Abstract
Background
Fibrostenosis and stricture are well-recognized endpoints in Crohn’s disease (CD). We hypothesized that stricturing-CD is characterized by eosinophilia and epithelial-IL-33. We proposed that eosinophil exposure to IL-33 would perpetuate inflammatory chronicity and subsequent fibrostenosis.
Methods
We performed a retrospective study of 74 children with inflammatory and stricturing ileal-CD comparing clinicopathological features to immunohistochemical measures of eosinophilia and IL-33. To scrutinize eosinophil patterns we developed a novel eosinophil-peroxidase (EPX)-score encompassing number, distribution and degranulation. Human eosinophils and intestinal fibroblasts were cultured with IL-33 and IL-13 and inflammatory and remodeling parameters were assessed. Anti-eosinophil therapy was also administered to the Crohn’s-like ileitis model (SAMP1/SkuSlc).
Results
Our novel EPX-score was more sensitive than H&E-staining, revealing significant differences in eosinophil patterns, comparing inflammatory and stricturing pediatric CD. A significant relationship between ileal-eosinophilia and complicated clinical/histopathological phenotype including fibrosis was determined. IL-33 induced significant eosinophil EPX-secretion and IL-13 production. Exposure to eosinophils in the presence of IL-33, ‘primed’ fibroblasts to increase pro-inflammatory cytokines (TNF-α, IL-1β, IL-6), eosinophil-associated chemokines (CCL24, CCL26) and IL-13Rα2 production. Production of fibrogenic molecules (collagen 1A2, fibronectin and periostin) increased following exposure of ‘primed’-fibroblasts to IL-13. Epithelial-IL-33 was increased in pediatric Crohn’s-ileitis and strongly associated with clinical and histopathological activity, ileal eosinophilia and complicated, fibrostenotic disease. SAMP1/SkuSlc eosinophil-targeted treatment resulted in significant improvements in inflammation and remodeling.
Conclusions
Our study of specimens from pediatric patients with ileal CD linked eosinophil patterns and IL-33 to fibrosis; and suggested these may contribute to the perpetuation of inflammation and subsequent stricture in pediatric CD.
Keywords: Pediatric Crohn’s Disease, Eosinophil, Fibrosis, Stricture
Introduction
The underlying pathogenesis of Crohn’s disease (CD) relates to the complex interactions between genetic, immunologic and environmental factors. In some cases, chronic inflammation leads to stricture formation and surgical resection of diseased tissue. While not all patients develop intestinal strictures, ileal location and duration of disease pose specific risks.(1) In fact, the cumulative incidence of stricturing disease has been reported as 27% at 5 years and 38% at 10 years.(2) Although certain similarities exist between pediatric and adult onset disease, pediatric disease is distinct, with more extensive and rapidly progressing pathology.(3) Thus studies focusing specifically on pediatric patients are of great value to gain a better understanding of cause and improved treatments or preventions. Without an exact understanding of the underlying pathogenesis of stricturing disease, the identification of biomarkers, therapeutic targets and thus Food and Drug Administration approved treatments remains undiscovered.
Previous studies link CD-related strictures with a number of different cell types including fibroblasts and eosinophils. With respect to eosinophils, a large body of evidence supports their role in tissue remodeling and fibrosis. For instance, eosinophils and eosinophil derived products induced the activation of fibroblasts in vitro, resulting in fibroblast proliferation, collagen gel contraction and production of fibrogenic molecules, remodeling proteases, cytokines and chemokines.(4–6) However, to date no study has examined the relationship between eosinophil patterns in stricturing pediatric CD compared to those with inflammatory pediatric CD. In addition, IL-33 has not been examined comparing stricturing to inflammatory pediatric CD populations. Using a mouse model of Crohn’s disease, others and we previously reported the significant association of eosinophils with mouse ileitis and identified their potential as a novel therapeutic target.(7, 8) Since these results provided support for a clinically relevant role for eosinophils in fibrotic-CD, and because of the identified role of eosinophils in other fibrotic diseases, we wondered whether eosinophil patterns were different in complicated pediatric CD such as stricturing disease compared to inflammatory CD. To address this, we hypothesized that eosinophils were associated with strictured CD in children and that the pro-fibrogenic cytokines, IL-33 and IL-13, stimulated a remodeling response from human eosinophils and intestinal fibroblasts.
Methods
Assessment of anti-eosinophil treatment in mouse ileitis
Original SAMP1 strain of the mouse model of Crohn’s-like ileitis, SAMP1/SkuSlc female mice 4–10-weeks-of-age, were purchased from Japan SLC (Hamamatsu, Japan).(9) Mice were maintained under specific pathogen free conditions with ad libitum access to food and water. Anti-CCR3 rat anti-mouse MAb 6S2-19-4 and monoclonal antibody specific for Interleukin-5 (TRFK-5; Cayman Chemical, Ann Arbor, MI) both selectively deplete murine eosinophils.(7, 10, 11) Experimental animals were injected intraperitoneal (i.p.) with 200μg of TRFK-5 antibody, 200μg of anti-CCR3 and 200μg of TRFK-5 antibodies once weekly from 20 to 30-weeks-of-age and sacrificed 24 hours following final administration. Inflamed control mice were treated i.p. with a non-specific isotype control antibody with equal dose and duration. The last 10 cm of the ileum was removed, processed and scored by a pathologist blinded to the experimental conditions (PJ) as previously described.(7, 12)
Briefly, three histological parameters were assessed with equal weight for each parameter: 1. active inflammation (granulocytes), 2. chronic inflammation (lymphoplasmacytic infiltrates), and 3. villus distortion (architectural disruption, separation of villi, crypts and muscularis). Infiltrating eosinophils were identified by immunostaining with rat anti-mouse MBP monoclonal antibody (Clone MT-14.7) and quantified as previously described.(7) Ileal sections were stained with periodic acid-Schiff (PAS) and goblet cells were quantified as previously described as a measure of remodeling.(7) The University of Colorado School of Medicine Institutional Animal Care and Use Committee approved these studies.
Subject selection
A retrospective chart review was performed of patients who were evaluated in the Digestive Health Institute, Section of Pediatric Gastroenterology, Hepatology, and Nutrition at the Children’s Hospital Colorado from 2002–2011 who had received a diagnosis of CD and had undergone surgical resection (stricturing) or biopsy (inflammatory) of the terminal ileum. Subjects were excluded from this analysis if they had incomplete treatment records. Based on review of the clinical record, subjects were subdivided into either stricturing or non-stricturing ileal disease. Subjects were defined as follows; stricturing CD subjects exhibited symptoms and radiological features consistent with partial obstruction that lead to surgical resection; inflammatory CD subjects had symptoms and clinical evidence of inflammation (labs and /or radiographs) without evidence of obstruction; control subjects had symptoms of gastrointestinal dysfunction, no clinical evidence of inflammation (labs and /or radiographs) and normal ileal mucosa. Patients were considered under CD treatment if they were prescribed either 5-aminosalicylates (5-ASAs), corticosteroids, immunomodulators, biologic-therapies or antibiotics alone or in combination. Clinical features recorded included location(s) of activity, previous treatments, ESR, CRP and HgB Units and calculation of Pediatric Crohn’s Disease Activity Index (PCDAI) (Table 1).(13)
Table 1.
Clinical characteristics of study subjects
| Control | Inflammatory | Stricturing | |
|---|---|---|---|
| Number (n) | 39 | 17 | 18 |
| Sex (% male) | 44 | 59 | 67 |
| Age range years (median) | 2–17 (14) | 6–17 (14) | 8–18 (14) |
| Disease duration (months) | N/A | 8.0 ± 2.9 | 11.3 ± 2.5 |
| ESR (mm/hr) | 10.0 ± 1.7 | 32.3 ± 6.7 | 35.7 ± 6.8 |
| CRP (mg/dL) | 0.5 ± 0.04 | 6.2 ± 3.7 | 9.3 ± 2.1 |
| Hgb (g/dL) | 13.6 ± 0.4 | 12.0 ± 0.4 | 11.4 ± 0.5 |
| Previous treatments | Corticosteroid – 0 5-ASA – 0 Immunomodulator – 0 Biologic – 0 Antibiotic – 0 |
Corticosteroid – 6 5-ASA – 4 Immunomodulator – 6 Biologic – 1 Antibiotic – 5 |
Corticosteroid – 15 5-ASA – 4 Immunomodulator – 17 Biologic – 4 Antibiotic – 2 |
| PCDAI | N/A | 35.2 ± 1.9 | 37.6 ± 2.3 |
All values mean ± SEM.
