Abstract
Inflammation is one of the predisposing factors known to be associated with Epstein Barr Virus (EBV) mediated tumorigenesis. However it is not well understood whether inflammation in itself plays a role in regulating the life cycle of this infectious agent. COX-2, a key mediator of the inflammatory processes is frequently over-expressed in EBV positive cancer cells. In various tumours, PGE2 is the principle COX-2 regulated downstream product which exerts its effects on cellular processes through the EP1-4 receptors. In this study, we further elucidated how upregulated COX-2 levels can modulate the events in EBV life cycle related to latency-lytic reactivation. Our data suggest a role for upregulated COX-2 on modulation of EBV latency through its downstream effector PGE2. This study demonstrates a role for increased COX-2 levels in modulation of EBV latency. This is important for understanding the pathogenesis of EBV-associated cancers in people with chronic inflammatory conditions.
Keywords: EBV, inflammation, reactivation, COX-2, PGE2
INTRODUCTION
Epstein Barr Virus (EBV) is a gamma herpesvirus ubiquitous in humans, and is widely known for its oncogenic properties. After primary infection, EBV follows two distinct life cycles in humans, a lytic form of infection with production of virion particles, or long-term latency. The switch between the latent and the lytic life cycle of EBV is the result of highly regulated interaction of EBV with its host and can be divided into three stages: (i) EBV infects human B lymphocytes resulting in proliferation of the infected cells, (ii) it can enter its latent phase with stringent expression of its latent genes as the cell proliferate, and (iii) it can be reactivated resulting in the release of infectious viral progeny for infection of new cells or transmission of the virus to other individuals. Lytic infection leads to extensive viral gene expression, production of new infectious virions and finally death of the infected host cell (Chang et al., 2012). A latent form of infection allows the virus to persist in a dormant form for the life of the host through its tightly restricted gene expression (Woellmer, Arteaga-Salas, and Hammerschmidt, 2012). The latently infected cells can also undergo lytic reactivation in response to certain stimuli. Importantly, EBV lytic reactivation is linked to development and progression of EBV associated malignancies including nasopharyngeal carcinoma and lymphoma (Liu et al., 2012). Interestingly, triggering EBV lytic reactivation has also generated much attention as a potential therapeutic strategy as expression of lytic antigens in EBV positive tumour cells will induce strong immune response to the viral lytic proteins, thus killing tumour cells (Giunco et al., 2013).
One of the predisposing factors known to be associated with EBV mediated tumorigenesis is inflammation. A connection between inflammation and cancer has been long suspected (Sgambato and Cittadini, 2010). Epidemiological studies have established that many tumours occur in association with chronic infections (Ziegler and Buonaguro, 2009). However it is not well understood whether inflammation in itself plays a role in regulating the life cycle of infectious agents. This is important specifically for infections like EBV whose life cycle involves long periods of latency during which the virus is immunologically undetectable and remains dormant. Inflammatory response against virus infection has been linked to virus pathogenesis leading to tumorigenesis, which includes cellular transformation, promotion, survival, proliferation, invasion, angiogenesis, and metastasis (Chen et al., 2013; Taniguchi et al., 2013). Thus, it is essential to identify the changes in the life cycle of the virus due to inflammation and their significance for driving the oncogenic process.
COX-2, a key mediator of the inflammatory processes, is frequently expressed in EBV positive nasopharyngeal tumours as well as detected at higher levels in EBV positive Lymphoblastoid cell lines when compared to EBV negative nasopharyngeal tumours or EBV negative Burkitt’s lymphoma cell lines. These observations suggest a role for COX-2 in EBV pathogenesis. Cyclooxygenase (COX) is a key enzyme in the biosynthetic pathway of prostaglandin (PG) synthesis. COX-1 and COX-2 are the two isoforms of COX (O'Neill and Ford-Hutchinson, 1993). COX-1 is constitutively expressed while COX-2 is an inducible early response gene (Satoh et al., 2012). Over-expression of COX-2 catalyzes the synthesis of PGE2 whose downstream target molecules contribute to physiological processes including transformation, survival, proliferation, metastasis and angiogenesis by up-regulating several signalling pathways and down-regulating apoptotic proteins (Satoh et al., 2012). COX-2 and PGE2 have been reported to have a positive correlation with various types of cancers including colon cancer, lung cancer, and gastric cancer (Nadda et al., 2012; Shin et al., 2012). The depth of invasion and carcinoma development has been shown to parallel the increase in expression of COX-2 (Coussens and Werb, 2002; Fujita et al., 2002). COX-2 can be induced by several intracellular and extracellular factors including lipopolysaccharide (LPS), epidermal growth factor (EGF), and tumour necrosis factor (Font-Nieves et al., 2012; Medeiros et al., 2010; Yucel-Lindberg et al., 1999). Published studies have revealed a close association between COX-2 over-expression and EBV associated cancers (Michelow, Wright, and Pantanowitz, 2012; Murray and Young, 2001). A convincing positive correlation has been reported between COX-2 and the EBV viral protein LMP1. LMP1 can up-regulate COX-2 which accelerates lymph node metastasis in NPC (Bai and Tang, 2009). We have earlier shown that the EBV latent antigen EBNA3C, can upregulate COX-2 in association with the metastasis suppressor Nm23-H1 (Kaul et al., 2006).
In various tumours, PGE2 is the principle COX-2 regulated downstream product. PGE2 exerts its cellular effect through the EP1-4 receptors (Konger et al., 2005). The EP receptors belong to a family of G-protein coupled receptors (Narumiya, Sugimoto, and Ushikubi, 1999; Tober et al., 2006). Despite close resemblance among all EP receptors, they exhibit differential binding affinities for PGE2 molecule and all four receptors are associated with different intracellular signalling cascades. Recently, the explicit roles of each EP receptors in tumorigenesis and malignancies have begun to draw tremendous attention. The EP1 receptor is specifically known to be associated with distinct signalling cascades, as compared to the other three EP receptors and behaves differently in cancer progression (Ma et al., 2013b). EP1 is also closely related to inflammation, skin cancer, colon carcinogenesis and late stage breast cancer progression. EP1 receptor induces elevation and mobilization of intracellular Ca+2 (Irie et al., 1994; Sugimoto and Narumiya, 2007). EP4 is a 65KDa protein which is coupled with phosphatidylinositol kinase and elevated cAMP level (Hull, Ko, and Hawcroft, 2004; Luschnig-Schratl et al., 2011). It is associated with various human cancer and its elevated mRNA level has been detected in breast cancer (Xia and Kirkman, 1990), colorectal cancer (Chell et al., 2006), and Prostate cancer (Biedrzycka et al., 2013). EP4 antagonists are also used for treatment of various immune diseases (Yao et al., 2009). It is used as an anti-inflammatory agent against diseases associated with inflammation (Luschnig-Schratl et al., 2011).
In nature, the biological processes associated with inflammation are required to extend defence to host against microbial infection by mediating cell growth, tissue repair and regeneration. Several metabolites secreted by infectious agents are responsible for inflammation thereby facilitating transformation and progression towards cancer (Antonic et al., 2013). Still, it is very difficult to predict how inflammation and associated pathways can modulate the life cycle of infectious agents. This is especially true for infections associated with viruses like EBV closely related to several human cancers. The life cycle of EBV is divided into latent and lytic phases, and the latent genes expressed during the latent cycle of the virus cleverly escape the immune system and remain virtually undetectable. However a number of conditions can induce the virus to undergo lytic reactivation. This transition from latent to lytic phase has not been completely explored. The lytic phase involves production of infectious viral particles which are important for infecting new host as well as new cells in same host. In this study, we further strive to understand the events related to inflammation characterized by upregulated COX-2 levels to modulate events in the EBV life cycle related to lytic reactivation. This adds to our understanding of how these host-viral interactions modulate the microenvironment contributing to transformation and cancer.
MATERIAL AND METHODS
Ethics Statement
Blood samples for isolation of peripheral blood mononuclear cells (PBMC) from healthy donors were collected after getting the protocol approval by the Institute Review Board of University of Delhi South Campus. Every donor gave written and informed consent and the samples were coded so as not to reveal any personally identifiable information.
Constructs and Cell lines
B95-8 (marmoset lymphoid cells) and LCL2 (lymphoblastoid cell line) are latently infected with EBV (Shaw, Petit, and Leung, 1987). Akata cell line is of Burkitt’s lymphoma origin and is latently infected with EBV (Takada et al., 1991). Human Embryonic Kidney HEK293, 293T and HeLa are EBV negative cell lines. B95-8 cells, LCL2 and Akata cells were maintained in RPMI-1640 (Lonza, Basel, Switzerland, Cat no. 12-702F). HEK 293 and HEK 293T cells were maintained in DMEM (Lonza, Basel, Switzerland, Cat no. BE12-604F). The COX-2 expression plasmid was a gift from Timothy Hla (Cornell University, Ithaca, NY).
Transfection
HEK293T cells were transfected by electroporation with a Bio-Rad Gene Pulser electroporator. Ten million transfected cells were plated in 100 mm dishes with 10 ml of growth medium and incubated at 37°C in 5% CO2. The cells were then harvested and assayed for the experiment.
Real-time RT-PCR
Total RNA was collected from cells using TRI reagent (Sigma Aldrich, St. Louis, MO, Cat. no. T9424) following the manufacturer’s instructions. RNA was used to prepare cDNA using Superscript first strand reverse transcription kit (Life technologies, Grand Island, NY, Cat. no. 11904-018) following manufacturer’s instructions. Specific primers were used to amplify 90–100 bp regions of EP1, EP4 genes. GAPDH was used as an internal control for normalization. The experiment was performed in triplicates. For EP1, forward primer 5’ATGGTGGGCCAGCTTGTC3’ and reverse primer 5’GCCACCAACACCAGCATT3’ were used. For EP4, forward primer 5’CGACCTTCTACA CGCTGGTATG3’ and reverse primer 5’CCGGGCTCACCAACAAAGT3’ were used. For GAPDH, forward primer 5’ AAGGTGAAGGTCGGAGTCAACG 3’ and reverse primer 5’ CCTTCTCCATGGTGGTGAAGAC 3’ were used.
EBV lytic reactivation and virus detection
Sodium butyrate (NaB), 12-tetra- decanoyl phorbol-13-acetate (TPA) and Lipopolysaccharide (LPS) were used to induce lytic reactivation of EBV in latently infected cells. TPA 1.6mM (Sigma Aldrich, St. Louis, MO, Cat. no. 79346), Sodium butyrate 2.5mM (Sigma Aldrich, St. Louis, MO, Cat. no. B588714) and LPS (Sigma Aldrich, St. Louis, MO, Cat. no. L6143) was used at a concentration of 1ug/ml. The supernatant from induced cells was collected after six days and centrifuged at 2,000x g for 5 minutes to remove any cellular debris. It was then filtered through 0.22u filter and was incubated with 20 units of DNaseI (New England Biolabs, Ipswich, MA, Cat. no. MO303L for 1 hour at 37°C to allow digestion of any virus free DNA. The supernatant was then incubated for 10 min at 70°C for heat inactivation of DNaseI. The DNaseI treated supernatant was then centrifuged at 200,000x g for 1 hour at 4°C in ultra-centrifuge to pellet the virus. The virus pellet was lysed with lysis buffer (3%SDS, 75mM Tris, 25mM EDTA) and proteinase K. The viral DNA was purified by phenol extraction and the presence of virus was confirmed by EBV-DNA specific real time PCR, targeting a 298 bp sequence from the Bam-W fragment of the EBV genome using the following primers: forward 5’ CTTTAGAGGCGAATGGGCGC 3’, and reverse 5’ AGGACCACTTTATACCAGGG 3’. The Bam-W fragment was amplified from viral DNA using Brilliant III Ultra-Fast SYBR QPCR Master mix (Agilent Technologies, Santa Clara, CA), 1 mM of each primer and 1 µl of the viral DNA product in a total volume of 20µl. Following cycling conditions were used in an 7900-HT Fast Real Time PCR system (Applied Biosystem, Waltham, MA): 10 min at 95°C for initial denaturation, 40 cycles each at 15 sec at 95°C for denaturation followed by 1 min at 60°C for annealing and polymerization. Data were collected twice during every cycle after the polymerization step and denaturation step. A melting curve analysis was performed to verify the specificity of the products, and the values for the relative quantitation were calculated by the ΔΔCt method. The experiment was performed in triplicate.
