Abstract
This study presents a progressive FastICA peel-off (PFP) framework for high density surface electromyogram (EMG) decomposition. The novel framework is based on a shift-invariant model for describing surface EMG. The decomposition process can be viewed as progressively expanding the set of motor unit spike trains, which is primarily based on FastICA. To overcome the local convergence of FastICA, a “peel off” strategy (i.e. removal of the estimated motor unit action potential (MUAP) trains from the previous step) is used to mitigate the effects of the already identified motor units, so more motor units can be extracted. Moreover, a constrained FastICA is applied to assess the extracted spike trains and correct possible erroneous or missed spikes. These procedures work together to improve the decomposition performance. The proposed framework was validated using simulated surface EMG signals with different motor unit numbers (30, 70, 91) and signal to noise ratios (SNRs) (20, 10, 0 dB). The results demonstrated relatively large numbers of extracted motor units and high accuracies (high F1-scores). The framework was also tested with 111 trials of 64-channel electrode array experimental surface EMG signals during the first dorsal interosseous (FDI) muscle contraction at different intensities. On average 14.1 ± 5.0 motor units were identified from each trial of experimental surface EMG signals.
Index Terms: FastICA, constrained FastICA, high density surface EMG, decomposition, motor unit spike train, MUAP waveform estimation
I. Introduction
The electromyogram (EMG) signal is the algebraic summation of motor unit action potential (MUAP) trains from different active motor units within electrode recording range. The motor unit is the smallest organizational and functional element of the neuromuscular system, and provides a fundamental framework for investigation of neuromuscular control. EMG decomposition involves breaking down the multiunit EMG signal into the contributions of the underlying MUAP trains. EMG decomposition makes it possible to estimate MUAP waveforms which can be used to detect characteristic changes associated with particular neuromuscular diseases (through quantitative MUAP analysis [1]). EMG decomposition also provides timing information of motor unit discharges, making it possible to examine the behavior of individual motoneurons in the human central nervous system (through monitoring motor unit recruitment and discharge rate). Such information leads to an understanding of neural control strategies prevailing in normal conditions, as well as their alterations in pathological conditions.
Since the concept of EMG decomposition arose in the early 1970s, a series of computer-based decomposition studies has been reported, including studies by De Luca and co-workers [2–5 and others], Stashuk and co-workers [6–9 and others], McGill and co-workers [10–13 and others], and different investigators [14–19 and others] (refer to [20] for a comprehensive review). These studies were mainly based on intramuscular EMG recordings using selective indwelling electrodes. For intramuscular EMG, the MUAPs from different motor units tend to have distinctive spikes that remain relatively constant from discharge to discharge. Therefore, morphological information of the MUAP waveforms can be used to sort action potentials from different active motor units. Tremendous efforts have been focused on separating the waveforms generated by the superposition of several MUAP spikes. The timing information of motor unit discharge can be helpful during this process [12].
In contrast to the many studies on intramuscular EMG decomposition, there have been fewer attempts reported regarding decomposition of surface EMG, which will do much to overcome the disadvantages (such as pain, risk of infection, etc.) of invasive indwelling EMG recording. This is primarily due to the heavy MUAP superposition routinely existing in surface EMG signals. Furthermore, the volume conductor effects of skin and subcutaneous body tissues can cause surface MUAP shapes from different motor units to be less distinguishable than in intramuscular recordings [21]. All of these make surface EMG decomposition a very challenging task, especially at relatively high force levels.
In the past decade, significant advances in both surface EMG recording and processing techniques have greatly promoted the development in surface EMG decomposition [22–27]. In particular, the design of high density surface EMG electrode arrays (comprised of a number of tiny electrode probes with small inter-probe distance) provides spatial information to supplement temporal information for motor unit discrimination. These arrays make it feasible to extract single motor unit activity from surface EMG at very low force levels with two-dimensional template matching methods [28] [29]. More complicated multiple channel signal processing techniques were also proposed for high density surface EMG decomposition [30–35]. For example, among these efforts (with high density surface EMG) a most distinguished achievement was by Holobar and colleagues, who developed decomposition methods based on convolution kernel compensation (CKC) [26] [36–38], which allowed extraction of a number of simultaneously discharged motor units from high density surface EMG.
Independent component analysis (ICA) was among the early efforts for high density surface EMG decomposition, but it met with only limited success (i.e., limited to extraction of a very small number of motor units) [31–34]. This is primarily due to the fact that ICA algorithms addressing an instantaneous mixing model are not most appropriate for decomposition of high density surface EMG [39]. In the current study, we propose a novel progressive FastICA peel-off (PFP) framework which can overcome the limitations of the previously used ICA methods for high density surface EMG decomposition. The performance of the proposed method was assessed by both simulated and experimental high density surface EMG signals. The results demonstrate that the framework can successfully extract activity of a number of simultaneously discharged motor units from high density surface EMG.
II. Methods
A. Introduction of FastICA
On account of its high convergence speed (at least quadratic convergence) and ease of use (no need to set the step size parameter), FastICA is one of the most popular and effective methods to solve problems in blind source separation. This technique measures non-Gaussianity as a separation criterion using negentropy to find the independent sources from their mixtures. As introduced in [40] [41], negentropy of a random variable y with zero mean and unit variance can be approximated with non-polynomial functions:
| (1) |
where G is a nonquadratic function (a default use can be G (x) = log (cosh(x)) ), υ is a standard normal random variable.
For the pre-whitened signal x, to find one independent component y= wTx, the following optimization problem needs to be solved:
| (2) |
Then an updating rule based on a fixed-point algorithm is presented below (refer to [40] for details):
| (3) |
In order to reduce the accumulation of errors, a parallel version using a symmetric orthogonalization step to replace the normalization step in (3) can handle n independent components in the same time, as illustrated below:
| (4) |
where W = [w1, w2, …, wn]T.
B. Data Model
A shift-invariant model can be used to describe multi-channel surface EMG signal [36]. If MUAP waveforms from different active motor units remain constant for a specific channel, the signal of this channel can be viewed as a sparse combination of these waveforms. Assuming N active motor units recorded by M surface electrodes: x = [x1, x2, …, xM]T, the signal on each channel can be described as:
| (5) |
where aij stands for the waveform of j th motor unit in the i th channel, L is the length of the waveform. sj (t) = Σkδ(t − Tj(k)) is a binary variable (i.e. either 0 or 1) that indicates whether the j th motor unit discharges at a specific time t. Tj(k) is the k th discharge time of the j th motor unit, δ represents Dirac Delta function. It is assumed that Tj(k+1) − Tj(k) > L for ∀k ∈ N. ni (t) represents the additive white-zero-mean Gaussian noise in i th channel.
Note that Equation (5) is a convolution model. To facilitate application of FastICA, we can use a “convolution to linearization” strategy to express (5) as a matrix form [41]. Define the following extended vectors:
where K is a proper delay. Then the convolutive mixing model can be written as:
| (6) |
where à is a matrix containing all the waveform coefficients aij in a proper order (for details, please refer to [36]).
C. Application of FastICA
After expressing (5) in the form of (6), it would be appropriate to apply FastICA. It should be noted that the output of FastICA is actually a filtering result of all the channel signals and their delays. So the waveforms do not necessarily convey physiologically meaningful information the way the MUAP waveforms themselves do. The primary aim of this step is to obtain the discharge time information of the different motor units (i.e. s in (5)) from the outputs of FastICA.
