Abstract
Enteroviruses (EV) uridylylate a peptide, VPg, as the first step in their replication. VPgpUpU, found free in infected cells, serves as the primer for RNA elongation. The abilities of four polymerases (3Dpol), from EV-species A-C, to uridylylate VPgs that varied by up to 60% of their residues were compared. Each 3Dpol was able to uridylylate all five VPgs using polyA RNA as template, while showing specificity for its own genome encoded peptide. All 3Dpol uridylylated a consensus VPg representing the physical chemical properties of 31 different VPgs. Thus the residues required for uridylylation and the enzymatic mechanism must be similar in diverse EV. As VPg-binding sites differ in co-crystal structures, the reaction is probably done by a second 3Dpol molecule. The conservation of polymerase residues whose mutation reduces uridylylation but not RNA elongation is compared.
Keywords: Peptide priming, nucleotide transfer reaction, RNA polymerase, Coxsackie virus, EV-71, poliovirus, PCP-consensus sequence, Metal ion dependent phosphotransfer
The human enteroviruses (EV), which include the polioviruses (PV), coxsackie viruses (CVA, CVB) and many other pathogens, cause febrile rash, respiratory illness, and neurologic disease (Eyckmans et al., 2014; Pallansch et al., 2013). Although incidence of PV paralysis has been reduced by >99% globally through routine immunization and mass vaccination campaigns, there continue to be cases in areas where vaccine campaigns have been inhibited by social unrest (Moturi et al., 2014). Non-polio EV, such as EV A71 (Chan et al., 2011; Wang et al., 2014; Yu et al., 2014; Zheng et al., 2014) and EV 68(Stephenson, 2014) (Jacobson et al., 2012; Tokarz et al., 2012), can spread rapidly among children. These can cause severe respiratory illness and a range of neurological diseases, from aseptic meningitis to encephalitis and paralysis(Kreuter et al., 2011; Pallansch et al., 2013; Tao et al., 2014). Infections with other EV, such as CVB3, may contribute to diabetes (Salvatoni et al., 2013; Yeung et al., 2011) and heart disease(Chapman and Kim, 2008; Cooper, 2009).
There are currently no drugs approved for the treatment of the many different enterovirus infections (Abzug, 2014). As EV are omnipresent in the intestinal tract of humans and animals, there is little way to prevent occasional infections. Their antigenic diversity (Acevedo et al., 2014; Blomqvist et al., 2008) make it difficult to develop vaccines to protect against the many different enterovirus pathogens. To aid in developing more widespread treatments for EV infections (Campagnola et al., 2011), it is important to identify common properties of the viral proteins involved in replication.
Early studies of poliovirus replication revealed that the 5’ end of the RNA was covalently bound to a small peptide, called VPg (for viral protein linked to the genome), which was essential for PV replication (Ambros and Baltimore, 1978; Lee et al., 1977). A uridylylated form of VPg, VPgpUpU, was shown to be present in the cytoplasm of infected cells(Crawford and Baltimore, 1983). Subsequently, it was shown that VPgpU could be formed in an in vitro reaction containing the polymerase (3Dpol) and a template RNA. The uridylylated peptides, VPgpU or VPgpUpU, prime viral RNA synthesis (Paul et al., 1998). VPg sequences are present in the genomes of all picornaviruses. Larger VPg proteins were also identified in caliciviruses and other families that were even more distinct from the picornaviruses (Goodfellow, 2011) but which may have arisen from combinations of picornavirus gene sequences during evolution of the eukaryotic cell (Koonin et al., 2008).
A wealth of data indicate that mutations throughout the 22 amino acid sequence of PV1- VPg reduce uridylylation in vitro and lower or eliminate the formation of infectious virus (Kuhn et al., 1988a; Kuhn et al., 1988b; Paul et al., 2003). However, there are many gene sequences known for EV VPgs, which differ at positions (when aligned with PV-VPg) that are known to affect uridylylation (Figure 1). Deep sequencing of viral isolates may reveal even more diversity (Acevedo et al., 2014), introduced through the high mutation rate of viral 3Dpol (Gnadig et al., 2012). Indeed, VPg seems to be evolving at a very rapid rate, as the sequences of the four EV 3Dpol included here are much more conserved, ranging from 67-74% identity.
Figure 1.
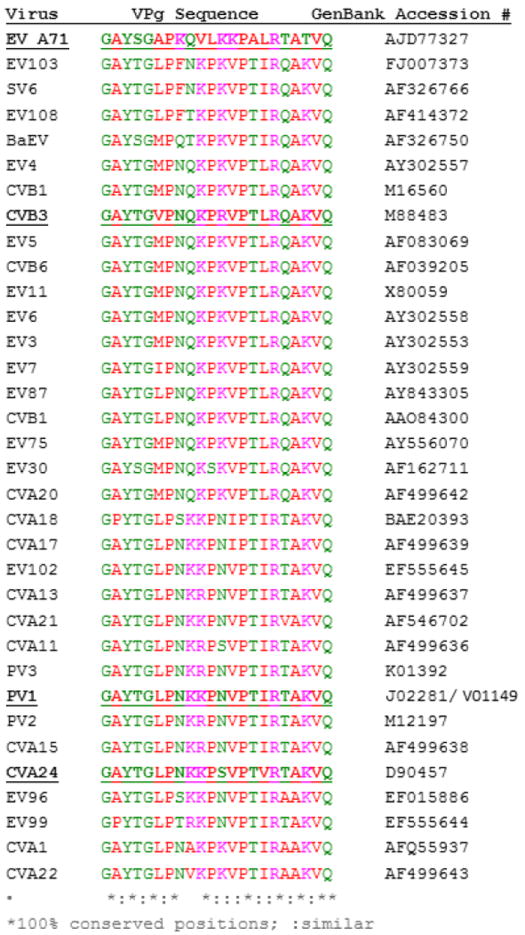
Sequences of EV VPgs used to design the PCP-consensus VPg with their gene bank accession numbers. Only the unique sequences were used in calculating the consensus.
