Abstract
Neutrophils are first responders of the immune system, rapidly migrating into affected tissues in response to injury or infection. To effectively call in this first line of defense, strategically placed cells within the vasculature and tissue respond to noxious stimuli by sending out coordinated signals that recruit neutrophils. Regulation of organ-specific neutrophil entry occurs at two levels. First, the vasculature supplying the organ provides cues for neutrophil egress out of the bloodstream in a manner dependent upon its unique cellular composition and architectural features. Second, resident immune cells and stromal cells within the organ send coordinated signals that guide neutrophils to their final destination. Here, we review recent findings that highlight the importance of these tissue-specific responses in the regulation of neutrophil recruitment and the initiation and resolution of inflammation.
Introduction
Neutrophils play a critical role in the early inflammatory response to infection or injury. Neutrophils comprise the majority of circulating peripheral blood leukocytes in humans and are exquisitely sensitive to inciting stimuli, able to be rapidly and efficiently mobilized in great numbers within minutes to areas of inflammation. While neutrophils have been considered to be foot soldiers of the immune system, dutifully performing their functions en masse with minimal instruction, more recent work has found that the regulation of their movement into organ compartments is more complex and dynamic than previously appreciated. Of note, the specific location and tissue environment where the particular inciting insult takes place, as well as the nature of the original insult, result in unique pathways by which neutrophil recruitment is regulated. In this Review, we will discuss recent studies that demonstrate how tissue resident cells facilitate the movement of neutrophils into target organs. This organ-specific recruitment occurs at two different levels – first, the architecture and cellular composition of the organ-specific vasculature provides cues for neutrophil exit from the bloodstream, and second, sentinel organ-specific tissue resident cells provide additional organized signals to promote their entry into tissues. Understanding how site-specific neutrophil recruitment occurs may help develop more targeted strategies that either strengthen host responses to infection or dampen inappropriate inflammatory processes.
Level 1: The blood vessel -- endothelium and associated perivascular cells
Within the vascular space, neutrophils comprise the largest pool of circulating white blood cells (40–80%) in the bloodstream in humans, where they may persist for hours to days until they reach senescence and are cleared by the bone marrow, spleen, and liver.1 Therefore, the endothelial cell layer lining the vascular lumen serves as an initial tissue barrier that limits leukocyte infiltration into tissues under homeostatic conditions, and then selectively recruits leukocytes during times of injury or infection. Under inflammatory conditions, cytokines, such as tumor necrosis factor (TNF) or IL-1β activate endothelial cells (EC), leading to increased expression of adhesion molecules, such as selectins and integrin ligands, and presentation of chemokines upon their surfaces, which participate in the neutrophil adhesion-recruitment cascade (recently reviewed).2,3 In this review, we will discuss the role of the vasculature in neutrophil recruitment from the perspective of its surrounding organ context, and in particular how associated cells of the vasculature (pericytes, perivascular macrophages, and perivascular mast cells) and cells resident within the tissue influence the manner in which neutrophils are recruited into sites of inflammation.
Endothelial cells
The endothelium has been proposed to be the body’s largest endocrine organ, covering a surface area of about 400 square meters and comprised of approximately 1.2 trillion EC.4 Within this endothelial “organ,” there exists significant heterogenicity, in terms of the type of blood flow that it supports (arterial vs. venular) and organ site that it supplies. It is well-known that the endothelium within different organs is structured differently, and that this architecture relates directly to its function. For example, the brain and retinal vasculature is characterized by EC linked by numerous tight junctions, which contribute to their immune privilege from patrolling leukocytes, the reticuloendothelial system (liver, spleen, and bone marrow) are lined by discontinuous ECs that more readily permit cellular trafficking, and organs such as the kidneys and intestine are lined by fenestrated ECs that permit the passage of macromolecules and nutrients.5,6 A recent study demonstrated that microvascular ECs from different organs have distinct gene signatures,7 although how these tissue-specific developmental programs influence neutrophil recruitment within these separate microenvironments is not yet fully studied. Therefore, understanding the organ-specific particularities of the vascular supply should be taken into consideration when interpreting previous findings from in vitro studies or from other body sites.
The development of the classical paradigm of rolling-adhesion-transmigration during leukocyte recruitment through endothelium has been defined either through in vitro models utilizing a flow chamber simulating physiologic blood flow over cultured endothelium, or through animal intravital microscopy visualizing leukocyte migration within easily accessible vascular areas, such as the skin, mesentery, and cremaster muscle. However, different patterns of leukocyte behavior and recruitment appear to occur in physiologically important organs, such as the liver, kidney, and lungs. The vasculature of the liver is characterized by sinusoids, comprised of fenestrated endothelium that connects the circulation of the hepatic artery to the portal vein. Under inflammatory conditions, neutrophils adhere and tether to the walls of the sinusoids in addition to post-capillary venules, and unlike the classical transmigration model, neutrophil recruitment in acute LPS-induced liver injury model is independent of selectins and integrins,8 and instead utilizes hepatic sinusoidal hyaluronan and neutrophil CD44 to mediate neutrophil adherence and transmigration through the sinusoids. In the lung, in vivo two-photon imaging demonstrates under normal conditions, weakly motile pulmonary neutrophils are present intravascularly and extravascularly in the lung, but upon ischemia-reperfusion (I/R) injury, they rapidly arrest and extravasate through the capillary network.9 A somewhat similar pattern of neutrophil behavior is seen in the kidney glomerulus. In uninflamed kidneys, neutrophils in glomerular capillaries are retained upon the capillary wall for prolonged periods of time (~5 minutes), termed dwell time. After induction of inflammation with anti-glomerular basement membrane antibody treatment, neutrophils increase their dwell time to over 20 minutes in a Mac-1 dependent fashion.10 These studies suggest that neutrophil behavior and receptor usage in organs with more porous endothelium will occur differently than in post-capillary venules in superficial connective tissue.
