Abstract
Nonalcoholic fatty livery disease (NAFLD) is the primary cause of chronic liver disease in the United States, afflicting an estimated 80–100 million Americans. NAFLD is a spectrum of liver diseases comprised of nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH). Whereas NAFL has negligible risk of progression, NASH patients often develop cirrhosis or hepatocellular carcinoma. Although liver biopsy is required to diagnose NASH, only patients with a high risk of NASH or advanced fibrosis require this evaluation. Despite the high prevalence of NAFLD, well-defined screening recommendations are currently lacking. In this review, suggestions for screening, diagnosis and initial work-up of NAFLD are given based on established guidelines and recent publications. Proposed drug treatments for NASH are also discussed, highlighting the study outcomes, as well as proposed uses and limitations of these drugs. PubMed was used with search terms nonalcoholic fatty liver disease and nonalcoholic steatohepatitis with filters of “English language.” A date range of January 1, 2000 to May 1, 2015 was used for the search. The bibliographies of key references were also manually searched and seminal publications before the year 2000 were included.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in the United States (US) and its prevalence and clinical importance is increasing worldwide.1, 2 Recent studies estimate that between 30 and 40% of the population in the US, 80–100 million Americans, is affected by NAFLD.3–6 The number of people at risk for NAFLD is even greater given the increasing prevalence of obesity, diabetes and metabolic syndrome6. Nonalcoholic steatohepatitis (NASH) is a frequently progressive subset of NAFLD that can be complicated by cardiovascular disease, cirrhosis and hepatocellular carcinoma (HCC).7, 8 Although there are no Federal Drug Administration approved drugs for NASH, there are several medications that have shown benefits in clinical trials. The uses and limitations of these medications will be discussed in detail (Table 1). Prompt diagnosis, timely referrals and effective treatments are necessary to improve the long-term outcomes of patients with NAFLD and NASH in the setting of primary care and general gastroenterology practices. This review will focus on these important aspects of patient care.
Table 1.
Medications for Use in Patients with NAFLDa
| Medication | Indications | Contraindications | Limitations | Side Effects |
|---|---|---|---|---|
| Pioglitazone | Primary treatment of biopsy-proven NASH in patients with or without DM Treatment of DM in NAFLD patients |
Symptomatic heart failure | May increase risk of bladder cancer | Weight gain, bone loss, GI upset, fatigue, lower extremity edema |
| Vitamin E | Primary treatment of biopsy-proven NASH in patients without DM | History of prostate cancer, bleeding disorder | May increase all-cause mortality, risk of prostate cancer Not tested in patients with DM |
Increased risk of bleeding and hemorrhagic stroke |
| Metformin | Treatment of DM and insulin resistance in NAFLD patients | Renal failure | Not a primary treatment for NASH | Diarrhea, lactic acidosis, GI upset |
| Obeticholic acid | Primary treatment of biopsy-proven NASH in DM and non-DM patients | Not currently commercially available | Not FDA approved or available outside of clinical trials Long-term safety is not known |
Pruritus, hypercholesterolemia |
| Statin | Treatment of hyperlipidemia in NAFLD patients | Excessive alcohol use, hypersensitivity to statin class | Not a primary treatment for NASH | Myalgias, GI upset, mild transaminitis, rare liver injury or myopathy |
DM = diabetes; GI = gastrointestinal; FDA = Federal Drug Administration; NAFLD = nonalcoholic fatty liver disease; NASH = nonalcoholic steatohepatitis
The content of this review is based upon a search of the literature performed in PubMed using the following search terms: nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Studies published in the non-English scientific literature were excluded. A date range of January 1, 2000 to May 1, 2015 was used for the search. The bibliographies of key references were also manually searched and seminal publications before the year 2000 were included.
Histology, Epidemiology and Disease Course
NAFLD is characterized by hepatic steatosis, without a history of excessive alcohol use, in the absence of other known liver disease.1 NAFLD is categorized into two subtypes: NAFL, which is usually non-progressive, and NASH, which is often progressive and can lead to cirrhosis and HCC.3 NAFL and NASH have traditionally been considered two separate clinical entities, rather than two points on a disease continuum.9 Recent studies evaluating sequential liver biopsies are challenging this notion.10–12 A systematic review and meta-analysis of paired biopsy studies found that both patients with NAFL and NASH have the potential to develop progressive liver disease.13 The fibrosis progression rate from stage 0 to stage 1 for NAFL versus NASH is 14 versus 7 years, providing suggestive evidence that NAFL, NASH and fibrosis progression are a continuum rather than separate diagnoses.13 Patients with NAFL and mild lobular inflammation, without ballooning, had increased risk of disease progression compared to those without inflammation.13 Another retrospective study evaluated serial liver biopsies in 108 patients and found no significant difference in the proportion of fibrosis progression between patients with NAFL and NASH at index biopsy (37% vs. 43%, p = 0.65).12 Similarly, a recent study analyzing paired liver biopsies over time showed that even patients with bland steatosis can progress to NASH, especially in the setting of metabolic risk factors14
Establishing an accurate diagnosis of NASH is of major clinical importance. A histologic diagnosis of NASH is associated with cardiovascular disease15 and more rapid progression of liver disease.13 To accurately distinguish NASH from NAFL requires a liver biopsy. NAFL is defined as bland steatosis with minimal or no inflammation, while NASH is characterized by macrovesicular steatosis, ballooning, and mixed lobular inflammation with or without zone-3 perisinusoidal fibrosis16 (fig. 1). Steatosis and ballooning in adult NASH are most commonly zone 3 predominant or panacinar.17, 18 When advanced fibrosis develops, the zonal distribution of steatosis and ballooning is often lost.17, 18 Acidophil bodies (compact eosinophilic cells representing apoptotic hepatocytes), and Mallory-Denk bodies (ropey intracytoplasmic inclusions composed of damaged intermediate filaments) are also frequently seen on biopsies from patients with NASH but are not required for the diagnosis.16
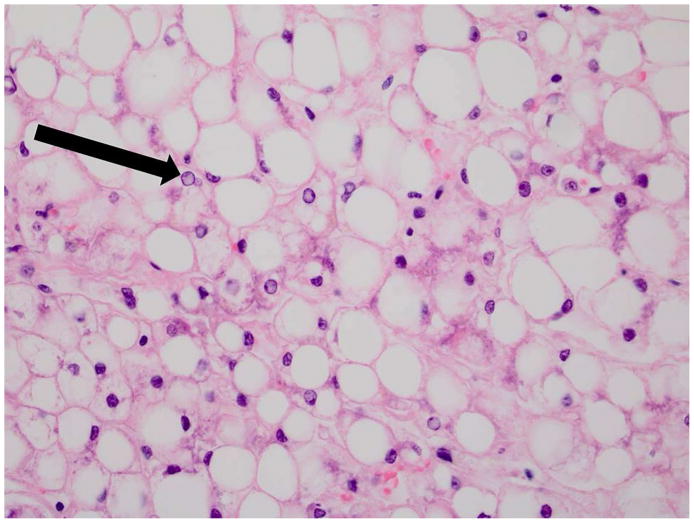
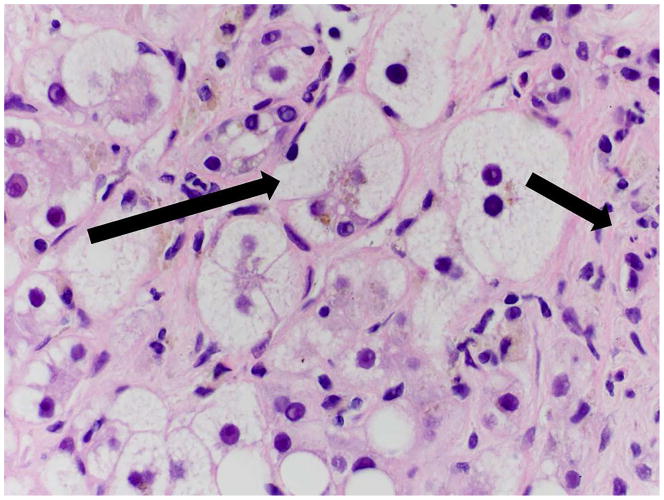
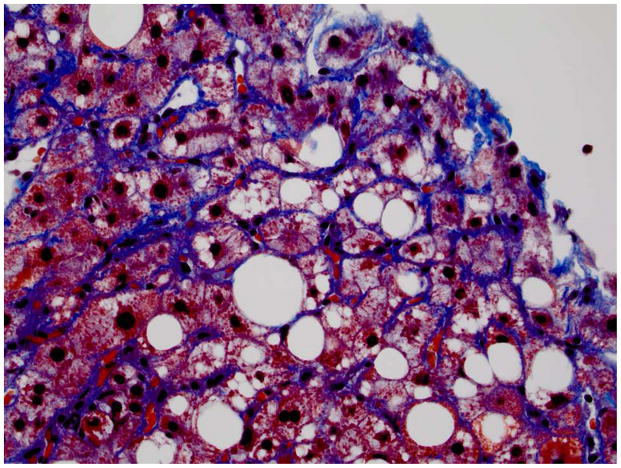
Figure 1. Histopathology of NAFLD.