No statistical difference between Inflammatory and Stricturing disease was obtained.
Human subject specimen histological assessments
To allow for equal comparison between biopsies and full thickness resected tissues all histological assessments were performed on mucosal tissue alone and not on deeper resected tissues. Hematoxylin and Eosin (H&E) stained ileal tissue sections were assessed by board certified pathologist (KC) for 1.) pathological activity (mild active, mild-, moderate- or severe-chronic active), 2.) eosinophil numbers (mean of 5 random high powered fields (hpf) and peak number counted in single most densely inflamed hpf at 40 X magnification-field size 0.26 mm2, data presented as mean ± range), and 3.) lamina propria fibrosis (mild, loose collagen matrix fibrils; moderate, condensed collagen matrix fibrils; or severe, substantially condensed collagen matrix fibrils) (Supplemental Figure 1A). Measurement of lamina propria fibrosis was also performed on Masson’s Trichrome stained tissue sections.
Eosinophil peroxidase (EPX) immunostaining was completed to determine the pattern of eosinophils within tissue specimens. Briefly, each section underwent staining with a monoclonal antibody for EPX (hybridoma MM25-82.2.1) as previously described.(11, 14) Slides were assessed for eosinophil patchiness/localization, degranulation, and intact eosinophil numbers to determine an ileal EPX index adapted from that previously described (Supplemental Table 1 and Supplemental Figure 1B).(14) Priority factors were assigned based on those indices that gave then greatest and the least differences when comparing inflammatory to stricturing specimens allowing stratification of these two disease types. On this basis, priorities were assigned to patchiness/localization (priority score-4), average numbers of eosinophil infiltrating the tissues (priority score-3), peak eosinophil number infiltrating the tissue (priority score-2) and degranulation (priority score-1). Tissue sections were assessed independently and in a blinded manner by a pathologist (KC) and two other research investigators (JM & LH).
Immunostaining was completed for Interleukin-33 (Polyclonal goat anti-IL-33; R&D Systems, Minneapolis, MN). Slides were assessed for IL-33 staining as follows: none-score 0, present in epithelial cells-score 1, or increased numbers of / presence in epithelial cells-score 2. IL-33 stained tissue sections were assessed independently by three research investigators (LH, RH, SDF) blinded to specimen demographics. The Institutional Review Board at the University of Colorado (COMIRB) approved this study.
Human eosinophil isolation
Human eosinophils were isolated from healthy volunteers. Briefly, white blood cells were isolated from whole blood and anticoagulated with 5% citrate buffer. Plasma was removed following centrifugation. Red blood cells (RBCs) were pelleted from leukocytes using 6% Dextran/0.9% saline sedimentation (Fluka, Sigma, MO). Granulocytes were isolated from discontinuous Percol gradients (42% and 51%) (Sigma, MO), and monocytes discarded. Residual erythrocytes were lysed with ice-cold water, and the remaining cells were washed. Eosinophils were purified by negative selection using anti-CD16 and anti-CD14 MACS microbeads and AutoMACS separation (Miltenyi, CA). Eosinophils were >95% pure as assessed by fast green (4%). The Institutional Review Board at the University of Colorado (COMIRB) approved this study.
Assessment of Eosinophil Activation by IL-33
Viable single cell suspensions were subjected to culture in the presence or absence of 100 ng/ml IL-33 (R&D Systems, Minneapolis, MN) for 3 hours. Cells were subjected to flow cytometric analysis with FITC labeled anti-ST2 or isotype control antibodies (MBL International, Woburn, MA). Eosinophil peroxidase (EPX) release into cell supernatants was quantified as a marker of eosinophil activation and granule protein release by peroxidase assay, modified from that previously described.(15) Eosinophils were also pelleted by centrifugation and harvested for electron microscopic analysis in glutaraldehyde fixative as previously described (16) or harvested for total cell RNA isolation with RNeasy Mini Kits (Qiagen, Valencia, CA). Taqman gene expression assays (Applied Biosystems, Foster City, CA) were performed on whole cell RNA, processed as previously described.(11) Data was normalized to 18S and calculated as Relative Expression (2−ΔΔ Ct).
Eosinophil and 18Co fibroblast co-culture
Intestinal myofibroblasts (18Co-ATCC) were cultured as previously described (4) in the presence of freshly isolated eosinophils at a ratio of 1:1. Following 24 or 48 hours co-culture, eosinophils were completely removed and 18Co cells harvested for total cell RNA isolation with RNeasy Mini Kits (Qiagen, Valencia, CA). For IL-13 stimulation experiments 18Co cells were co-cultured with eosinophils for 48 hours, eosinophils were removed and 18Co cells were returned to culture in the presence of IL-13 (100 ng/ml, R&D Systems, Minneapolis, MN) for an additional 72 hours, at which point 18Co’s were harvested for total cell RNA isolation with RNeasy Mini Kits (Qiagen, Valencia, CA).
Statistical analysis
Statistical analyses of data outcomes were performed by Mann-Whitney test, 1-way ANOVA (Kruskal Wallis) test with Dunns correction for multiple comparisons, 1-way ANOVA (Neuman Keuls) test, Spearman rank correlation test and Student’s t-test where appropriate. Data are expressed as means ± SEM. A P-value of ≤0.05 was considered as statistical significance although in some cases higher levels of significance are noted and described in the figure legends where applicable. *P≤0.05, **P≤0.01, ***P≤0.001.
Results
Eosinophil targeted therapies diminished chronic inflammation and remodeling in a mouse model of Crohn’s-like ileitis
To examine the causative effects of eosinophils on tissue remodeling, the SAMP1/SkuSlc mouse model of Crohn’s-like disease was treated with eosinophil targeting antibodies (TRFK-5 or anti-CCR3 & TRFK-5 in combination). A significant improvement in overall inflammation and tissue architecture was determined by examining H&E stained ileal tissue sections (14.8±1.8 vs 4.6±0.5; P<0.001 vs 4.7±1.7 Total Inflammatory Score; P<0.01) (Supplemental Figure 2A). As expected, treatment with both antibody regimens led to a significant reduction in ileal tissue eosinophils as quantified on major basic protein (MBP) stained sections (200±22 vs 82±14; P<0.01 vs 46±3 Eosinophils / HPF; P<0.001) (Supplemental Figure 2B). As a measure of remodeling, goblet cell hyperplasia assessed by Periodic-Acid Schiff (PAS) staining was significantly decreased in these anti-eosinophil treated mice compared to age matched IgG-treated controls (30±3 vs 13±1; P<0.001 vs 11±1 Percent Goblet Cells per Villus; P<0.001) (Supplemental Figure 2C).
