Abstract
Despite the tremendous progress made in the treatment of cerebrovascular occlusive diseases, many patients suffering from ischemic brain injury still experience dismal outcomes. Although rehabilitation contributes to post stroke functional recovery, there is no doubt that interventions that promote the restoration of blood supply are proven to minimize ischemic injury and improve recovery. In response to the acutely decreased blood perfusion during arterial occlusion, arteriogenesis, the compensation of blood flow through the collateral circulation during arterial obstructive diseases can act not only in a timely fashion but also much more efficiently compared to angiogenesis, the sprouting of new capillaries, and a mechanism occurring in a delayed fashion while increases the total resistance of the vascular bed of the affected territory. Interestingly, despite the vast differences between the two vascular remodeling mechanisms, some crucial growth factors and cytokines involved in angiogenesis are also required for arteriogenesis. Understanding the mechanisms underlying vascular remodeling after ischemic brain injury is a critical step towards the development of effective therapies for ischemic stroke. The present article will discuss our current views in vascular remodeling acutely after brain ischemia, namely arteriogenesis, and some relevant clinical therapies available on the horizon in augmenting collateral flow that hold promise in treating ischemic brain injury.
Keywords: arteriogenesis, angiogenesis, stroke, carotid diseases, anastomosis, vascular remodeling
Collateral circulation, the conduit for arteriogenesis, is a specialized vascular network with a distinct phenotype from the regular arteries, veins or capillaries (Faber et al., 2014) and plays an important role in the development and outcome of ischemic diseases. Native collaterals are naturally occurring artery-to-artery or arteriole-to-arteriole anastomoses present in healthy tissue, whereas they undergo outward remodeling in order to compensate for reduced blood flow in response to vascular obstructive diseases. However, the development of collaterals over time in the adult CNS can paradoxically be linked to chronic pathophysiology such as hypoperfusion.
1. The role of collateral circulation in the risk and outcome of ischemic stroke
In addition to factors involved in cerebral autoregulation, the extent of cerebral collateralization directly contributes to the cerebrovascular reserve capacity, which in turn, affecting the hemodynamics (Vernieri et al., 2001). For this very reason, cerebral collateral circulation has long been reported to alter the risk of stroke (Norris et al., 1990, Schomer et al., 1994). In patients with symptomatic severe internal carotid artery (ICA) stenosis, the risk at 2 years of stroke or TIA is significantly reduced among those with angiographically defined collaterals compared to those without (Henderson et al., 2000). Ample clinical evidences also suggest that the collateral status is an independent predictor of outcome and response to thrombolytic therapies in patients with ischemic stroke, and a good collateral status can lower the rate of hemorrhagic transformation after thrombolytic and endovascular therapies (Liebeskind, 2003, Shuaib et al., 2011b, Liu et al., 2014).
A number of collateral circuits exist within and between the extracranial and intracranial circulations, making the external carotid artery (ECA) a potential source of collateral flow when the ICA experiences chronic stenosis or occlusion. The intracranial collateral circuits are further divided into primary (i.e., circle of Willis) or secondary (e.g., ophthalmic artery and leptomenigeal vessels) collateral pathways (Liebeskind, 2003, Shuaib et al., 2011b, Liu et al., 2014). Unilateral ICA occlusion often led to enhanced collateral flow through the anterior Circle of Willis as well as an increase in the diameter of the anterior communicating arteries (AComA) (Hartkamp et al., 1999), possibly due to the relatively short distance between the AComA and the Circle of Willis. In contrast, bilateral ICA occlusion led to both increased collateral flow through the posterior circle of Willis and an increase in the diameter of the posterior communicating artery (Hartkamp et al., 1999), likely due to the increased demand for collateral compensation. Furthermore, in patients with ICA occlusion, sometimes vertebrobasilar arteries appeared to be responsible for blood flow in the territory of the MCA ipsilateral to the occluded ICA, whereas the interhemispheric cross flow from the contralateral ICA provided collateral flow to the ACA territory of the ischemic side (van Laar et al., 2007), which could potentially reduce the risk of internal hemodynamic watershed infarction (Hendrikse et al., 2002, Bisschops et al., 2003). In patients with complete occlusion of the intracranial ICA and/or the MCA (M1 or M2 segments), enhancement of leptomeningeal collaterals on CT angiography was correlated with lower post-stroke mortality (Lima et al., 2010). Nonetheless, patients with both MCA occlusion and rapid recruitment of secondary collateral pathways usually fared more favorably in the progression of symptoms post-stroke (Maas et al., 2009). In the patients who received endovascular revascularization therapy, angiographically defined collateral grade determined the recanalization rate and clinical outcome (Bang et al., 2008, Bang et al., 2011a, Bang et al., 2011b, Liebeskind, 2013).
2. Genetics governing variance in collateral circulation
It has long been recognized that significant variation in infarct volume exist in inbred mouse strains. Using quantitative trait locus (QTL) analysis, a number of loci were mapped from independent laboratories and were found to modulate the stroke severity in mice. Among which, two overlapping loci, namely Civq1 (cerebral infarct volume QTL1) (Keum and Marchuk, 2009)) and Canq1 (collateral artery number QTL1) (Zhang et al., 2010, Wang et al., 2012) on mouse chromosome #7, contributes to greater than 50% of the variation in infarct volume among 15–16 inbred strains of mice and 37% of the phenotypic variance in pial collateral number and the extent of collateral remodeling between C57BL/6 and BALB/c respectively. In an elegant follow-up study, among a series of congenic mouse strains in the gene Dce1, a refined region of Candq1, from C57BL/6 were introgressed into the genome, there was an allele dose-dependent rescue of collateral extent, blood flow and reduction of ischemic severity (Sealock et al., 2014). Although these studies provide indisputable relationship between the extent of native collaterals and ischemic injury, genes modulating infarct volume independently of the extent of collateral circulation have also been identified (Chu et al., 2013), adding to the complexity of biological response to ischemic.
3. Regulation of collateral flow and arteriogenesis
The native collateral system is determined during the embryonic stages and is fully developed by the third week of life in mice (Chalothorn and Faber, 2010). VEGF levels contribute to collateral formation in the embryo, as evidenced by the fewer pial collaterals found in the VEGFlo/+ mice compared to wild type or VEGFhi/+ mice, or in BALB/c mice compared to C57BL/6 mice (Clayton et al., 2008). A reduction in the number and diameter of collaterals in the adult, also known as collateral rarefaction, occurs with aging (Faber et al., 2011, Hecht et al., 2012), eNOS-dependent endothelial dysfunction (Dai and Faber, 2010) the presence of vascular risk factors (Menon et al., 2013). Similar to humans with metabolic syndrome, type II diabetes mice db/db also exhibit impaired leptomeningeal collateral flow poor outcome after MCA stroke (Akamatsu et al., 2015 In press), coincided with diminished eNOS phosphorylation (Li et al., 2013).
Arteriogenesis is triggered by fluid shear stress (FSS) following a sudden occlusion or a chronically progressing stenosis in the artery, resulting in the activation of endothelium, monocyte invasion and recruitment, activation of additional inflammatory responses, secretion of growth factors and cytokines, followed by matrix digestion and ultimately proliferation of smooth muscle cells involved in the outward remodeling of collateral vessels and the increase of collateral flow (Heil and Schaper, 2004, van Oostrom et al., 2008, Fung and Helisch, 2012, la Sala et al., 2012, Troidl and Schaper, 2012, Liu et al., 2014). The molecular mechanisms involved in this complex process are discussed below.
3.1 Arteriogenesis is triggered by fluid shear stress rather than hypoxia
FSS refers to the tangential component of frictional forces generated at the vessel wall by the flow of a viscous fluid such as blood. Because arterial occlusion lowers the pressure in the distal vasculature, it increases flow through pre-existing collaterals due to the resulting pressure gradient. The initial increase of FSS exerted by the blood on the endothelium as it flows by activates the endothelium and stimulates a cascade of signaling events (Pipp et al., 2004, Schierling et al., 2009a, Schierling et al., 2011), leading to the conductance of the collateral vessels. As the collateral vessels grow in diameter, fluid shear stress falls and collateral flow decreases, providing a self-regulating mechanism. The collateral flow developed proximal to the occlusion does not require the local expression of either vascular endothelial growth factor (VEGF) or hypoxia-inducible factor 1 (HIF1) (Lee et al., 2004), although VEGF is known to play a role in the formation of collateral vessels during development and the continuing remodeling of collateral vessels. It is this mechanosensitive feature of arteriogenesis that prominently differentiates itself from other types of vascular growth such as angiogenesis. By maximizing FSS via an artificial arteriovenous (AV) shunt, collateral artery growth can be induced beyond the physiological extent and can overcome anatomical restrictions (Eitenmuller et al., 2006). FSS can induce arteriogenesis in both peripheral and cerebral circulations. In the latter, Schierling and colleagues created an AV fistula between the distal stump of the ligated carotid artery and the adjacent jugular vein following the ligation of bilateral common carotid arteries. They have observed considerable arteriogenesis and vessel growth in the FSS stimulated arteries in this ligature-shunt model (Schierling et al., 2009a).
