Abstract
Study Design
In vivo cat model study.
Objective
To determine whether intervertebral facet joint fixation and segmental thrust level alter paraspinal muscle spindle activity during simulated spinal manipulation.
Summary of Background Data
Intervertebral motion is commonly assessed by manual therapy practitioners during clinical evaluation and treatment. Mechanoreceptor activity elicited during spinal manipulation has been theorized as a potential mechanism of its efficacy. The degree to which intervertebral fixation and segmental thrust level alter paraspinal muscle spindle activity during high velocity low amplitude spinal manipulation (HVLA-SM) is unclear.
Methods
Intervertebral fixation was created by inserting facet screws through the left L5–6, L6–7 and left L4–5, L5–6, L6–7, facet joints of a cat spine. Changes in the mean instantaneous frequency of L6 muscle spindle discharge were determined during five HVLA-SM thrust durations ((0-control, 75, 100, 150, 250ms) delivered at the L4 or L6 spinous process in each of 3 conditions within the same preparation: laminectomy-only (surgical control; n=23), L5–6 and L6–7 fixations (n=20), and L4–5, L5–6, and L6–7 fixations (n=7). Comparisons were made between thrust levels, thrust durations and spinal joint conditions using a linear mixed model.
Results
Insertion of facet screws compared to laminectomy-only significantly increased (P<.001) lumbar spinal stiffness during L6 HVLA-SM. Compared to laminectomy-only, both the 2 facet screw (100ms; P<.05) and 3 screw conditions [75 and 100ms (P<.001), 150 ms (P<.005), and 250 ms (P<.05)] significantly decreased L6 spindle response during the L6 HVLA-SM. HVLA-SM delivered 2 segments rostral to the level of muscle spindle input significantly decreases spindle response compared to HVLA-SM delivered at-level, however non-target HVLA-SM still elicits 60–80% of at-level muscle spindle response.
Conclusions
Intervertebral fixation decreases paraspinal muscle spindle response during L6 HVLA-SM in a cat model. While HVLA-SM target accuracy maximizes spindle response, non-target HVLA-SM still elicits substantial levels of muscle spindle activity.
Keywords: afferent, cat, facet joint, fixation, intervertebral, muscle spindle, lumbar spine, low back, neurophysiology, proprioception, spinal manipulation, specificity, trunk
Introduction
At any given time 15–30% of adults will have low back pain (LBP) with a majority experiencing recurrent episodes within a 12-month period.1 Although aberrant spinal joint motion has yet to be established as causative for LBP,2 altered intervertebral motion (hypo- or hypermobility) is associated with neck and LBP.3–11 There is evidence to suggest that when clinical identification of aberrant spinal joint motion is accompanied by a correspondingly tailored manual therapy treatment approach therapeutic outcomes improve.7,11–17
High velocity low amplitude spinal manipulation (HVLA-SM) is a commonly used noninvasive form of manual therapy recommended by both clinical guidelines and evidence reports as a treatment for neck and low back pain.18–21 HVLA-SM is typically applied to reduce clinically-identified intervertebral joint fixation/hypomobility with the goals of normalizing intervertebral motion, reducing pain and/or improving function. Physical exam and clinical diagnostic methods traditionally used to identify the optimal site for manual therapeutic intervention typically focus on joint malalignment, joint fixation/hypomobility, pain provocation, and static or dynamic findings of paraspinal tissue abnormality.22–25 The physical contact site for an HVLA-SM is usually intended to target a specific vertebra. However the clinician’s ability to locate and deliver substantial forces to, and/or cavitate intended target levels has been questioned.24,26–34 It has been shown that by the time the manipulative thrust is delivered, the area of peak pressure for the applied force may have migrated up to 10mm from the intended target.33 Similarly, the mean discrepancy from the intended targeted lumbar vertebra for an HVLA-SM and the resulting joint cavitation location was determined to be 5.29cm (at least one vertebra away) and could be as great as 14cm (two to three vertebrae away).31 A more recent study found that only 71.7% of HVLA-SM related cavitations are confined to a 3 vertebral segment area.29 Despite the importance typically imputed to clinically identifying the precise level of spinal joint fixation and contacting that target level for an HVLA-SM, it is not clear to what extent fixated joints (targeted) would respond differently from non-fixated joints (non-targeted) to HVLA-SM.
It has long been postulated that manual therapy interventions, including spinal manipulation provide benefit by disrupting joint adhesions, musculoskeletal pain cycles and/or muscle hypertonicity.35–38 Spinal manipulation is thought to elicit a barrage of sensory activity from a diverse set of spinal joint and paraspinal tissue mechanoreceptors which in turn influence spinal reflexes and/or subcortical processing to alter motoneuron output resulting in positive clinical outcomes.36–46 We previously showed that only when the thrust of an HVLA-SM is delivered at a clinically relevant duration (≤150ms) does a very high frequency discharge occur from paraspinal muscles. This occurs in both a laminectomy-only45,47 and single (L5–6) lumbar facet joint fixation48 cat model. The purpose of the current study was two-fold. First, we wanted to determine how intervertebral facet joint dysfunction created by multiple unilateral facet joint fixations alters muscle spindle discharge during HVLA-SM. Second, we sought to determine how muscle spindles respond to HVLA-SM thrusts that were delivered two vertebral segments rostral in both functionally intact facet joint preparations and in the presence of multiple unilateral intervertebral facet joint fixations. Both objectives are clinically relevant. Individuals undergoing spinal manipulation often present with intervertebral joint dysfunction at one or more segmental levels,4,7,13,49–51 and manipulative thrust force may not be delivered accurately to the clinically identified site of spinal joint dysfunction.29–34