Eosinophil mucosal distribution pattern differentiates stricturing from non-stricturing pediatric ileal Crohn’s disease
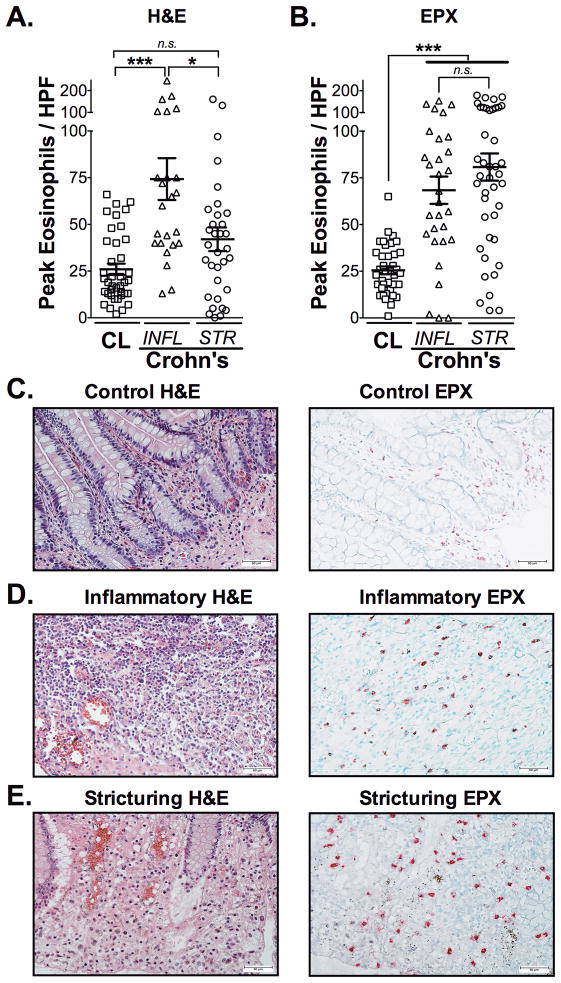
Next, we determined whether any histopathological features present in the mucosal surface of clinical specimens from pediatric subjects permitted the distinction of inflammatory from stricturing CD. Clinical characteristics of stricturing CD subjects (n=18), inflammatory Crohn’s subjects (n=17) and control subjects (n=39) are presented in Table 1. Subjects were included in this study that were both treatment naïve and those undergoing medical treatments for CD (5-aminosalicylates (5-ASAs), corticosteroids, immunomodulators, biologic-therapies or antibiotics alone or in combination). No statistical differences were identified between stricturing and inflammatory CD subjects when comparing mean disease duration and laboratory markers of inflammation (ESR, CRP and HgB) (Table 1). We next determined whether the number of eosinophils, as traditionally counted on H&E stained tissues, were able to provide distinguishing histological features of these two phenotypes. Whereas peak eosinophil counts, as measured by H&E, were increased in inflammatory CD compared to control subjects (P<0.001), they were not significantly increased comparing control subjects to stricturing CD specimens (Figure 1A, C–E).
Figure 1. Eosinophil patterns are distinguishable between mucosa of inflammatory and stricturing Crohn’s disease.
Peak numbers of eosinophils were enumerated in (A) H&E and (B) Eosinophil Peroxidase (EPX)-stained ileal tissues from patients with CD. Subjects were divided into those with strictures (STR) and those without strictures that had inflammatory CD (INFL). Representative photomicrographs comparing H&E (C, E, G) to EPX-stained (D, F, H) tissues from control (CL), inflammatory and stricturing Crohn’s-ilea. Scale bars represent 50μM. Data are expressed as means ± SEM. Statistical significance was assessed by 1-way ANOVA with Newman-Keuls multiple comparisons test. *P≤0.05, ***P≤0.001.
On H&E staining, the tinctural features of eosinophils and their granules, in the context of an inflamed mucosa, can make enumeration challenging. Based on this, we questioned whether a more comprehensive assessment of mucosal eosinophil patterns might be beneficial. To address this, we took advantage of our previously developed eosinophilic peroxidase (EPX) immunohistochemical staining protocol using this on these ileal tissue specimens. Results from this more sensitive and specific staining could now identify a significant increase in peak eosinophil number comparing stricturing CD specimens to control subjects (P<0.001) (Figure 1B, C–E).
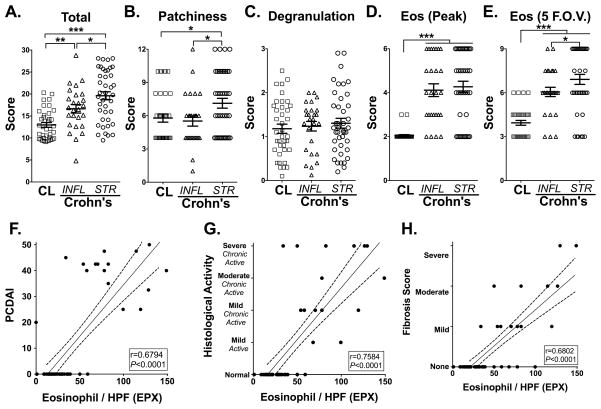
To further address this difference in sensitivity between H&E and EPX analysis, a four-point EPX immunohistochemistry-based indexed scoring system was adapted from our previous work(14) to better characterize eosinophil patterns in the ileal mucosa of stricturing and inflammatory CD specimens (Table 1). EPX-scoring analysis revealed a significant increase in eosinophil burden when comparing controls (12.97±0.54; EPX-score) to inflammatory (16.64±0.97; P<0.01) and to stricturing CD specimens (19.61±0.9; P<0.001) (Figure 2A). In contrast to using just peak eosinophil numbers (Figure 2D), a significant increase in eosinophil pattern as assessed by total EPX-score (patchiness/distribution, degranulation & numbers combined) was captured in the stricturing CD specimens as compared to inflammatory CD (P<0.05) (Figure 2A). Further dissection of differences between inflammatory and structuring CD mucosal eosinophilia, revealed differences in patchiness or distribution/localization of eosinophils (7.12±0.44 vs. 5.52±0.46; P<0.05, stricturing vs inflammatory) (Figure 2B) and average number of eosinophils counted across 5 fields of view (6.92±0.34 vs. 6.06±0.32, stricturing vs inflammatory) (Figure 2E). Neither peak number of eosinophils nor degranulation could distinguish between stricturing and inflammatory CD specimens (Figure 2C–D). Analysis of tissue from treatment-naïve subjects showed similar patterns; eosinophil EPX-score was increased in stricturing and inflammatory CD subjects compared to control subjects (Supplemental Figure 3).
Figure 2. Distinguishing stricturing Crohn’s-ileitis using eosinophil peroxidase (EPX) immunohistochemistry based scoring system. Eosinophil infiltration correlates with disease activity, chronicity and fibrosis.
EPX immunohistochemistry provides a quantitatively sensitive strategy to distinguish inflammatory from stricturing CD specimens. Examination of the scores for individual EPX parameters associated with all control (CL), inflammatory (INFL) or stricturing (STR) CD patients found in Table 1 demonstrated differences between (A) their overall scores, in addition to individual parameters comprising the EPX algorithm: (B) Patchiness, (C) Degranulation, (D) Peak eosinophils / hpf and (E) average eosinophils across 5 random fields-of-view (F.O.V.). Statistical assessments (ANOVA with Newman-Keuls) of the EPX-staining scores (means ± SEM) demonstrated the utility of this algorithm to distinguish between stricturing and inflammatory CD mucosal specimens. *P≤0.05, **P≤0.01, ***P≤0.001. Peak numbers of ileal eosinophils / hpf (EPX) were analyzed for relationships to subjects’ (F) pediatric Crohn’s disease activity index (PCDAI), (G) histological activity and (H) fibrosis score measured in H&E stained tissues for subjects defined in Table 1 that were not considered under treatment relevant to their CD. The Pearson correlation coefficient (r) and its associated statistical significance are shown.
Ileal eosinophilia correlates with clinicopathological features of pediatric Crohn’s disease
To address the potential association of eosinophils with disease activity, we next correlated the peak number of eosinophils as identified by EPX staining with the pediatric Crohn’s disease activity index (PCDAI) as well as histological features of chronicity and fibrosis. In order to exclude any potential treatment-effect bias, only treatment-naïve specimens were examined. This comparison revealed a highly significant relationship between the peak numbers of eosinophils / hpf and PCDAI (r=0.68, P<0.0001; Figure 2F). In addition, a highly significant association was identified between eosinophilia and histological chronicity (r=0.76, P<0.0001; Figure 2G) as well as fibrosis (r=0.68, P<0.0001; Figure 2H). The relationship between eosinophilia and fibrosis was also confirmed when examining fibrosis assessed by Masson’s Trichrome stain (r=0.33; P<0.01). Together, these results indicate that mucosal eosinophilia is associated with chronicity and complications in pediatric Crohn’s ileitis and that detailed analysis of mucosal eosinophil patterns provides significant benefit in clinically and histologically characterizing inflammatory versus stricturing pediatric CD.
