Abstract
Background & Aims
Current clinical assays for total 25-hydroxy(OH) vitamin D measure vitamin D bound to vitamin D binding protein (DBP) and albumin plus unbound (“free”) D. We investigated the relationship between total and free 25(OH)D with bone metabolism markers in normal(>3.5g/dL) versus low(≤3.5g/dL) albumin cirrhotics.
Methods
82 cirrhotics underwent measurement of free and total 25(OH)D by immunoassay, DBP, and markers of bone metabolism (intact parathyroid hormone(iPTH), C-telopeptide(CTX), bone-specific alkaline phosphatase(BSAP), osteocalcin, amino-terminal pro-peptide of type 1-collagen(P1NP)). Pearson coefficients assessed relevant associations.
Results
Cirrhotics with low(n=54) vs. normal(n=28) albumin had lower total 25(OH)D(12.1vs.21.7ng/mL), free 25(OH)D(6.2vs.8.6pg/mL), and DBP(91.4vs.140.3ug/mL) [p<0.01 for each]. iPTH was similar in low and normal albumin groups(33vs.28pg/mL; p=0.38), although serum CTX(0.46vs.0.28ng/mL) and BSAP(31.7vs.24.8ug/L) were increased (p<0.01). An inverse relationship was observed between total 25(OH)D and iPTH in normal(r=−0.47, p=0.01) but not low albumin cirrhotics(r=0.07, p=0.62). Similar associations were seen between free 25(OH)D and iPTH(Normal: r=−0.46, p=0.01; Low: r=−0.03, p=0.84). BSAP, osteocalcin, and P1NP were elevated above the normal range in all cirrhotics but not consistently associated with total or free 25(OH)D.
Conclusions
Cirrhotics with low versus normal albumin have lower levels of DBP, total and free 25(OH)D. The expected relationship between total or free 25(OH)D with iPTH was observed in normal but not low albumin cirrhotics, demonstrating that total 25(OH)D is not an accurate marker of bioactive vitamin D status in cirrhotics with synthetic dysfunction. Additional investigation into the role of vitamin D supplementation and its impact on bone mineral homeostasis in this population is needed.
Keywords: Metabolic bone disease, parathyroid hormone, free hormone hypothesis, vitamin D binding protein, albumin
INTRODUCTION
Vitamin D is essential for mineralization of bone. Vitamin D deficiency, defined as a total 25-hydroxy (OH) D level <20 ng/mL1, has been reported to be highly prevalent in patients with cirrhosis, with rates reaching as high as 86%2. Thirty percent have levels <7 ng/mL3. The reasons for such a high prevalence of vitamin D deficiency in these patients is poorly understood, but possible mechanisms include intestinal fat malabsorption due to cholestasis and gut edema, limited sun exposure due to relative immobility, reduced dietary intake, and decreased hepatic hydroxylation of vitamin D1,4–6.
While metabolic bone disease is also common in patients with cirrhosis, studies have shown an inconsistent relationship between total 25(OH)D levels and bone-related outcomes. Rates of osteoporosis in cirrhotics approach 55%7, and fragility fractures occur in at least 20% of liver transplant recipients within the first year of transplantation8. However, in patients with cirrhosis, total 25(OH)D levels have not been associated with bone mineral density or fracture risk8,9, and repletion of total 25(OH)D levels does not improve bone mineral density10–12. Despite a high prevalence of total 25(OH)D deficiency, osteomalacia in adults with cirrhosis is exceedingly rare10,11,13. Furthermore, although total 25(OH)D levels decrease as severity of cirrhosis increases3,14,15, intact parathyroid hormone (iPTH) levels do not vary with total 25(OH)D levels14. These data suggest that, in patients with cirrhosis, total 25(OH)D levels may not be the most accurate marker of physiologically active vitamin D.
Current assays to assess vitamin D status in the clinical setting measure total 25(OH)D that reflects total serum vitamin D in three forms: 1) bound to vitamin D binding protein, accounting for 85% of the total, 2) bound to albumin, accounting for 15% of the total, and 3) unbound (“free”), accounting for 0.03% of the total16. Only free 25(OH)D is available for conversion to the 1,25(OH)2D, the bioactive form of vitamin D17. We hypothesized that patients with cirrhosis and evidence of hepatic synthetic dysfunction would have lower vitamin D binding protein compared to those without hepatic synthetic dysfunction and thus have higher free 25(OH)D levels and lower rates of bone turnover than predicted by their total 25(OH)D level. Therefore, the aim of this study was to measure and determine the relationship between free 25(OH)D levels, total 25(OH)D, vitamin D binding protein, and serum markers of bone turnover in cirrhotic patients with and without hepatic synthetic dysfunction.