Inhibitors
COX-2 specific inhibitor NS398 was used at a concentration of 50 uM (Sigma Aldrich, St. Louis, MO, Cat. no. N194). AH6808 is a specific inhibitor of EP1/EP2 receptors, and was used at a concentration of 40 uM (Sigma Aldrich, St. Louis, MO, Cat. no. A1221). The EP4 specific inhibitor AH23848 was used at concentration of 40 uM (Sigma Aldrich, St. Louis, MO, Cat. no. A8227).
PGE2 ELISA
In order to estimate the concentration of PGE2 in cell culture supernatant, ELISA (Enzyme-linked immunosorbent assay) was performed using Prostaglandin E2 EIA monoclonal kit (Cayman Chemicals, Ann Arbor, MI, Cat no. 514010) following manufacturer’s instructions. The ELISA was performed in triplicate, and the results are presented as the means of standard errors of means (SEMs).
Western Blot
Prior to separation on SDS polyacrylamide gels, cell lysates were mixed with 2x SDS loading buffer and heated to 95°C for 5 min. Equal amounts of total protein was loaded in all wells. After electrophoresis, the resolved proteins were transferred onto nitrocellulose membrane. The membranes were blocked in 5% skimmed milk powder in PBS at room temperature for 30 min, and then washed with TBST. The membranes were incubated with primary antibody overnight at 4°C. The membranes were washed again and incubated with secondary antibody for 30 minutes at room temperature. After washing, the membrane was scanned using Typhoon scanner (GE Healthcare, Pittsburgh, PA). COX-2 was detected in blots with anti-COX-2 mouse monoclonal antibody (Cayman Chemicals, Ann Arbor, MI, Cat no. 160112) used at dilution 1:1000, then probed with Alexa fluor 488 goat anti-mouse secondary antibody. COX-2 was detected as 72 KDa band in western blots. For detection of the EP1 receptor we used anti-EP1 rabbit polyclonal antibody (Cayman Chemicals, Ann Arbor, MI, Cat no. 101740) at dilution 1:1000, which was probed with Alexa fluor goat anti-rabbit 488 secondary antibody (Life technologies, Grand Island, NY, Cat. no. 11034). EP1 was detected at 42 KDa. For EP4 we used anti-EP4 rabbit polyclonal antibody (Cayman Chemicals, Ann Arbor, MI, Cat no. 101775) at dilution 1:1000. It was probed with Alexa fluor goat anti-rabbit secondary antibody (Life technologies, Grand Island, NY, Cat. no. A11034). For detection of EBV glycoprotein gp350, anti-gp350 mouse monoclonal antibody (Santa Cruz, Dallas, TX, Cat. no. SC57724) was used at a dilution of 1:500. It was probed with Alexa fluor goat anti-mouse secondary antibody. EBV glycoprotein gp350 was detected at 113 KDa. GAPDH was used as internal loading control.
Immunofluorescence
Induced and control cells were grown on poly-L-lysine coated coverslips. Cells were washed with 1x PBS and fixed with acetone: methanol (1:1) at −20° C for 20 minutes followed by washing with 1x PBS. The cells were permeabilized using 0.5% Triton X 100 (Sigma Aldrich, St. Louis, MO, Cat. no. T8787) and blocked with cold fish gelatin (1% BSA, 1% cold fish skin gelatin, 0.5% Triton X-100, 0.01% PBS pH 7.2–7.4) for 30 minutes at room temperature. This was followed by incubation with primary antibody (described in Western blot section) overnight at 4°C. After three washings with PBS, cells were incubated with secondary antibody for 30 minutes followed by DAPI to stain the nuclei of the cells. The cover slips were mounted using fluoromount (90% glycerol & 0.1% p-phenylenediamine in PBS pH 9.0). The presence of the protein of interest was detected by confocal microscopy and signal intensity was quantitated using Image J software (Schneider, Rasband, and Eliceiri, 2012).
Co-cultivation of COX-2 expressing adherent cells with EBV latently infected cells
Ten million 293T cells were grown in 100 mm cell culture dish and incubated with LPS (1ug/ml) to induce COX-2 expression. The media was replaced with fresh media after 6 hours after washing the cells with DPBS to remove any traces of LPS. These COX-2 expressing cells were then co-cultivated with 10 million EBV infected LCLs. Both the cells were co-cultivated by incubating for 6 days after which the culture supernatant was collected and processed for detection and presence of EBV as described earlier. Supernatant from uninduced 293T cells co-cultivated with LCLs was used as a control. The 293T cells transfected with COX-2 expression plasmid or cell culture supernatant alone from such cells were also used for co-cultivation with LCLs. Mock transfected 293T cells or cell culture supernatant from mock transfected cells were used as controls.
Infection of PBMC with EBV particles
EBV was re-activated from the EBV positive cell line B95-8 using LPS (1ug/ ml). Exponentially growing B95-8 cells were induced with LPS for 24 hours. Then cells were washed with phosphate buffer saline (PBS) three times to remove any residual LPS, re-suspended in 10% RPMI and incubated for 4 days. The cells were then centrifuged at 1,000xg to pellet them and the culture supernatant was collected. The supernatant was filtered through a 0.22 micron filter and stored at −80°C until used. To confirm the presence of EBV, we processed 1ml of culture supernatant for viral DNA extraction followed by real time PCR amplification to detect the BamW fragment of EBV as described earlier. For Peripheral blood mononuclear cells (PBMC) isolation, blood was collected from volunteers after obtaining informed consent from them. Eight ml of blood was drawn using BD safety-lok blood collection set (BD Biosciences, San Hose, CA, Cat. no. 367283) in BD vacutainer CPT cell preparation tube with sodium heparin (BD Biosciences, San Hose, CA, Cat. no.362753). The BD vacutainer with blood was centrifuged at 1,500x g for 15 minutes at room temperature. After centrifugation, mononuclear cells and platelets separated in whitish layer of buffy coat under plasma layer. The mononuclear cells were collected and washed with PBS, then re-suspended in 20% RPMI at concentration of 1×106 cells/ ml. Cyclosporine A (Sigma Aldrich, St. Louis, MO, Cat. no. C3662) was added at 2 ug/ml concentration to prevent activation of T cells. To test the biological activity of virus reactivated in response to COX-2 expression, the culture supernatant from uninduced B95-8 cells or from LPS induced B95-8 cells prepared previously was added to an equal volume of mononuclear cells and incubated at 37°C. The cells were observed daily until aggregates of cells started appearing indicating infection and transformation of infected B cells. After 2–3 days post infection, clusters of cells were visible by light microscopy. Fresh media was added subsequently to the culture as needed.
RESULTS
LPS induces COX-2 expression in EBV positive lymphoblastoid cells
To investigate the role of inflammation on EBV latency we used purified LPS as an extracellular inducer to modulate COX-2 up-regulation in EBV latently infected cells. LPS is a biologically active compound found in the outer membrane of gram negative bacteria (Noguchi et al., 2001). In our study, we found that the addition of LPS correlated with COX-2 over-expression at the protein level in two EBV positive cell lines (LCL2, B95-8) resulting in approximately 2 to 4 fold increase (Fig. 1A, 1B) (p<0.001). GAPDH was used as an internal control for normalization. The experiments were performed three times independently and the data presented as the mean with standard error. The COX-2 overexpression was not as high as seen in cell of epithelial origin such as HeLa (> 10 fold) (data not shown). We had earlier shown that LPS does not result in COX-2 upregulation in EBV negative Burkitt’s lymphoma cells, whereas it is significantly upregulated in latently infected EBV-positive cells (Kaul et al., 2006). These results indicate that LPS induces EBV positive lymphoid cells resulting in enhanced COX-2 expression in the absence of an overwhelming pro-inflammatory response as seen in other cell types. This may be either because the LPS receptor TLR4 is expressed in only a small percentage of EBV infected cells (Komai-Koma et al., 2004), or by some alternate mechanism.
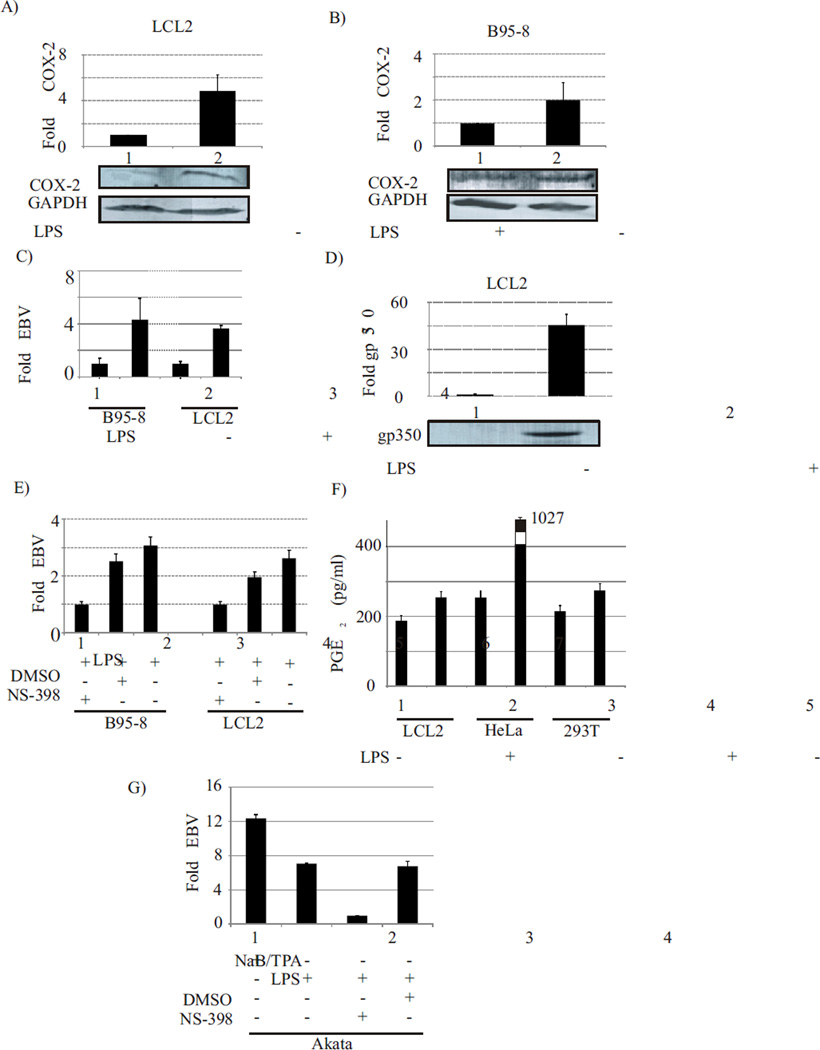
Fig. 1. LPS mediated COX-2 upregulation is coincident with EBV lytic reactivation, gp350 and PGE2 expression, and is inhibited by the COX-2 inhibitor NS-398.