D. “Peel off” Strategy with Estimation of MUAP Waveforms
FastICA usually converges to local solutions. In EMG decomposition, some solution regions are relatively small and difficult to be found, particularly given random initialization. Although the orthogonalization steps in FastICA can help avoid repeating the same solutions, such processing is not sufficient for extracting a higher number of motor units.
Mitigating the influence of the already identified MUAP trains (i.e. those easily convergent solutions with FastICA) turns out to be helpful for extracting more motor units. Therefore, it is important to estimate the MUAP waveforms with the identified spike trains (from FastICA) and subtract them from the original signal. Then the residual signal can be further processed with FastICA. Such a “peel off” strategy helps extract more motor units. The MUAP waveform estimation process is introduced below.
For simplicity, considering only one channel in (5):
| (7) |
Without loss of generality, suppose we have obtained q(q<N) spike trains of different motor units: sj(j=1, 2, …, q). In order to estimate the waveforms of these q motor units in this channel, a straightforward approach is to solve the following least squares problem [42]:
| (8) |
where X denotes the vector containing all the sample points of x, A(q) = [a1(0), a1(1),…,a1(L − 1),…, aq(L−1)T is a vector containing all the waveforms of q motor units, and S(q) is the vector containing the full content of sj across time and the q motor units. A(q) * S(q) denotes a vector of the same size with X, formed by convolving aj with sj for each motor unit and then summing across the q motor units. The analytical solution can be expressed as [42]:
| (9) |
where S̑ is a matrix formed by all the elements of S(q), which satisfies S̑A(q) = A(q) * S(q). The solution contains the best estimation of MUAP waveforms from q motor units in the least squares sense. Then the residual signal (i.e. can be obtained.
E. Constrained FastICA for Assessment of Spike Trains
To ensure the decomposition performance of the “peel off” strategy, two issues need to be considered. First, we should verify that the spike trains from the residual signal are not “fake” spike trains induced by cumulative errors. Instead, they are valid independent components corresponding to active motor units. Second, for each of the already confirmed valid spike trains, we should double-check that its discharge times are complete and accurate. This is particularly important for reliable estimation of MUAP waveforms to be subtracted. Therefore, for each valid spike train, a procedure is required to correct erroneous or missed spikes. Such spikes may be induced by noise or estimation error (particularly when processing residual signals).
We propose the use of constraint FastICA [43] [44] to address the above two issues. In more details, we can use a candidate spike train as a constraint to drive FastICA processing of the original signal to converge toward an independent component mostly similar to the spike train. If the constrained FastICA fails to achieve this, it is assumed that the candidate spike train under testing is not valid (and therefore rejected). For the validated spike train, we further compare its discharge times with the constrained FastICA output (which usually contains more definite information), and update the spike train by correcting erroneous or missed spikes. For elusive spikes, this process can be repeated to help decision making.
Compared to FastICA, the optimization problem of the constrained FastICA [43] [44] can be expressed as follows:
For the pre-whitened data x,
| (10) |
where g(y) is a measure of closeness between output y= wTx and reference signal r in the sense of correlation. In our case r is a spike train to be assessed, ξ(0≤ξ≤1) is a preset lower bound of the optimum correlation. Since the constraint E {r2}−1 = 0 can be dealt with separately, it can be removed from the constraints.
The above problem can be solved by an augmented Lagrangian function:
| (11) |
where μ and λ are Lagrange multipliers, γ is penalty factor. The Newton-like learning gradient can be used directly to update w [44]:
| (12) |
Note that λ has been canceled, which simplifies the algorithm structure, facilitating its easy use.
F. Decomposition Framework
Given the above, the PFP framework for high density surface EMG decomposition can be summarized below.
Let x = [x1, x2, …, xM]T represent the original signal, and v̂ = [v̂1, v̂2, …, v̂M]T represent the residual signal. At the beginning, set v̂ = x.
Step 1. Express v̂ in a proper extended form.
Step 2. Apply FastICA on extended v̂ to extract non-repetitive spike trains. If no new results emerge, go to step 5.
Step 3. For each spike train obtained in step 2, apply constrained FastICA on x (also applied to the extended form) using the spike train as a reference signal to test the reliability and correct possible erroneous or missed spikes. Store the reliable spike trains to the set ψ. If no reliable spike trains emerge, go to step 5.
Step 4. For each channel of x, use all of the spike trains belonging to ψ to estimate the MUAP waveforms, which are used to update residual signal v̂. Then go to Step 1.
Step 5. Output ψ, and the algorithm ends.
G. Tips for Framework Implementation
Since our algorithm is not completely automatic, below we provide some suggestions or tips on setting or adjusting relevant parameters.
Tip 1 (on the output number n of FastICA)
The whole framework is indeed a deflationary process. However, in order to reduce the accumulation of errors and accelerate the process of decomposition, we use a symmetric version of FastICA (i.e. Equation (4)), which can handle n independent components in the same time. At the beginning we can set the output number n to be a relatively large value (such as 10), and then it can be decreased in the subsequent period to search for more accurate solutions (note that when n=1, Equation (4) will degenerate to Equation (3)).
Tip 2 (on the delay factor K)
In FastICA, we can set K to be a relatively large value (e.g. the sample length corresponding to 2–4 ms) at the beginning and then gradually reduce it during the process because the residual signal tends to be more and more whitened. A large K value will facilitate precise estimate of the outputs, but meanwhile impose calculation cost and convergence difficulty. In constrained FastICA as a test tool, we set K to be a larger value (default value 15 ms).
Tip 3 (on the lower bound of the correlation coefficient ξ in the constrained FastICA)
It can be difficult to estimate how much the optimum correlation coefficient should be. For robustness, ξ can be set close to 1 in the beginning and then gradually decrease (e.g. in each iteration it can be multiplied by 0.97, or by other proper approaches).
Tip 4 (on the length of the waveform L)
Note that there may be a little time shift between the estimated and actual spike trains (associated with the delay factor K). In order to accurately implement the “peel off” step, the spike trains extracted from FastICA should be shifted back a short period (usually 5–10 ms). Meanwhile L should be slightly longer than the real waveform duration (L can be usually set to be 25–40 ms, depending on the preset time shift).
Tip 5 (on the thresholds used to extract spike trains from the outputs of FastICA)
This threshold needs manual adjustment. Taking advantage of the orthogonalization step in the FastICA (which implies E{(wTx)} = 1), we can set the threshold to be a relatively uniform value. In this study the absolute values of the thresholds were usually set between 3 and 5. The values were slightly adjusted depending on specific circumstances.
Tip 6 (toward more automatic threshold setting for spike train extraction from FastICA outputs)
A nonlinear energy operator (NEO) [45], defined as Φ[x(t)] = x2(t)−x(t−1)x(t+1), can be used to facilitate automatic threshold setting. NEO processing can result in signals with positive and more conspicuous or striking spikes. Therefore, it is an appropriate tool for spike detection and consequently reducing the uncertainty of threshold setting. After the NEO processing, we recommend setting the threshold as mean ± 2~3 SD (standard deviation) of the spike sequences to more automatically or robotically extract spike trains from the outputs of FastICA.