A. 40x H&E image of NAFL Note the severe fatty change, numerous glycogenated nuclei (arrow) and lack of inflammation or balloon degeneration.
B. 60x H&E image of NASH In addition to significant steatosis, there is evidence of ballooned hepatocytes (long arrow) and mixed inflammation including neutrophils (short arrow).
C. 40x Klatskin trichrome stain image of NASH On this Klatskin stain the sinusoidal fibrosis characteristic of NASH is evident (zone 3).
H&E = hematoxylin and eosin; NAFL = nonalcoholic fatty liver; NAFLD = nonalcoholic fatty liver disease; NASH = nonalcoholic steatohepatitis
In the western world, NAFLD is most commonly associated with obesity, metabolic syndrome and diabetes.19 As with other metabolic conditions, NAFLD appears to have a strong genetic component. Both family history of diabetes and Hispanic ethnicity have been identified as risk factors.19 The first genome-wide association study on NAFLD identified that the variant I148M (rs738409) located in human patatin-like phospholipase domain containing 3 gene (PNPLA3) was associated with increased hepatic fat content and hepatic inflammation.20 This allele was found at higher frequency in Hispanic patients, providing one possible reason for increased susceptibility in this population. Metabolic syndrome, diabetes and advanced age have all been shown to increase the risk of liver disease progression in NAFLD patients.19, 21, 22
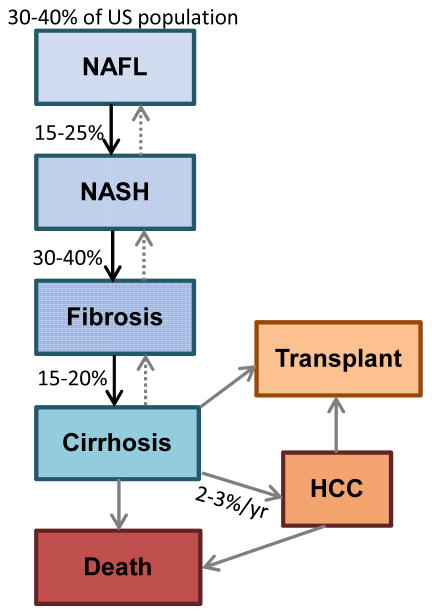
It is estimated that NASH occurs in 20% of patients with NAFLD (3–12% of the US population).5, 6 Approximately 30–40% of patients with NASH will develop fibrosis3, 23–25 (fig. 2)5, 12, 13, 26–28. Although fibrosis regresses in some patients,6, 25, 29 others progress to advanced fibrosis or cirrhosis.25, 30 In fact, NASH is the third leading cause of cirrhosis in the US, and the third most common indication for liver transplant.31, 32 In addition to cirrhosis, and the complications that accompany it, NASH places patients at risk for HCC.33 There have been recent reports of HCC developing in NASH patients without cirrhosis; however the risk appears to be low and routine surveillance for HCC in non-cirrhotic NASH patients is not recommended until further evidence is available.27, 28
Figure 2. Epidemiology of NAFLD.
It is estimated that 3–12% of the US population is affected by NASH. 5 Although NASH patients have a higher risk of progression, it should be noted that some patients may progress directly from NAFL to advanced fibrosis. 12, 13 Approximately 2–3% of patients with NASH cirrhosis will develop HCC each year.26 Some NASH patients without cirrhosis may develop HCC, but the magnitude of risk is not clear.27, 28
sHCC = hepatocellular carcinoma; NAFL = nonalcoholic fatty liver; NAFLD = nonalcoholic fatty liver disease; NASH = nonalcoholic steatohepatitis; US = United States
Pathogenesis
Nonalcoholic fatty liver (NAFL) can be seen in any setting in which there is an excess of energy intake and increased hepatic lipid storage in the form of triglycerides.34 Steatosis may be worsened by de novo lipogenesis in the liver and decreased export of triglycerides from the liver in the form of very-low density lipoproteins.35 NASH occurs in only a subset of patients with NAFLD. The “two-hit” hypothesis of NAFLD suggests that an increase in oxidative stress leads to overwhelming lipid peroxidation and resultant necroinflammatory injury to the fatladen hepatocytes.34, 36 A more recent view of NAFLD pathogenesis identifies lipotoxicity as the primary driver of cellular injury and death in NASH.37 In the lipotoxicity model of NASH, free fatty acid metabolites cause endoplasmic reticular stress, hepatocyte apoptosis, necrosis and inflammation leading to the histologic findings that characterize this disease.38, 39 The hepatocellular injury in turn triggers fibrogenesis and inflammation, hastening disease progression.40 In this model, triglyceride accumulation may actually be protective against hepatocellular injury.37
Like most chronic medical conditions, NASH involves a complex interplay between genetics and the environment. There are strong correlations among NASH, obesity, diabetes, and metabolic syndrome,19 suggesting a mechanistic link between these diseases. Recent investigations have revealed interplay between the liver, innate immune system, gut and adipose tissue. As a result, the farnesoid X receptor (FXR) has become a target of interest.41 FXR plays an important role in modulating metabolism and insulin sensitivity through binding of lipophilic bile acids.41, 42 Dysregulation of the gut-liver axis has also been implicated as a contributor to NASH pathogenesis through derangement of the intestinal microbiome, production of gut-derived endotoxin and altered intestinal permeability.43–45 Chemokines, such as adiponectin46, 47 and TNF-α (tumor necrosis factor alpha),48, 49 may be mediators of some of these systemic changes. A thorough discussion of NAFLD pathogenesis is outside the scope if this review, but is addressed in several recent publications.40, 50
Clinical Presentation
NAFLD is most commonly asymptomatic, although some patients have non-specific complaints, such as fatigue, right upper quadrant discomfort or epigastric fullness. In the absence of advanced liver disease, hepatomegaly may be the only physical finding. Once cirrhosis develops, splenomegaly, spider angiomata, palmar erythema or ascites may be identified. NAFLD is most commonly recognized through abnormal liver chemistries or incidental ultrasound findings and should be considered in the differential of any patient with elevated transaminases.51, 52 Most commonly patients have a mildly elevated aspartate transaminase (AST) and/or alanine transaminase (ALT), with an AST:ALT ratio <1.51 In later stages of the disease this ratio may reverse, so AST:ALT >1 does not exclude NAFLD.53 Although patients with NASH commonly come to medical attention because of an elevated ALT, a normal or near normal ALT level does not exclude NASH.54 Alkaline phosphatase and/or gamma glutamyltransferase may be mildly elevated, but bilirubin typically remains normal unless advanced disease is present. Elevated international normalized ratio, hypoalbuminemia or thrombocytopenia often indicate cirrhosis or portal hypertension, and may be the primary lab findings in patients with advanced fibrosis.
