Abstract
Little is known about whether emotion regulation can have lasting effects on the ability of a stimulus to continue eliciting affective responses in the future. To address this issue, participants cognitively reappraised negative images once or four times. One week later they passively viewed old and new images to identify lasting effects of prior reappraisal. As in prior work, active reappraisal increased prefrontal responses while decreasing amygdala responses and self-reported emotion. At one week, amygdala responses remained attenuated for images that had been repeatedly reappraised compared to images reappraised once, new control images, and control images seen as many times but were never reappraised. Prefrontal activation was not selectively elevated for repeatedly reappraised images and was not related to long-term amygdala attenuation. These results suggest that reappraisal can exert long-lasting “dose-dependent” effects on amygdala response that may cause lasting changes in the neural representation of an unpleasant event's emotional value.
Keywords: emotion regulation, reappraisal, amygdala, fMRI, long-term
Introduction
In our everyday sea of emotional waves and swells, the ability to exert top-down regulatory control over emotion helps us maintain an even keel. So important is this ability that problems with it are hallmarks of numerous clinical disorders (Berking et al., 2008; Kring & Sloan, 2009). Accordingly, experimental research on the behavioral and neural mechanisms of emotion regulation has grown enormously in the past decade. The scope of this work is limited, however, by its almost exclusive focus on the regulation of emotions as they happen.
For many events, however, it matters a great deal whether the effects of regulation endure over time. For example, consider how prior attempts to get over a bad break-up are tested in a chance encounter with the old flame. It matters whether that flame still burns, takes continued effort to extinguish, or has truly gone out – and as such – we could be said to have truly, “gotten over it.” Parallel examples abound, including in clinical contexts, where the efficacy of cognitive behavioral therapies turns not just on a patient's ability control his fears and anxieties in a given moment, but on whether those feelings return when emotional triggers are encountered in the future (Berking et al., 2008; Butler, Chapman, Forman, & Beck, 2006; Dobson, 2010; Hollon & Beck, 1994).
At present, very little is known about the neural mechanisms determining when, how and why the effects of emotion regulation will be long-lasting. We addressed this issue by testing for lasting effects of a well-studied cognitive regulatory strategy known as reappraisal. Reappraisal involves changing one's emotional response by changing one's interpretation of a stimulus/situation's meaning (Gross, 1998b, 2013). When used to down-regulate negative emotion, reappraisal can effectively attenuate self-report, physiological and neural markers of affective response, particularly in the amygdala, by recruiting prefrontal systems implicated in domain-general cognitive control functions (Davidson, 2002; Denny, Silvers, & Ochsner, 2009; Gross, 1998a; Gross & John, 2003; Ochsner & Gross, 2008; Ochsner, Silvers, & Buhle, 2012; Silvers, Buhle, & Ochsner, 2014; Walter et al., 2009). Although two recent studies found that amygdala responses to previously reappraised stimuli may remain attenuated at the end of a single experimental session – 10-15 minutes after regulation took place (Erk et al., 2010; Walter et al., 2009) – whether and under what circumstances this attenuation can endure over longer periods of time – and involving what neural mechanisms – is unknown.
Using a novel variant of an established method, we asked three novel questions about the long-lasting effects of using reappraisal to successfully attenuate negative emotion. First, we asked whether the down-regulatory effects of reappraisal on amygdala response can last for one week. To address this question participants first completed a reappraisal task with aversive images where we expected successful reappraisal would be accompanied by greater activity in lateral prefrontal cortex and lesser activity in the amygdala. One week later participants passively viewed brief re-presentations of previously seen images. This allowed us to ask whether amygdala attenuation would endure over time.
Second, we asked whether the durability of amygdala attenuation depends on how many attempts one makes at regulating a response. Here, we compared the long-term effects of reappraising stimuli one time as compared to four times, building on clinical findings that long-lasting regulatory effects may follow from repeated attempts to reappraise a given stimulus (Dobson, 2010; Feske & Chambless, 1995). If repetition (i.e. higher, “doses,” of reappraisal) matters, then we expected to see longer-lasting amygdala attenuation for stimuli reappraised four times.
Third, as a means of probing the mechanisms that underlie the maintenance of regulatory effects, we asked whether long-term attenuation of amygdala responses could occur without the continued need for top-down prefrontal regulatory control, thereby reflecting an enduring change in one's initial affective response tendency (McRae, Misra, Prasad, Pereira, & Gross, 2012; Ochsner & Gross, 2007; Ochsner et al., 2009). If reappraisal can change this response tendency – and active top-down regulation is no longer required for amygdala responses to be diminished – then sustained amygdala attenuation should be observed in the absence of a relationship with prefrontal activity. In the colloquial terms of the relationship example offered earlier, such findings would support the idea that reappraisal can help you “get over” an emotional upset such that you no longer need to exert top-down control to regulate responses to it.
Method
Participants
Twenty-two healthy adult participants (mean age = 24.0 years; 15 female) were recruited and gave informed consent to participate in accordance with the human subjects regulations of New York University. Participants were paid approximately $120 for the entire experiment ($50 for each of two scanning sessions plus $10/hour for the Pre-exposure session). Five exclusions were made for the following reasons: one participant had incorrect images shown at the final scanning session; one participant was a behavioral outlier (>3 standard deviations from the mean for negative affect reports during the Pre-exposure session), and there was evidence that the participant had not been engaged in performing the task; due to a technical problem, one participant had an unbalanced number of regulation versus no regulation training blocks; one participant repeatedly fell asleep during the task, including for entire runs; and one participant showed unacceptably large functional image distortions due in part to repeated repositioning in the scanner. Thus, the present results reflect data from 17 participants (mean age = 24.1 years; 12 female). A priori sample size and data-collection stopping targets were based on sample and effect sizes reported in the extant reappraisal literature (i.e. commonly around 16-18 participants; for a meta-analysis, see Buhle et al., 2014).