III. Performance Evaluation with Simulated Signals
A. Surface EMG Simulation
Our surface EMG simulation primarily relies on a motoneuron pool model [46] describing motor unit recruitment and discharge patterns, and a surface EMG model [47] describing MUAP waveforms from different motor units. The different components for this simulation are briefly presented below.
MUAP simulation
The simulation of MUAPs was mainly based on a tripole model as described in [47], and the generation and extinction of the action potentials at the fiber end-plate and tendon were also considered. A cylindrical muscle with a radius of 8 mm was simulated. The thickness of fat and skin layers was set to be 2.5 mm, but for simplicity their volume conductor effects were not considered in the simulation. There were approximately 70,000 muscle fibers from 120 motor units distributed in parallel within the muscle. The fibers of each motor unit were randomly scattered in a circular territory, with a density of approximately 20 fibers/mm2 (can be higher with muscle boundary). Following an exponential function, the number of muscle fibers per motor unit or the motor unit territory was simulated to have a wide range (resulting in a 100-fold range of twitch force as simulation in [46]). The muscle conduction velocity was correlated to the diameter of fibers as described in [48]. Other parameters primarily followed [46–48], as summarized in Table 1.
Table 1.
Parameters setting used for surface EMG simulation
| Distribution | Mean | SD | Range | |
|---|---|---|---|---|
| Fiber number | Uniform | 70000 | ±0.5 mean | |
| MU fiber endplate center position | Uniform | 0 | ±8 mm | |
| Fiber endplate position variation | Uniform | 0 | ±2 mm | |
| Half fiber length | Gaussian | 40 mm | 4 mm | ±2 SD |
| Mean fiber diameter for a MU | Gaussian | 55 μm | 10 μm | ±2 SD |
| Fiber diameter variation within a MU | Gaussian | 0 μm | 1 μm | ±2 SD |
| ISI variation | Gaussian | 0 | 0.2* instant mean ISI | ±2 SD |
Motor unit recruitment and discharge patterns
The distribution of motoneuron or motor unit recruitment thresholds was modelled by an exponential function [46]. Therefore, many motor units had low thresholds and few had relatively high thresholds. Each motor unit discharged at 8 Hz once excitation exceeded its recruitment threshold. The peak discharge rate was 25 Hz for the first (lowest threshold) motor unit and 35 Hz for the last (highest threshold) one. The last motor unit was recruited at 50% maximal excitation. The firing rate of each motor unit increased linearly with the excitation level until the peak value was reached. In [46], different motor unit discharge patterns were simulated (including those following or violating the “onion-skin” phenomenon). For evaluation of our decomposition framework, this is not a critical issue. So, only one motor unit discharge pattern was used. Three excitation levels (2.7%, 10%, 20% maximum excitation) were simulated, which corresponded to 30, 70 and 91 active motor units. For a 64-channel recording system (as mentioned in next paragraph), the three excitation levels provided an opportunity to model and examine different situations in terms of the number of sources and the number of available observations, namely over-determined, mild under-determined, and serious under-determined situations.
Surface EMG and recording configuration
For each excitation level, a 10 s contraction was simulated. The excitation level increased from 0 to a specific excitation level in the first 2 s, remained constant for 7 s, and decreased to 0 in the last 1 s. The inter-spike intervals (ISI) for each motor unit were simulated as a random process with a Gaussian probability function. The simulated surface EMG was recorded by a 64-channel surface electrode array (arranged in 8 by 8 channels, with inter-electrode distance of 4 mm for both horizontal and vertical directions). The electrode array was placed with its columns aligned parallel to the muscle fiber direction and its center electrodes located approximately over the innervation zones. The simulated signals were recorded at a sampling rate of 2 kHz per channel. Different SNRs ranging from 0 dB, 10 dB and 20 dB were also simulated with additive zero-mean Gaussian noise (spatially independent). For each condition, 5 trials of simulation were performed. Thus a total of 45 surface EMG signals were simulated for decomposition performance evaluation (3 excitation levels × 3 SNR levels × 5 trials).
B. Evaluation Criteria
In order to assess decomposition performance, the decomposition Recall and Precision were calculated as follows [49]:
| (13) |
where TPj (true positives) denotes the number of correctly identified discharges for the j th motor unit, FNj (false negatives) is the number of unidentified discharges, and FPj (false positives) is the number of misplaced discharges.
Note that there may be a little time shift between the true spike trains and the estimated ones. A cross-correlation function can be used to facilitate their coupling. First, calculate the following parameters:
| (14) |
where R•,• (t) represents cross-correlation function. ρ* is the maximum cross-correlation coefficient between the estimated spike train s̃ and the set of simulated spike trains. If ρ*≥0.3, we can accept existence of a simulated spike train corresponding to s̃ (in fact, if there is no corresponding spike train, ρ* will generally be less than 0.1; otherwise it will generally be greater than 0.5). Then j* is the indicator of the corresponding spike train. Meanwhile, the corresponding delay t* can also be estimated. After such a time shift, all the parameters can be calculated easily and the discharge time tolerance was set equal to ± 3 ms.
One should note that Recall and Precision are mutually restrained. Therefore, F1-score was used as a measure of decomposition performance. F1-score is the harmonic mean of Recall and Precision [49]:
| (15) |
C. Evaluation Results
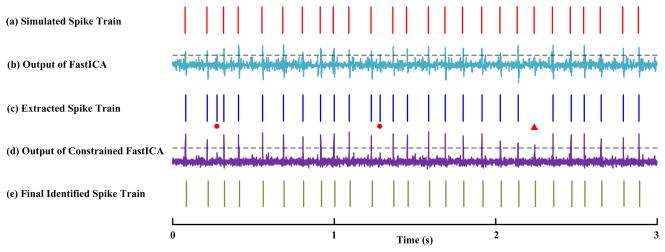
Figure 1 shows how the decomposition process can benefit from the constrained FastICA, where a specific step using constrained FastICA during the decomposition process is demonstrated. A surface EMG with SNR of 0 dB and 10% excitation level (70 active motor units) was simulated. Figure 1(a) shows the true spike train used for simulation, while correspondingly Figure 1(b) is the output of applying FastICA to the residual signal from the previous step. After a threshold was applied, a spike train can be identified, but with erroneous spikes (as indicated by
 ) and missed spikes (indicated by
) and missed spikes (indicated by
 ) (Figure 1(c)). Figure 1(d) shows the output of constrained FastICA (using the spike train shown by Figure 1(c) as a constraint). Finally, with the extra information from Figure 1(d), the corrected spike train can be obtained as shown in Figure 1(e), which matches well with Figure 1(a).
) (Figure 1(c)). Figure 1(d) shows the output of constrained FastICA (using the spike train shown by Figure 1(c) as a constraint). Finally, with the extra information from Figure 1(d), the corrected spike train can be obtained as shown in Figure 1(e), which matches well with Figure 1(a).
Fig. 1.
A demonstration of constrained ICA for correcting possible erroneous or missed spikes during the decomposition process. The processed surface EMG was simulated in a condition of 0 dB SNR and 10% maximum excitation (70 active motor units). This figure shows a specific step using constrained FastICA during the decomposition process. (a) A simulated spike train; (b) The output of applying FastICA to the residual signal from the previous step; (c) The extracted spike train from applying a threshold (the dashed line) to (b); (d) The output of constrained FastICA using (c) as a constraint, which allows correction of possible erroneous (demonstrated by
 ) or missed spikes (demonstrated by
) or missed spikes (demonstrated by
 ). (e) The final identified spike train which matches the model input (a).