The addition of LPS correlated with COX-2 over-expression at the protein level in two EBV positive cell lines (LCL2, B95-8) resulting in approximately 2 to 4 fold increase (Fig. 1A, 1B) (p<0.001). Significantly higher amounts of EBV genome were detected from supernatant of induced cells as compared to uninduced cells (Fig. 1C) (p<0.01). The EBV late lytic protein gp350 protein was detected at a significantly higher levels (p<0.001) as compared to uninduced cells (Fig. 1D). Addition of NS-398 resulted in a significant reduction of 2.6 to 3 fold (p<0.01) in the amount of EBV copies detected in cell culture supernatant (Fig. 1E). The mean PGE2 concentration in the supernatant of LPS treated EBV latently infected LCL2 was 254 pg/ml as compared to un-induced LCL2 at 187 pg/ml indicating a 35% increase (p<0.001) in PGE2 levels (Fig. 1F). In case of HeLa cells there was an approximately 4-fold increase (from 265 pg/ml to 1027 pg/ml) (p<0.001) in PGE2 levels. The overall increase in PGE2 levels in treated 293T cells was not so robust (from 214 pg/ml to 275 pg/ml) (p<0.01).The induction of Burkitt lymphoma Akata cells by LPS resulted in detection of EBV genome from supernatant of induced cells, at levels comparable to that in supernatant of NaB/TPA induced Akata cells (Fig. 1G). Addition of NS-398 resulted in a significant reduction of about 7 fold (p<0.01) in the amount of EBV copies detected in cell culture supernatant (Fig. 1E).
COX-2 over-expression results in lytic reactivation of EBV in latently infected cells
To study the effect of COX-2 up-regulation on EBV latency, we treated the EBV latently infected LCL2, B95-8 and Burkitt’s lymphoma Akata cells with LPS. We tested different concentrations of LPS for different time points and the optimum concentration of LPS required for detectable reactivation of EBV from B95-8 and LCL2 cell lines was found to be 1ug/ml. Detection of EBV in the cell culture supernatant of induced cells peaked at 6 days post-induction based on EBV genome copies as determined by the real time PCR targeting BamW fragment of EBV. Significantly higher amounts of EBV genome were detected from supernatant of induced cells as compared to uninduced cells (Fig. 1C) (p<0.01). The EBV genome was undetectable from supernatant of uninduced Akata cells. Whereas when the supernatant of induced Akata cells were tested, the level of EBV genome detected were comparable to that detected from Nab/TPA induced Akata cells (Fig. 1G). To confirm the role of COX-2 in EBV lytic reactivation we used COX-2 specific inhibitor NS-398 (Copeland et al., 1994). NS-398 has been reported to act through direct interaction with COX-2. This results in structural transition of the enzyme leading to a very tight association with the inhibitor (Copeland et al., 1994). EBV latently infected LCL2 and B95-8 cells were treated with LPS and incubated for 6 days in the presence or absence of the COX-2 specific inhibitor NS-398. The supernatant was harvested after six days and tested for the presence of EBV by real time PCR targeting the BamW region of EBV (see materials and methods). Addition of NS-398 resulted in a significant reduction of 2.6 to 3 fold (p<0.01) in the amount of EBV copies detected in cell culture supernatant (Fig. 1E) indicating that-LPS mediated lytic reactivation of EBV occurs predominantly through COX-2 regulated pathways and its downstream effector molecules. Similar results were observed when experiments were performed using Burkitt’s lymphoma Akata cells resulting in up to 7 fold reduction (p<0.01) in amount of EBV genome detected from cell culture supernatant, when NS-398 was added along with LPS (Fig. 1G). The lytic reactivation of EBV in latently infected cells is accompanied by an increased expression of the EBV late lytic protein gp350 (Gong and Kieff, 1990). When LPS treated EBV latently infected LCL2 were tested for gp350 expression levels, the gp350 protein was detected at a significantly higher levels (p<0.001) as compared to uninduced cells (Fig. 1D).
The COX-2 downstream effector prostaglandin E2 (PGE2) and prostanoid receptors EP1 and EP4 are induced during COX-2 mediated EBV lytic reactivation
An increase in COX-2 levels in response to various inflammatory stimuli simultaneously leads to a dramatic increase in production of pro-inflammatory molecule PGE2 (Satoh et al., 2012). Among these, the PGE2 is a crucial COX-2 mediated metabolic product of arachidonic-acid (Satoh et al., 2012). It is also a key molecule involved in inflammation and exerts its action through the membrane receptors EP1-4 located on the surface of target cells (Konger et al., 2005). These receptors belong to a family of G- protein coupled receptors (GPCR) (Ahmad et al., 2013; Ma et al., 2013a; Narumiya, Sugimoto, and Ushikubi, 1999; O'Banion et al., 1991; O'Callaghan et al., 2013). To understand the role of these COX-2 downstream effector molecules, we determined if there was any modulation of their expression patterns which occurred simultaneously to EBV lytic reactivation. The results showed that 24 hours post induction, the level of PGE2 in the supernatant collected from the induced cells, was significantly higher (p<0.001) as compared to the un-induced control samples (Fig. 1F). This correlated with the amount of virus detected in the supernatant of treated cells. The mean PGE2 concentration in the supernatant of LPS treated EBV latently infected LCL2 was 254 pg/ml as compared to un-induced LCL2 at 187 pg/ml indicating a 35% increase (p<0.001) in PGE2 levels (Fig. 1F). Similar results were observed for EBV negative HeLa and 293T cells. In case of HeLa cells there was an approximately 4-fold increase (from 265 pg/ml to 1027 pg/ml) (p<0.001) in PGE2 levels. Interestingly, overall increase in PGE2 levels in treated 293T cells was not so robust (from 214 pg/ml to 275 pg/ml) (p<0.01). This is most likely due to the low expression levels of TLR4 receptors on 293T cells which are critical for LPS signaling pathway (Medvedev and Vogel, 2003). Therefore LPS-mediated COX-2 upregulation and modulation of COX-2 downstream effector molecules can occur irrespective of EBV infection status of the cell. Interestingly, such upregulation is more significant in cells of epithelial origin as compared to EBV infected lymphoid cells.
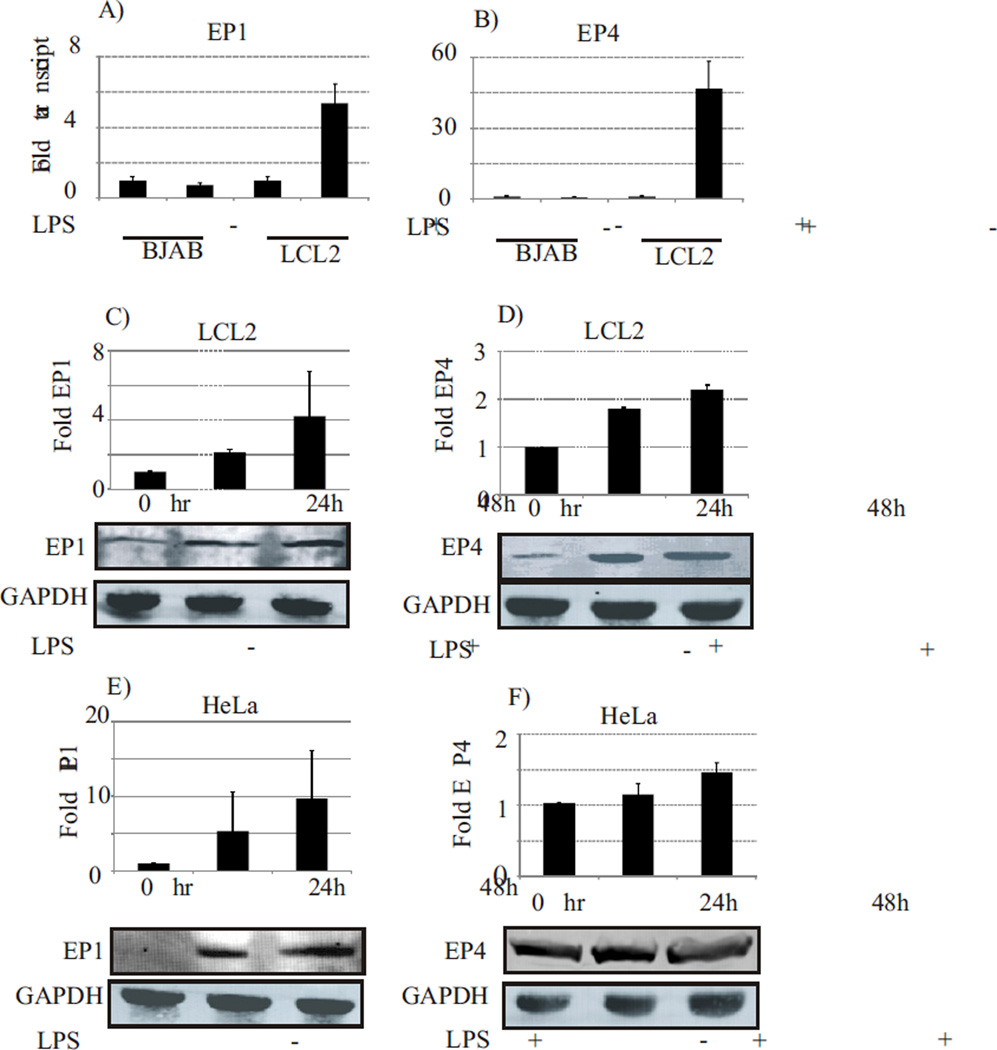
We examined whether or not, there was any difference in the expression of EP receptors which was concurrent with EBV lytic reactivation as a result of COX-2 upregulation. The expression levels of EP receptors transcripts in EBV positive cell lines (LCL2, B95-8) was analyzed in cells after treatment with LPS. The cells were assayed using real time RT-PCR to determine the relative changes in mRNA transcript level of the EP receptors. The results showed that both EP1 and EP4 transcripts were significantly up-regulated in response to COX-2 upregulation by LPS (Fig. 2A and 2B). The EP1 mRNA transcripts showed a 5.4-fold upregulation (p<0.001) as compared to uninduced cells when tested in LCL2 (Fig. 2A, compare lanes 3 and 4). Whereas, EP4 transcripts were increased greater than 40 fold (p<0.001) compared to uninduced LCL2 (Fig. 2B, compare lanes 3 and 4). Interestingly, the EBV negative BJAB cells did not show any significant changes in the transcripts levels of the EP1 or EP4 receptors even when treated with LPS. Importantly, the transcript levels of the EP2 and EP3 receptors did not show any significant changes (data not shown) when LCL2 were treated with LPS when compared to uninduced cells and BJAB. To determine whether changes in EP1 and EP4 transcript levels correlated to a corresponding increase in protein expression levels, we treated LCL2 with LPS and assayed the EP1 and EP4 levels by western blotting using EP1 and EP4 specific antibodies, respectively. The expression levels of the receptors were normalized using GAPDH as a loading control. We found elevated expression of EP1 up to 4-fold (Fig. 2C) (p<0.05) and EP4 up to 2.5-fold (Fig. 2D) (p<0.05) in EBV positive cells (LCL2) in response to LPS treatment. This was coincident with EBV lytic reactivation, as compared to the uninduced control samples. Similar up regulation was observed in EBV negative HeLa cells as well (Fig. 2E and 2F) (p<0.05).
Fig. 2. LPS mediated COX-2 upregulation results in upregulation of the EP1 and EP4 receptors.
Both EP1 and EP4 receptors were transcriptionally upregulated in LCL2 but not in EBV negative BJAB cells in response to LPS induction (panel A and B). The EP1 mRNA transcripts showed a 5.4-fold upregulation (p<0.001) as compared to uninduced cells when tested in LCL2 (Fig. 2A, compare lanes 3 and 4). Whereas, EP4 transcripts were increased greater than 40 fold (p<0.001) compared to uninduced LCL2 (Fig. 2B, compare lanes 3 and 4). LPS mediated COX-2 upregulation of EBV latently infected cells as well as EBV negative cancer cells of epithelial origin results in over-expression of EP1 and EP4 receptors. The expression of EP1 was elevated up to 4-fold (Fig. 2C) (p<0.05) and EP4 up to 2.5-fold (Fig. 2D) (p<0.05) in EBV positive cells (LCL2) in response to LPS treatment. This was coincident with EBV lytic reactivation, as compared to the uninduced control samples. Similar up regulation was observed in EBV negative HeLa cells as well (Fig. 2E and 2F) (p<0.05).
