Abstract
The classic view of sensorineural hearing loss (SNHL) is that the “primary” targets are hair cells, and that cochlear-nerve loss is “secondary” to hair cell degeneration. Our recent work in mouse and guinea pig has challenged that view. In noise-induced hearing loss, exposures causing only reversible threshold shifts (and no hair cell loss) nevertheless cause permanent loss of >50% of cochlear-nerve / hair-cell synapses. Similarly, in age-related hearing loss, degeneration of cochlear synapses precedes both hair cell loss and threshold elevation. This primary neural degeneration has remained hidden for three reasons: 1) the spiral ganglion cells, the cochlear neural elements commonly assessed in studies of SNHL, survive for years despite loss of synaptic connection with hair cells, 2) the synaptic terminals of cochlear nerve fibers are unmyelinated and difficult to see in the light microscope, and 3) the degeneration is selective for cochlear-nerve fibers with high thresholds. Although not required for threshold detection in quiet (e.g. threshold audiometry or auditory brainstem response threshold), these high-threshold fibers are critical for hearing in noisy environments. Our research suggests that 1) primary neural degeneration is an important contributor to the perceptual handicap in SNHL, and 2) in cases where the hair cells survive, neurotrophin therapies can elicit neurite outgrowth from spiral ganglion neurons and re-establishment of their peripheral synapses.
A. Primary vs. Secondary Neural Degeneration in Sensorineural Hearing Loss
Sensorineural hearing loss (SNHL), as a category of hearing impairment, includes those etiologies in which the underlying pathology involves the sensory cells and/or the sensory neurons of the inner ear. Although primary neural degeneration, i.e. neural loss without hair cell loss, is recognized as a subclass of SNHL, it has been considered rare, comprising mainly cases of congenital and/or hereditary defects (Starr et al., 2000; Starr et al., 1996). As a clinical entity, auditory neuropathy, as it is called, is defined by normal hair cell function (as seen in normal otoacoustic emissions) despite absent or grossly abnormal cochlear neural responses, such as the auditory brainstem response (ABR). Although the underlying histopathology is poorly understood, the dysfunction can theoretically originate anywhere from hair cell synaptic transmission to the conduction of action potentials in auditory nerve fibers (ANFs). One clearcut etiology is a genetic mutation in otoferlin (Santarelli et al., 2009), a protein expressed in inner hair cells (IHCs) and thought to control vesicle release at the synapses with ANFs (Beurg et al., 2010).
In cases of acquired SNHL, far and away the most common form of SNHL, it has been widely believed that the hair cells are the primary targets, and that the degeneration of sensory neurons occurs almost exclusively as a secondary consequence of the loss of their hair cell targets (Bohne et al., 2000; Johnsson, 1974). This view arises from observing the time course of histopathology in the two most common animal models of acquired SNHL i.e. acoustic trauma and ototoxic antibiotics. Numerous studies over the last 5 decades have shown that within 24 hrs, or less, after a noise exposure or drug treatment, there can be massive hair cell loss, whereas the first degeneration of spiral ganglion neurons (SGNs), the cell bodies of the ANFs, is not detectable for weeks to months post treatment (Johnsson, 1974; Liberman et al., 1978; Spoendlin, 1975). At these longer post-treatment times, SGN loss tends to be maximal in the regions of hair cell loss, particularly in regions of inner hair cell (IHC) loss. This seemed reasonable, because 95% of ANFs contact IHCs only (Spoendlin, 1972), and naturally led to the idea that SGNs degenerate as a consequence of losing an important source of a key trophic factor generated by the IHCs. Some studies suggested that the presence or absence of supporting cells in the IHC area was actually the key determinant of neural survival (Sugawara et al., 2005; Suzuka et al., 1988). Regardless, the consensus was that hair cell loss in acquired SNHL is primary and SGN loss is secondary.
B. Noise-induced Cochlear Synaptopathy without Hair Cell Loss
There is extensive and longstanding evidence that cochlear neurons are directly targeted by noise. Morphological studies in cats, guinea pigs and mice have shown that a prominent component of the cochlea’s acute response to acoustic overexposure is the swelling of ANF terminals in the region of their synaptic contact with the hair cells (Liberman et al., 1982; Robertson, 1983; Spoendlin, 1971). This noise-induced swelling is seen only in the IHC area, not the OHC area, and, although prominent during the 24–48 hours post exposure, tends to disappear at longer post-exposure survival times. A similar swelling of synaptic terminals can be seen in cochleas perfused with glutamate agonists, or after hypoxia, and can be at least partially blocked by perfusion of glutamate antagonists during acoustic overstimulation (Pujol et al., 1999; Pujol et al., 1985). These observations suggest that the noise-induced acute neuropathy is a kind of glutamate excitotoxicity. The fact that it is not observed in the OHC area is consistent with the observation that AMPA-type glutamate receptors are highly expressed at the IHC/ANF synapses, but not the OHC/ANF synapses, in the adult ear (Liberman et al., 2011; Matsubara et al., 1996; Ottersen et al., 1998).