3.2 Activation of endothelium and nitric oxide
Endothelial cells lining the blood vessel lumen can directly sense changes in flow shear stress and transmit signals to smooth muscle cells in the media and fibroblast cells in the adventitia that are normally only exposed to transmural interstitial flow. Microarray data identified a potential role for the shear stress sensitive gene transient receptor potential cation channel subfamily V member 4 (Trpv4), a Ca2+ channel in endothelial cells, in transducing the stimuli induced by FSS. Pharmacological activation of Trpv4 strongly increased cerebral arteriogenesis and collateral flow (Schierling et al., 2011), while Trpv-channel blocker reduced collateral flow (Troidl et al., 2009a). Complementary to these findings, arteriogenesis was found impaired in mice lacking Trpv4 (Troidl et al., 2009a). Additional evidence suggested that actin-binding Rho activating protein might also be involved in FSS-induced arteriogenesis (Troidl et al., 2009b). Although the precise downstream signaling of Trpv4 has yet to be identified, Ca2+-dependent mechanisms including the activation of endothelial-derived hyperpolarization factor (EDHF), cyclooxygenase, as well as NOS are potential candidates (Troidl et al., 2009a, Schierling et al., 2011). Among these, the most likely molecule transmitting the signal triggered by FSS is NO, which can diffuse through basement membrane that separates the endothelial cells and smooth muscle cells and can also cross talk with prostacyclin (Osanai et al., 2001). Targeted deletion of the eNOS gene led to a loss of vasodilation but not of arteriogenesis, while targeted deletion of iNOS led to partial but not complete impairment of arteriogenesis. Only the deletion of both eNOS and iNOS resulted in a complete loss of collateral vessel remodeling during arterial occlusion (Troidl et al., 2010). On the other hand, contradictory evidence suggests that eNOS deficiency causes collateral vessel rarefaction and reduced expression of cell cycle genes in the eNOS-KO mice, associated with impaired proliferation of vascular wall cells involved in collateral remodeling (Dai and Faber, 2010). In addition to the key players mentioned above, FSS also upregulates endothelial microRNA-21 which suppresses its target gene phosphatase and tensin homolog (PTEN), contributing to increased eNOS phosphorylation and NO production (Weber et al., 2010).
3.3 Regulation of collateral flow by the immune system
Although the initiation of arteriogenesis does not require the presence of HIFs, hypoxia activates signaling pathways that lead to the production of crucial factors and regulators for collateral growth including VEGF and inflammatory cytokines (Rey and Semenza, 2010, Silvestre et al., 2013). HIF-1 can also induce integrin β2, which in turn induces adhesion and infiltration of leukocytes (Kong et al., 2007) and vascular progenitor cells into the hypoxic sites (Rey et al., 2009). On the other hand, the HIFs themselves are also subjected to regulation, in which the hydroxylation of HIFs for example, by the prolyl hydroxylase domain-containing proteins (PHDs), leads to further degradation of the proteins by proteases (Loinard et al., 2009). Conversely, downregulation of Phd2 expression in macrophages enhances collateral growth via TIE2-dependent signaling (Takeda et al., 2011, Hamm et al., 2013).
Emerging studies have identified functional roles for various innate and adaptive immune cell subsets as regulators of arteriogenesis, including monocytes/macrophages, T helper 17 (Th17) cells, regulatory T lymphocytes (Tregs), and natural killer (NK) cells (la Sala et al., 2012). Several lines of direct or indirect evidence support the critical role of monocytes and monocyte signaling in arteriogenesis. Pharmacological depletion of monocytes impairs arteriogenesis in rabbit and mouse models of hindlimb ischemia (Heil et al., 2002). Consistent results were also obtained in osteopetrotic mice with natural monocytopenia using a hindlimb ischemia model (Bergmann et al., 2006). In addition, adoptive transfer of wild type bone marrow mononuclear cells enhanced reperfusion following hindlimb ischemia (Capoccia et al., 2008). The improved reperfusion following wild type adoptive transfer was absent in mice defective in MCP-1/CCL2, suggesting that the recruitment of the inflammatory subset of monocytes to sites of ischemia is a critical step in collateral growth (Capoccia et al., 2008). As an indirect evidence for the crucial role of monocytes, mice lacking urokine plasminogen activator (uPA) showed less postischemic collateralization than wild type mice, reflected by an uPA-dependent reduction of leukocyte infiltration (Deindl et al., 2003). In additional to the presence of monocytes, collateral flow is also regulated by the phenotypes of infiltrated monocytes. The M1-type macrophages express iNOS and proinflammatory cytokines such as IL-1 and IL-12, whereas the M2-subset expresses arginase 1, anti-inflammatory cytokine IL-10 and VEGF (Silvestre et al., 2013, Rath et al., 2014). Deletion of one allele of the phd2 gene not only polarized macrophages toward a M2 phenotype but also promoted arteriogenesis after hindlimb ischemia, which was associated with an increased production of SDF-1 and PDGF-B (Takeda et al., 2011). The Phd2 gene regression was apparently mediated via Angiopoietin-1 (ANG1)/TIE2 because blockade of ANG1 by a soluble trap prevented the downregulation of Phd2 expression in macrophages and their phenotype switch, consequently impeding collateral growth (Hamm et al., 2013). A recent study by Troidl and colleagues determined the temporal and spatial distribution of macrophage subpopulation during arteriogenesis in a rat model of chronic FSS (Troidl et al., 2013). In this model, M2 macrophages appeared early following reperfusion and maintained up to 28 days, concentrated particularly in the region of collateral growth. M1 macrophages were also present, but to a lesser extent. In keeping with data from experimental studies, impaired chemotaxis of monocytes appeared to account for the impaired formation of collaterals in patients with diabetes (Waltenberger et al., 2000, Tchaikovski et al., 2009).
In some ways, arteriogenesis shares similarities with an active inflammatory process. For example, growing collaterals are characterized by the presence of infiltrating monocytes/macrophages in the lumen and in the perivascular space (Arras et al., 1998). However, it is unclear whether a specific type of inflammatory response is needed for arteriogenesis following an ischemic insult, or a usual ischemia-elicited inflammation suffices to induce collateral development if works in concert with sheer stress and gene products of activated endothelium. Gene expression profiling during collateral development in a mouse model of hindlimb ischemia indicated a prominent role for inflammatory response-related genes, including CXCL5, MCP-1, CXCL9, and CXCL10. Furthermore, the nature of a coordinated appearance of proinflammatory genes followed by anti-inflammatory genes suggests that inflammation contributes to the initiation of collateral development (Meisner and Price, 2010). Apart from cells contributing to the innate immunity, reduced infiltrating T lymphocytes were found in apoE−/− mice along with an impaired collateral flow following limb ischemia. In support of the role of T lymphocytes in arteriogenesis, asthymic nude mice had increased macrophage infiltrating the ischemic limb in the absence of T cells and a concurrently decreased VEGF expression and impaired recovery of blood flow following limb ischemia compare to C57 strain with normal T cells (Couffinhal et al., 1999).
Lastly, the infiltrating macrophages that are more commonly associated with inflammatory response also produce a number of angiogenic growth factors including MCP-1, VEGF, FGF, GM-CSF, HGF, TNF-α, TGF-β and PDGF (Schierling et al., 2009b) that are critical for the development and maturation of the collaterals. The infusion of some of the most potent angiogenic factors at high pharmacological doses achieved only a fraction of the maximum conductance obtained by high FSS in a rabbit model of femoral artery occlusion, suggesting that these factors alone cannot fully account for the arteriogenic effects. Nonetheless, the findings implicate potential in using these cytokines or growth factors to promote arteriogenesis.
As a rich source of cytokines and growth factors needed for vessel growth and repair, stem- and progenitor cell-based therapies have gained considerable acceptance in treating ischemic diseases. Endothelial progenitor cells (EPC), derived from either peripheral blood or bone marrow, can respond to shear stress and contribute to collateral formation when transplanted into animals with either limb or cardiac ischemia (Kamihata et al., 2001, Deindl et al., 2006). Other indirect revascularization procedures, such as encephalomyosynangiosis (EMS) followed by implantation of myoblast expressing VEGF164 has also shown to improve functional collateralization in chronic cerebral hypoperfusion (Hecht et al., 2015).
3.4 Collateral vessel remodeling involves the activation of proteases that degrade basement membrane and reorganize the extracellular matrix
Following the infiltration of monocytes and macrophages into the sites of collateral vessel, collateral growth and enlargement requires the proliferation of endothelial and mural cells and remodeling of the extracellular matrix. The orchestrated production of cytokines and growth factors induces the degradation of the extracellular matrix and stimulates the proliferation of endothelial (EC) and smooth muscle cells (SMC) in preparation for the remodeling of extracellular matrix and the expansion of the collateral diameter. However, the mechanisms leading to the production of cytokines and growth factors by monocytes and macrophages at sites of collateral growth are not well understood.
The turnover and remodeling of the extracellular matrix is carried out by the matrix metalloproteinases (MMPs) and their inhibitors, namely tissue inhibitor of metalloproteinases (TIMPs). During arteriogenesis, the external elastic lamina and elastin are broken down by MMPs and plasmin, creating space for the expanding vessel (Fung and Helisch, 2012, la Sala et al., 2012). Earlier studies indicated that MMP-2, MMP-9 and TIMP-1 were up-regulated in the intima during collateral remodeling (Kadoglou et al., 2005), suggesting that it is the balance between MMPs and TIMPs which is critical for the maintenance and remodeling of the vessel wall. Metabolic disorders such as diabetes disrupts this balance during arteriogenesis (Schatteman et al., 2000). For example, Lepr-db/db mutation blunted the ischemia-induced up-regulation of MMP-2, MMP-12 and MMP-16 in the murine hindlimb ischemia model (Schiekofer et al., 2005), contributed to the impaired arteriogenesis. To the contrary, serum level of MMP-9 but not MMP-2 was found to be significantly higher in patients with Moyamoya disease (Fujimura et al., 2009), a chronic cerebrovascular illness that is associated with pathological instability in the vessel wall and abnormal growth of some collaterals.
