Abstract
The translocator protein 18 kD (TSPO) has been the focus of intense research by the biomedical community and the pharmaceutical industry because of its apparent involvement in many disease-related processes. These include steroidogenesis, apoptosis, inflammation, neurological disease and cancer, resulting in the use of TSPO as a biomarker and its potential as a drug target. Despite more than 30 years of study, the precise function of TSPO remains elusive. A recent breakthrough in determining the high-resolution crystal structures of bacterial homologs of mitochondrial TSPO provides new insight into the structural and functional properties at a molecular level and new opportunities for investigating the significance of this ancient and highly conserved protein family. The availability of atomic level structural information from different species also provides a platform for structure-based drug development. Here we briefly review current knowledge regarding TSPO and the implications of the new structures with respect to hypotheses and controversies in the field.
Keywords: Translocator protein 18 kD (TSPO), crystal structure, ligand binding, porphyrin, cholesterol, benzodiazepine drugs, steroid hormones, VDAC, stress response
1. Introduction
Translocator protein 18 kDa (TSPO), previously known as the peripheral benzodiazepine receptor (PBR), has consistently been a research focus across multiple disciplines since its first discovery in 1977 as the peripheral binding site for the anti-anxiety drugs, benzodiazepines [1]. TSPO is located in the contact region of outer and inner membranes of mitochondria in human [2] and there is considerable evidence that TSPO is involved in translocating cholesterol into mitochondria, the first and rate-limiting step of steroidogenesis [3]. Recent studies suggest that TSPO forms part of a large complex that facilitates the import of cholesterol [4,5]; however, the precise mechanism of its involvement remains unclear [6,7]. TSPO has also received considerable attention as an important player in neurological disease, since the discovery that brain produces steroids locally [8–10] and that a human single polymorphism of TSPO is associated with anxiety disorders [11–13]. A role of TSPO in other processes, including inflammation, apoptosis, mitophagy, cancer, transport of porphyrin and stress sensing [10,14–19] has also been extensively documented. But it remains a challenge, and an essential prerequisite for drug development, to establish the precise nature of TSPO’s involvement in these apparently disparate processes.
An ortholog of mammalian TSPO was independently discovered in the gram negative photosynthetic bacterium Rhodobacter, considered one of the closest living relatives to the mitochondrion [20]. In Rhodobacter, TSPO regulates the transition between respiration and photosynthesis in response to changes in oxygen levels and light. Detailed genetic and biochemical analysis indicates that TSPO in Rhodobacter acts as part of a regulatory pathway that senses an altered environment and that involves translocation of porphyrins [21–23]. Notably, the bacterial homolog in Rhodobacter sphaeroides can be functionally replaced by rat TSPO [24], indicating conserved functions of TSPO beyond its ability to bind drug ligands. Our group has developed methodologies to purify TSPO from R. sphaeroides (RsTSPO) in a native state and to characterize its ligand binding properties with various endogenous and drug ligand [25]. Using this well characterized protein, high resolution crystal structures were obtained for RsTSPO wild-type as well as a mutant mimic of the disease-related human single polymorphism, allowing correlation of structure and function in TSPO at the molecular level for the first time [26]. Another crystal structure of TSPO from a gram positive bacterium Bacillus cereus (BcTSPO) [27] and an NMR structure of the mouse TSPO (mTSPO) [28] were also recently reported. The structures show similar overall topology of the monomer, but dramatic differences between the crystallographic and NMR structures, including the ligand binding residues and oligomeric states. These differences and hypotheses regarding ligand binding and functional attributes derived from these structures need further investigation. Fundamental questions remain to be answered, as to whether TSPO is a receptor, a transporter or even an enzyme, and the extent to which it acts alone or with protein partners.
2. The functional unit of TSPO
The oligomeric state of purified RsTSPO in solution was determined to be a dimer by light scattering and mass spectrometry [25]. In fact, no monomer species was observed for RsTSPO in detergent solution. In three different crystal forms of RsTSPO that were obtained in the lipidic cubic phase, the identical dimeric structure was observed [26], strongly suggesting that the minimum structural unit of RsTSPO is a dimer (Figure 1B). The same dimer form gives the best fit for a lower resolution cryo-EM structure [26,29]. But more questions need to be answered: 1) is the dimer observed in the crystal structure the same as the solution dimer and 2) is the dimer also the minimum functional unit? The first question can be addressed by mutagenesis and distance measurements between selected residues by cross-linking or DEER spectroscopy. But regarding function, a conclusive test is much more challenging, due in part to the fact that the functions of TSPO remain to be clearly defined. Although a monomer is seen in the relatively severe conditions needed for refolding and NMR analysis of mouse TSPO [28], the Rhodobacter form does not appear to exist as a monomer in the purified state when sustained by lipids or mild detergents. But it is possible that different oligomeric states have distinct functions, for example, signaling vs. transport, and that evolutionary changes in this ancient protein have favored different types of association. Indeed, the Bacillus version of TSPO, more distantly related to the mitochondrial form than Rhodobacter, adopts both a monomer and a different, less strongly interacting dimer [27].