Pericytes
Pericytes are mesenchymally derived cells that are embedded in the basement membrane surrounding the endothelial cell layer of the blood vessel. They are found around smaller vessels, such as blood capillaries, precapillary arterioles, postcapillary venules, and collecting venules.11 Pericytes are elongated cells with multiple cytoplasmic processes, and a single pericyte may contact multiple EC, with pericyte coverage over the abluminal blood vessel area between 10% to 50%. In addition to being largely restricted to small vessels, pericyte distribution varies between organs, with the highest coverage in the central nervous system and retina (1:1 ratio pericyte:endothelial cell), the lung at a ratio of 1:10, while skeletal muscle has much sparser coverage at a ratio of 1:100.11,12 While the reasons for this differential distribution is not clear, it is likely that the increased pericyte density within the brain contributes to its protection from patrolling leukocytes and pathogens as a physical barrier under homeostasis and as an active participant against infection.13 Pericytes are integrators of signals between multiple cells, providing nutrients and maturation signals for vascular development and regulating the transit of immune cells from the bloodstream into underlying tissues. They express cytokine receptors and toll-like receptors and release cytokines and chemokines in response to stimulation.14–16
Understanding role of pericytes in regulating leukocyte recruitment has been greatly advanced by the use of intravital microscopy. Investigation of the migrating behavior of neutrophils in IL-1β-stimulated cremaster muscle showed that they preferentially migrate through areas around the blood vessel that are low in basement membrane components, such as laminin 10 and collagen IV. These low expression regions (LER) were ultimately found to be gaps in between neighboring pericytes, and after neutrophil transmigration occurs, these gaps enlarge in size although not in number,17 a phenomenon not seen in monocyte transmigration.18 This remodeling of the basement membrane after neutrophil migration facilitates subsequent populations of neutrophils to migrate afterwards, increasing the efficiency of the immune response. While neutrophil transmigration through the endothelial wall occurs rather rapidly (~4 minutes), neutrophils spend considerably more time (~20 minutes) crawling in the abluminal space between the endothelial and pericyte layers in a manner that is ICAM-1, LFA-1, and Mac-1 dependent.15
Numerous studies have shown that dynamic changes in pericyte shape and function regulate neutrophil migration through the basement membrane. Pericytes express receptors for IL-1 and TNF,15 and can be induced to express neutrophil-active chemokines, such as CXCL115 and CXCL8.19,20 While pericytes express chemokines primarily on their cell bodies, allowing for prolonged contact between neutrophil as they crawl along the pericyte layer, there are also areas of high CXCL1 expression in a few border regions that correspond with areas of neutrophil emigration.15 Stimulation of pericytes with cytokines can also induce a change in shape, leading to a more elongated shape and an increase in gap size between venules15 through inhibition of the RhoA/Rho Kinase (ROCK) signaling pathway,21 thereby increasing the total LER area available for neutrophil migration. After neutrophils migrate through LERs, there is decreased density of ECM components, such as laminin-α522. This LER remodeling is inhibitable either by neutrophil depletion or administration of a neutrophil elastase inhibitor, implying that a neutrophil-dependent proteolytic event is responsible for this process. However, one study found that ECM produced by cytokine- or bleomycin-activated pericytes increased the efficiency of transendothelial migration in vitro through upregulation of endothelial ICAM-1 and redistribution to cellular borders, indicating that pericytes may also regulate neutrophil migration through differences in the content of the ECM they produce.23
While the majority of neutrophil transmigration occurs at the level of the postcapillary venules, where pericytes are neural/glial antigen 2 (NG2)--smooth muscle α-actin (α-SMA)+, neutrophil adhesion may occur at the arteriolar level24, and a different subset of NG2+α-SMA+ pericytes resides in the arteriolar and capillary vessels.16 These pericytes express TLR2, TLR4, TNFR1 and FPR2, and upon stimulation pericytes increases ICAM-1 expression.16 Stimulation of pericytes also increases the expression of chemoattractants CXCL1, CXCL8, and MIF and guide extravasating monocytes and neutrophils. Through direct interactions mediated by chemokines and ICAM-1, these pericytes promote neutrophilic inflammation by providing “tracks” for the neutrophils to migrate along, increasing their velocity and directionality of movement, as well as their survival and activation.
Perivascular macrophages
In addition to pericytes, perivascular macrophages (PVM) are intimately associated with the abluminal surface of blood vessels and may also serve as rapid responders to surrounding noxious stimuli.25 The role of PVM specifically in neutrophil recruitment was recently studied using transgenic DPE-GFP mice, in which GFP is expressed under the control of the Cd4 promoter, allowing for the in vivo visualization of PVM, which are adjacent to postcapillary venules and confirmed to be CD45+CD3−CD326−CD11bhiF4/80+.26 These PVM cover approximately one-third of the length of these vessels in the skin in close association with pericytes, although PVM themselves do not directly contact EC. PVMs are differentially distributed throughout organs, as they are found in connective tissues, such as the skin, cremaster muscle, and meninges, but not in the lung or spleen.26 Upon inoculation of the ear skin with Staphylococcus aureus, neutrophils rapidly extravasate preferentially towards PVM and exit within 0–20 micron of PVM. Gene expression analysis of sorted skin immune cells confirmed that DPE-GFP+ PVMs had the highest expression of chemokines, such as Cxcl1, Cxcl2, Ccl2, Ccl3, and Ccl4, while GFP− macrophages had lower expression of these chemokines but higher expression of IL-1β. This study suggests that the ability of PVMs to rapidly call in circulating neutrophils by producing chemokines is an important means of host defense against bacteria.
Alternatively, PVM may play protective roles in response to other types of tissue injury. CD169, or sialic acid-binding IgG-like lectin (Siglec)-1, is expressed on macrophages of secondary lymphoid organs, such as the lymph node and spleen. CD169 positive macrophages within the kidney medullary region are also CX3CR1hiLy6Clo and importantly closely associated with the vasculature.27 Upon induction of renal I/R injury, CD169-deficient mice had increased neutrophil infiltration and tissue necrosis along with greater renal function deterioration and death. This phenotype was rescued either by depletion of neutrophils or through the adoptive transfer of Ly6Clo monocytes. Examination of kidneys showed increased Cxcl1, Cxcl2, and ICAM-1 expression in CD169-deficient mice, and co-culture of endothelium with CD169-deficient leukocytes increased ICAM-1 expression on endothelium, a finding also seen in kidney endothelium of CD169-deficient mice even before renal I/R injury.27 The authors hypothesize that in the kidney, CD169 perivascular macrophages serve to tonically suppress baseline endothelial ICAM-1 expression, preventing excessive neutrophil infiltration upon local tissue damage.