). (e) The final identified spike train which matches the model input (a).
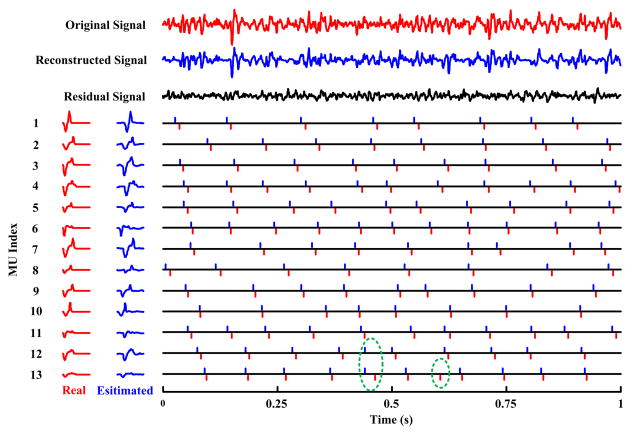
Figure 2 demonstrates an example of one channel simulated surface EMG and the decomposed results (MUAP waveforms and discharge time). The processed 64-channel surface EMG was simulated with SNR of 20 dB and 10% excitation level (70 active motor units). The (one channel) simulated surface EMG, the reconstructed signal using all identified motor units (with Recall ≥90% and Precision ≥90%) and the residual signal after decomposition are all demonstrated (Top Panel). In this example in total 13 motor units were identified. The index beside each motor unit indicates the order in which it was extracted from the signal. We can observe a good matching between the decomposed results and the “real” MUAP waveforms (Left-Bottom Panel), as well as between the estimated and actual discharge times (Right-Bottom Panel).
Fig. 2.
An example of decomposition of simulated surface EMG signal. The processed surface EMG was simulated in a condition of 20 dB SNR and 10% maximum excitation (70 active motor units). Top Panel: The three signals from top to bottom show one channel simulated surface EMG (of a 64-channel electrode array), the signal reconstructed using all identified motor units and the residual signal after decomposition. Left-Bottom Panel: The waveforms on the left (in red) show the MUAP templates on this specific channel used for surface EMG simulation, whereas the waveforms on the right (in blue) show the estimated MUAP templates from the identified spike train. Right-Bottom Panel: The comparison of the real spike train (red bars in the bottom) used in simulation and the identified spike train (blue bars on the top). Note there might be a slight time shift between true and estimated waveforms and spike trains. In this figure, the blue bars indicate the instants of 10 ms before the FastICA outputs. Setting a relatively long shifting back period can facilitate reliable MUAP waveform estimation.
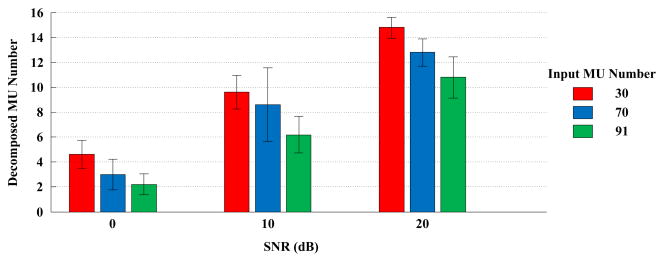
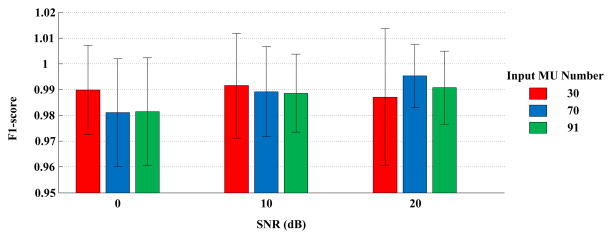
The PFP decomposition framework was tested with all the simulated surface EMG signals. Figure 3 summarizes the average number of motor units with Recall ≥90% and Precision ≥90%. Figure 4 presents the average F1-score of the identified motor units.
Fig. 3.
Average number of identified motor units (decomposed MU number) with recall ≥90% and precision ≥90%, depending on different input motor unit numbers and SNR levels. The results are averaged over 5 simulation runs.
Fig. 4.
Average F1-score per reconstructed motor unit, depending on different input motor unit numbers and SNR levels. The results are averaged over 5 simulation runs.
IV. Testing With Experimental Signals
A. Description of Experimental Signals
The experimental surface EMG signals used for testing the proposed framework were acquired from the first dorsal interosseous (FDI) muscle of twelve healthy subjects. The procedures were approved by the Institutional Review Board of Northwestern University (Chicago, USA). All the subjects gave their written consent before the experiment. Subjects were seated upright in a mobile Biodex chair (Biodex, Shirley, NY). A standard 6 degrees-of-freedom load cell (ATI Inc, Apex, NC) setup was used to accurately record the isometric contraction force of the FDI muscle during index finger abduction. Standard procedures were followed to minimize spurious force contributions from unrecorded muscles as described in [50]. Surface EMG signals were recorded from the FDI muscle using a flexible 2-dimensional 64-channel (8 × 8, individual recording probe 1.2 mm in diameter, center-to-center distance 4 mm) surface electrode array (TMS International BV, The Netherlands). The skin of the tested muscle was carefully prepared and the electrode array was attached to the FDI muscle with a double adhesive sticker and further secured with medical tapes. The maximum voluntary contraction (MVC) was first measured; after that, each subject was asked to generate an isometric contraction force of the FDI muscle at different contraction levels. The subject was asked to maintain the force as stable as possible for at least 5 s. The actual percent MVC for each contraction was calculated afterwards by normalizing the force measurement (averaged from the stable force period) to each subject’s MVC. A Refa128 amplifier (TMS International BV, The Netherlands) was used to record surface EMG signals. The signals were sampled at 2 kHz per channel, with a band pass filter setting at 10–500 Hz.
B. Decomposition Outcomes
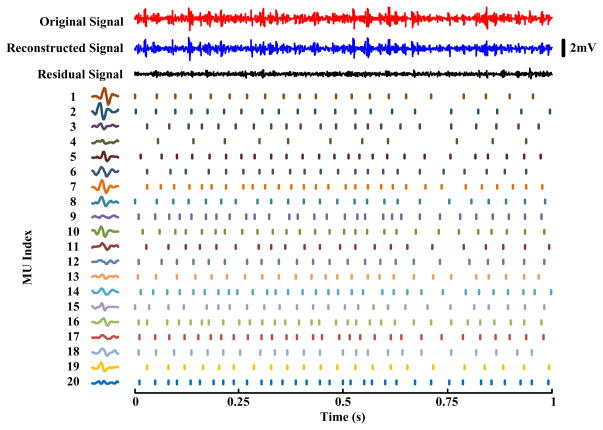
Figure 5 shows an example of experimental surface EMG decomposition at a constant FDI muscle contraction level (approximately 55% MVC). One channel experimental surface EMG signal, the reconstructed signal using all identified motor units and the residual signal after decomposition are all demonstrated (Top Panel). Twenty motor units were extracted. In the figure, the MUAP waveform (on this specific channel) and time instants of each motor unit are also presented. The index along with each motor unit indicates its order extracted from the signal.
