Abstract
Objective
To determine whether individualized gait training is more effective than usual care for reducing mobility disability and pain in individuals with symptomatic knee osteoarthritis (OA).
Design
Adults age ≥60 with symptomatic knee OA and mobility limitations were randomized to physical therapist-directed gait training on an instrumented treadmill, with biofeedback individualized to optimize knee movements, biweekly for 3 months or usual care (control). Mobility disability was defined by LLFDI Basic Lower Limb Function score (primary); limitations by timed 400m walk, chair-stand, and stair-climb tests at baseline, 3, 6 and 12 months; and symptoms by the Knee Injury/Osteoarthritis Outcome Score. Analyses used longitudinal mixed models.
Results
There were no significant inter-group differences between the 35 gait-training (74.3% women; age 69.7±8.2 years) and 21 control (57.1% women; age 68.9±6.5 years) participants at baseline. At 3 months, gait-training participants had greater improvement in mobility disability (4.3±1.7; p=0.0162) and symptoms (8.6±4.1; p=0.0420). However, there were neither intergroup differences detected for pain, 400m walk, chair-stand or stair-climb times at 3 months nor for any outcomes at 6 or 12 months.
Conclusions
Compared with usual care, individualized gait-training resulted in immediate improvements in mobility disability and knee symptoms in adults with symptomatic knee OA, but these effects were not sustained.
Keywords: Knee Osteoarthritis, rehabilitation, gait, mobility
INTRODUCTION
Limited mobility is one of the most disabling problems facing the growing population of older adults. Osteoarthritis (OA) is the most prevalent joint disorder, resulting in substantial mobility limitations1 and a significant financial impact2, and the knee is the most commonly affected weight-bearing joint3. Risk of disability attributable to knee OA is equal to that for cardiac disease and greater than that due to any other medical disorder in older adults4, 5. Impaired mobility due to knee OA will have an increasingly significant societal impact, with a lifetime risk of symptomatic knee OA between 14–45%6, 7, and substantial association with activity limitations8.
In order to compensate for pain or joint instability, patients alter their walking strategies by shifting loads to joints such as the hip and ankle and alter patterns of muscle activation9. This can lead to increased energy expenditure as well as increased torque on other joints, contributing to functional limitations in these activities10. Rising from a chair11, community mobility, and ascending stairs are frequently limited with symptomatic knee OA and these limitations are associated with physical dependence12 and earlier death.13
In our prior study, we discovered differences in mechanical energy and power (the rate of energy use) in older adults with symptomatic knee OA with higher in comparison with those with lower levels of physical function9. These findings beg the question regarding the extent to which targeted rehabilitation interventions can transition lower functioning older adults to a higher functioning status through targeting the characteristic motion strategies previously identified. To assess this question most effectively, a specific training program would be ideal.
The principle of specificity of training indicates that exercises that closely approximate the goal functional activity are most effective in improving physical performance during that activity. Gait training is most specific for improving gait and evidence suggests that gait analysis in older adults with knee OA can identify specific changes in mechanical energy14, and differentiate people with symptomatic knee OA with higher vs. lower mobility levels9. Therefore, rehabilitation that targets kinetic chain compensations may be most effective in reducing functional limitations through augmenting adaptive and reducing maladaptive compensations. Computerized motion analysis enables assessment of compensatory patterns that may not otherwise be detected, providing a potential means of attenuating functional limitations.
The purpose of this study was to assess the extent to which intensive gait training, informed by computerized motion analysis, can reduce mobility disability, impairments, and functional limitations in older adults with symptomatic knee OA. We hypothesized that, in comparison with usual care (control group), a 3-month participant-specific gait training intervention would improve mobility disability (Basic Lower Limb Function score on the Late Life Function and Disability Index: LLFDI) and that this benefit would be maintained at 6- and 12-month follow-up. Our secondary hypotheses were that, in comparison to baseline measures, at 3-, 6-, and 12-month follow-up, participants in the gait-training intervention would demonstrate improvements in a) knee pain and symptoms (KOOS), and b) functional limitations (timed 400m walk, chair rise and stair climb tests).
PARTICIPANTS AND METHODS
Participants
Men and women age 60 years or older with symptomatic knee OA9, 15 (defined by a definite osteophyte or joint space narrowing in either tibiofemoral compartment on posteroanterior knee radiographs16 and an affirmative response to “Have you had pain or stiffness in one or both knees on most of the past 30 days” on both the telephone screen and screening visit15, 17) and mobility disability (LLFDI advanced lower limb function score below 32 points) were recruited at the University of Iowa. This threshold for functional limitations was used due to its correlation with a Short Physical Performance Battery Score of ≤ 9, a cut-off for ADL disability in community-dwelling older adults18. All participants were able to walk without an assistive device and ascend at least two stairs. Given that women over age 60 have 1.35-fold risk for knee OA as men,19 and women make up 55% of the population over age 60 years in developed nations,20 the aim was to recruit approximately 65% women to include representative proportions by sex.
The primary recruitment strategy was targeted mailings to patients with ICD-9 codes relevant to knee OA (715.96, 715.16, 715.36), while excluding those with a code indicating lower limb surgery in the past 6 months. Orthopaedics, rheumatology and internal medicine clinics within a 40-mile radius also were targeted with mailings and fliers. In addition, study notices were posted in local senior centers and assisted living centers. Prior to enrollment of each participant, the principal investigator confirmed the presence of symptomatic knee OA, as defined above.