Screening, Diagnosis and Initial Management
The prevalence of NAFLD and NASH is high in the general population, but there are no accepted screening regimens, even in high-risk patients. Despite poor sensitivity and specificity, serum ALT and AST are the most readily available and commonly used tests to evaluate for asymptomatic liver disease. Obtaining a random ALT and AST in patients with metabolic syndrome or diabetes may be reasonable given the high disease burden in this population. Although 50% of patients with NAFLD have normal liver chemistries, up to 80% of patients with NASH may be identified on the basis of elevated transaminases.54
There are several noninvasive scoring systems designed to increase the detection of NAFLD and advanced fibrosis,55, 56 but the best way to incorporate these into a screening model is not clear. The fatty liver index, for example, uses triglycerides, waist circumference, body mass index and gamma-glutamyltransferase to improve the sensitivity and specificity of diagnosing NAFLD,57 but there is no consensus on which, or if, patients should be screened using this or similar models. Biomarkers, such as cytokeratin 18, currently lack the sensitivity needed to be clinically useful screening tests.58 A recent study from the NASH Clinical Research Network proposed and validated a model designed to quantify the likelihood of NASH or advanced fibrosis in adult diabetic patients using routinely available clinical variables and labs.59 The model for NASH identification included white race, body mass index (BMI), waist circumference, ALT, AST, albumin, hemoglobin A1c, Homeostasis Model Assessment of insulin resistance, and ferritin. Although sensitivity was poor (56.8%), the model demonstrated excellent specificity (90.0%) and had a positive predictive value of 93.2%, making it a helpful clinical tool to predict the presence of NASH in adult diabetics. Despite improvements in predictive models, screening is not recommended at this time in any population.
A baseline liver evaluation and cardiovascular risk assessment should be considered in all patients who have a presumptive diagnosis of NAFLD (Table 2)59. A liver ultrasound, full liver chemistry panel, international normalized ratio, creatinine and complete blood count should be obtained to characterize the pattern of liver injury and to assess the severity of disease. Other causes of liver disease should be investigated and excluded, and medications known to worsen steatosis should be discontinued.60 Common comorbidities, such as diabetes and dyslipidemia, should be identified and treated. Higher quantity of fat in the liver is associated with increased cardiovascular risk,15 but there are no consensus on routine cardiovascular screening in this population. Patients with cirrhosis should be screened for HCC and esophageal varices according to current American Association of Liver Disease guidelines.30 There are no current HCC screening guidelines for NASH patients without cirrhosis.61
Table 2.
Recommended Management of Patients with NAFLDa
|
AASLD = American Association of Liver Disease; ALT = alanine transferase; AST = aspartate aminotransferase; BMI = body mass index; CBC = complete metabolic panel; EGD = esophagoduodenoscopy; HCC = hepatocellular carcinoma; Hgb A1c = hemoglobin A1c; INR = international normalized ratio; NAFLD = nonalcoholic fatty liver disease; NASH = nonalcoholic steatohepatitis
Biopsy should be considered in diabetics with other risk factors for advanced fibrosis.59
Imaging
Ultrasonography is the most inexpensive and widely available imaging test for NAFLD.51, 62 Typical sonographic findings of NAFLD are hepatomegaly and increased echogenicity. Unfortunately, ultrasonography is not sensitive if less than 30% of the liver is involved by steatosis.63 Variability in operator skill and the limiting body habitus of the typical NAFLD patient can lead to inadequate or inconsistent results.64 Although changes of cirrhosis and portal hypertension may be identified on ultrasound, it is neither a sensitive nor specific modality for this diagnosis.62 Given these limitations, other imaging studies are being investigated for the diagnosis and risk stratification of NAFLD patients. Magnetic resonance imaging, including magnetic resonance spectroscopy, has shown good sensitivity and specificity in detecting and quantifying steatosis,65 but is it expensive and not widely utilized for this purpose.66–68 Transient elastography has been used with moderate success to evaluate the degree of fibrosis and cirrhosis in NAFLD patients, but is limited by body habitus and degree of steatosis.69, 70 Magnetic resonance elastography (MRE) provides an accurate non-invasive measure of fibrosis in NAFLD, and may be particularly helpful in identifying patients with advanced fibrosis.71, 72 Among all non-invasive imaging modalities, MRE appears to be the most accurate test for fibrosis assessment in NAFLD.72, 73 Despite growing evidence of its potential clinical utility, MRE is not widely available and data are needed to show its performance and cost-effectiveness as a fibrosis screening test in routine clinical practice. If validated in prospective studies, MRE may be utilized to screen for advanced fibrosis in high-risk patients, such as older diabetics. A novel quantitative ultrasound technique using ultrasound imagingbased biomarkers is being developed for diagnosis and quantification of hepatic steatosis.74 Although not yet commercially available, in the future this could provide a low-cost method of identifying patients with probable NAFLD. As with all diagnostic modalities, advanced imaging studies should be ordered in a facility equipped to accurately perform and interpret the test. With the development of more specialized and complex testing, it is important that the ordering physician understand the indications for the study as well as the clinical application of specific results.
Referral for Consideration of Liver Biopsy
Liver biopsy is the gold standard for diagnosing NAFL and NASH, but is not indicated in all patients with suspected disease. It is invasive, expensive and not without risk. Liver biopsy should be considered in all patients with persistently elevated aminotransferases in whom the diagnosis remains uncertain.60 Biopsy is also important in ruling out the presence of other concomitant liver disease, such as in the setting of elevated serum ferritin or the presence of autoantibodies. Furthermore, the biopsy has prognostic value in NAFLD as presence of NASH and/or fibrosis provides information regarding future risk of progression to cirrhosis and risk of liver-related mortality.