Fig. 5.
An example of experimental surface EMG decomposition. The signal was recorded with a 64-channel electrode array during a healthy subject’s isometric contraction of FDI muscle at approximately 55% MVC. Top Panel: The three signals from top to bottom show one channel surface EMG, the signal reconstructed using all identified motor units and the residual signal after decomposition. Left-Bottom Panel: The estimated MUAP waveform of each motor unit on this specific channel. Right-Bottom Panel: The identified spike train for each motor unit. The motor unit index indicates the order of the motor units extracted from the signal. The ratio of the residual signal energy to original signal energy for this channel is 21%. The ratio averaged from all the channels was 23% ± 8%.
In total 111 trials of experimental high density surface EMG signals were tested. Most of the tested signals were recorded at relatively small to middle muscle contraction levels while few were at very strong levels. The tested trials had an average muscle contraction level of 35% MVC (range: 1.5%–100% MVC). After processing all the tested experimental signals, we found on average 14.1±5.0 motor units were extracted from each trial. The coefficient of variation (CoV) of the inter-spike interval was calculated for each motor unit, and the average CoV was 0.23 ± 0.07. For each tested signal, the residual energy after the decomposition was also calculated across all the channels by subtracting the contribution of the decomposed motor units. On average, the energy of the residual signal was 37% ± 16% of the original signal.
V. Discussion
Surface EMG decomposition has been a research focus in the past decade, and progress has been made with advancement in both surface EMG acquisition and processing techniques [22–27]. In particular, the development of high density surface EMG techniques (i.e. using electrode arrays comprised of a number of recording probes with tiny skin-electrode contact area and small inter-probe distance) has allowed application of different multi-channel signal processing techniques for surface EMG decomposition [26–38].
Among different multiple signal processing methods, independent component analysis (ICA) has been used for surface EMG decomposition based on the inversion of the instantaneous mixing model. For example, Nakamura et al. applied FastICA to extract MUAP trains from electrode array EMG signals [31] [32]. Different ICA algorithms have been tested for separating the MUAP trains [33–35]. Unfortunately, previous ICA-based methods demonstrated limited success for surface EMG decomposition, because the primary effect of ICA processing appeared to be increasing the sparseness of the signal, rather than separating the activity from single motor units (see a recent review [39]). This is due to the fact that ICA is most appropriate for dealing with instantaneous data mixtures, ideally assuming that the mixed components detected by different sensors vary only in amplitude. In such a case, a variety of methods have been well developed to separate the sources. However, for surface EMG, due to different volume conductor effects (i.e. low pass filter effects) of the skin and subcutaneous body tissues between a motor unit and the different recording electrodes over the skin, the MUAP has a different shape at each recording site [51]. Therefore, the limited success of previous ICA applications for EMG decomposition lies in the over-simplified assumption of surface EMG signal as instantaneous data mixture models (rather than convolutive data models).
So far, more successful developments in high density surface EMG decomposition have been based on the convolutive models (that allow for the spatial variation of MUAP shapes), among which the most distinguished achievement is a convolution kernel compensation (CKC) approach, proposed by Holobar and Zazula [36], to sequentially identifying single motor unit activity. This technique has been validated in the past decade under different situations, including in muscles with highly diverse anatomy, and in patients with neurodegenerative diseases (Parkinson, type II diabetes) [52–55].
All the work presented in this study is based on a shift-invariant model (Equation (5), also used in the CKC approach [36]), which can well describe surface EMG. In the PFP framework, the most important step (indeed, also the ultimate goal of the whole processing) is to estimate each motor unit’s spike train (i.e. s in Equation (5)). Once a spike train can be correctly estimated, the information of its origin motor unit will become available, since the MUAP waveform can be reliably estimated from Equation (9). In this regard, the PFP surface EMG decomposition framework can be viewed as a process of progressively expanding the set of spike trains.
In this study, the primary purpose of FastICA is to estimate motor unit spike trains. A “convolution to linearization” strategy can be used to convert Equation (5) to an instantaneous mixture model (Equation (6)), thus facilitating application of FastICA. It is noteworthy that in such case, the independent component obtained by FastICA does not contain morphological information of the MUAP, whereas the key information derived here is the motor unit spike train. This is an important improvement over previous ICA-based approaches for surface EMG decomposition [31–35].
The lack of satisfactory performance of previous FastICA methods for surface EMG decomposition also lies in the local convergence of FastICA, which limits the number of decomposed motor units to a great extent. In order to extract more motor units, one strategy used in our framework is to make full use of already identified motor units. Therefore, the MUAP waveforms are estimated and a “peel off” procedure is employed to subtract the already identified MUAP trains from the original signal. Such processing facilitates FastICA to find more solutions (i.e. extraction of more motor units). Conversely, FastICA does not require pre-determination of the number of independent components, which is difficult to know but acts as an important factor in many other ICA algorithms [41]. Different independent components can be estimated individually with FastICA, which also facilitates the “peel off” procedure.
As mentioned earlier, progressive expansion of the set of motor unit spike trains is the ultimate goal of the whole processing. Correct estimation of spike trains is very important to ensure smooth and effective implementation of the framework. Therefore, a constrained FastICA is introduced to assess the extracted spike trains and correct possible erroneous or missed spikes. Such a procedure further improves the decomposition performance.
To sum up, in the PFP framework, FastICA is used to search motor unit spike trains. A “peel off” procedure is employed to mitigate the effects of the already identified motor units on the FastICA convergence, so more motor units can emerge. Constrained FastICA is applied to assess the extracted spike trains and correct possible erroneous or missed spikes. All these factors together result in a good decomposition performance. As demonstrated in our simulation study, when the SNR level is relatively high, on average the developed framework can decompose more than 10 motor units with F1-scores close to 1 for different numbers of input motor units (Figures 2–4). The experimental study also demonstrated higher decomposition yield (Figure 5) compared with the previous ICA-based methods.
Study Limitations
Motor unit synchronization
ICA is founded on the assumption that sources within a mixture are independent. A classical view based largely on visual inspection of needle EMG recordings for coincident motor units is that they discharge almost independently [56] [57]. Such a view has been revised in the light of the findings of a number of studies in which rigorous statistical analyses of motor unit data have been performed [58–61 and many others]. FastICA used in the developed framework applies a sub-optimal “non-Gaussian” criterion (rather than the “independence” in a mathematically strict sense) as a signal separation criterion. Its objective is to find statistically independent components in the general “non-Gaussian” case. If MUAP trains remain super-Gaussian variables (or if they are sparse), FastICA can capture sufficient information through the sparsity of MUAP trains for separating them. This is similar to the CKC algorithm which also depends on the sparse model. However, it should be acknowledged that the sparsity of MUAP trains tends to be affected with motor unit synchronization (particularly at relative high synchronization levels). If the sparsity of MUAP trains is destroyed by motor unit synchronization, the appropriateness of the FastICA based framework is questioned. Therefore, further studies are needed to quantitatively examine how different motor unit synchronization levels may influence the performance of the proposed framework for surface EMG decomposition.