Materials
180 negative images (mean normative valence = 2.42, mean normative arousal = 5.75) and 36 neutral images (mean normative valence = 5.51, mean normative arousal = 3.29) were selected on the basis of normative ratings from the International Affective Picture System (Lang, Greenwald, Bradley, & Hamm, 1993). An additional set of 12 similarly valenced and arousing negative images were used during training and Pre-Exposure (described below).
Procedure
Participants completed 3 sessions over the course of 9 days, which included a behavior-only training session (Pre-exposure), and two fMRI scanning sessions (Figure 1). These sessions are detailed below.
Figure 1.
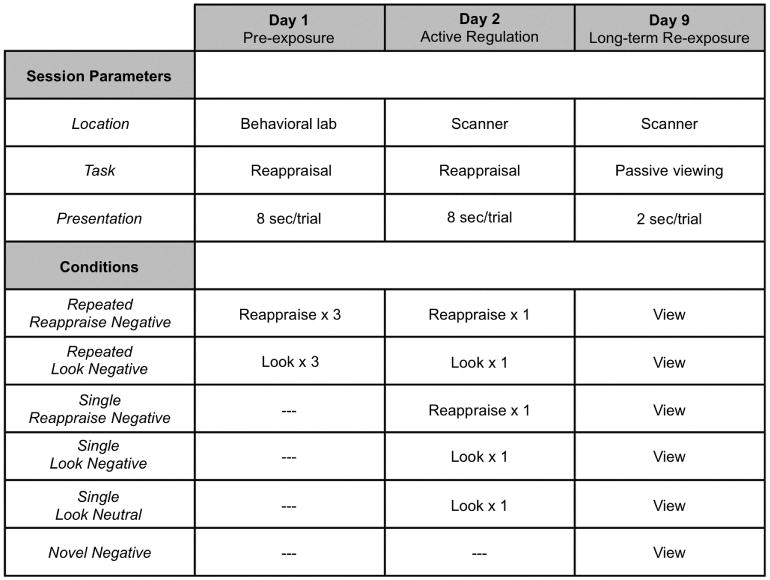
Task paradigm. On Day 1, the Pre-exposure session, participants complete a standard reappraisal task in the behavioral laboratory. On Reappraise trials participants down-regulate the negative emotions elicited by negative images and on Look trials they respond naturally to a matched set of images. This task is completed three times in succession with participants repeatedly Reappraising or Looking at the same images each time. On Day 2, the Active Regulation session, participants complete the reappraisal task in the scanner. On Repeated presentation trials, they once again Reappraise or Look at the images they had Reappraised or Looked at in the Pre-exposure session. On Single presentation trials, they Reappraise or Look at images seen for the first time in the scanning session. On Day 9, the Long-term Re-exposure session, participants passively view all images from the Active Regulation session along with Novel, never before seen, negative images. Inclusion of these images allows determination (see Figure 2) of whether amygdala responses to Repeatedly or Singly reappraised stimuli remain attenuated – as they were during Active Regulation – or whether amygdala responses have returned to their level of response to novel aversive events.
I. Pre-exposure
On the first day (Pre-exposure), participants first received training in reappraisal, specifically using psychological distancing (Ochsner & Gross, 2008; Trope & Liberman, 2010). Participants were told that they would see a number of trials, each beginning with an instruction cue word presented in the center of a computer screen: either LOOK or DECREASE. For LOOK trials, participants were asked to look and respond naturally to the forthcoming image. For DECREASE trials, participants were walked through how to reappraise the forthcoming image as a detached, objective impartial observer, and/or imagine that the pictured events occurred far away or a long time ago. In the presence of an experimenter, participants were asked to self-generate appropriate reappraisals in response to two sample reappraisal trials. The experimenter did not proceed until the participant could adequately self-generate a reappraisal. Participants then completed a fixed-timing set of practice trials with 3 LOOK and 3 DECREASE trials.
Participants then completed a computerized image-based reappraisal task similar to one described previously (Ochsner, Bunge, Gross, & Gabrieli, 2002; Ochsner et al., 2004). For each trial, the instruction cue was presented for 2 s, followed by presentation of an image for 8 s, a jittered fixation interval of between 3 and 7 s (average = 4 s), a negative affect rating period of 3 s (on a scale of 1 [weak] to 5 [strong]), and a final jittered inter-stimulus fixation interval of between 3 and 7 s (average = 4 s). During image presentation, participants were instructed to keep their eyes on the image for the entire time that it was on the screen. For the negative affect rating period, participants were instructed that it was important to be as honest as they could be about how they felt at that moment regardless of whether or not any attempts to decrease their negative emotion were successful. Participants completed 6 runs of trials that were blocked by condition, and images were repeated in like blocks. Specifically, participants completed 3 runs of DECREASE trials and 3 runs of LOOK trials. Each run contained 36 trials, all with negative images, and the blocks were repeated such that each LOOK and DECREASE trial was presented 3 separate times. The block presentation order always alternated between LOOK and DECREASE blocks, and whether the first block was LOOK or DECREASE was counterbalanced across participants. Within each run, trials were presented in randomized order. Further, negative images were separately and randomly assigned to condition prior to generating the task scripts for each participant, for this and all subsequent conditions in the experiment, with the stipulation that the randomized condition assignments could not result in any pair-wise significant or marginal (p<0.10, two-tailed) differences between normative valences and arousals across all conditions in the experiment. Following the completion of the sixth task block, the participant was reminded of the next session.