However, function eventually constrains sequence variability. To determine the minimum requirements for uridylylation, we analyzed the sequences, the underlying conservation of physicochemical properties, and the structures of VPg and their binding sites on the polymerases in co-crystal structures. VPg, before uridylylation, in solution has a flexible, or even disordered structure (Schein et al., 2006b), which might also be stabilized by binding to cellular components or the polymerase. In contrast, chemically synthesized, uridylylated PV-VPgpU has a very stable structure in solution (Schein et al., 2010). The NMR structure indicated that the positively charged residues directly coordinate with the UMP moiety of the modified tyrosine. Such a stable structure is probably needed for VPgpU to effectively prime RNA synthesis.
To determine the specificity endowed within the diversity of sequences of VPg, we chose four diverse EV polymerases and determined whether they could recognize VPgs that differed greatly in sequence from their own encoded peptide. We purified the 3Dpol of three important human pathogens, from EV-A71 (species A), CVB3 (species B), and CVA24 (species C, and closely related in sequence to PV-3Dpol) (Smura et al., 2014). Our results indicate that the diversity in the sequences of the VPg of species A-C correlates with their different binding sites for uridylylation on the 3Dpol. The underlying physical chemical properties of the VPgs were captured in a single consensus sequence. All four of the polymerases tested could uridylylate this artificial sequence, while still showing preference for their own VPg. The ability of all to uridylylate a consensus peptide, coupled with evidence that VPg-based replication can be done in trans (Chen et al., 2013), suggest there is indeed a common mechanism for VPg uridylylation. However, the specificity we show here, coupled with the different binding sites seen in co-crystal structures, supports a “2 molecule mechanism” (Sun et al., 2012), where the VPg can be located at different positions on one polymerase molecule, and uridylylated by a second polymerase molecule.
Methods
VPg Synthesis and Quantification
VPgs were produced synthetically, using normal FMOC-based amino acid derivatives. Synthetic VPgpU, used for calibrating the position of VPgpU on gels in early stages of this work, was generated as described previously (Schein et al., 2010; van der Heden van Noort et al., 2013). VPgs were dissolved in water and their concentration determined using the extinction coefficients for tyrosine (the only UV-absorbing amino acid in the peptide) in the range of 220-280 nm.
Determining a PCP-consensus VPg
The PCP-consensus method determines the sequence that is most similar in its physical chemical properties to all others in a set. It is designed to be useful for sets with many sequences (such as viral isolates) that have a high superficial redundancy (i.e., nearly identical sequences that differ at only a few positions). The rationale and details of the method are described in detail elsewhere (Bowen et al., 2012; Danecek et al., 2010; Danecek and Schein, 2010; Schein et al., 2012). Here, the PCP-con program was used to determine a consensus of 31 of the most diverse EV VPg sequences (Figure 1). The resulting PCP-consensus sequence is compared to the four wild type VPgs that were used in this study in Table 1.
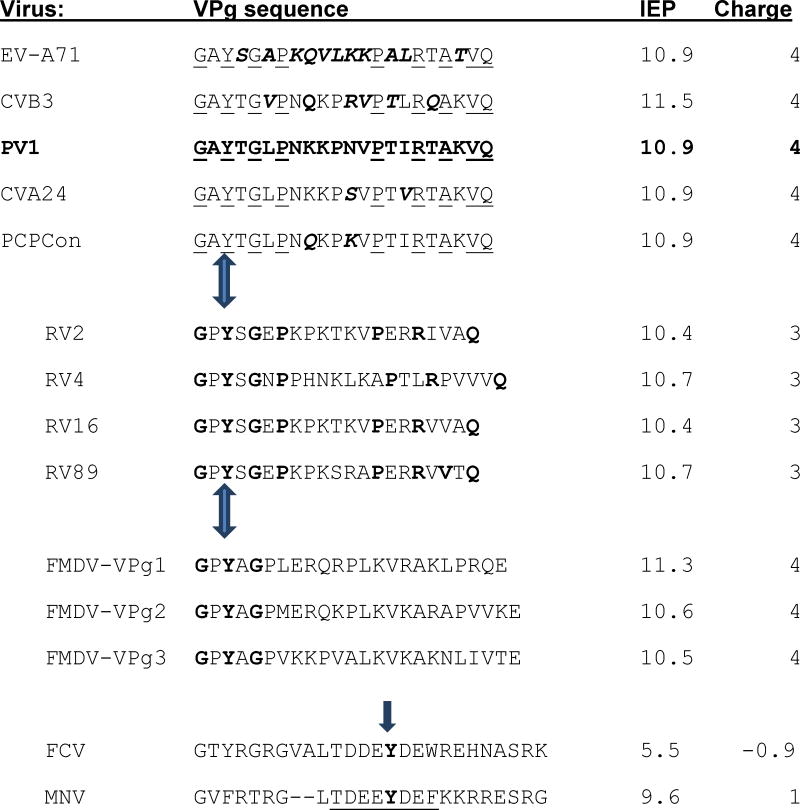
Table 1. Comparison of the isoelectric points (IEP) and net charges at pH 7 of VPgs.
The top five VPgs were used in this study. PV1 VPg sequence is bold; residues that differ from it in the other EV sequences chosen for study are bold and italicized. Residues conserved in EV species A-C are underlined. These are followed by VPg sequences from RV and the 3 genome encoded VPgs of the distantly related picornavirus FMDV. The conserved residues in RV that they share with other enteroviruses are bold. The bold residues in the FMDV sequences are those identical to PV1 VPg.
In contrast, as the last two sequences illustrate, the conserved areas (residues 10-30) around the uridylylated Tyr (bold) from the VPg proteins of feline calicivirus (FCV) and murine norovirus (MNV) VPgs (Leen et al., 2013) have completely different IEP and charge, suggesting two different mechanisms for uridylylation. Mutation of the underlined residues in MNV- VPg greatly reduce or prevent VPg uridylylation, VPg-RNA synthesis by the MNV polymerase and virus recovery (Leen et al., 2013).
|
Arrows indicate the uridylylated Tyrosine. The charges and IEP were calculated with the Peptide property calculator from Innovagen (http://pepcalc.com/ppc.php)).