Potential participants were screened by phone, including the LLFDI as well as assessment of the inclusion and exclusion criteria. Conditions other than knee OA, which could affect walking, were exclusionary (e.g. amputation, severe back pain, severe peripheral vascular or heart disease and neurological or developmental disease including multiple sclerosis, Parkinson's disease, myositis, rickets, or lower limb musculoskeletal surgery in the past 6 months). In addition, participants who had undergone corticosteroid injection into either a peripheral joint or into the spine in the past 3 months (which could threaten internal validity of assessing the independent effect of the intervention) or who anticipated inability to return for follow-up were excluded. Other prospective exclusion criteria that no volunteers met were: medical conditions that may preclude safe participation in the study protocol, including but not limited to acute or terminal illness or unstable cardiovascular condition (e.g. New York Heart Association Class III or IV congestive heart failure, clinically significant aortic stenosis, history of cardiac arrest, use of a cardiac defibrillator, uncontrolled angina); report of medical conditions that may impair ability to participate including but not limited to pulmonary disease requiring the use of supplemental oxygen; inability or unwillingness to comply with the study protocol or be randomized; inability to obtain written clearance for participation in the study by a physician; concurrent participation in another observational or interventional research study; current consumption of more than 14 alcoholic drinks per week; judgment of the principal investigator that participation would endanger the safety of an individual.
This study was registered at clinicaltrials.gov (NCT00844558). All participants completed an IRB-approved consent process, culminating in providing written consent. On the screening Long Distance Corridor Walk (LDCW) test, no participants developed chest pain, severe shortness of breath, sustained heart rate >135 BPM or <40 BPM and all participants provided a written release to participate in the study from their physicians.
After completion of baseline outcome measures, participants who qualified were 2:1 randomized to either the gait training or the control group (randomization.com). Sequentially-numbered, opaque, sealed envelopes that contained the assignment for each study participant were prepared by a research assistant not involved with the study measurements or interventions.
OUTCOME MEASURES
Outcomes were measured at baseline and 3, 6, and 12 months later. Participants first completed questionnaires and then completed physical performance tests in the same order at each visit. Specifically, questionnaires were followed by the timed walk, chair rise, and stair climb tests.
Mobility Disability
The LLFDI Basic Lower Limb Function score (Primary Outcome) was included in addition to the performance-based outcome measures to allow the results to be compared with other studies of mobility in older adults21, 22, and also due to the relevance of self-report of mobility disability in addition to measurement of functional limitations by physical performance testing18, 23. Study staff administered the LLFDI,24 a 32-item questionnaire on functional limitations in performing activities, which was created to fit within the same disablement model used in the design of this study.
Functional Limitations
Performance tests provide measurements of functional limitations18, which interact with people's environment and expectations to contribute to disability18. Results of performance tests have been indicators of falls, nursing home admission and mortality23, 25, 26, and gait speed as a measure of functional limitations has been found to correlate well with self-reported disability23. As self-reported mobility disability and performance measures provide complimentary insights into the mobility status of older adults, both were measured.
The LDCW included both 2-minute walk distance as well as 400m-walk time. This measure has been shown to be predictive of changes in community mobility25. Participants wore a heart rate monitor during the LDCW and there were no episodes of sustained heart rate >135 BPM or <40 BPM or symptoms such as chest pain or shortness of breath. For participants unable to walk 400m, gait speed was estimated from the 2-minute walk distance.
Objective functional limitations were assessed with a timed chair-stand test, measured as the time (in seconds) required to stand from a seated position in a standardized chair five times without using arms23, and found to be reliable in our laboratory27.
Functional limitations specific to ascending stairs were assessed with a timed stair climb, using a standard 8-stair flight (stair height = 19cm). Participants were instructed to ascend the stairs safely as quickly as possible. If necessary for safety, the handrails could be used. The stopwatch was started when the participant initiated foot movement and was stopped when both feet arrived on the top (eighth) step. Times for two trials, attempted on the same day, were averaged and recorded (to the nearest 0.01 second) and left/right handrail use was recorded. The reliability for this test has been reported to be excellent (ICC = 0.97)28.
Knee Pain and Symptoms
The Knee injury and Osteoarthritis Outcome Score (KOOS) is a 42-item self-administered questionnaire that covers five patient-relevant dimensions, including pain and knee-related symptoms. This instrument has been found to be a reliable, and responsive measure in older adults with knee OA, and sensitive to changes in pain and knee-related symptoms over 6- and 12-month periods29.
Participant Characteristics
To confirm adequacy of randomization of factors that may influence mobility or pain, participants completed the Physical Activity Score for the Elderly questionnaire (PASE), a self- administered physical activity questionnaire that covers leisure and work-related activities30, as well as the Center for Epidemiological Studies Depression Scale (CESD) questionnaire31 .
Gait Analysis
Gait biomechanics over a level surface were evaluated using a three-dimensional (3D) motion analysis system (Optotrack, Model 3020, Northern Digital, Inc, Waterloo, Ontario; force plates, Kistler Model 9286, Amherst, NY) at baseline and during the 8th, 16th, and 24th visits. Participants walked along a 10-meter walkway several times at their self-selected walking velocity and at 1.12 m/s. Three non-collinear markers, placed on the pelvis and trunk, and bilaterally on the feet, shanks, and thighs were used to generate rigid body representations of each body segment as previously described9. Gait data were processed using Visual 3D software (C-Motion, Inc., Germantown, MD). The marker data, collected at 60 Hz, and the force plate data, collected at 300 Hz, were filtered with a second order Butterworth low-pass filter with a conventional cut-off frequency of 6 Hz 32. Inverse dynamics was used to calculate the net joint moments in 3D.