Perivascular mast cells
Perivascular mast cells comprise another important tissue resident cell population closely associated with the vasculature.28 Mast cells are widely distributed throughout vascular beds, particularly in organs where constant contact with the environment occurs, such as the skin, intestine, and lungs, as well as the brain and meninges. In addition to being sentinel cells of the IgE-mediated allergic response, mast cells play important roles in infections and sterile inflammation.29 Upon activation, mast cells release granule-associated mediators, such as vasoactive amines, proteases, and cytokines, and rapidly synthesize bioactive lipids (leukotrienes and prostaglandins) and cytokines. Intravital, high-resolution confocal microscopy of the ears in MCPT5cre mast cell reporter mice demonstrated that perivascular mast cells reside in SMA− negative capillary and venular beds.30 While nonmotile, these perivascular mast cells form stable projections into the lumen of blood vessels that gradually retract, and they also make direct contact with the interior of the vessel with their cell bodies, where they may sample the luminal contents of the bloodstream and even bind and phagocytose circulating IgE. While the skin is considered the body’s largest organ, there is considerable site-to-site variability in terms of cellular composition, and a recent study enumerating resident immune cell populations in different locations of the skin further reiterated that fact. In mice, the dermis of the ear contains significantly higher numbers of mast cells when compared with the back, footpad, or tail, and 50% of the mast cells in the ear are located perivascularly, within 10 microns of a blood vessel.31 This difference in mast cell density was phenotypically apparent in a passive cutaneous anaphylaxis model with antigen-specific IgE, as the skin of the ear developed greater histamine release and vascular leakage upon challenge, when compared with the skin of the back, in a manner that was mast cell-dependent. Differences in mast cell density in rats have also been observed in two commonly used sites for in vivo imaging, as the mesentery has a higher number of mast cells while cremaster venules are more sparsely populated.32
The use of mast cell stabilizers and mast cell-deficient animals has helped to determine the roles of mast cells in pathophysiologic states. Perivascular mast cells are critical for neutrophil recruitment in animal models of vasculitis and arthritis33 and cerebral ischemia.34 Mast cells are important producers of TNF, IL-1, and IL-6, which promote subsequent neutrophil recruitment by inducing chemokine production in surrounding cells and by increasing ICAM-1 expression on endothelium. In experimental allergic encephalomyelitis, a model of multiple sclerosis, meningeal mast cells release TNF in response to immunization, leading to both T cell and neutrophil entry into the CNS.35 In a mouse model of I/R injury, circulating plasmin was found to activate perivascular mast cells, resulting in the generation of leukocytes and platelet activating factor and subsequent neutrophil recruitment, a process inhibited by the administration of plasmin inhibitors.36 Tissue plasminogen activator, a naturally occurring serine protease capable of converting plasminogen to plasmin, also has this same effect upon perivascular mast cells and resultant neutrophil recruitment.37
Mast cells also contain preformed neutrophil chemokines such as CXCL1 and CXCL2 that are released on LPS stimulation.38 In the peritoneum, mast cells are required for initial recruitment of neutrophils out of the blood vessels and into the peritoneal tissue, but tissue-resident macrophages are then needed for subsequent neutrophil migration through the interstitum and into the peritoneal cavity, as clodronate-treated macrophage-depleted mice had decreased neutrophils in the peritoneal cavity but an accumulation of neutrophils within the tissue. In this model, perivascular mast cells quickly call in circulating neutrophils from the bloodstream into the surrounding tissue, but the tissue-resident macrophages are then responsible for guiding the neutrophils toward the organ lumen and site of initial challenge.
Level 2: Organ-specific resident cell activation and neutrophil recruitment
After traversing the vessel wall, neutrophils need additional signals to guide them efficiently through the interstitial space and into organ cavities. The cellular composition of individual organs are unique, comprised of a combination of stromal cells such as fibroblasts and epithelium, as well as immune cells such as macrophages and innate lymphoid cells. In addition, the presence of microbiota either under homeostasis (such as in mucosal surfaces) or during infection of normally sterile sites may provide additional cues.39 Therefore, the process of neutrophil recruitment within individual organs is dictated by the inciting insult and the response of organ-specific tissue-resident cells and newly-recruited immune cells, culminating in the resultant inflammatory response.
Tissue macrophages
It is widely acknowledged that macrophages serve as key sentinels of tissue injury, as they express numerous innate immune receptors, such as toll-like receptors, Fc receptors, and scavenger receptors, that recognize and phagocytose pathogens and cellular debris, and produce large amounts of proinflammatory cytokines in order to mobilize surrounding cells to respond in concert. Tissue-resident macrophages are generally comprised of two populations: embryonically derived resident macrophages that persist and replenish in situ through cellular proliferation, and macrophages originally derived from circulating Ly6Chi monocytes, which are recruited from the circulation upon inflammatory cues and differentiate into macrophages once established within the tissues.40–42 Most organs have differing proportions of macrophages of embryonic and adult hematopoietic stem cell (HSC)-monocytic origin, with the exception of the microglia of the brain, which are solely of embryonic yolk sac origin. However, the proportion of embryonic- and adult HSC-derived macrophages within a given tissue may vary throughout time depending upon the stressors that the organ encounters. After depletion, tissue macrophages self-generate through in situ proliferation, but under conditions of acute stress (such as with lethal irradiation), circulating monocytes may seize advantage by migrating into inflammatory or injured tissues and establishing themselves as tissue macrophages. Within the heart, Ly6ChiCCR2+ macrophages differentially express critical components of the inflammasome, implying that they have a lower threshold to respond to stimuli when compared with embryonically derived macrophages and could influence the outcome of inflammatory responses.43 Indeed, the Immunological Genome Project performed a gene expression atlas of tissue macrophages from various organ sites (microglia, peritonea, lung, spleen, etc.) to define a universal set of genes specifically expressed by tissue macrophages, as well as uncover differences between different subpopulations.44 While certain genes were expressed uniformly in all macrophages regardless of tissue origin, notably CD16 (high-affinity Fcγ receptor) and MerTK (kinase involved in efferocytosis), differences were seen in other important cell surface receptors, such as chemokine receptors, C-type lectins, and toll-like receptors, indicating diverse and specialized functions from site to site.