II. Active Regulation
Participants returned for an fMRI scan one day later. Participants were first given an additional walk-through training of the reappraisal instructions. This walk-through used unique images not shown in the actual task. This set of unique images was counterbalanced across participants with the set given at Pre-exposure. Next, participants entered the scanner and completed an 8 minute resting state scan during which they were instructed to have whatever thoughts and feelings they naturally have, to keep their eyes closed, but to remain awake. Data from this and subsequent resting state scans were not examined in the present analyses. Then, participants completed the Active Regulation task using the same trial structure and same two instruction cues (LOOK or DECREASE) as during the Pre-exposure session. At Active Regulation, 180 total image trials were shown. The 36 Reappraise and 36 Look Negative images from Pre-exposure (“Repeated Reappraise Negative” and “Repeated Look Negative” trials, respectively) were presented along with 36 novel Look Negative (“Single Look Negative”) and 36 novel Reappraise Negative trials (“Single Reappraise Negative”), and 36 novel Look Neutral trials (“Single Look Neutral”). These 180 trials were evenly distributed into 6 functional runs, with 6 trials/condition/run. Within each run, trials were presented in randomized order.
Immediately following Active Regulation, participants completed another 8 minute resting scan. Immediately following the resting scan, participants completed a passive viewing scan in which half of the images presented during Active Regulation plus 18 novel negative images were presented. This initial passive viewing scan on the same day as Active Regulation is not the focus of the current study.
III. Long-term Re-exposure
One week after Active Regulation, participants returned for an fMRI scan. Participants first underwent an 8 minute resting state scan. This was followed by a passive viewing scan in which the other half of images from Active Regulation were shown (i.e. 90 images, consisting of 18 Single Look Neutral images, 18 Single Look Negative images, 18 Single Reappraise Negative images, 18 Repeated Look Negative images, and 18 Repeated Reappraise Negative images), along with 18 novel negative (“Novel Negative”) images, for a total of 108 images. The instructions were to simply view the images, keeping their eyes on them the entire time; no instruction cues were presented. The trial structure consisted of image presentation for 2 s, followed by a jittered inter-stimulus fixation interval of between 3 and 7 s (average = 4 s). All 108 images were presented in a single run, in a novel randomized order for each participant. Following this last passive viewing scan, a final 8 minute resting state scan was performed.
Data Acquisition and Analysis
I. Behavioral
Stimulus presentation and behavioral data acquisition were controlled using E-Prime software (Psychology Software Tools, Inc.). Behavioral data were analyzed using linear mixed models incorporating fixed effects for valence (negative versus neutral), instruction type (Reappraise versus Look), and number of presentations (repeated versus single), and a random effect consisting of an intercept for each participant.
II. fMRI
Whole-brain fMRI data were acquired on a 3.0T Siemens Allegra MRI system. Anatomical and functional images were acquired with a T2*-sensitive EPI BOLD sequence with a TR of 2000 ms, TE of 15 ms, flip angle of 82°, 34 slices, with 3mm isometric voxels, no interslice gap. Functional images were preprocessed using SPM8 software (Wellcome Department of Cognitive Neurology, UCL), including slice-timing correction, realignment, and coregistration between each participant's functional and anatomical data, normalization to a standard template (Montreal Neurological Institute; MNI) using 3mm isometric voxels, and spatial smoothing using a Gaussian kernel (full-width at half-maximum = 6 mm).
A random-effects GLM was then run using Neuroelf v0.9c software (neuroelf.net) incorporating task regressors for Active Regulation and both passive viewing scans. For Active Regulation, separate regressors for fMRI responses to cue (differentiated by whether the instruction cue was LOOK or DECREASE), stimulus presentation (differentiated by 5 conditions: Single Look Neutral, Single Look Negative, Single Reappraise Negative, Repeated Look Negative, and Repeated Reappraise Negative), and rating period (undifferentiated by condition) were specified. For the passive viewing scan, regressors for each stimulus presentation period were specified (i.e. Single Look Neutral, Single Look Negative, Single Reappraise Negative, Repeated Look Negative, Repeated Reappraise Negative, and Novel Negative). All task regressors were convolved with a canonical hemodynamic response function (HRF). Participants' six motion parameter estimates were also entered into the GLM as covariates of no interest. Participant time courses underwent percent signal change transformation. The GLM was computed using ordinary least squares regression and random effects modeling. Contrasts were then performed among various conditions at Active Regulation and at Long-term Re-exposure. Inter-session contrasts between conditions at Active Regulation and Long-term Re-exposure were not performed, however, given that differences in stimulus presentation duration (i.e. 8 s versus 2 s) and task (i.e. reappraisal versus passive viewing) limit the interpretability of the results.
Data were visualized and thresholded using Neuroelf, and beta estimates were extracted for a priori regions-of-interest (ROI's; i.e. amygdala and vlPFC) and analyzed using linear mixed models as described above. All amygdala functional ROI's were masked with a Brodmann atlas-based anatomical boundary using Neuroelf. Whole-brain familywise error (FWE) multiple comparison correction thresholds were determined using Alphasim (Ward, 2000). In regions of a priori interest, including amygdala, FWE extent thresholds were small volume-corrected using a bilateral Brodmann atlas-based anatomical amygdala mask. Anatomical labels were determined by converting MNI coordinates to Talairach space (Talairach & Tournoux, 1988) and using the Talairach Daemon brain atlas (Lancaster et al., 2000). Reported coordinates are in MNI space.