During the late phase of arteriogenesis, ECs and SMCs proliferate and migrate (Scholz et al., 2000). SMCs account for a large part of the production of new tissue, changing their phenotype from contractile to a synthetic and proliferative phenotype (Schaper, 2009). As the collateral vessels grow in diameter with cell proliferation, FSS falls and collateral flow decreases as a feedback autoregulatory mechanism.
4. Therapies to enhance collateral flow in cerebrovascular diseases
The only approved treatment of acute ischemic stroke at this time is the use of recombinant tissue plasminogen activator (rt-PA) within 3 hours or 4.5 hours in selected patients from the onset (Kwiatkowski et al., 1999, Hacke et al., 2008). However, only a small number of patients with acute ischemic stroke receive rt-PA therapy mainly due to its brief therapeutic time window (Adeoye et al., 2011) and the increased risk of intracranial hemorrhage (Hacke et al., 2008). Therefore, other safer treatments that have longer therapeutic time window are required.
Recently collateral perfusion including pial or leptomeningeal anastomosis is widely recognized as a key element that has significant effects on various aspects of clinical practice or consideration including the time course of ischemic injury, stroke severity and therapeutic opportunities. Collaterals have also been recognized to influence recanalization, reperfusion, hemorrhagic transformation, and neurological outcomes after stroke (Shuaib et al., 2011b, Liebeskind, 2013). Therefore, it is considered that collaterals are the potential therapeutic targets and many clinical investigations intended to increase collateral perfusion have been attempted and tested.
4.1 Pharmacologic approaches via volume expansion, hemodilution, vasodilation, and induced hypertension
Several studies have investigated the efficacy of volume expanders, hemodilutors and vasodilators, on increasing collateral circulation to improve outcome after stroke (Shuaib et al., 2011b). Previous volume expansion and hemodilution studies with dextran or hydoxyethyl starch have not shown any improvement in outcome or reduction in mortality in acute ischemic stroke (Schneider et al., 1985, Hartmann et al., 1987). Based on these available evidences, the American Heart Association’s 2007 guidelines (Adams et al., 2007) conclude that volume expansion or hemodilution is not recommended in patients with acute stroke. However, the use of albumin has provided some early promising results. In addition to its hypervolemic effects, it also has antioxidant, antithrombotic, and anti-inflammatory properties (Prajapati et al., 2011). Base on these findings, high-dose albumin (2 g/kg) treatment was tested in large prospective, double blind, placebo-controlled trials known as ALIAS (albumin in Acute Stroke). Unfortunately, this trial failed to demonstrate an overall clinical benefit among the participants (Ginsberg et al., 2013).
Dilation of cerebral arteries has the potential to increase flow through collaterals. However, some previous small trials with vasodilator have not shown any consistent benefit in neurological outcome (Hsu et al., 1988, Chan and Kay, 1993). In light of these outcomes, it is considered that the possibility of steal phenomenon at ischemic region after using vasodilator could be harmful to ischemic tissue (Kuwabara et al., 1995). Consequently, the American Heart Association’s 2007 guidelines also advise against the use of vasodilators in acute ischemic stroke.
A rise in systemic blood pressure could improve blood flow to the brain, possibly through increased collateral flow because cerebrovascular autoregulation is impaired during stroke and changes in mean arterial blood pressure have a linear effect on cerebral blood flow (Wityk, 2007). Some preliminary clinical data suggest that induced mild hypertension may improve NIHSS score with an acceptable degree of safety (Rordorf et al., 2001, Hillis et al., 2003), although there has not been a large, randomized clinical trial of this treatment and many questions still remain unanswered about the safety and potential benefits of pressure therapy (Mistri et al., 2006). The American Heart Association’s 2007 guidelines recommend that induced hypertension can be used in “exceptional cases” and that cardiac and neurological status should be closely monitored (Adams et al., 2007).
4.2 Non -Pharmacologic Approaches
4.2.1 External counterpulsation (ECP)
External counterpulsation (ECP) is a noninvasive and well-established treatment for ischemic heart disease with sustained long-term effects (Arora et al., 1999, Michaels et al., 2004). It operates by applying ECG-triggered diastolic pressure of ~250 mm Hg to the lower extremities by using air-filled cuffs. The diastolic augmentation of the blood flow and the simultaneously decreasing systolic afterload therefore increases blood flow to the heart, brain, and kidneys (Bonetti et al., 2003). Recently ECP has been investigated for ischemic stroke (Han and Wong, 2008), and a recent review suggests that ECP is associated with a remarkable increase in the number of ischemic stroke patients with clinical improvement (Han et al., 2008, Lin et al., 2012). However, at this time, a recent review showed that there are no clear evidence to support ECP therapy from previous RCTs and well-designed and large scale RCTs are needed (Lin et al., 2012).
4.2.2 Partial Aortic Occlusion
Partial occlusion of the abdominal aorta increases the blood volume beyond the occlusion site and augments cerebral blood flow in animal studies (Hammer et al., 2009). Experimental data suggest that the cerebral blood flow increase persists even after the end of the occlusion procedure (Stokland et al., 1980, Saether et al., 1996, Hammer et al., 2009). NeuroFlo device (CoAxia, Maple Grove, MN, USA) was developed with two balloons inflated in the abdominal aorta to occupy 70% of the vessel lumen above and below the renal arteries for 45 min. The Safety and Efficacy of NeuroFlo Technology in Ischemic Stroke (SENTIS) trial is the largest randomized controlled trial of device therapy to date and tested the potential augmentation of collateral perfusion (Shuaib et al., 2011a). A total of 515 acute stroke patients between 0 and 14 hours after symptom onset were randomized to partial aortic occlusion or standard care. Unfortunately the primary efficacy endpoint did not differ between the groups, although safety of this novel treatment was confirmed. However, some findings of subgroup analyses indicate that patients treated in early time-windows (≤6 h) with a moderate stroke severity (NIHSS score of 8–14) might have gained most benefit from aortic occlusion (Shuaib et al., 2013). In addition, in patients older than 70 years, those treated with the NeuroFlo device showed favorable response to this treatment compared with those who were not treated with NeuroFlo (Leker et al., 2012). These findings showed that partial aortic occlusion might still remain a potential tool for treatment of stroke in acute phase at least in selected patients.
4.2.3 Sphenopalatine Ganglion Stimulation
Several animal studies have demonstrated that stimulation of the sphenopalatine ganglion activates the parasympathetic innervation of the intracranial blood vessels and causes vasodilation and increased cerebral blood flow (Seylaz et al., 1988, Suzuki et al., 1990, Toda et al., 2000, Yarnitsky et al., 2005). Based on such findings, a prospective open-label study ‘Implant for Augmentation of CBF trial 1’ (ImpACT-1) is ongoing to investigate the efficacy, safety, and tolerability of the ischemic stroke system in patients with acute ischemic stroke within 24 hours of stroke onset (Khurana et al., 2009). The implantable neural stimulator (1 inch in size) is implanted by using oral procedure with local anesthesia, and the sphenopalatine ganglion will be stimulated for 4 h per day for up to 5 days.
4.2.4 Transcranial Laser Therapy
Transcranial laser therapy (TLT) is a noninvasive technology that uses near-infrared laser energy delivered transcranially to modulate biochemical changes within neural cells. In vivo studies have suggested that infrared laser therapy could be beneficial for the treatment of acute ischemic stroke (Lampl et al., 2007). The mechanisms of this method are not fully clear, however, it is considered that absorption of infrared laser energy leads to stimulate mitochondrial energy production and increase in ATP production, then suppress apoptosis in ischemic tissue. In addition to the effect of increasing mitochondrial function, in one study using mice, increased CBF was also observed after laser therapy via increasing NO level in the brain (Uozumi et al., 2010).
In prospective, double-blind NeuroThera Effectiveness and Safety Trial 1, 2 (NEST trial 1, 2) (Lampl et al., 2007, Zivin et al., 2009, Stemer et al., 2010, Huisa et al., 2013), the safety and efficacy of TLT were tested. On these studies, patients received transcranial near-infrared wavelength laser treatment within 24 h of symptom onset by using NeuroThera Laser System (Photothera, Carlsbad, California). The NEST-1 trial showed a statistically significant improvement in efficacy with TLT vs. sham in both the 90-day binary NIHSS (success defined as NIHSS 0–1 or decrease of at least nine points vs. baseline) and 90-day binary modified Rankin Scale (mRS) (success defined as mRS 0–2) without any safety concerns (Lampl et al., 2007). In the NEST-2, the primary efficacy endpoint, the mRS score at 90 days dichotomized as 0–2 for success, did not reach statistical significance (P = 0.09) (Zivin et al., 2009). However, further analysis showed that by excluding severe stroke subgroup (NIHSS 16–22), the efficacy end point became statistically significant in patients treated with TLT (Zivin et al., 2009). This led to the next step and currently, the NEST-3 clinical trial is ongoing.
4.3 Improving collateral flow in chronic cerebrovascular ischemic diseases
Chronic progressive steno-occlusive changes of extra or intracranial major arteries in the absence of compensatory collateral flow can result in hemodynamic insufficiency and lead to hemodynamic infarction (Vilela and Newell, 2008). Previous studies have shown that elevated oxygen extraction fraction (OEF) and reduced cerebrovascular reactivity (CVR) to acetazolamide may predict the risk of recurrent ischemic stroke in patients with hemodynamic compromise (Grubb et al., 1998, Yamauchi et al., 1999, Kuroda et al., 2001).