Synaptic swelling can be seen immediately post-exposure in noise-exposed ears regardless of whether there will be only a temporary threshold shift (TTS) or a permanent threshold shift (PTS). The acute ultrastructural pathology in the ANF terminals is dramatic: numerous swollen profiles are seen opposite normal-looking pre-synaptic ribbon complexes. These swollen terminals are often devoid of cytoplasm and show widespread rupture of the cell membrane, suggesting that degeneration must soon follow (Liberman et al., 1982; Robertson, 1983). The fact that the swelling disappears with post-exposure time, as thresholds recover, suggested that 1) the swollen terminals were responsible for the TTS, and 2) that the terminals must fully regenerate if the thresholds recover. The appearance of growth-cone like structures in the IHC neuropil also supported the idea that ANF terminals regenerate after noise (Puel et al., 1998; Ruel et al., 2007). However, none of these ultrastructural studies counted synapses in recovered ears.
Two physiological observations are relevant to interpreting this correlation between threshold recovery and neural recovery. First, in noise-induced PTS as well as TTS, the threshold shifts in neural responses, either ABR or the compound action potential (CAP) recorded from the round window, are very similar in magnitude to the threshold shifts in otoacoustic emissions (Darrow et al., 2007; Mills, 2003). Given that the emissions reflect the integrity of cochlear processes “upstream” of synaptic transmission, i.e. cochlear micromechanics, endocochlear potential and OHC forward and reverse transduction, the similarity between OAE and ABR threshold shifts suggests that one need look no farther than OHC damage to “explain” the threshold shift in moderate (~40 – 50 dB) TTS or PTS induced by noise. Based on prior studies of acoustic trauma, we believe that moderate PTS is typically due to stereocilia damage on OHCs (Liberman et al., 1984; Wang et al., 2002), while TTS might be due to reversible collapse of the supporting cells of the organ of Corti (Liberman et al., 1982; Wang et al., 2002). Second, several lines of evidence suggest that pure-tone thresholds, whether behavioral (the audiogram) or electrophysiological (CAP or ABR), are remarkably insensitive to diffuse neural degeneration (see below), as long as OHC function is normal (Bourien et al., 2014; Liberman et al., 1997; Lobarinas et al., 2013; Schuknecht et al., 1955). Thus, the synaptic swelling is likely not responsible for the transient threshold elevation, and the full recovery of thresholds need not be indicative of full neural recovery.
Direct evidence for lingering post-exposure neural damage came to light in a study designed to probe the interactions between early noise-exposure and aging (Kujawa et al., 2006). In that study, we found that mice exposed to a noise designed to cause a moderate (30–40 dB) PTS in both ABRs and DPOAEs, suffered a delayed loss of SGNs (after months to years) despite no initial, or delayed, loss of IHCs or OHCs. To gain further insight into the mechanisms of this primary neural degeneration, we applied immunostaining protocols to allow us to evaluate and quantify the IHC/ANF synapses at early post-exposure times.
