. 2002;18(2):99–106. doi: 10.1097/00024382-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, Rahman I. Regulation of SIRT1 in cellular function: role of polyphenols. Arch Biochem Biophys. 2010;501:79–90. doi: 10.1016/j.abb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simao F, Matte A, Pagnussat AS, Netto CA, Salbego CG. Resveratrol prevents CA1 neurons against ischemic injury by parallel modulation of both GSK3β and CREB through PI3K/Akt pathway. Eur J Neuroscience. 2012;36:2899–2905. doi: 10.1111/j.1460-9568.2012.08229.x. [DOI] [PubMed] [Google Scholar]
- 30.Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, et al. Repeat dose study of the cancer chemopreventative agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70 (22):9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao PC, Ng LT, Lin LT, Richardson CD, Wang GH, Lin CC. Resveratrol arrests cell cycle and induces apoptosis in human hepatocellular carcinoma Huh-7 cells. J Med Food. 2010;13(6):1415–1423. doi: 10.1089/jmf.2010.1126. [DOI] [PubMed] [Google Scholar]
- 32.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Disp. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]