Polymerase purification
Genes for the CVA24 and EV A71 polymerases were obtained from EV collections at the CDC. The CVB3 polymerase gene was obtained from the cloned cDNA of the strain CVB3/28 (Tracy et al., 2002). This strain induces myocarditis and pancreatitis in susceptible mice and accelerates the development of T1 diabetes in older non-obese diabetic mice (Tu et al., 1995). The three 3Dpol genes were subcloned into pET30 in the Recombinant DNA Laboratory at UTMB, so that the resulting protein would have a C-terminal hexahistidine tag. PV 3Dpol was expressed in E. coli from plasmid pT5-3D (a gift of Dr. Karla Kirkegaard).
Plasmids containing the respective 3Dpol gene were transformed into the Rosetta DE3 strain of E. coli that has been optimized for the codon usage of higher organisms. Protein expression was induced with 1 mM isopropyl β-D-thiogalactopyranoside (IPTG) for 16 hours at 18°C (with shaking). The 3Dpol was in the soluble fraction of the lysate and was purified using Talon metal affinity resin (Clontech) with a 5-100 mM imidazole gradient. Protein-containing fractions were concentrated to ~2 mL, and further purified on a Superdex 75 (GE Healthcare) gel filtration column. Protein-containing fractions were pooled and concentrated to 2-7 mg/mL (Supplementary Figure S1). Dengue virus polymerase was expressed and purified as described previously (Bussetta and Choi, 2012).
Assay for uridylylation
The reaction mixtures (10 μl) contained 50 mM HEPES, pH 7.5, 8% glycerol, 0.5 μg of the template RNA: polyA (Sigma); 0.5 mM manganese(II) acetate, 1-2 μg purified 3Dpol, 1 μg synthetic VPg, and 10 μM UTP (+α-UT32P (Amersham)) (Paul et al., 1998). Except where noted otherwise (Fig. 3), multiplex assays of the polymerases with the five VPgs were done in siliconized PCR plates and incubated for 2 h at 30°C. They were stopped by addition of SDS containing gel loading buffer and heated at 60 °C for 3-4 minutes before applying to TGX-any KD minigels (15 slot) or Criterion (26 slot), Tris-Tricine/SDS-PAGE (10-20%, Biorad Criterion Peptide). The uridylylated VPg32PU products were quantified with a Phosphorimager (PMI; Biorad).
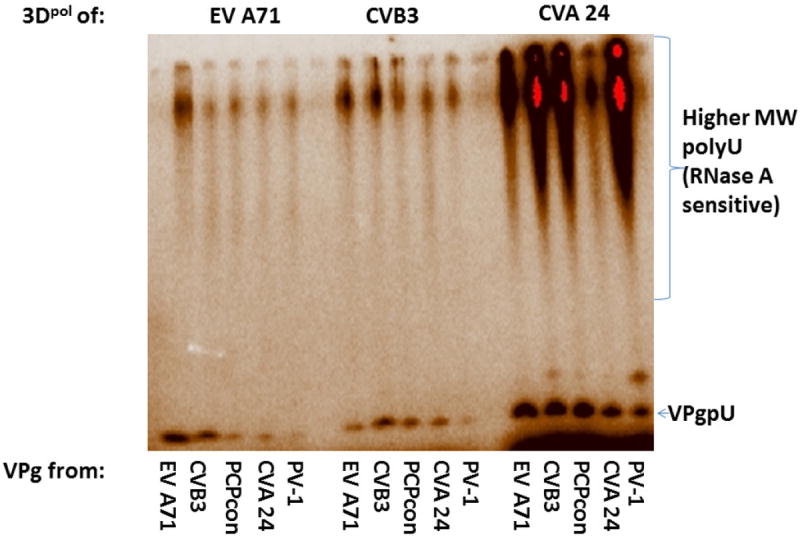
Figure 3. Uridylation of 5 diverse VPgs by Enteroviral polymerases from species A-C.
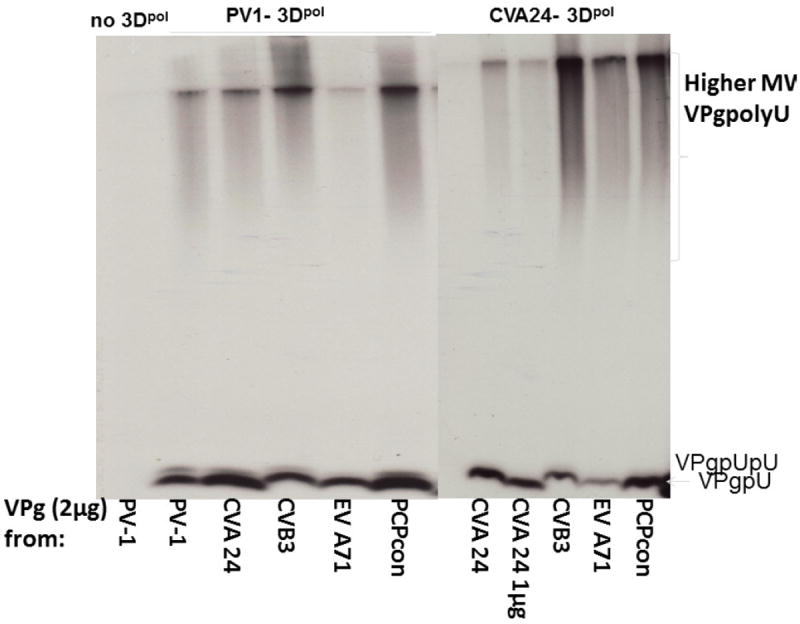
Sequences of VPgs are shown in Table 1. a) PV and CVA24 3Dpol efficiently uridylylate all five VPgs. The assay was incubated for 1h at 34°C, and reactions were run on two-13.5% PAG (aligned next to one another). Both polymerases from species C (Brown et al., 2003) uridylylate a PCP-consensus VPg (PCPcon) as efficiently as their respective wild-type VPgs and those from EV-B Species B (represented by CVB3 VPg) and species A (EV-A71). b. Comparison of uridylylation of 5 different VPgs by 3Dpol from EV-A71, CVB3, and CVA24. The reactions were incubated for 2h at 30°C and run on a 26 slot Criterion Tris-Tricine 10-20% gel (Bio-Rad). The three polymerases were purified within a few weeks of each other (Figure S1). The reactions were run simultaneously with the same amounts of each VPg in the assay. The quantification is shown in Table 2. products.