Direct bypass surgery is a surgical strategy to enhance the compensation collateral flow and improve the hemodynamic compromise mainly caused by ICA occlusion, MCA occlusion or severe stenosis (Rodriguez-Hernandez et al., 2011). Extracranial-intracranial (EC-IC) bypass with superficial temporal artery to middle cerebral artery cortical branch (STA-MCA) anastomosis is the most common bypass for stroke patients with hemodynamic compromise. However, recent randomized clinical trials showed that clinical indications and efficacy of bypass surgery were controversial. The Japan EC/IC Bypass Trial (JET) study demonstrates in their interim that STA-MCA anastomosis improves the 2-year outcome in patients with reduced CBF and CVR on SPECT (Ogasawara and Ogawa, 2006). However, as a major impediment in validating the benefit of EC/IC bypass in improving hemodynamics, the final results of JET study have yet to be published in a peer review journal. On the other hand, the Carotid Occlusion Surgery Study (COSS) in North America proves no beneficial effects at the 2-year outcome in patients with elevated OEF due to ICA occlusion even though they had achieved excellent bypass graft patency, improved cerebral hemodynamics (Powers et al., 2011, Grubb et al., 2013) and low rate of perioperative ischemic strokes caused by technical problems of bypass surgery (Reynolds et al., 2013). The discrepancies between the two studies are mainly due to the differences in 30-day postoperative stroke rates (estimated 0% in JET study, 15% in COSS), although the perioperative complication rate in JET remains unknown. These two studies may indicate the benefit of the STA-MCA bypass surgery after perioperative periods (Amin-Hanjani et al., 2012). However, at this point, given the higher stroke rate in perioperative periods in COSS, it is difficult to recommend the bypass surgery for the patients with carotid occlusion.
Moyamoya disease (MMD) is a another chronic cerebrovascular ischemic disease in which progressive bilateral steno-occlusive changes occur at the terminal portion of the internal carotid artery (ICA), leading to an abnormal vascular network at base of the brain (Suzuki and Takaku, 1969). This abnormal vascular network is named as ‘moyamoya’, a Japanese term depicting the hazy appearance of blood vessels on angiograms that bears resemblance to a puff of cigarette smoke. Clinical manifestations include ischemia, hemorrhage, and epilepsy, etc., in which young patients usually present with ischemic symptoms while adult patients with either ischemia or hemorrhage (Kuroda and Houkin, 2008). The abnormal angiogenesis at base of the brain may be specific to MMD because non-MMD patients exhibiting steno-occlusive changes in the intracranial major arteries rarely have collateral vessels similar to moyamoya vessels (Yoshihara et al., 2008, Achrol et al., 2009).
Among the research aimed at understanding the genetics, development and progression of moyamoya disease, investigating mechanisms by which the moyamoya induces pathological changes in vessel wall and aberrant vessel development have become the main focus. As a unique characteristic of MMD, it was noticed that spontaneous leptomeningeal anastomoses developed after placing donor materials supplied by external carotid arteries directly onto the surface of the ischemic brain in both adults and children (Houkin et al., 2000, Isono et al., 2002, Scott et al., 2004). Therefore, various kinds of indirect non-anastomotic bypass procedures have been developed. On the other hand there were few studies showing the efficacy of indirect revascularization to enhance collateral angiogenesis in cases of non-MMD intracranial atherosclerotic arterial stenosis. In addition, previous study showed that indirect bypass surgery did not promote adequate pial collateral artery development in patients with atherosclerotic occlusive cerebrovascular diseases (Komotar et al., 2009).
In indirect revascularization surgery for MMD, angiogenesis is induced in association with wound-healing and the repair process (Nakamura et al., 2009), which is presumably related to the underlying high levels of various pro-angiogenic growth factors such as basic fibroblast growth factor, transforming growth factor beta, platelet-derived growth factor, or hepatocyte growth factor etc. in MMD patients (Weinberg et al., 2011). The efficacy of these treatments is currently assessed by the outcome of perfusion improvement or new vessel formation using perfusion SPECT, cerebral angiography, or MR imaging (Kuroda and Houkin, 2008). The beneficial effects are not immediate because surgical collaterals require 3–4 months to develop (Houkin et al., 2004, Veeravagu et al., 2008). Interestingly, collateral pathways through indirect bypass do not develop in about 40–50% of adult patients, although indirect bypass provides extensive surgical collaterals in almost all pediatric patients (Mizoi et al., 1996). Careful evaluation of new vessel growth after indirect surgery in MMD patient would be insightful in understanding the shared mechanisms between collateral growth and angiogenesis in the ischemic brain.
5. Conclusions
Vascular remodeling of various mechanisms occurs in acute and chronic cerebrovascular occlusive diseases. It is commonly accepted that arteriogenesis, the engagement of collateral flow, is a critical determinant of stroke risk and outcome. On the other hand, angiogenesis, sprouting of new capillaries from postcapillary venules, is active during the chronic process of hypoxia and a driving force for post ischemic neovascularization. Although cell based therapeutic angiogenesis still holds promise for the treatment of ischemic diseases, delivering angiogenic factors in the form of protein or gene therapy has not resulted in clinical benefit (Chu and Wang, 2012, Said et al., 2013). Understanding the mechanisms underlying each remodeling process, regardless whether unique or shared, is the first step towards the development of effective revascularization therapy. In addition, it will also shed light on how vascular risk factors impair vascular homeostasis and remodeling in ischemic diseases.
Figure.
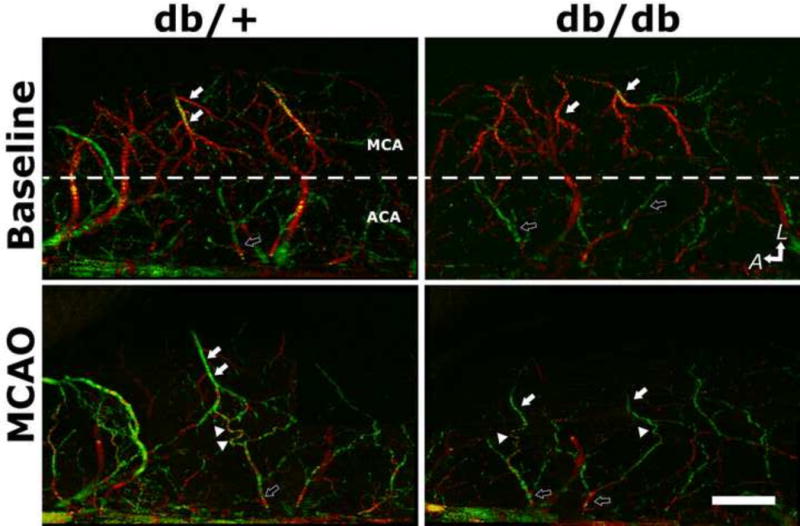
Type-II diabetes db/db mice exhibit impaired collateral flow recruitment after experimental stroke compared to their normoglycemic control db/+ mice. Retrograde collateral flow dynamics was assessed by Doppler optical coherent tomography (DOCT), a non-invasive high-resolution three-dimensional optical imaging technique for microangiography. Representative DOCT images of db/+ and db/db mice at baseline and immediately after MCAO were shown. The anatomic orientation is labeled with arrows pointing to the lateral (L) and anterior (A) directions. Dotted white lines mark the divide between MCA and ACA territory. White- and black-filled arrows indicate MCA and ACA branches, respectively. The direction of blood flow is color-coded, with the blood flowing towards the scanning probe beam or towards ACA territory coded as red, and the retrograde flow towards proximal MCA as green. Arrowheads indicate the tortuous anastomoses between MCA and ACA. Scale bar: 1 mm.