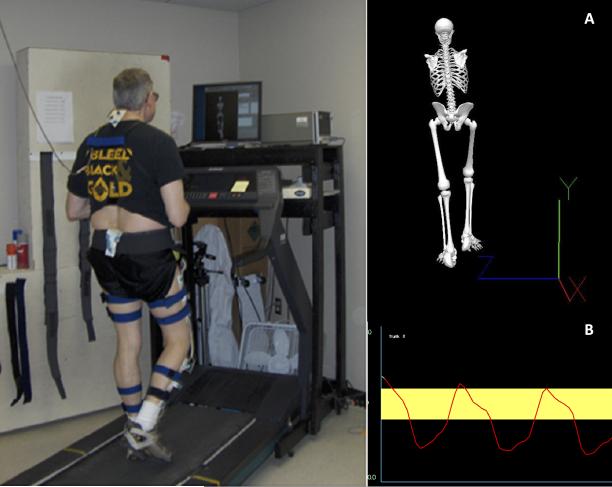
Gait Training Intervention
Participants randomized to the gait-training group attended 24 biweekly 45-minute sessions directed by a physical therapist (PT) that were comprised of guided strategies to optimize knee movements during treadmill walking, using computerized motion analysis with visual biofeedback (Figure 1). This frequency was selected in consideration of skeletal muscle recovery from exercise and evidence that biweekly is an appropriate exercise frequency for older adults33. Two physical therapists were involved with the study from initiation to completion and each worked with the same participants throughout the entirety of the study. In addition to the supervised gait training, based on evaluation of strength, flexibility, trunk and lower limb range of motion and gait at the first gait-training visit, a physical therapist instructed participants in individualized home programs. Following the initial 3-month intervention, participants were encouraged to continue the intervention at home through scripted telephone-based motivational interviewing and a tracking component.
Figure 1.
Participant-specific biofeedback during instrumented treadmill gait training. Example of real-time biofeedback provided during gait training for correction of kinematic patterns. Visual 3D was used to provide feedback to participants either as a (A) skeleton model which introduced gross concepts of body movements or (B) target area which was used for more specific feedback.
For the supervised training, information from the initial gait assessments and physical evaluations of strength and ROM) were reviewed. Given the bias towards high external knee adduction moment (EKAM) (based on our normative database, 0.35±0.15 Nm/kg), kinematic and spatial measures were assessed for asymmetries and abnormal magnitudes that in previous work were thought to affect knee frontal plane kinetics34-37. The assumption that defined the training goals was that the EKAM, in individuals without knee OA, is achieved via good alignment and control of the pelvis (i.e. reduce pelvic drop) and reduction in truncal lean or rotation. Therefore, the major goals in retraining gait were to move participants toward symmetrical and typical displacements of the trunk and pelvis about neutral frontal (X) and transverse (Y) axes. Correcting motion deviations about the frontal plane was prioritized in setting goals for the subjects. While the majority of training time and primary focus for all subjects was trunk and pelvic kinematics, additional feedback, regarding secondary concerns— width of base of support and knee hyperextension during walking— was provided to those participants with identified abnormalities in those parameters. During training the physical therapist and participant monitored the reduction of the EKAM and pelvic control and the physical therapist confirmed that moments at other joints (e.g. the hips and the contralateral knee) were not negatively affected.
Measurable outcomes, including range of motion measures and progress in ability to complete sets of repetitions with an elastic cord, were used to monitor the effectiveness of the home exercise component. These programs were progressed with the goal of enabling participants to develop gait patterns more characteristic of higher functioning older adults with knee OA9.
The kinematic and kinetic information in combination with clinical assessments of range of motion (ROM) and strength were used to establish participant-specific goals for gait training. The same marker system and model generation was used to generate anatomical models of the participants during treadmill walking (Gaitway, h/p/cosmos sports & medical gmbh, Nussdorf-Traunstein, Germany). Treatment involved providing visual feedback for correction of motor performance. The initial gait training intervention was governed by the participant's experience walking on a treadmill and by their conditioning. The a priori training goal was for all participants, by the end of the second week, to be capable of walking on the instrumented treadmill at a self-selected speed for three 8-minute intervals, with 3- to 5-minute rest periods. Due to moderate to severe mobility limitations, this was not achieved for some participants.
To correct kinematic patterns based on participant-specific goals, during gait training, participants were provided with intermittent real-time biofeedback on a computer screen placed on a table (150 cm in height) about 1m in front of them. This feedback consisted of a skeletal image or specific kinematic measures represented in a line tracing over several gait cycles with a target area representing a neutral position (Figure 1). The real-time feedback enabled participants to visualize body movements and make postural adjustments during treadmill walking. Feedback was also provided during rest periods to reinforce corrected patterns.
Following the 3-month intervention, participants were encouraged by phone to continue the training on their own. Researchers contacted participants in the gait-training group by telephone at 4, 5, 8, and 10 months, using a scripted motivational interview regarding participants’ knee OA and the walking program. In addition, participants returned for level surface gait analysis at 3, 6, and 12 months. The same modeling approach that was used during the initial level gait evaluation was used at the follow-up gait evaluations.
Osteoarthritis Self-Care (Control Group)
Participants in the control group received the usual care for symptomatic knee OA through their usual health care providers and were not asked to make changes to their lifestyle. Usual care for these subjects may have included a yearly visit with their physician, use of pain medications for knee symptoms, knee surgery, and/or physical therapy. To provide a similar frequency of study contact as was provided to the gait-training participants, the control participants were given an Arthritis Foundation symptom diary and instructed to record twice each week for the first 3 months (Sunday and Wednesday) and once a week (Sunday) for the following 9 months: their knee symptoms, healthcare appointments related to their knee OA, or any changes in the way in which they treated their knee OA. Researchers contacted the control participants by telephone at 1, 2, 4, 5, 8, and 10 months in addition to meeting with them at 3, 6, and 12 months for collection of outcome measures. The scripted telephone contacts and study visits ensured that participants in both of the groups received a similar degree of attention throughout the trial.
Statistical Analyses
The study design was a two-group randomized controlled trial (RCT) with pairwise comparisons between the groups at baseline, 3, 6, and 12 months. Longitudinal mixed models for repeated measures were constructed to test the hypotheses. For the primary outcome measure, we tested whether the estimate of the difference in mean LLFDI Basic Lower Limb Function score for the gait-training group from baseline to 3 months was significantly higher than the estimate of the difference for the control group over the same time period. Each model was based on three main effects, a fixed group effect, a random participant effect, and a fixed time effect consisting of four levels (baseline, 3, 6, and 12 months). We also included an interaction effect for group and time, to determine whether temporal changes differed between groups. All analyses were conducted using SAS (Version 9.1.2, SAS Institute, Inc., Cary, NC).