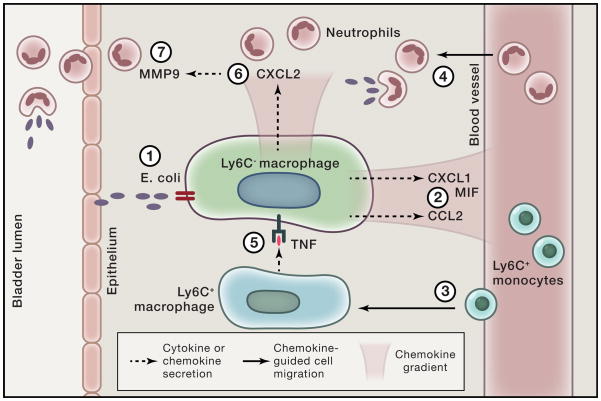
A recent study nicely illustrates how tissue resident and recruited macrophages work in concert to orchestrate an effective neutrophilic response to infection.45 A mouse model of urinary tract infection using intraureteral instillation of uropathogenic E. coli was used to study the roles of macrophages and neutrophils in host defense. Intracellular cytokine staining of Ly6C− and Ly6C+ macrophage populations from the bladder demonstrated that the former produced the chemoattractants CXCL1, CXCL2, CXCL6, and MIF, while the latter were the main producers of TNF. Interestingly, when the infection model was reproduced in mice deficient in the TNF receptor, the investigators observed that neutrophils, which were present in the bladder epithelium and urine in wild-type mice, were no longer able to migrate through the interstitial tissue into the epithelium in the absence of TNF signaling. They subsequently found that TNF produced by Ly6C+ macrophages was critical for Ly6C− macrophages to produce CXCL2, which induced neutrophils to produce the matrix metalloproteinase 9, enabling neutrophils to cross the epithelial layer and phagocytose these pathogens. Therefore, they identified three phagocytic populations with distinct and critical roles in the fight against bacterial infection: 1) Ly6C− tissue resident macrophages that attract the first wave of neutrophils by producing neutrophil-active chemokines, 2) Ly6C+ macrophages, which assist neutrophils by giving them the ability to cross epithelial barriers, and 3) neutrophils that travel from the vasculature through the interstitum and epithelial layer into the bladder lumen, where they phagocytose and kill bacteria (Fig. 2).
Figure 2. Tissue resident cells coordinate neutrophil recruitment through epithelial cell layers.
Resident Ly6C− macrophages sense infection by E. coli (1) and produce MIF and CXCL1 (2), which recruit monocytes (3) and neutrophils (4), respectively, from the circulation into the interstitial space of the bladder. Newly recruited Ly6C+ monocytes release TNF (5), which induces Ly6C− macrophages to release CXCL2 (6). CXCL2 stimulates neutrophils to produce MMP9 (7), which enables them to cross the uroepithelium and reach the bladder lumen to control infection from invading pathogens.
Inflammatory arthritis is a common and debilitating affliction characterized by neutrophil-predominant infiltration within the joint space. It has long been recognized that macrophages play a central role in the pathogenesis of autoimmune inflammatory arthritis, as inhibition of a key macrophage-derived cytokine (TNF) has become a mainstay of treatment.46 However, the complex roles of the origins of macrophages and their pro- and anti-inflammatory effects have been recently studied in mouse models of inflammatory arthritis. In the K/BxN serum transfer induced model of arthritis (STIA), Ly6C− monocytes, rather than Ly6C+ monocytes, are necessary and sufficient for neutrophil recruitment and STIA after clodronate-mediated global monocyte depletion. 47. Gout is another type of inflammatory arthritis triggered by monosodium urate (MSU) crystals that activate the inflammasome in resident macrophages,48 thereby releasing IL-1β and inducing local neutrophil chemokine production and intense neutrophilic infiltration. A gout model using mouse peritoneum for MSU crystal challenge demonstrated that resident macrophages, rather than infiltrating monocytes, were responsible for IL-1β, TNF, and IL-6 production upon initial challenge with MSU crystals.49 However, upon a rapid second rechallenge with MSU crystals, monocytes that had previously been recruited and still present then established themselves as proinflammatory tissue resident macrophages, developing components of the inflammasome and phagocytic capacity,50 suggesting that prior monocytic infiltration provides a new pool of tissue macrophages that supports further neutrophilic inflammation. These studies illustrate that even within a newly recruited macrophage population, there exists phenotypic plasticity during the course of disease that can influence neutrophil trafficking.