III. Functional Connectivity
Finally, as part of an assessment of whether results at Long-term Re-exposure were driven by top-down versus bottom-up mechanisms, functional connectivity (PPI (Friston et al., 1997)) analyses were performed using the 40 voxel right amygdala cluster that showed long-term attenuation of activity to Repeated Reappraise Negative trials (Table S2) as a seed region. A GLM was then computed incorporating regressors for the within-participant coupling of activity between this right amygdala seed region and other brain areas, as well as a PPI term representing the within-participant coupling of the seed region and other brain areas modulated by the psychological context of interest, which in this case was the difference between Repeated Reappraise Negative trials and all other negative conditions at Long-term Re-exposure (i.e. Repeated Reappraise Negative – 0.25*[Repeated Look Negative + Single Reappraise Negative + Single Look Negative + Novel Negative]). Participants' six motion parameter estimates were also entered into the GLM as covariates of no interest. Beta estimates underwent percent signal change transformation. Following GLM estimation, random effects analyses were performed as above, with contrasts performed for regions showing a significant PPI effect. Results were statistically thresholded as described above.
Results
Overview
Seventeen healthy adults completed a three-session paradigm (see Figure 1). The first Pre-exposure session involved completing three repetitions of a standard reappraisal task with aversive images (Denny & Ochsner, 2014; McRae et al., 2010; Ochsner et al., 2002; Ochsner & Gross, 2008; Ochsner et al., 2004; Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008). This task included “Reappraise” trials on which participants reappraised the meaning of each aversive image in order to diminish their negative response to it, as well as baseline “Look” trials where participants were instructed to look at and respond naturally to images. Different sets of valence, arousal, and content matched images were used for each condition (counterbalanced across participants) and these same sets were used for each of the three Reappraise or Look repetitions. One day later was the Active Regulation session where participants completed a reappraisal task in a functional MRI scanner with both Reappraise and Look trials. Here, half of the images were “Repeated” images from the prior day's Pre-exposure session and half were never before seen “Single” images. One week later, at Long-term Re-exposure, participants passively viewed previously presented stimuli while in the functional magnetic resonance imaging (fMRI) scanner, along with Novel aversive images.
Pre-exposure and Active Regulation Sessions
As the focus of the report is the Long-term Re-exposure session, the results of the Pre-exposure and Active Regulation sessions are briefly summarized here and described in detail in the Supplemental Material. For present purposes, three findings are important to document here. First, during the Pre-exposure session, reappraisal was effective in decreasing negative affect (Figure S1A). Second, during the Active Regulation session, reappraisal was effective in decreasing negative affect for both Repeated stimuli (that had been presented in the Pre-exposure session) and Single stimuli (i.e. that were presented for the first time; Figure S1B). The fMRI data mirrored these effects, with attenuation of right amygdala activity and engagement of left ventrolateral prefrontal (vlPFC) regions typical of reappraisal for both Repeated and Single presentation stimuli (Figure S2; Table S1).
Apart from largely replicating prior work on the neural correlates of reappraisal implementation in individual sessions (Buhle et al., 2014), these data are important because they set the stage for asking whether and how effective reappraisals – that by the conclusion of the Active Regulation session have been repeated four times for Repeated stimuli and have occurred just once for Single stimuli – lead to long-lasting effects on amygdala responsivity one week later. In addition, because only self-report data were collected during the Pre-exposure session, it was important to perform a manipulation check for the validity of these self-report data. To do this, we correlated regulatory success for Repeated stimuli during Pre-exposure and Active Regulation (i.e. the within-participant difference between average ratings of negative affect for Look Negative versus Reappraise Negative trials) with the magnitude of amygdala attenuation for Repeated Reappraise Negative trials one week later at Long-term Re-exposure. As noted in the Supplemental Material (Figure S3; for full analysis, see Supplemental Material), this correlation was significant, suggesting that regulatory success during the Pre-exposure session contributes to the long-term durability of reappraisal-related amygdale modulation.
Long-term Re-exposure Session
In the Long-term Re-exposure session, participants passively viewed images from six conditions (see Figure 1). Four conditions were of primary interest because they would allow us to directly address the question of whether and why reappraisal may have long-lasting effects on amygdala response. On Repeated Reappraise Negative and Repeated Look Negative trials, participants viewed images that had been previously presented in both the Pre-exposure Session and the Active Regulation session, for a total of 4 prior presentations per image. And on Single Reappraisal and Single Look Negative trials participants viewed images that had been previously presented only in the Active Regulation session, for only 1 prior presentation each.
In addition, two other conditions were included. On Single Look Neutral trials participants viewed neutral images seen once in the Active regulation session, which would allow us to determine whether any long-lasting attenuation of amygdala responses to aversive images on Repeated vs. Single presentation trials would be similar to the response to a previously seen stimulus that did not elicit an aversive response. By contrast, on Novel Negative trials participants viewed never before seen images that would allow us determine whether responses to previously seen negative images had returned to the level of responses to images that had never been seen before.
Can amygdala attenuation be long-lasting, and if so, under what conditions?