Materials and Methods
All experiments were approved by the Institutional Animal Care and Use Committee. Electrophysiological activity in single primary afferent fibers from paraspinal muscle spindles was obtained during simulated HVLA-SM in 23 deeply anesthetized male cats weighing an average of 5.4 kg (SD 0.55). All general surgical and electrophysiological procedures have been previously described in detail elsewhere.47,48,52–55
Preparation & Procedures
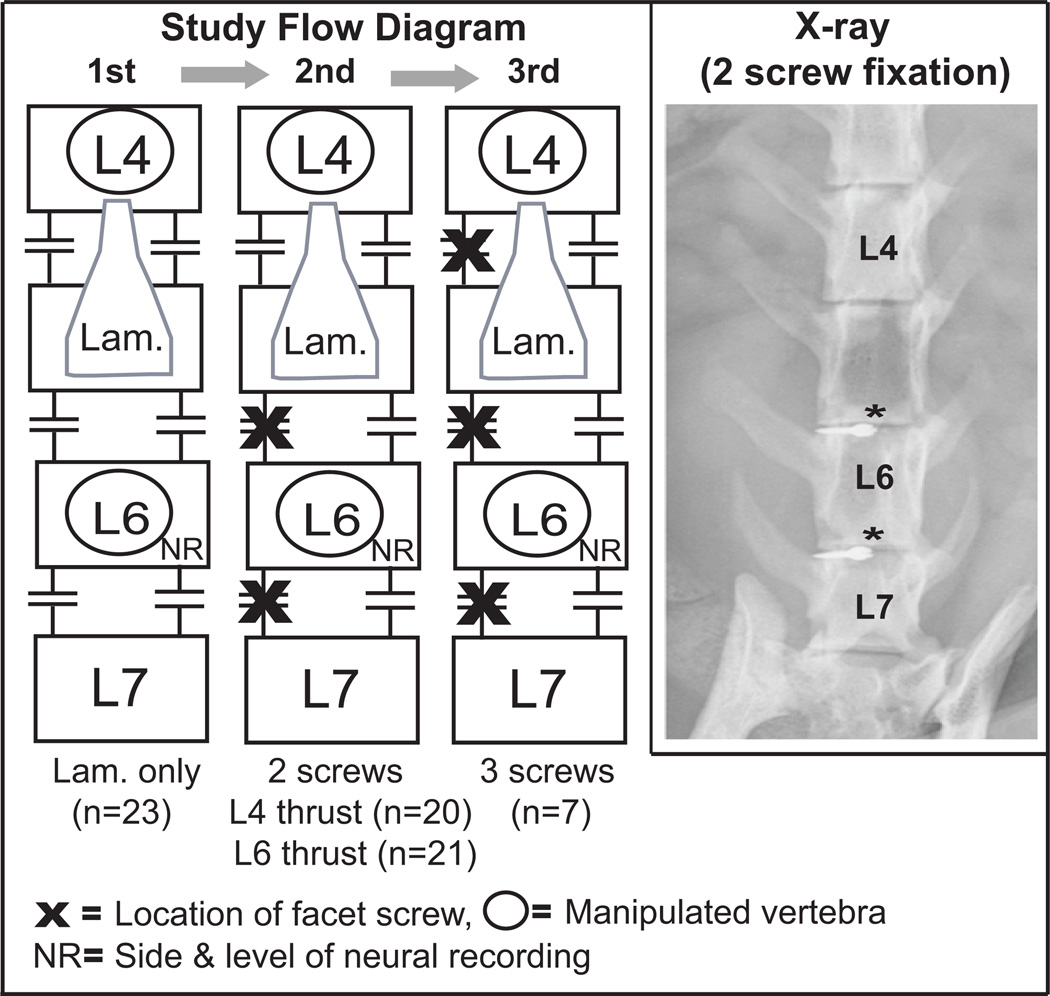
Anesthesia was induced using isoflurane. Catheters were placed in a carotid artery and external jugular vein to monitor blood pressure, introduce fluids, and maintain deep anesthesia with Nembutal (35 mg/kg, iv; Oak Pharmaceuticals, Lake Forest, IL). The trachea was intubated and the cat was artificially ventilated. Arterial pH, PCO2, and PO2 were maintained within the normal range (pH 7.32–7.43; PCO2, 32–37 mmHg; PO2, >85 mmHg). Since our focus was on low back afferents, the right sciatic nerve was cut to reduce afferent input from the hindlimb. A laminectomy was performed at L5 exposing L6 dorsal rootlets. Finely teased L6 dorsal root filaments were placed on a monopolar electrode until the recording contained a single unit that responded only to mechanical pressure applied directly to multifidus or longissimus muscles in the lumbar spine. Afferent fibers remained positioned on the recording electrode while facet screws were carefully placed unilaterally into the left L5–6 & L6–7 and left L4–5, L5–6, & L6–7 facet joints. Titanium endosteally anchored miniscrews (10 mm tomas-pin; Dentaurum, Ispringen, Germany) were inserted through the articular pillars48,52 (Fig. 1). At times, the afferent recording was lost during facet screw placement. Paraspinal muscle tissues on the right side remained intact with the exception of a small slit made for attaching toothed forceps to apply the HVLA-SM.
Figure 1.
Diagram and x-ray showing the anatomical location and order of surgical/electrophysiological procedures (laminectomy-only, laminectomy & 2 facet screws*, laminectomy & 3 facet screws) performed in the same animal while maintaining a primary afferent recording. Lam., represents the extent of the surgical laminectomy performed, n = number of preparations tested.
Afferents were identified as muscle spindles by their increased discharge to succinylcholine (100 mg/kg; Butler Schein, OH), sustained response to a fast vibratory stimulus (~70 Hz) and/or decreased discharge to muscle twitch caused by bipolar direct muscle stimulation (0.2–0.3 mA; 50 µs).48,53,56,57 One spindle afferent was investigated per cat because removing and re-inserting facet joint screws multiple times would likely reduce the lumbar spinal joint stiffness the screws were intended to impart.
Simulated HVLA Spinal Manipulation
Simulated HVLA-SM thrusts were applied in a dorsal-ventral direction either at the L4 (non-target) or L6 (target) spinous process under 3 spinal joint conditions in the same animal: laminectomy-only, 2 level fixation (L5–6, L6–7), or 3 level fixation (L4–5, L5–6, L6–7). HVLA-SMs were delivered via forceps attached to and controlled by a feedback motor system.48,52,54 Peak manipulative forces of 3.95 kg (55% of an average cat body weight as determined in larger studies47,57) were applied to the spinous process under force control. Five HVLA-SM thrust durations (0-control, 75, 100, 150, 250ms) were applied. Spinal manipulations were separated by 5 minutes.48,57 L6 muscle spindle responses during L4 and L6 HVLA-SM thrusts were determined in the following order: laminectomy-only, 2 level fixation, and 3 level fixation condition (Fig. 1). The order of thrust duration was randomized within each of the 3 spinal joint conditions. Lumbar spinal stiffness was determined during each HVLA-SM. Forces and displacements were measured simultaneously by the feedback control system. Stiffness was calculated as the slope of the force-displacement curve from thrust onset to peak thrust amplitude.
Data analysis
As previously described,47,48,52,57 neural discharge was quantified as instantaneous frequency (IF) by taking the reciprocal of the time interval between successive action potentials. Muscle spindle responses during HVLA-SM protocols were obtained by subtracting the mean IF (MIF) of a 2s baseline preceding the HVLA-SM from the MIF during the HVLA-SM’s thrust. The difference in MIF (∆MIF) constituted the response measure. All neural activity is reported in impulses per second (imp/s).
Of the 23 animals used in this study, laminectomy-only data were obtained in all preparations. Data were obtained in 20/23 preparations following the placement of 2 facet screws (L5–6 & L6–7), and in 7/23 preparations following placement of 3 facet screws (L4–5, L5–6, L6–7). Placement of the 3rd facet screw was less successful due to technical/device-related space constraints. Comparisons among manipulative thrust levels, thrust durations, spinal joint conditions and their interactions were tested using a linear mixed model repeated measures ANOVA with spinal joint conditions as the repeated factor. Individual comparisons following significant main effects were performed using Bonferroni post hoc t-tests. Statistical significance was set at .05.