METHODS
Subjects
This was a prospective single-center cohort study of adult patients with cirrhosis who were seen as outpatients from October 2012 through March 2014. The presence of cirrhosis was confirmed by clinical evidence of portal hypertension, laboratory evidence of hepatic synthetic dysfunction, and radiographic evidence of cirrhosis and/or portal hypertension. Given the association between secondary hyperparathyroidism and renal insufficiency, patients were excluded if they had a serum creatinine >1.5 mg/dL within the three months prior to enrollment. Subjects who had a serum creatinine >1.5 mg/dL based on the results from the study phlebotomy were excluded from the analyses.
Study procedures, sample processing, and assays
Subjects completed a questionnaire regarding supplemental calcium and vitamin D intake, average daily sun exposure, and use of daily sunscreen. They subsequently underwent a single venous blood draw. Demographics and etiology of liver disease were obtained from the patients’ electronic health records.
Once obtained, the patient samples were transferred on ice within an hour to the laboratory, centrifuged for 15 minutes at 3500rpm, serum aliquoted into 3 individual 2 mL cryogenic vials, and stored at −80°C until batched analysis.
Direct measurement of free 25(OH)D levels was made by immunoassay (Future Diagnostics B.V., Wijchen, The Netherlands, http://www.future-diagnostics.nl/). In this assay, antibodies reactive to 25(OH)D are immobilized in a microtiter well (solid phase). Standards, controls, and patient samples are exposed to these antibodies, which capture free 25(OH)D in a buffer solution. The solid phase is then washed and a labeled analog of 25(OH)D is allowed to react with the remaining antibody in a second incubation. This incubation is followed by another wash and quantitation of the signal, which is inversely proportional to the level of free 25(OH)D in the sample. Assay characteristics have previously been described18.
Additional laboratory tests were provided by Heartland Assays, Inc. (Ames, Iowa). Total 25(OH)D levels were measured using the DiaSorin Liaison assay, an FDA-approved, direct, competitive chemiluminescence immunoassay that is co-specific for both 25(OH)D3 and 25(OH)D2. Also obtained from the serum samples were: Vitamin D binding protein (R&D Systems, Inc.), albumin (Pointe Scientific, Inc.), 1,25(OH)2D (DiaSorin), intact parathyroid hormone (DiaSorin), calcium (Pointe Scientific, Inc.), inorganic phosphorus (Pointe Scientific, Inc.), total alkaline phosphatase (Pointe Scientific), bone-specific alkaline phosphatase (DiaSorin), C-telopeptide (CTX; Immunodiagnostic Systems), amino-terminal pro-peptide of type 1 collagen (P1NP; My BioSource), and osteocalcin (Ouidel). Serum calcium was corrected for albumin (g/dL).
Statistical Analysis
For the primary analyses, the cohort was divided into two groups by serum albumin: “normal” (>3.5 g/dL) and “low albumin” (≤3.5 g/dL). A sensitivity analysis was performed comparing markers of vitamin D status and bone turnover by vitamin D binding protein levels >55 ug/mL versus ≤55 ug/mL. We compared the two groups using the Chi-square test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Linear regression was used to quantify the relationship between total or free 25(OH)D and markers of bone turnover. Pearson correlation coefficients (r) measured the degree of linear dependence between total or free 25(OH)D and markers of bone turnover. Using a range of values of correlation between free 25(OH)D and iPTH based on previously published data18, we estimated that a sample size of 80 subjects had up to 93% power to detect a clinically significant difference between those with low versus normal albumin.
Participants gave informed consent to the study approved by the UCSF Committee for Human Research #10-01437.
RESULTS
Baseline characteristics (Table 1)
Table 1.
Baseline characteristics of the 82 patients with cirrhosis.
| Characteristic* | All n=82 |
Normal albumin (>3.5 g/dL) n=28 |
Low albumin (≤3.5 g/dL) n=54 |
p-value |
|---|---|---|---|---|
| Age, years | 59.9 (53.9–63.9) | 59.9 (53.9–63.4) | 59.8 (54.3–64.5) | 0.72 |
| Women | 33 (40%) | 14 (50%) | 19 (35%) | 0.20 |
| Non-Hispanic White | 46 (56%) | 20 (71%) | 26 (48%) | 0.05 |
| Etiology of liver disease | 0.65 | |||
| Chronic hepatitis C | 48 (59%) | 15 (54%) | 33 (61%) | |
| Alcohol | 13 (16%) | 5 (18%) | 8 (15%) | |
| Primary biliary cirrhosis or primary sclerosing cholangitis | 7 (8%) | 4 (14%) | 3 (5%) | |
| Other | 14 (17%) | 4 (14%) | 10 (19%) | |
| BMI, kg/m2 | 27.8 (25.2–32.4) | 27.1 (24.4–30.8) | 28.0 (25.2–33.7) | 0.19 |
| MELD | 12 (9–16) | 9 (7–12) | 14 (12–17) | <0.01 |
| Total bilirubin, mg/dL | 2.2 (1.3–3.3) | 1.5 (1.1–2.2) | 2.5 (1.9–4.5) | <0.01 |
| Creatinine, mg/dL | 0.8 (0.6–1.0) | 0.7 (0.6–0.9) | 0.9 (0.7–1.0) | <0.01 |
| eGFR†, ml/min | 95 (79–105) | 100 (91–106) | 91 (74–100) | 0.01 |
| INR | 1.3 (1.2–1.5) | 1.1 (1.0–1.2) | 1.5 (1.3–1.6) | <0.01 |
| Alkaline phosphatase, IU/L | 176 (120–241) | 117 (96–182) | 208 (144–261) | <0.01 |
| Albumin, g/dL | 3.2 (2.7–3.8) | 4.1 (3.8–4.4) | 2.8 (2.5–3.2) | <0.01 |
| Vitamin D binding protein, ug/mL | 100.6 (63.3–157.1) | 140.3 (89.4–202.7) | 91.4 (55.1–129.7) | <0.01 |
| Taking D supplement | 38 (46%) | 15 (54%) | 23 (43%) | 0.52 |
| Vitamin D dose, units per day | 1000 (400–1000) | 1000 (400–2000) | 1000 (400–1000) | 0.62 |
| Sun exposure per day, hours | 1.4 (0.8–2.7) | 1.3 (0.7–2.9) | 1.7 (1.0–2.6) | 0.81 |
| Use of sunscreen | 15 (19%) | 8 (29%) | 7 (14%) | 0.12 |
Abbreviations: MELD, Model for End-Stage Liver Disease; eGFR, estimated glomerular filtration rate; INR, international normalized ratio for prothrombin time.