With this in mind, we then addressed our three questions about the long-term effects of reappraisal. The first two were whether long-lasting attenuation of amygdale response may be observed one week after having regulated, and whether such effects could follow from just a single attempt at reappraisal or required repeated opportunities to reappraise a given stimulus. To address these questions, we asked whether there were main effects of instruction type (i.e., Reappraise vs. Look Negative trials, collapsing across number of presentations) or number of presentations (collapsing across instruction type) on the amygdala response (within an anatomically-defined ROI) at Long-term Re-exposure. Neither effect exceeded small volume-corrected FWE thresholds.
We then asked whether there was a lasting effect of instruction type for either Repeated or Single presentation stimuli considered separately that may not have been detected in the overall main effect of instruction type. Here we found that right amygdala activity remained attenuated in the Reappraise versus Look Negative contrast for Repeated trials (73 voxels, peak at [27, -9, -27], FWE small volume-corrected, p<0.05, two-tailed). However, a contrast of Single Reappraise Negative versus Single Look Negative trials did not yield any results that exceeded small volume-corrected FWE thresholds in amygdala or any whole brain FWE-corrected results.
Further, a direct comparison of Repeated Reappraise Negative versus Single Reappraise Negative trials revealed reduced activity in right amygdala for Repeated Reappraise Negative trials (21 voxels, peak at [24, -6, -24], FWE small volume-corrected, p<0.05, two-tailed). Taken together, these results suggested that amygdala responses showed long-term attenuation for negative stimuli only if they had been repeatedly reappraised. To confirm this, we tested the 2 × 2 instruction type × number of presentations interaction (i.e. including only Repeated Reappraise Negative, Repeated Look Negative, Single Reappraise Negative, and Single Look Negative trials) within the ROI defined above based on the contrast of Repeated Reappraise Negative versus Single Reappraise Negative trials. Importantly, this ROI definition is independent of Look Negative trials and their contribution to the 2 × 2 interaction. We found that the 2 × 2 interaction is significant within this ROI (F(1,48)=5.22, p<0.03), with activity significantly lower for Repeated Reappraise Negative trials relative to all other negative image conditions and not significantly different between Repeated Look Negative and Single Look Negative trials (Figure S4).
Further, in order to provide a complementary, single-step, and direct test of the existence of this critical 2 × 2 “dose-dependence” interaction effect in amygdala, we computed the 2 × 2 interaction contrast (i.e. (Repeated Reappraise Negative – Repeated Look Negative) – (Single Reappraise Negative – Single Look Negative)). This contrast yielded a significant result in right amygdala, again showing lowest activity for Repeated Reappraise Negative trials (30 voxels, peak at [27, -9, -21], FWE small volume-corrected, p<0.05, two-tailed).
Finally, in order to further assess the extent to which this effect of relative attenuation for Repeated Reappraise Negative trials extended to all negative image conditions at Long-term Re-exposure (i.e. by including Novel Negative trials), we computed the corresponding contrast (i.e. Repeated Reappraise Negative– 0.25*[Repeated Look Negative + Single Reappraise Negative + Single Look Negative + Novel Negative]; Figure S5 and Table S2). This contrast showed that responses for Repeated Reappraise Negative trials were significantly lower than all other negative image conditions in right amygdala (40 voxels, peak at [30, -3, -21], FWE small volume-corrected, p<0.05, two-tailed; shown in Figure 2A (top)).
Figure 2.
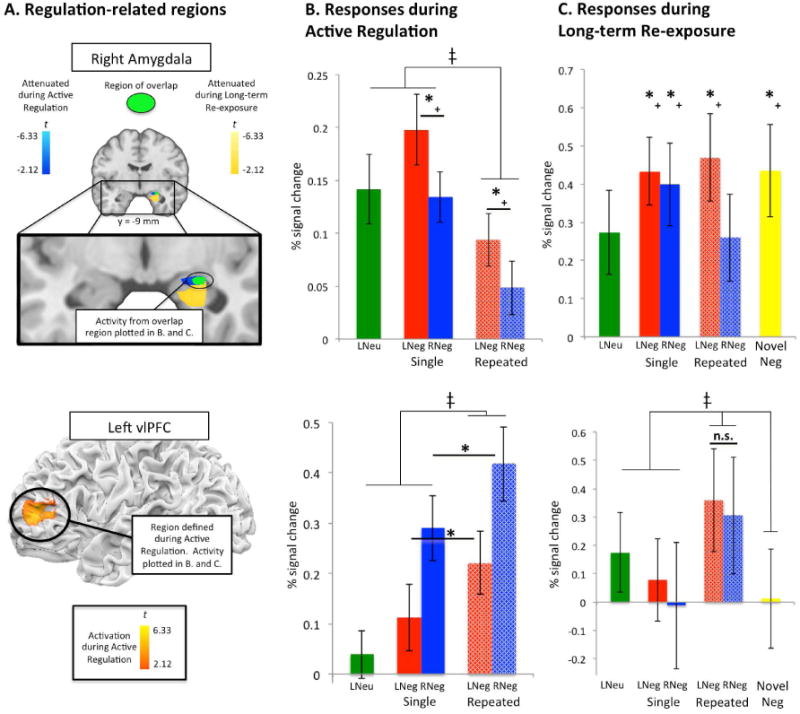
(top row) (A) Conjunction region-of-interest (ROI; shown in green) of right amygdala ROI's showing regulation-related attenuation during Active Regulation (shown in blue; see Table S1) and at Long-term Re-Exposure (shown in yellow; see Table S2). (B) Activity from this overlap right amygdala ROI at Active Regulation (*+ reflects p<0.05, two-tailed). Comparisons marked with *+ are non-independent of the selection criteria for activity during the Active Regulation phase of the study (i.e. Reappraise Negative > Look Negative trials, collapsed across number of presentations) and are shown for illustration of the selection criteria only. ‡ reflects a main effect of number of presentations, p<0.01). (C) Activity from the overlap right amygdala ROI at Long-term Re-exposure (*+ reflects a significant difference from Repeated Reappraise Negative trials, p<0.05, one-tailed), indicating that amygdala attenuation was long-lasting only for repeatedly reappraised images. Comparisons marked with *+ are non-independent of the selection criteria for activity during the Long-term Re-exposure phase of the study (i.e. Repeated Reappraise Negative trials versus all negative image conditions) and are shown for illustration of the selection criteria only. (bottom row) (A) Left vlPFC ROI defined during Active Regulation for the contrast of Reappraise Negative > Look Negative trials (collapsed across number of presentations). (B) Activity from this left vlPFC ROI at Active Regulation. * reflects p<0.05, two-tailed. ‡ reflects a main effect of number of presentations (p<0.01). (C) Activity from this left vlPFC ROI at Long-term Re-exposure is not greater for repeatedly reappraised images, but is instead greater for all repeatedly presented stimuli. This suggests that at Long-term Re-exposure, vlPFC supports retrieval of mnemonic (e.g. semantic) information but does not directly play a role in the lasting attenuation of amygdala responses shown in the row above. ‡ reflects a main effect of number of presentations (p<0.01). All error bars represent ±standard error (SEM).