Results
Deriving a PCP-consensus VPg for EV species A-C
The sequences of 31 diverse EV were used to derive a PCP-consensus for VPg (Figure 1). Only 9 residues (without inserting gaps) of the 22 are conserved across EV species A, B, and C. Seven of these residues are also conserved in analogous positions in diverse Rhinoviruses (RV, enterovirus species D; Table 1). The conservation of G1 and Q22 reflects the sequence needed for protease cleavage of the P3 domain of the polyprotein (Pathak et al., 2007). Despite the relatively low absolute identity, the physical chemical properties at each position are more conserved. For example, there is always a positively charged residue at positions 8-10 in the sequences, and arginine is always present at position 17. The absolute sequence number of the positively charged residues is not conserved (e.g., K9 is a Q, N, R or T in the different sequences). However, all 5 VPg sequences synthesized for this study have the same predicted IEP (10.9) and charge (+4) at pH 7.
The unique VPg sequences (from species A, B, and C) chosen for this study are compared in Table 1 with those of other picornavirus sequences and the uridylylation site of larger VPgs from other virus families. The IEP and net charges for the sequences of RV VPgs is somewhat lower. The sequences of the three genome encoded VPgs of the distantly related Foot and mouth disease virus (FMDV; genus Aphthovirus) are, like FMDV polymerase (see the alignments in Supplementary materials), significantly different from those of the Enteroviruses used in this study. For example, the FMDV VPgs contain at least one negatively charged amino acid. However, their overall IEP and net charge are similar to those of the EVs.
Figure 2 shows the absolutely conserved residues of the EV-VPgs (maroon) mapped on the NMR structure of VPg (PDB accession code 2BBL), with the uridylylated Tyr3 residues in turquoise. Residues K9/10 are circled, as mutation of both of these residues is lethal for PV replication in culture.
Figure 2.
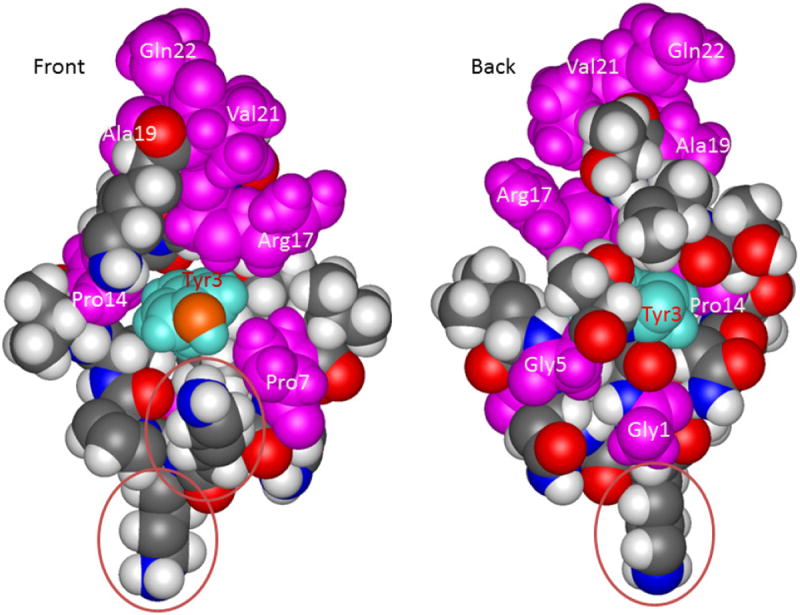
NMR structure of VPg (2BBL.pdb; structure 1)(Schein et al., 2006a) showing residues conserved in all EV species A-C sequences (maroon) and the uridylylated Tyr3 (in cyan, its phenolic O is in orange red). The other residues are “CPK” colored according to atom type (H=gray, O=red, black=C, Blue=N). Residue positions where positive charge is conserved are circled. Here, Front indicates the (positively charged) face of VPg on which the Tyr3-OH is located, and Back indicates the side of VPg that docks to the polymerase at the indicated binding site for VPg (Schein et al., 2006b).
Comparision of uridylyation activities of EV species A-C
Three 3Dpol were purified similarly and their ability to uridylylate the VPgs was compared to that of PV-3Dpol (purified in another lab) in the most permissive assay for uridylylation, using a polyA template RNA and Mn2+ (Figure 3). Quantitative comparison of the PAGE assays of representative experiments for uridylylation of the 4 natural VPgs (CVA24, CVB3, EV A71, and PV1), and the PCP-consensus VPg, by three polymerases from the three species are shown in Table 2. Within the accuracy of this assay, we can say that each of the polymerases did show preference for its own VPg, with CVA24 consistently being more accepting of sequences that differed from its own. All of the VPgs, including the consensus, were uridylylated to at least 25% of the efficiency of the cognate encoded VPg.
Table 2. Volumes of the VPg-pU bands and the relative incorporation of P32-UMP into VPgpU/VPgpUpU bands.