MUAP shape alteration
The PFP framework requires the assumption that MUAPs from the same motor unit remain stationary over time, which might not always be the case in reality, even during constant force isometric contractions. For example, previous studies reported that the MUAP waveforms undergo alteration in shape with muscle fatigue [62], temperature changes [63], or in other situations such as during dynamic contractions [64]. Because of the shift-invariant model assumption, MUAP waveform alterations during a contraction may influence the decomposition performance. Therefore, it is important to minimize the factors contributing to MUAP waveform changes for the best capacity of applying the developed framework.
Underdetermined problem
Like most blind source separation algorithms, in order to recover all the sources, ICA typically requires that the number of sources is less than or equal to the number of observations. For an underdetermined problem, many blind source separation algorithms (including FastICA) have also demonstrated effectiveness for extracting at least a portion of the sources [41] [65]. The practical limitation in surface electrode array recording determines that surface EMG decomposition is often an underdetermined problem. In such a situation, the developed framework can still be able to discriminate a certain number of motor units (with F1-scores close to 1) through iteratively detecting the most sparse components, as demonstrated in our simulation study. We also observed that for a given electrode array, the decomposition yield in underdetermined situations tends to be affected by the increased MUAP superposition, due to higher input motor unit numbers (i.e. increased extent of underdetermination) and their firing rates (Figure 3). The issue of decomposing all the constituent motor units reaches beyond the current state of the art in surface EMG decomposition. Nonetheless, considering maximum possible recording channels in an electrode array design, how to increase decomposition yield remains an important research topic to be addressed in the future, especially for an underdetermined situation.
Performance validation
For experimental surface EMG data, one approach to quantifying decomposition performance is “2-source” validation using simultaneous intramuscular and high density surface EMG recordings [66]. The agreement on the discharge time instants of the common motor units decomposed independently from both intramuscular and surface EMG will provide a validation of the decomposition performance. This approach is difficult to perform in the current study due to the lack of simultaneous intramuscular EMG recording. An alternative approach is to divide 64 channels’ surface EMG to two groups with equal numbers of channels and perform the “2-source” validation by decomposing each group separately using the proposed framework. However, under the proposed ICA-based framework, it is predictable that such an evaluation approach would result in an agreement of the common motor units from the two groups. Indeed, we performed such an analysis and confirmed this prediction. Therefore, “2-source” validation with simultaneous intramuscular EMG recording remains our future work for quantification of experimental high density surface EMG decomposition performance. In addition, the proposed decomposition framework was only tested in the FDI muscle in this study. Further testing with different muscles would be necessary for an extensive validation.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China under Grant 81271658, and in part by the National Institutes of Health of the U.S. Department of Health and Human Services under Grant R01NS080839. The authors would like to thank Faezeh Jahanmiri-Nezhad, PhD and Xiaoyan Li, PhD for helping collection of experimental high density surface EMG data used in this study.
Biographies

Maoqi Chen received the B.S. degree in electronic science and technology from the University of Science and Technology of China, Hefei, China, in 2013. He is currently a graduate student in the biomedical engineering program of the same university. His research interests include blind source separation and biomedical signal processing.

Ping Zhou (S’01 M’05 SM’07) received the B.S. degree in electrical engineering and the M.S. degree in biomedical engineering from the University of Science and Technology of China, Hefei, China, in 1995 and 1999, respectively, and the Ph.D. degree in biomedical engineering from Northwestern University, Evanston, IL, USA, in 2004.
From 1999 to 2014, he was progressively a Research Assistant, Research Associate, full time and adjunct research faculty at the Rehabilitation Institute of Chicago, Chicago, IL, USA. He was also an Adjunct Research Assistant and later Associate Professor in Physical Medicine and Rehabilitation of Northwestern University, Chicago, IL, USA, from 2006 to 2014. He currently holds an Adjunct Associate Professor position in Physical Medicine and Rehabilitation at the University of Texas Health Science Center at Houston, TX, USA. He directs the NeuroMyo Engineering for Rehabilitation laboratory housed in the outpatient clinic at TIRR Memorial Hermann Research Center. He is also an affiliated Professor in the biomedical engineering program of the University of Science and Technology of China. His research interests include biomedical signal (in particular, EMG) processing, motor unit pathophysiology and electrodiagnosis, myoelectric control, and assistive devices for neurorehabilitation.
Contributor Information
Maoqi Chen, Email: hiei@mail.ustc.edu.cn, Biomedical Engineering Program, University of Science and Technology of China, Hefei, 230027 China.
Ping Zhou, Email: dr.ping.zhou@ieee.org, Department of Physical Medicine and Rehabilitation, University of Texas Health Science Center and TIRR Memorial Hermann Research Center, Houston, TX, 77030 USA, and also with the Biomedical Engineering Program, University of Science and Technology of China, Hefei, 230027 China.
References
- 1.Phongsamart G, Wertsch JJ. Quantitative electromyography. Phys Med Rehabil Clin N Am. 2003 May;14(2):231–41. doi: 10.1016/s1047-9651(02)00124-9. [DOI] [PubMed] [Google Scholar]
- 2.LeFever RS, De Luca CJ. A procedure for decomposing the myoelectric signal into its constituent action potentials--Part I: Technique, theory, and implementation. IEEE Trans Biomed Eng. 1982 Mar;29(3):149–57. doi: 10.1109/tbme.1982.324881. [DOI] [PubMed] [Google Scholar]
- 3.LeFever RS, Xenakis AP, De Luca CJ. A procedure for decomposing the myoelectric signal into its constituent action potentials--Part II: Execution and test for accuracy. IEEE Trans Biomed Eng. 1982 Mar;29(3):158–64. doi: 10.1109/TBME.1982.324882. [DOI] [PubMed] [Google Scholar]
- 4.De Luca CJ. Decomposition of the EMG signal into constituent motor unit action potentials. Muscle Nerve. 1995;18(12):1492–4. [PubMed] [Google Scholar]