Eosinophils are activated by IL-33 and prime intestinal fibroblasts in the perpetuation of eosinophil recruitment and exacerbated fibrosis via IL-13
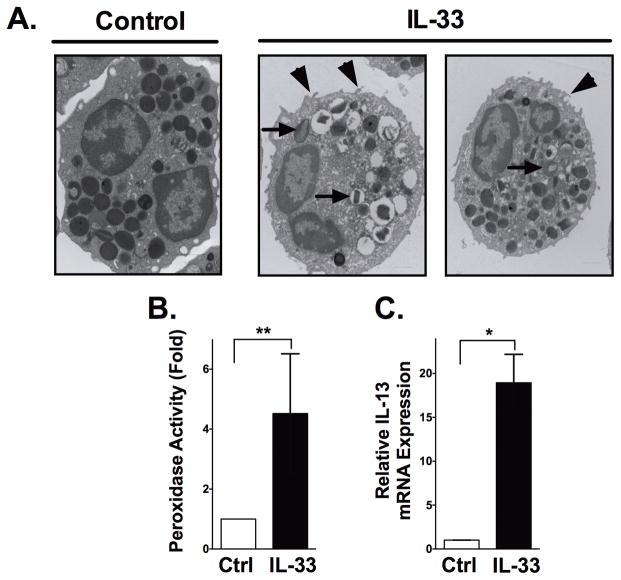
We next took a reductionist approach to determine the impact of eosinophils in intestinal fibrosis. Based on previous work suggesting a role for IL-33 in eosinophil and fibrosis related diseases, we first determined the ability of IL-33 to induce eosinophil degranulation and the production of fibrogenic cytokines associated with inflammatory bowel disease (IBD) such as IL-13.(17) We first confirmed that human eosinophils expressed the cognate IL-33 receptor ST2 by flow cytometry; similar to previously published studies, we show that 18.3±6.9% of resting human eosinophils express ST2 (n=3 donors). Next, we found that exposure of human eosinophils to IL-33 resulted in eosinophil activation observed by electron micrograph, including membrane ruffling and reversal of granule staining consistent with degranulation (Figure 3A). IL-33 stimulated eosinophils also resulted in significant release of EPX compared to controls, as captured by immunofluorescence assay (Figure 3B). Finally, we examined the ability of IL-33 to induce human eosinophil derived fibrogenic IL-13, as had previously been demonstrated for mouse eosinophils. These results demonstrated a significant increase in IL-13 mRNA synthesis (19-fold; P<0.01) (Figure 3C).
Figure 3. Circulating eosinophils are activated by IL-33.
Circulating eosinophils were isolated and subjected to IL-33 stimulation (100ng/ml) for 3 hours, then assessed for evidence of activation. (A) Representative electron micrographs provide evidence of eosinophil activation (right) compared to untreated controls (left), including reversal of granule staining consistent with degranulation (arrows) and membrane ruffling (arrowheads). (B) Biochemical measures of peroxidase activity in cell free supernatants from control and IL-33-stimulated eosinophils (3hrs, 100ng/ml) were performed. (C) Eosinophil IL-13 mRNA expression analysis was performed by real-time PCR on extracted RNA from control and IL-33-stimulated eosinophils (3hrs, 100ng/ml). Data are expressed as means ± SEM of 4 individual donors. Statistical significance was assessed using the non-parametric Mann-Whitney test compared with untreated controls. *P≤0.05, **P≤0.01.
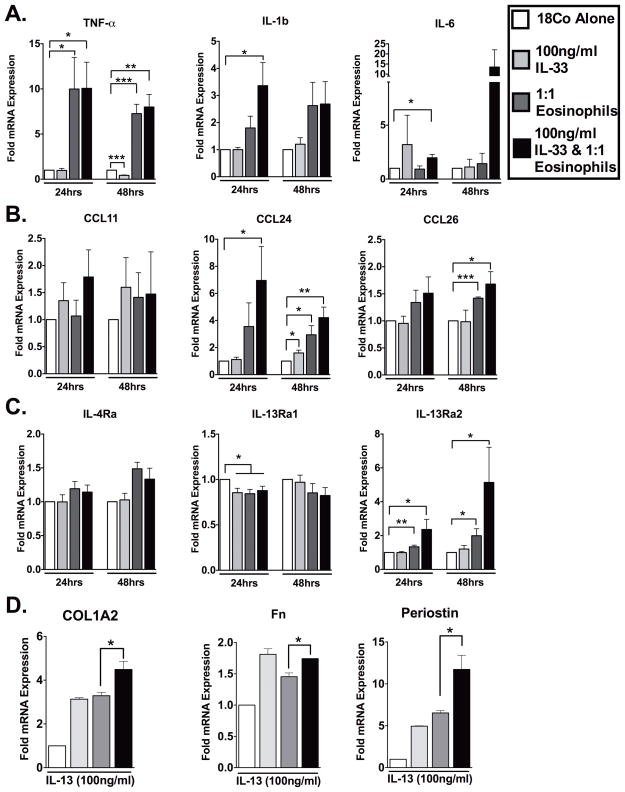
To further determine the downstream impact of IL-33-stimulated eosinophils, we examined whether these activated leukocytes could induce a pro-inflammatory response from intestinal fibroblasts (Figure 4). Our results showed that, co-culture of IL-33-stimulated eosinophils with intestinal fibroblasts led to increased production of a number of inflammatory (TNF-α, IL-1β and IL-6) and eosinophil chemotactic (CCL24 and CCL26) molecules (Figure 4A–B). For instance, compared to control fibroblasts, TNF-α was significantly increased by the presence of IL-33-stimulated eosinophils (10-fold; P<0.05 [24hr]) (7-fold; P<0.01 [48hr])) (Figure 4A). IL-1β was increased 3.4-fold (P<0.05 [24hrs]) (Figure 4A), while IL-6 was increased 2-fold (P<0.05 [24hrs]) and 13.5-fold (P=0.18; [48hrs]) (Figure 4A).
Figure 4. IL-33-activated eosinophils induce pro-inflammatory intestinal fibroblast activation and perpetuation of eosinophil recruitment in vitro.
Confluent 18Co intestinal fibroblasts cells were co-cultured with IL-33 (100ng/ml) and eosinophils. As controls 18Co cells treated with IL-33 alone (100ng/ml), 18Co cells co-cultured with eosinophils in the absence of IL-33 or 18Co cells alone were used. (A) Pro-inflammatory cytokine, (B) eosinophilic chemokine and (C) cytokine receptor mRNA analysis of 18Co fibroblasts was performed by real-time PCR on extracted RNA following 24 and 48 hours of co-culture. In a second series of experiments cells were treated in the 4 groups described above. Following 48 hours of co-culture 18Co cells were washed and IL-13 (100ng/ml) was placed into each of the wells. (D) Fibrosis-associated molecule mRNA analysis of IL-13-stimulated 18Co fibroblasts was performed by real-time PCR on extracted RNA following an additional 72 hours of culture. Data are expressed as means ± SEM of 2–6 individual donors. Statistical significance was assessed using 1-way ANOVA with Newman-Keuls multiple comparisons test. *P≤0.05, **P≤0.01, ***P≤0.001.
In addition, the increased production of the eosinophil-associated chemokines CCL24 (Eotaxin-2) and CCL26 (Eotaxin-3) also demonstrated the potential for further eosinophil recruitment by fibroblasts. Compared to control fibroblasts, CCL24 was significantly increased by the presence of IL-33-stimulated eosinophils (7-fold; P<0.01 [24hr]) (4.2-fold; P<0.01 [48hr])) (Figure 4B). CCL26 was increased 1.7-fold (P<0.05 [24hrs]) (Figure 4B) and changes in CCL11 (Eotaxin-1) production were not found.
The expression of the cytokine receptor IL-13Rα2 was also significantly increased (Figure 4Ciii). IL-13Rα2 has been described as a mediator of IL-13–induced intestinal fibrosis or as an IL-13-antagonist in other organs.(18, 19) To investigate the role of increased fibroblast IL-13Rα2 expression following IL-33-stimulated eosinophil co-culture, we followed this co-culture by removing the eosinophils and secreted-mediators and subsequently stimulated these ‘primed’ intestinal fibroblasts with IL-13 in fresh media. Fibroblasts first cultured in the presence of IL-33-stimulated eosinophils and subsequently stimulated with IL-13 induced significant production of COL1A2 when compared to fibroblasts first cultured in the presence of eosinophils alone with subsequent IL-13 stimulation (4.5-fold versus 3.3-fold; P<0.05) (Figure 4D). A similar induction of Fibronectin (1.7-fold versus 1.5-fold; P<0.05), and Periostin (11.7-fold versus 6.5-fold; P<0.05) was also established (Figure 4D). Here we determined a clear two-step method by which IL-33-stimulated eosinophils mediate the ‘priming’ of intestinal fibroblasts for subsequent IL-13 induced pro-fibrotic activity.