Distinct functional roles of arteriogenesis in the risk and outcome of ischemic diseases are delineated
Genetics and cellular mechanisms regulating arteriogenesis
Clinical mplications of arteriogenesis in cerebrovascular diseases
Potential therapies in promoting collateral flow including those in trials
Acknowledgments
This work was supported by NIH grant R01 NS071050 (JL), VA merit award I01RX000655 (JL) AHA EIA 0940065N (JL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: None
References
- Achrol AS, Guzman R, Lee M, Steinberg GK. Pathophysiology and genetic factors in moyamoya disease. Neurosurgical focus. 2009;26:E4. doi: 10.3171/2009.1.FOCUS08302. [DOI] [PubMed] [Google Scholar]
- Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF, American Heart A, American Stroke Association Stroke C, Clinical Cardiology C, Cardiovascular R, Intervention C, Atherosclerotic Peripheral Vascular D, Quality of Care Outcomes in Research Interdisciplinary Working G Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke; a journal of cerebral circulation. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke; a journal of cerebral circulation. 2011;42:1952–1955. doi: 10.1161/STROKEAHA.110.612358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akamatsu Y, Nishijima Y, Lee CC, Yang SY, Shi L, An L, Wang RK, Tominaga T, Liu J. Impaired leptomeningeal collateral flow contributes to the poor outcome following experimental stroke in the type-II-diabetic mice Journal of Neuroscience. 2015 doi: 10.1523/JNEUROSCI.3838-14.2015. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin-Hanjani S, Barker FG, 2nd, Charbel FT, Connolly ES, Jr, Morcos JJ, Thompson BG, Cerebrovascular Section of the American Association of Neurological S, Congress of Neurological S Extracranial-intracranial bypass for stroke-is this the end of the line or a bump in the road? Neurosurgery. 2012;71:557–561. doi: 10.1227/NEU.0b013e3182621488. [DOI] [PubMed] [Google Scholar]
- Arora RR, Chou TM, Jain D, Fleishman B, Crawford L, McKiernan T, Nesto RW. The multicenter study of enhanced external counterpulsation (MUST-EECP): effect of EECP on exercise-induced myocardial ischemia and anginal episodes. Journal of the American College of Cardiology. 1999;33:1833–1840. doi: 10.1016/s0735-1097(99)00140-0. [DOI] [PubMed] [Google Scholar]
- Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. The Journal of clinical investigation. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, Kim D, Jahan R, Duckwiler GR, Yoon SR, Vinuela F, Liebeskind DS, Investigators UC Impact of collateral flow on tissue fate in acute ischaemic stroke. Journal of neurology, neurosurgery, and psychiatry. 2008;79:625–629. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, Lee KH, Liebeskind DS. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke; a journal of cerebral circulation. 2011a;42:693–699. doi: 10.1161/STROKEAHA.110.595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, Lee KH, Liebeskind DS, Collaborators UC-SS Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke; a journal of cerebral circulation. 2011b;42:2235–2239. doi: 10.1161/STROKEAHA.110.604603. [DOI] [PubMed] [Google Scholar]
- Bergmann CE, Hoefer IE, Meder B, Roth H, van Royen N, Breit SM, Jost MM, Aharinejad S, Hartmann S, Buschmann IR. Arteriogenesis depends on circulating monocytes and macrophage accumulation and is severely depressed in op/op mice. Journal of leukocyte biology. 2006;80:59–65. doi: 10.1189/jlb.0206087. [DOI] [PubMed] [Google Scholar]
- Bisschops RH, Klijn CJ, Kappelle LJ, van Huffelen AC, van der Grond J. Collateral flow and ischemic brain lesions in patients with unilateral carotid artery occlusion. Neurology. 2003;60:1435–1441. doi: 10.1212/01.wnl.0000061616.96745.90. [DOI] [PubMed] [Google Scholar]
- Bonetti PO, Holmes DR, Jr, Lerman A, Barsness GW. Enhanced external counterpulsation for ischemic heart disease: what’s behind the curtain? Journal of the American College of Cardiology. 2003;41:1918–1925. doi: 10.1016/s0735-1097(03)00428-5. [DOI] [PubMed] [Google Scholar]
- Capoccia BJ, Gregory AD, Link DC. Recruitment of the inflammatory subset of monocytes to sites of ischemia induces angiogenesis in a monocyte chemoattractant protein-1-dependent fashion. Journal of leukocyte biology. 2008;84:760–768. doi: 10.1189/jlb.1107756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalothorn D, Faber JE. Formation and maturation of the native cerebral collateral circulation. Journal of molecular and cellular cardiology. 2010;49:251–259. doi: 10.1016/j.yjmcc.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YW, Kay CS. Pentoxifylline in the treatment of acute ischaemic stroke–a reappraisal in Chinese stroke patients. Clinical and experimental neurology. 1993;30:110–116. [PubMed] [Google Scholar]
- Chu H, Wang Y. Therapeutic angiogenesis: controlled delivery of angiogenic factors. Therapeutic delivery. 2012;3:693–714. doi: 10.4155/tde.12.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu PL, Keum S, Marchuk DA. A novel genetic locus modulates infarct volume independently of the extent of collateral circulation. Physiological genomics. 2013;45:751–763. doi: 10.1152/physiolgenomics.00063.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circulation research. 2008;103:1027–1036. doi: 10.1161/CIRCRESAHA.108.181115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couffinhal T, Silver M, Kearney M, Sullivan A, Witzenbichler B, Magner M, Annex B, Peters K, Isner JM. Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in ApoE−/− mice. Circulation. 1999;99:3188–3198. doi: 10.1161/01.cir.99.24.3188. [DOI] [PubMed] [Google Scholar]
- Dai X, Faber JE. Endothelial nitric oxide synthase deficiency causes collateral vessel rarefaction and impairs activation of a cell cycle gene network during arteriogenesis. Circulation research. 2010;106:1870–1881. doi: 10.1161/CIRCRESAHA.109.212746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deindl E, Zaruba MM, Brunner S, Huber B, Mehl U, Assmann G, Hoefer IE, Mueller-Hoecker J, Franz WM. G-CSF administration after myocardial infarction in mice attenuates late ischemic cardiomyopathy by enhanced arteriogenesis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:956–958. doi: 10.1096/fj.05-4763fje. [DOI] [PubMed] [Google Scholar]
- Deindl E, Ziegelhoffer T, Kanse SM, Fernandez B, Neubauer E, Carmeliet P, Preissner KT, Schaper W. Receptor-independent role of the urokinase-type plasminogen activator during arteriogenesis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:1174–1176. doi: 10.1096/fj.02-0800fje. [DOI] [PubMed] [Google Scholar]
- Eitenmuller I, Volger O, Kluge A, Troidl K, Barancik M, Cai WJ, Heil M, Pipp F, Fischer S, Horrevoets AJ, Schmitz-Rixen T, Schaper W. The range of adaptation by collateral vessels after femoral artery occlusion. Circulation research. 2006;99:656–662. doi: 10.1161/01.RES.0000242560.77512.dd. [DOI] [PubMed] [Google Scholar]
- Faber JE, Chilian WM, Deindl E, van Royen N, Simons M. A brief etymology of the collateral circulation. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1854–1859. doi: 10.1161/ATVBAHA.114.303929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber JE, Zhang H, Lassance-Soares RM, Prabhakar P, Najafi AH, Burnett MS, Epstein SE. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:1748–1756. doi: 10.1161/ATVBAHA.111.227314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura M, Watanabe M, Narisawa A, Shimizu H, Tominaga T. Increased expression of serum Matrix Metalloproteinase-9 in patients with moyamoya disease. Surgical neurology. 2009;72:476–480. doi: 10.1016/j.surneu.2008.10.009. discussion 480. [DOI] [PubMed] [Google Scholar]
- Fung E, Helisch A. Macrophages in collateral arteriogenesis. Frontiers in physiology. 2012;3:353. doi: 10.3389/fphys.2012.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MD, Palesch YY, Hill MD, Martin RH, Moy CS, Barsan WG, Waldman BD, Tamariz D, Ryckborst KJ, Alias, Neurological Emergencies Treatment Trials I High-dose albumin treatment for acute ischaemic stroke (ALIAS) Part 2: a randomised, double-blind, phase 3, placebo-controlled trial. Lancet neurology. 2013;12:1049–1058. doi: 10.1016/S1474-4422(13)70223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb RL, Jr, Derdeyn CP, Fritsch SM, Carpenter DA, Yundt KD, Videen TO, Spitznagel EL, Powers WJ. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. Jama. 1998;280:1055–1060. doi: 10.1001/jama.280.12.1055. [DOI] [PubMed] [Google Scholar]
- Grubb RL, Jr, Powers WJ, Clarke WR, Videen TO, Adams HP, Jr, Derdeyn CP, Carotid Occlusion Surgery Study I Surgical results of the Carotid Occlusion Surgery Study. Journal of neurosurgery. 2013;118:25–33. doi: 10.3171/2012.9.JNS12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D, Investigators E Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. The New England journal of medicine. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- Hamm A, Veschini L, Takeda Y, Costa S, Delamarre E, Squadrito ML, Henze AT, Wenes M, Serneels J, Pucci F, Roncal C, Anisimov A, Alitalo K, De Palma M, Mazzone M. PHD2 regulates arteriogenic macrophages through TIE2 signalling. EMBO molecular medicine. 2013;5:843–857. doi: 10.1002/emmm.201302695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer M, Jovin T, Wahr JA, Heiss WD. Partial occlusion of the descending aorta increases cerebral blood flow in a nonstroke porcine model. Cerebrovascular diseases. 2009;28:406–410. doi: 10.1159/000235628. [DOI] [PubMed] [Google Scholar]