A prospective sample size was estimated, using a one-way ANOVA with three factor levels for the three groups, where the standard deviation for LLFDI Basic Lower Limb Function score was set to 7.44, based on communication with Suzanne C. Olarsch at Boston University on 2/19/08, as the SD for change in the LLFDI in similar populations had not been published prior to conduct of our study. To detect a difference in LLFDI Basic Lower Limb Function score of 5, with 70% power (for a two-sided test with alpha = 0.05), a minimum sample size of 28 is required. Due to slow recruitment of older adults with symptomatic knee OA and moderate-to-severe mobility limitations in a pilot study, a need to maximize statistical power, and desire to best characterize the gait training intervention, participants were 2:1 randomized to gait training and control groups.
RESULTS
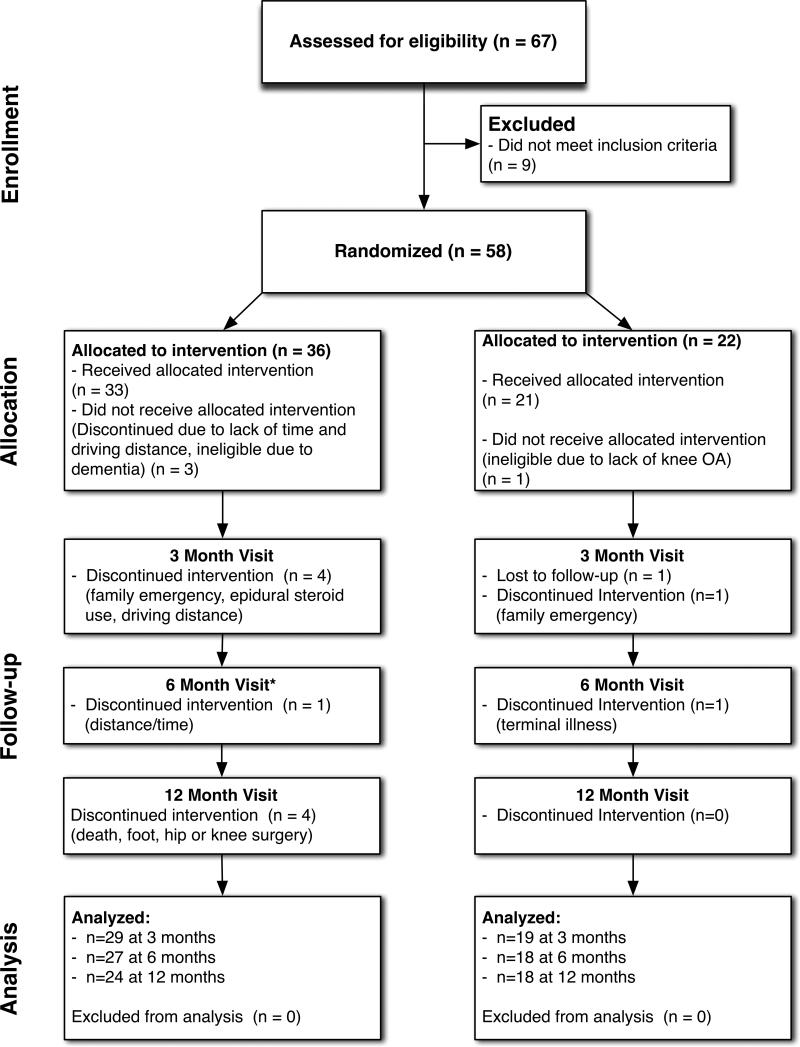
A total of 18 men and 38 women enrolled in this study and were randomized to the gait training intervention (n=35; 26 women) or control group (n=21; 12 women) and timing and reasons for dropouts are detailed in Figure 2. At baseline, there were no significant differences between groups of participants who completed at least one follow-up visit (Table 1). Baseline visits began on September 7, 2008. The last 6-month visit took place March 18, 2010 and the last 12-month visit took place September 29, 2010.
Figure 2.
Participant Inclusion Diagram *1 missed visit due to minor surgery
TABLE 1.
Baseline Characteristics of Participants with Any Follow-up
| Variable | Gait Group N=29 | Control Group N=19 | P-Value |
|---|---|---|---|
| Age (years) | 69.1±7.3 | 69.6±6.4 | 0.8110 |
| Sex (number women, %) | 22/29 (75.9 %) | 10/19 (52.6 %) | 0.0950 |
| CESD score | 5.5±3.9 | 7.3±4.4 | 0.2296 |
| PASE score | 136.1±103.3 | 136.9±80.1 | 0.9779 |
| *OA Severity: | |||
| KL=2 | 5 (17.9%) | 5 (26.3%) | |
| KL=3 | 13 (46.4%) | 9 (47.4%) | 0.7061 |
| KL=4 | 10 (35.7%) | 5 (26.3%) | |
| LLFDI Basic Lower Limb Function score | 65.8 ± 9.2 | 63.5 ± 6.1 | 0.3505 |
| KOOS Pain | 62.7 ± 10.8 | 59.8 ± 13.1 | 0.4073 |
| KOOS Symptoms | 60.1 ± 16.8 | 63.0 ± 13.6 | 0.5380 |
| LDCW (sec) | 371.9 ± 78.5 | 381.3 ± 140.6 | 0.7928 |
| Stair-Climb (sec) | 5.9 ± 2.8 | 6.9 ± 5.0 | 0.3960 |
| Chair Stand (sec) | 15.1 ± 3.8 | 14.1 ± 3.1 | 0.4495 |
one gait participant with missing Kellgren-Lawrence (KL) grade information
Recruitment was successful in selecting a group of participants with moderate to severe mobility limitations. Due to this, only 10 participants were able to tolerate treadmill training set durations in excess of 7 minutes. For 7 of the participants, average treadmill speed during training sessions was less than 0.7 meters per second. In addition, 20 of the 29 participants used bilateral handrail contact for safety when walking on the treadmill.