- 5.Nawab SH, Wotiz RP, De Luca CJ. Decomposition of indwelling EMG signals. J Appl Physiol (1985) 2008 Aug;105(2):700–10. doi: 10.1152/japplphysiol.00170.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stashuk D, de Bruin H. Automatic decomposition of selective needle-detected myoelectric signals. Biomedical Engineering, IEEE Transactions on. 1988;35(1):1–10. doi: 10.1109/10.1330. [DOI] [PubMed] [Google Scholar]
- 7.Stashuk DW, Naphan RK. Probabilistic inference-based classification applied to myoelectric signal decomposition. IEEE Trans Biomed Eng. 1992 Apr;39(4):346–55. doi: 10.1109/10.126607. [DOI] [PubMed] [Google Scholar]
- 8.Stashuk DW. Decomposition and quantitative analysis of clinical electromyographic signals. Medical Engineering & Physics. 1999;21(6):389–404. doi: 10.1016/s1350-4533(99)00064-8. [DOI] [PubMed] [Google Scholar]
- 9.Parsaei H, Stashuk DW. EMG signal decomposition using motor unit potential train validity. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2013;21(2):265–74. doi: 10.1109/TNSRE.2012.2218287. [DOI] [PubMed] [Google Scholar]
- 10.McGill KC, Dorfman LJ. High-resolution alignment of sampled waveforms. IEEE Trans Biomed Eng. 1984 Jun;31(6):462–8. doi: 10.1109/TBME.1984.325413. [DOI] [PubMed] [Google Scholar]
- 11.McGill KC. Optimal resolution of superimposed action potentials. IEEE Trans Biomed Eng. 2002 Jul;49(7):640–50. doi: 10.1109/TBME.2002.1010847. [DOI] [PubMed] [Google Scholar]
- 12.McGill KC, Lateva ZC, Marateb HR. EMGLAB: an interactive EMG decomposition program. Journal of Neuroscience Methods. 2005;149(2):121–33. doi: 10.1016/j.jneumeth.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Florestal JR, Mathieu PA, McGill KC. Automatic decomposition of multichannel intramuscular EMG signals. J Electromyogr Kinesiol. 2009 Feb;19(1):1–9. doi: 10.1016/j.jelekin.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Loudon GH, Jones NB, Sehmi AS. New signal processing techniques for the decomposition of EMG signals. Med Biol Eng Comput. 1992 Nov;30(6):591–9. doi: 10.1007/BF02446790. [DOI] [PubMed] [Google Scholar]
- 15.Fang J, Agarwal GC, Shahani BT. Decomposition of multiunit electromyographic signals. IEEE Trans Biomed Eng. 1999 Jun;46(6):685–97. doi: 10.1109/10.764945. [DOI] [PubMed] [Google Scholar]
- 16.Gut R, Moschytz GS. High-precision EMG signal decomposition using communication techniques. IEEE Transactions on Signal Processing. 2000;48(9):2487–94. [Google Scholar]
- 17.Zennaro D, Wellig P, Koch VM, Moschytz GS, Laubli T. A software package for the decomposition of long-term multichannel EMG signals using wavelet coefficients. IEEE Trans Biomed Eng. 2003 Jan;50(1):58–69. doi: 10.1109/TBME.2002.807321. [DOI] [PubMed] [Google Scholar]
- 18.Erim Z, Lin W. Decomposition of intramuscular EMG signals using a heuristic fuzzy expert system. IEEE Transactions on Biomedical Engineering. 2008;55(9):2180–9. doi: 10.1109/TBME.2008.923915. [DOI] [PubMed] [Google Scholar]
- 19.Florestal JR, Mathieu PA, Malanda A. Automated decomposition of intramuscular electromyographic signals. IEEE Trans Biomed Eng. 2006 May;53(5):832–9. doi: 10.1109/TBME.2005.863893. [DOI] [PubMed] [Google Scholar]
- 20.Parsaei H, Stashuk DW, Rasheed S, Farkas C, Hamilton-Wright A. Intramuscular EMG signal decomposition. Crit Rev Biomed Eng. 2010;38(5):435–65. doi: 10.1615/critrevbiomedeng.v38.i5.20. [DOI] [PubMed] [Google Scholar]
- 21.Zhou P, Rymer WZ. MUAP number estimates in surface EMG: template-matching methods and their performance boundaries. Ann Biomed Eng. 2004 Jul;32(7):1007–15. doi: 10.1023/b:abme.0000032463.26331.b3. [DOI] [PubMed] [Google Scholar]
- 22.Merletti R, Aventaggiato M, Botter A, Holobar A, Marateb H, Vieira TM. Advances in surface EMG: recent progress in detection and processing techniques. Crit Rev Biomed Eng. 2010;38(4):305–45. doi: 10.1615/critrevbiomedeng.v38.i4.10. [DOI] [PubMed] [Google Scholar]
- 23.Rau G, Schulte E, Disselhorst-Klug C. From cell to movement: to what answers does EMG really contribute? Journal of Electromyography and Kinesiology. 2004;14(5):611–617. doi: 10.1016/j.jelekin.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Lapatki BG, Van Dijk JP, Jonas IE, Zwarts MJ, Stegeman DF. A thin, flexible multielectrode grid for high-density surface EMG. Journal of Applied Physiology. 2004;96(1):327–36. doi: 10.1152/japplphysiol.00521.2003. [DOI] [PubMed] [Google Scholar]
- 25.Nawab SH, Chang SS, De Luca CJ. High-yield decomposition of surface EMG signals. Clin Neurophysiol. 2010 Oct;121(10):1602–15. doi: 10.1016/j.clinph.2009.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holobar A, Farina D, Gazzoni M, Merletti R, Zazula D. Estimating motor unit discharge patterns from high-density surface electromyogram. Clin Neurophysiol. 2009 Mar;120(3):551–62. doi: 10.1016/j.clinph.2008.10.160. [DOI] [PubMed] [Google Scholar]
- 27.Farina D, Holobar A, Merletti R, Enoka RM. Decoding the neural drive to muscles from the surface electromyogram. Clin Neurophysiol. 2010 Oct;121(10):1616–23. doi: 10.1016/j.clinph.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 28.Kleine BU, van Dijk JP, Lapatki BG, Zwarts MJ, Stegeman DF. Using two-dimensional spatial information in decomposition of surface EMG signals. Journal of electromyography and kinesiology. 2007;17(5):535–48. doi: 10.1016/j.jelekin.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Gligorijevic I, van Dijk JP, Mijovic B, Van Huffel S, Blok JH, De Vos M. A new and fast approach towards sEMG decomposition. Med Biol Eng Comput. 2013 May;51(5):593–605. doi: 10.1007/s11517-012-1029-y. [DOI] [PubMed] [Google Scholar]
- 30.Zazula D, Holobar A. An approach to surface EMG decomposition based on higher-order cumulants. Comput Methods Programs Biomed. 2005 Dec;80(Suppl 1):S51–60. doi: 10.1016/s0169-2607(05)80006-9. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura H, Yoshida M, Kotani M, Akazawa K, Moritani T. The application of independent component analysis to the multi-channel surface electromyographic signals for separation of motor unit action potential trains: part I-measuring techniques. J Electromyogr Kinesiol. 2004 Aug;14(4):423–32. doi: 10.1016/j.jelekin.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura H, Yoshida M, Kotani M, Akazawa K, Moritani T. The application of independent component analysis to the multi-channel surface electromyographic signals for separation of motor unit action potential trains: part II-modelling interpretation. J Electromyogr Kinesiol. 2004 Aug;14(4):433–41. doi: 10.1016/j.jelekin.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Garcia GA, Okuno R, Akazawa K. A decomposition algorithm for surface electrode-array electromyogram. A noninvasive, three-step approach to analyze surface EMG signals. IEEE Eng Med Biol Mag. 2005 Jul-Aug;24(4):63–72. doi: 10.1109/memb.2005.1463398. [DOI] [PubMed] [Google Scholar]
- 34.Jiang N, Farina D. Covariance and time-scale methods for blind separation of delayed sources. IEEE Trans Biomed Eng. 2011 Mar;58(3):550–6. doi: 10.1109/TBME.2010.2084999. [DOI] [PubMed] [Google Scholar]