The volumes for the VPgpU bands (see Figure 4) are given in total units (to allow comparison from one polymerase to the other) and then relative to the wild type VPg for each 3Dpol (shown bold). Results with the consensus VPg are underlined for each 3Dpol.
| 3Dpol/VPg: | VPg-pU | |
|---|---|---|
| CVA24/CVA24VPg | 54134 | 1.00 |
| CVA24/CVB3VPg | 82568 | 1.53 |
| CVA24/EV A71VPg | 106020 | 1.96 |
| CVA24/PCPconVPg | 99805 | 1.84 |
| CVA24/PV1-VPg | 33164 | 0.61 |
| CVB3/CVA24VPg | 17090 | 0.54 |
| CVB3/CVB3VPg | 31852 | 1.00 |
| CVB3/EV A71VPg | 11040 | 0.35 |
| CVB3/PCPconVPg | 15722 | 0.49 |
| CVB3/PV1-VPg | 13223 | 0.42 |
| EV A71/CVA24VPg | 12208 | 0.25 |
| EV A71/CVB3VPg | 23309 | 0.48 |
| EV A71/EV A71VPg | 48621 | 1.00 |
| EV A71/PCPconVPg | 16776 | 0.35 |
| EV A71/PV1-VPg | 13903 | 0.29 |
As a control, we also tested another RNA dependent, RNA polymerase, from the Flavivirus Dengue (DENV), in the uridylylation assay with and without consensus VPg. As Figure S2 shows, DENV polymerase produced large amounts of RNase A-sensitive, poly U RNA in the uridylylation assay. As expected, it did not produce VPgpU, which was produced by all three enteroviral 3Dpol in the same assay mix.
Discussion
We show here that EV 3Dpol, chosen from the most diverse members of EV species A-C, are able to uridylylate all four wild type VPgs as well as a PCP-consensus VPg. The efficiency was 25%-100% of that seen for their cognate VPgs. Surprisingly, the two most divergent 3Dpol, from EV A71 (EV-A representative) and CVB3 (EV-B), still uridylylated the PCP-consensus VPg as well as or better than the wild type VPgs of CVA24 or PV1 (both EV-C). This indicates there is a common framework for uridylylation by even the most divergent EV-3Dpol.
These results mirror to some extent the specificity of RV-VPgs. The rhinovirus VPgs differ from those of EV species A-C in length, and the presence of negatively charged amino acids (Table 1). Mutations to insert negative charge into PV1 VPg, such as replacing Leu6 with glutamic acid, greatly reduce replication (Cheney et al., 2003). Previous studies have shown that RV2 3Dpol can uridylylate the VPgs of diverse RV (Gerber et al., 2001) and PV VPg, but PV 3Dpol cannot uridylylate the VPgs of RV2 or RV89 (Paul et al., 2003). The 3Dpol of RV16 (Cheney et al., 2003) also uridylylates PV VPg and the reaction is inhibited by the same mutations that inhibit uridylylation by PV 3Dpol (Gerber et al., 2001). This again implies a basic common underlying mechanism, with specificity encoded by both the enzyme and the peptide.
Diverse VPg binding sites on the 3Dpol of EV A71 (Chen et al., 2013) and CVB3 (Gruez et al., 2008b) suggest a second polymerase molecule may catalyze uridylylation. These sites are both on the “reverse surface” of the polymerase (Fig. S3 and S4). The site identified for CVB3 is near that identified for PV 3Dpol by mutagenesis (Lyle et al., 2002) and docking studies (Schein et al. 2006). Since the site for EV A71 is so different, yet both polymerases are able to uridylylate the same VPgs, this suggests that uridylylation is done by a second polymerase molecule, with the VPg bound to the surface of the first. Assuming the VPgs of species B and C continue to bind to approximately the same region on EV A71, alteration of R379 to L, F377 to G and V391 to T on the protein surface could greatly destabilize the binding site, thus lowering the reaction rate. The need for two polymerase molecules to catalyze the reaction has been suggested by several other authors based on different types of data (Gruez et al., 2008a; Sun et al., 2012; Tellez et al., 2006).
The similarity in overall charge of the 3 encoded FMDV VPgs (Table 1) does suggest that this Apthovirus should have the same basic mechanism as the EV for uridylylation. However, the sequence similarity between the two sets of VPgs is very low (3/22 identical, 3/9 identical for the absolutely conserved amino acids). A Blast search of the Refseq database starting from PV1 VPg brings only VPgs for EV species A-D and J within the first 10 sequences, but no FMDV VPg in the top 100 sequences. The same search, beginning with FMDV VPg2 finds FMDV but no EV VPg.
Further, the polymerases diverge in both sequence and structure. The amino acids of the surface sites for the EV VPgs are not present in FMDV 3Dpol (Fig. S3). In co-crystal structures of the 3Dpol of FMDV with both its free and uridylylated VPg1 (Ferrer-Orta et al., 2006), both VPg and VPgpU were seen fully extended near the active site of the polymerase. Thus the Apthoviruses may indeed have a different mechanism for uridylylation.
Alternatively, much of the data could be explained if the surface site for VPg is simply to aid in cleavage of VPg from the 3BC protein. Higher resolution crystal structures of the EV-polymerases with their uridylylated VPgs might help to resolve these possibilities.
Essential, negatively charged amino acids in larger VPgs of caliciviruses suggest a different uridylylation mechanism. It is clear simply from sequence conservation (Table 1), as well as mutation studies, that a net positive charge on the peptide is essential for uridylylation of EV VPgs, and probably for those of FMDV. The positively charged residues could bind the incoming UTP residue during uridylylation, as well as stabilize the position of the bound Tyr-UMP conjugate during priming (Schein et al., 2010).
Although the small picornaviral VPgs are positively charged, the reactive tyrosine in the NMR structures of feline calicivirus (FCV) and murine norovirus (MNV) projects from a protein helix, and is surrounded by negatively charged residues in the linear sequence (last lines of Table 1). As Table 1 shows, the charges of the sequences surrounding the reactive Tyrosine of the FCV and MNV VPgs are “polar opposites”. While the positive charges of PV-VPg are essential, mutation of negatively charged residues near the uridylylated Tyr in MNV VPgs prevents formation of the VPg-RNA covalent complex and virus replication (Leen et al., 2013). These residues could stabilize the structure through salt bridges (formed perhaps to residues not included in the NMR structure). Alternatively, they could bind metal ions as part of the catalytic mechanism. Negatively charged amino acids, particularly aspartates, capable of tightly binding metal ions, play an important role in nuclease and phosphatase common mechanisms (Braun and Schein, 2014) (Oezguen et al., 2007).
The differences in the environment of the uridylylated tyrosine in different VPgs suggests that the “big bang of picornavirus evolution” (Koonin et al., 2008) that gave rise to so many diverse viruses, also gave different solutions to the problem of generating a stable surface to prime RNA synthesis.