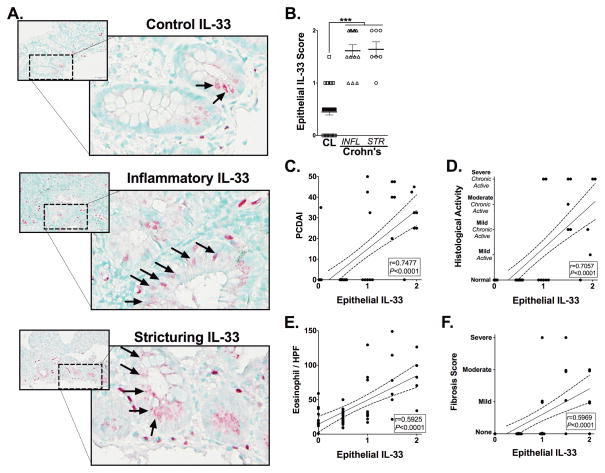
IL-33 is increased in pediatric ileal Crohn’s disease, is associated with eosinophilia and histological evidence of disease chronicity
Since IL-33 can stimulate the influx of eosinophils into inflamed tissues and elicit their activation, we next examined intestinal epithelial-IL-33 in pediatric tissues affected by CD using immunohistochemistry (Figure 5). IL-33’s was most strongly detected in endothelial cell nuclei, as well as in infiltrating lamina propria cells, epithelial cells, Paneth cell-granules and could sometimes be detected as cell free cytokine within extracellular spaces. Epithelial-IL-33 was detected more frequently in epithelial cells of the crypts than villi, and was located in both the cytoplasm and nucleus of these cells, consistent with previous reports examining Crohn’s and Ulcerative colitis specimens.(20–22) Epithelial-IL-33 was significantly increased in untreated inflammatory (1.6±0.08; P<0.001) and stricturing (1.3±0.06; P<0.001) CD subjects’ specimens compared to controls’ (0.45±0.05) (Figure 5A–B). Interestingly, Crohn’s disease-related treatments had no effect on epithelial-IL-33 levels in either group. Correlation analysis determined a significant relationship between the increasing presence of epithelial-IL-33 and PCDAI (r=0.75, P<0.001), histologic activity (r=0.71, P<0.001) and numbers of eosinophils (r=0.6: P<0.001) (Figure 5C–E). A significant correlation was also detected between epithelial-IL-33 and fibrosis (r=0.5969; P<0.001) (Figure 5F). A modest but statistically significant relationship existed between cell-free/extracellular-IL-33 and eosinophil degranulation (r=0.2760; P<0.05) in addition to fibrosis (r=0.4529; P=0.001).
Figure 5. Epithelial-IL-33 expression is associated with disease progression and eosinophilia in complicated Crohn’s disease.
Representative photomicrographs of IL-33 immuohistochemically stained ileal tissues from (Ai) control (CL) subjects or from patients with (Aii) inflammatory (INFL) or (Aiii) stricturing (STR) CD. Arrows indicate epithelial-IL-33. (B) A score was generated to quantify the absence (0), presence (1) or increased presence (2) of epithelial-IL-33 staining. Epithelial-IL-33 scores were analyzed for relationships to subjects’ (C) pediatric Crohn’s disease activity index (PCDAI), (D) histological activity and (E) peak eosinophils / hpf (EPX) and (F) fibrosis score measured in H&E stained tissues. All analyses were performed on subjects defined in Table 1 that were not considered under treatment relevant to their CD. Statistical significance was assessed for epithelial-IL-33 scores by using 1-way ANOVA with Newman-Keuls multiple comparisons test. ***P≤0.001. For all other analysis the Pearson’s correlation coefficient (r) and its associated statistical significance are shown.
Discussion
Fibrostenotic ileal CD represents a poorly understood pediatric patient phenotype that frequently requires surgical resection. Identification of predisposing factors, novel biomarkers and therapeutic targets associated with this group of patients could provide earlier diagnosis and medical intervention. Because of an increasing body of work that supports a role for eosinophils in fibrotic diseases outside of the gastrointestinal tract, we wondered whether accumulation and activation of this cell was associated with pediatric ileal stricturing CD. To address this, we utilized immunohistochemical staining for eosinophil peroxidase (EPX) to fully capture a number of key features related to eosinophil infiltration and translated our findings to assess the role of IL-33, a key fibrogenic molecule, in activating eosinophils to promote a stenotic phenotype. Histological assessments with EPX revealed that the degree and localization pattern of eosinophilic inflammation distinguished stricturing pediatric CD compared to inflammatory pediatric CD. In addition, our data elucidate the lack of sensitivity, specifically in fibrostenotic pediatric CD, of H&E staining for the study of eosinophils’ role(s) in intestinal inflammation. Activation of eosinophils by IL-33 led to not only degranulation, but also production of IL-13 and fibroblast production of a number of downstream molecules associated with fibrosis. Together these findings provide support for a possible pathogenetic role of eosinophils in the remodeling events that lead to pathological fibrostenosis in pediatric CD.
Clinical studies examining eosinophils’ role in IBD, especially those focusing on complicated pediatric CD, are limited due to a number of factors including small numbers of patients, inadequate methodologies to detect eosinophils, variability of eosinophil number along the gastrointestinal tract and lack of information with respect to treatments taken by study subjects prior to specimen procurement. In fact, few studies have defined a ‘normal’ number of eosinophils along the gastrointestinal tract of children thus making comparisons between control and diseased tissues difficult. With respect to our overall hypothesis, we carefully defined this unique and large group of ileal stenotic pediatric patients and compared them to relevant pediatric non-inflamed controls. To address a central issue of capturing the full content of eosinophilic inflammation, we used an eosinophil granule specific immunohistochemical stain to develop a scoring index rather than just using the single metric of eosinophil number. Careful and thorough patient selection and tissue analysis created the unique set of findings presented here.
A number of previous studies have documented the profound accumulation of eosinophils in mucosal tissues from patients with pathologically-phenotypically undefined IBD, but their role within this microenvironment remains uncertain. An urgent need exists to begin targeting specific mechanisms including eosinophils’ role in specific aspects of mucosal dysfunction. In this regard, eosinophils have been shown to participate directly, as an effector cell in barrier dysfunction, goblet cell hyperplasia and muscle contraction.(7, 23, 24) In the intestine, eosinophils in vitro have been directly implicated in the activation of intestinal fibroblasts, resulting in fibroblast proliferation, the production of fibronectin, collagen, collagen gel contraction, IL-6 and IL-8 secretion.(4–6) In addition, eosinophils may communicate with other resident cells to initiate or perpetuate an inflammatory response. In support of our results, recent in vitro studies examined the impact of IL-33 on not only fibrosis, but also eosinophil activation. For instance, IL-33-stimulated eosinophils adhere to matrix and connective tissue proteins and secrete significant quantities of IL-8 compared to control eosinophils.(25–29) Exposure of mouse bone-marrow-derived eosinophils to IL-33 results in the production of IL- 13.(30) Since human eosinophils are one of the key sources of IL-13, we sought to determine whether IL-33 could directly stimulate IL-13 production and for the first time show this in human eosinophils.(30–38) In addition, co-culture of ‘primed’ fibroblasts with IL-33-activated eosinophils, led to increased IL13Rα2 expression and downstream to IL-13 induced pro-fibrotic molecule expression. These findings bear relevance since Fichtner-Feigl et al previously defined the crucial role of IL-13 signaling via IL-13Rα2 in intestinal fibrosis using chronic mouse models.(18, 39–41)
To date eosinophil production of IL-13 has not been studied in the context of eosinophil-fibroblast interactions and the subsequent activation and perpetuation of fibrogenic processes in the intestines. Here we show for the first time in a reductionist setting that eosinophil-fibroblast or IL-33-eosinophil-fibroblast co-cultures leads to the induction of IL-13Rα2. Subsequent exposure of these intestinal fibroblasts to IL-13 leads to the production of fibrogenic molecules Collagen 1a2, Fibronectin and Periostin. Thus eosinophils may contribute in a two-step manner towards both perpetuation of inflammation and the potentiation of pro-fibrotic activation of intestinal fibroblasts in vitro.