Fibroblasts
Tissue fibroblasts provide critical structural support for organs through the synthesis of extracellular matrix components and are critical mediators of wound healing. Under inflammatory conditions, they also participate in leukocyte recruitment by producing a variety of chemokines and proteases.51,52 Fibroblast-endothelial interactions and their effects upon neutrophil transmigration in vitro have been studied using co-culture methods consisting of an endothelial cell layer grown to confluence on the apical side of a Transwell system, with a supporting basement membrane-like matrix, with a stromal cell line grown on the basal side of the Transwell with or without flow conditions. Stromal cells that have been studied this way include epithelial cells,53 pericytes,19,20 and fibroblasts (synovial and skin).54 Co-culture of rheumatoid arthritis (RA) synovial fibroblasts with human umbilical vein endothelial cells (HUVEC) support neutrophil adhesion in a CXCL5-dependent manner under flow conditions, while dermal fibroblasts minimally support neutrophil adhesion,54 indicating that the organ of fibroblast origin could influence neutrophil migration through endothelium. Indeed, co-culture of HUVEC and rheumatoid synovial fibroblasts induce neutrophil-active chemokine expression in both cell lines, but much more robustly so in the rheumatoid fibroblasts (~70–80 fold increase in CXCL5 and CXCL8), but this same effect was not seen in dermal fibroblasts.55
In autoimmune tissue inflammation, fibroblasts may support neutrophil-active chemokine production upon cytokine stimulation. In a model of experimental autoimmune myocarditis, abrogation of the IL-17 signaling pathway protected mice from developing dilated cardiomyopathy, characterized by intracardiac fibrosis and reduced ventricular function.56 Although Il17a−/− and Il17ra−/− mice mounted autoimmune responses to cardiac myosin and equivalent numbers of intracardiac CD45 cells, their hearts had fewer neutrophils and monocytes infiltrating the tissues. In vitro and in vivo studies identified IL17A-stimulated cardiac fibroblasts as the most robust producers of proinflammatory mediators such as CXCL1, CCL2, GM-CSF, and IL-6, and could therefore promote the proinflammatory phenotype of Ly6Chi monocytes by inducing monocytes to upregulate IL1β, IL6, and IL12 expression. In the STIA model of arthritis, neutrophil-derived IL-1β induces neutrophil-active chemokine production by fibroblasts, which then recruit additional IL-1β-expressing neutrophils into the joint space, generating a positive feedback loop.57
Epithelium
Epithelial cells, upon stimulation with cytokines, also produce chemokines that promote neutrophil infiltration.58 Cxcl5 production by lung alveolar type II cells is induced by synergistic actions of IL-17A and TNF,59 and its production by Clara cells is regulated by the circadian clock gene BmaI.60 However, epithelial cells may interact with other tissue cells to downregulate neutrophil recruitment. For example, co-culture of kidney glomerular podocytes with EC demonstrated that podocytes diminished neutrophil recruitment over TNF-stimulated glomerular EC, although interestingly this same effect was not seen with HUVEC, indicating that the source of the endothelial cell affected neutrophil function.53 Cells may also interact with each other through gap junctions. Connexin 43 (Cx43) is a gap junction molecule expressed on arterial endothelium and in cardiac tissue that facilitates spread of Ca2+-intracellular spikes between cells. Interestingly, while Cx43 is not normally found in capillaries or venules, Cx43 exists in pulmonary capillaries and spreads intracellular Ca2+ levels throughout the endothelium and increases endothelial P-selectin expression, suggesting an important role in endothelial activation.61 In addition, Cx43 mediates gap junction connections between alveolar macrophages and epithelium, and LPS challenge induces synchronous spikes in Ca2+ levels not only in responding alveolar macrophages but throughout epithelium of adjoining alveoli.62 Mice whose alveolar macrophages are deficient in Cx43 have increased lung injury and mortality after intranasal LPS challenge, and broncheolar lavage fluid has higher levels of CXCL1, CXCL5, IL-6, CCL3, CCL5, IL-1β, and TNF, indicating that alveolar macrophage-lung epithelial communication through gap junctions protect against excessive inflammation.
Innate lymphoid cells
Innate lymphoid cells (ILCs) are an emerging heterogeneous class of CD45+lin-Thy1+ immune cells of lymphoid origin, but are innate-like in that they lack antigen specificity. ILCs are found within mucosal tissues, such as the intestine and respiratory tract, in addition to the skin and lymphoid organs, where they respond to pathogens through innate pattern recognition sensors.63 Upon activation, ILCs rapidly synthesize and release important immunoregulatory cytokines associated with Th1, Th2, or Th17 responses upon activation, and are thus grouped as ILC1, ILC2, or ILC3, respectively.64 ILC3 and closely related lymphoid-tissue inducer cells (LTi) are found in the intestine and lymphoid tissue/Peyer’s patches, respectively, where they play an important role in maintaining tissue homeostasis with commensals and other microbes. ILC3 and LTi cells both share the ability to produce IL-17 and IL-22 and influence Th17-like responses. One of the hallmark features of IL-17-mediated diseases is the regulation of neutrophil homeostasis and neutrophilic inflammation, therefore, communication between microbiota and ILC3 have significant potential to influence neutrophil trafficking.65
Several studies support the notion that the gut microbiome helps to maintain neutrophil counts in the periphery, as germ-free mice have lower peripheral neutrophil counts.66 In perinatal mice treated with broad-spectrum antibiotics, both the number and diversity of intestinal microbes were reduced, and correspondingly, post-natal granulocytosis was suppressed as reflected by lower plasma G-CSF levels and heightened susceptibility to E. coli sepsis. These mice had reduced intestinal levels of IL-17A, and the majority of IL-17-producing cells within the intestine were found to be CD4-TCR-RORgt+NKp46+ ILC3s.67 ILC3s have also been implicated in the pathogenesis of inflammatory bowel disease (IBD), as they play an important role in innate animal models of IBD68 and are found in increased numbers in human IBD intestinal samples.69 Tbet−/−Rag2−/− (TRUC) mice develop spontaneous colitis resembling human ulcerative colitis that is dependent upon IL-23 signaling as well as the presence of neutrophils.70 ILC3 are the critical IL-23R- and IL-17A-expressing cells in this model, and IL-17 in combination with TNF induce Cxcl1, Cxcl2, and Cxcl5 production from intestinal epithelial cells. Through their positioning in the intestine and sensing of the microbial and tissue environment, ILCs are poised to regulate neutrophil counts in the blood under homeostasis and can promote extravasation through the tissues under inflammatory conditions.
Concluding remarks
Clearly, recent key technological and intellectual advances have caused major shifts in perspective regarding the roles of tissue resident cells in the fundamental process of neutrophil migration. In vivo microscopy has provided the classical foundation for explicating the roles of cell-cell interactions critical for the process of leukocyte recruitment, but the development of transgenic reporter mice and novel imaging techniques have revealed the diversity of leukocyte and tissue cellular responses between different organs, as well as the relative importance of critically associated cellular players. In vitro assays recapitulating aspects of physiologic processes have historically utilized a core set of cell lines, but recent work demonstrating the tissue-specific imprinting of a given cell lineage under homeostasis, let alone under disease conditions, has challenged the concept that findings from an individual cell line may be generalizable to processes that occur in different sites and under different conditions. In addition, the emergence of diverse innate immune cell populations, such as macrophages and innate lymphoid cells, continue to be refined and redefined using cellular markers, and future studies utilizing these criteria to define these populations will help standardize future experimental design in order to bring clarity to the individual roles of these cell populations in immune responses. Defining these organ-specific requirements for neutrophil trafficking will assist in more precisely targeting immune responses for enhancement of host defense or suppression of pathologic inflammation.