The American Association of Liver Disease practice guidelines for NAFLD recommend considering a liver biopsy in NAFLD patients who are at increased risk of NASH and advanced fibrosis.1 These guidelines suggest that the presence of metabolic syndrome and the NAFLD fibrosis score be used to identify these high-risk patients.1 A recent study by the NASH Clinical Research Network used data on 435 patients with NAFLD and diabetes to develop and verify a model to predict the presence of NASH and advanced fibrosis in diabetic NAFLD patients.59 Among this large cohort of patients, 69.2% were found to have NASH and 41.0% were found to have advanced fibrosis,59 highlighting the high risk of progressive disease in this population. The model used to predict advanced fibrosis in diabetics included age, BMI, wait-to-hip ratio, Hispanic ethnicity, hypertension, ALT-to-AST ratio, bilirubin (total and direct), alkaline phosphatase, isolated abnormal alkaline phosphatase, globulin, albumin, serum insulin, hematocrit, international normalized ratio, and platelet count.59 This proposed model predicted advanced fibrosis better than the NAFLD fibrosis score, and may be used to guide liver biopsy referral in patients with type II diabetes. New evidence suggests that advanced age or a strong family history of NASH may also be associated with a more aggressive disease course5 but there are not adequate data to support biopsy in all patients with these risk factors. Patients undergoing bariatric surgery have a high prevalence of NASH and should strongly consider having an intraoperative liver biopsy for diagnosis and staging.75 Lab parameters suggesting cirrhosis, such as thrombocytopenia, hypoalbuminemia and AST>ALT should also prompt consideration of liver biopsy referral.76
In addition to proving a definitive diagnosis, liver biopsy is useful in NAFLD to identify the stage of liver disease and assess the likelihood of progression. Liver biopsies are subject to significant sampling error that may lead to inaccurate staging or disease classification,77 but liver biopsy remains the gold standard for diagnosis and staging. Patients with bland steatosis without necroinflammation on biopsy are unlikely to develop cirrhosis, assuming metabolic parameters remain unchanged. The severity of steatosis, however, may correlate with the development of metabolic syndrome and the risk of cardiovascular disease independent of the presence of NASH.15 The presence of NASH on index biopsy predicts a more aggressive disease course with a higher rate of progression to cirrhosis.11 In addition to informing patient discussions about prognosis, this information helps identify which patients should be considered for more aggressive therapy. All currently recommended pharmacologic treatments for NASH require a histologic diagnosis prior to initiation of therapy.1
Lifestyle Interventions
Weight loss is the cornerstone of NAFLD treatment. Clinical trials have shown that weight loss reduces hepatic steatosis78 and that exercise itself may improve histology regardless of weight change.79, 80 Even resistance training led to improvements in hepatic steatosis in a recent randomized controlled trial.81 A weight loss of at least 3–5% of total body weight has been found to improve steatosis, and weight loss of more than 7% of total body weight is associated with a decrease in necroinflammation.82 A small randomized control trial (RCT) of 31 patients showed that an intensive weight loss program led to more weight loss, improvement in ALT and histology when compared to general education alone.78 Two-thirds of patients in the intervention group no longer met the definition of NASH after the 48-week study period. This suggests that patients may reap more benefit from a structured weight loss regimen than from physician education alone. Encouraging patients to seek additional training and assistance with weight loss may be worthwhile.
Bariatric Surgery
Although bariatric surgery has been shown to help morbidly obese patients lose weight, there are limited data supporting its use as a treatment for NASH. A Cochrane review of 21 prospective and retrospective studies showed an improvement in steatosis and/or inflammation scores following bariatric surgery, but the authors were unable to recommend its use for this indication as none of the studies were adequately randomized or controlled.75 A recent study from France looked prospectively at 109 obese patients with biopsy-proven NASH who underwent bariatric surgery.83 Nearly 85% of patients had histologic resolution of NASH at one year. Histologic resolution was most common in patients with mild NASH prior to surgery and in patients who underwent gastric bypass rather than vertical gastric banding.83 The study suggests bariatric surgery may be a promising treatment for NASH, but is limited by the lack of randomization or a control group.
In a large Cochrane review, four studies found worsening fibrosis in NAFLD patients who underwent bariatric surgery.75 The finding of worsening disease was seen primarily in patients with very high BMI or advanced fibrosis,75 indicating that this surgery may not be safe in all patients. Other studies evaluating the risk of bariatric surgery in NAFLD patients have found no increase in post-operative complications.84, 85 The Mayo Clinic published a review of 14 patients with compensated cirrhosis who underwent bariatric surgery at their center.86 Although there was no increase in perioperative complications, the authors noted that the results only apply to well-compensated cirrhotic patients treated in a large referral center.86 Patients with NAFLD who meet other medical criteria for surgery should be referred for bariatric procedures,87 but data to support bariatric surgery as a specific treatment for NASH remain limited.1 A prospective RCT is needed to further investigate the safety and efficacy of this promising intervention.
Pioglitazone
Although the pathogenesis of NASH has not been fully elucidated, there is a clear association with insulin resistance and sensitization of the liver to metabolic injury and inflammation. Several insulin sensitizing medications have been tested for the treatment of NASH, with mostly limited success. Although no medications are Federal Drug Administration approved for NASH treatment, pioglitazone appears to have a beneficial effect on steatosis, necroinflammation and possibly even fibrosis.88, 89 Pioglitazone and other thiazolidinediones (TZDs) are selective peroxisome proliferator-activated receptor-gamma (PPAR-γ) agonists. They modify the adipocytokine profile by stimulating adipocyte maturation. This in turn increases beta-oxidation of fatty acids and reduces pro-inflammatory cytokines. PPAR-γ agonists also work at the level of the muscle, liver and adipose tissue to improve insulin sensitivity.
The largest RCT of pioglitazone use in NASH is the PIVENS trial. This study included 247 nondiabetic patients with biopsy-proven NASH randomized to treatment with 96 weeks of placebo, pioglitazone 30 mg or vitamin E 800 IU.90 The primary end point was histologic improvement, which required improvement by 1 or more points in the hepatocellular ballooning score, no increase in the fibrosis score and either a decrease in the NAFLD activity score (NAS) to a score of 3 points or less or a decrease in the NAS of at least 2 points, with at least a 1-point decrease in either the lobular inflammation or steatosis score.91 The NAS is calculated using the grade of steatosis (grade 0–3), ballooning (grade 0–2) and lobular inflammation (grade 0–3),1 and serves as a relatively objective manner of standardizing NASH histology and allowing comparison of biopsies in clinical trials. Although the pioglitazone arm did not meet the primary endpoint (P=0.04), there was a significant reduction in steatosis (P<0.001) and lobular inflammation in this group (P=0.004).90 The most common adverse events were gastrointestinal upset, lower extremity edema and fatigue.