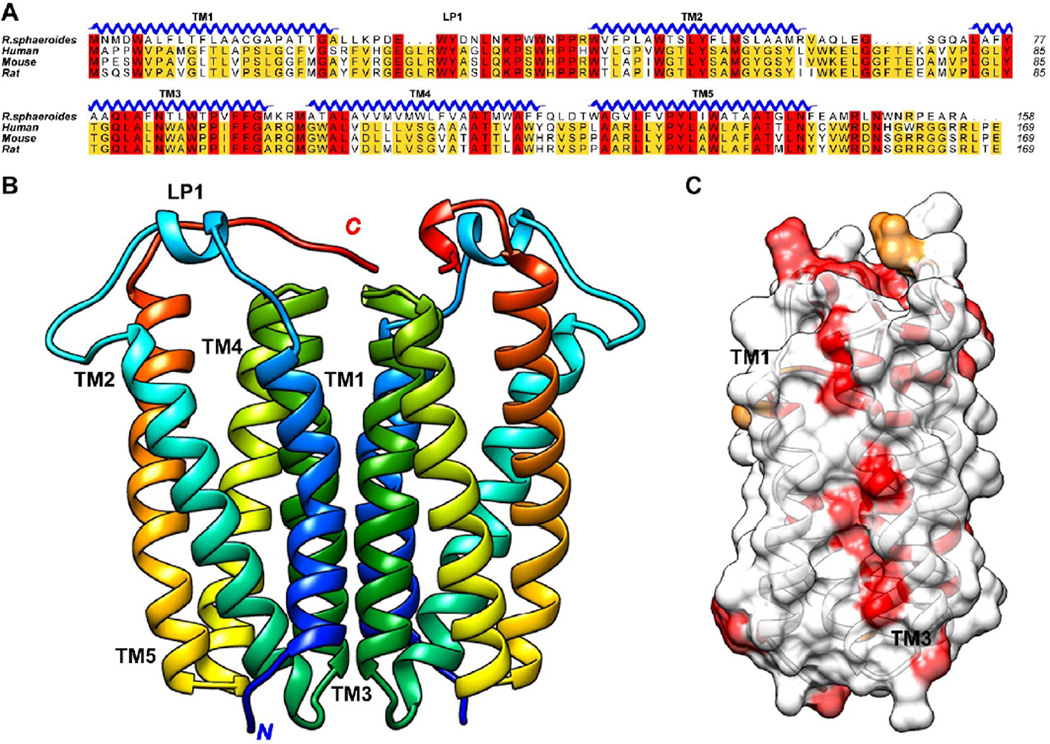
Figure 1. Dimeric structure of RsTSPO and the conservation of the dimer interface.
(A) Sequence alignment showing the most conserved residues in red, less conserved in yellow, unconserved in white. The alignment demonstrates that R. sphaeroides TSPO is closely related to its mammalian homologs. (B) RsTSPO crystal structure shows a dimer composed of two monomers of 5 transmembrane helices (TM), with TM-III (green) contributing most strongly to the interface interaction. (C) Highly conserved surface residues in TM-III at the dimer interface, indicated in red. (Made in Chimera from PDB#: 4UC1)
While RsTSPO appears to form an obligate dimer, mouse TSPO in mitochondrial membranes and reconstituted liposomes was reported to undergo an equilibrium between monomer, dimer, and higher-ordered oligomeric forms [30,31], while the NMR structure of mTSPO shows only a monomeric form with PK11195 bound [28]. Additional studies in native membranes indicate that a 800 kD hetero-oligomeric complex is required for transporting and processing cholesterol, involving TSPO along with VDAC and other components [5]. These results again suggest that different oligomeric states of TSPO may be associated with different functions.
Beyond the question of whether a dimer or higher order oligomer is functionally important, is whether the observed dimer interface is conserved through evolution, i.e. does knowledge of the dimer interface of RsTSPO apply to the human protein. The crystal structure of RsTSPO provides a first clue. As shown in Figure 1A&C, the dimer interface is quite conserved between R. sphaeroides and mammalian proteins, particularly with respect to the residues of TM-III, the critical player in the dimer interface. In the crystal structure of BcTSPO [27], a different dimer is observed which could reflect crystal packing or a separate path during evolution. In general, the dimer appears to be an important conserved species, whether stable as in RsTSPO or perhaps inducible in the case of mouse. But the precise influence of the dimer or oligomer state on the various TSPO interactions and functions, including cholesterol metabolism, remains to be further investigated.
3. TSPO ligand interactions
3.1 Observed drug and porphyrin binding sites
A central cavity surrounded by the five transmembrane (TM) helices and capped by a long loop between TM I and TM II is observed in all structures of TSPO and appears to be an important ligand binding region (Figure 2). An endogenous porphyrin ligand [26] and the drug ligand PK11195 [27] were observed in the high resolution crystal structures, though neither were completely resolved, suggesting incomplete occupancy. In the crystal structure of BcTSPO [27], a PK11195 was fit in the central cavity. It was also identified in the NMR structure in a similar location but with significantly different side chain interactions [28]. One explanation for this discrepancy is that the NMR structure was determined by refolding denatured TSPO in a zwitterionic detergent that could cause some distortion and rotation of the helices. Thus, it is unclear whether the NMR structure of mouse TSPO reveals a native binding mode for PK11195. We presume that the crystal structure is more reliable, due to the good correspondence between the crystal structures from two different species obtained under different experimental conditions [26,27]. There remains a possibility that some of the anomolous features of the mouse NMR structure relate to it being a mammalian TSPO rather than a bacterial homolog, but a number of considerations argue against that [26,27,31].