Fibrosis is considered a sequela to chronic inflammation, thus cytokines are an important factor in this process. IL-33 is produced by a wide range of cells in IBD including colonocytes(20, 22) and colitis associated myofibroblasts.(42, 43) Previous studies have defined that IL-33 is increased in active IBD samples compared to uninvolved or control specimens.(20–22, 42–45) Not all studies refer to the site of specimen collection, however there is enough evidence currently to state that IL-33 expression is increased in colonic IBD, and seems most prominent in ulcerative colitis. Studies of Crohn’s disease and IL-33 have been limited in specimen number and mainly to colonic location. IL-33 has been implicated in the fibrotic process in other organs.(17, 46–50) Thus, ours is the first significantly powered study of IL-33 comparing inflammatory and stricturing disease in pediatric Crohn’s ileitis specimens. We report here that epithelial-IL-33 is increased in ileal localized pediatric Crohn’s IBD. This IL-33 is associated with increased Pediatric Crohn’s Disease Activity Index (PCDAI), histological activity, eosinophilic infiltration and more severe fibrosis score.
The clinical relevance of these findings is based on two highly significant correlations. First, while our novel metrics of eosinophilic inflammation was able to distinguish inflammatory from structuring pediatric CD, peak eosinophil values were also highly correlative with Pediatric Crohn’s Disease Activity Index (PCDAI) and histological features of chronicity and fibrosis. Second, epithelial IL-33 expression in ileal tissues was also highly correlative with eosinophilia, Pediatric Crohn’s Disease Activity Index (PCDAI) and histological evidence of chronicity. (51, 52)
Our findings are consistent with a growing body of interest and that of our previous study that suggested the association between eosinophils and bowel wall thickening in pediatric patients with eosinophilic colitis and with esophageal remodeling in EoE.(53, 54) Here, using a new and more potent double-antibody eosinophil-targeted treatment, we reinforce our previous findings demonstrating the positive impact of single-antibody eosinophil-targeted treatment on ileal inflammation and remodeling.(7)
The pathogenesis of CD-related strictures is not fully understood but ileal disease and duration of disease are considered risk factors. The cells most implicated in this process are those of mesenchymal origin including fibroblasts, smooth muscle cells and recruited or trans-differentiated mesenchymal-like cells.(55) Once activated by profibrogenic mediators such as TGF-β and IL-13 these cells proliferate and produce fibrotic molecules such as collagen, fibronectin and periostin. The production of an excessive amount of these cells and molecules leads to tissue thickening and stiffness and eventually to stricture. Growing evidence supports a new paradigm whereby fibroblasts contribute to initiation and perpetuation of inflammatory processes independent of their structural and fibrogenic roles during chronic disease.(56) Another study of colonic myofibroblasts has shown that type-2 cytokines regulate the eosinophil-chemokine eotaxin-3 expression.(57) Here we provide evidence for the first time that eosinophils and in addition IL-33-stimulated eosinophils induce intestinal fibroblast production of proinflammatory cytokines TNF-α, IL-1β and IL-6 and eosinophilic-chemokines eotaxin-2 (CCL24) and eotaxin-3 (CCL26). Thus IL-33-eosinophil-fibroblast interactions may act to perpetuate intestinal inflammation and eosinophilia by both cytokine and chemokine production.
Our findings are limited by the lack of longitudinal data from our specimens. Future studies should consider examining eosinophilia over the course of disease in paired samples pre and post-stricturing in addition to increased numbers of specimens with and without treatment. Due to the rare nature of these specimens this should be a heavily powered long-term study. Similar to co-culture experiments reported here, studies examining eosinophils’ role in esophageal and dermal fibroblast activation determined the necessity for contact between eosinophils and fibroblasts.(29, 31) Future studies should examine the role of soluble mediators versus juxtacrine signalling between eosinophils and fibroblasts in mediating inflammatory and fibrogenic processes. Previous studies have associated epithelil-IL-33 with inflammatory ulcerative colitis in adults. Here we report associations between epithelial-IL-33 and fibrostenotic pediatric Crohn’s ileitis. Future studies need to consider dissection of IL-33 in fibrotic clinical complications of chronic disease, including quantitative comparisons between these two anatomical sites. Clinical implications of our study now highlight the need to examine eosinophilia when histologically assessing specimens from CD patients. Our data demonstrate that altered mucosal eosinophil patterns determined by our EPX-scoring system can differentiate inflammatory versus stricturing ileal mucosal specimens, and that this is independent of disease duration.
Here, we have added to previous studies in mouse models indicating that eosinophils play a significant role in ileal remodeling and fibrosis in specimens from pediatric patients with CD. These studies established that fibrosis occurring in pediatric ileal CD is associated with greater eosinophilic inflammation, epithelial-IL-33 expression and mediated in part by IL-33, eosinophils and IL-13 signaling in intestinal fibroblasts. Further studies are planned to address a longitudinal examination of eosinophils in the progression toward fibrosis in pediatric and adult CD. Our data add to a growing body of literature dissecting the mechanisms of fibrostenosis in CD, implicating eosinophils and eosinophil-derived mediators as viable targets in CD-related strictures.
Supplementary Material
(A) Representative photomicrographs of H&E stained ileal tissue sections representing subjects with (i) mild, (ii) moderate or (iii) severe fibrosis. (B) Representative photomicrographs of EPX-immuohistochemically stained ileal tissue sections from (i) control subjects or from patients with (ii) inflammatory or (iii) stricturing CD. Scale bars represent 50μM.
20-week-old SAMP1 mice received 10 injections (i.p.) of anti-IL-5 (TRFK-5; 200μg) or TRFK5 and anti-CCR3 (200μg) combined (or IgG control antibody (200μg)) once per week for 10-weeks. Representative photomicrographs of (Ai) Hematoxylin and Eosin (H&E), (Bi) Major Basic Protein (MBP) and (Ci) Periodic Acid Schiff (PAS) immunohistochemistry on mouse ilea are presented. (Aii) Histologic-indices of inflammation were assessed post treatment. (Bii) Average number of eosinophils per high-powered field (hpf) was quantified. (Cii) Percent of goblet cells per villus were enumerated. Scale bars represent 100μM. Data are expressed as means ± SEM of 4–6 individual mice per group and represent 2-independent experimental repeats. Statistical significance was assessed by 1-way ANOVA with Newman-Keuls multiple comparisons test. **P≤0.01, ***P≤0.001.
EPX-immunohistochemistry provides a quantitatively sensitive strategy to distinguish inflammatory from stricturing CD specimens independently of treatment effects. Examination of the scores for individual EPX parameters for all control, inflammatory or stricturing CD patients found in Table 1 that were not considered under treatment relevant to their CD demonstrated differences between (A) their overall scores; in addition to individual parameters comprising the EPX algorithm: (B) Patchiness, (C) Degranulation, (D) Peak eosinophils / hpf and (E) average eosinophils across 5 random fields-of-view (F.O.V.). Statistical assessments (ANOVA with Newman-Keuls) of the EPX-staining scores (means ± SEM) demonstrated the utility of this algorithm to distinguish between stricturing and inflammatory CD mucosal specimens independent of treatment effects. *P≤0.05, **P≤0.01, ***P≤0.001.
Acknowledgments
Funding Support
This work was supported by grants from the United States National Institutes of Health (R01-DK62245 and K24-DK 100303) (GTF), and (R01-HL065228 and K26-RR0109709) (JJL), (NIH/NCRR Colorado CTSI Grant Number UL1 RR025780) (JCM) were sources of funding used in the performance of studies including data analysis and manuscript preparation. Its contents are the authors’ sole responsibility and do not necessarily represent official NIH views. Grants from Crohn’s and Colitis Foundation of America (#3760 & #3047 JCM, #2570195 EMcN), the North American Society for Pediatric Gastroenterology Hepatology and Nutrition (JCM), the American Partnership for Eosinophilic Disorders (JCM), the Mayo Foundation for Medical Education and Research (JJL) were the sources of funding used in the performance of studies including data analysis and manuscript preparation. The study sponsors palyed no role in the study design in the collection, analysis, and interpretation of data.