- Han JH, Leung TW, Lam WW, Soo YO, Alexandrov AW, Mok V, Leung YF, Lo R, Wong KS. Preliminary findings of external counterpulsation for ischemic stroke patient with large artery occlusive disease. Stroke; a journal of cerebral circulation. 2008;39:1340–1343. doi: 10.1161/STROKEAHA.107.500132. [DOI] [PubMed] [Google Scholar]
- Han JH, Wong KS. Is counterpulsation a potential therapy for ischemic stroke? Cerebrovascular diseases. 2008;26:97–105. doi: 10.1159/000139655. [DOI] [PubMed] [Google Scholar]
- Hartkamp MJ, van Der Grond J, van Everdingen KJ, Hillen B, Mali WP. Circle of Willis collateral flow investigated by magnetic resonance angiography. Stroke; a journal of cerebral circulation. 1999;30:2671–2678. doi: 10.1161/01.str.30.12.2671. [DOI] [PubMed] [Google Scholar]
- Hartmann A, Tsuda Y, Lagreze H. Effect of hypervolaemic haemodilution of regional cerebral blood flow in patients with acute ischaemic stroke: a controlled study with hydroxyethylstarch. Journal of neurology. 1987;235:34–38. doi: 10.1007/BF00314195. [DOI] [PubMed] [Google Scholar]
- Hecht N, He J, Kremenetskaia I, Nieminen M, Vajkoczy P, Woitzik J. Cerebral hemodynamic reserve and vascular remodeling in C57/BL6 mice are influenced by age. Stroke; a journal of cerebral circulation. 2012;43:3052–3062. doi: 10.1161/STROKEAHA.112.653204. [DOI] [PubMed] [Google Scholar]
- Hecht N, Marushima A, Nieminen M, Kremenetskaia I, von Degenfeld G, Woitzik J, Vajkoczy P. Myoblast-mediated gene therapy improves functional collateralization in chronic cerebral hypoperfusion. Stroke; a journal of cerebral circulation. 2015;46:203–211. doi: 10.1161/STROKEAHA.114.006712. [DOI] [PubMed] [Google Scholar]
- Heil M, Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis) Circulation research. 2004;95:449–458. doi: 10.1161/01.RES.0000141145.78900.44. [DOI] [PubMed] [Google Scholar]
- Heil M, Ziegelhoeffer T, Pipp F, Kostin S, Martin S, Clauss M, Schaper W. Blood monocyte concentration is critical for enhancement of collateral artery growth. American journal of physiology Heart and circulatory physiology. 2002;283:H2411–2419. doi: 10.1152/ajpheart.01098.2001. [DOI] [PubMed] [Google Scholar]
- Henderson RD, Eliasziw M, Fox AJ, Rothwell PM, Barnett HJ. Angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Stroke; a journal of cerebral circulation. 2000;31:128–132. doi: 10.1161/01.str.31.1.128. [DOI] [PubMed] [Google Scholar]
- Hendrikse J, Eikelboom BC, van der Grond J. Magnetic resonance angiography of collateral compensation in asymptomatic and symptomatic internal carotid artery stenosis. Journal of vascular surgery. 2002;36:799–805. doi: 10.1067/mva.2002.127346. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Ulatowski JA, Barker PB, Torbey M, Ziai W, Beauchamp NJ, Oh S, Wityk RJ. A pilot randomized trial of induced blood pressure elevation: effects on function and focal perfusion in acute and subacute stroke. Cerebrovascular diseases. 2003;16:236–246. doi: 10.1159/000071122. [DOI] [PubMed] [Google Scholar]
- Houkin K, Kuroda S, Ishikawa T, Abe H. Neovascularization (angiogenesis) after revascularization in moyamoya disease. Which technique is most useful for moyamoya disease? Acta neurochirurgica. 2000;142:269–276. doi: 10.1007/s007010050035. [DOI] [PubMed] [Google Scholar]
- Houkin K, Nakayama N, Kuroda S, Ishikawa T, Nonaka T. How does angiogenesis develop in pediatric moyamoya disease after surgery? A prospective study with MR angiography. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2004;20:734–741. doi: 10.1007/s00381-004-0971-x. [DOI] [PubMed] [Google Scholar]
- Hsu CY, Norris JW, Hogan EL, Bladin P, Dinsdale HB, Yatsu FM, Earnest MP, Scheinberg P, Caplan LR, Karp HR. Pentoxifylline in acute nonhemorrhagic stroke. A randomized, placebo-controlled double-blind trial. Stroke; a journal of cerebral circulation. 1988;19:716–722. doi: 10.1161/01.str.19.6.716. [DOI] [PubMed] [Google Scholar]
- Huisa BN, Stemer AB, Walker MG, Rapp K, Meyer BC, Zivin JA, Nest investigators Transcranial laser therapy for acute ischemic stroke: a pooled analysis of NEST-1 and NEST-2. International journal of stroke : official journal of the International Stroke Society. 2013;8:315–320. doi: 10.1111/j.1747-4949.2011.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isono M, Ishii K, Kamida T, Inoue R, Fujiki M, Kobayashi H. Long-term outcomes of pediatric moyamoya disease treated by encephalo-duro-arterio-synangiosis. Pediatric neurosurgery. 2002;36:14–21. doi: 10.1159/000048343. [DOI] [PubMed] [Google Scholar]
- Kadoglou NP, Daskalopoulou SS, Perrea D, Liapis CD. Matrix metalloproteinases and diabetic vascular complications. Angiology. 2005;56:173–189. doi: 10.1177/000331970505600208. [DOI] [PubMed] [Google Scholar]
- Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- Keum S, Marchuk DA. A locus mapping to mouse chromosome 7 determines infarct volume in a mouse model of ischemic stroke. Circulation Cardiovascular genetics. 2009;2:591–598. doi: 10.1161/CIRCGENETICS.109.883231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana D, Kaul S, Bornstein NM, Imp ACTSG Implant for augmentation of cerebral blood flow trial 1: a pilot study evaluating the safety and effectiveness of the Ischaemic Stroke System for treatment of acute ischaemic stroke. International journal of stroke : official journal of the International Stroke Society. 2009;4:480–485. doi: 10.1111/j.1747-4949.2009.00385.x. [DOI] [PubMed] [Google Scholar]
- Komotar RJ, Starke RM, Otten ML, Merkow MB, Garrett MC, Marshall RS, Elkind MS, Connolly ES. The role of indirect extracranial-intracranial bypass in the treatment of symptomatic intracranial atheroocclusive disease. Journal of neurosurgery. 2009;110:896–904. doi: 10.3171/2008.9.JNS17658. [DOI] [PubMed] [Google Scholar]
- Kong T, Scully M, Shelley CS, Colgan SP. Identification of Pur alpha as a new hypoxia response factor responsible for coordinated induction of the beta 2 integrin family. Journal of immunology. 2007;179:1934–1941. doi: 10.4049/jimmunol.179.3.1934. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Houkin K. Moyamoya disease: current concepts and future perspectives. Lancet neurology. 2008;7:1056–1066. doi: 10.1016/S1474-4422(08)70240-0. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Houkin K, Kamiyama H, Mitsumori K, Iwasaki Y, Abe H. Long-term prognosis of medically treated patients with internal carotid or middle cerebral artery occlusion: can acetazolamide test predict it? Stroke; a journal of cerebral circulation. 2001;32:2110–2116. doi: 10.1161/hs0901.095692. [DOI] [PubMed] [Google Scholar]
- Kuwabara Y, Ichiya Y, Sasaki M, Akashi Y, Yoshida T, Fukumura T, Masuda K. A comparison of the cerebrovascular responses to CO2 and Diamox in patients with unilateral occlusive cerebral arteries: a H2(15)O PET study. Kaku igaku The Japanese journal of nuclear medicine. 1995;32:569–577. [PubMed] [Google Scholar]
- Kwiatkowski TG, Libman RB, Frankel M, Tilley BC, Morgenstern LB, Lu M, Broderick JP, Lewandowski CA, Marler JR, Levine SR, Brott T. Effects of tissue plasminogen activator for acute ischemic stroke at one year. National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Study Group. The New England journal of medicine. 1999;340:1781–1787. doi: 10.1056/NEJM199906103402302. [DOI] [PubMed] [Google Scholar]
- la Sala A, Pontecorvo L, Agresta A, Rosano G, Stabile E. Regulation of collateral blood vessel development by the innate and adaptive immune system. Trends in molecular medicine. 2012;18:494–501. doi: 10.1016/j.molmed.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Lampl Y, Zivin JA, Fisher M, Lew R, Welin L, Dahlof B, Borenstein P, Andersson B, Perez J, Caparo C, Ilic S, Oron U. Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1) Stroke; a journal of cerebral circulation. 2007;38:1843–1849. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]
- Lee CW, Stabile E, Kinnaird T, Shou M, Devaney JM, Epstein SE, Burnett MS. Temporal patterns of gene expression after acute hindlimb ischemia in mice: insights into the genomic program for collateral vessel development. Journal of the American College of Cardiology. 2004;43:474–482. doi: 10.1016/j.jacc.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Leker RR, Molina C, Cockroft K, Liebeskind DS, Concha M, Shuaib A, De Deyn PP, Burgin WS, Gupta R, Dillon W, Diener HC. Effects of age on outcome in the SENTIS trial: better outcomes in elderly patients. Cerebrovascular diseases. 2012;34:263–271. doi: 10.1159/000342668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Atochin D, Kashiwagi S, Earle J, Wang A, Mandeville E, Hayakawa K, d’Uscio LV, Lo EH, Katusic Z, Sessa W, Huang PL. Deficient eNOS phosphorylation is a mechanism for diabetic vascular dysfunction contributing to increased stroke size. Stroke; a journal of cerebral circulation. 2013;44:3183–3188. doi: 10.1161/STROKEAHA.113.002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebeskind DS. Collateral circulation. Stroke; a journal of cerebral circulation. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- Liebeskind DS. Trials of endovascular therapies or collaterals? International journal of stroke : official journal of the International Stroke Society. 2013;8:258–259. doi: 10.1111/ijs.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima FO, Furie KL, Silva GS, Lev MH, Camargo EC, Singhal AB, Harris GJ, Halpern EF, Koroshetz WJ, Smith WS, Yoo AJ, Nogueira RG. The pattern of leptomeningeal collaterals on CT angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke; a journal of cerebral circulation. 2010;41:2316–2322. doi: 10.1161/STROKEAHA.110.592303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Liu M, Wu B, Hao Z, Yang J, Tao W. External counterpulsation for acute ischaemic stroke. The Cochrane database of systematic reviews. 2012;1:CD009264. doi: 10.1002/14651858.CD009264.pub2. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang Y, Akamatsu Y, Lee CC, Stetler RA, Lawton MT, Yang GY. Vascular remodeling after ischemic stroke: mechanisms and therapeutic potentials. Prog Neurobiol. 2014;115:138–156. doi: 10.1016/j.pneurobio.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loinard C, Ginouves A, Vilar J, Cochain C, Zouggari Y, Recalde A, Duriez M, Levy BI, Pouyssegur J, Berra E, Silvestre JS. Inhibition of prolyl hydroxylase domain proteins promotes therapeutic revascularization. Circulation. 2009;120:50–59. doi: 10.1161/CIRCULATIONAHA.108.813303. [DOI] [PubMed] [Google Scholar]
- Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, Harris GJ, Halpern E, Kemmling A, Koroshetz WJ, Furie KL. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke; a journal of cerebral circulation. 2009;40:3001–3005. doi: 10.1161/STROKEAHA.109.552513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisner JK, Price RJ. Spatial and temporal coordination of bone marrow-derived cell activity during arteriogenesis: regulation of the endogenous response and therapeutic implications. Microcirculation. 2010;17:583–599. doi: 10.1111/j.1549-8719.2010.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon BK, Smith EE, Coutts SB, Welsh DG, Faber JE, Goyal M, Hill MD, Demchuk AM, Damani Z, Cho KH, Chang HW, Hong JH, Sohn SI. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Annals of neurology. 2013 doi: 10.1002/ana.23906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels AD, Linnemeier G, Soran O, Kelsey SF, Kennard ED. Two-year outcomes after enhanced external counterpulsation for stable angina pectoris (from the International EECP Patient Registry [IEPR]) The American journal of cardiology. 2004;93:461–464. doi: 10.1016/j.amjcard.2003.10.044. [DOI] [PubMed] [Google Scholar]
- Mistri AK, Robinson TG, Potter JF. Pressor therapy in acute ischemic stroke: systematic review. Stroke; a journal of cerebral circulation. 2006;37:1565–1571. doi: 10.1161/01.STR.0000222002.57530.05. [DOI] [PubMed] [Google Scholar]
- Mizoi K, Kayama T, Yoshimoto T, Nagamine Y. Indirect revascularization for moyamoya disease: is there a beneficial effect for adult patients? Surgical neurology. 1996;45:541–548. doi: 10.1016/0090-3019(95)00475-0. discussion 548–549. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Imai H, Konno K, Kubota C, Seki K, Puentes S, Faried A, Yokoo H, Hata H, Yoshimoto Y, Saito N. Experimental investigation of encephalomyosynangiosis using gyrencephalic brain of the miniature pig: histopathological evaluation of dynamic reconstruction of vessels for functional anastomosis. Laboratory investigation. Journal of neurosurgery Pediatrics. 2009;3:488–495. doi: 10.3171/2008.6.PEDS0834. [DOI] [PubMed] [Google Scholar]
- Norris JW, Krajewski A, Bornstein NM. The clinical role of the cerebral collateral circulation in carotid occlusion. Journal of vascular surgery. 1990;12:113–118. doi: 10.1067/mva.1990.21479. [DOI] [PubMed] [Google Scholar]
- Ogasawara K, Ogawa A. JET study (Japanese EC-IC Bypass Trial) Nihon rinsho Japanese journal of clinical medicine. 2006;64(Suppl 7):524–527. [PubMed] [Google Scholar]
- Osanai T, Akutsu N, Fujita N, Nakano T, Takahashi K, Guan W, Okumura K. Cross talk between prostacyclin and nitric oxide under shear in smooth muscle cell: role in monocyte adhesion. American journal of physiology Heart and circulatory physiology. 2001;281:H177–182. doi: 10.1152/ajpheart.2001.281.1.H177. [DOI] [PubMed] [Google Scholar]
- Pipp F, Boehm S, Cai WJ, Adili F, Ziegler B, Karanovic G, Ritter R, Balzer J, Scheler C, Schaper W, Schmitz-Rixen T. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:1664–1668. doi: 10.1161/01.ATV.0000138028.14390.e4. [DOI] [PubMed] [Google Scholar]
- Powers WJ, Clarke WR, Grubb RL, Jr, Videen TO, Adams HP, Jr, Derdeyn CP, Investigators C Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the Carotid Occlusion Surgery Study randomized trial. Jama. 2011;306:1983–1992. doi: 10.1001/jama.2011.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajapati KD, Sharma SS, Roy N. Current perspectives on potential role of albumin in neuroprotection. Reviews in the neurosciences. 2011;22:355–363. doi: 10.1515/RNS.2011.028. [DOI] [PubMed] [Google Scholar]
- Rath M, Muller I, Kropf P, Closs EI, Munder M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Frontiers in immunology. 2014;5:532. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey S, Lee K, Wang CJ, Gupta K, Chen S, McMillan A, Bhise N, Levchenko A, Semenza GL. Synergistic effect of HIF-1alpha gene therapy and HIF-1-activated bone marrow-derived angiogenic cells in a mouse model of limb ischemia. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20399–20404. doi: 10.1073/pnas.0911921106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey S, Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovascular research. 2010;86:236–242. doi: 10.1093/cvr/cvq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MR, Grubb RL, Jr, Clarke WR, Powers WJ, Zipfel GJ, Adams HP, Jr, Derdeyn CP, Carotid Occlusion Surgery Study I Investigating the mechanisms of perioperative ischemic stroke in the Carotid Occlusion Surgery Study. Journal of neurosurgery. 2013;119:988–995. doi: 10.3171/2013.6.JNS13312. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Hernandez A, Josephson SA, Langer D, Lawton MT. Bypass for the prevention of ischemic stroke. World neurosurgery. 2011;76:S72–79. doi: 10.1016/j.wneu.2011.06.032. [DOI] [PubMed] [Google Scholar]
- Rordorf G, Koroshetz WJ, Ezzeddine MA, Segal AZ, Buonanno FS. A pilot study of drug-induced hypertension for treatment of acute stroke. Neurology. 2001;56:1210–1213. doi: 10.1212/wnl.56.9.1210. [DOI] [PubMed] [Google Scholar]
- Saether OD, Juul R, Aadahl P, Stromholm T, Myhre HO. Cerebral haemodynamics during thoracic- and thoracoabdominal aortic aneurysm repair. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 1996;12:81–85. doi: 10.1016/s1078-5884(96)80280-2. [DOI] [PubMed] [Google Scholar]
- Said SS, Pickering JG, Mequanint K. Advances in growth factor delivery for therapeutic angiogenesis. Journal of vascular research. 2013;50:35–51. doi: 10.1159/000345108. [DOI] [PubMed] [Google Scholar]
- Schaper W. Collateral circulation: past and present. Basic research in cardiology. 2009;104:5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. The Journal of clinical investigation. 2000;106:571–578. doi: 10.1172/JCI9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiekofer S, Galasso G, Sato K, Kraus BJ, Walsh K. Impaired revascularization in a mouse model of type 2 diabetes is associated with dysregulation of a complex angiogenic-regulatory network. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:1603–1609. doi: 10.1161/01.ATV.0000171994.89106.ca. [DOI] [PubMed] [Google Scholar]
- Schierling W, Troidl K, Apfelbeck H, Troidl C, Kasprzak PM, Schaper W, Schmitz-Rixen T. Cerebral arteriogenesis is enhanced by pharmacological as well as fluid-shear-stress activation of the Trpv4 calcium channel. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2011;41:589–596. doi: 10.1016/j.ejvs.2010.11.034. [DOI] [PubMed] [Google Scholar]
- Schierling W, Troidl K, Mueller C, Troidl C, Wustrack H, Bachmann G, Kasprzak PM, Schaper W, Schmitz-Rixen T. Increased intravascular flow rate triggers cerebral arteriogenesis. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009a;29:726–737. doi: 10.1038/jcbfm.2008.165. [DOI] [PubMed] [Google Scholar]
- Schierling W, Troidl K, Troidl C, Schmitz-Rixen T, Schaper W, Eitenmuller IK. The role of angiogenic growth factors in arteriogenesis. Journal of vascular research. 2009b;46:365–374. doi: 10.1159/000189797. [DOI] [PubMed] [Google Scholar]
- Schneider R, Hacke W, Kiesewetter H, Jung F. Hemodilution in acute ischemic insults. Comparative study on the clinical and hemorheologic effectiveness of 10% HES 200/0.5 and 10% dextran 40. Fortschritte der Medizin. 1985;103:1031–1034. [PubMed] [Google Scholar]
- Scholz D, Ito W, Fleming I, Deindl E, Sauer A, Wiesnet M, Busse R, Schaper J, Schaper W. Ultrastructure and molecular histology of rabbit hind-limb collateral artery growth (arteriogenesis) Virchows Archiv : an international journal of pathology. 2000;436:257–270. doi: 10.1007/s004280050039. [DOI] [PubMed] [Google Scholar]
- Schomer DF, Marks MP, Steinberg GK, Johnstone IM, Boothroyd DB, Ross MR, Pelc NJ, Enzmann DR. The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. The New England journal of medicine. 1994;330:1565–1570. doi: 10.1056/NEJM199406023302204. [DOI] [PubMed] [Google Scholar]
- Scott RM, Smith JL, Robertson RL, Madsen JR, Soriano SG, Rockoff MA. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. Journal of neurosurgery. 2004;100:142–149. doi: 10.3171/ped.2004.100.2.0142. [DOI] [PubMed] [Google Scholar]
- Sealock R, Zhang H, Lucitti JL, Moore SM, Faber JE. Congenic fine-mapping identifies a major causal locus for variation in the native collateral circulation and ischemic injury in brain and lower extremity. Circulation research. 2014;114:660–671. doi: 10.1161/CIRCRESAHA.114.302931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seylaz J, Hara H, Pinard E, Mraovitch S, MacKenzie ET, Edvinsson L. Effect of stimulation of the sphenopalatine ganglion on cortical blood flow in the rat. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1988;8:875–878. doi: 10.1038/jcbfm.1988.145. [DOI] [PubMed] [Google Scholar]