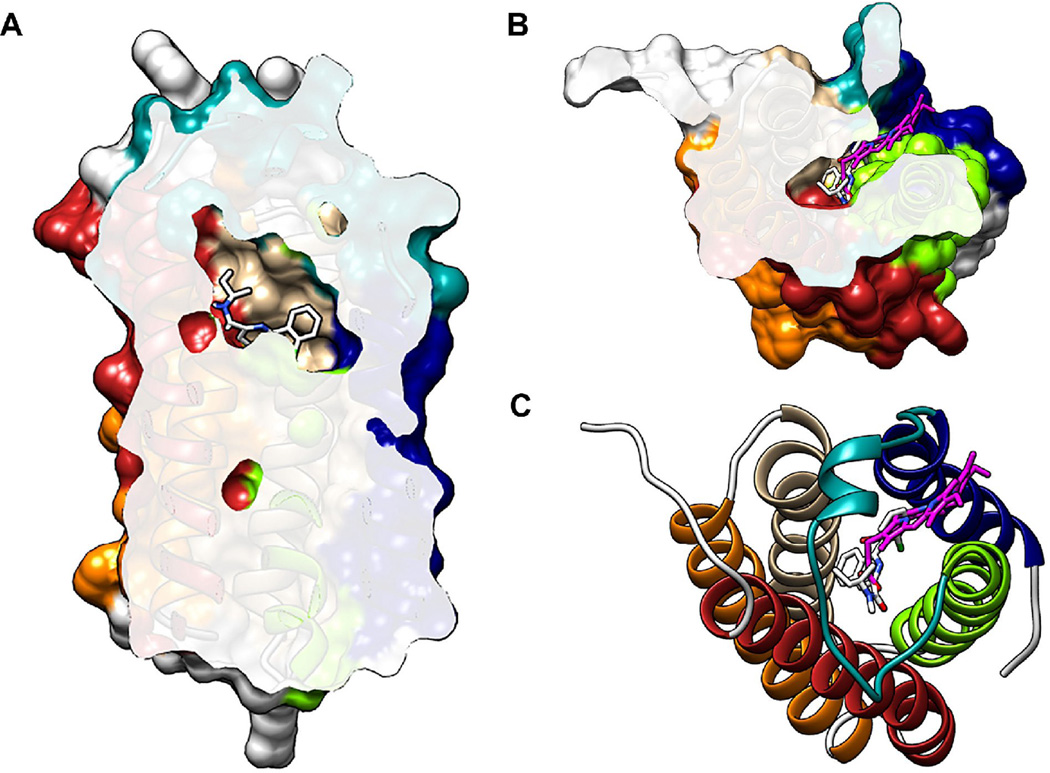
Figure 2. Ligand binding sites in the crystal structure of RsTSPO.
A highly similar central cavity is seen in the crystal structures of both Rhodobacter and Bacillus TSPO with a loop (teal) covering the top. (A) Cutaway of RsTSPO showing the central cavity with PK11195 (white) from BcTSPO modeled in the cavity. (B, C) The porphyrin (magenta) resolved in RsTSPO binds in the same cavity as PK11195, in a partially overlapping site. The protein surface is shown in A and B and a cartoon representation in C. (Figures were made in Chimera from PDB# 4UC1 and PDB# 4RY1.)
The endogenous ligand, porphyrin, is also partially resolved in the central cavity in the crystal structure of RsTSPO, but in a different position than PK11195 (Figure 2B,C). While PK11195 is located right in the middle of the cavity, the porphyrin is shifted toward an opening between TM I and TM II. Previous binding analyses show that PK11195 and porphyrin influence the binding of each other [25], consistent with the crystal structures showing that the binding sites are partially overlapping (Figure 2B, C). Both ligands interact with several conserved tryptophans in the central cavity. Interestingly, a histidine residue has been identified as playing a critical role in binding of heme in the TSPO from Arabidopsis thaliana [32]. When the Arabidopsis sequence is modeled on the Rhodobacter structure, this histidine position would readily allow it to ligand an iron atom, if heme were bound in the same location as the porphyrin observed in RsTSPO. This correspondence strongly suggests that the porphyrin binding site that we interpret in the Rhodobacter structure is in fact the correct conserved binding site for porphyrin or heme. Converting the proline in RsTSPO to a histidine and observing an increased affinity for heme, would provide confirmation of this conclusion.
Despite some significant discrepancies, all three structures (Rhodobacter, Bacillus and mouse) suggest that the central cavity provides important binding sites for drug ligands and porphyrins. These structures can now give valuable clues for further investigation of the TSPO-ligand interactions in the search for more specific ligands targeting TSPO. Further, it is noteworthy that the capping loop region between TM-I and TM-II is resolved in several distinct positions in the different RsTSPO crystal structures, suggesting that conformational changes may be induced by ligand binding and may be important in determining interactions with self and other proteins. Mutations in this loop in the Rhodobacter protein have already been shown to impact ligand binding and oligomeric state [23].