For the main analyses, estimates and 95% confidence intervals for the change in outcomes between the baseline visit and the 3-, 6-, and 12-month visits, comparing the gait training and control group participants, are presented in Table 2. LLFDI Basic lower limb function score (effect size 0.7) and KOOS symptoms (effect size 0.6) improved in the gait training versus control group at 3 months, but these differences were not sustained at either 6- or 12-month follow-up.
Table 2.
Inter-Group Comparison in Mean Change in Outcome Measures With Respect to Baseline {95% CI for Mean Change}
| Variable | Month 3 | Month 6 | Month 12 | |||
|---|---|---|---|---|---|---|
| Estimate | p-value | Estimate | p-value | Estimate | p-value | |
| LLFDI Basic Lower Limb Function score | 4.3 {0.8, 7.8} | 0.0161** | 2.3 {−1.8, 6.3} | 0.2649 | 1.0 {−7.4, 9.4} | 0.8091 |
| KOOS Pain | 7.0 {−1.5, 15.6} | 0.1036 | 3.7 {−4.7, 12.1} | 0.3840 | 7.2 {−2.0, 16.5} | 0.1202 |
| KOOS Symptoms | 8.6 {0.3, 16.8} | 0.0420** | 6.2 {−2.9, 15.4} | 0.1754 | 6.0 {−6.2, 18.2} | 0.3269 |
| LDCW time (sec.) | 0.7 {−28.9, 30.2} | 0.9644 | 5.4 {−38.5, 49.4} | 0.8053 | 7.6 {−32.0, 47.1} | 0.7023 |
| Stair Climb Time (sec.) | 0.2 {−0.9, 1.3} | 0.6641 | 1.3 {−0.6, 3.2} | 0.1765 | 0.8 {−0.7, 2.4} | 0.2892 |
| Chair Stand Time (sec.) | 0.1 {−2.1, 2.3} | 0.9111 | 1.1 {−1.5, 3.7} | 0.4104 | 0.5 {−1.5, 2.5} | 0.6435 |
P-values are for change in the gait training versus control group, comparing follow-up visit measures to baseline and controlling for repeated measures.
Intra-group analyses (Table 3) revealed improvement in the LLFDI Basic Lower Limb Function scores in the Gait group at 3 and 6 months. In the Gait group, there also were improvements in knee pain and knee symptoms at 3, 6, and 12 months. However, in the control group, there were no significant changes in the LLFDI Basic Lower Limb Function score, knee pain or knee symptoms at any time point.
Table 3.
Intra-Group Comparison in Mean Change in Outcome Measures With Respect to Baseline {95% CI for Mean Change}
| Variable | Month 3 | Month 6 | Month 12 | |||
|---|---|---|---|---|---|---|
| Gait | Control | Gait | Control | Gait | Control | |
| LLFDI Basic Lower Limb Function score | 3.3 {1.1, 5.5} p=0.0040 | −1.0 {−3.7, 1.7} p=0.4480 | 3.1 {0.5, 5.7} p=0.0183 | 0.8 {−2.3, 4.0} p=0.5963 | 3.9 {−1.3, 9.1} p=0.1344 | 2.9 {−3.7, 9.5} p=0.3798 |
| KOOS Pain | 8.2 {2.8, 13.5} p=0.0037 | 1.1 {−5.5, 7.7} p=0.7384 | 5.4 {0.1, 10.7} p=0.0464 | 1.7 {−4.8, 8.2} p=0.5960 | 10.1 {4.4, 15.8} p=0.0009 | 2.8 {−4.4, 10.1} p=0.4330 |
| KOOS Symptoms | 11.5 {6.3, 16.7} p<0.0001 | 3.0 {−3.4, 9.4} p=0.3551 | 8.1 {2.3, 13.8} p=0.0072 | 1.8 {−5.3, 8.9} p=0.6075 | 8.5 {0.9, 16.0} p=0.0298 | 2.4 {−7.1, 12.0} p=0.6082 |
| LDCW time (sec.) | −10.1 {−28.7, 8.5} p=0.2790 | −10.8 {−33.7, 12.2} p=0.3499 | −16.4 {−44.1, 11.4} p=0.2412 | −21.8 {−55.8, 12.3} p=0.2047 | −9.6 {−34.5, 15.2} p=0.4395 | −17.2 {−47.9, 13.5} p=0.2656 |
| Stair Climb Time (sec.) | 0.2 {−0.5, 0.9} p=0.5729 | 0.0 {−0.9, 0.8} p=0.9208 | −0.2 {−1.3, 1.0} p=0.7910 | −1.4 {−2.9, 0.0} p=0.0528 | −0.3 {−1.3, 0.7} p=0.5572 | −1.1 {−2.4, 0.1} p=0.0683 |
| Chair Stand Time (sec.) | −0.9 {−2.2, 0.4} p=0.1832 | −1.0 {−2.8, 0.8} p=0.2693 | −1.9 {−3.5, −0.3} p=0.0233 | −3.0 {−5.0, −0.9} p=0.0061 | −2.5 {−3.7, −1.3} p=0.0002 | −2.9 {−4.5, −1.3} p=0.0006} |
*P-values are for change within each group, comparing follow-up visit measures to baseline and controlling for repeated measures.
For the walk and stair climb times, there were no significant differences detected in either group at any time point. For the chair stand time, there were no significant changes at 3 months in either group, but there were improvements at 6 and 12 months in both the Gait and the Control groups.
For participants in the Gait training group, who were still enrolled at each time point, compliance with gait training instructions following completion of the 3-month intervention, was reported by 87.5% at Month 4, 91.7% at Month 5, 94.2% at Month 8, 94.3% at Month 10, and 94.1% at Month 12. Structured monthly assessments, reviewed by the data safety monitoring board, detected no adverse events in either group.