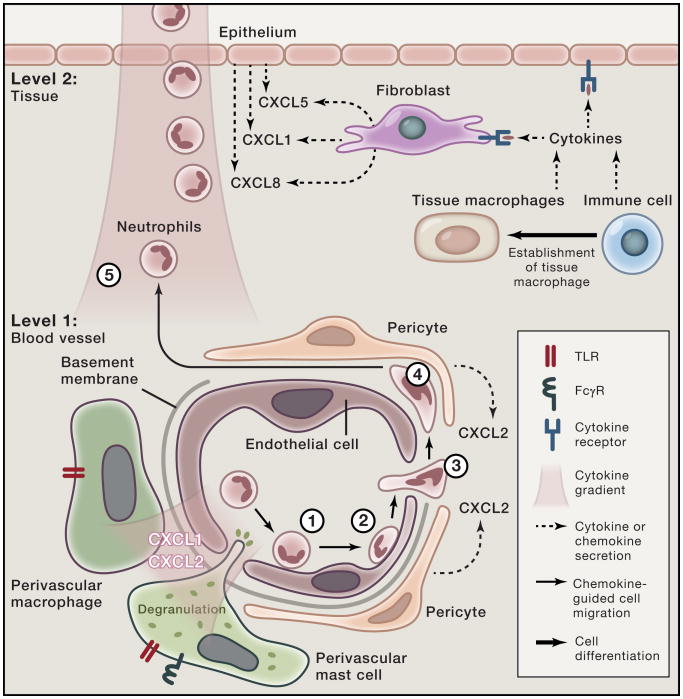
Figure 1. Tissue-resident cellular players in neutrophil extravasation through the vessel wall into tissues.
Regulation of tissue-specific neutrophil recruitment into organs occurs at two levels. Level 1 consists of the vasculature supplying the organ. First, endothelial cells induce neutrophil rolling (1) through selectin expression and next induce neutrophil arrest (2) through chemokine-induced activation of integrins. It should be noted that the cell surface receptors required for these processes differ depending upon the organ the blood vessel is supplying. Neutrophils then transmigrate (3) through the endothelial cell layer into the abluminal space. Pericytes encase the abluminal side of capillaries and post-capillary venules. Upon stimulation by TLR ligands or cytokines, pericytes promote neutrophil recruitment by producing neutrophil-active chemokines and upregulating surface ICAM-1, enabling neutrophils to crawl on their surfaces in the abluminal space (4) towards basement membrane low-expression regions (LER). In addition, upon activation pericytes undergo shape change and contract, enlarging areas of exposed basement membrane and available space for neutrophils to pass through. Perivascular macrophages exist in close contact with pericytes and express toll-like receptors and lectins that enable them to produce neutrophil-active chemokines upon stimulation. Perivascular mast cells contain preformed TNF and neutrophil-active chemokines in their granules, which may be rapidly released upon stimulation with immune complexes or TLRs. The distribution of perivascular cells is site- and organ-specific. Level 2 consists of the nature of the tissue resident cells that provide further guidance for neutrophils (5) into the interstitium and in some tissues across the epithelium. Macrophages and immune cells produce cytokines that activate resident tissue fibroblasts and epithelial cells to produce neutrophil-active chemokines. Previously recruited monocytes may also differentiate into tissue macrophages, secreting cytokines and chemokines that further influence neutrophil recruitment.
Box 1. Neutrophil recruitment by tumor stroma and associated cells.
The development and recent success of immune-modulating therapies in cancer has breathed new life into the field of immunooncology. While the primary therapeutic focus has been on modulating T cell responses to boost tumor immunity, the literature is filled with studies that indicate that neutrophils (both circulating and intratumoral) contribute to poor prognosis in multiple non-hematologic cancers.71 Neutrophils possess many attributes that may contribute to cancer progression. They produce multiple proteases that may promote tumor cell motility and invasion as well as angiogenesis, they produce reactive oxygen species that are genotoxic, and have been shown to variably induce cellular proliferation. Neutrophil extracellular traps (NETs) may trap circulating tumor cells and promote metastasis. Tumors formed under inflammatory circumstances, such as UV-radiation-induced melanoma, may be accompanied by neutrophilic inflammation as a result of HMGB1 released by damaged keratinocytes leading to neutrophil activation by TLR4/MyD88 and release of TNF, promoting angiotropism and metastasis.72 In fact, the GRO chemokines (CXCL1, CXCL2, CXCL3) are produced by melanoma cells and have potent mitogenic activity,73 in addition to neutrophil chemotactic activity.74 Several recent studies have pointed to an important and emerging role of CXCR2-mediated neutrophilic inflammation in carcinogenesis. In an intestinal polyp model, invasion by Clostridium species into intestinal epithelial barriers led to enhanced IL-1, TNF, Cxcl2, and IL-17 expression and increased numbers of neutrophils, promoting the formation of serrated polyps in the cecum of mice, the size of which were reduced with neutrophil depletion.75 In inflammation-induced models of skin papillomas, CXCR2 ligands are produced by both the tumor cells and the surrounding stroma, with neutrophils infiltrating the tumor. CXCR2 deficiency and CXCR2 inhibitor treatment reduced the development of inflammation-induced skin papillomas and intestinal adenomas. In addition, CXCR2 ligands are formed by spontaneously occurring intestinal tumor models, and CXCR2 deficiency abrogates carcinogenesis.76 In a mouse lymphoma model, tumor-derived oxysterols, such 22R-HC(22-hydroxycholesterol) represent novel ligands for CXCR2 on neutrophils and induce their migration into tumor microenvironments.77 BLT1, another neutrophil chemoattractant receptor, promotes tumorigenesis in a model of inflammation-exacerbated lung cancer, with mast cell- and macrophage-derived-LTB4 primarily responsible for drawing in neutrophil towards the inflamed lung.78 It remains to be seen whether inhibition of neutrophil-specific chemoattractant receptors may represent a novel immune modulating strategy to be used in combination with existing therapies. Another unknown question is how neutrophils may traverse the altered vasculature of the tumor, which is disorganized, immature, and infamously leaky and permeable. However, these studies lend support to the concept that neutrophilic inflammation is deleterious in cancer biology and may provide new directions for further investigation.
Highlights.