Results
Recordings were obtained from 23 single L6 muscle spindle afferents. Seventeen had receptive fields in the longissimus and 6 in the multifidus muscle. All afferents increased their mean discharge frequency following succinylcholine injection and had sustained responses to fast vibratory stimuli. All afferents, with the exception of 2 whose recordings were lost prior to muscle stimulation, were silenced by muscle twitch.
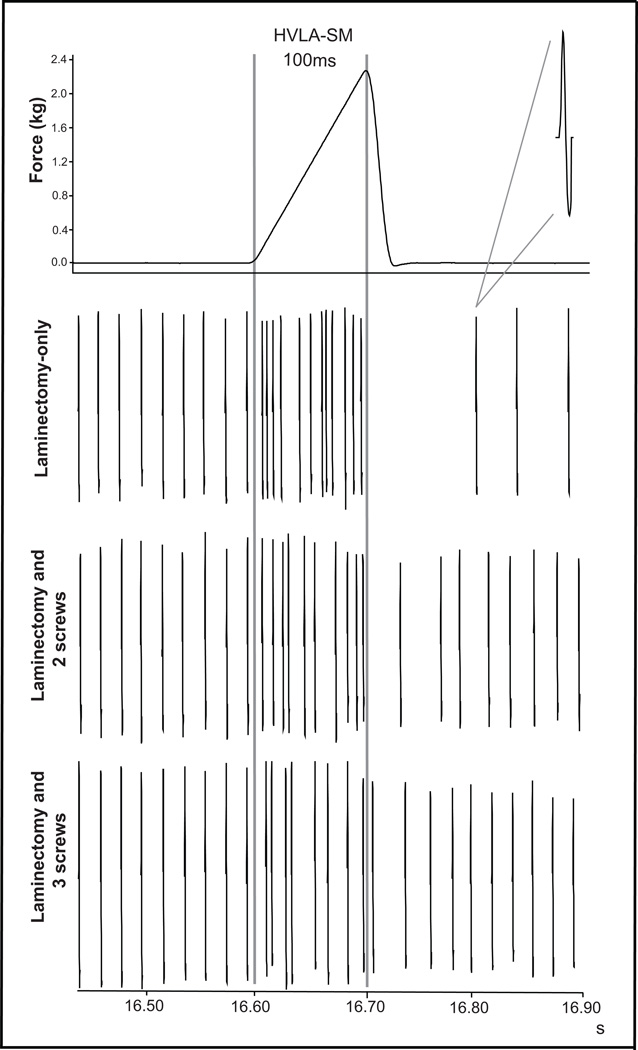
During the thrust phase of an HVLA-SM, resting muscle spindle discharge frequency increased. This was typically followed by a period of silence due to spindle unloading and subsequent resumption of resting spindle discharge. Representative examples from the same muscle spindle afferent responding to 100ms L6 HVLA-SMs under the three different spinal joint conditions are shown in Fig. 2. The laminectomy-only condition exhibited the greatest increase in response during the manipulative thrust, whereas there was a relative decrease in response proportional to the number of intervertebral facet fixations (Fig. 2).
Figure 2.
Representative example of a simulated L6 HVLA-SM with a 100ms thrust duration and paraspinal muscle spindle recordings from the same L6 afferent in 3 spinal joint conditions. Note the stability of baseline discharge between conditions and the decrease in afferent response during the HVLA-SM thrust with increased intervertebral joint fixation.
Intervertebral Fixation and HVLA-SM Thrusts at L4 or L6
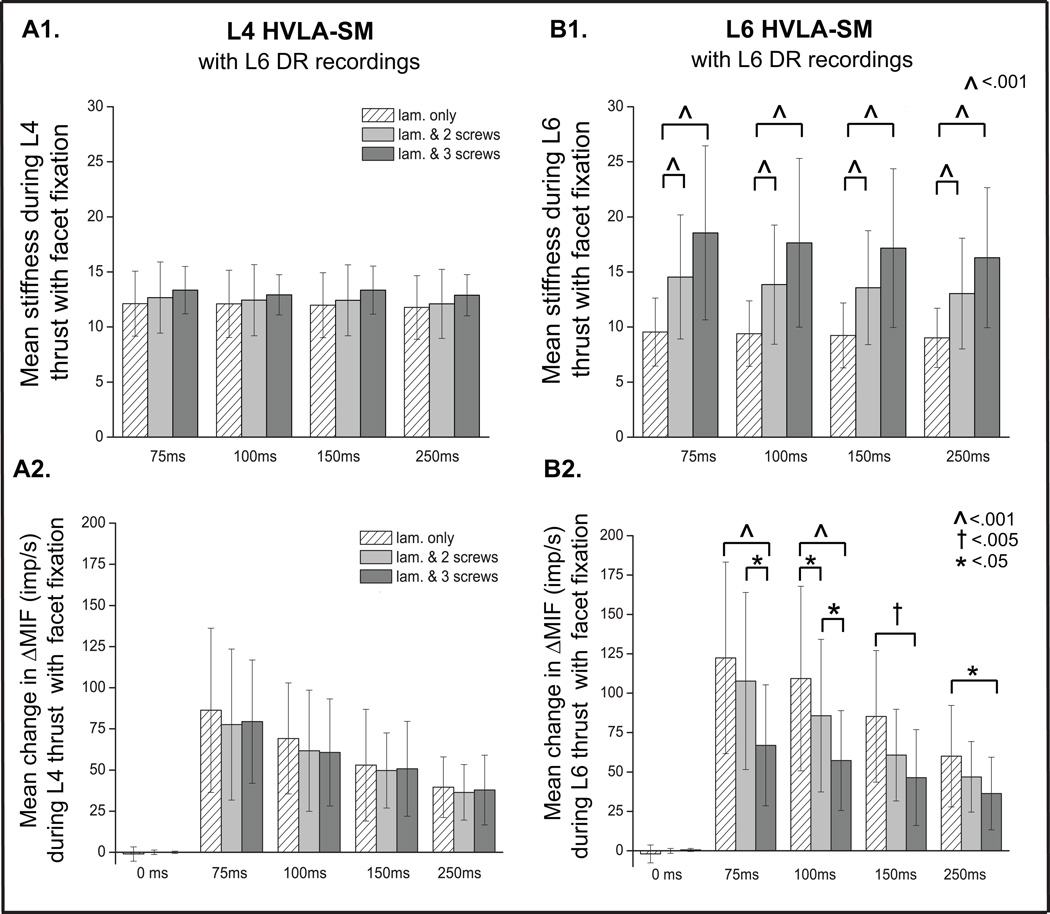
Facet screws were placed unilaterally at the left L5–6 and L6–7 (2 level fixation) and at the left L4–5, L5–6 and L6–7 (3 level fixation). Placement of these screws did little to alter lumbar spinal stiffness during the L4 HVLA-SM (Fig. 3A1). During non-target L4 HVLA-SM, muscle spindle response from L6 afferents clearly demonstrated a pattern in which shorter thrust durations caused graded increases in spindle response, however there were no significant changes across conditions at any of the L4 thrust durations (75–250ms) (Fig. 3A2). On the other hand, the 2 level and 3 level fixations increased L6 spinal stiffness during the L6 HVLA-SM compared to the laminectomy-only condition (P≤.001) (Fig. 3B1). Addition of the third facet screw at L4–5 did not significantly increase stiffness compared to the 2 level fixation during L6 HVLA-SM (Fig. 3B1). During target L6 HVLA-SM, the 2 level fixation compared to the laminectomy-only condition significantly decreased muscle spindle response at 100ms HVLA-SM thrust duration (Fig. 3B2). After placement of the 3rd screw, and compared to the laminectomy-only condition, muscle spindle response significantly decreased at all target L6 HVLA-SM thrust durations (75, 100, 150, 250ms). The largest decreases in spindle response occurred with L6 HVLA-SMs whose thrust durations were 75 and 100ms (Fig. 3B2). In addition, the 3 level fixation condition produced greater decreases in spindle response compared to the 2 level fixation condition only at the two shorter (75 and 100ms) L6 HVLA-SM thrust durations (Fig. 3B2).