DISCUSSION
In this RCT, 3 months of biweekly, intensive PT-directed gait training with continuous lower limb kinematic biofeedback significantly improved mobility disability and knee symptoms in adults with symptomatic knee OA and moderately severe mobility limitations. However, improvements in mobility limitations when walking, standing from a chair and ascending stairs were not significant in comparison with usual care. Furthermore, the improvements in mobility, knee pain and knee symptoms in the Gait training group were not significant in comparison with the Control group at 6- or 12-month follow-up, indicating a lack of sustained benefit despite monthly motivational interviewing by telephone.
The goal of our intervention was to improve lower limb mobility, based on observations that, during walking, altered gait mechanics may contribute to worsening of symptoms and physical function in people with knee OA9, 38, 39. According to a disablement model for knee OA, impairments such as pain and muscle weakness, mediate the effects of pathology on development of functional limitations, such as reduced community mobility40. Reduced activity can lead to further weakness or loss of range of motion, thereby altering walking mechanics and contributing to further joint pathology. Based on this model, interventions should be designed to reduce functional limitations as well as impairments, with the goal of interrupting this cycle and reducing risk for disability. As hypothesized, gait training reduced mobility disability (e.g. stair ascent and vehicle transfers) and improved knee pain. However, at greater than 3-month follow-up, Gait training participants did not significantly differ in outcome measures in comparison with those in the Control group. Furthermore, walk and stair climb times did not change in either group.
Despite endeavoring to maximize the effect size and duration of effect through maximizing specificity of training and basing the training on previously identified parameters in a similar population, our results did not differ substantially from those of non-specific exercise training. As summarized in three recent meta-analyses, a wide variety of exercise interventions improve pain and function in individuals with knee OA41-43. Fransen and McConnell found moderate effect sizes in 32 studies of knee OA exercise programs—standardized mean difference (SMD) of 0.40 for improved pain and 0.37 for improved function43. Tanaka et al found larger effect sizes for pain reduction, ranging from -1.42 for non-weight-bearing strengthening, to -0.70 for weight-bearing strengthening and only −0.45 for aerobic exercise in RCT that involved training the equivalent of 3 times per week for 8 weeks (n=8)42.
When studies with a broader range of training frequencies were included, Wang et al found smaller effect sizes for improved pain with aerobic exercise (SMD=−0.21) and improved pain (SMD=−0.68) and function (SMD=−1.00) with strengthening exercise in a review of 84 RCT of physical therapy interventions for knee OA41, and characterized the results as having low strength of evidence (due to risk of bias and lower precision of estimated treatment effects). Thus, effect sizes for improving functional limitations appear to be moderate at best. In fact, Fransen and McConnell estimated the result of short-term supervised exercise programs for knee OA may lead to a reduction of only 1 point on the WOMAC pain index (scale 0-20) and 3 points on the WOMAC function index (scale of 0-68)43.
Several lines of evidence also indicate this low effect size may be because exercise interventions that improve impairments, such as lower limb weakness, may not necessarily reduce functional limitations44-46 and therefore task specificity or training for a specific functional activities may be more appropriate for older adults with knee OA. In a RCT comparing functional-task exercise with resistance exercise and a control group in women over age 70 without mobility limitations, De Vreede et al found that performance improved significantly more in the functional-task exercise group, despite significant knee extensor and elbow flexor strength gains in the resistance exercise group44. For this reason, Teixeira et al recommended function-specific interventions for people with knee OA, rather than focusing on correction of impairments, which may not be sufficient to improve physical performance46. Thus, these findings may explain, in part, why the treadmill gait-training intervention with kinematic foci did not improve performance on timed mobility tests.
We found a reduction in mobility disability and knee symptoms at 3 months, but no statistically significant improvement in mobility performance in comparison with usual care. These findings are in line with previous studies of gait interventions. Indeed, older adults with knee OA who participated in walking training have improved their walking speed and activity level, but not other functional limitations, such as chair stand or stair ascent, or impairments such as pain or muscular weakness47. Though participants in our gait-training intervention did not improve their walking times, this could be related to not focusing on increasing gait speed in their training. Thus, this particular intervention would be unlikely to improve gait speed, despite a short-term improvement in knee pain and symptoms. The frequency (twice per week) and duration (3 months) of the intervention is unlikely to account for the absence of positive findings. Prior studies provide evidence that this was a sufficient frequency and duration for improvement. For example, Shull et al evaluated 6 weeks of once weekly gait training and found significant improvements in pain and function upon completion and at 1-month follow-up48.
Despite being a well-controlled study over a clinically useful duration of for assessment of outcomes, there were several potential limitations. Our study included individuals with moderately severe mobility disability. However, individuals varied in knee OA radiographic severity and it is possible that those with more severe or long-standing mobility limitations may have been less likely to demonstrate improvement in mobility. Furthermore, participants with LLFDI Basic Lower Limb Function scores below a threshold for disability were recruited in order to target a population with greatest need for an intervention to improve mobility. However, this group may have been too severely limited to maximally benefit from the intensive gait-training intervention. Recruitment also resulted in a greater percentage of women than anticipated. Given that women over age 60 have 1.35-fold risk for knee OA as men,19 and women make up 55% of the population over age 60 years in developed nations,20 our aim was to recruit approximately 63% women to include representative proportions by sex. However, due to the multiple inclusion criteria, a higher proportion was recruited (70.4%). It is unclear whether this could have an impact on the findings or the direction of such an impact as contrary effects (e.g. women having lower physical function, but being more compliant with the intervention) or unmeasured factors could account for an effect or lack of effect on the results.
Statistical power also was an important limitation in that 28 participants were required, but a greater number of dropouts than anticipated (7 by Month 6 and 11 by Month 12), may have led to inability to detect statistically significant inter-group differences at these later time points. An important finding was that, despite the improvement in self-reported function at the end of the 3-month gait-training intervention, results were not sustained. This suggests that the motivational interviewing by telephone may have been insufficient and that booster sessions may be necessary to sustain improvement.