- 35.Theis FJ, Garcia GA. On the use of sparse signal decomposition in the analysis of multi-channel surface electromyograms. Signal Processing. 2006;86(3):603–23. [Google Scholar]
- 36.Holobar A, Zazula D. Multichannel blind source separation using convolution kernel compensation. IEEE Transactions on Signal Processing. 2007;55(9):4487–96. [Google Scholar]
- 37.Holobar A, Zazula D. Correlation-based decomposition of surface electromyograms at low contraction forces. Medical and Biological Engineering and Computing. 2004;42(4):487–95. doi: 10.1007/BF02350989. [DOI] [PubMed] [Google Scholar]
- 38.Glaser V, Holobar A, Zazula D. Real-time motor unit identification from high-density surface EMG. IEEE Trans Neural Syst Rehabil Eng. 2013 Nov;21(6):949–58. doi: 10.1109/TNSRE.2013.2247631. [DOI] [PubMed] [Google Scholar]
- 39.Holobar A, Farina D. Blind source identification from the multichannel surface electromyogram. Physiol Meas. 2014 Jul;35(7):R143–65. doi: 10.1088/0967-3334/35/7/R143. [DOI] [PubMed] [Google Scholar]
- 40.Hyvarinen A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Netw. 1999;10(3):626–34. doi: 10.1109/72.761722. [DOI] [PubMed] [Google Scholar]
- 41.Hyvarinen A, Karhunen J, Oja E. Independent Component Analysis. John Wiley & Sons; 2001. [Google Scholar]
- 42.Pillow JW, Shlens J, Chichilnisky EJ, Simoncelli EP. A model-based spike sorting algorithm for removing correlation artifacts in multi-neuron recordings. PLoS One. 2013;8(5):e62123. doi: 10.1371/journal.pone.0062123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.James CJ, Gibson OJ. Temporally constrained ICA: an application to artifact rejection in electromagnetic brain signal analysis. IEEE Transactions on Biomedical Engineering. 2003;50(9):1108–16. doi: 10.1109/TBME.2003.816076. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z. Fixed-point algorithms for constrained ICA and their applications in fMRI data analysis. Magnetic Resonance Imaging. 2011;29(9):1288–303. doi: 10.1016/j.mri.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maragos P, Kaiser JF, Quatieri TF. On amplitude and frequency demodulation using energy operators. IEEE Trans Signal Processing. 1993 Apr;41:1532 50. [Google Scholar]
- 46.Fuglevand AJ, Winter DA, Patla AE. Models of recruitment and rate coding organization in motor-unit pools. J Neurophysiol. 1993 Dec;70(6):2470–88. doi: 10.1152/jn.1993.70.6.2470. [DOI] [PubMed] [Google Scholar]
- 47.Merletti R, Lo Conte L, Avignone E, Guglielminotti P. Modeling of surface myoelectric signals. I. Model implementation. IEEE Transactions on Biomedical Engineering. 1999;46(7):810–20. doi: 10.1109/10.771190. [DOI] [PubMed] [Google Scholar]
- 48.Duchene J, Hogrel JY. A model of EMG generation. IEEE Trans Biomed Eng. 2000 Feb;47(2):192–201. doi: 10.1109/10.821754. [DOI] [PubMed] [Google Scholar]
- 49.Goutte C, Gaussier E. A probabilistic interpretation of precision, recall and F-score, with implication for evaluation. Lecture Notes in Computer Science. 2005;3408:345–59. [Google Scholar]
- 50.Li X, Suresh A, Zhou P, Rymer WZ. Alterations in the peak amplitude distribution of the surface electromyogram poststroke. IEEE Trans Biomed Eng. 2013 Mar;60(3):845–52. doi: 10.1109/TBME.2012.2205249. [DOI] [PubMed] [Google Scholar]
- 51.Farina D, Cescon C, Merletti R. Influence of anatomical, physical, and detection-system parameters on surface EMG. Biological Cybernetics. 2002;86(6):445–56. doi: 10.1007/s00422-002-0309-2. [DOI] [PubMed] [Google Scholar]
- 52.Marateb HR, McGill KC, Holobar A, Lateva ZC, Mansourian M, Merletti R. Accuracy assessment of CKC high-density surface EMG decomposition in biceps femoris muscle. J Neural Eng. 2011 Dec;8(6):066002. doi: 10.1088/1741-2560/8/6/066002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holobar A, Minetto MA, Botter A, Negro F, Farina D. Experimental analysis of accuracy in the identification of motor unit spike trains from high-density surface EMG. IEEE Trans Neural Syst Rehabil Eng. 2010 Jun;18(3):221–9. doi: 10.1109/TNSRE.2010.2041593. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe K, Gazzoni M, Holobar A, Miyamoto T, Fukuda K, Merletti R, Moritani T. Motor unit firing pattern of vastus lateralis muscle in type 2 diabetes mellitus patients. Muscle Nerve. 2013 Nov;48(5):806–13. doi: 10.1002/mus.23828. [DOI] [PubMed] [Google Scholar]
- 55.Holobar A, Glaser V, Gallego JA, Dideriksen JL, Farina D. Non-invasive characterization of motor unit behaviour in pathological tremor. J Neural Eng. 2012 Oct;9(5):056011. doi: 10.1088/1741-2560/9/5/056011. [DOI] [PubMed] [Google Scholar]
- 56.Taylor A. The significance of grouping of motor unit activity. J Physiol. 1962;162:259–69. doi: 10.1113/jphysiol.1962.sp006930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kranz H, Baumgartner G. Human alpha motoneurone discharge, a statistical analysis. Brain Res. 1974;67(2):324–9. doi: 10.1016/0006-8993(74)90282-0. [DOI] [PubMed] [Google Scholar]
- 58.Datta AK, Stephens JA. Synchronization of motor unit activity during voluntary contraction in man. J Physiol. 1990;422:397–419. doi: 10.1113/jphysiol.1990.sp017991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmied A, Descarreaux M. Influence of contraction strength on single motor unit synchronous activity. Clin Neurophysiol. 2010;121(10):1624–32. doi: 10.1016/j.clinph.2010.02.165. [DOI] [PubMed] [Google Scholar]
- 60.Winges SA, Santello M. Common input to motor units of digit flexors during multi-digit grasping. J Neurophysiol. 2004;92(6):3210–20. doi: 10.1152/jn.00516.2004. [DOI] [PubMed] [Google Scholar]
- 61.De Luca CJ, Kline JC. Statistically rigorous calculations do not support common input and long-term synchronization of motor-unit firings. J Neurophysiol. 2014;112(11):2729–44. doi: 10.1152/jn.00725.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fortune E, Lowery MM. Effect of extracellular potassium accumulation on muscle fiber conduction velocity: a simulation study. Ann Biomed Eng. 2009;37(10):2105–17. doi: 10.1007/s10439-009-9756-4. [DOI] [PubMed] [Google Scholar]
- 63.Bertram MF, Nishida T, Minieka MM, Janssen I, Levy CE. Effects of temperature on motor unit action potentials during isometric contraction. Muscle Nerve. 1995;18(12):1443–6. doi: 10.1002/mus.880181215. [DOI] [PubMed] [Google Scholar]
- 64.De Luca CJ, Chang SS, Roy SH, Kline JC, Nawab SH. Decomposition of surface EMG signals from cyclic dynamic contractions. J Neurophysiol. 2014 Dec 24; doi: 10.1152/jn.00555.2014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomas J, Deville Y, Hosseini S. Differential fast fixed-point algorithms for underdetermined instantaneous and convolutive partial blind source separation. IEEE Transactions on Signal Processing. 2007;55(7):3717–29. [Google Scholar]
- 66.Mambrito B, De Luca CJ. A technique for the detection, decomposition and analysis of the EMG signal. Electroencephalography and Clinical Neurophysiology. 1984;58(2):175–88. doi: 10.1016/0013-4694(84)90031-2. [DOI] [PubMed] [Google Scholar]