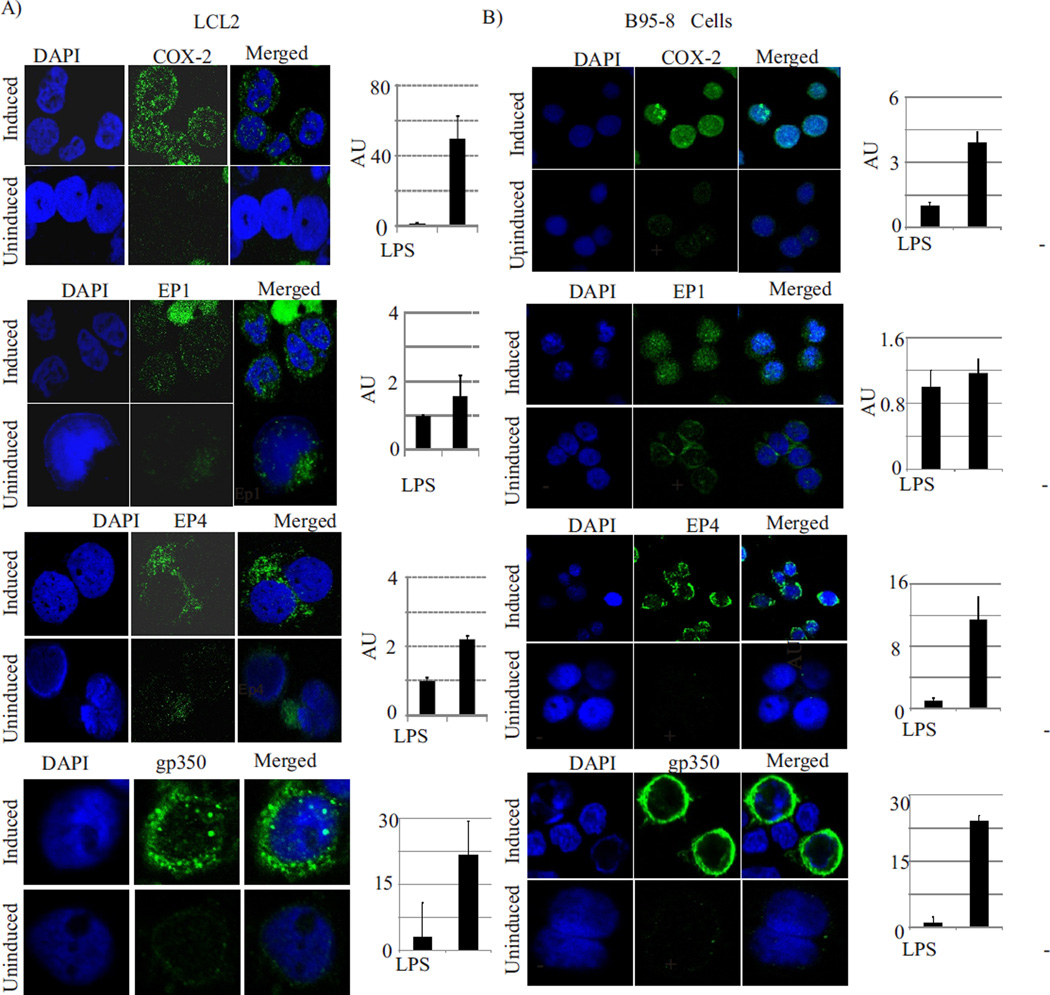
Immunofluorescence studies on EBV latently infected cells also showed that LPS induction of these cells resulted in lytic reactivation of EBV and is accompanied with elevated COX-2, EP1 and EP4 expression (Fig. 3). We used LCL2 (human B-cell) as well as B95-8 (marmoset B cells) for these studies. The COX-2 immunofluorescence signals in induced cells were much stronger as compared to uninduced cells, and were localized both in the cytoplasm and nucleus (Fig. 3 A and B, top panel). COX-2 has been previously reported to be localized in nucleus and function as transcriptional factor (78). EP1 and EP4 signals were also much stronger in LPS treated LCL2 and B95-8 (Fig. 3A and B, middle panels). Both EP1 and EP4 were predominantly localized in the cytoplasm and mainly on the cell membrane (Fig. 3A and B, middle panels). Thus, our data clearly showed that induction with LPS resulted not only in enhanced expression of COX-2, but was also concurrent with increased extra-cellular PGE2 levels along with increased expression of the prostaglandin specific downstream effectors EP1 and EP4 at the mRNA and protein expression levels. The induced cells also showed significantly (up to 20-fold) (p<0.001) increased expression of EBV late lytic protein gp350 (Fig. 3A and B, bottom panel). These data strongly suggest a direct role of EP1 and EP4 receptors in COX-2 mediated lytic reactivation of EBV.
Fig. 3. LPS induction of EBV positive cells results in overexpression and intracellular accumulation of COX-2 as well as its downstream target receptors EP1 and EP4.
Panel 3A: LCL2 were induced with LPS (1ug/ml) for 24 hours and harvested for immunofluorescence assays using anti-COX-2, anti-EP1, anti EP4, and anti-gp350 antibodies. COX-2, EP1, EP4 and gp350 expression were significantly upregulated (p<0.001) in induced cells as compared to un-induced cells. The images were analysed using Image J software for quantification of immunofluorescence signals in at least 3 different microscopic fields for each. The mean values and standard error of three independent experiments are presented as bar graph in right panel. Panel 3B: B95-8 cells were induced with LPS (1ug/ml) for 24 hours and then processed for immunofluorescence assays as described for LCLs.
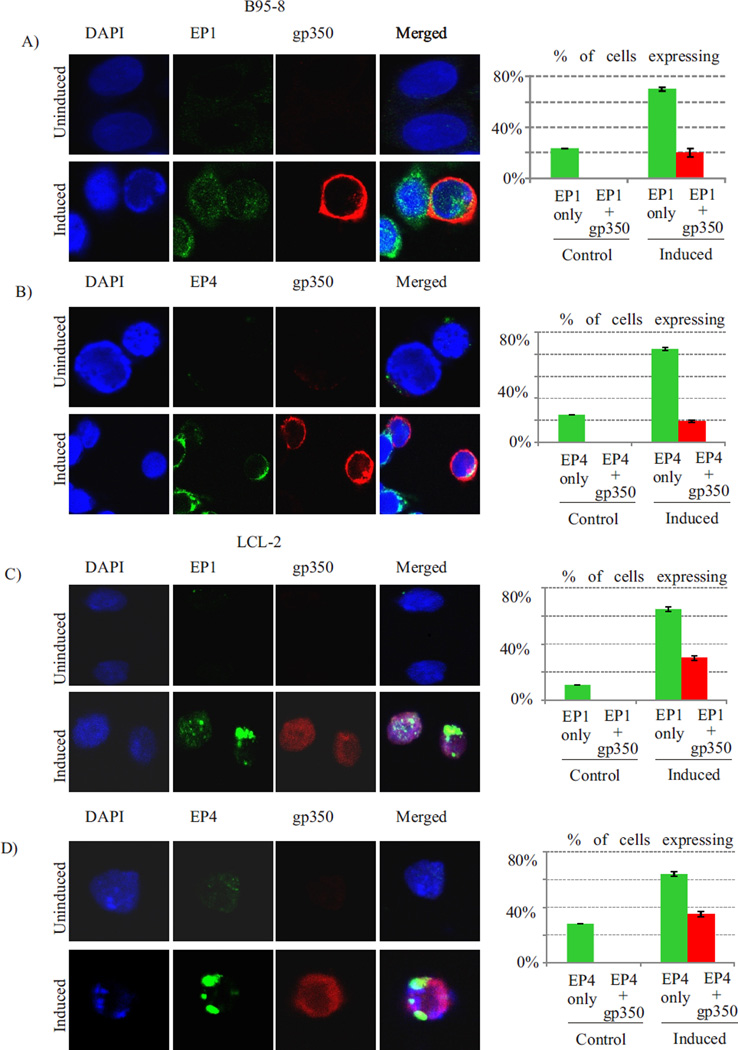
A fraction of cells with enhanced EP1 or EP4 expression undergo lytic reactivation
To understand the role of EP1 and EP4 over expression in induction of EBV lytic reactivation in latently infected cells, we examined what percentage of EP1 or EP4 over expressing cells co-expressed with EBV late lytic protein gp350. Our data showed that EP1 or EP4 signals were detected in approximately 23–25% of uninduced B95-8 cells as compared to 70–85% in induced cells (p<0.01) (fig. 4A, 4B). The gp350 expression was undetectable in uninduced B95-8 cells, whereas approximately 29% of EP1 expressing and 23% of EP4 expressing B95-8 cells were also positive for gp350 (fig. 4A, 4B) (p<0.01). Similar results were observed when experiment was repeated in LCL2 cells. The EP1 or EP4 signals were detected in approximately 11–28% of uniduced LCL2 cells as compared to 64–65% in induced cells (p<0.01) (fig. 4C, 4D). The gp350 expression was undetectable in uninduced LCL2 cells, whereas approximately 46% of EP1 expressing and 54% of EP4 expressing LCL2 cells were also positive for gp350 (fig. 4C, 4D) (p<0.01).
Fig. 4. A fraction of cells with enhanced EP1 or EP4 expression undergo lytic reactivation.
EP1 or EP4 signals were detected in approximately 23–25% of uninduced B95-8 cells as compared to 70–85% in induced cells (p<0.01) (fig. 4A, 4B). The gp350 expression was undetectable in uninduced B95-8 cells, whereas approximately 29% of EP1 expressing and 23% of EP4 expressing B95-8 cells were also positive for gp350 (fig. 4A, 4B) (p<0.01). Similar results were observed when experiment was repeated in LCL2 cells. The EP1 or EP4 signals were detected in approximately 11–28% of uniduced LCL2 cells as compared to 64–65% in induced cells (p<0.01) (fig. 4C, 4D). The gp350 expression was undetectable in uninduced LCL2 cells, whereas approximately 46% of EP1 expressing and 54% of EP4 expressing LCL2 cells were also positive for gp350 (fig. 4C, 4D) (p<0.01). The mean values and standard error of three independent experiments are presented as bar graph in right panel.