Supplementary Material
Figure 4.
Acknowledgments
Special appreciation and thanks are due to Kaija Maher, CDC, for obtaining the genes for CVA24 and EV-A71 3Dpol and staff at FfAME, particularly Ryan Shaw, Lucy Glushacova, Ozlem Yaren, Jennifer Moses, Nicole Leal, Nidhi Sharma, Andrea Bradley and Dianne Rowold for help in establishing the assay methods and Steve Benner for helpful discussions. This work was supported by grants from the NIH to CHS (1R21AI105985), and in part by grant RO1AI0152235 (PI: Eckard Wimmer). Synthesis of VPgs and VPg-pUs was supported by VIDI grant from the Dutch organization for Scientific Council (NWO).
Abbreviations used
3B, etc.: Enteroviruses express one long polyprotein. This is cleaved into three fragments that are further cleaved to yield precursor and mature viral proteins. The third fragment is cleaved to form 3AB (3B is VPg), 3BC, 3CD (where 3C is a protease, and 3CD accelerates the uridylylation assay using cre RNA as template), and 3Dpol (the RNA polymerase).
Other abbreviations include:
- CV
coxsackievirus
- DENV
Dengue virus
- FMDV
foot and mouth disease virus
- EV
enterovirus
- FCV
feline calicivirus
- IEP
isoelectric point
- MNV
murine norovirus
- RV
rhinovirus
- PAGE
polyacrylamide gel electrophoresis
- PCP
physical chemical properties
- PCP-consensus
consensus sequence based on conservation of PCPs in each column of a multiple sequence alignment
- pU
Uridylylated (i.e., VPgpU, VPgpUpU)
- PV
poliovirus
- VPg
viral peptide linked to the genome
- VPgpU
uridylylated VPg
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Abzug MJ. The enteroviruses: Problems in need of treatments. Journal of Infection. 2014;68(Supplement 1):S108–S114. doi: 10.1016/j.jinf.2013.09.020. [DOI] [PubMed] [Google Scholar]
- Acevedo A, Brodsky L, Andino R. Mutational and fitness landscapes of an RNA virus revealed through population sequencing. Nature. 2014;505:686–690. doi: 10.1038/nature12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V, Baltimore D. Protein is linked to the 5’ end of poliovirus RNA by a phosphodiester linkage to tyrosine. J Biol Chem. 1978;253:5263–5266. [PubMed] [Google Scholar]
- Blomqvist S, Paananen A, Savolainen-Kopra C, Hovi T, Roivainen M. Eight years of experience with molecular identification of human enteroviruses. J Clin Microbiol. 2008;46:2410–2413. doi: 10.1128/JCM.00313-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen DM, Lewis JA, Lu W, Schein CH. Simplifying complex sequence information: a PCP-consensus protein binds antibodies against all four Dengue serotypes. Vaccine. 2012;30:6081–6087. doi: 10.1016/j.vaccine.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun W, Schein Catherine H. Membrane Interaction and Functional Plasticity of Inositol Polyphosphate 5-Phosphatases. Structure. 2014;22:664–666. doi: 10.1016/j.str.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Brown B, Oberste MS, Maher K, Pallansch MA. Complete Genomic Sequencing Shows that Polioviruses and Members of Human Enterovirus Species C Are Closely Related in the Noncapsid Coding Region. Journal of Virology. 2003;77:8973–8984. doi: 10.1128/JVI.77.16.8973-8984.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussetta C, Choi KH. Dengue virus nonstructural protein 5 adopts multiple conformations in solution. Biochemistry. 2012;51:5921–5931. doi: 10.1021/bi300406n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnola G, Gong P, Peersen OB. High throughput screening identification of poliovirus RNA-dependent RNA polymerase inhibitors. Antiviral Research. 2011;91:241–251. doi: 10.1016/j.antiviral.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YF, Sam IC, Wee KL, Abubakar S. Enterovirus 71 in Malaysia: A decade later. Neurol Asia. 2011;16:1–15. [Google Scholar]
- Chapman NM, Kim KS. Persistent coxsackievirus infection: enterovirus persistence in chronic myocarditis and dilated cardiomyopathy. Curr Top Microbiol Immunol. 2008;323:275–292. doi: 10.1007/978-3-540-75546-3_13. [DOI] [PubMed] [Google Scholar]
- Chen C, Wang Y, Shan C, Sun Y, Xu P, Zhou H, Yang C, Shi P-Y, Rao Z, Zhang B, Lou Z. Crystal Structure of Enterovirus 71 RNA-Dependent RNA Polymerase Complexed with Its Protein Primer VPg: Implication for a trans Mechanism of VPg Uridylylation. Journal of Virology. 2013;87:5755–5768. doi: 10.1128/JVI.02733-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney IW, Naim S, Shim JH, Reinhardt M, Pai B, Wu JZ, Hong Z, Zhong W. Viability of Poliovirus/Rhinovirus VPg Chimeric Viruses and Identification of an Amino Acid Residue in the VPg Gene Critical for Viral RNA Replication. Journal of Virology. 2003;77:7434–7443. doi: 10.1128/JVI.77.13.7434-7443.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper LT., Jr Myocarditis. N Engl J Med. 2009;360:1526–1538. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford NM, Baltimore D. Genome-linked protein VPg of poliovirus is present as free VPg and CPgpUpU in poliovirus-infected cells. Proc Natl Acad Sci USA. 1983;80:7452–7455. doi: 10.1073/pnas.80.24.7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danecek P, Lu W, Schein CH. PCP consensus sequences of flaviviruses: correlating variance with vector competence and disease phenotype. J Mol Biol. 2010;396:550–563. doi: 10.1016/j.jmb.2009.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danecek P, Schein CH. Flavitrack analysis of the structure and function of West Nile non-structural proteins. Int J Bioinform Res Appl. 2010;6:134–146. doi: 10.1504/IJBRA.2010.032117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyckmans T, Wollants E, Janssens A, Schoemans H, Lagrou K, Wauters J, Maertens J. Coxsackievirus A16 encephalitis during obinutuzumab therapy, Belgium. Emerging Infectious Diseases. 