Figure 3.
Comparisons of lumbar spinal stiffness from thrust onset to thrust peak during an HVLA-SM delivered at L4 (A1) or L6 (B1) between 3 joint conditions (laminectomy-only, laminectomy & 2 facet screws, laminectomy & 3 facet screws). Comparisons of the mean change in MIF (ΔMIF) during 5 manipulative thrust durations applied at L4 (A2) and L6 (B2) in 3 spinal joint conditions. 0ms thrust duration represents a time control. Data reported as means and SD. Lam., laminectomy, MIF, mean instantaneous frequency.
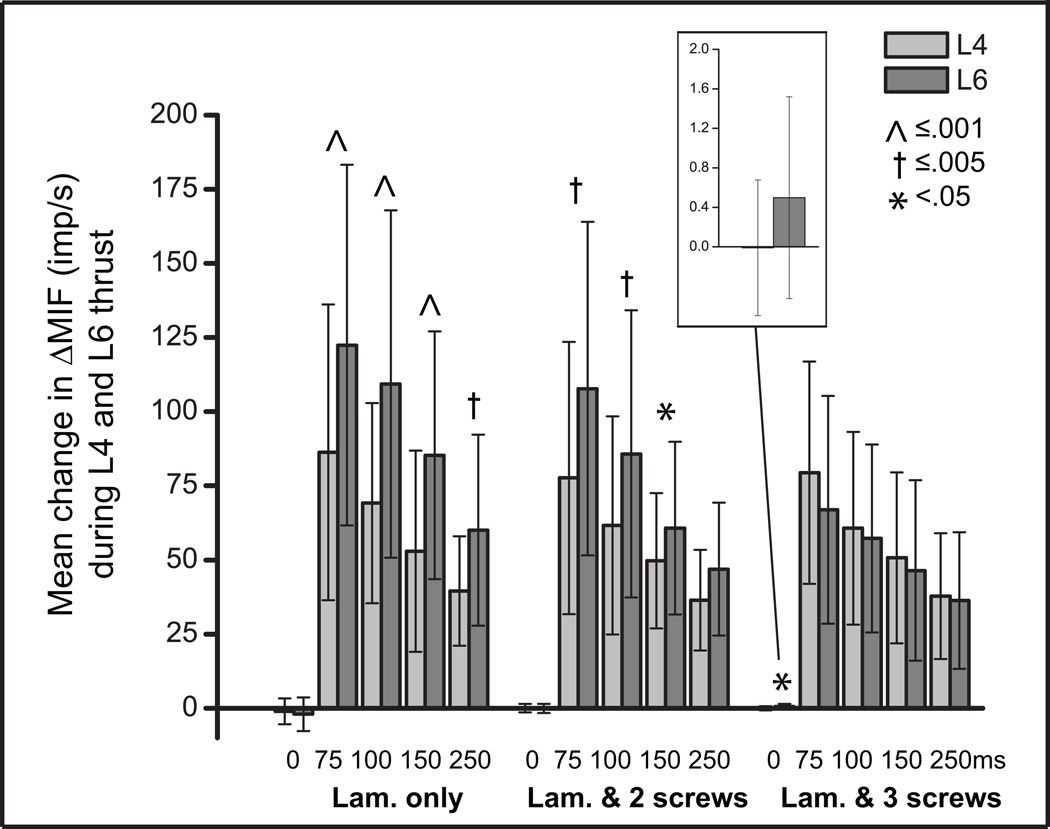
Comparisons between spindle responses to HVLA-SMs delivered at L4 (non-target) vs L6 (target) for each thrust duration and joint condition are shown in Figure 4. In the laminectomy-only and 2 level fixation, L6 spindle responses to the L4 HVLA-SM were significantly less than the L6 HVLA-SM at thrust durations ≤ 150ms. There was a 20–40% decrease in L6 spindle response with L4 HVLA-SM compared to L6 HVLA-SM in the laminectomy-only and 2 level fixation conditions (Fig. 4). Whereas with the 3 level fixation, there were no differences in L6 spindle response between L4 and L6 HVLA-SM at any thrust duration ≥75ms. In the 3 level fixation during the two control protocols (0ms, non-thrust), the significant difference in spindle response was small representing less than 1 imp/s (Fig. 4, inset).
Figure 4.
Comparisons of the mean change in MIF (ΔMIF) during 5 manipulative thrust durations applied at L4 and L6 in 3 spinal joint conditions. 0ms thrust duration represents a time control. Data reported as means and SD. Lam., laminectomy, MIF, mean instantaneous frequency.
Discussion
This animal study demonstrates important findings regarding two aspects of a commonly used noninvasive therapeutic intervention (spinal manipulation). First, during clinically relevant spinal manipulative thrust durations (≤ 150ms), unilateral intervertebral joint fixation significantly decreases paraspinal muscle spindle response compared to non-fixated conditions. Second and perhaps more importantly, this study shows that while L6 muscle spindle response decreases with L4 HVLA-SM, 60–80% of a L6 HVLA-SM muscle spindle response is still elicited from an HVLA-SM delivered 2 segments away in both the absence and presence of intervertebral joint fixation. These findings may have clinical implications concerning specific (targeted) vs non-specific (non-targeted) HVLA-SM.