A meta-analysis from Boettcher et. al. reviewed four good quality RCTs, including the PIVENS trial, addressing treatment of NASH with TZDs.88 When both rosiglitazone and pioglitazone were included in the analysis, there was a statistically significant improvement in necroinflammation when compared to placebo, but again no improvement in fibrosis. A subgroup analysis of pioglitazone versus placebo, however, did show an improvement in fibrosis.88
Although pioglitazone treatment has been associated with histologic improvement in NASH, there are drawbacks to its use. TZDs commonly cause a significant increase in body weight with an average increase of 4 kg.88 Rosiglitazone is no longer available in the US due to an association with coronary events and decompensation of heart failure. Due to similar concerns about pioglitazone, a large meta-analysis was performed which included 16,390 patients with type two diabetes.92 Although pioglitazone was associated with a statistically significant increase in congestive heart failure, from 1.8 to 2.3 % (p=0.002), all-cause mortality, MI and stroke were reduced in patients taking pioglitazone.92 Unfortunately, since only diabetic patients were included in these safety studies, it is not known if the findings are applicable to all patients with NASH. Considering only diabetic NASH patients for treatment with this agent may be overly restrictive, since all of the studies which investigated histologic improvement were performed in non-diabetic patients. However, only patients with biopsy-proven NASH should be considered for treatment with pioglitazone, as these are the patients who are at greatest risk of progression and thus most likely to benefit from a pharmacologic intervention.60 This medication should be avoided in patients with known heart failure, a history of bladder cancer or an increased risk of bone loss, as use may increase risk of these conditions.
Vitamin E
The pathogenesis of NASH is felt to be at least in part due to damage from oxidative stress induced by reactive oxygen species. Vitamin E (alpha-tocopherol) is a naturally occurring antioxidant thought to mitigate oxidative stress and reactive oxygen species formation through suppression of lipid peroxidation, and has been investigated as a possible therapy for NASH. In the TONIC trial, 173 children and adolescents with biopsy-proven NASH were randomized to treatment with vitamin E 800 IU, metformin or placebo.93 At the end of the 96-week trial, the vitamin E group had achieved a significant improvement in NAS (P=0.02) and hepatocellular ballooning score (P=0.006), but no significant change in ALT (P=0.07) compared to placebo. The PIVENS trial, which included only non-diabetic patients with biopsy-proven NASH and no evidence of cirrhosis, remains the largest RCT to investigate the effect of vitamin E on NASH.90 After 96 weeks of treatment, patients in the vitamin E arm achieved a significant improvement in NASH histology (P=0.001). Furthermore, both vitamin E and pioglitazone led to resolution of NASH in approximately one-third of the patients. Vitamin E use was also associated with a decrease in ALT (P=0.001) and NAS (P=<0.001), but fibrosis scores were not changed when compared to placebo (P=0.24).90
Vitamin E is well-tolerated with minimal side effects, but the safety of long-term use is not known.94 A large meta-analysis of randomized antioxidant supplement trials suggested an increase in all-cause mortality in patients taking vitamin E (RR 1.04; 95% CI, 1.01–1.07), or other antioxidant supplements.95 Vitamin E use is also associated with an increased risk of prostate cancer with an absolute risk of 1.6 per 1000 person year.96 A meta-analysis investigating the effect of vitamin E on stroke subtypes found a decrease in ischemic stroke (RR 0.90, P=0.02), but an increase in hemorrhagic stroke (RR 1.22, P=0.045).97 Although the absolute risk increase for these conditions remains low, it is recommended that these potential health concerns be discussed with patients prior to initiating treatment. At this time, there is no evidence for use in patients with diabetes, cirrhosis or NAFLD without biopsy-proven NASH. Given the promising study results and lack of alternative therapies, it is reasonable to start 800 IU of vitamin E in non-diabetic, non-cirrhotic patients with biopsy-confirmed NASH.60 It is not recommended to treat with vitamin E unless the patient has a biopsy-proven diagnosis of NASH.1
Emerging Therapies
Obeticholic acid, a synthetic derivative of the naturally occurring bile salt chenodeoxycholic acid, is a FXR agonist that has recently been evaluated as a potential therapy for NASH.98 When bound to the FXR, lipophilic bile acids such as obeticholic acid have been shown to decrease hepatic gluconeogenesis and improve insulin sensitivity.99 In the FLINT trial, 283 patients were randomized to receive obeticholic acid or placebo with biopsies performed at baseline and after 72 weeks of treatment.42 After a planned interim analysis of histologic changes demonstrated benefit from treatment, the decision was made to complete the 72 week biopsy in only 200 of the patients. The final analysis demonstrated a statistically significant improvement in histologic features of NASH, including ballooning, inflammation, steatosis and fibrosis.42 This study included both diabetic and non-diabetic patients, as well as non-responders to vitamin E who were randomized to either obeticholic acid or placebo. Although nearly a quarter of patients treated with obeticholic acid developed pruritus, only one patient had to discontinue therapy due to severe pruritus. Due to its effect on bile acid synthesis and cholesterol disposal, obeticholic acid was predictably associated with a significant increase in total cholesterol and low-density lipoprotein (LDL) with a decrease in high density lipoprotein. Future studies are needed to examine the long-term effects of these alterations on the cardiovascular risk in patients with NASH. Mechanistic studies are underway to better understand the pathogenesis of obeticholic acid-induced pruritus and LDL increase, and determine if co-treatment with other pharmacologic agents can modify the pruritus or risks of LDL increase. It is important to note that ursodeoxycholic acid, a bile acid derivative that is currently in clinical use, failed to show convincing benefit in NASH.100, 101 However, unlike obeticholic acid, ursodeoxycholic acid is not an effective FXR agonist.
Pentoxifylline (PTX) is a phosphodiesterase inhibitor that has been shown to inhibit the proinflammatory cytokine TNF-α.102 TNF-α has been implicated in the pathogenesis of NASH in animal models,45 although its specific contribution in human NASH is not entirely clear.103 PTX also increases hepatic glutathione production, which may contribute to its hepatoprotective effect. Human studies investigating PTX therapy in NASH are limited. Only two RCTs to date have investigated histologic end points of treatment.104, 105 Zein et al conducted a placebocontrolled trial including 55 patients with biopsy-proven NASH who were randomized to either PTX 400 mg three times daily orally or placebo for 12 months.105 Liver histology was assessed at the end of the treatment. The PTX group demonstrated significant improvement in serum ALT, histologic features of NASH and fibrosis. There was histologic resolution of NASH in 25% of the treatment arm, compared to 3.9% of those receiving placebo.105 Cirrhotic patients were excluded from the study and less than 10% of the study group had diabetes. A smaller RCT including 26 patients who were randomized to either PTX or placebo did not show improvement in histology or ALT.104 Although the dosing and treatment duration were the same as the other RCT, this study included patients with cirrhosis, which may account for some of the differences in outcomes. Nausea and vomiting were the most common side effects in those receiving PTX. Although these studies indicate that PTX may have therapeutic potential, there are insufficient data to recommend its use in NASH at this time. Additional studies investigating the use of PTX are underway.
Metformin increases peripheral glucose and fatty acid uptake while inhibiting lipogenesis and gluconeogenesis. It has been shown to improve insulin resistance and transaminitis, while inducing weight loss.106 In two pilot studies, metformin improved liver histology93, 107, 108 but subsequent RCTs did not support this finding.93, 108–110 Metformin has been shown to be very effective in treating diabetes and insulin resistance and is safe for use in patients with NAFLD. Physicians are encouraged to use metformin for the management of pre-diabetes or diabetes in those who have co-existing NAFLD. Recent case-control and cohort studies have shown the benefit of metformin in reducing the risk of liver decompensation, death, and HCC in patients with cirrhosis.111 Further RCTs are needed to assess the effect of metformin in reducing the risk of hepatic decompensation and HCC in patients with NASH cirrhosis. There are several other therapies outside the scope of this review that are in clinical trials for the treatment of NAFLD and NASH.112 Descriptions of ongoing trials are available through the National Institute of Health website at Clinicaltrials.gov.