This study focused on the effects of a highly resource-intensive gait-training program on impairments and functional limitations. Further analyses should assess whether such a program may result in biomechanical changes in gait that could protect against structural or symptomatic progression of knee OA.
CONCLUSIONS
In comparison with usual care, 3 months of individualized physical therapist-supervised gait training reduced self-reported mobility limitations in older adults with symptomatic knee OA immediately, but a prolonged effect was not detected at 6 or 12 months.
Supplementary Material
Acknowledgments
This research was supported by a Paul B. Beeson Career Development Award in Aging Research (K23AG030945). Investigators retained full independence in the conduct of this research. There was no commercial support for this research and the authors have no interests that could create a potential or apparent conflict of interest with regard to this work. A portion of the research was presented at the American Geriatrics Society 2010 Annual Scientific Meeting (Orlando, FL), May 12–15, 2010.
Footnotes
Supplemental DigitalContent: CONSORT Checklist
References
- 1.Nevitt MC, Lane N. Body weight and osteoarthritis. Am J Med. 1999;107(6):632–3. doi: 10.1016/s0002-9343(99)00297-1. [DOI] [PubMed] [Google Scholar]
- 2.March LM, Bachmeier CJ. Economics of osteoarthritis: a global perspective. Baillieres Clin Rheumatol. 1997;11(4):817–34. doi: 10.1016/s0950-3579(97)80011-8. [DOI] [PubMed] [Google Scholar]
- 3.Davis MA. Epidemiology of osteoarthritis. Clin Geriatr Med. 1988;4(2):241–55. [PubMed] [Google Scholar]
- 4.Guccione AA. Arthritis and the process of disablement. Phys Ther. 1994;74(5):408–14. doi: 10.1093/ptj/74.5.408. [DOI] [PubMed] [Google Scholar]
- 5.Kramer JS, Yelin EH, Epstein WV. Social and economic impacts of four musculoskeletal conditions. A study using national community-based data. Arthritis Rheum. 1983;26(7):901–7. doi: 10.1002/art.1780260712. [DOI] [PubMed] [Google Scholar]
- 6.Losina E, Weinstein AM, Reichmann WM, Burbine SA, Solomon DH, Daigle ME, et al. Lifetime Risk and Age at Diagnosis of Symptomatic Knee Osteoarthritis in the US. Arthritis Care Res (Hoboken) 2013;65(5):703–11. doi: 10.1002/acr.21898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59(9):1207–13. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226–9. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 9.Segal NA, Yack HJ, Brubaker M, Torner JC, Wallace R. Association of dynamic joint power with functional limitations in older adults with symptomatic knee osteoarthritis. Arch Phys Med Rehabil. 2009;90(11):1821–8. doi: 10.1016/j.apmr.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGibbon CA, Puniello MS, Krebs DE. Mechanical energy transfer during gait in relation to strength impairment and pathology in elderly women. Clin Biomech (Bristol, Avon) 2001;16(4):324–33. doi: 10.1016/s0268-0033(01)00004-3. [DOI] [PubMed] [Google Scholar]
- 11.Pai YC, Chang HJ, Chang RW, Sinacore JM, Lewis JL. Alteration in multijoint dynamics in patients with bilateral knee osteoarthritis. Arthritis Rheum. 1994;37(9):1297–304. doi: 10.1002/art.1780370905. [DOI] [PubMed] [Google Scholar]
- 12.Ettinger WH, Jr., Afable RF. Physical disability from knee osteoarthritis: the role of exercise as an intervention. Med Sci Sports Exerc. 1994;26(12):1435–40. [PubMed] [Google Scholar]
- 13.Hirvensalo M, Rantanen T, Heikkinen E. Mobility difficulties and physical activity as predictors of mortality and loss of independence in the community-living older population. J Am Geriatr Soc. 2000;48(5):493–8. doi: 10.1111/j.1532-5415.2000.tb04994.x. [DOI] [PubMed] [Google Scholar]
- 14.McGibbon CA, Krebs DE, Scarborough DM. Rehabilitation effects on compensatory gait mechanics in people with arthritis and strength impairment. Arthritis Rheum. 2003;49(2):248–54. doi: 10.1002/art.11005. [DOI] [PubMed] [Google Scholar]
- 15.Segal NA, Torner JC, Felson DT, Niu J, Sharma L, Lewis CE, et al. Knee extensor strength does not protect against incident knee symptoms at 30 months in the multicenter knee osteoarthritis (MOST) cohort. PM R. 2009;1(5):459–65. doi: 10.1016/j.pmrj.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segal NA, Nevitt MC, Gross KD, Hietpas J, Glass NA, Lewis CE, et al. The Multicenter Osteoarthritis Study: opportunities for rehabilitation research. PM R. 2013;5(8):647–54. doi: 10.1016/j.pmrj.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–31. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 19.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006;33(11):2271–9. [PubMed] [Google Scholar]
- 20.United Nations. Department of Economic and Social Affairs . Population Division. World population ageing, 1950-2050. United Nations; New York: 2002. [Google Scholar]
- 21.Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Jr., Walston JD. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52(4):625–34. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 22.Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol A Biol Sci Med Sci. 2003;58(8):728–33. doi: 10.1093/gerona/58.8.m728. [DOI] [PubMed] [Google Scholar]
- 23.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 24.Haley SM, Jette AM, Coster WJ, Kooyoomjian JT, Levenson S, Heeren T, et al. Late Life Function and Disability Instrument: II. Development and evaluation of the function component. J Gerontol A Biol Sci Med Sci. 2002;57(4):M217–22. doi: 10.1093/gerona/57.4.m217. [DOI] [PubMed] [Google Scholar]