Neutrophil recruitment into tissues is orchestrated by tissue-resident cells.
Transendothelial migration of neutrophils is influenced by organ-specific vessels and associated cells.
Tissue immune and stromal cells work in concert to regulate neutrophil recruitment.
Acknowledgments
NDK was supported by a grant from the Rheumatology Research Foundation and ADL was supported by grants from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Summers C, et al. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41:694–707. doi: 10.1016/j.immuni.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 4.Anggard EE. The endothelium--the body’s largest endocrine gland? J Endocrinol. 1990;127:371–375. doi: 10.1677/joe.0.1270371. [DOI] [PubMed] [Google Scholar]
- 5.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 6.Cines DB, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 7.Nolan DJ, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell. 2013;26:204–219. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong J, et al. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J Clin Invest. 1997;99:2782–2790. doi: 10.1172/JCI119468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreisel D, et al. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc Natl Acad Sci U S A. 2010;107:18073–18078. doi: 10.1073/pnas.1008737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devi S, et al. Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat Med. 2013;19:107–112. doi: 10.1038/nm.3024. [DOI] [PubMed] [Google Scholar]
- 11.Shepro D, Morel NM. Pericyte physiology. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- 12.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circulation research. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 13.Hurtado-Alvarado G, Cabanas-Morales AM, Gomez-Gonzalez B. Pericytes: brain-immune interface modulators. Front Integr Neurosci. 2014;7:80. doi: 10.3389/fnint.2013.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovac A, Erickson MA, Banks WA. Brain microvascular pericytes are immunoactive in culture: cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide. J Neuroinflammation. 2011;8:139. doi: 10.1186/1742-2094-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Proebstl D, et al. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med. 2012;209:1219–1234. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stark K, et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol. 2013;14:41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- 17.Voisin MB, Probstl D, Nourshargh S. Venular basement membranes ubiquitously express matrix protein low-expression regions: characterization in multiple tissues and remodeling during inflammation. The American journal of pathology. 2010;176:482–495. doi: 10.2353/ajpath.2010.090510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voisin MB, Woodfin A, Nourshargh S. Monocytes and neutrophils exhibit both distinct and common mechanisms in penetrating the vascular basement membrane in vivo. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1193–1199. doi: 10.1161/ATVBAHA.109.187450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayres-Sander CE, et al. Transendothelial migration enables subsequent transmigration of neutrophils through underlying pericytes. PloS one. 2013;8:e60025. doi: 10.1371/journal.pone.0060025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pieper C, Pieloch P, Galla HJ. Pericytes support neutrophil transmigration via interleukin-8 across a porcine co-culture model of the blood-brain barrier. Brain Res. 2013;1524:1–11. doi: 10.1016/j.brainres.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 21.Wang S, et al. Pericytes regulate vascular basement membrane remodeling and govern neutrophil extravasation during inflammation. PloS one. 2012;7:e45499. doi: 10.1371/journal.pone.0045499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, et al. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sava P, Cook IO, Mahal RS, Gonzalez AL. Human microvascular pericyte basement membrane remodeling regulates neutrophil recruitment. Microcirculation. 2015;22:54–67. doi: 10.1111/micc.12173. [DOI] [PubMed] [Google Scholar]
- 24.Sumagin R, Sarelius IH. Emerging understanding of roles for arterioles in inflammation. Microcirculation. 2013;20:679–692. doi: 10.1111/micc.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. Journal of leukocyte biology. 2004;75:388–397. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- 26.Abtin A, et al. Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat Immunol. 2014;15:45–53. doi: 10.1038/ni.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karasawa K, et al. Vascular-Resident CD169-Positive Monocytes and Macrophages Control Neutrophil Accumulation in the Kidney with Ischemia-Reperfusion Injury. J Am Soc Nephrol. 2015;26:896–906. doi: 10.1681/ASN.2014020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunder CA, St John AL, Abraham SN. Mast cell modulation of the vascular and lymphatic endothelium. Blood. 2011;118:5383–5393. doi: 10.1182/blood-2011-07-358432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galli SJ, Tsai M. Mast cells in allergy and infection: versatile effector and regulatory cells in innate and adaptive immunity. European journal of immunology. 2010;40:1843–1851. doi: 10.1002/eji.201040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng LE, Hartmann K, Roers A, Krummel MF, Locksley RM. Perivascular mast cells dynamically probe cutaneous blood vessels to capture immunoglobulin E. Immunity. 2013;38:166–175. doi: 10.1016/j.immuni.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong PL, et al. The skin immune atlas: three-dimensional analysis of cutaneous leukocyte subsets by multiphoton microscopy. J Invest Dermatol. 2015;135:84–93. doi: 10.1038/jid.2014.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Megens RT, Kemmerich K, Pyta J, Weber C, Soehnlein O. Intravital imaging of phagocyte recruitment. Thrombosis and haemostasis. 2011;105:802–810. doi: 10.1160/TH10-11-0735. [DOI] [PubMed] [Google Scholar]
- 33.Johnston B, Burns AR, Kubes P. A role for mast cells in the development of adjuvant-induced vasculitis and arthritis. The American journal of pathology. 1998;152:555–563. [PMC free article] [PubMed] [Google Scholar]
- 34.Strbian D, Karjalainen-Lindsberg ML, Tatlisumak T, Lindsberg PJ. Cerebral mast cells regulate early ischemic brain swelling and neutrophil accumulation. J Cereb Blood Flow Metab. 2006;26:605–612. doi: 10.1038/sj.jcbfm.9600228. [DOI] [PubMed] [Google Scholar]
- 35.Sayed BA, Christy AL, Walker ME, Brown MA. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: a role for neutrophil recruitment? J Immunol. 2010;184:6891–6900. doi: 10.4049/jimmunol.1000126. [DOI] [PubMed] [Google Scholar]
- 36.Reichel CA, et al. Plasmin inhibitors prevent leukocyte accumulation and remodeling events in the postischemic microvasculature. PloS one. 2011;6:e17229. doi: 10.1371/journal.pone.0017229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uhl B, et al. Tissue plasminogen activator promotes postischemic neutrophil recruitment via its proteolytic and nonproteolytic properties. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1495–1504. doi: 10.1161/ATVBAHA.114.303721. [DOI] [PubMed] [Google Scholar]