The laminectomy-only condition elicited the most change in muscle spindle response during targeted (L6) HVLA-SM and non-targeted (L4) HVLA-SM. This indicates that the change in paraspinal muscle length was greatest during the manipulative thrust in the laminectomy-only condition as one might have expected. Despite the additional 3rd screw placed at a distal joint (L4–5) not significantly increasing lumbar spinal stiffness above that of the 2 screw fixation during the targeted L6 HVLA-SM (Fig. 3B1), mean L6 HVLA-SM muscle spindle response was consistently less at all thrust durations for the 3 screw versus 2 screw fixation condition (Fig. 3B2).
The shortest L6 HVLA-SM thrust durations elicited the greatest change in mean spindle response regardless of the degree of unilateral facet joint fixation (Fig. 3B2). This finding supports earlier findings that larger changes in paraspinal muscle spindle response occur as thrust durations become more clinically relevant (≤150ms, manually-delivered34,58) in laminectomy-only47,59 and single facet (L5–6) fixated preparations.48 Shorter duration non-target L4 HVLA-SMs failed to significantly increase L6 muscle spindle response more than longer durations but a pattern of increasing L6 response with decreasing thrust duration regardless of facet fixation condition is clearly evident (Fig. 3A2).
The finding that non-target HVLA-SM delivered 2 segments away elicited significantly less but yet a substantial percentage (60–80%) of the neural response elicited during target HVLA-SM may have important clinical implications with regards to HVLA-SM thrust accuracy/specificity requirements. It may explain how target vs non-target site manual therapy interventions can show similar clinical efficacy.13,60–62 In a recent study using the same model as the current study, the increase in L6 muscle spindle response caused by an HVLA-SM is not different between 3 anatomical thrust contact sites (spinous process, lamina, mammillary body) on the target L6 vertebra but is significantly less when the contact site is located 1 segment caudal at L7 (Reed et al. submitted). The current study confirms that a non-target HVLA-SM compared to a target HVLA-SM decreases spindle response but adds the caveat that a substantial percentage (60–80%) of afferent response can be elicited from an HVLA-SM delivered 2 segments away irrespective of the absence or presence of intervertebral fixation.
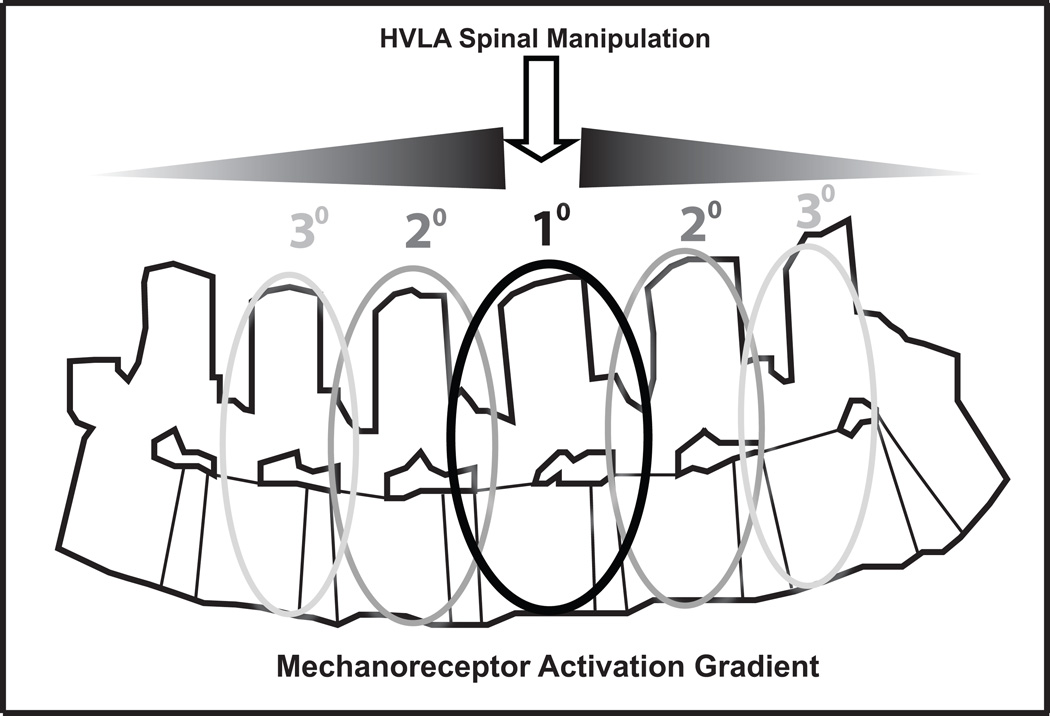
Together these studies provide a strong argument for a regional model of an HVLA-SM mechanoreceptor activation gradient such as depicted in Figure 5. The greatest mechanoreceptor discharge would occur at the anatomical site of peak force delivery with a diminution of mechanoreceptor activation propagating bi-directionally to adjacent and to non-adjacent vertebrae. This concept of a mechanoreceptor activation gradient is supported by biomechanical studies showing that while a majority of HVLA-SM related cavitations are typically confined to a 3 contiguous vertebra area, more distant cavitations do occur albeit with less frequency.29,31 In addition, HVLA-SM has been shown to produce measurable in vivo intervertebral motions at remote vertebra63 and less forceful grade IV posterior-to-anterior spinal mobilizations applied at each lumbar spinous process elicit vertebral movement at all levels of the lumbar spine as demonstrated using in vivo dynamic MRI studies.64,65 While it remains mechanistically unclear, various theories have been proposed of how HVLA-SM mechanoreceptor stimulation could produce sustained physiological changes.66,67 If the underlying mechanism(s) of HVLA-SM requires a certain mechanoreceptor activation threshold for altering central motoneuronal or nociceptor excitability then a mechanoreceptor activation gradient in which 60–80% of paraspinal mechanoreceptor activity could be generated by an HVLA-SM applied as far as 2 segments away from the intended target vertebra suggests that precise segmental accuracy may be less important to HVLA-SM clinical efficacy than commonly believed. To date, at least 2 randomized clinical trials involving HVLA-SM support the concept that while precise segmental level accuracy may be ideal, it is not an absolute prerequisite for clinical efficacy.13,62
Figure 5.
Schematic of a proposed spinal manipulation neural mechanoreceptor activation gradient model in which maximum afferent activity occurs at the site of peak thrust force and decreasing, yet substantial levels of afferent activity is elicited from this point bi-directionally. While not reducing the emphasis on clinical HVLA-SM thrust accuracy, this gradient may account for clinical efficacy despite technique diversity and poor inter-examiner reliability for optimal thrust site determination.