Median (interquartile range) or n (%)
Calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation25.
Reference range: 55.9–475.0 ug/mL
A total of 88 subjects enrolled in this study. 6 (7%) subjects with serum creatinine >1.5 mg/dL on the day of enrollment (based on laboratory results performed specifically for the study) were excluded. Therefore, data from 82 subjects were analyzed. Baseline characteristics of the entire cohort are listed in Table 1. Median age was 59.9 years, 40% were women, 56% were non-Hispanic White, 59% had chronic hepatitis C (HCV) as the primary etiology of their liver disease, and 46% were taking a vitamin D supplement at the time of the study visit. Median body mass index (BMI) was 27.8 kg/m2. The median value for total bilirubin was 2.2 mg/dL and for creatinine was 0.8 mg/dL. The median Model for End-Stage Liver Disease (MELD) score for the cohort was 12.
Compared to cirrhotics with normal albumin, patients with low albumin had significantly lower vitamin D binding protein levels (91.4 vs. 140.3 ug/mL, p<0.01). For every one unit g/dL decrease in serum albumin, vitamin D binding protein decreased by 42.5 ug/mL (p<0.01).
The cohort was categorized into two groups by serum albumin: 28 (34%) had serum albumin >3.5 g/dL (“normal”) and 54 (66%) ≤3.5 g/dL (“low”). Median albumin in the normal albumin group was 3.8 and in the low albumin group was 2.6. Compared to those with normal albumin, subjects with low albumin were less likely to be non-Hispanic White (48 vs. 71%, p=0.05) and had higher MELD scores (14 vs. 9, p<0.01). The two groups were otherwise similar by age, % women, % with chronic hepatitis C, body mass index (BMI), % taking a vitamin D supplement, average sun exposure per day, and use of daily sunscreen.
Markers of vitamin D status and bone turnover (Table 2)
Table 2.
25(OH)D levels, vitamin D binding protein, and markers of bone metabolism in 82 patients with cirrhosis with normal (>3.5 g/dL) and low (≤3.5 g/dL) albumin.
| Characteristic* | Reference range |
All n=82 |
Normal albumin (>3.5 g/dL) n=28 |
Low albumin (≤3.5 g/dL) n=54 |
p-value |
|---|---|---|---|---|---|
| Total 25(OH)D, ng/mL | 32.0–100.0 | 15.5 (10.2–23.8) | 21.7 (16.4–30.8) | 12.1 (8.4–16.8) | <0.01 |
| Free 25(OH)D, pg/mL | 4.5±1.6 † | 6.8 (5.0–9.1) | 8.6 (6.4–10.6) | 6.2 (4.5–8.1) | <0.01 |
| % free 25(OH)D | 0.043 (0.037–0.053) | 0.039 (0.031–0.045) | 0.046 (0.039–0.058) | <0.01 | |
| 1,25(OH)2D, pg/mL | 16.0–56.0 | 19.0 (11.2–33.5) | 33.0 (16.2–42.9) | 13.8 (9.9–21.7) | <0.01 |
| Calcium, mg/dL (corrected for albumin g/dL) | 8.5–10.4 | 9.5 (9.1–9.7) | 9.3 (8.9–9.6) | 9.5 (9.2–9.8) | 0.08 |
| Phosphorus, mg/dL | 2.5–4.8 | 3.1 (2.7–3.3) | 3.1 (2.8–3.3) | 3.1 (2.6–3.3) | 0.78 |
| iPTH, pg/mL | 13.0–54.0 | 29.2 (21.5–41.6) | 27.7 (22.8–33.3) | 32.7 (19.5–45.5) | 0.38 |
| Serum C-telopeptide, ng/mL | 0.11–1.35 | 0.39 (0.24–0.62) | 0.28 (0.18–0.44) | 0.46 (0.31–0.72) | <0.01 |
| Bone-specific alkaline phosphatase, ug/L | 5.5–24.6 | 29.3 (22.1–46.3) | 24.8 (15.7–34.8) | 31.7 (25.1–52.2) | <0.01 |
| Amino-terminal pro-peptide of type 1 collagen, ng/mL | 16.0–96.0 | 155.7 (118.8–206.0) | 139.5 (117.6–176.6) | 167.4 (119.2–211.9) | 0.55 |
| Osteocalcin, ng/mL | 3.7–10.0 | 10.4 (7.7–13.6) | 10.1 (7.7–13.2) | 10.4 (7.9–14.1) | 0.98 |
Abbreviations: iPTH, intact parathyroid hormone.