COX-2 mediated EBV reactivation is restricted in the presence of EP1 and EP4 specific inhibitors
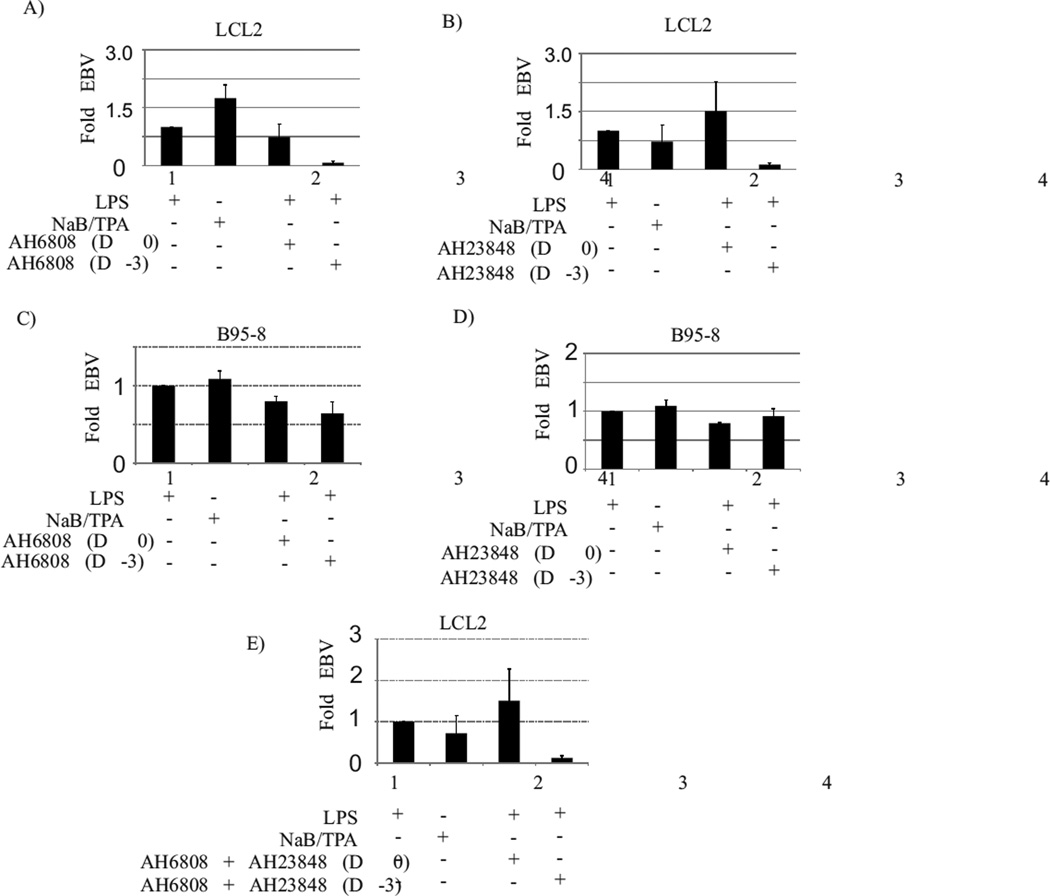
To validate the role of EP1 and EP4 receptors in COX-2 mediated lytic reactivation of EBV, we then used specific inhibitors against the EP receptors. The specific inhibitor AH6809 was used for blocking EP1 receptor (Zhao et al., 2011). AH6809 (6-isopropoxy-9-oxoxanthene-2-carboxylic acid) is a known EP1 antagonist and is also reported to work against EP2 and DP receptors (Zhao et al., 2011). We used AH23848 to inhibit the EP4 receptor (Vo et al., 2013). EBV latently infected LCL2 cells were treated with LPS and incubated with or without EP1 and EP4 specific inhibitors at non-cytotoxic doses described in material and method section. The EP inhibitors were either added 3 days prior to LPS treatment, or simultaneous to LPS treatment, or 3 days post LPS treatment. The cell culture supernatant was harvested six days after LPS treatment and tested for the presence of EBV by real time PCR using Bam W primers as described in material and methods section. For the EP1 receptor, the addition of the inhibitor 3 days prior to LCL2 induction resulted in a 14-fold reduction (p<0.001) in virus detection as compared to no inhibitor control (Fig. 5A, compare lanes 1 and 4). The addition of the EP1 inhibitor simultaneously to LPS induction of LCL2 also reduced the virus detection by 1.35 fold (Fig. 5A, compare lanes 1 and 3) (p<0.05). For EP4 receptor, the addition of the inhibitor 3 days prior to LCL2 induction resulted in a 10-fold reduction (p<0.001) in virus detection as compared to no inhibitor control (Fig. 5B compare lanes 1 and 4). The addition of EP4 inhibitor simultaneously with LPS treatment of LCL2 did not block virus lytic reactivation (Fig. 5B, compare lanes 1 and 3).
Fig. 5. EBV lytic reactivation in response to LPS mediated COX-2 upregulation in latently infected cells was blocked in the presence of specific chemical inhibitors of EP1 and EP4.
Ten million EBV latently infected LCL2 (panel A) or B95-8 (panel C) were induced with LPS (1ug/ml) and treated with EP1 specific inhibitor AH6809 either simultaneous with LPS induction (panel A and C, lane 3) or 3 days before LPS induction (panel A & C, lane 4). Similarly the effect of EP4 inhibitor (AH23848) was tested on LPS mediated EBV lytic reactivation in LCL2 (panel B) and B95-8 cells (panel D). The effect of simultaneous addition of EP1 and EP4 inhibitors on LPS treatment mediated EBV lytic reactivation was tested in B95-8 cells (panel E). For the EP1 receptor, the addition of the inhibitor 3 days prior to LCL2 induction resulted in a 14-fold reduction (p<0.001) in virus detection as compared to no inhibitor control (Fig. 5A, compare lanes 1 and 4). The addition of the EP1 inhibitor simultaneously to LPS induction of LCL2 also reduced the virus detection by 1.35 fold (Fig. 5A, compare lanes 1 and 3) (p<0.05). For EP4 receptor, the addition of the inhibitor 3 days prior to LCL2 induction resulted in a 10-fold reduction (p<0.001) in virus detection as compared to no inhibitor control (Fig. 5B compare lanes 1 and 4). The addition of EP4 inhibitor simultaneously with LPS treatment of LCL2 did not block virus lytic reactivation (Fig. 5B, compare lanes 1 and 3). These experiments above were repeated in B95-8 cells, and a similar pattern was observed (Fig. 5C, D), although the degree of inhibition of lytic reactivation (1.2 to 1.5 fold) (p<0.05) was not as dramatic as seen in LCL2. When the inhibitors against the EP1 and EP4 receptors were added together prior to LPS treatment, a complete inhibition of EBV reactivation was observed (Fig. 5E, compare lanes 1 and 4) (p<0.001).
These experiments above were repeated in B95-8 cells, and a similar pattern was observed (Fig. 5C, D), although the degree of inhibition of lytic reactivation (1.2 to 1.5 fold) (p<0.05) was not as dramatic as seen in LCL2 indicating some redundancy in downstream pathways in these cells. Moreover, when the inhibitors against the EP1 and EP4 receptors were added together prior to LPS treatment, a complete inhibition of EBV reactivation was observed (Fig. 5E, compare lanes 1 and 4) (p<0.001). It is also important to mention that addition of inhibitors at any time simultaneous or post LPS induction did not result in any significant inhibition of EBV reactivation (Fig. 5A, B, C, D, and E, compare lanes 3 and 4). Our results clearly indicated a role for the EP1 and EP4 receptors in COX-2 mediated lytic reactivation of EBV.
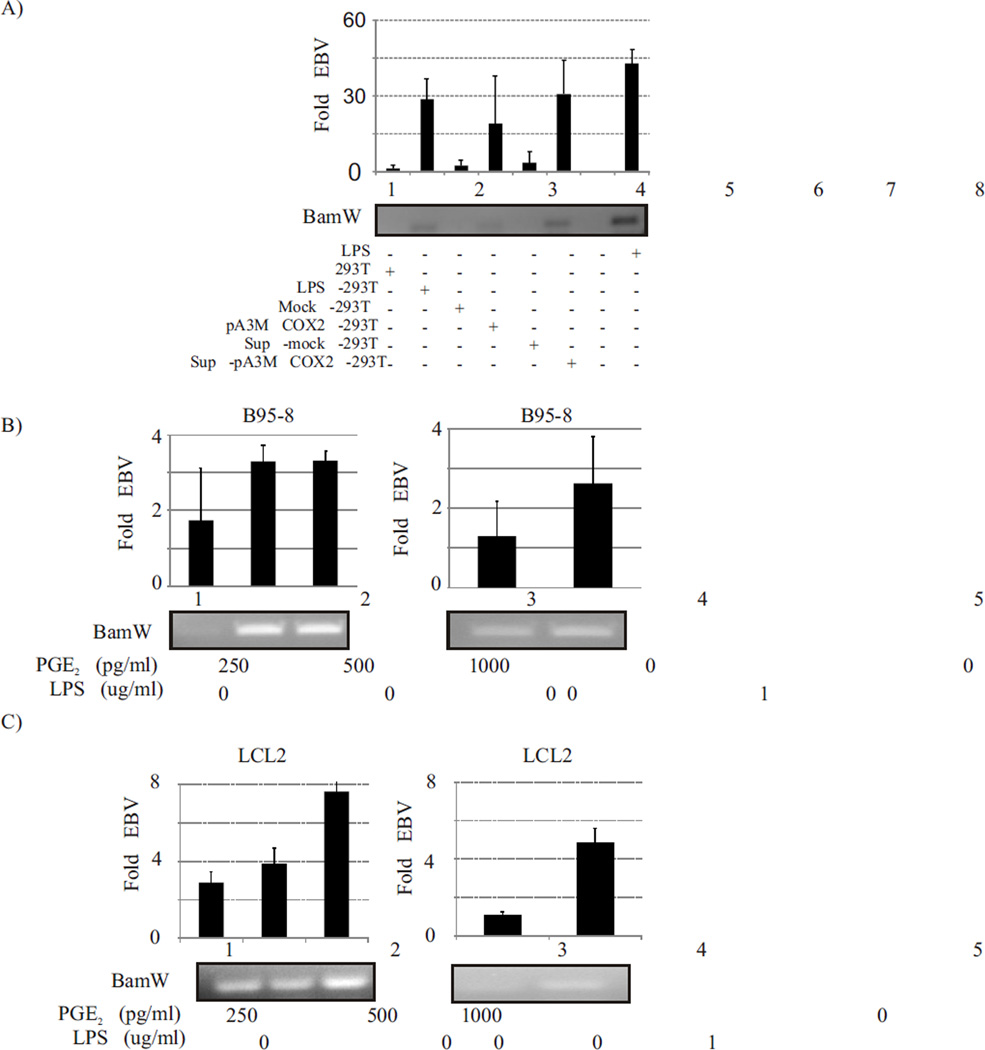
Co-cultivation of COX-2 expressing cells with EBV latently infected cells can induce EBV lytic reactivation
The COX-2 downstream effector molecule PGE2 is known to act on target cells in autocrine as well as paracrine mode (Dohadwala et al., 2002). PGE2 is a critical molecule in amplifying and generating a self-perpetuating cycle that induces inflammation and cancer (Dagouassat et al., 2013). It has been previously reported that patients suffering from chronic inflammatory conditions like rheumatoid arthritis have a high incidences of EBV associated malignancies (Dagouassat et al., 2013; Kondo et al., 2013; Lossius et al., 2013). Our data in this manuscript have shown that PGE2 released in response to COX-2 upregulation in EBV latently infected cells can result in lytic reactivation most likely through an autocrine mode of action. We have also shown that LPS induction of EBV negative cells such as 293T and HeLa can also upregulate COX-2, EP1, EP4 expression and PGE2 release from these cells. We hypothesize that a chronic inflammatory condition characterized by increased COX-2 expression in inflamed cells can induce lytic reactivation of EBV in latently infected cells even at far off sites in vivo through paracrine activities of extracellular secreted PGE2 functioning as the effector molecule. To determine if COX-2 upregulation can act in paracrine mode and cause EBV lytic reactivation, we treated 10 million adherent 293T cells to increase COX-2 expression and co-cultivated these cells or their culture supernatant, with an equal number of latently infected EBV-positive cells. The upregulated PGE2 levels in the supernatant of LPS treated 293T were closer to upregulated levels observed in the supernatant of LPS treated latently infected EBV-positive cells. Therefore we determined if it resulted in lytic reactivation of EBV. Our results clearly showed that up-regulation of COX-2 level in 293T cells could induce lytic reactivation of EBV in co-cultivated LCL2 cells (Fig. 6A, Lane 2) (p<0.001). The lytic reactivation observed in LCL2 co-cultivated with induced 293T cells was comparable to that in LPS induced LCL2 (Fig. 6A, compare lanes 2 and 8). Similar results were obtained when COX-2 overexpressing 293T cells (transfected with a COX-2 expression plasmid) were co-cultivated with LCL2 (Fig. 6A, lane 4) (p<0.05). When only the supernatant from COX-2 expressing 293T cells was added to LCL2, this also resulted in lytic reactivation of EBV (Fig. 6A, lane 6) (p<0.001). These data clearly indicate that soluble factors released from COX-2 expressing cells in the culture supernatant were enough to induce lytic reactivation of EBV in latently infected cells. To establish the specific role of PGE2, we tested the effect of addition of exogenous purified PGE2 (Cayman Chemicals, Ann Arbor, MI, Cat no. 414014) on latently infected EBV-positive cells. The addition of increasing amounts of exogenous purified PGE2 at levels within biological range from 250 to 1000 pg/ml resulted in increased lytic reactivation in EBV latently infected B95-8 cells (Fig. 6B). The amount of virus detected in cell culture supernatant of PGE2 treated cells was equivalent to the virus detected in supernatant of LPS treated cells (Fig. 6B, compare lane 1, 2, 3 with 5). A similar pattern was observed when LCL2 was treated with exogenous PGE2 (Fig. 6C). Our data clearly indicated that latently infected EBV-positive cells can initiate lytic reactivation in response to COX-2 up regulation in neighboring cells in a paracrine mode of action. This finding may be very significant in addressing clinical conditions associated with chronic inflammation. These observations are useful in explaining the higher incidence of EBV associated cancers in people suffering from chronic inflammatory conditions characterized by elevated COX-2 levels (Kondo et al., 2013; Lossius et al., 2013).