Referral for Consideration of Liver Transplantation
Despite optimal medical management, many patients with NASH will develop cirrhosis. In fact, NASH often goes unrecognized until patients present with symptoms of decompensated liver disease. Cirrhosis due to NASH is currently the third most common indication for liver transplant in the US and the second most common indication for liver transplant listing.32 UNOS data have shown that the number of patients with NASH listed for liver transplant has tripled in the last 10 years.32 NASH is also the most rapidly growing etiology of liver disease among patients transplanted for HCC.31 Despite their elevated BMIs and medical comorbidities, patients transplanted for NASH cirrhosis have no worse 90-day survival than those transplanted for hepatitis C or alcoholic liver disease.32 Studies evaluating long-term posttransplant survival in NASH patients show that survival is similar, or even superior, to those transplanted for other etiologies.113, 114 Although graft loss is significantly lower in NASH patients, risk of cardiovascular death is much higher,114 highlighting the importance of excellent preventative care following transplant. Patients transplanted for NASH cirrhosis also have a higher rate of chronic kidney disease after transplantation.115 Any patient developing signs of decompensated cirrhosis, such as ascites, hepatic encephalopathy or variceal bleed, and/or a model for end-stage liver disease score ≥10 should be referred to a transplant center for evaluation.116
Future Directions and Conclusion
NAFLD is one of the leading causes of chronic liver disease worldwide. The obesity epidemic has lead to an ever-increasing number of patients at risk for NAFLD and NASH. Liver biopsy is costly and invasive, but it remains the only definitive means to diagnose NASH. Therefore, the development of non-invasive methods of diagnosing NASH is a major unmet need in the field. Although weight loss and other lifestyle modifications remain the foundation of treatment, there are now good data to support the use of pioglitazone and vitamin E in specific patients.60, 90 Unfortunately, fewer than 50% of patients with NASH respond to current therapies, which underlines the importance of continued research and the need for improved therapies for NASH and NASH-related fibrosis. There are several promising therapeutic modalities and targets currently under investigation. The field is at a tipping point of discovery and innovation. With continued interest in research efforts, improvements in the diagnosis and management of NAFLD and NASH will continue in coming years.
Article Highlights.
Nonalcoholic fatty liver disease (NAFLD) is a spectrum of liver diseases comprised of nonalcoholic fatty liver (NAFL), which has a negligible risk of progression, and nonalcoholic steatohepatitis (NASH), which has a higher risk of liver disease progression. NASH is a histologic diagnosis based on liver biopsy findings of steatosis, ballooning and lobular inflammation. NASH is associated with an increased risk of cardiovascular death, cirrhosis, end-stage liver disease and hepatocellular carcinoma.
Patients with suspected or known NAFLD and a high risk for NASH or advanced fibrosis should be referred for consideration of liver biopsy.
Lifestyle modifications, including weight loss and exercise, form the cornerstone of NAFLD treatment and should be strongly encouraged. Vitamin E and pioglitazone have been shown to benefit select patients with biopsy-proven NASH.
Statins and metformin therapy are not indicated for the treatment of NASH, but are safe and effective in NASH patients with other clinical indications for their use, such as dyslipidemia and diabetes.
Acknowledgments
Leana Guerin, MD from the University of Iowa Hospitals & Clinics, Department of Pathology for preparing the histology figures.
Financial support: RL is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award; Funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Association of Specialty Professors, and the American Gastroenterological Association and grant K23-DK090303-02.
Abbreviations
- ALT
alanine transferase
- AST
aspartate aminotransferase
- BMI
body mass index
- EGD
esophagoduodenoscopy
- FXR
farnesoid X receptor
- GGT
gammaglutamyltransferase
- HCC
hepatocellular carcinoma
- Hgb A1c
hemoglobin A1c
- LDL
lowd-ensity lipoprotein
- MRE
magnetic resonance elastography
- NAFL
nonalcoholic fatty liver
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD activity score
- NASH
nonalcoholic steatohepatitis
- RCT
randomized control trial
- RR
relative risk
- TZD
thiazolidinediones
- TNF-α
tumor necrosis factor alpha
- US
United States
Footnotes
Disclosures: The study sponsor(s) had no role in the study design, collection, analysis, interpretation of the data, and/or drafting of the manuscript. EKS has no financial support to disclose. All authors report that no conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Erin K Spengler, Division of Gastroenterology, Department of Medicine, University of Iowa Hospitals and Clinics.
Rohit Loomba, Translational Research Unit, Division of Gastroenterology, Department of Medicine, Division of Epidemiology, Department of Family and Preventive Medicine, UC San Diego School of Medicine.
References
- 1.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 2.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 3.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 4.Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999–2004. Gastroenterology. 2007;133:1814–1820. doi: 10.1053/j.gastro.2007.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 6.Wong VW, Wong GL, Choi PC, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969–974. doi: 10.1136/gut.2009.205088. [DOI] [PubMed] [Google Scholar]
- 7.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Bhala N, Angulo P, van der Poorten D, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54:1208–1216. doi: 10.1002/hep.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22:1714–1719. [PubMed] [Google Scholar]
- 10.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 12.McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J Hepatol. 2015;62:1148–1155. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 13.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis Progression in Nonalcoholic Fatty Liver vs Nonalcoholic Steatohepatitis: A Systematic Review and Meta-analysis of Paired-Biopsy Studies. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pais R, Charlotte F, Fedchuk L, et al. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol. 2013;59:550–556. doi: 10.1016/j.jhep.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Arulanandan A, Ang B, Bettencourt R, et al. Association Between Quantity of Liver Fat and Cardiovascular Risk in Patients with Non-alcoholic Fatty Liver Disease Independent of Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 16.Yeh MM, Brunt EM. Pathological features of fatty liver disease. Gastroenterology. 2014;147:754–764. doi: 10.1053/j.gastro.2014.07.056. [DOI] [PubMed] [Google Scholar]
- 17.Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis. 2012;32:3–13. doi: 10.1055/s-0032-1306421. [DOI] [PubMed] [Google Scholar]
- 18.Chalasani N, Wilson L, Kleiner DE, et al. Relationship of steatosis grade and zonal location to histological features of steatohepatitis in adult patients with non-alcoholic fatty liver disease. J Hepatol. 2008;48:829–834. doi: 10.1016/j.jhep.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loomba R, Abraham M, Unalp A, et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943–951. doi: 10.1002/hep.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pirazzi C, Adiels M, Burza MA, et al. Patatin-like phospholipase domain-containing 3 (PNPLA3) I148M (rs738409) affects hepatic VLDL secretion in humans and in vitro. J Hepatol. 2012;57:1276–1282. doi: 10.1016/j.jhep.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 21.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 22.Noureddin M, Yates KP, Vaughn IA, et al. Clinical and histological determinants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology. 2013;58:1644–1654. doi: 10.1002/hep.26465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fassio E, Alvarez E, Dominguez N, Landeira G, Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40:820–826. doi: 10.1002/hep.20410. [DOI] [PubMed] [Google Scholar]