3.2 Cholesterol binding to TSPO
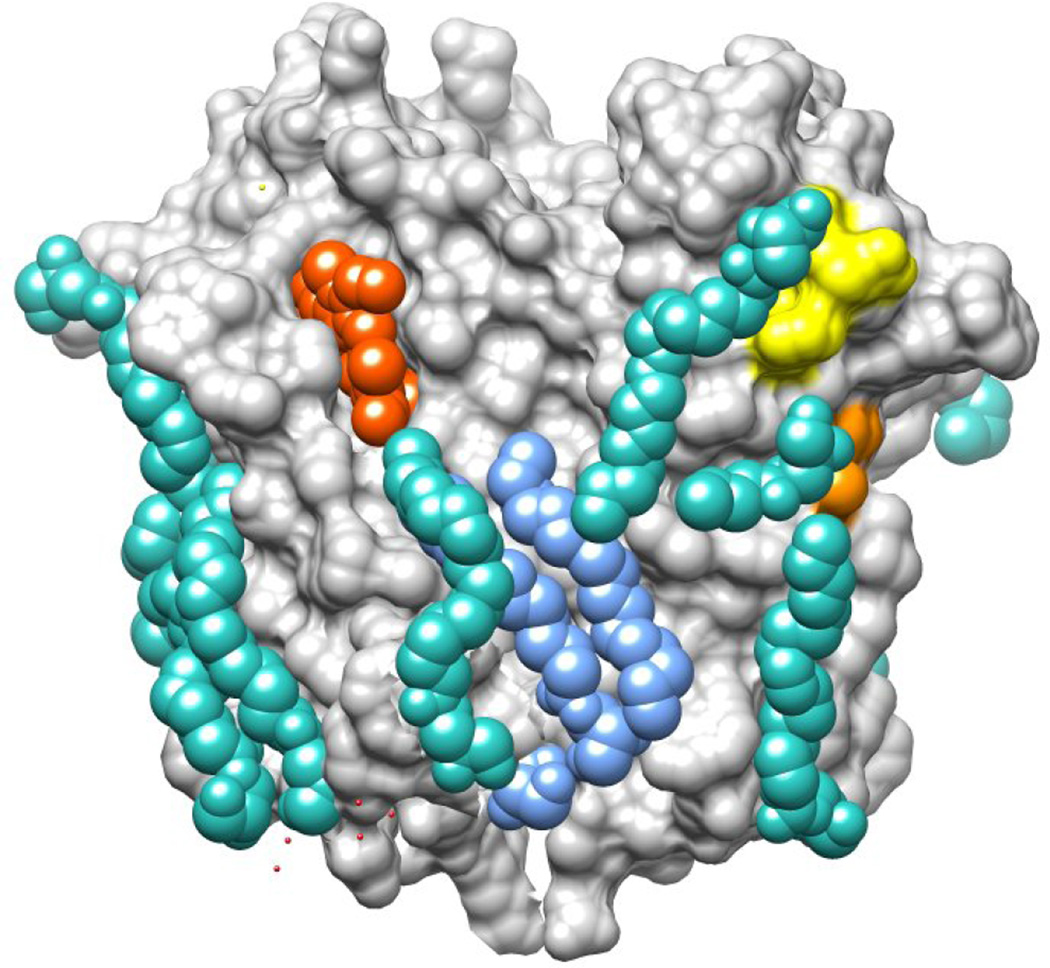
Cholesterol is not resolved in any of the crystal structures, which is not unexpected since bacterial TSPO has relatively low affinity for cholesterol [25]. But unlike the other ligands that appear to reside in the central pocket, cholesterol binding studies over the years have given many clues that its site is spatially distinct. The location that was originally proposed by Papadopoulos and colleagues, the CRAC motif [33] and a more recently identified binding enhancement motif, LAF [34], can be seen in the structures of RsTSPO [26] to be on the outer surface of the five helical bundle and not occluded by the dimer interface (Figure 3). Mutational analyses support the importance of this region for cholesterol binding [34]. It is striking that covariance analysis [35,36] shows a close interaction between TM-IV and V, precisely as seen in the crystal structure. The strongest residue pairs identified by covariance analysis coincide with the LAF-CRAC motif, especially the LAF region, emphasizing its importance. Indeed, mutagenesis shows that substituting the human sequence in this position in the bacterial TSPO confers high affinity for cholesterol. The crystal structure of RsTSPO (Figure 3) shows the location of the LAF-CRAC site on TM-V. Tightly bound lipid molecules (cyan and blue), occupying sites in the vicinity of LAF (orange) and CRAC (yellow), suggest that the high concentration of monoolein needed to crystallize RsTSPO may have displaced the cholesterol present in the crystallization medium.
Figure 3. Crystal structure of RsTSPO with bound lipids and ligands.
RsTSPO crystal structure is shown in a surface rendering. Phospholipid is shown in light blue, monooleins in cyan, prophyrin in red, the CRAC site in yellow and the LAF site in orange. The human polymorphism A147T (A139T in RsTSPO) is located immediately above the LAF site. (Produced in Chimera from PDB# 4UC1.)
Of considerable additional interest is the observation that a human single nucleotide polymorphism in TSPO is associated with anxiety-related diseases [11–13]. The polymorphism causes one amino acid substitution, A147T, in the LAF-CRAC region. It was discovered that this change lowered the affinity for certain TSPO ligands that are used for PET imaging of the brain [37], a technique that relies on the high localized expression of TSPO in regions of inflammation [38]. Recreating this mutation in RsTSPO allowed us to study its ligand binding behavior, revealing that cholesterol binding was decreased as well as other ligands [26]. The mutant form gave well-diffracting crystals that show a more tightly packed structure than the WT in the upper half of the protein containing the ligand pocket, suggesting a more closed configuration. Key residues in the LAF-CRAC region are also rearranged, possibly accounting for the altered cholesterol binding. The relationship of this relatively common polymorphism (~9% of a sample Caucasian population were observed to be homozygous for this mutation [39]) to anxiety disorders that are also very prevalent (~ 18% of the American population [National Institute of Mental Health1]), is still a subject of debate, but could involve an effect on cholesterol transport and steroidogenesis in mitochondria [8].
4. Porphyrin degradation activity of TSPO
TSPO from all species studied bind porphyrins, and this function is proposed to play a role in a variety of processes in which TSPO is involved [15,25,32,40,41]. The idea of TSPO being a porphyrin degrading enzyme was recently suggested based on the observation of time and light-dependent color change of porphyrin molecules when mixed with purified TSPO from the Chlorobium tepidum, an anaerobic phototrophic bacterium [42]. Our preliminary tests with purified RsTSPO indicate a similar ability to decrease the Soret band absorption as a signal of oxidation. However this activity is quite slow and highly dependent on light, raising the question of whether the activity is physiologically significant in multicellular organism. Several purified bacterial and eukaryotic TSPOs have been tested for this activity [27,42]. Almost 1:1 protein to substrate ratios were used in most experiments, and the turnover rates vary from several minutes to half an hour. These observations suggest that augmenting porphyrin breakdown can occur in certain species, but may have become less significant during the evolution of multicellular organisms in which light is not a usual reactant in most tissues. In the absence of light, porphyrin could potentially be oxidized by ROS generated by other reactions; if this “dark” reaction were facilitated by TSPO it might have some role in stress sensing or stress response. So far, however, there is no report of TSPO enhancing the breakdown of porphyrin in the dark.