Fig. 6. EBV latently infected cells undergo lytic reactivation when co-cultivated with COX-2 expressing cells.
The up-regulation of COX-2 level in 293T cells could induce lytic reactivation of EBV in co-cultivated LCL2 cells (Fig. 6A, Lane 2) (p<0.001). The lytic reactivation observed in LCL2 co-cultivated with induced 293T cells was comparable to that in LPS induced LCL2 (Fig. 6A, compare lanes 2 and 8). Similar results were obtained when COX-2 overexpressing 293T cells (transfected with a COX-2 expression plasmid) were co-cultivated with LCL2 (Fig. 6A, lane 4) (p<0.05). When only the supernatant from COX-2 expressing 293T cells was added to LCL2, this also resulted in lytic reactivation of EBV (Fig. 6A, lane 6) (p<0.001). The addition of increasing amounts of exogenous purified PGE2 at levels within biological range from 250 to 1000 pg/ml resulted in increased lytic reactivation in EBV latently infected B95-8 cells (Fig. 6B). A similar pattern was observed when LCL2 was treated with exogenous PGE2 (Fig. 6C).
EBV progeny virions produced as a result of COX-2-mediated lytic reactivation are biologically active
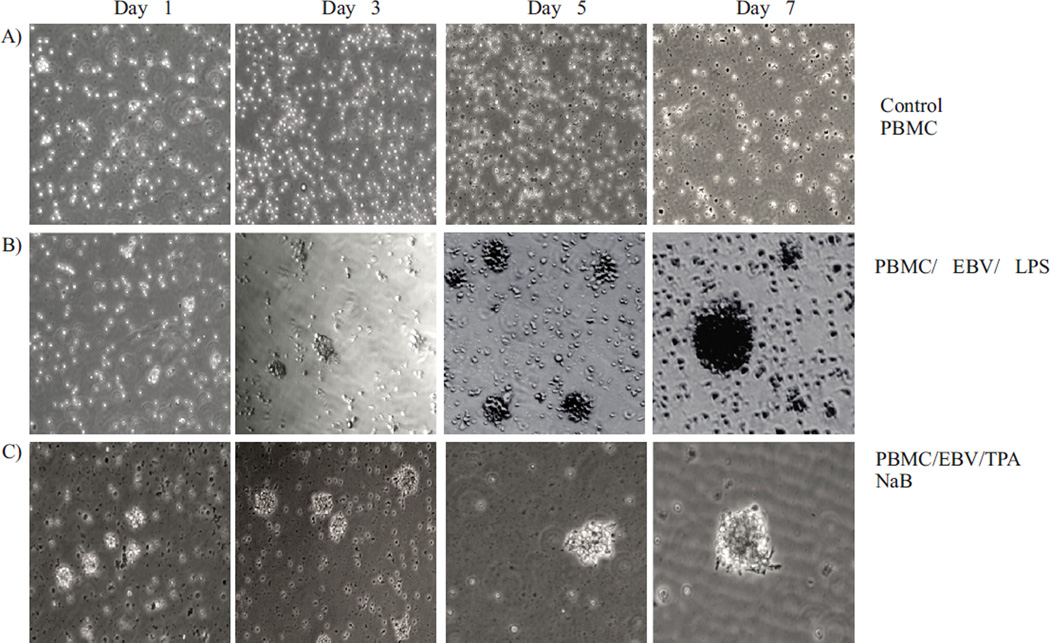
To investigate the biological activity of EBV progeny generated in response to COX-2 up regulation, freshly isolated peripheral blood mononuclear cell (PBMC) were treated with the virus obtained from cell culture supernatant of LPS induced EBV infected cells. To investigate if the virus was biologically functional, ten million PBMCs were infected with the virus. The PBMCs were monitored daily by brightfield microscopy. The results of the infection showed that the EBV progeny virus had successfully infected the primary B cells which are transformed leading to immortalization and generation of Lymphoblastoid cell lines. All cells in the uninfected control and those exposed to supernatant from uninduced B95-8 control died, but the primary B-cells infected with EBV generated lymphoblasts which were similar to those seen in LCLs confirming the ability of EBV infectious virions to infect and immortalize the naïve B cells (Fig. 7).
Fig. 7. EBV virions harvested from lytic reactivation by LPS induced EBV latently infected cells are biologically active.
Human PBMCs infected with EBV harvested from LPS induced B95-8 cells resulted in infection and transformation of B-cells. Uninfected PBMCs and PBMCs added with supernatant from uninduced B95-8 cells were used as a negative control which did not show any aggregation, whereas PBMCs infected with EBV harvested from NaB/ TPA induced B95-8 cells were used as positive controls to assay the biological activity of EBV.
DISCUSSION AND CONCLUSION
A number of studies have shown that the inflammatory response against virus infection is linked to virus associated pathogenesis which leads to cellular transformation, promotion, survival, proliferation, invasion, angiogenesis, and metastasis (Woller and Kuhnel, 2013). However, the direct role of inflammation in modulating life cycle events of infectious viral agents is yet to be understood. In the case of virus like EBV whose life cycle involves long periods of latency and subsequent reactivation, the significance of the inflammatory response needs to be carefully evaluated. This is especially important as lytic reactivation is a critical step in EBV life cycle and is very important for virus dissemination to new hosts and infection of nascent cells. Reactivation provides new opportunities for the virus to not only spread to other host but also to infect naïve cells in infected host, which in immuno-compromised patients may result in transformation and tumorigenesis. Previous studies have demonstrated that up regulation of the modulator of inflammation COX-2 and its downstream effector molecules has a key role in EBV (Kaul et al., 2006; Murono et al., 2001), as well as KSHV life cycle events (Paul, Chandran, and Sharma-Walia, 2013b; Paul, Sharma-Walia, and Chandran, 2011).
In the present study, we investigated a link between chronic inflammation characterized by upregulated COX-2 levels and induction of EBV lytic reactivation. We used LPS mediated up regulation of COX-2 in latently infected EBV cells as a model system. Our studies now showed that LPS addition to latently infected EBV positive cells resulted not only in up regulation of COX-2 but also an increased expression of its downstream effector PGE2. The elevated COX-2 and PGE2 levels were coincident with expression of EBV late lytic protein gp350 and detection of EBV in cell culture supernatant indicating lytic reactivation at least in a sub-population of infected cells. The addition of a COX-2 specific inhibitor NS-398 resulted in a dramatic reduction in the amount of virus detected in the cell culture supernatant indicating that LPS mediated EBV lytic reactivation is mainly because of upregulated COX-2 levels. Burkitt’s lymphoma Akata cells also showed a similar pattern indicating the phenomenon is likely to have biological relevance in vivo. The increased expression of EBV lytic protein gp350 from LPS treated cells indicate that EBV genome detected in cell culture supernatant is from progeny virions released from cells as a result of lytic reactivation. This along with DNaseI treatment of supernatant before virus concentration and viral genome extraction clearly indicate that the detected EBV sequence is not from episomal DNA released due to cell lysis. The overexpression of COX-2 and PGE2 in infected cells was also coincident with overexpression of PGE2 receptors EP1 and EP4. The significant biological functions governed by COX-2/ PGE2/ EP receptors pro-inflammatory axis is described in several viral-linked tumors especially in cancer related with the family of oncogenic human herpes virus (Paul, Chandran, and Sharma-Walia, 2013a). The G-protein coupled receptors (EP1-4) govern several biochemical changes and administer processes involving the immune system (De Keijzer et al., 2013). Our data show that significant overexpression of EP1 and EP4 was detected not only at transcriptional levels but also at the protein levels. The expression levels of both receptors were increased several fold in treated cell lines compared to the untreated cells. Also, the expression profile of the EP4 receptor was significant with a dramatic rise in EP4 expression. This supports the hypothesis that among all four EP receptors, EP4 may prove to be the most versatile and important receptor. EP4 is widely known for its cancer promoting and pro-angiogenic activities (Konya et al., 2013). It is important to note that these receptors did not get upregulated in response to LPS treatment in EBV negative Burkitt’s Lymphoma cells. Moreover the functional inhibition of EP1 and EP4 receptors using chemical inhibitors dramatically reduced COX-2 mediated lytic reactivation of EBV even when COX-2 levels were upregulated. In fact the combined usage of inhibitors against EP1 and EP4 completely blocked the lytic reactivation suggesting that the majority of COX-2 mediated regulation of EBV latency is via the EP1 and EP4 receptors. The observation that the effective blockage of lytic reactivation required addition of EP inhibitors prior to LPS, and did not work as efficiently when the inhibitors were added simultaneously or after LPS induction, suggests that prior blockage of EP receptors was necessary to block lytic reactivation. It is possible that prior inhibition of EP receptors can result in dysfunction of their downstream signalling important for lytic reactivation of EBV. This clearly points to a specific role of EP1/4 receptors and their downstream effectors in modulation of EBV infection cycle. It is important to point out that Sodium butyrate (NaB) which is a histone deacetylase inhibitor and TPA which is a histone acetyltransferase inducer are known to activate lytic viral replication in EBV latently infected cells (Daibata et al., 1998; Luka, Kallin, and Klein, 1979). Sodium butyrate and TPA activates pathways that have been linked to EP receptors (Shelby, Nelson, and Morris, 2005). Sodium butyrate activates the PKA (EP2 and EP4) pathway, while TPA activates PKC (EP1) (Shelby, Nelson, and Morris, 2005). It has been previously reported that EP receptors have distinct binding characteristics and are coupled to different intracellular signalling pathways resulting in the increase in levels of intracellular calcium (Reader, Holt, and Fulton, 2011). The EP receptors have also been shown to be functionally regulated epigenetically (Gray et al., 2009). The intracellular PGE2 can also have pro-apoptotic affect in cancer cells (Lalier et al., 2011). Therefore it is possible that COX-2/ PGE2 mediated lytic reactivation of EBV may also be using similar pathways downstream of EP1 and EP4 receptors, and needs further investigations.