Median (interquartile range)
Mean ± standard deviation, which was obtained from samples drawn from 107 medically stable, community-dwelling adults18.
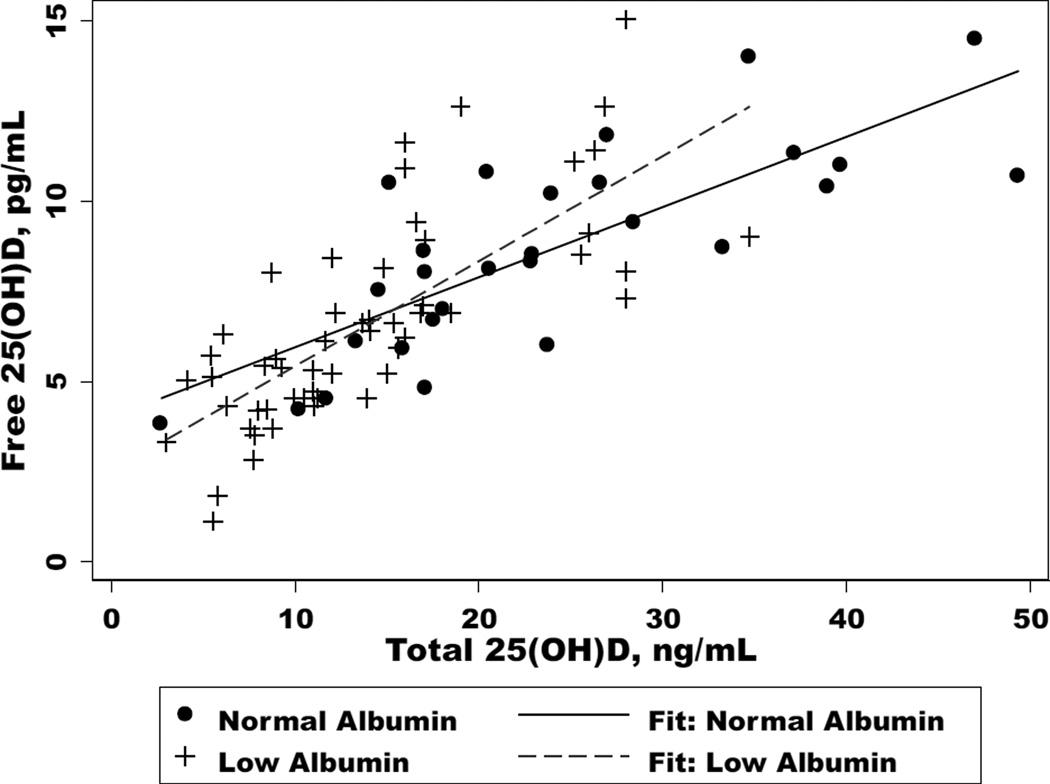
Total 25(OH)D levels were significantly lower in subjects with low albumin compared to normal albumin (12.1 vs. 21.7 ng/mL, p<0.01). There was a positive correlation between total 25(OH)D and serum albumin (r=0.52); for every one unit decrease in albumin, total 25(OH)D levels increased by 6.9 ng/mL (p<0.01). Similarly, free 25(OH)D levels were also significantly lower in subjects with low versus normal albumin (6.2 vs. 8.6 pg/mL, p<0.01), but % free 25(OH)D was higher (0.046 vs. 0.039%, p<0.01). The two markers of vitamin D were strongly correlated in both low [r=0.75, coefficient (coeff)=−0.29, p<0.01] and normal (r=0.78, coeff=0.19, p<0.01) albumin groups (Figure 1). Subjects with low albumin also had significantly lower levels of 1,25(OH)2D (13.8 vs. 33.0 pg/mL, p<0.01). When expressed as a ratio of 1,25(OH)2D pg/ml to total 25(OH)D ng/ml, those with albumin >3.5 g/dL had a ratio of 1.52 compared to 1.14 in subjects with albumin ≤3.5 g/dl.
Figure 1.