- Shuaib A, Bornstein NM, Diener HC, Dillon W, Fisher M, Hammer MD, Molina CA, Rutledge JN, Saver JL, Schellinger PD, Shownkeen H, Investigators ST Partial aortic occlusion for cerebral perfusion augmentation: safety and efficacy of NeuroFlo in Acute Ischemic Stroke trial. Stroke; a journal of cerebral circulation. 2011a;42:1680–1690. doi: 10.1161/STROKEAHA.110.609933. [DOI] [PubMed] [Google Scholar]
- Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet neurology. 2011b;10:909–921. doi: 10.1016/S1474-4422(11)70195-8. [DOI] [PubMed] [Google Scholar]
- Shuaib A, Schwab S, Rutledge JN, Starkman S, Liebeskind DS, Bernardini GL, Boulos A, Abou-Chebl A, Huang DY, Vanhooren G, Cruz-Flores S, Klucznik RP, Saver JL, investigators St Importance of proper patient selection and endpoint selection in evaluation of new therapies in acute stroke: further analysis of the SENTIS trial. Journal of neurointerventional surgery. 2013;5(Suppl 1):i21–24. doi: 10.1136/neurintsurg-2012-010562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre JS, Smadja DM, Levy BI. Postischemic revascularization: from cellular and molecular mechanisms to clinical applications. Physiological reviews. 2013;93:1743–1802. doi: 10.1152/physrev.00006.2013. [DOI] [PubMed] [Google Scholar]
- Stemer AB, Huisa BN, Zivin JA. The evolution of transcranial laser therapy for acute ischemic stroke, including a pooled analysis of NEST-1 and NEST-2. Current cardiology reports. 2010;12:29–33. doi: 10.1007/s11886-009-0071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokland O, Miller MM, Ilebekk A, Kiil F. Mechanism of hemodynamic responses to occlusion of the descending thoracic aorta. The American journal of physiology. 1980;238:H423–429. doi: 10.1152/ajpheart.1980.238.4.H423. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Archives of neurology. 1969;20:288–299. doi: 10.1001/archneur.1969.00480090076012. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Hardebo JE, Kahrstrom J, Owman C. Selective electrical stimulation of postganglionic cerebrovascular parasympathetic nerve fibers originating from the sphenopalatine ganglion enhances cortical blood flow in the rat. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1990;10:383–391. doi: 10.1038/jcbfm.1990.68. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Costa S, Delamarre E, Roncal C, Leite de Oliveira R, Squadrito ML, Finisguerra V, Deschoemaeker S, Bruyere F, Wenes M, Hamm A, Serneels J, Magat J, Bhattacharyya T, Anisimov A, Jordan BF, Alitalo K, Maxwell P, Gallez B, Zhuang ZW, Saito Y, Simons M, De Palma M, Mazzone M. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature. 2011;479:122–126. doi: 10.1038/nature10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchaikovski V, Olieslagers S, Bohmer FD, Waltenberger J. Diabetes mellitus activates signal transduction pathways resulting in vascular endothelial growth factor resistance of human monocytes. Circulation. 2009;120:150–159. doi: 10.1161/CIRCULATIONAHA.108.817528. [DOI] [PubMed] [Google Scholar]
- Toda N, Tanaka T, Ayajiki K, Okamura T. Cerebral vasodilatation induced by stimulation of the pterygopalatine ganglion and greater petrosal nerve in anesthetized monkeys. Neuroscience. 2000;96:393–398. doi: 10.1016/s0306-4522(99)00557-6. [DOI] [PubMed] [Google Scholar]
- Troidl C, Jung G, Troidl K, Hoffmann J, Mollmann H, Nef H, Schaper W, Hamm CW, Schmitz-Rixen T. The temporal and spatial distribution of macrophage subpopulations during arteriogenesis. Current vascular pharmacology. 2013;11:5–12. [PubMed] [Google Scholar]
- Troidl C, Troidl K, Schierling W, Cai WJ, Nef H, Mollmann H, Kostin S, Schimanski S, Hammer L, Elsasser A, Schmitz-Rixen T, Schaper W. Trpv4 induces collateral vessel growth during regeneration of the arterial circulation. Journal of cellular and molecular medicine. 2009a;13:2613–2621. doi: 10.1111/j.1582-4934.2008.00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troidl K, Ruding I, Cai WJ, Mucke Y, Grossekettler L, Piotrowska I, Apfelbeck H, Schierling W, Volger OL, Horrevoets AJ, Grote K, Schmitz-Rixen T, Schaper W, Troidl C. Actin-binding rho activating protein (Abra) is essential for fluid shear stress-induced arteriogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2009b;29:2093–2101. doi: 10.1161/ATVBAHA.109.195305. [DOI] [PubMed] [Google Scholar]
- Troidl K, Schaper W. Arteriogenesis versus angiogenesis in peripheral artery disease. Diabetes/metabolism research and reviews. 2012;28(Suppl 1):27–29. doi: 10.1002/dmrr.2232. [DOI] [PubMed] [Google Scholar]
- Troidl K, Tribulova S, Cai WJ, Ruding I, Apfelbeck H, Schierling W, Troidl C, Schmitz-Rixen T, Schaper W. Effects of endogenous nitric oxide and of DETA NONOate in arteriogenesis. Journal of cardiovascular pharmacology. 2010;55:153–160. doi: 10.1097/FJC.0b013e3181c9556f. [DOI] [PubMed] [Google Scholar]
- Uozumi Y, Nawashiro H, Sato S, Kawauchi S, Shima K, Kikuchi M. Targeted increase in cerebral blood flow by transcranial near-infrared laser irradiation. Lasers in surgery and medicine. 2010;42:566–576. doi: 10.1002/lsm.20938. [DOI] [PubMed] [Google Scholar]
- van Laar PJ, Hendrikse J, Klijn CJ, Kappelle LJ, van Osch MJ, van der Grond J. Symptomatic carotid artery occlusion: flow territories of major brain-feeding arteries. Radiology. 2007;242:526–534. doi: 10.1148/radiol.2422060179. [DOI] [PubMed] [Google Scholar]
- van Oostrom MC, van Oostrom O, Quax PH, Verhaar MC, Hoefer IE. Insights into mechanisms behind arteriogenesis: what does the future hold? Journal of leukocyte biology. 2008;84:1379–1391. doi: 10.1189/jlb.0508281. [DOI] [PubMed] [Google Scholar]
- Veeravagu A, Guzman R, Patil CG, Hou LC, Lee M, Steinberg GK. Moyamoya disease in pediatric patients: outcomes of neurosurgical interventions. Neurosurgical focus. 2008;24:E16. doi: 10.3171/FOC/2008/24/2/E16. [DOI] [PubMed] [Google Scholar]
- Vernieri F, Pasqualetti P, Matteis M, Passarelli F, Troisi E, Rossini PM, Caltagirone C, Silvestrini M. Effect of collateral blood flow and cerebral vasomotor reactivity on the outcome of carotid artery occlusion. Stroke; a journal of cerebral circulation. 2001;32:1552–1558. doi: 10.1161/01.str.32.7.1552. [DOI] [PubMed] [Google Scholar]
- Vilela MD, Newell DW. Superficial temporal artery to middle cerebral artery bypass: past, present, and future. Neurosurgical focus. 2008;24:E2. doi: 10.3171/FOC/2008/24/2/E2. [DOI] [PubMed] [Google Scholar]
- Waltenberger J, Lange J, Kranz A. Vascular endothelial growth factor-A-induced chemotaxis of monocytes is attenuated in patients with diabetes mellitus: A potential predictor for the individual capacity to develop collaterals. Circulation. 2000;102:185–190. doi: 10.1161/01.cir.102.2.185. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang H, Wiltshire T, Sealock R, Faber JE. Genetic dissection of the Canq1 locus governing variation in extent of the collateral circulation. PloS one. 2012;7:e31910. doi: 10.1371/journal.pone.0031910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Baker MB, Moore JP, Searles CD. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochemical and biophysical research communications. 2010;393:643–648. doi: 10.1016/j.bbrc.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg DG, Arnaout OM, Rahme RJ, Aoun SG, Batjer HH, Bendok BR. Moyamoya disease: a review of histopathology, biochemistry, and genetics. Neurosurgical focus. 2011;30:E20. doi: 10.3171/2011.3.FOCUS1151. [DOI] [PubMed] [Google Scholar]
- Wityk RJ. Blood pressure augmentation in acute ischemic stroke. Journal of the neurological sciences. 2007;261:63–73. doi: 10.1016/j.jns.2007.04.033. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Fukuyama H, Nagahama Y, Nabatame H, Ueno M, Nishizawa S, Konishi J, Shio H. Significance of increased oxygen extraction fraction in five-year prognosis of major cerebral arterial occlusive diseases. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1999;40:1992–1998. [PubMed] [Google Scholar]
- Yarnitsky D, Lorian A, Shalev A, Zhang ZD, Takahashi M, Agbaje-Williams M, Macdonald RL. Reversal of cerebral vasospasm by sphenopalatine ganglion stimulation in a dog model of subarachnoid hemorrhage. Surgical neurology. 2005;64:5–11. doi: 10.1016/j.surneu.2004.09.029. discussion 11. [DOI] [PubMed] [Google Scholar]
- Yoshihara T, Taguchi A, Matsuyama T, Shimizu Y, Kikuchi-Taura A, Soma T, Stern DM, Yoshikawa H, Kasahara Y, Moriwaki H, Nagatsuka K, Naritomi H. Increase in circulating CD34-positive cells in patients with angiographic evidence of moyamoya-like vessels. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008;28:1086–1089. doi: 10.1038/jcbfm.2008.1. [DOI] [PubMed] [Google Scholar]
- Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:923–934. doi: 10.1038/jcbfm.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivin JA, Albers GW, Bornstein N, Chippendale T, Dahlof B, Devlin T, Fisher M, Hacke W, Holt W, Ilic S, Kasner S, Lew R, Nash M, Perez J, Rymer M, Schellinger P, Schneider D, Schwab S, Veltkamp R, Walker M, Streeter J, NeuroThera E, Safety Trial I Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke; a journal of cerebral circulation. 2009;40:1359–1364. doi: 10.1161/STROKEAHA.109.547547. [DOI] [PubMed] [Google Scholar]