Because we scanned participants at both the Active Regulation and Long-Term Re-exposure sessions, we then used a conjunction analysis to determine whether the amygdala region showing these long-term effects of repeated reappraisal overlapped with the region showing the concurrent effects of reappraisal during Active Regulation. Although the two regions had different peak foci, they showed significant overlap in the right dorsal amygdala (9 voxels, peak at [24, -9, -15], FWE small volume-corrected for right amygdala, p<0.05, two-tailed; overlap shown in green in Figure 2A (top), with Active Regulation effects shown in blue and Long-term Re-exposure effects shown in yellow). Crucially, these overlap voxels showed the main effects of reappraisal and repeated presentation during Active Regulation (Figure 2B (top)) and showed long-lasting attenuation for Repeated Reappraise Negative trials during Long-term Re-exposure (Figure 2C (top)).
Does long-lasting amygdala attenuation require continued prefrontal engagement?
Our third question concerned the potential mechanisms underlying amygdala attenuation at Long-term Re-exposure – in particular, whether attenuation reflected a lasting change in the amygdala's bottom-up, stimulus-driven response profile (McRae et al., 2012; Ochsner et al., 2009) as opposed to on-going prefrontally-mediated top-down regulation. Although the Long-term Re-exposure session did not involve active demands to reappraise the presented negative images, it is possible that these images may have triggered spontaneous reappraisal, particularly for those that had been already reappraised four times. By contrast, it is possible that repeated reappraisal can result in long-term attenuation in the image-evoked amygdala response even without invoking active reappraisal. Four analyses were performed to address this question.
First, we sought to determine whether a critical cognitive control-related region that had been recruited during initial reappraisal showed a response profile suggestive of on-going regulation at Long-term Re-exposure. For this analysis we focused on a region of left vlPFC that meta-analyses have shown is the region most typically associated with reappraisal (Buhle et al., 2014; Ochsner & Gross, 2008) and also was active during reappraisal in the Active Regulation session. If regulation was occurring at Long-term re-exposure, we would expect left vlPFC activity to be the mirror image of what was observed for the amygdala, i.e., greatest activity on trials where long-term amygdala attenuation was shown (Repeated Reappraise Negative trials) and lowest activity on trials where attenuation was not observed (trials with all other types of negative images). Using the left vlPFC ROI defined at Active Regulation (Figure 2A-B (bottom)), we extracted beta estimates from the Long-term Re-exposure session (Figure 2C (bottom)). As shown, in contrast to amygdala, vlPFC activity did not show a significant difference between Repeated and Single Reappraisal trials at Long-term Re-exposure, but rather a main effect of number of repetitions (F(1,80)=8.55, p<0.01), with activity greatest for repeated presentation trials overall. This pattern is consistent with left vlPFC's role in retrieval of semantic information about stimuli (Badre, Poldrack, Pare-Blagoev, Insler, & Wagner, 2005; Badre & Wagner, 2007; Thompson-Schill, Bedny, & Goldberg, 2005), which may have been more likely for repeatedly presented stimuli.
Second, we followed-up this targeted search with a whole-brain analysis using the contrast that showed that long term attenuation of amygdala response was observed only for repeatedly reappraised stimuli (i.e. Repeated Reappraise Negative trials vs. all other negative image conditions) to determine whether there were any regions at Long-term Re-exposure whose activity might be indicative of prefrontally-mediated regulation using this contrast. Figure S5 and Table S2 show that at Long-term Re-exposure no regions showed activity that was greatest for Repeated Reappraise Negative trials.
Although these first two analyses showed that no control-related regions exhibit greater average levels of activity when long-lasting amygdala attenuation was observed, it is still possible that lasting attenuation may involve differential patterns of functional connectivity between the amygdala and control regions. Our third and fourth analyses addressed these possibilities.
The third analysis examined between-participants connectivity. For this analysis we first extracted activation from the right amygdala region showing long-term attenuation for repeatedly reappraised stimuli (shown in yellow and green in Figure 2A (top)). A whole-brain correlational analysis was then used to ask whether participants showing greater attenuation in this seed region showed greater activity in any other brain regions at Long-term Re-exposure. No FWE-corrected effects were observed in PFC. This analysis was then repeated using activity for Repeated Reappraise Negative trials alone rather than relative to all other negative image conditions, and again no FWE-corrected PFC effects were observed.