5. Functional roles of TSPO: hypotheses and controversies
Despite more than 30 years of study, the function(s) of TSPO under physiological conditions remain controversial. The apparent involvement of TSPO in multiple processes, including cholesterol and porphyrin transport, cancer, apoptosis, autophagy and inflammation [8,10,14,15,18,32,40,43–46], make it difficult to discern its precise role(s), especially since conclusions are often based on the effects of TSPO ligands whose specificity cannot be guaranteed in the complex cellular milieu. Although originally proposed to be the transporter of cholesterol into mitochondria and the rate-limiting step of steroidogenesis, it is clear that TSPO alone may not fulfill this function. Evidence from knock out mouse studies [6,47] yields conflicting evidence as to the importance of TSPO in embryonic development, while the characterization of the cholesterol translocon [4,5] indicates the involvement of a variety of players in the regulation of cholesterol movement into mitochondria. This conclusion is further supported by the crystal structures of RsTSPO, which appear to excluded an internal transport pathway within a monomer or between monomers in a dimer, but pose the possibility of an external transport route that requires a partner [26]. Nevertheless, the very high affinity (nanomolar) for cholesterol in mammalian TSPO [34], and the ability of TSPO specific ligands to influence cholesterol metabolism [30,48] indicate a likely important role of TSPO in cholesterol homeostasis. The correlation of the human polymorphism, A147T, with reduced cholesterol conversion to steroid hormones [39] along with an association with anxiety disorders, further strengthens the functional relationship of TSPO with cholesterol metabolism, as does the altered structure and binding properties of the mimic of the human polymorphism [26].
In another controversial area, TSPO has been proposed to be a part of the mitochondrial permeability transition pore (MPTP) since mammalian TSPO was initially co-isolated with the voltage gated anion channel (VDAC) and the adenine nucleotide translocator (ANT) [49]. Despite the fact that the molecular composition of the permeability transition pore is still hotly debated [50–52], considerable evidence suggests a role of TSPO in its regulation. Indeed, a variety of drug ligands that bind TSPO appear to regulate apoptosis through effects on the permeability transition [53–55]. This association could explain TSPO’s influence on cancer cell growth and proliferation [44]. However, the permeability transition pore and the cholesterol translocon complex are not sufficiently well defined to allow a clear interpretation of the effects of TSPO and its ligands on either.
Beyond its potential role as a transporter, TSPO could also act as a receptor or sensor, in line with its function in Rhodobacter where RsTSPO is part of a regulatory process that facilitates the switch between photosynthesis and respiration in response to changes in light and oxygen conditions. The role of RsTSPO in this signaling path appears to involve porphyrin transport and regulation of photosynthetic genes [21,22]; however, given the location of TSPO in the outer membrane of mitochondria in higher organisms, it is hard to visualize a similar stress response mechanism. In plants and cyanobacteria, it has been shown that the knockout of a TSPO homolog is significantly less sensitive to salt stress [56]. In contrast, when Arabidopsis is challenged with oxidative stress by porphyrin-induced cytotoxicity, TSPO overexpression protects against chlorosis [32, 57]. The role of TSPO in these stress responses remains to be determined. The elevated expression level of TSPO under various stressful conditions, such as oxidative stress, salt stress and inflammation in bacteria, plants and animals, suggests an evolutionarily conserved stress sensing or stress combating role for TSPO. Considering the ability of TSPO to bind and potentially transport porphyrin compounds, high levels of TSPO could provide a mechanism for alleviating oxidative stress, through favoring the removal [21,58] or degradation of porphyrin [27].
Another stress related phenomenon is the process of autophagy, where involvement of TSPO has recently been demonstrated [59]. Association of TSPO and VDAC1 was documented by co-immunoprecipitation and an increased ratio of TSPO to VDAC1 correlated with increased ROS production, inhibition of mitophagy and accumulation of damaged mitochondria. The inhibition of mitophagy was found to be dependent on both VDAC1 and TSPO, raising interesting questions regarding the precise mechanism of TSPO involvement in ROS production or release.
It is interesting to note that the structure of TSPO has some resemblance to a G-protein coupled receptor with the vase shaped structure and the central binding site capped by a long loop, suggesting that TSPO could act as a ligand-activated receptor. But a mechanism of conformational transmission that would equate to GPCR interactions with G-proteins is not obvious from the current crystal structures. Certainly VDAC or the MPTP complex or the cholesterol translocon are likely downstream players in any signaling pathway, considering the substantial evidence of effects of TSPO ligands on these proteins and processes. Additional structures in different liganded and oligomeric states will be needed to address this question.
6. Conclusions
TSPO is clearly involved in a number of important but complex functions that remain to be further elucidated. The new structural information provides the basis for proposing and testing hypotheses regarding the functional interactions and partners of TSPO, bringing new momentum to the field. However, to understand these functions, a multidisciplinary approach will be required that brings together the tools of structure biology, cell biology, genetics, and physiology. Much work is needed to solve the puzzle that TSPO still presents and to determine its physiological roles in health and disease.
ACKNOWLEDGEMENTS
Funding was provided by NIH GM26916 (SF-M) and Michigan State University Strategic Partnership Grant, Mitochondrial Science & Medicine (SF-M).