The authors wish to thank the members of all the participating laboratories and the members of the Gastrointestinal Eosinophilic Diseases Program Children’s Hospital Colorado for insightful discussions and critical comments. We also wish to acknowledge the invaluable assistance in animal husbandry and care at University of Colorado School of Medicine (Kristann Magee) and the administrative support provided by Joshua Rosenfeld, Linda Mardel and Shirley (“Charlie”) Kern. We gratefully thank Eric Wartchow for technical assistance on electron microscopy. We thank the physicians (Robert Kramer, Edward Hoffenberg, Edwin Liu, Edwin de Zoeten, David Brumbaugh, Christine Waasdorp, Shikha Sundaram, Cara Mack, Michael Narkewicz, Ronald Sokol, Jason Soden, Deborah Neigut,) and nurses (Tammy Armstrong, Sharon Mooney and Jo Anne Newton), research assistants (Zachary Robinson and Joseph Ruybal), pathology staff (Sara Garza), research coordinators (Wendy Moore and Alyson Yeckes) and endoscopy technical staff (Bill Markovich) who contributed to this work by helping to provide and collect samples. We are grateful to our patients and families who were part of this study.
Footnotes
Conflict of Interests
Authors have no conflicts of interest to report.
References
- 1.Thia KT, Sandborn WJ, Harmsen WS, et al. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology. 2010;139:1147–1155. doi: 10.1053/j.gastro.2010.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta N, Bostrom AG, Kirschner BS, et al. Incidence of stricturing and penetrating complications of Crohn’s disease diagnosed in pediatric patients. Inflamm Bowel Dis. 2010;16:638–644. doi: 10.1002/ibd.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henderson P, van Limbergen JE, Wilson DC, et al. Genetics of childhood-onset inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:346–361. doi: 10.1002/ibd.21283. [DOI] [PubMed] [Google Scholar]
- 4.Furuta GT, Ackerman SJ, Varga J, et al. Eosinophil granule-derived major basic protein induces IL-8 expression in human intestinal myofibroblasts. Clin Exp Immunol. 2000;122:35–40. doi: 10.1046/j.1365-2249.2000.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomes I, Mathur SK, Espenshade BM, et al. Eosinophil-fibroblast interactions induce fibroblast IL-6 secretion and extracellular matrix gene expression: implications in fibrogenesis. J Allergy Clin Immunol. 2005;116:796–804. doi: 10.1016/j.jaci.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 6.Xu X, Rivkind A, Pikarsky A, et al. Mast cells and eosinophils have a potential profibrogenic role in Crohn disease. Scand J Gastroenterol. 2004;39:440–447. doi: 10.1080/00365520310008566. [DOI] [PubMed] [Google Scholar]
- 7.Masterson JC, McNamee EN, Jedlicka P, et al. CCR3 Blockade Attenuates Eosinophilic Ileitis and Associated Remodeling. Am J Pathol. 2011;179:2302–2314. doi: 10.1016/j.ajpath.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takedatsu H, Mitsuyama K, Matsumoto S, et al. Interleukin-5 participates in the pathogenesis of ileitis in SAMP1/Yit mice. Eur J Immunol. 2004;34:1561–1569. doi: 10.1002/eji.200324680. [DOI] [PubMed] [Google Scholar]
- 9.Takeda T, Hosokawa M, Takeshita S, et al. A new murine model of accelerated senescence. Mech Ageing Dev. 1981;17:183–194. doi: 10.1016/0047-6374(81)90084-1. [DOI] [PubMed] [Google Scholar]
- 10.Justice JP, Borchers MT, Crosby JR, et al. Ablation of eosinophils leads to a reduction of allergen-induced pulmonary pathology. American journal of physiology Lung cellular and molecular physiology. 2003;284:L169–178. doi: 10.1152/ajplung.00260.2002. [DOI] [PubMed] [Google Scholar]
- 11.Masterson JC, McNamee EN, Fillon SA, et al. Eosinophil-mediated signalling attenuates inflammatory responses in experimental colitis. Gut. 2014 doi: 10.1136/gutjnl-2014-306998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNamee EN, Wermers JD, Masterson JC, et al. Novel model of TH2-polarized chronic ileitis: the SAMP1 mouse. Inflamm Bowel Dis. 2010;16:743–752. doi: 10.1002/ibd.21148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439–447. [PubMed] [Google Scholar]
- 14.Protheroe C, Woodruff SA, de Petris G, et al. A novel histologic scoring system to evaluate mucosal biopsies from patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2009;7:749–755. e711. doi: 10.1016/j.cgh.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colgan SP, Dzus AL, Parkos CA. Epithelial exposure to hypoxia modulates neutrophil transepithelial migration. J Exp Med. 1996;184:1003–1015. doi: 10.1084/jem.184.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capocelli KE, Fernando SD, Menard-Katcher C, et al. Ultrastructural features of eosinophilic oesophagitis: impact of treatment on desmosomes. J Clin Pathol. 2015;68:51–56. doi: 10.1136/jclinpath-2014-202586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rankin AL, Mumm JB, Murphy E, et al. IL-33 induces IL-13-dependent cutaneous fibrosis. J Immunol. 2010;184:1526–1535. doi: 10.4049/jimmunol.0903306. [DOI] [PubMed] [Google Scholar]
- 18.Fichtner-Feigl S, Strober W, Kawakami K, et al. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 19.Mentink-Kane MM, Wynn TA. Opposing roles for IL-13 and IL-13 receptor alpha 2 in health and disease. Immunological reviews. 2004;202:191–202. doi: 10.1111/j.0105-2896.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- 20.Beltran CJ, Nunez LE, Diaz-Jimenez D, et al. Characterization of the novel ST2/IL-33 system in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1097–1107. doi: 10.1002/ibd.21175. [DOI] [PubMed] [Google Scholar]
- 21.Pastorelli L, Garg RR, Hoang SB, et al. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci U S A. 2010;107:8017–8022. doi: 10.1073/pnas.0912678107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seidelin JB, Bjerrum JT, Coskun M, et al. IL-33 is upregulated in colonocytes of ulcerative colitis. Immunology letters. 2010;128:80–85. doi: 10.1016/j.imlet.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Furuta GT, Nieuwenhuis EE, Karhausen J, et al. Eosinophils alter colonic epithelial barrier function: role for major basic protein. Am J Physiol Gastrointest Liver Physiol. 2005;289:G890–897. doi: 10.1152/ajpgi.00015.2005. [DOI] [PubMed] [Google Scholar]
- 24.Jacoby DB, Gleich GJ, Fryer AD. Human eosinophil major basic protein is an endogenous allosteric antagonist at the inhibitory muscarinic M2 receptor. J Clin Invest. 1993;91:1314–1318. doi: 10.1172/JCI116331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cherry WB, Yoon J, Bartemes KR, et al. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008;121:1484–1490. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chow JY, Wong CK, Cheung PF, et al. Intracellular signaling mechanisms regulating the activation of human eosinophils by the novel Th2 cytokine IL-33: implications for allergic inflammation. Cellular & molecular immunology. 2010;7:26–34. doi: 10.1038/cmi.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, et al. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–1534. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzukawa M, Koketsu R, Iikura M, et al. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab Invest. 2008;88:1245–1253. doi: 10.1038/labinvest.2008.82. [DOI] [PubMed] [Google Scholar]
- 29.Wong CK, Leung KM, Qiu HN, et al. Activation of eosinophils interacting with dermal fibroblasts by pruritogenic cytokine IL-31 and alarmin IL-33: implications in atopic dermatitis. PLoS One. 2012;7:e29815. doi: 10.1371/journal.pone.0029815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouffi C, Rochman M, Zust CB, et al. IL-33 markedly activates murine eosinophils by an NF-kappaB-dependent mechanism differentially dependent upon an IL-4-driven autoinflammatory loop. J Immunol. 2013;191:4317–4325. doi: 10.4049/jimmunol.1301465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rieder F, Nonevski I, Ma J, et al. T-helper 2 cytokines, transforming growth factor beta1, and eosinophil products induce fibrogenesis and alter muscle motility in patients with eosinophilic esophagitis. Gastroenterology. 2014;146:1266–1277. e1261–1269. doi: 10.1053/j.gastro.2014.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmid-Grendelmeier P, Altznauer F, Fischer B, et al. Eosinophils express functional IL-13 in eosinophilic inflammatory diseases. J Immunol. 2002;169:1021–1027. doi: 10.4049/jimmunol.169.2.1021. [DOI] [PubMed] [Google Scholar]