2014;20:913–915. doi: 10.3201/eid2005.131766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Orta C, Arias A, Agudo R, Perez-Luque R, Escarmis C, Domingo E, Verdaguer N. The structure of a protein primer-polymerase complex in the initiation of genome replication. Embo Journal. 2006;25:880–888. doi: 10.1038/sj.emboj.7600971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber K, Wimmer E, Paul AV. Biochemical and Genetic Studies of the Initiation of Human Rhinovirus 2 RNA Replication: Purification and Enzymatic Analysis of the RNA-Dependent RNA Polymerase 3Dpol. Journal of Virology. 2001;75:10969–10978. doi: 10.1128/JVI.75.22.10969-10978.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnadig NF, Beaucourt S, Campagnola G, Borderia AV, Sanz-Ramos M, Gong P, Blanc H, Peersen OB, Vignuzzi M. Coxsackievirus B3 mutator strains are attenuated in vivo. Proc Natl Acad Sci U S A. 2012;109:E2294–2303. doi: 10.1073/pnas.1204022109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow I. The genome-linked protein VPg of vertebrate viruses - a multifaceted protein. Curr Opin Virol. 2011;1:355–362. doi: 10.1016/j.coviro.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruez A, Selisko B, Roberts M, Bricogne G, Bussetta C, Jabafi I, Coutard B, De Palma AM, Neyts J, Canard B. The crystal structure of coxsackievirus B3 RNA-dependent RNA polymerase in complex with its protein primer VPg confirms the existence of a second VPg binding site on Picornaviridae polymerases. Journal of Virology. 2008a;82:9577–9590. doi: 10.1128/JVI.00631-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruez A, Selisko B, Roberts M, Bricogne G, Bussetta C, Jabafi I, Coutard B, De Palma AM, Neyts J, Canard B. The crystal structure of coxsackievirus B3 RNA-dependent RNA polymerase in complex with its protein primer VPg confirms the existence of a second VPg binding site on Picornaviridae polymerases. J Virol. 2008b;82:9577–9590. doi: 10.1128/JVI.00631-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LM, Redd JT, Schneider E, Lu X, Chern SW, Oberste MS, Erdman DD, Fischer GE, Armstrong GL, Kodani M, Montoya J, Magri JM, Cheek JE. Outbreak of lower respiratory tract illness associated with human enterovirus 68 among American Indian children. Pediatr Infect Dis J. 2012;31:309–312. doi: 10.1097/INF.0b013e3182443eaf. [DOI] [PubMed] [Google Scholar]
- Koonin EV, Wolf YI, Nagasaki K, Dolja VV. The Big Bang of picorna-like virus evolution antedates the radiation of eukaryotic supergroups. Nat Rev Micro. 2008;6:925–939. doi: 10.1038/nrmicro2030. [DOI] [PubMed] [Google Scholar]
- Kreuter JD, Barnes A, McCarthy JE, Schwartzman JD, Oberste MS, Rhodes CH, Modlin JF, Wright PF. A fatal central nervous system enterovirus 68 infection. Arch Pathol Lab Med. 2011;135:793–796. doi: 10.5858/2010-0174-CR.1. [DOI] [PubMed] [Google Scholar]
- Kuhn RJ, Tada H, Ypma-Wong MF, Dunn JJ, Semler BL, Wimmer E. Construction of a “mutagenesis cartridge” for poliovirus genome-linked viral protein: isolation and characterization of viable and nonviable mutants. Proc Natl Acad Sci USA. 1988a;85:519–523. doi: 10.1073/pnas.85.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn RJ, Tada H, Ypma-Wong MF, Semler BL, Wimmer E. Mutational analysis of the genome-linked protein VPg of poliovirus. J Virol. 1988b;62:4207–4215. doi: 10.1128/jvi.62.11.4207-4215.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YF, Nomoto A, Detjen BM, Wimmer E. A protein covalently linked to poliovirus genome RNA. Proc Natl Acad Sci U S A. 1977;74:59–63. doi: 10.1073/pnas.74.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen EN, Kwok KYR, Birtley JR, Simpson PJ, Subba-Reddy CV, Chaudhry Y, Sosnovtsev SV, Green KY, Prater SN, Tong M, Young JC, Chung LMW, Marchant J, Roberts LO, Kao CC, Matthews S, Goodfellow IG, Curry S. Structures of the Compact Helical Core Domains of Feline Calicivirus and Murine Norovirus VPg Proteins. Journal of Virology. 2013;87:5318–5330. doi: 10.1128/JVI.03151-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyle JM, Clewell A, Richmond K, Richards OC, Hope DA, Schultz SC, Kirkegaard K. Similar structural basis for membrane localization and protein priming by an RNA-dependent RNA polymerase. J Biol Chem. 2002;277:16324–16331. doi: 10.1074/jbc.M112429200. [DOI] [PubMed] [Google Scholar]
- Moturi EK, Porter KA, Wassilak SG, Tangermann RH, Diop OM, Burns CC, Jafari H. Progress toward polio eradication--Worldwide, 2013-2014. MMWR Morb Mortal Wkly Rep. 2014;63:468–472. [PMC free article] [PubMed] [Google Scholar]
- Oezguen N, Schein CH, Peddi SR, Power TD, Izumi T, Braun W. A “moving metal mechanism” for substrate cleavage by the DNA repair endonuclease APE-1. Proteins. 2007;68:313–323. doi: 10.1002/prot.21397. [DOI] [PubMed] [Google Scholar]
- Pallansch MA, Oberste MS, Whitton JL. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Knipe DM, H P, Cohen JI, Griffin DE, Lamb RA, Martin MA, Roizman B, editors. Fields Virology. 6. Lippincott Williams & Wilkins; Philadelphia: 2013. [Google Scholar]