The fourth analysis used a within-subjects measure of connectivity implemented as a psychophysiological interaction, or PPI analysis (Friston et al., 1997). This analysis asked whether the amygdala region used in the preceding analysis (shown in yellow and green in Figure 2A (top)) showed a stronger timeseries correlation with PFC on Repeated Reappraise Negative trials as compared to other negative image trials types at Long-term Re-exposure. No FWE-corrected PFC regions showed a significant PPI effect. Thus, taken together, these analyses yielded no evidence that top-down prefrontally-mediated control is required at long-term re-exposure in order for amygdala attenuation to endure.
Discussion
This study began with the question of whether we could find neural evidence that individuals can use cognitive regulatory strategies to, “get over,” unpleasant events such that subsequent emotional responses to them remain diminished. One week after successfully using cognitive reappraisal to diminish behavioral (negative affect) and neural (right amygdala) markers of emotional response, we found that the amygdala's response remained attenuated for images that had been reappraised four times, but not for images that had been reappraised only once. Critically, we found no evidence that these enduring changes in amygdala response required on-going recruitment of prefrontal regions involved in top-down control (including those that were engaged during Active Regulation). Taken together, these findings provide evidence that cognitive regulation can create long-lasting changes in the ability of stimuli to elicit affective responses, and as such, have important implications for both basic and translational research.
On the basic side, this study builds on prior research in three ways. First, it builds on studies showing that reappraisal-related effects on the amygdala can last for periods of up to fifteen minutes (Erk et al., 2010; Walter et al., 2009) by elucidating conditions sufficient for, and the mechanisms underlying, regulatory effects that can last over many days. Second, these results suggest that there may be different routes by which cognitive forms of regulation – as opposed to related, but distinct, forms of affective learning – exert lasting changes on affective response. For example, extinction leads to lasting reductions in amygdala responses to stimuli that previously elicited conditioned fear responses via top-down signals from ventromedial prefrontal regions thought to inhibit amygdala-mediated responding (Phelps, Delgado, Nearing, & LeDoux, 2004; Quirk, Garcia, & Gonzalez-Lima, 2006; Sotres-Bayon, Cain, & LeDoux, 2006). By contrast, while lateral prefrontal regions associated with cognitive control are important for initially reappraising a stimulus in an effective manner, we found that lasting effects on amygdala response occurred in the absence of continued prefrontal recruitment. Future research could ask whether and how different kinds of affective responses require continued involvement of prefrontal control systems as a function of the specific regulatory strategies one deploys. Third, given that the amygdala has multiple sub-nuclei, it is tempting to ask which sub-nuclei and their associated functions are reflected in the overlapping right dorsal amygdala region that showed concurrent and lasting effects of reappraisal. Because the present study did not acquire functional data of sufficient resolution to draw strong inferences on this point, interpretation of our dorsal amygdala findings must remain speculative until tested using high-resolution imaging that could more precisely determine what amygdala nuclei are impacted by reappraisal in the short and long-term.
On the translational side, two kinds of connections to clinical contexts can be made. First, the present results may provide insights into some of the mechanisms by which cognitive therapies can result in lasting changes in affective responses. We found that four, but not one, attempts to reappraise the meaning of an aversive stimulus led to a lasting change in affective responding. To the extent that reappraisal provides a laboratory model of the cognitive regulatory processes involved in cognitive behavioral therapy, then the present study suggests that changes in amygdala responses could be a “dose-dependent” marker for successful therapeutic outcomes (Dobson, 2010; Feske & Chambless, 1995). This leads to the second point. The methods and results of this study could serve as a new tool for probing regulatory ability in clinical, developmental or aging populations where these abilities are not yet mature or have broken down. For example, future work could ask under what conditions a given population can effectively reappraise in the moment – as well as how long the effects of reappraisal last. Reports that the effects of a single reappraisal on amygdala responses lasted 15 minutes for healthy adults (Walter et al., 2009), but didn't last for individuals with major depressive disorder (Erk et al., 2010), highlight how valuable such work could be.
Overall, there remain important questions about the boundary conditions for the present results. At present we know that the effects of cognitive regulation on amygdala response can last a week if one has reappraised a stimulus four times. But we don't yet know whether these effects can last for even longer time periods, how fewer or greater numbers of reappraisal attempts may impact durability, or how much the detectability of long-term effects depends how one is re-exposed (e.g. for shorter or greater amounts of time; during passive viewing or active judgments) to previously reappraised stimuli. Future research could address these issues. Another issue for future work is whether it may in some cases be necessary for the prefrontal involvement seen during active regulation to be re-evoked during subsequent re-exposures in order for long-term effects of regulation to be observed. To address this issue it could be useful, for example, to directly compare prefrontal activity during active regulation and re-exposure, which we could not do in the present study because of differences in stimulus presentation duration and task across testing sessions. Further, in the present study, our inferences about long-term changes in the emotional value of negative stimuli are qualified by the fact that we measured only neural responses at Long-term Re-exposure (albeit with a focus on neural activity with clearly established links to negative affective responses). Future research may clarify whether effects of repeated reappraisal on amygdala response are paralleled by long-term changes in other response channels, like self-reported emotional experience, facial expressive behavior, autonomic responses or memory for regulated events. Finally, while participants were instructed to fixate on presented images during the entire time they were presented, future work assessing eye gaze patterns will be important to perform in order to substantiate the unique effects of reappraisal after controlling for eye gaze; promisingly, such effects have been reported previously in single session reappraisal studies for amygdala activity (van Reekum et al., 2007) as well as self-reported emotional experience and psychophysiology (Urry, 2010).