It is evident from this current and previous work48 that spinal joint fixation which decreases intervertebral mobility also decreases paraspinal muscle spindle responses during simulated spinal manipulation. Therefore it is possible that in order to achieve positive clinical outcomes, purposed or intuitive modifications of the HVLA-SM’s biomechanical parameters (preload, thrust magnitude, thrust duration, etc.) are required on the part of the manual therapy practitioner. These modifications are most likely determined consciously or unconsciously during manual physical assessment of the patient (which typically includes evaluation of segmental stiffness, muscle hypertonicity and mechanical pain response levels).7,24,68,69
The experimental preparation was considered functionally de-efferented because the deep level of Nembutal anesthesia, evidenced by the need for ventilation and absence of withdrawal reflexes, likely caused little to no γ-motoneuron activity.70–72 Although the methods used to create intervertebral fixation in this study were invasive, the purpose of the model was to produce a moderate degree of segmental dysfunction, less than what would be achieved using greater intervertebral body instrumentation such as steel rods and/or intervertebral cages. The anterior lumbar vertebral bodies were not fixated and thereby this model of posterior spinal joint dysfunction may provide greater similarity to the degree of overall intervertebral dysfunction most commonly encountered by manual therapy clinicians. Study limitations include the use of healthy animals without confounding factors such as degenerative and/or inflammatory joint changes and the exclusion of rotary and/or non-posterior-anterior thrust vectors which are commonly used in clinical settings. These factors could alter the present findings.
Conclusion
Intervertebral fixation decreases muscle spindle discharge during target HVLA-SM in a cat model. While HVLA-SM target accuracy maximizes spindle response, non-target thrust muscle spindle response is substantial and possibly provides a neurophysiological rationale for clinical efficacy despite low levels of inter-examiner reliability in determining optimal specific sites for HVLA-SM.
Acknowledgements
The manuscript submitted does not contain information about medical device(s)/drug(s). The NIH National Center for Complementary and Alternative Medicine (K01AT005935) grant in a facility with support from the NIH National Center for Research Resources under Research Facilities Improvement Grant Number C06RR15433 funds were received in support of this work.
Relevant financial activities outside the submitted work: grants.
The authors thank Darlene Burke (University of Louisville-KSCIRC) of the Neuroscience Core (supported by grant 8P30GM103507-National Institute of General Medical Sciences, NIH) for statistical analyses support, Randall Sozio for surgical assistance, and Drs. Robert Vining for x-ray assistance, Stephen Onifer and Robert Cooperstein for their helpful suggestions and critical manuscript review.
Reference List
- 1.Cassidy JD, Cote P, Carroll L, et al. Incidence and course of low back pain episodes in the general population. Spine. 2005;30:2817–2823. doi: 10.1097/01.brs.0000190448.69091.53. [DOI] [PubMed] [Google Scholar]
- 2.Mulholland RC. The myth of lumbar instability: the importance of abnormal loading as a cause of low back pain. Eur Spine J. 2008;17:619–625. doi: 10.1007/s00586-008-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mellin G. Decreased joint and spinal mobility associated with low back pain in young adults. J Spinal Disord. 1990;3:238–243. [PubMed] [Google Scholar]
- 4.Abbott JH, Fritz JM, McCane B, et al. Lumbar segmental mobility disorders: comparison of two methods of defining abnormal displacement kinematics in a cohort of patients with non-specific mechanical low back pain. BMC Musculoskeletal Disorders. 2006;7:45. doi: 10.1186/1471-2474-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickey JP, Pierrynowski MR, Bednar DA, et al. Relationship between pain and vertebral motion in chronic low-back pain subjects. Clin Biomech. 2002;17:345–352. doi: 10.1016/s0268-0033(02)00032-3. [DOI] [PubMed] [Google Scholar]
- 6.Dvorak J, Panjabi MM, Novotny JE, et al. Clinical validation of functional flexion-extension roentgenograms of the lumbar spine. Spine. 1991;16:943–950. doi: 10.1097/00007632-199108000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fritz JM, Whitman JM, Childs JD. Lumbar spine segmental mobility assessment: an examination of validity for determining intervention strategies in patients with low back pain. Arch Phys Med Rehabil. 2005;86:1745–1752. doi: 10.1016/j.apmr.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 8.Klein AB, Snyder-Mackler L, Roy SH, et al. Comparison of spinal mobility and isometric trunk extensor forces with electromyographic spectral analysis in identifying low back pain. Phys Ther. 1991;71:445–454. doi: 10.1093/ptj/71.6.445. [DOI] [PubMed] [Google Scholar]
- 9.Kulig K, Powers CM, Landel RF, et al. Segmental lumbar mobility in individuals with low back pain: in vivo assessment during manual and self-imposed motion using dynamic MRI. BMC Musculoskelet Disord. 2007;8:8. doi: 10.1186/1471-2474-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panjabi MM. Clinical spinal instability and low back pain. J Electromyogr Kinesiol. 2003;13:371–379. doi: 10.1016/s1050-6411(03)00044-0. [DOI] [PubMed] [Google Scholar]
- 11.Ssavedra-Hernandez M, Castro-Sanchez AM, Fernandez-de-Las-Penas C, et al. Predictors for identifying patients with mechanical neck pain who are likely to achieve short-term success with mechanical neck pain who are likely to achieve short-term success with manipulative interventions directed at the cervical and thoracic spine. J Manipulative Physiol Ther. 2011;34:144–152. doi: 10.1016/j.jmpt.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Fritz JM, Childs JD, Flynn TW. Pragmatic application of a clinical prediction rule in primary care to identify patients with low back pain with a good prognosis following a brief spinal manipulation intervention. BMC Fam Pract. 2005;6:29. doi: 10.1186/1471-2296-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleland JA, Fritz JM, Kulig K, et al. Comparison of the effectiveness of three manual physical therapy techniques in a subgroup of patients with low back pain who satisfy a clinical prediction rule: a randomized clinical trial. Spine. 2009;34:2720–2729. doi: 10.1097/BRS.0b013e3181b48809. [DOI] [PubMed] [Google Scholar]
- 14.Flynn T, Fritz J, Whitman J, et al. A clinical prediction rule for classifying patients with low back pain who demonstrate short-term improvement with spinal manipulation. Spine. 2002;27:2835–2843. doi: 10.1097/00007632-200212150-00021. [DOI] [PubMed] [Google Scholar]