The relationship total 25(OH)D and free 25(OH)D in cirrhotics with normal (>3.5 g/dL) and low (≤3.5 g/dL) albumin (p<0.01 for each).
Intact PTH levels were similar among those with low versus normal albumin (32.7 vs. 27.7 pg/mL, p=0.38), as were albumin-corrected serum calcium (9.5 vs. 9.3 mg/dL, p=0.08) and serum phosphorus (3.1 vs. 3.1 mg/dL, p=0.78). Both serum CTX (0.46 vs. 0.28 ng/mL; p<0.01) and bone-specific alkaline phosphatase (31.7 vs. 24.8 ug/L, p<0.01; Table 2) were significantly elevated in low versus normal albumin subjects, while P1NP (167.4 vs. 139.5 ng/mL; p=0.55) and osteocalcin (10.4 vs. 10.1 ng/mL; p=0.98) were not. However, in both groups the levels of bone alkaline phosphatase, P1NP, and osteocalcin were at or above the upper limits of normal suggesting a high bone turnover state in these subjects despite normal levels of PTH.
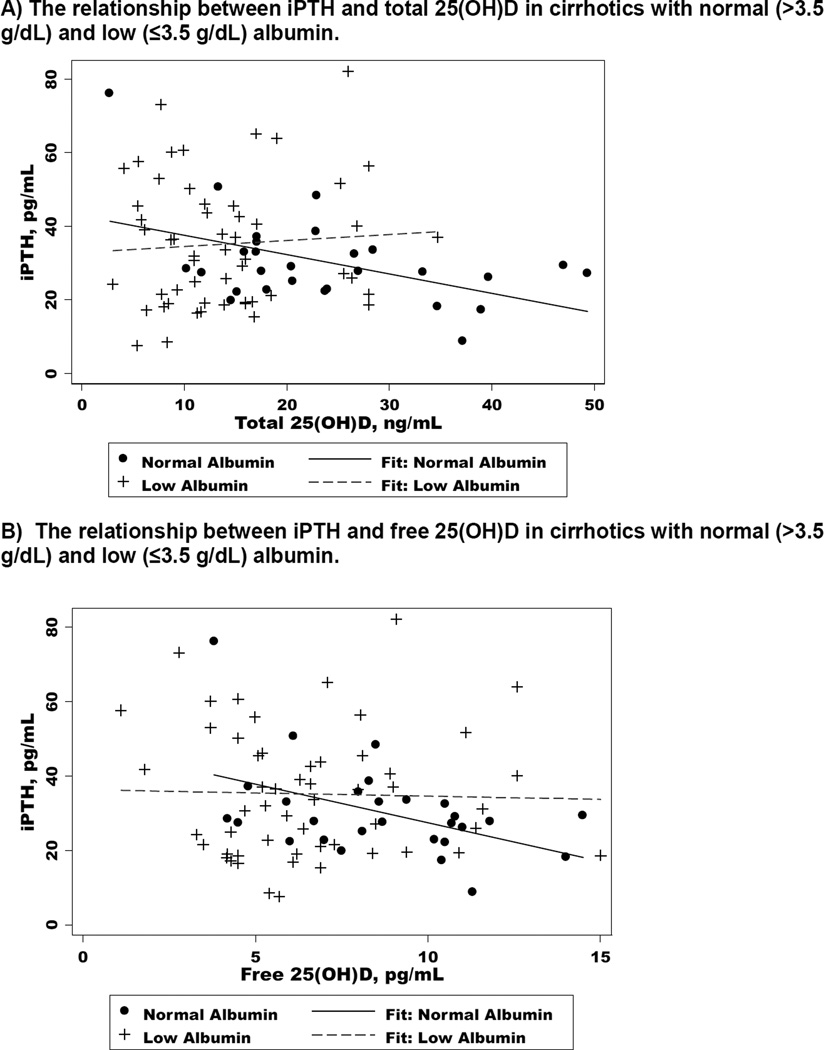
Associations between vitamin D levels and iPTH (Figure 2, Table 3)
Figure 2.
The relationship between iPTH total or free 25(OH)D in cirrhotics with normal (>3.5 g/dL) and low (≤3.5 g/dL) albumin.
Table 3.
The relationship between total 25(OH)D or free 25(OH)D and select markers of bone turnover.