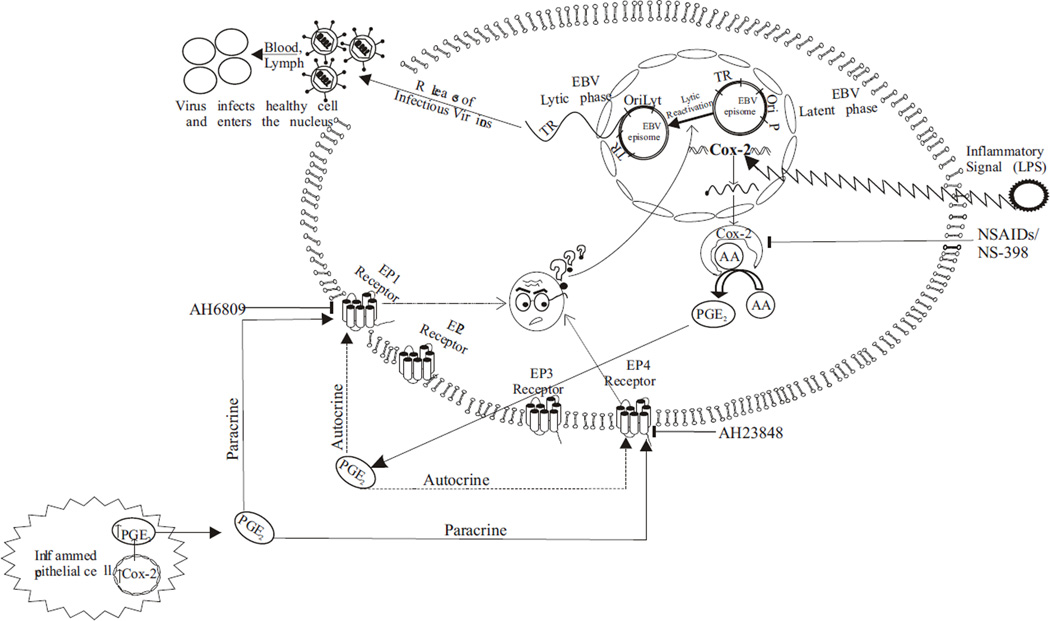
It was earlier reported that patients suffering from chronic inflammatory conditions characterized by upregulated COX-2 levels have high incidences of EBV associated malignancy (Kondo et al., 2013; Lossius et al., 2013). Our data from co-cultivation experiments demonstrate that upregulated COX-2 levels in epithelial cells can induce lytic reactivation in co-cultured EBV latently infected lymphoid cells. The addition of culture supernatant from COX-2 expressing cells of epithelial origin or even exogenous purified PGE2 was enough to trigger virus reactivation. The reactivated virus was also fully functional and biologically active as demonstrated by its transforming potential when infected to fresh PBMCs. These observations now provide an explanation for COX-2 to be a possible contributor to the incidences of EBV associated cancers in people with chronic inflammatory conditions. It is possible that upregulated COX-2 levels in such patients may affect latently infected EBV positive resting B-cells at peripheral sites in the body by paracrine mode of action via PGE2 (Fig. 8). Lytic reactivation in such patients will not only allow the progeny virions to infect naïve cells but also increase the probability of transformation of de novo infected cells. If such patients are also immuno-compromised, the probability of transformation and tumourigenesis will also be increased as is clinically evident. This study now adds another dimension to our understanding of the role of inflammation in the progression of cancers mediated by the oncogenic virus EBV.
Fig. 8. Schematic model shows that COX-2 upregulation in response to an inflammatory signal such as LPS results in EBV lytic reactivation from latently infected cells.
The upregulation of COX-2 is associated with EBV lytic cycle reactivation. Inhibition of COX-2 with specific inhibitor NS-398 blocks lytic reactivation. The up regulation of COX-2 enzyme results in increased production of the effector molecule PGE2 which has autocrine and paracrine action facilitated through EP1, EP2, EP3 and EP4 receptors. The EP1 and EP4 receptors are also upregulated in response to LPS induction and their inhibition directly reduces EBV lytic reactivation suggesting their possible involvement in transition from the latent to lytic cycle in response to inflammatory signals. Also, PGE2 released from inflamed EBV negative epithelial cell can act via a paracrine mode of action and lead to EBV lytic reactivation from latently infected cell.
Research Highlights.
Increased COX-2 levels in latently infected cells induce lytic reactivation of EBV
The virus reactivation occurs via PGE2 and EP1/ EP4 receptors
The reactivated progeny virions are biologically active and can transform cells
This can help explain EBV cancers in people with chronic inflammatory conditions
ACKNOWLEDGEMENT
This work was supported by grants from the Department of Biotechnology of Government of India (BT/PR15109/GBD/27/320/2011), MRP grant funded by UGC (FN-41-1144/2012), and R&D Grant from University of Delhi. JG is Senior Research Fellow funded by UGC, NG is Project Fellow funded by UGC. LK is Senior Research Fellow funded by CSIR. E.S.R. is a Scholar of the Leukemia and Lymphoma Society of America and is funded by public health grants (CA137894-05, DK050306-17, R01-CA-137894, R01-CA-171979, P01-CA-174439, R01-CA-177423). We are thankful to Timothy Hla (Cornell University, Ithaca, NY) for providing COX-2 expression plasmid as a gift.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahmad AS, Maruyama T, Narumiya S, Dore S. PGE2 EP1 Receptor Deletion Attenuates 6-OHDA-Induced Parkinsonism in Mice: Old Switch, New Target. Neurotox Res. 2013;23(3):260–266. doi: 10.1007/s12640-013-9381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonic V, Stojadinovic A, Kester KE, Weina PJ, Brucher BL, Protic M, Avital I, Izadjoo M. Significance of infectious agents in colorectal cancer development. J Cancer. 2013;4(3):227–240. doi: 10.7150/jca.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai W, Tang J. [The expression and relationship of cyclooxygenase-2 and latent membrane protein-1 in nasopharyngeal carcinoma] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke ZaZhi. 2009;23(3):105–108. [PubMed] [Google Scholar]
- Biedrzycka A, Kloch A, Migalska M, Bielanski W. Molecular characterization of putative Hepatozoon sp. from the sedge warbler (Acrocephalus schoenobaenus) Parasitology. 2013;140(6):695–698. doi: 10.1017/S0031182012002004. [DOI] [PubMed] [Google Scholar]
- Chang LS, Wang JT, Doong SL, Lee CP, Chang CW, Tsai CH, Yeh SW, Hsieh CY, Chen MR. Epstein-Barr virus BGLF4 kinase downregulates NF-kappaB transactivation through phosphorylation of coactivator UXT. J Virol. 2012;86(22):12176–12186. doi: 10.1128/JVI.01918-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chell SD, Witherden IR, Dobson RR, Moorghen M, Herman AA, Qualtrough D, Williams AC, Paraskeva C. Increased EP4 receptor expression in colorectal cancer progression promotes cell growth and anchorage independence. Cancer Res. 2006;66(6):3106–3113. doi: 10.1158/0008-5472.CAN-05-3702. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Hsu WL, Yang HI, Lee MH, Chen HC, Chien YC, You SL. Epidemiology of virus infection and human cancer. Recent Results Cancer Res. 2013;193:11–32. doi: 10.1007/978-3-642-38965-8_2. [DOI] [PubMed] [Google Scholar]
- Copeland RA, Williams JM, Giannaras J, Nurnberg S, Covington M, Pinto D, Pick S, Trzaskos JM. Mechanism of selective inhibition of the inducible isoform of prostaglandin G/H synthase. Proc Natl Acad Sci U S A. 1994;91(23):11202–11206. doi: 10.1073/pnas.91.23.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagouassat M, Gagliolo JM, Chrusciel S, Bourin MC, Duprez C, Caramelle P, Boyer L, Hue S, Stern JB, Validire P, Longrois D, Norel X, Dubois-Rande JL, Le Gouvello S, Adnot S, Boczkowski J. The cyclooxygenase-2-prostaglandin e2 pathway maintains senescence of chronic obstructive pulmonary disease fibroblasts. Am J Respir Crit Care Med. 2013;187(7):703–714. doi: 10.1164/rccm.201208-1361OC. [DOI] [PubMed] [Google Scholar]
- Daibata M, Taguchi T, Taguchi H, Miyoshi I. Integration of human herpesvirus 6 in a Burkitt's lymphoma cell line. Br J Haematol. 1998;102(5):1307–1313. doi: 10.1046/j.1365-2141.1998.00903.x. [DOI] [PubMed] [Google Scholar]
- De Keijzer S, Meddens MB, Torensma R, Cambi A. The Multiple Faces of Prostaglandin E2 G-Protein Coupled Receptor Signaling during the Dendritic Cell Life Cycle. Int J Mol Sci. 2013;14(4):6542–6555. doi: 10.3390/ijms14046542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohadwala M, Batra RK, Luo J, Lin Y, Krysan K, Pold M, Sharma S, Dubinett SM. Autocrine/paracrine prostaglandin E2 production by non-small cell lung cancer cells regulates matrix metalloproteinase-2 and CD44 in cyclooxygenase-2-dependent invasion. J Biol Chem. 2002;277(52):50828–50833. doi: 10.1074/jbc.M210707200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Font-Nieves M, Sans-Fons MG, Gorina R, Bonfill-Teixidor E, Salas-Perdomo A, Marquez-Kisinousky L, Santalucia T, Planas AM. Induction of COX-2 enzyme and down-regulation of COX-1 expression by lipopolysaccharide (LPS) control prostaglandin E2 production in astrocytes. J Biol Chem. 2012;287(9):6454–6468. doi: 10.1074/jbc.M111.327874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Koshida K, Keller ET, Takahashi Y, Yoshimito T, Namiki M, Mizokami A. Cyclooxygenase-2 promotes prostate cancer progression. Prostate. 2002;53(3):232–240. doi: 10.1002/pros.10152. [DOI] [PubMed] [Google Scholar]
- Giunco S, Dolcetti R, Keppel S, Celeghin A, Indraccolo S, Dal Col J, Mastorci K, De Rossi A. hTERT Inhibition Triggers Epstein-Barr Virus Lytic Cycle and Apoptosis in Immortalized and Transformed B Cells: A Basis for New Therapies. Clin Cancer Res. 2013;19(8):2036–2047. doi: 10.1158/1078-0432.CCR-12-2537. [DOI] [PubMed] [Google Scholar]
- Gong M, Kieff E. Intracellular trafficking of two major Epstein-Barr virus glycoproteins, gp350/220 and gp110. J Virol. 1990;64(4):1507–1516. doi: 10.1128/jvi.64.4.1507-1516.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SG, Al-Sarraf N, Baird AM, Cathcart MC, McGovern E, O'Byrne KJ. Regulation of EP receptors in non-small cell lung cancer by epigenetic modifications. Eur J Cancer. 2009;45(17):3087–3097. doi: 10.1016/j.ejca.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Hull MA, Ko SC, Hawcroft G. Prostaglandin EP receptors: targets for treatment and prevention of colorectal cancer? Mol Cancer Ther. 2004;3(8):1031–1039. [PubMed] [Google Scholar]
- Irie A, Sugimoto Y, Namba T, Asano T, Ichikawa A, Negishi M. The C-terminus of the prostaglandin-E-receptor EP3 subtype is essential for activation of GTP-binding protein. Eur J Biochem. 1994;224(1):161–166. doi: 10.1111/j.1432-1033.1994.tb20007.x. [DOI] [PubMed] [Google Scholar]
- Kaul R, Verma SC, Murakami M, Lan K, Choudhuri T, Robertson ES. Epstein-Barr virus protein can upregulate cyclo-oxygenase-2 expression through association with the suppressor of metastasis Nm23-Hl. J Virol. 2006;80(3):1321–1331. doi: 10.1128/JVI.80.3.1321-1331.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci U S A. 2004;101(9):3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Tanimoto K, Yamada K, Yoshimoto G, Suematsu E, Fujisaki T, Oshiro Y, Tamura K, Takeshita M, Okamura S. Mature T/NK-cell lymphoproliferative disease and Epstein-Barr virus infection are more frequent in patients with rheumatoid arthritis treated with methotrexate. Virchows Arch. 2013;462(4):399–407. doi: 10.1007/s00428-013-1389-1. [DOI] [PubMed] [Google Scholar]
- Konger RL, Billings SD, Thompson AB, Morimiya A, Ladenson JH, Landt Y, Pentland AP, Badve S. Immunolocalization of low-affinity prostaglandin E receptors, EP and EP, in adult human epidermis. J Invest Dermatol. 2005;124(5):965–970. doi: 10.1111/j.0022-202X.2005.23658.x. [DOI] [PubMed] [Google Scholar]