- 15.Raney N, Peterson E, Smith T, et al. Development of a clinical prediction rule to identify patients with neck pain likely to benefit from cervical traction and exercise. Eur Spine J. 2009;18:382–391. doi: 10.1007/s00586-008-0859-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Childs JD, Fritz JM, Flynn TW, et al. A clinical prediction rule to identify patients with low back pain most likely to benefit from spinal manipulation: a validation study. Annals of Internal Medicine. 2004;141:920–928. doi: 10.7326/0003-4819-141-12-200412210-00008. [DOI] [PubMed] [Google Scholar]
- 17.Hicks GE, Fritz JM, Delitto A, et al. Preliminary development of a clinical prediction rule for determining which patients with low back pain will respond to a stabilization exercise program. Arch Phys Med Rehabil. 2005;86:1753–1762. doi: 10.1016/j.apmr.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 18.Bronfort G, Haas M, Evans R, et al. Effectiveness of manual therapies: the UK evidence report. Chiropr Osteopat. 2010;18:3. doi: 10.1186/1746-1340-18-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Childs JD, Cleland JA, Elliott JM, et al. Neck pain: Clinical practice guidelines linked to the International Classification of Functioning, Disability, and Health from the Orthopedic Section of the American Physical Therapy Association. J Orthop Sports Phys Ther. 2008;38:A1–A34. doi: 10.2519/jospt.2008.0303. [DOI] [PubMed] [Google Scholar]
- 20.Dagenais S, Tricco AC, Halderman S. Synthesis of recommendations for the assessment and management of low back pain from recent clinical practice guidelines. Spine J. 2010;10:514–529. doi: 10.1016/j.spinee.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 21.Koes BW, van Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the mangement of non-specific low back pain in primary care. Eur Spine J. 2010;19:2075–2094. doi: 10.1007/s00586-010-1502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jull G, Bogduk N, Marsland A. The accuracy of manual diagnosis for cervical zygapophysial joint pain syndromes. Med J Aust. 1988;148:233–236. doi: 10.5694/j.1326-5377.1988.tb99431.x. [DOI] [PubMed] [Google Scholar]
- 23.Bergmann TF, Finer BA. Joint Assessment-P.A.R.T.S. Top Clin Chiropr. 2000;7:1–10. [Google Scholar]
- 24.Triano J, Budgell B, Bagnulo A, et al. Review of methods used by chiropractors to determine the site for applying manipulation. Chiropr Man Ther. 2013;21:36. doi: 10.1186/2045-709X-21-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Licciardone JC, Nelson KE, Glonek T, et al. Osteopathic manipulative treatment of somatic dysfunction amoung patients in the family practice clinic setting: a retrospective analaysis. J Am Osteopath Assoc. 2005;105:537–544. [PubMed] [Google Scholar]
- 26.Haneline MT, Young M. A review of intraexaminer and interexaminer reliability of static spinal palpation: a literature synthesis. J Manipulative Physiol Ther. 2009;32:379–386. doi: 10.1016/j.jmpt.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Stochkendahl MJ, Christensen HW, Hartvigsen J, et al. Manual examination of the spine: a systematic critical literature review of reproducibility. J Manipulative Physiol Ther. 2006;29:475–485. doi: 10.1016/j.jmpt.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 28.vanTrijffel E, Anderegg Q, Bossuyt PM, et al. Inter-examiner reliability of passive assessment of intervertebral motion in the cervical and lumbar spine: a systematic review. Man Ther. 2005;10:256–269. doi: 10.1016/j.math.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Cramer GD, Ross JK, Raju PK, et al. Distribution of cavitations as identified with accelerometry during lumbar spinal manipulation. J Manipulative Physiol Ther. 2011;34:572–583. doi: 10.1016/j.jmpt.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beffa R, Mathews R. Does the adjustment cavitate the targeted joint? An investigation into the location of cavitation sounds. J Manipulative Physiol Ther. 2004;27:e2. doi: 10.1016/j.jmpt.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Ross JK, Bereznick DE, McGill SM. Determining cavitation location during lumbar and thoracic spinal manipulation: is spinal manipulation accurate and specific? Spine. 2004;29:1452–1457. doi: 10.1097/01.brs.0000129024.95630.57. [DOI] [PubMed] [Google Scholar]
- 32.Perle S, Kawchuk GN. Pressures generated during spinal manipulation and their association with hand anatomy. J Manipulative Physiol Ther. 2005;28:265.e1–265.e7. doi: 10.1016/j.jmpt.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Herzog W, Kats M, Symons B. The effective forces transmitted by high-speed, low-amplitude thoracic manipulation. Spine. 2001;26:2105–2110. doi: 10.1097/00007632-200110010-00012. [DOI] [PubMed] [Google Scholar]
- 34.Hessell BW, Herzog W, Conway PJW, et al. Experimental measurement of the force exerted during spinal manipulation using the Thompson technique. J Manipulative Physiol Ther. 1990;13:448–453. [PubMed] [Google Scholar]
- 35.Cramer GD, Gregerson DM, Knudsen JT, et al. The effects of side-posture positioning and spinal adjusting on the lumbar Z joints: a randomized controlled trial with sixty-four subjects. Spine. 2002;27:2459–2466. doi: 10.1097/00007632-200211150-00008. [DOI] [PubMed] [Google Scholar]
- 36.Pickar JG. Neurophysiological effects of spinal manipulation. Spine J. 2002;2:357–371. doi: 10.1016/s1529-9430(02)00400-x. [DOI] [PubMed] [Google Scholar]
- 37.Clark BC, Thomas JS, Walkowski SA, et al. The Biology of Manual Therapies. J Am Osteopath Assoc. 2012;112:617–629. [PubMed] [Google Scholar]
- 38.Korr IM. The Neurobiologic Mechanisms in Manipulative Therapy. New York: Plenum Press; 1978. [Google Scholar]
- 39.Bialosky JE, Bishop MD, Price DD, et al. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther. 2009;14:531–538. doi: 10.1016/j.math.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dishman JD, Ball KA, Burke J. Central motor excitability changes after spinal manipulation: a transcranial magnetic stimulation study. J Manipulative Physiol Ther. 2002;25:1–9. [PubMed] [Google Scholar]
- 41.Dishman JD, Greco DS, Burke JR. Motor-evoked potentials recorded from lumbar erector spinae muscles: a study of corticospinal excitability changes associated with spinal manipulation. J Manipulative Physiol Ther. 2008;31:258–270. doi: 10.1016/j.jmpt.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Knutson GA. The role of the gamma-motor system in increasing muscle tone and muscle pain syndromes: a review of the Johansson/Sojka hypothesis. J Manipulative Physiol Ther. 2000;23:564–572. doi: 10.1067/mmt.2000.109674. [DOI] [PubMed] [Google Scholar]
- 43.Korr IM. Proprioceptors and somatic dysfunction. J Am Osteopath Assoc. 1975;74:638–650. [PubMed] [Google Scholar]
- 44.Pickar JG, McLain RF. Responses of mechanosensitive afferents to manipulation of the lumbar facet in the cat. Spine. 1995;20:2379–2385. doi: 10.1097/00007632-199511001-00002. [DOI] [PubMed] [Google Scholar]