| Marker | Normal Albumin (>3.5 g/dL) | Low Albumin (≥3.5 g/dL) | ||||
|---|---|---|---|---|---|---|
| r | Coefficient | p-value | r | Coefficient | p-value | |
| iPTH, pg/mL | ||||||
| Total 25(OH)D | −0.47 | −0.53 | 0.01 | 0.07 | 0.16 | 0.62 |
| Free 25(OH)D | −0.46 | −2.08 | 0.01 | −0.03 | −0.17 | 0.84 |
| Serum C-telopeptide, ng/mL | ||||||
| Total 25(OH)D | −0.52 | −0.02 | <0.01 | 0.03 | 0.002 | 0.82 |
| Free 25(OH)D | −0.49 | −0.06 | <0.01 | 0.24 | 0.04 | 0.09 |
| Bone-specific alkaline phosphatase, ug/L | ||||||
| Total 25(OH)D | −0.22 | −0.34 | 0.26 | 0.09 | 0.29 | 0.51 |
| Free 25 (OHD) | −0.04 | −0.25 | 0.84 | −0.06 | −0.46 | 0.68 |
| Amino-terminal pro-peptide of type 1 collagen, ng/mL | ||||||
| Total 25(OH)D | −0.06 | −1.46 | 0.76 | −0.26 | 4.58 | 0.06 |
| Free 25(OH)D | 0.07 | 7.3 | 0.71 | 0.01 | 0.55 | 0.93 |
| Osteocalcin, ng/mL | ||||||
| Total 25(OH)D | −0.52 | −0.18 | <0.01 | 0.15 | 0.13 | 0.29 |
| Free 25(OH)D | −0.33 | −0.47 | 0.08 | 0.34 | 0.79 | 0.01 |
A significant inverse relationship was observed between total 25(OH)D and iPTH in patients with normal albumin [r=−0.47, coefficient (coeff)= −0.53, p=0.01], but not among those with low albumin (r=0.07, coeff=0.16, p=0.62) [Figure 2A]. Similar associations were seen between free 25(OH)D and iPTH (Normal albumin: r=−0.46, coeff= −0.10, p=0.01; Low albumin: r=−0.03, coeff = −0.005, p=0.84) [Figure 2B]. For serum CTX, the association with total 25(OH)D was statistically significant for subjects with normal (r=−0.52, p=<0.01) but not low (r=0.03, p=0.82) albumin. There were no significant relationships between bone-specific alkaline phosphatase and total or free 25(OH)D in either group (p>0.05). There was a trend toward a weak negative correlation between P1NP and total 25(OH)D in the low albumin group (r=−0.26, p=0.06). For osteocalcin, there was an inverse relationship that was moderate with total 25(OH)D (r=−0.52, p<0.01), with a trend toward a weak inverse relationship with free 25(OH)D (r=−0.33, p=0.08) among subjects with normal albumin. For those with low albumin, there was a weak positive correlation between osteocalcin and free 25(OH)D (r=0.34, p=0.01).
DISCUSSION
Given its role in bone metabolism, muscle function, cancer prevention, and innate immune function1, vitamin D is a particularly important prohormone for patients with cirrhosis, who suffer from accelerated loss of bone mineral density, sarcopenia, high risk for hepatocellular carcinoma, and infections. This has led the American Gastroenterological Association to recommend vitamin D supplementation to a goal of 25–30 ng/mL to all patients with cirrhosis19. In clinical practice, repletion of vitamin D, which requires frequent monitoring of levels and prescribing of high-dose vitamin D, can result in significant resource-utilization in this population of patients.
In this study evaluating vitamin D status in patients with cirrhosis, total 25(OH)D deficiency was highly prevalent in cirrhotics, confirming data from prior studies2–4,20. However, in our cohort, total 25(OH)D levels were substantially lower in those with low albumin levels despite a similar proportion taking vitamin D supplementation, average daily sun exposure, and use of sunscreen. Importantly, while total 25(OH)D levels were inversely correlated with iPTH in those with normal synthetic function, as is expected in adults without chronic liver disease1,21,22, we observed no significant association between total 25(OH)D and iPTH among those with serum albumin levels ≤3.5 g/dL. Similar relationships were observed between total 25(OH)D and serum CTX, a marker of bone resorption, in subjects with normal but not low albumin, further strengthening our findings. Our data challenge the accuracy of total 25(OH)D as a marker of vitamin D status in the subgroup of cirrhotics with synthetic dysfunction.
Not surprisingly, we found that total vitamin D binding protein levels were substantially lower in cirrhotics with synthetic dysfunction. However, while % free 25(OH)D was slightly – but statistically significantly – higher in subjects with albumin ≤3.5 g/dL, we did not observe higher free 25(OH)D concentrations in the low compared to normal albumin patients. Subjects with albumin >3.5g/dl showed the expected inverse relationship between free 25(OH)D and iPTH, but the group with low albumin (and low vitamin D binding protein) did not, although in neither group were the iPTH levels out of the normal range. One possible clue for this unexpected result comes from the substantially higher 1,25(OH)2D levels in subjects with normal versus low albumin levels. This suggests an impaired efficiency in the conversion of 25(OH)D to 1,25(OH)2D in the subjects with low albumin and is consistent with their lower free 25(OH)D concentrations (6.2 vs. 8.6 pg/ml). Whether the subjects with more advanced cirrhosis, as evidenced by greater synthetic dysfunction, had substances in their circulation that altered the availability of free 25(OH)D to suppress iPTH or facilitate 1,25(OH)2D production remains to be seen.