- 38.De Filippo K, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121:4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 39.Goldszmid RS, Trinchieri G. The price of immunity. Nat Immunol. 2012;13:932–938. doi: 10.1038/ni.2422. [DOI] [PubMed] [Google Scholar]
- 40.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashimoto D, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yona S, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Epelman S, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gautier EL, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiwon M, et al. Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell. 2014;156:456–468. doi: 10.1016/j.cell.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davignon JL, et al. Targeting monocytes/macrophages in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2013;52:590–598. doi: 10.1093/rheumatology/kes304. [DOI] [PubMed] [Google Scholar]
- 47.Misharin AV, et al. Nonclassical Ly6C(−) monocytes drive the development of inflammatory arthritis in mice. Cell Rep. 2014;9:591–604. doi: 10.1016/j.celrep.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 49.Martin WJ, Walton M, Harper J. Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal-induced murine peritoneal model of acute gout. Arthritis Rheum. 2009;60:281–289. doi: 10.1002/art.24185. [DOI] [PubMed] [Google Scholar]
- 50.Martin WJ, Shaw O, Liu X, Steiger S, Harper JL. Monosodium urate monohydrate crystal-recruited noninflammatory monocytes differentiate into M1-like proinflammatory macrophages in a peritoneal murine model of gout. Arthritis Rheum. 2011;63:1322–1332. doi: 10.1002/art.30249. [DOI] [PubMed] [Google Scholar]
- 51.Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nature reviews. Rheumatology. 2012 doi: 10.1038/nrrheum.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flavell SJ, et al. Fibroblasts as novel therapeutic targets in chronic inflammation. British journal of pharmacology. 2008;153(Suppl 1):S241–246. doi: 10.1038/sj.bjp.0707487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuravi SJ, et al. Podocytes regulate neutrophil recruitment by glomerular endothelial cells via IL-6-mediated crosstalk. J Immunol. 2014;193:234–243. doi: 10.4049/jimmunol.1300229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lally F, et al. A novel mechanism of neutrophil recruitment in a coculture model of the rheumatoid synovium. Arthritis Rheum. 2005;52:3460–3469. doi: 10.1002/art.21394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith E, et al. Duffy antigen receptor for chemokines and CXCL5 are essential for the recruitment of neutrophils in a multicellular model of rheumatoid arthritis synovium. Arthritis Rheum. 2008;58:1968–1973. doi: 10.1002/art.23545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baldeviano GC, et al. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circulation research. 2010;106:1646–1655. doi: 10.1161/CIRCRESAHA.109.213157. [DOI] [PubMed] [Google Scholar]
- 57.Chou RC, et al. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity. 2010;33:266–278. doi: 10.1016/j.immuni.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol. 2011;45:189–201. doi: 10.1165/rcmb.2011-0011RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, et al. IL-17A and TNF-alpha exert synergistic effects on expression of CXCL5 by alveolar type II cells in vivo and in vitro. J Immunol. 2011;186:3197–3205. doi: 10.4049/jimmunol.1002016. [DOI] [PubMed] [Google Scholar]
- 60.Gibbs J, et al. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat Med. 2014;20:919–926. doi: 10.1038/nm.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parthasarathi K, et al. Connexin 43 mediates spread of Ca2+-dependent proinflammatory responses in lung capillaries. The Journal of clinical investigation. 2006;116:2193–2200. doi: 10.1172/JCI26605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Westphalen K, et al. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature. 2014;506:503–506. doi: 10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crellin NK, et al. Regulation of cytokine secretion in human CD127(+) LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity. 2010;33:752–764. doi: 10.1016/j.immuni.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 64.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells--how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 65.Wirths S, Bugl S, Kopp HG. Neutrophil homeostasis and its regulation by danger signaling. Blood. 2014;123:3563–3566. doi: 10.1182/blood-2013-11-516260. [DOI] [PubMed] [Google Scholar]
- 66.Balmer ML, et al. Microbiota-derived compounds drive steady-state granulopoiesis via MyD88/TICAM signaling. J Immunol. 2014;193:5273–5283. doi: 10.4049/jimmunol.1400762. [DOI] [PubMed] [Google Scholar]
- 67.Deshmukh HS, et al. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med. 2014;20:524–530. doi: 10.1038/nm.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buonocore S, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geremia A, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208:1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ermann J, Staton T, Glickman JN, de Waal Malefyt R, Glimcher LH. Nod/Ripk2 signaling in dendritic cells activates IL-17A-secreting innate lymphoid cells and drives colitis in T-bet−/−.Rag2−/− (TRUC) mice. Proc Natl Acad Sci U S A. 2014;111:E2559–2566. doi: 10.1073/pnas.1408540111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dumitru CA, Lang S, Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Semin Cancer Biol. 2013;23:141–148. doi: 10.1016/j.semcancer.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 72.Bald T, et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature. 2014;507:109–113. doi: 10.1038/nature13111. [DOI] [PubMed] [Google Scholar]
- 73.Richmond A, et al. Molecular characterization and chromosomal mapping of melanoma growth stimulatory activity, a growth factor structurally related to beta-thromboglobulin. The EMBO journal. 1988;7:2025–2033. doi: 10.1002/j.1460-2075.1988.tb03042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moser B, Clark-Lewis I, Zwahlen R, Baggiolini M. Neutrophil-activating properties of the melanoma growth-stimulatory activity. J Exp Med. 1990;171:1797–1802. doi: 10.1084/jem.171.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bongers G, et al. Interplay of host microbiota, genetic perturbations, and inflammation promotes local development of intestinal neoplasms in mice. J Exp Med. 2014;211:457–472. doi: 10.1084/jem.20131587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jamieson T, et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. The Journal of clinical investigation. 2012;122:3127–3144. doi: 10.1172/JCI61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raccosta L, et al. The oxysterol-CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J Exp Med. 2013;210:1711–1728. doi: 10.1084/jem.20130440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Satpathy SR, et al. Crystalline silica-induced leukotriene B-dependent inflammation promotes lung tumour growth. Nat Commun. 2015;6:7064. doi: 10.1038/ncomms8064. [DOI] [PMC free article] [PubMed] [Google Scholar]