- Konya V, Marsche G, Schuligoi R, Heinemann A. E-type prostanoid receptor 4 (EP4) in disease and therapy. Pharmacol Ther. 2013 doi: 10.1016/j.pharmthera.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalier L, Pedelaborde F, Braud C, Menanteau J, Vallette FM, Olivier C. Increase in intracellular PGE2 induces apoptosis in Bax-expressing colon cancer cell. BMC Cancer. 2011;11:153. doi: 10.1186/1471-2407-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Li H, Chen L, Yang L, Li L, Tao Y, Li W, Li Z, Liu H, Tang M, Bode AM, Dong Z, Cao Y. (−)-Epigallocatechin-3-gallate inhibition of Epstein-Barr virus spontaneous lytic infection involves ERK1/2 and PI3-K/Akt signaling in EBV-positive cells. Carcinogenesis. 2012;34(3):627–637. doi: 10.1093/carcin/bgs364. [DOI] [PubMed] [Google Scholar]
- Lossius A, Johansen JN, Torkildsen O, Vartdal F, Holmoy T. Epstein-Barr virus in systemic lupus erythematosus, rheumatoid arthritis and multiple sclerosis-association and causation. Viruses. 2013;4(12):3701–3730. doi: 10.3390/v4123701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luka J, Kallin B, Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979;94(1):228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- Luschnig-Schratl P, Sturm EM, Konya V, Philipose S, Marsche G, Frohlich E, Samberger C, Lang-Loidolt D, Gattenlohner S, Lippe IT, Peskar BA, Schuligoi R, Heinemann A. EP4 receptor stimulation down-regulates human eosinophil function. Cell Mol Life Sci. 2011;68(21):3573–3587. doi: 10.1007/s00018-011-0642-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Holt D, Kundu N, Reader J, Goloubeva O, Take Y, Fulton AM. A prostaglandin E (PGE) receptor EP4 antagonist protects natural killer cells from PGE-mediated immunosuppression and inhibits breast cancer metastasis. Oncoimmunology. 2013a;2(1):e22647. doi: 10.4161/onci.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Kundu N, loffe OB, Goloubeva O, Konger R, Baquet C, Gimotty P, Reader J, Fulton AM. Prostaglandin E receptor EP1 suppresses breast cancer metastasis and is linked to survival differences and cancer disparities. Mol Cancer Res. 2013b;8(10):1310–1318. doi: 10.1158/1541-7786.MCR-10-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros R, Figueiredo CP, Pandolfo P, Duarte FS, Prediger RD, Passos GF, Calixto JB. The role of TNF-alpha signaling pathway on COX-2 upregulation and cognitive decline induced by beta-amyloid peptide. Behav Brain Res. 2010;209(1):165–173. doi: 10.1016/j.bbr.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Medvedev AE, Vogel SN. Overexpression of CD14, TLR4, and MD-2 in HEK 293T cells does not prevent induction of in vitro endotoxin tolerance. J Endotoxin Res. 2003;9(1):60–64. doi: 10.1179/096805103125001360. [DOI] [PubMed] [Google Scholar]
- Michelow P, Wright C, Pantanowitz L. A review of the cytomorphology of Epstein-Barr virus-associated malignancies. Acta Cytol. 2012;56(1):1–14. doi: 10.1159/000334235. [DOI] [PubMed] [Google Scholar]
- Murono S, Inoue H, Tanabe T, Joab I, Yoshizaki T, Furukawa M, Pagano JS. Induction of cyclooxygenase-2 by Epstein-Barr virus latent membrane protein 1 is involved in vascular endothelial growth factor production in nasopharyngeal carcinoma cells. Proc Natl Acad Sci U S A. 2001;98(12):6905–6910. doi: 10.1073/pnas.121016998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PG, Young LS. Epstein-Barr virus infection: basis of malignancy and potential for therapy. Expert Rev Mol Med. 2001;3(28):1–20. doi: 10.1017/S1462399401003842. [DOI] [PubMed] [Google Scholar]
- Nadda N, Setia S, Vaish V, Sanyal SN. Role of cytokines in experimentally induced lung cancer and chemoprevention by COX-2 selective inhibitor, etoricoxib. Mol Cell Biochem. 2012;372(1–2):101–112. doi: 10.1007/s11010-012-1451-3. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79(4):1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Iwasaki K, Shitashige M, Umeda M, Izumi Y, Murota S, Ishikawa I. Downregulation of lipopolysaccharide-induced intercellular adhesion molecule-1 expression via EP2/EP4 receptors by prostaglandin E2 in human fibroblasts. Inflammation. 2001;25(2):75–81. doi: 10.1023/a:1007110304044. [DOI] [PubMed] [Google Scholar]
- O'Banion MK, Sadowski HB, Winn V, Young DA. A serum- and glucocorticoid-regulated 4-kilobase mRNA encodes a cyclooxygenase-related protein. J Biol Chem. 1991;266(34):23261–23267. [PubMed] [Google Scholar]
- O'Callaghan G, Ryan A, Neary P, O'Mahony C, Shanahan F, Houston A. Targeting the EP1 receptor reduces Fas ligand expression and increases the antitumor immune response in an in vivo model of colon cancer. Int J Cancer. 2013 doi: 10.1002/ijc.28076. [DOI] [PubMed] [Google Scholar]
- O'Neill GP, Ford-Hutchinson AW. Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissues. FEBS Lett. 1993;330(2):156–160. doi: 10.1016/0014-5793(93)80263-t. [DOI] [PubMed] [Google Scholar]
- Paul AG, Chandran B, Sharma-Walia N. COX-2-PGE2-EP receptor inflammatory axis: a key player in KSHV associated malignancies. Transl Res. 2013a doi: 10.1016/j.trsl.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul AG, Chandran B, Sharma-Walia N. Cyclooxygenase-2-prostaglandin E2-eicosanoid receptor inflammatory axis: a key player in Kaposi's sarcoma-associated herpes virus associated malignancies. Transl Res. 2013b;162(2):77–92. doi: 10.1016/j.trsl.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul AG, Sharma-Walia N, Chandran B. Targeting KSHV/HHV-8 latency with COX-2 selective inhibitor nimesulide: a potential chemotherapeutic modality for primary effusion lymphoma. PLoS One. 2011;6(9):e24379. doi: 10.1371/journal.pone.0024379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader J, Holt D, Fulton A. Prostaglandin E2 EP receptors as therapeutic targets in breast cancer. Cancer Metastasis Rev. 2011;30(3–4):449–463. doi: 10.1007/s10555-011-9303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Amagase K, Ebara S, Akiba Y, Takeuchi K. Cyclooxygenase (COX)-1 and COX-2 both play an important role in the protection of the duodenal mucosa in cats. J Pharmacol Exp Ther. 2012;344(1):189–195. doi: 10.1124/jpet.112.199182. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgambato A, Cittadini A. Inflammation and cancer: a multifaceted link. Eur Rev Med Pharmacol Sci. 2010;14(4):263–268. [PubMed] [Google Scholar]
- Shaw JE, Petit RG, Leung K. Growth of B95-8 cells and expression of Epstein-Barr virus lytic phase in serum-free medium. J Virol. 1987;61(12):4033–4037. doi: 10.1128/jvi.61.12.4033-4037.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby BD, Nelson A, Morris C. Gamma-herpesvirus neoplasia: a growing role for COX-2. Microsc Res Tech. 2005;68(3–4):120–129. doi: 10.1002/jemt.20226. [DOI] [PubMed] [Google Scholar]
- Shin WG, Kim HJ, Cho SJ, Kim HS, Kim KH, Jang MK, Lee JH, Kim HY. The COX-2-1195AA Genotype Is Associated with Diffuse-Type Gastric Cancer in Korea. Gut Liver. 2012;6(3):321–327. doi: 10.5009/gnl.2012.6.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282(16):11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- Takada K, Horinouchi K, Ono Y, Aya T, Osato T, Takahashi M, Hayasaka S. An Epstein-Barr virus-producer line Akata: establishment of the cell line and analysis of viral DNA. Virus Genes. 1991;5(2):147–156. doi: 10.1007/BF00571929. [DOI] [PubMed] [Google Scholar]
- Taniguchi A, Hashida Y, Nemoto Y, Taguchi T, Iwahara Y, Daibata M. Pyothorax-associated lymphoma (PAL) with biclonal Epstein-Barr virus infection: characterization of a novel PAL cell line with unique features. Leuk Res. 2013;37(11):1545–1550. doi: 10.1016/j.leukres.2013.07.033. [DOI] [PubMed] [Google Scholar]
- Tober KL, Wilgus TA, Kusewitt DF, Thomas-Ahner JM, Maruyama T, Oberyszyn TM. Importance of the EP(1) receptor in cutaneous UVB-induced inflammation and tumor development. J Invest Dermatol. 2006;126(1):205–211. doi: 10.1038/sj.jid.5700014. [DOI] [PubMed] [Google Scholar]
- Vo BT, Morton D, Jr, Komaragiri S, Millena AC, Leath C, Khan SA. TGF-beta Effects on Prostate Cancer Cell Migration and Invasion Are Mediated by PGE2 through Activation of PI3K/AKT/mTOR Pathway. Endocrinology. 2013;154(5):1768–1779. doi: 10.1210/en.2012-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woellmer A, Arteaga-Salas JM, Hammerschmidt W. BZLF1 governs CpG-methylated chromatin of Epstein-Barr Virus reversing epigenetic repression. PLoS Pathog. 2012;8(9):el002902. doi: 10.1371/journal.ppat.1002902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woller N, Kuhnel F. Virus infection, inflammation and prevention of cancer. Recent Results Cancer Res. 2013;193:33–58. doi: 10.1007/978-3-642-38965-8_3. [DOI] [PubMed] [Google Scholar]
- Xia W, Kirkman RL. Immune function in transplanted small intestine. II: sIgA production in cholera toxin-primed rats. Transplant Proc. 1990;22(6):2481–2482. [PubMed] [Google Scholar]
- Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K, Sugimoto Y, Narumiya S. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Thl7 cell expansion. Nat Med. 2009;15(6):633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- Yucel-Lindberg T, Ahola H, Carlstedt-Duke J, Modeer T. Involvement of tyrosine kinases on cyclooxygenase expression and prostaglandin E2 production in human gingival fibroblasts stimulated with interleukin-lbeta and epidermal growth factor. Biochem Biophys Res Commun. 1999;257(2):528–532. doi: 10.1006/bbrc.1999.0523. [DOI] [PubMed] [Google Scholar]
- Zhao L, Wu Y, Xu Z, Wang H, Zhao Z, Li Y, Yang P, Wei X. Involvement of COX-2/PGE2 signalling in hypoxia-induced angiogenic response in endothelial cells. J Cell Mol Med. 2011;16(8):1840–1855. doi: 10.1111/j.1582-4934.2011.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler JL, Buonaguro FM. Infectious agents and human malignancies. Front Biosci. 2009;14:3455–3464. doi: 10.2741/3464. [DOI] [PubMed] [Google Scholar]