ABBREVIATIONS
- TSPO
Translocator Protein 18 kDa
- PBR
peripheral-type benzodiazepine receptor
- RsTSPO
TSPO from Rhodobacter sphaeroides
- BcTSPO
TSPO from Bacillus cereus
- mTSPO
TSPO from mouse
- MPTP
mitochondrial permeability transition pore
- VDAC
voltage dependent anion channel
- ANT
adenine nucleotide translocator
- CRAC
Cholesterol Recognition/interaction Amino acid Consensus
- PET
positron emission tomography
- PpIX
porphyrin/tetrapyrrole
- PK11195
N-butan-2-yl-1-(2-chlorophenyl)-N-methylisoquinoline-3-carboxamide
- WT
wild-type
- PDB
Protein Data Bank
- NMR
Nuclear magnetic resonance
- DEER
Double Electron Electron Resonance
- EM
electron microscopy
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Braestrup C, Squires RF. Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H) diazepam binding. Proc Natl Acad Sci U S A. 1977;74:3805–3809. doi: 10.1073/pnas.74.9.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snyder SH, Verma A, Trifiletti RR. The peripheral-type benzodiazepine receptor: A protein of mitochondrial outer membranes utilizing porphyrins as endogenous ligands. FASEB J. 1987;1:282–288. doi: 10.1096/fasebj.1.4.2820823. [DOI] [PubMed] [Google Scholar]
- 3.Papadopoulos V, Amri H, Boujrad N, Cascio C, Culty M, Garnier M, Hardwick M, Li H, Vidic B, Brown AS, Reversa JL, Bernassau JM, Drieu K. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids. 1997;62 doi: 10.1016/s0039-128x(96)00154-7. [DOI] [PubMed] [Google Scholar]
- 4.Fan J, Papadopoulos V. Evolutionary origin of the mitochondrial cholesterol transport machinery reveals a universal mechanism of steroid hormone biosynthesis in animals. PLoS One. 2013;8:e76701. doi: 10.1371/journal.pone.0076701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rone MB, Midzak AS, Issop L, Rammouz G, Jagannathan S, Fan J, Ye X, Blonder J, Veenstra T, Papadopoulos V. Identification of a dynamic mitochondrial protein complex driving cholesterol import, trafficking, and metabolism to steroid hormones. Mol Endocrinol. 2012;26:1868–1882. doi: 10.1210/me.2012-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stocco DM. The role of pbr/tspo in steroid biosynthesis challenged. Endocrinology. 2014;155:6–9. doi: 10.1210/en.2013-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papadopoulos V. On the role of the translocator protein (18-kDa) TSPO in steroid hormone biosynthesis. Endocrinology. 2014;155:15–20. doi: 10.1210/en.2013-2033. [DOI] [PubMed] [Google Scholar]
- 8.Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, Schumacher M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 2010;9:971–988. doi: 10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- 9.Papadopoulos V, Lecanu L, Brown RC, Han Z, Yao ZX. Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience. 2006;138:749–756. doi: 10.1016/j.neuroscience.2005.05.063. [DOI] [PubMed] [Google Scholar]
- 10.Papadopoulos V, Lecanu L. Translocator protein (18 kDa) TSPO: An emerging therapeutic target in neurotrauma. Exp Neurol. 2009;219:53–57. doi: 10.1016/j.expneurol.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colasanti A, Owen DR, Grozeva D, Rabiner EA, Matthews PM, Craddock N, Young AH. Bipolar disorder is associated with the rs6971 polymorphism in the gene encoding 18 kDa translocator protein (TSPO) Psychoneuroendocrinology. 2013;38:2826–2829. doi: 10.1016/j.psyneuen.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa B, Pini S, Martini C, Abelli M, Gabelloni P, Landi S, Muti M, Gesi C, Lari L, Cardini A, Galderisi S, Mucci A, Lucacchini A, Cassano GB. Ala147thr substitution in translocator protein is associated with adult separation anxiety in patients with depression. Psychiatr Genet. 2009;19:110–111. doi: 10.1097/YPG.0b013e32832080f6. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura K, Yamada K, Iwayama Y, Toyota T, Furukawa A, Takimoto T, Terayama H, Iwahashi K, Takei N, Minabe Y, Sekine Y, Suzuki K, Iwata Y, Pillai A, Nakamoto Y, Ikeda K, Yoshii M, Fukunishi I, Yoshikawa T, Mori N. Evidence that variation in the peripheral benzodiazepine receptor (PBR) gene influences susceptibility to panic disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:222–226. doi: 10.1002/ajmg.b.30211. [DOI] [PubMed] [Google Scholar]
- 14.Batarseh A, Papadopoulos V. Regulation of translocator protein 18 kDa (TSPO) expression in health and disease states. Mol Cell Endocrinol. 2010;327:1–12. doi: 10.1016/j.mce.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wendler G, Lindemann P, Lacapère JJ, Papadopoulos V. Protoporphyrin IX binding and transport by recombinant mouse PBR. Biochem Biophys Res Commun. 2003;311:847–852. doi: 10.1016/j.bbrc.2003.10.070. [DOI] [PubMed] [Google Scholar]
- 16.Katz Y, Ben-Baruch G, Kloog Y, Menczer J, Gavish M. Increased density of peripheral benzodiazepine-binding sites in ovarian carcinomas as compared with benign ovarian tumours and normal ovaries. Clin Sci (Lond) 1990;78:155–158. doi: 10.1042/cs0780155. [DOI] [PubMed] [Google Scholar]
- 17.Decaudin D, Castedo M, Nemati F, Beurdeley-Thomas A, De Pinieux G, Caron A, Pouillart P, Wijdenes J, Rouillard D, Kroemer G, Poupon MF. Peripheral benzodiazepine receptor ligands reverse apoptosis resistance of cancer cells in vitro and in vivo. Cancer Res. 2002;62:1388–1393. [PubMed] [Google Scholar]