We are cautious about over-interpretation of our analyses of multiple markers of bone turnover. However, we note that all three of the bone formation markers that we measured – bone-specific alkaline phosphatase, P1NP, and osteocalcin – were elevated in both groups of cirrhotics, while the bone resorption marker, serum CTX, was not. This provides novel evidence for a potential pathophysiologic mechanism – increased osteoblast function – underlying the high rates of low bone mineral density reported in cirrhotics7,8, but stands in contrast to to prior work that has demonstrated uncoupled activity of osteoclasts and osteoblasts resulting in increased osteoclast and depressed osteoblast function23,24. Clearly, this is an area in need of additional investigation to better understand the mechanism of osteoporosis in patients with cirrhosis.
One limitation of this study is that we recruited patients for this study year-round, potentially limiting the data by seasonal variation in vitamin D levels. However, the primary intent of our study was to evaluate vitamin D binding protein levels and the intra-subject relationship between total and free 25(OH)D, which should be preserved regardless of the time of the year. A second limitation is that we used iPTH as a serum marker of bone metabolism rather than actual measurements of bone mineral density. Clinically detectable changes in bone mineral density occur after long-standing vitamin D deficiency, so future investigation evaluating the association between vitamin D and bone mineral density in patients with normal and low albumin will require longitudinal measurements of vitamin D with long-term follow-up. We acknowledge that the use of albumin, a “negative” acute-phase reactant, to categorize our cohort subgroups might have resulted in misclassification of patients into the low albumin group. However, a sensitivity analysis categorizing the cohort by low versus normal vitamin D binding protein levels rather than serum albumin levels did not alter the qualitative relationships between total 25(OH)D or free 25(OH)D and iPTH (data not shown).
In conclusion, cirrhotics with synthetic dysfunction as evidenced by low serum albumin have lower levels of vitamin D binding protein, total 25(OH)D and free 25(OH)D compared to cirrhotics with normal albumin. The expected relationship between total 25(OH)D and iPTH was observed in cirrhotics with normal but not low albumin. These results demonstrate that total 25(OH)D is not an accurate marker of bioactive vitamin D status in cirrhotics with low albumin and call into question our current practices of measuring and aggressively repleting vitamin D in this population.
Supplementary Material
KEY POINTS.
-
-
Total 25(OH)D deficiency is highly prevalent in cirrhotics, but few are free 25(OH)D deficient.
-
-
Cirrhotics with synthetic dysfunction, characterized by low albumin, have lower levels of vitamin D binding protein and, thus, lower total and free 25(OH)D levels compared to cirrhotics without synthetic dysfunction.
-
-
The expected relationship between total or free 25(OH)D with iPTH was observed in normal but not low albumin cirrhotics.
-
-
Total 25(OH)D is not an accurate marker of bioactive vitamin D status in cirrhotics with synthetic dysfunction, raising questions regarding the best way to assess and manage vitamin D status in this population.
Acknowledgements
There are no acknowledgements.
Financial support: This study was funded by NIH P30 DK026743 (UCSF Liver Center) and an American College of Gastroenterology Clinical Research Award, NIH R21 AG041660 and NIH RO1 AR050023.
Abbreviations
- OH
hydroxyl
- iPTH
intact parathyroid hormone
- CTX
C-telopeptide
- P1NP
amino-terminal pro-peptide of type 1 collagen
- HCV
hepatitis C virus
- BMI
body mass index
- MELD
model for end-stage liver disease
Footnotes
Disclosures: The authors of this manuscript have no conflicts of interest to disclose as described by Liver International.
Writing Assistance: None.
Author contributions:
Lai: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; study supervision
Bikle: Study concept and design; interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content
Lizaola: Acquisition of data; drafting of the manuscript; study supervision
Hayssen: Acquisition of data; drafting of the manuscript; study supervision
Terrault: Study concept and design; critical revision of the manuscript for important intellectual content
Schwartz: Study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis
REFERENCES
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Fisher L, Fisher A. Vitamin D and Parathyroid Hormone in Outpatients With Noncholestatic Chronic Liver Disease. Clinical gastroenterology and Hepatology. 2007;5(4):513–520. doi: 10.1016/j.cgh.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Arteh J, Narra S, Nair S. Prevalence of Vitamin D Deficiency in Chronic Liver Disease. Dig Dis Sci. 2009;55(9):2624–2628. doi: 10.1007/s10620-009-1069-9. [DOI] [PubMed] [Google Scholar]