- 24.Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371–379. doi: 10.1016/j.jhep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis Progression in Nonalcoholic Fatty Liver versus Nonalcoholic Steatohepatitis: A Systematic Review and Metaanalysis of Paired-Biopsy Studies. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 27.Takuma Y, Nouso K. Nonalcoholic steatohepatitis-associated hepatocellular carcinoma: our case series and literature review. World J Gastroenterol. 2010;16:1436–1441. doi: 10.3748/wjg.v16.i12.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 29.Hamaguchi E, Takamura T, Sakurai M, et al. Histological course of nonalcoholic fatty liver disease in Japanese patients: tight glycemic control, rather than weight reduction, ameliorates liver fibrosis. Diabetes Care. 2010;33:284–286. doi: 10.2337/dc09-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 31.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–2195. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 32.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic Steatohepatitis Is the Second Leading Etiology of Liver Disease Among Adults Awaiting Liver Transplantation in the United States. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto E, Yatsuji S, Tobari M, et al. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol. 2009;44(Suppl 19):89–95. doi: 10.1007/s00535-008-2262-x. [DOI] [PubMed] [Google Scholar]
- 34.Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 35.Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM. 2010;103:71–83. doi: 10.1093/qjmed/hcp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tariq Z, Green CJ, Hodson L. Are oxidative stress mechanisms the common denominator in the progression from hepatic steatosis towards non-alcoholic steatohepatitis (NASH)? Liver Int. 2014;34:e180–190. doi: 10.1111/liv.12523. [DOI] [PubMed] [Google Scholar]
- 37.Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–788. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 38.Feldstein AE. Novel insights into the pathophysiology of nonalcoholic fatty liver disease. Semin Liver Dis. 2010;30:391–401. doi: 10.1055/s-0030-1267539. [DOI] [PubMed] [Google Scholar]
- 39.Loomba R, Quehenberger O, Armando A, Dennis EA. Polyunsaturated fatty acid metabolites as novel lipidomic biomarkers for noninvasive diagnosis of nonalcoholic steatohepatitis. J Lipid Res. 2015;56:185–192. doi: 10.1194/jlr.P055640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuppan D, Schattenberg JM. Non-alcoholic steatohepatitis: pathogenesis and novel therapeutic approaches. J Gastroenterol Hepatol. 2013;28(Suppl 1):68–76. doi: 10.1111/jgh.12212. [DOI] [PubMed] [Google Scholar]
- 41.Trauner M, Claudel T, Fickert P, Moustafa T, Wagner M. Bile acids as regulators of hepatic lipid and glucose metabolism. Dig Dis. 2010;28:220–224. doi: 10.1159/000282091. [DOI] [PubMed] [Google Scholar]
- 42.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2014 doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mouzaki M, Comelli EM, Arendt BM, et al. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120–127. doi: 10.1002/hep.26319. [DOI] [PubMed] [Google Scholar]
- 44.Raman M, Ahmed I, Gillevet PM, et al. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2013;11:868–875. e861–863. doi: 10.1016/j.cgh.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 45.Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–211. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Musso G, Gambino R, De Michieli F, Durazzo M, Pagano G, Cassader M. Adiponectin gene polymorphisms modulate acute adiponectin response to dietary fat: Possible pathogenetic role in NASH. Hepatology. 2008;47:1167–1177. doi: 10.1002/hep.22142. [DOI] [PubMed] [Google Scholar]
- 47.Musso G, Gambino R, Durazzo M, et al. Adipokines in NASH: postprandial lipid metabolism as a link between adiponectin and liver disease. Hepatology. 2005;42:1175–1183. doi: 10.1002/hep.20896. [DOI] [PubMed] [Google Scholar]
- 48.Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880–885. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- 49.Maher JJ, Leon P, Ryan JC. Beyond insulin resistance: Innate immunity in nonalcoholic steatohepatitis. Hepatology. 2008;48:670–678. doi: 10.1002/hep.22399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725. e716. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Dowman JK, Tomlinson JW, Newsome PN. Systematic review: the diagnosis and staging of nonalcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2011;33:525–540. doi: 10.1111/j.1365-2036.2010.04556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000;342:1266–1271. doi: 10.1056/NEJM200004273421707. [DOI] [PubMed] [Google Scholar]
- 53.Sorbi D, Boynton J, Lindor KD. The ratio of aspartate aminotransferase to alanine aminotransferase: potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am J Gastroenterol. 1999;94:1018–1022. doi: 10.1111/j.1572-0241.1999.01006.x. [DOI] [PubMed] [Google Scholar]
- 54.Yan E, Durazo F, Tong M, Hong K. Nonalcoholic fatty liver disease: pathogenesis, identification, progression, and management. Nutr Rev. 2007;65:376–384. doi: 10.1301/nr.2007.aug.376-384. [DOI] [PubMed] [Google Scholar]
- 55.Angulo P, Bugianesi E, Bjornsson ES, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:782–789. e784. doi: 10.1053/j.gastro.2013.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 57.Koehler EM, Schouten JN, Hansen BE, Hofman A, Stricker BH, Janssen HL. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin Gastroenterol Hepatol. 2013;11:1201–1204. doi: 10.1016/j.cgh.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 58.Cusi K, Chang Z, Harrison S, et al. Limited Value of Plasma Cytokeratin-18 as a Biomarker for NASH and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease (NAFLD) J Hepatol. 2013 doi: 10.1016/j.jhep.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 59.Bazick J, Donithan M, Neuschwander-Tetri BA, et al. Clinical Model for NASH and Advanced Fibrosis in Adult Patients With Diabetes and NAFLD: Guidelines for Referral in NAFLD. Diabetes Care. 2015 doi: 10.2337/dc14-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 61.White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342–1359. e1342. doi: 10.1016/j.cgh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Festi D, Schiumerini R, Marzi L, et al. Review article: the diagnosis of non-alcoholic fatty liver disease -- availability and accuracy of non-invasive methods. Aliment Pharmacol Ther. 2013;37:392–400. doi: 10.1111/apt.12186. [DOI] [PubMed] [Google Scholar]
- 63.Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21:87–97. doi: 10.1007/s00330-010-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Idilman IS, Keskin O, Celik A, et al. A comparison of liver fat content as determined by magnetic resonance imaging-proton density fat fraction and MRS versus liver histology in non-alcoholic fatty liver disease. Acta Radiol. 2015 doi: 10.1177/0284185115580488. [DOI] [PubMed] [Google Scholar]
- 66.Tang A, Desai A, Hamilton G, et al. Accuracy of MR Imaging-estimated Proton Density Fat Fraction for Classification of Dichotomized Histologic Steatosis Grades in Nonalcoholic Fatty Liver Disease. Radiology. 2014:140754. doi: 10.1148/radiol.14140754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang A, Tan J, Sun M, et al. Nonalcoholic Fatty Liver Disease: MR Imaging of Liver Proton Density Fat Fraction to Assess Hepatic Steatosis. Radiology. 2013 doi: 10.1148/radiol.12120896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Permutt Z, Le TA, Peterson MR, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22–29. doi: 10.1111/j.1365-2036.2012.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–462. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 70.Naveau S, Lamouri K, Pourcher G, et al. The diagnostic accuracy of transient elastography for the diagnosis of liver fibrosis in bariatric surgery candidates with suspected NAFLD. Obes Surg. 2014;24:1693–1701. doi: 10.1007/s11695-014-1235-9. [DOI] [PubMed] [Google Scholar]
- 71.Loomba R, Wolfson T, Ang B, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: A prospective study. Hepatology. 2014 doi: 10.1002/hep.27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cui J, Ang B, Haufe W, et al. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Aliment Pharmacol Ther. 2015;41:1271–1280. doi: 10.1111/apt.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh S, Venkatesh SK, Wang Z, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015;13:440–451. e446. doi: 10.1016/j.cgh.2014.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin SC, Heba E, Wolfson T, et al. Noninvasive Diagnosis of Nonalcoholic Fatty Liver Disease and Quantification of Liver Fat Using a New Quantitative Ultrasound Technique. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, Mendez-Sanchez N, Lizardi-Cervera J, Uribe M. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010:CD007340. doi: 10.1002/14651858.CD007340.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372–384. doi: 10.1016/j.jhep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 77.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 78.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sullivan S, Kirk EP, Mittendorfer B, Patterson BW, Klein S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology. 2012;55:1738–1745. doi: 10.1002/hep.25548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80..