- 18.Kreisl WC, Jenko KJ, Hines CS, Lyoo CH, Corona W, Morse CL, Zoghbi SS, Hyde T, Kleinman JE, Pike VW, McMahon FJ, Innis RB Team BCPRP. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab. 2013;33:53–58. doi: 10.1038/jcbfm.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic parkinson's disease. Neurobiol Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Bui ET, Bradley PJ, Johnson PJ. A common evolutionary origin for mitochondria and hydrogenosomes. Proc Natl Acad Sci U S A. 1996;93:9651–9656. doi: 10.1073/pnas.93.18.9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeliseev AA, Kaplan S. A novel mechanism for the regulation of photosynthesis gene expression by the TSPO outer membrane protein of Rhodobacter sphaeroides 2.4.1. J Biol Chem. 1999;274:21234–21243. doi: 10.1074/jbc.274.30.21234. [DOI] [PubMed] [Google Scholar]
- 22.Yeliseev AA, Kaplan S. A sensory transducer homologous to the mammalian peripheral-type benzodiazepine receptor regulates photosynthetic membrane complex formation in Rhodobacter sphaeroides 2.4.1. J Biol Chem. 1995;270:21167–21175. doi: 10.1074/jbc.270.36.21167. [DOI] [PubMed] [Google Scholar]
- 23.Yeliseev AA, Kaplan S. Tspo of rhodobacter sphaeroides. A structural and functional model for the mammalian peripheral benzodiazepine receptor. J Biol Chem. 2000;275:5657–5667. doi: 10.1074/jbc.275.8.5657. [DOI] [PubMed] [Google Scholar]
- 24.Yeliseev AA, Krueger KE, Kaplan S. A mammalian mitochondrial drug receptor functions as a bacterial "Oxygen" Sensor. Proc Natl Acad Sci U S A. 1997;94:5101–5106. doi: 10.1073/pnas.94.10.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li F, Xia Y, Meiler J, Ferguson-Miller S. Characterization and modeling of the oligomeric state and ligand binding behavior of purified translocator protein 18 kDa from Rhodobacter sphaeroides. Biochemistry. 2013;52:5884–5899. doi: 10.1021/bi400431t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li F, Liu J, Zheng Y, Garavito RM, Ferguson-Miller S. Protein structure. Crystal structures of translocator protein (TSPO) and mutant mimic of a human polymorphism. Science. 2015;347:555–558. doi: 10.1126/science.1260590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo Y, Kalathur RC, Liu Q, Kloss B, Bruni R, Ginter C, Kloppmann E, Rost B, Hendrickson WA. Protein structure. Structure and activity of tryptophan-rich TSPO proteins. Science. 2015;347:551–555. doi: 10.1126/science.aaa1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaremko L, Jaremko M, Giller K, Becker S, Zweckstetter M. Structure of the mitochondrial translocator protein in complex with a diagnostic ligand. Science. 2014;343:1363–1366. doi: 10.1126/science.1248725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korkhov VM, Sachse C, Short JM, Tate CG. Three-dimensional structure of TSPO by electron cryomicroscopy of helical crystals. Structure. 2010;18:677–687. doi: 10.1016/j.str.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delavoie F, Li H, Hardwick M, Robert JC, Giatzakis C, Péranzi G, Yao ZX, Maccario J, Lacapère JJ, Papadopoulos V. In vivo and in vitro peripheral-type benzodiazepine receptor polymerization: Functional significance in drug ligand and cholesterol binding. Biochemistry. 2003;42:4506–4519. doi: 10.1021/bi0267487. [DOI] [PubMed] [Google Scholar]
- 31.Lacapère JJ, Delavoie F, Li H, Péranzi G, Maccario J, Papadopoulos V, Vidic B. Structural and functional study of reconstituted peripheral benzodiazepine receptor. Biochem Biophys Res Commun. 2001;284:536–541. doi: 10.1006/bbrc.2001.4975. [DOI] [PubMed] [Google Scholar]
- 32.Vanhee C, Zapotoczny G, Masquelier D, Ghislain M, Batoko H. The Arabidopsis multistress regulator TSPO is a heme binding membrane protein and a potential scavenger of porphyrins via an autophagy-dependent degradation mechanism. Plant Cell. 2011;23:785–805. doi: 10.1105/tpc.110.081570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Papadopoulos V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology. 1998;139:4991–4997. doi: 10.1210/endo.139.12.6390. [DOI] [PubMed] [Google Scholar]
- 34.Li F, Liu J, Valls L, Hiser C, Ferguson-Miller SM. Identification of a key cholesterol binding enhancement motif in translocator protein 18 kDa. Biochemistry. 2015;54:1441–1443. doi: 10.1021/bi5015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones DT, Buchan DW, Cozzetto D, Pontil M. Psicov: Precise structural contact prediction using sparse inverse covariance estimation on large multiple sequence alignments. Bioinformatics. 2012;28:184–190. doi: 10.1093/bioinformatics/btr638. [DOI] [PubMed] [Google Scholar]
- 36.de Juan D, Pazos F, Valencia A. Emerging methods in protein co-evolution. Nat Rev Genet. 2013;14:249–261. doi: 10.1038/nrg3414. [DOI] [PubMed] [Google Scholar]
- 37.Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, Rhodes C, Pulford DJ, Bennacef I, Parker CA, StJean PL, Cardon LR, Mooser VE, Matthews PM, Rabiner EA, Rubio JP. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the pet radioligand pbr28. J Cereb Blood Flow Metab. 2012;32:1–5. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen MK, Guilarte TR. Translocator protein 18 kDa (TSPO): Molecular sensor of brain injury and repair. Pharmacol Ther. 2008;118:1–17. doi: 10.1016/j.pharmthera.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa B, Pini S, Gabelloni P, Da Pozzo E, Abelli M, Lari L, Preve M, Lucacchini A, Cassano GB, Martini C. The spontaneous ala147thr amino acid substitution within the translocator protein influences pregnenolone production in lymphomonocytes of healthy individuals. Endocrinology. 2009;150:5438–5445. doi: 10.1210/en.2009-0752. [DOI] [PubMed] [Google Scholar]