In summary, the capacity for emotion regulation is critical for responding adaptively to life's stressors. While our understanding of the behavioral consequences and neural bases of emotion regulation has grown tremendously in the past decade, in this study we addressed unanswered questions concerning how long such effects last, and what mechanisms underlie this durability. By showing that regulation can cause lasting changes in emotional responses, these findings deepen understanding of when and why regulation is successful in both everyday and clinical contexts.
Supplementary Material
Acknowledgments
We would like to thank Jacqueline McDougall for assistance with pilot data collection. This work was supported by NIMH grant R01 MH076137 and NICHD grant R01 HD069178 to K. N. O. and NIMH grant R01 MH074692 to L. D.
Footnotes
Author Contributions: B. T. Denny, L. Davachi, and K. N. Ochsner designed research; B. T. Denny, M. Inhoff, and N. Zerubavel performed research; B. T. Denny analyzed data; and B. T. Denny, L. Davachi, and K. N. Ochsner wrote the paper. All authors approved the final version of the manuscript for submission.
References
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47(6):907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Berking M, Wupperman P, Reichardt A, Pejic T, Dippel A, Znoj H. Emotion-regulation skills as a treatment target in psychotherapy. Behav Res Ther. 2008;46(11):1230–1237. doi: 10.1016/j.brat.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex. 2014;24(11):2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin Psychol Rev. 2006;26(1):17–31. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51(1):68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Denny BT, Ochsner KN. Behavioral effects of longitudinal training in cognitive reappraisal. Emotion. 2014;14(2):425–433. doi: 10.1037/a0035276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Silvers JA, Ochsner KN. How we heal what we don't want to feel: The functional neural architecture of emotion regulation. In: Kring AM, Sloan DM, editors. Emotion regulation and psychopathology: A transdiagnostic approach to etiology and treatment. New York: Guilford Press; 2009. pp. 59–87. [Google Scholar]
- Dobson KS, editor. Handbook of cognitive-behavioral therapies. 3rd. New York: Guilford Press; 2010. [Google Scholar]
- Erk S, Mikschl A, Stier S, Ciaramidaro A, Gapp V, Weber B, et al. Acute and sustained effects of cognitive emotion regulation in major depression. J Neurosci. 2010;30(47):15726–15734. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske U, Chambless DL. Cognitive behavioral versus exposure only treatment for social phobia: A meta-analysis. Behav Therapy. 1995;26:695–720. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J Pers Soc Psychol. 1998a;74(1):224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: An integrative review. Review of General Psychology. 1998b;2(3):271–299. [Google Scholar]
- Gross JJ. Emotion Regulation: Taking Stock and Moving Forward. Emotion. 2013 doi: 10.1037/a0032135. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Hollon SD, Beck AT. Cognitive and cognitive-behavioral therapies. In: Bergin AE, Garfield SL, editors. Handbook of psychotherapy and behavior change. 4th. Oxford: John Wiley & Sons; 1994. pp. 428–466. [Google Scholar]
- Kring AM, Sloan DM, editors. Emotion Regulation and Psychopathology: A Transdiagnostic Approach to Etiology and Treatment. New York: Guilford Press; 2009. [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30(3):261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JD, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. J Cogn Neurosci. 2010;22(2):248–262. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Misra S, Prasad AK, Pereira SC, Gross JJ. Bottom-up and top-down emotion generation: implications for emotion regulation. Soc Cogn Affect Neurosci. 2012;7(3):253–262. doi: 10.1093/scan/nsq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The neural architecture of emotion regulation. In: Gross JJ, editor. Handbook of emotion regulation. New York: Guilford Press; 2007. pp. 87–109. [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17(2):153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, et al. For better or for worse: neural systems supporting the cognitive down-and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RR, Hughes B, McRae K, Cooper JC, Weber J, et al. Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychol Sci. 2009;20(11):1322–1331. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60(4):337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Silvers JA, Buhle JT, Ochsner KN. The neuroscience of emotion regulation: Basic mechanisms and their role in development, aging and psychopathology. In: Ochsner KN, Kosslyn SM, editors. The handbook of cognitive neuroscience, Vol 2: The cutting edges. New York: Oxford University Press; 2014. pp. 53–78. [Google Scholar]
- Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry. 2006;60(4):329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system – An approach to cerebral imaging. New York: Thieme; 1988. [Google Scholar]
- Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol. 2005;15(2):219–224. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Trope Y, Liberman N. Construal-level theory of psychological distance. Psychol Rev. 2010;117(2):440–463. doi: 10.1037/a0018963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL. Seeing, thinking, and feeling: emotion-regulating effects of gaze-directed cognitive reappraisal. Emotion. 2010;10(1):125–135. doi: 10.1037/a0017434. [DOI] [PubMed] [Google Scholar]
- van Reekum CM, Johnstone T, Urry HL, Thurow ME, Schaefer HS, Alexander AL, et al. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. Neuroimage. 2007;36(3):1041–1055. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, von Kalckreuth A, Schardt D, Stephan A, Goschke T, Erk S. The temporal dynamics of voluntary emotion regulation. PLoS ONE. 2009;4(8):e6726. doi: 10.1371/journal.pone.0006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for FMRI data. 2000 Retrieved July 3, 2011, from http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