- 81.Bacchi E, Negri C, Zanolin ME, et al. Metabolic effects of aerobic training and resistance training in type 2 diabetic subjects: a randomized controlled trial (the RAED2 study) Diabetes Care. 2012;35:676–682. doi: 10.2337/dc11-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lazo M, Solga SF, Horska A, et al. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care. 2010;33:2156–2163. doi: 10.2337/dc10-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lassailly G, Caiazzo R, Buob D, et al. Bariatric Surgery Reduces Features of Non-alcoholic Steatohepatitis in Morbidly Obese Patients. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 84.Ribeireiro T, Swain J, Sarr M, et al. NAFLD and insulin resistance do not increase the risk of postoperative complications among patients undergoing bariatric surgery--a prospective analysis. Obes Surg. 2011;21:310–315. doi: 10.1007/s11695-010-0228-6. [DOI] [PubMed] [Google Scholar]
- 85.Weingarten TN, Swain JM, Kendrick ML, et al. Nonalcoholic steatohepatitis (NASH) does not increase complications after laparoscopic bariatric surgery. Obes Surg. 2011;21:1714–1720. doi: 10.1007/s11695-011-0521-z. [DOI] [PubMed] [Google Scholar]
- 86.Pestana L, Swain J, Dierkhising R, Kendrick ML, Kamath PS, Watt KD. Bariatric surgery in patients with cirrhosis with and without portal hypertension: a single-center experience. Mayo Clin Proc. 2015;90:209–215. doi: 10.1016/j.mayocp.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 87.Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient--2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Endocr Pract. 2013;19:337–372. doi: 10.4158/EP12437.GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35:66–75. doi: 10.1111/j.1365-2036.2011.04912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bell LN, Wang J, Muralidharan S, et al. Relationship between adipose tissue insulin resistance and liver histology in nonalcoholic steatohepatitis: a pioglitazone versus vitamin E versus placebo for the treatment of nondiabetic patients with nonalcoholic steatohepatitis trial follow-up study. Hepatology. 2012;56:1311–1318. doi: 10.1002/hep.25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sanyal AJ, Brunt EM, Kleiner DE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344–353. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. Jama. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 93.Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. Jama. 2011;305:1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bjelakovic G, Nikolova D, Gluud C. Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm? PLoS One. 2013;8:e74558. doi: 10.1371/journal.pone.0074558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 96.Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schurks M, Glynn RJ, Rist PM, Tzourio C, Kurth T. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702. doi: 10.1136/bmj.c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cariou B. The farnesoid X receptor (FXR) as a new target in non-alcoholic steatohepatitis. Diabetes Metab. 2008;34:685–691. doi: 10.1016/S1262-3636(08)74605-6. [DOI] [PubMed] [Google Scholar]
- 99.Cariou B, van Harmelen K, Duran-Sandoval D, et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem. 2006;281:11039–11049. doi: 10.1074/jbc.M510258200. [DOI] [PubMed] [Google Scholar]
- 100.Ratziu V, de Ledinghen V, Oberti F, et al. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011–1019. doi: 10.1016/j.jhep.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 101.Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 102.Ward A, Clissold SP. Pentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs. 1987;34:50–97. doi: 10.2165/00003495-198734010-00003. [DOI] [PubMed] [Google Scholar]
- 103.Zein CO, Lopez R, Fu X, et al. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: new evidence on the potential therapeutic mechanism. Hepatology. 2012;56:1291–1299. doi: 10.1002/hep.25778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Van Wagner LB, Koppe SW, Brunt EM, et al. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. Ann Hepatol. 2011;10:277–286. [PubMed] [Google Scholar]
- 105.Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610–1619. doi: 10.1002/hep.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Group DPPDR. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nair S, Diehl AM, Wiseman M, Farr GH, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20:23–28. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 108.Loomba R, Lutchman G, Kleiner DE, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29:172–182. doi: 10.1111/j.1365-2036.2008.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garinis GA, Fruci B, Mazza A, et al. Metformin versus dietary treatment in nonalcoholic hepatic steatosis: a randomized study. Int J Obes (Lond) 2010;34:1255–1264. doi: 10.1038/ijo.2010.40. [DOI] [PubMed] [Google Scholar]
- 110.Shields WW, Thompson KE, Grice GA, Harrison SA, Coyle WJ. The Effect of Metformin and Standard Therapy versus Standard Therapy alone in Nondiabetic Patients with Insulin Resistance and Nonalcoholic Steatohepatitis (NASH): A Pilot Trial. Therap Adv Gastroenterol. 2009;2:157–163. doi: 10.1177/1756283X09105462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang X, Harmsen WS, Mettler TA, et al. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology. 2014;60:2008–2016. doi: 10.1002/hep.27199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pearlman M, Loomba R. State of the art: treatment of nonalcoholic steatohepatitis. Curr Opin Gastroenterol. 2014;30:223–237. doi: 10.1097/MOG.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 113.Wong RJ, Chou C, Bonham CA, Concepcion W, Esquivel CO, Ahmed A. Improved survival outcomes in patients with non-alcoholic steatohepatitis and alcoholic liver disease following liver transplantation: an analysis of 2002–2012 United Network for Organ Sharing data. Clin Transplant. 2014;28:713–721. doi: 10.1111/ctr.12364. [DOI] [PubMed] [Google Scholar]
- 114.Wang X, Li J, Riaz DR, Shi G, Liu C, Dai Y. Outcomes of liver transplantation for nonalcoholic steatohepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:394–402. e391. doi: 10.1016/j.cgh.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 115.Fussner LA, Charlton MR, Heimbach JK, et al. The impact of gender and NASH on chronic kidney disease before and after liver transplantation. Liver Int. 2014;34:1259–1266. doi: 10.1111/liv.12381. [DOI] [PubMed] [Google Scholar]
- 116.Murray KF, Carithers RL AASLD. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407–1432. doi: 10.1002/hep.20704. [DOI] [PubMed] [Google Scholar]