- 40.Verma A, Nye JS, Snyder SH. Porphyrins are endogenous ligands for the mitochondrial (peripheral-type) benzodiazepine receptor. Proc Natl Acad Sci U S A. 1987;84:2256–2260. doi: 10.1073/pnas.84.8.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rampon C, Bouzaffour M, Ostuni MA, Dufourcq P, Girard C, Freyssinet JM, Lacapere JJ, Schweizer-Groyer G, Vriz S. Translocator protein (18 kDa) is involved in primitive erythropoiesis in zebrafish. FASEB J. 2009;23:4181–4192. doi: 10.1096/fj.09-129262. [DOI] [PubMed] [Google Scholar]
- 42.Ginter C, Kiburu I, Boudker O. Chemical catalysis by the translocator protein (18 kDa) Biochemistry. 2013;52:3609–3611. doi: 10.1021/bi400364z. [DOI] [PubMed] [Google Scholar]
- 43.Fan J, Lindemann P, Feuilloley MG, Papadopoulos V. Structural and functional evolution of the translocator protein (18 kDa) Curr Mol Med. 2012;12:369–386. doi: 10.2174/1566524011207040369. [DOI] [PubMed] [Google Scholar]
- 44.Wu X, Gallo KA. The 18-kDa translocator protein (TSPO) disrupts mammary epithelial morphogenesis and promotes breast cancer cell migration. PLoS One. 2013;8:e71258. doi: 10.1371/journal.pone.0071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gatliff J, Campanella M. The 18 kda translocator protein (TSPO): A new perspective in mitochondrial biology. Curr Mol Med. 2012;12:356–368. doi: 10.2174/1566524011207040356. [DOI] [PubMed] [Google Scholar]
- 46.Azarashvili T, Stricker R, Reiser G. The mitochondria permeability transition pore complex in the brain with interacting proteins - promising targets for protection in neurodegenerative diseases. Biol Chem. 2010;391:619–629. doi: 10.1515/BC.2010.070. [DOI] [PubMed] [Google Scholar]
- 47.Tu LN, Morohaku K, Manna PR, Pelton SH, Butler WR, Stocco DM, Selvaraj V. Peripheral benzodiazepine receptor/translocator protein global knockout mice are viable with no effects on steroid hormone biosynthesis. J Biol Chem. 2014;289:27444–27454. doi: 10.1074/jbc.M114.578286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Yao Z, Degenhardt B, Teper G, Papadopoulos V. Cholesterol binding at the cholesterol recognition/ interaction amino acid consensus (CRAC) of the peripheral-type benzodiazepine receptor and inhibition of steroidogenesis by an hiv TAT-CRAC peptide. Proc Natl Acad Sci U S A. 2001;98:1267–1272. doi: 10.1073/pnas.031461598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McEnery MW, Snowman AM, Trifiletti RR, Snyder SH. Isolation of the mitochondrial benzodiazepine receptor: Association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc Natl Acad Sci U S A. 1992;89:3170–3174. doi: 10.1073/pnas.89.8.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabó I, Lippe G, Bernardi P. Dimers of mitochondrial atp synthase form the permeability transition pore. Proc Natl Acad Sci U S A. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krauskopf A, Eriksson O, Craigen WJ, Forte MA, Bernardi P. Properties of the permeability transition in vdac1(−/−) mitochondria. Biochim Biophys Acta. 2006;1757:590–595. doi: 10.1016/j.bbabio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 52.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341(Pt 2):233–249. [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao J, Liang D, Zhang H, Liu Y, Li F, Chen YH. 4'-chlorodiazepam, a translocator protein (18 kDa) antagonist, improves cardiac functional recovery during postischemia reperfusion in rats. Exp Biol Med (Maywood) 2010;235:478–486. doi: 10.1258/ebm.2009.009291. [DOI] [PubMed] [Google Scholar]
- 54.Azarashvili T, Grachev D, Krestinina O, Evtodienko Y, Yurkov I, Papadopoulos V, Reiser G. The peripheral-type benzodiazepine receptor is involved in control of ca2+-induced permeability transition pore opening in rat brain mitochondria. Cell Calcium. 2007;42:27–39. doi: 10.1016/j.ceca.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Hirsch T, Decaudin D, Susin SA, Marchetti P, Larochette N, Resche-Rigon M, Kroemer G. Pk11195, a ligand of the mitochondrial benzodiazepine receptor, facilitates the induction of apoptosis and reverses bcl-2-mediated cytoprotection. Exp Cell Res. 1998;241:426–434. doi: 10.1006/excr.1998.4084. [DOI] [PubMed] [Google Scholar]
- 56.Balsemão-Pires E, Jaillais Y, Olson BJ, Andrade LR, Umen JG, Chory J, Sachetto-Martins G. The arabidopsis translocator protein (AtTSPO) is regulated at multiple levels in response to salt stress and perturbations in tetrapyrrole metabolism. BMC Plant Biol. 2011;11:108. doi: 10.1186/1471-2229-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Busch AW, Montgomery BL. Interdependence of tetrapyrrole metabolism, the generation of oxidative stress and the mitigative oxidative stress response. Redox Biol. 2015;4C:260–271. doi: 10.1016/j.redox.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeno S, Veenman L, Katz Y, Bode J, Gavish M, Zaaroor M. The 18 kDa mitochondrial translocator protein (TSPO) prevents accumulation of protoporphyrin ix. Involvement of reactive oxygen species (ROS) Curr Mol Med. 2012;12:494–501. doi: 10.2174/1566524011207040494. [DOI] [PubMed] [Google Scholar]
- 59.Gatliff J, East D, Crosby J, Abeti R, Harvey R, Craigen W, Parker P, Campanella M. TSPO interacts with VDAC1 and triggers a ROS-mediated inhibition of mitochondrial quality control. Autophagy. 2014;10:2279–2296. doi: 10.4161/15548627.2014.991665. [DOI] [PMC free article] [PubMed] [Google Scholar]