- 25.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. Jama. 2006;295(17):2018–26. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 26.Reuben DB, Seeman TE, Keeler E, Hayes RP, Bowman L, Sewall A, et al. Refining the categorization of physical functional status: the added value of combining self-reported and performance-based measures. J Gerontol A Biol Sci Med Sci. 2004;59(10):1056–61. doi: 10.1093/gerona/59.10.m1056. [DOI] [PubMed] [Google Scholar]
- 27.Segal NA, Boyer ER, Wallace R, Torner JC, Yack HJ. Association between chair stand strategy and mobility limitations in older adults with symptomatic knee osteoarthritis. Arch Phys Med Rehabil. 2013;94(2):375–83. doi: 10.1016/j.apmr.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuoco A, Callahan DM, Sayers S, Frontera WR, Bean J, Fielding RA. Impact of muscle power and force on gait speed in disabled older men and women. J Gerontol A Biol Sci Med Sci. 2004;59(11):1200–6. doi: 10.1093/gerona/59.11.1200. [DOI] [PubMed] [Google Scholar]
- 29.Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS) - validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1(1):17. doi: 10.1186/1477-7525-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52(7):643–51. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 31.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 32.Winter DA. Biomechanics and motor control of human movement. 3rd ed. Wiley; Hoboken, N.J.: 2005. [Google Scholar]
- 33.Taaffe DR. Sarcopenia--exercise as a treatment strategy. Australian family physician. 2006;35(3):130–4. [PubMed] [Google Scholar]
- 34.Fregly BJ, Reinbolt JA, Rooney KL, Mitchell KH, Chmielewski TL. Design of patient539 specific gait modifications for knee osteoarthritis rehabilitation. IEEE Trans Biomed Eng. 2007;54(9):1687–95. doi: 10.1109/TBME.2007.891934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunt MA, Bennell KL. Predicting dynamic knee joint load with clinical measures in people with medial knee osteoarthritis. Knee. 2011;18(4):231–4. doi: 10.1016/j.knee.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Huang SC, Wei IP, Chien HL, Wang TM, Liu YH, Chen HL, et al. Effects of severity of degeneration on gait patterns in patients with medial knee osteoarthritis. Med Eng Phys. 2008;30(8):997–1003. doi: 10.1016/j.medengphy.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Jackson BD, Wluka AE, Teichtahl AJ, Morris ME, Cicuttini FM. Reviewing knee osteoarthritis--a biomechanical perspective. Journal of Science & Medicine in Sport. 2004;7(3):347–57. doi: 10.1016/s1440-2440(04)80030-6. [DOI] [PubMed] [Google Scholar]
- 38.Fisher NM, White SC, Yack HJ, Smolinski RJ. Pendergast DR: Muscle function and gait in patients with knee osteoarthritis before and after muscle rehabilitation. Disabil Rehabil. 1997;19(2):47–55. doi: 10.3109/09638289709166827. [DOI] [PubMed] [Google Scholar]
- 39.Hunt MA, Wrigley TV, Hinman RS, Bennell KL. Individuals with severe knee osteoarthritis (OA) exhibit altered proximal walking mechanics compared with individuals with less severe OA and those without knee pain. Arthritis Care & Research. 2010;62(10):1426–32. doi: 10.1002/acr.20248. [DOI] [PubMed] [Google Scholar]
- 40.Wang TJ, Chern HL, Chiou YE. A theoretical model for preventing osteoarthritis-related disability. Rehabil Nurs. 2005;30(2):62–7. doi: 10.1002/j.2048-7940.2005.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 41.Wang SY, Olson-Kellogg B, Shamliyan TA, Choi JY, Ramakrishnan R, Kane RL. Physical therapy interventions for knee pain secondary to osteoarthritis: a systematic review. Ann Intern Med. 2012;157(9):632–44. doi: 10.7326/0003-4819-157-9-201211060-00007. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka R, Ozawa J, Kito N, Moriyama H. Efficacy of strengthening or aerobic exercise on pain relief in people with knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Clinical Rehabilitation. 2013;27(12):1059–71. doi: 10.1177/0269215513488898. [DOI] [PubMed] [Google Scholar]
- 43.Fransen M, McConnell S. Exercise for osteoarthritis of the knee. The Cochrane database of systematic reviews. 2008;(4):CD004376. doi: 10.1002/14651858.CD004376.pub2. [DOI] [PubMed] [Google Scholar]
- 44.de Vreede PL, Samson MM, van Meeteren NL, van der Bom JG, Duursma SA, Verhaar HJ. Functional tasks exercise versus resistance exercise to improve daily function in older women: A feasibility study. Arch Phys Med Rehabil. 2004;85(12):1952–61. doi: 10.1016/j.apmr.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Rogind H, Bibow-Nielsen B, Jensen B, Moller HC, Frimodt-Moller H, Bliddal H. The effects of a physical training program on patients with osteoarthritis of the knees. Arch Phys Med Rehabil. 1998;79(11):1421–7. doi: 10.1016/s0003-9993(98)90238-6. [DOI] [PubMed] [Google Scholar]
- 46.Teixeira PE, Piva SR, Fitzgerald GK. Effects of impairment-based exercise on performance of specific self-reported functional tasks in individuals with knee osteoarthritis. Phys Ther. 2011;91(12):1752–65. doi: 10.2522/ptj.20100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Talbot LA, Gaines JM, Huynh TN, Metter EJ. A home-based pedometer-driven walking program to increase physical activity in older adults with osteoarthritis of the knee: a preliminary study. J Am Geriatr Soc. 2003;51(3):387–92. doi: 10.1046/j.1532-5415.2003.51113.x. [DOI] [PubMed] [Google Scholar]
- 48.Shull PB, Silder A, Shultz R, Dragoo JL, Besier TF, Delp SL, et al. Six-week gait retraining program reduces knee adduction moment, reduces pain, and improves function for individuals with medial compartment knee osteoarthritis. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2013;31(7):1020–5. doi: 10.1002/jor.22340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.