- 45.Pickar JG, Sung PS, Kang YM, et al. Response of lumbar paraspinal muscles spindles is greater to spinal manipulative loading compared with slower loading under length control. Spine J. 2007;7:583–595. doi: 10.1016/j.spinee.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gillette RG. A speculative argument for the coactivation of diverse somatic receptor populations by forceful chiropractic adjustments. Manual Med. 1987;3:1–14. [Google Scholar]
- 47.Reed WR, Cao DY, Long CR, et al. Relationship between biomechanical characteristics of spinal manipulation and neural responses in an animal model: effect of linear control of thrust displacement versus force, thrust amplitude, thrust duration and thrust rate. Evid Based Complement Alternat Med. 2013;2013:492039. doi: 10.1155/2013/492039. eCAM 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reed WR, Long CR, Pickar JG. Effects of unilateral facet fixation and facetectomy on muscle spindle responsiveness during simulated spinal manipulation in an animal model. J Manipulative Physiol Ther. 2013;36:585–594. doi: 10.1016/j.jmpt.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lundberg G, Gerdle B. Correlations between joint and spinal mobility, spinal sagittal configuration, segmental mobility, segmental pain, symptoms and disabilities in female homecare personnel. Scand J Rehab Med. 2000;32:124–133. doi: 10.1080/003655000750045479. [DOI] [PubMed] [Google Scholar]
- 50.McGregor A, Anderton L, Gedroyc W. The assessment of intersegmental motion and pelvic tilt in elite oarsmen. Med Sci Sports Exerc. 2002;34:1143–1149. doi: 10.1097/00005768-200207000-00015. [DOI] [PubMed] [Google Scholar]
- 51.Thompson RE, Pearcy MJ, Downing KJ, et al. Disc lesions and the mechanics of the intervertebral joint complex. Spine. 2000;25:3026–3035. doi: 10.1097/00007632-200012010-00010. [DOI] [PubMed] [Google Scholar]
- 52.Reed WR, Pickar JG, Long CR. Effect of changing lumbar stiffness by single facet joint dysfunction on the responsiveness of lumbar muscle spindles to vertebral movement. J Can Chiropr Assoc. 2014;58:160–169. [PMC free article] [PubMed] [Google Scholar]
- 53.Pickar JG. An in vivo preparation for investigating neural responses to controlled loading of a lumbar vertebra in the anesthetized cat. J Neurosci Methods. 1999;89:87–96. doi: 10.1016/s0165-0270(99)00060-6. [DOI] [PubMed] [Google Scholar]
- 54.Reed WR, Cao DY, Ge W, et al. Using vertebral movement and intact paraspinal muscles to determine the distribution of intrafusal fiber innervation of muscle spindle afferents in the anethesized cat. Exp Brain Res. 2013;225:205–215. doi: 10.1007/s00221-012-3362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sung PS, Kang YM, Pickar JG. Effect of spinal manipulation duration on low threshold mechanoreceptors in lumbar paraspinal muscles: a preliminary report. Spine. 2005;30:115–122. doi: 10.1097/01.brs.0000147800.88242.48. [DOI] [PubMed] [Google Scholar]
- 56.Brown MC, Engberg I, Matthews PB. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol. 1967;192:773–800. doi: 10.1113/jphysiol.1967.sp008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cao DY, Reed WR, Long CR, et al. Effects of thrust amplitude and duration of high-velocity, low-amplitude spinal manipulation on lumbar muscle spindle responses to vertebral position and movement. J Manipulative Physiol Ther. 2013;36:68–77. doi: 10.1016/j.jmpt.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Triano JJ. Biomechanics of spinal manipulative therapy. Spine J. 2001;1:121–130. doi: 10.1016/s1529-9430(01)00007-9. [DOI] [PubMed] [Google Scholar]
- 59.Pickar JG, Kang YM. Paraspinal muscle spindle responses to the duration of a spinal manipulation under force control. J Manipulative Physiol Ther. 2006;29:22–31. doi: 10.1016/j.jmpt.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 60.Slaven EJ, Goode AP, Coronado RA, et al. The relative effectiveness of segment specific level and non-specific level spinal joint mobilization on pain and range of motion: results of a systematic review and meta-analysis. J Man Manip Ther. 2013;21:7–17. doi: 10.1179/2042618612Y.0000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chiradejnant A, Maher CG, Latimer J, et al. Efficacy of "therapist-selected" versus "randomly selected" mobilisation techniques for the treatment of low back pain: a randomised controlled trial. Aust J Physiother. 2003;49:233–241. doi: 10.1016/s0004-9514(14)60139-2. [DOI] [PubMed] [Google Scholar]
- 62.Haas M, Groupp E, Panzer D, et al. Efficacy of cervical endplay assessment as an indicator for spinal manipulation. Spine. 2003;28:1091–1096. doi: 10.1097/01.BRS.0000067276.16209.DB. [DOI] [PubMed] [Google Scholar]
- 63.Nathan M, Keller TS. Measurement and analysis of the in vivo posteroanterior impulse response of the human thoracolumbar spine: a feasibility study. J Manipulative Physiol Ther. 1994;17:431–441. [PubMed] [Google Scholar]
- 64.Kulig K, Landel R, Powers CM. Assessment of lumbar spine kinematics using dynamic MRI: a proposed mechanism of sagittal plane motion induced by manual posterior-to-anterior mobilization. J Orthop Sports Phys Ther. 2004;34:57–64. doi: 10.2519/jospt.2004.34.2.57. [DOI] [PubMed] [Google Scholar]
- 65.Powers CM, Kulig K, Harrison J, et al. Segmental mobility of the lumbar spine during a posterior to anterior mobilization: assessment using dynamic MRI. Clin Biomech. 2003;18:80–83. doi: 10.1016/s0268-0033(02)00174-2. [DOI] [PubMed] [Google Scholar]
- 66.Pickar JG, Bolton PS. Spinal manipulative therapy and somatosensory activation. J Electromyogr Kinesiol. 2012;22:785–794. doi: 10.1016/j.jelekin.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bialosky JE, Bishop MD, Price DD, et al. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther. 2009;14:531–538. doi: 10.1016/j.math.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abbott JH, Flynn TW, Fritz JM, et al. Manual physical assessment of spinal segmental motion: intent and validity. Man Ther. 2009;14:36–44. doi: 10.1016/j.math.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 69.Murphy DR, Morris C. Manual examination of the patient. In: Haldeman S, Dagenais S, editors. Principles and practice of chiropractic. New York: McGraw-Hill; 2005. pp. 593–610. [Google Scholar]
- 70.Collins JG, Kendig JJ, Mason P. Anesthetic actions within the spinal cord: contributions to the state of general anesthesia. Trends Neurosci. 1995;18:549–553. doi: 10.1016/0166-2236(95)98377-b. [DOI] [PubMed] [Google Scholar]
- 71.Taylor A, Durbaba R, Rodgers JF. The classification of afferents from muscle spindles of the jaw-closing muscles of the cat. J Physiol. 1992;456:609–628. doi: 10.1113/jphysiol.1992.sp019356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taylor A, Rodgers JF, Fowle AJ, et al. The effect of succinylcholine on cat gastrocnemius muscle spindle afferents of different types. J Physiol. 1992;456:629–644. doi: 10.1113/jphysiol.1992.sp019357. [DOI] [PMC free article] [PubMed] [Google Scholar]