- 33.Aceves SS, Chen D, Newbury RO, et al. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-beta1, and increase esophageal smooth muscle contraction. J Allergy Clin Immunol. 2010;126:1198–1204. e1194. doi: 10.1016/j.jaci.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 34.Aceves SS, Newbury RO, Dohil R, et al. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:206–212. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Dyer KD, Percopo CM, Xie Z, et al. Mouse and human eosinophils degranulate in response to platelet-activating factor (PAF) and lysoPAF via a PAF-receptor-independent mechanism: evidence for a novel receptor. J Immunol. 2010;184:6327–6334. doi: 10.4049/jimmunol.0904043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spencer LA, Szela CT, Perez SA, et al. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85:117–123. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stolarski B, Kurowska-Stolarska M, Kewin P, et al. IL-33 exacerbates eosinophil-mediated airway inflammation. J Immunol. 2010;185:3472–3480. doi: 10.4049/jimmunol.1000730. [DOI] [PubMed] [Google Scholar]
- 38.Woerly G, Lacy P, Younes AB, et al. Human eosinophils express and release IL-13 following CD28-dependent activation. J Leukoc Biol. 2002;72:769–779. [PubMed] [Google Scholar]
- 39.Fichtner-Feigl S, Fuss IJ, Young CA, et al. Induction of IL-13 triggers TGF-beta1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol. 2007;178:5859–5870. doi: 10.4049/jimmunol.178.9.5859. [DOI] [PubMed] [Google Scholar]
- 40.Fichtner-Feigl S, Strober W, Geissler EK, et al. Cytokines mediating the induction of chronic colitis and colitis-associated fibrosis. Mucosal immunology. 2008;1 (Suppl 1):S24–27. doi: 10.1038/mi.2008.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fichtner-Feigl S, Young CA, Kitani A, et al. IL-13 signaling via IL-13R alpha2 induces major downstream fibrogenic factors mediating fibrosis in chronic TNBS colitis. Gastroenterology. 2008;135:2003–2013. 2013 e2001–2007. doi: 10.1053/j.gastro.2008.08.055. [DOI] [PubMed] [Google Scholar]
- 42.Sponheim J, Pollheimer J, Olsen T, et al. Inflammatory bowel disease-associated interleukin-33 is preferentially expressed in ulceration-associated myofibroblasts. Am J Pathol. 2010;177:2804–2815. doi: 10.2353/ajpath.2010.100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobori A, Yagi Y, Imaeda H, et al. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol. 2010;45:999–1007. doi: 10.1007/s00535-010-0245-1. [DOI] [PubMed] [Google Scholar]
- 44.Sedhom MA, Pichery M, Murdoch JR, et al. Neutralisation of the interleukin-33/ST2 pathway ameliorates experimental colitis through enhancement of mucosal healing in mice. Gut. 2013;62:1714–1723. doi: 10.1136/gutjnl-2011-301785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wakahara K, Baba N, Van VQ, et al. Human basophils interact with memory T cells to augment Th17 responses. Blood. 2012;120:4761–4771. doi: 10.1182/blood-2012-04-424226. [DOI] [PubMed] [Google Scholar]
- 46.Li D, Guabiraba R, Besnard AG, et al. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J Allergy Clin Immunol. 2014;134:1422–1432. e1411. doi: 10.1016/j.jaci.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luzina IG, Kopach P, Lockatell V, et al. Interleukin-33 potentiates bleomycin-induced lung injury. Am J Respir Cell Mol Biol. 2013;49:999–1008. doi: 10.1165/rcmb.2013-0093OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanchez-Mas J, Lax A, del Asensio-Lopez MC, et al. Modulation of IL-33/ST2 system in postinfarction heart failure: correlation with cardiac remodelling markers. European journal of clinical investigation. 2014;44:643–651. doi: 10.1111/eci.12282. [DOI] [PubMed] [Google Scholar]
- 49.Marvie P, Lisbonne M, L’Helgoualc’h A, et al. Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. Journal of cellular and molecular medicine. 2010;14:1726–1739. doi: 10.1111/j.1582-4934.2009.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McHedlidze T, Waldner M, Zopf S, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bailey JR, Bland PW, Tarlton JF, et al. IL-13 promotes collagen accumulation in Crohn’s disease fibrosis by down-regulation of fibroblast MMP synthesis: a role for innate lymphoid cells? PLoS One. 2012;7:e52332. doi: 10.1371/journal.pone.0052332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scharl M, Frei S, Pesch T, et al. Interleukin-13 and transforming growth factor beta synergise in the pathogenesis of human intestinal fistulae. Gut. 2013;62:63–72. doi: 10.1136/gutjnl-2011-300498. [DOI] [PubMed] [Google Scholar]
- 53.Brandon JL, Schroeder S, Furuta GT, et al. CT imaging features of eosinophilic colitis in children. Pediatric radiology. 2013;43:697–702. doi: 10.1007/s00247-012-2615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kagalwalla AF, Akhtar N, Woodruff SA, et al. Eosinophilic esophagitis: epithelial mesenchymal transition contributes to esophageal remodeling and reverses with treatment. J Allergy Clin Immunol. 2012;129:1387–1396. e1387. doi: 10.1016/j.jaci.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rieder F, Fiocchi C. Intestinal fibrosis in inflammatory bowel disease: progress in basic and clinical science. Curr Opin Gastroenterol. 2008;24:462–468. doi: 10.1097/MOG.0b013e3282ff8b36. [DOI] [PubMed] [Google Scholar]
- 56.Li M, Riddle SR, Frid MG, et al. Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension. J Immunol. 2011;187:2711–2722. doi: 10.4049/jimmunol.1100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takahashi K, Imaeda H, Fujimoto T, et al. Regulation of eotaxin-3/CC chemokine ligand 26 expression by T helper type 2 cytokines in human colonic myofibroblasts. Clin Exp Immunol. 2013;173:323–331. doi: 10.1111/cei.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Representative photomicrographs of H&E stained ileal tissue sections representing subjects with (i) mild, (ii) moderate or (iii) severe fibrosis. (B) Representative photomicrographs of EPX-immuohistochemically stained ileal tissue sections from (i) control subjects or from patients with (ii) inflammatory or (iii) stricturing CD. Scale bars represent 50μM.
20-week-old SAMP1 mice received 10 injections (i.p.) of anti-IL-5 (TRFK-5; 200μg) or TRFK5 and anti-CCR3 (200μg) combined (or IgG control antibody (200μg)) once per week for 10-weeks. Representative photomicrographs of (Ai) Hematoxylin and Eosin (H&E), (Bi) Major Basic Protein (MBP) and (Ci) Periodic Acid Schiff (PAS) immunohistochemistry on mouse ilea are presented. (Aii) Histologic-indices of inflammation were assessed post treatment. (Bii) Average number of eosinophils per high-powered field (hpf) was quantified. (Cii) Percent of goblet cells per villus were enumerated. Scale bars represent 100μM. Data are expressed as means ± SEM of 4–6 individual mice per group and represent 2-independent experimental repeats. Statistical significance was assessed by 1-way ANOVA with Newman-Keuls multiple comparisons test. **P≤0.01, ***P≤0.001.
EPX-immunohistochemistry provides a quantitatively sensitive strategy to distinguish inflammatory from stricturing CD specimens independently of treatment effects. Examination of the scores for individual EPX parameters for all control, inflammatory or stricturing CD patients found in Table 1 that were not considered under treatment relevant to their CD demonstrated differences between (A) their overall scores; in addition to individual parameters comprising the EPX algorithm: (B) Patchiness, (C) Degranulation, (D) Peak eosinophils / hpf and (E) average eosinophils across 5 random fields-of-view (F.O.V.). Statistical assessments (ANOVA with Newman-Keuls) of the EPX-staining scores (means ± SEM) demonstrated the utility of this algorithm to distinguish between stricturing and inflammatory CD mucosal specimens independent of treatment effects. *P≤0.05, **P≤0.01, ***P≤0.001.