- Pathak HB, Arnold JJ, Wiegand PN, Hargittai MRS, Cameron CE. Picornavirus genome replication - Assembly and organization of the VPg uridylylation ribonucleoprotein (initiaion) complex. Journal of Biological Chemistry. 2007;282:16202–16213. doi: 10.1074/jbc.M610608200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul AV, Peters J, Mugavero J, Yin J, van Boom JH, Wimmer E. Biochemical and genetic studies of the VPg uridylylation reaction catalyzed by the RNA polymerase of poliovirus. J Virol. 2003;77:891–904. doi: 10.1128/JVI.77.2.891-904.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul AV, van Boom JH, Filippov D, Wimmer E. Protein-primed RNA synthesis by purified poliovirus RNA polymerase. Nature. 1998;393:280–284. doi: 10.1038/30529. [DOI] [PubMed] [Google Scholar]
- Salvatoni A, Baj A, Bianchi G, Federico G, Colombo M, Toniolo A. Intrafamilial spread of enterovirus infections at the clinical onset of type 1 diabetes. Pediatric Diabetes. 2013;14:407–416. doi: 10.1111/pedi.12056. [DOI] [PubMed] [Google Scholar]
- Schein CH, Bowen DM, Lewis JA, Choi K, Paul A, van der Heden van Noort GJ, Lu W, Filippov DV. Physicochemical property consensus sequences for functional analysis, design of multivalent antigens and targeted antivirals. BMC Bioinformatics. 2012;13(Suppl 13):S9. doi: 10.1186/1471-2105-13-S13-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein CH, Oezguen N, van der Heden van Noort GJ, Filippov DV, Paul A, Kumar E, Braun W. NMR solution structure of poliovirus uridylyated peptide linked to the genome (VPgpU) Peptides. 2010;31:1441–1448. doi: 10.1016/j.peptides.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein CH, Oezguen N, Volk DE, Garimella R, Paul A, Braun W. NMR structure of the viral peptide linked to the genome (VPg) of poliovirus. Peptides. 2006a;27:1676–1684. doi: 10.1016/j.peptides.2006.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein CH, Volk DE, Oezguen N, Paul A. Novel, structure-based mechanism for uridylylation of the genome-linked peptide (VPg) of picornaviruses. Proteins. 2006b;63:719–726. doi: 10.1002/prot.20891. [DOI] [PubMed] [Google Scholar]
- Smura T, Blomqvist S, Vuorinen T, Ivanova O, Samoilovich E, Al-Hello H, Savolainen-Kopra C, Hovi T, Roivainen M. Recombination in the evolution of enterovirus C species sub-group that contains types CVA-21, CVA-24, EV-C95, EV-C96 and EV-C99. Plos One. 2014;9:e94579. doi: 10.1371/journal.pone.0094579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. CDC tracking enterovirus D-68 outbreak causing severe respiratory illness in children in the Midwest. JAMA. 2014;312:1290. doi: 10.1001/jama.2014.13256. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang Y, Shan C, Chen C, Xu P, Song M, Zhou H, Yang C, Xu W, Shi P-Y, Zhang B, Lou Z. Enterovirus 71 VPg Uridylation Uses a Two-Molecular Mechanism of 3D Polymerase. Journal of Virology. 2012;86:13662–13671. doi: 10.1128/JVI.01712-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z, Wang H, Li Y, Liu G, Xu A, Lin X, Song L, Ji F, Wang S, Cui N, Song Y. Molecular epidemiology of human enterovirus associated with aseptic meningitis in Shandong Province, China, 2006-2012. Plos One. 2014;9:e89766. doi: 10.1371/journal.pone.0089766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez AB, Crowder S, Spagnolo JF, Thompson AA, Peersen OB, Brutlag DL, Kirkegaard K. Nucleotide channel of RNA-dependent RNA polymerase used for intermolecular uridylylation of protein primer. J Mol Biol. 2006;357:665–675. doi: 10.1016/j.jmb.2005.12.044. [DOI] [PubMed] [Google Scholar]
- Tokarz R, Firth C, Madhi SA, Howie SR, Wu W, Sall AA, Haq S, Briese T, Lipkin WI. Worldwide emergence of multiple clades of enterovirus 68. J Gen Virol. 2012;93:1952–1958. doi: 10.1099/vir.0.043935-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy S, Drescher KM, Chapman NM, Kim KS, Carson SD, Pirruccello S, Lane PH, Romero JR, Leser JS. Toward testing the hypothesis that group B coxsackieviruses (CVB) trigger insulin-dependent diabetes: inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence. J Virol. 2002;76:12097–12111. doi: 10.1128/JVI.76.23.12097-12111.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Z, Chapman NM, Hufnagel G, Tracy S, Romero JR, Barry WH, Zhao L, Currey K, Shapiro B. The cardiovirulent phenotype of coxsackievirus B3 is determined at a single site in the genomic 5’ nontranslated region. J Virol. 1995;69:4607–4618. doi: 10.1128/jvi.69.8.4607-4618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heden van Noort GJ, Schein CH, Overkleeft HS, van der Marel GA, Filippov DV. A general synthetic method toward uridylylated picornavirus VPg proteins. J Pept Sci. 2013;19:333–336. doi: 10.1002/psc.2508. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhu Q, Zeng M, Altmeyer R, Zou G. Complete genome sequence of a human enterovirus 71 strain isolated from a fatal case in shanghai, china, in 2012. Genome Announc. 2014;2 doi: 10.1128/genomeA.00457-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ. 2011;342:d35. doi: 10.1136/bmj.d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Gao Z, Zong Y, Bao L, Xu L, Deng W, Li F, Lv Q, Xu Y, Yao Y, Qin C. Histopathological features and distribution of EV71 antigens and SCARB2 in human fatal cases and a mouse model of enterovirus 71 infection. Virus Res. 2014;189:121–132. doi: 10.1016/j.virusres.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Zheng XQ, Chen XQ, Gao Y, Fu M, Chen YP, Xu DP, Lin A, Yan WH. Elevation of human leukocyte antigen-G expression is associated with the severe encephalitis associated with neurogenic pulmonary edema caused by Enterovirus 71. Clin Exp Med. 2014;14:161–167. doi: 10.1007/s10238-013-0237-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