- 4.Lim LY, Chalasani N. Vitamin D Deficiency in Patients with Chronic Liver Disease and Cirrhosis. Curr Gastroenterol Rep. 2011;14(1):67–73. doi: 10.1007/s11894-011-0231-7. [DOI] [PubMed] [Google Scholar]
- 5.Hepner GW, Roginsky M, Moo HF. Abnormal vitamin D metabolism in patients with cirrhosis. Am J Dig Dis. 1976;21(7):527–532. doi: 10.1007/BF01464758. [DOI] [PubMed] [Google Scholar]
- 6.Crawford BA, Labio ED, Strasser SI, McCaughan GW. Vitamin D replacement for cirrhosis-related bone disease. Nat Clin Pract Gastroenterol Hepatol. 2006;3(12):689–699. doi: 10.1038/ncpgasthep0637. [DOI] [PubMed] [Google Scholar]
- 7.Gallego-Rojo FJ, Gonzalez-Calvin JL, Muñoz-Torres M, Mundi JL, Fernandez-Perez R, Rodrigo-Moreno D. Bone mineral density, serum insulin-like growth factor I, and bone turnover markers in viral cirrhosis. Hepatology. 1998;28(3):695–699. doi: 10.1002/hep.510280315. [DOI] [PubMed] [Google Scholar]
- 8.Carey EJ, Balan V, Kremers WK, Eileen HJ. Osteopenia and osteoporosis in patients with end-stage liver disease caused by hepatitis C and alcoholic liver disease: not just a cholestatic problem. Liver Transpl. 2003;9(11):1166–1173. doi: 10.1053/jlts.2003.50242. [DOI] [PubMed] [Google Scholar]
- 9.Diamond T, Stiel D, Mason R, et al. Serum vitamin d metabolites are not responsible for low turnover osteoporosis in chronic liver disease. J Clin Endocrinol Metab. 1989;69(6):1234. doi: 10.1210/jcem-69-6-1234. [DOI] [PubMed] [Google Scholar]
- 10.Matloff DS, Kaplan MM, Neer RM, Goldberg MJ, Bitman W, Wolfe HJ. Osteoporosis in primary biliary cirrhosis: effects of 25-hydroxyvitamin D3 treatment. Gastroenterology. 1982;83(1):97–102. [PubMed] [Google Scholar]
- 11.Herlong HF, Recker RR, Maddrey WC. Bone disease in primary biliary cirrhosis: histologic features and response to 25-hydroxyvitamin D. Gastroenterology. 1982;83(1):103–108. [PubMed] [Google Scholar]
- 12.Crippin JS, Jorgensen RA, Dickson ER, Lindor KD. Hepatic osteodystrophy in primary biliary cirrhosis: effects of medical treatment. Am J Gastroenterol. 1994;89(1):47–50. [PubMed] [Google Scholar]
- 13.Collier J. Bone disorders in chronic liver disease. Hepatology. 2007;46(4):1271–1278. doi: 10.1002/hep.21852. [DOI] [PubMed] [Google Scholar]
- 14.Miroliaee A, Nasiri-Toosi M, Khalilzadeh O, Esteghamati A, Abdollahi A, Mazloumi M. Disturbances of parathyroid hormone-vitamin D axis in non-cholestatic chronic liver disease: a cross-sectional study. Hepatol Int. 2010;4(3):634–640. doi: 10.1007/s12072-010-9194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crawford BAL, Kam C, Donaghy AJ, McCaughan GW. The heterogeneity of bone disease in cirrhosis: a multivariate analysis. Osteoporosis International. 2003;14(12):987–994. doi: 10.1007/s00198-003-1495-z. [DOI] [PubMed] [Google Scholar]
- 16.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the Free Fraction of 25-Hydroxyvitamin D in Serum and Its Regulation by Albumin and the Vitamin D-Binding Protein. Journal of Clinical Endocrinology and Metabolism. 1986;63(4):954–959. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- 17.Bikle DD, Gee E. Free, and not total, 1,25-dihydroxyvitamin D regulates 25-hydroxyvitamin D metabolism by keratinocytes. Endocrinology. 1989;124(2):649–654. doi: 10.1210/endo-124-2-649. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz JB, Lai J, Lizaola B, et al. A comparison of direct and calculated free 25(OH) Vitamin D levels in clinical populations. J Clin Endocrinol Metab. 2014 doi: 10.1210/jc.2013-3874. jc.2013–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Gastroenterological Association, Pressman A. American gastroenterological association medical position statement: Osteoporosis in hepatic disorders. Gastroenterology. 2003;125(1):937–940. doi: 10.1016/s0016-5085(03)01060-6. [DOI] [PubMed] [Google Scholar]
- 20.Malham M. Vitamin D deficiency in cirrhosis relates to liver dysfunction rather than aetiology. World J Gastroenterol. 2011;17(7):922. doi: 10.3748/wjg.v17.i7.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338(12):777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 22.Chapuy MC, Preziosi P, Maamer M, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporosis International. 1997;7(5):439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 23.Guichelaar MMJ, Malinchoc M, Sibonga JD, Clarke BL, Eileen HJ. Bone histomorphometric changes after liver transplantation for chronic cholestatic liver disease. J Bone Miner Res. 2003;18(12):2190–2199. doi: 10.1359/jbmr.2003.18.12.2190. [DOI] [PubMed] [Google Scholar]
- 24.Giouleme OI, Vyzantiadis TA, Nikolaidis NL, et al. Pathogenesis of osteoporosis in liver cirrhosis. Hepatogastroenterology. 2006;53(72):938–943. [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.