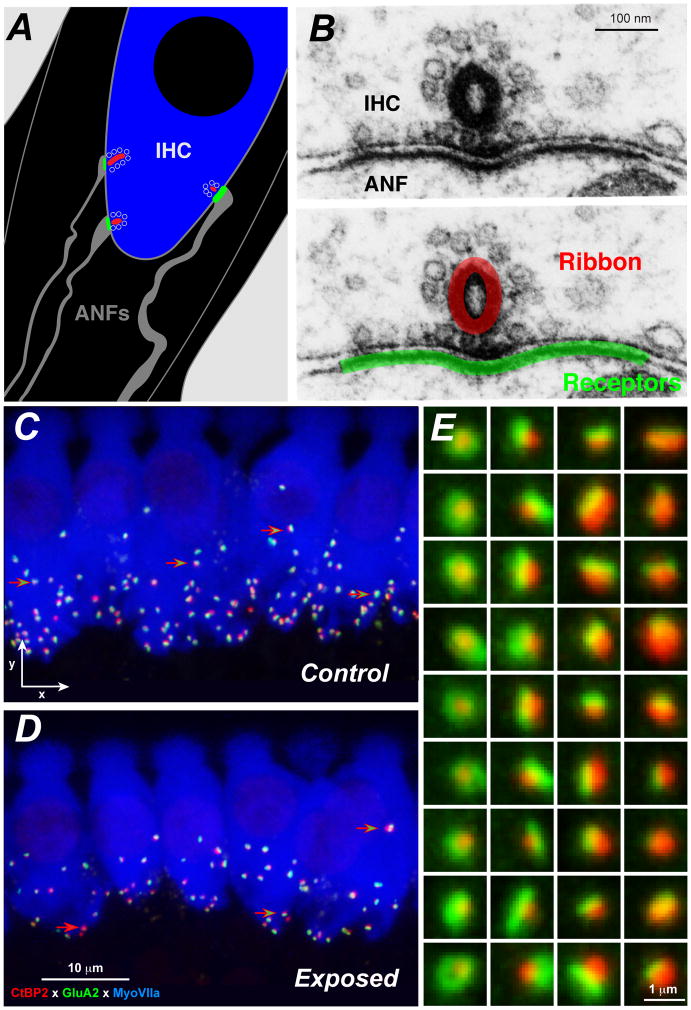
Each SGN sends a single peripheral axon to the organ of Corti, where it contacts a single IHC via a single unmyelinated terminal dendrite within the organ of Corti (Liberman, 1980; Liberman et al., 1990; Stamataki et al., 2006). As shown in Figure 1, the synapse between the ANF terminal and the IHC comprises a single active zone of pre- and post-synaptic specializations (Liberman, 1980). The pre-synaptic zone can be immunostained with antibodies to a protein called CtBP2, which is a prominent component of the synaptic ribbons anchored to these active zones (Khimich et al., 2005; Schmitz et al., 2000). The post-synaptic elements can be immunostained with antibodies to the AMPA-type glutamate receptors (e.g. GluA2), which are prominently expressed in the ANF terminals (Matsubara et al., 1996), or with antibodies to neurofilaments expressed in the axoplasm of ANFs or a Na+-K+ ATPase highly expressed in the membranes of ANF terminals (McLean et al., 2009). In the normal ear, the perfect one-to-one pairing between CTBP2- and GluA2-positive puncta (Figure 1E) provides compelling evidence that IHC synapses can be accurately counted at the light-microscopic level.
Figure 1. Immunostaining cochlear epithelial whole mounts to reveal primary cochlear synaptopathy.
A: Schematic of the basolateral membrane of an IHC showing three of the 10–20 synapses from ANF terminals that normally contact an IHC in the mouse cochlea. The color scheme for red, green and blue matches that used for the confocal images in Panels C and D. B: Electron micrographs of the active zone between an ANF and an IHC from cat (Liberman, 1980), showing the presynaptic ribbon, its halo of vesicle and the pre- and post-synaptic membrane thickening. In the lower panel of the pair of images, red and green have been superimposed on the micrograph to schematize the immunostained synaptic puncta we count in the confocal. C and D: Maximum projections from z-stacks of the IHC area from the 32 kHz region of a control and a noise-exposed mouse cochlea fixed 1 wk post exposure to the noise band described in Figure 2. Green-filled red arrows point to paired synaptic puncta in both images; red-filled arrow (D only) points to an orphan synaptic ribbon. E: High-power thumbnails of a selection of paired synaptic puncta, arrayed to illustrate the resolution achieved in the confocal and the trend that synapses with larger post-synaptic receptor patches (GluA2, green) tend to be paired with smaller pre-synaptic ribbons (CtBP2, red), and vice versa (Liberman et al., 2011).
Armed with these immunostaining protocols, we showed, in mouse, that noise exposures causing a large (35–45 dB) TTS, as measured 1 day post exposure, but no PTS, as measured 2 wks post exposure, nevertheless cause an immediate (within 24 hrs) synaptopathy in the IHC area (Kujawa et al., 2009). This synaptopathy is seen as a loss of 40–50% of the IHC/ANF synapses (Fig. 1C, D and Fig. 2A), despite no loss of IHCs or OHCs, despite full recovery of ABR and DPOAE thresholds, and despite no initial or delayed loss of IHCs. Loss of SGNs eventually matched the loss of ANF synapses, however the time course was extremely slow: the loss of SGNs slowly approached the magnitude of the acute loss of synapses over 1–2 years. Although the eventual loss of the SGN is very slow, the immediate post-exposure loss of an ANF’s sole synaptic connection to the IHC renders these fibers unresponsive to sound and only relevant to the sense of hearing in the presence of a cochlear implant.
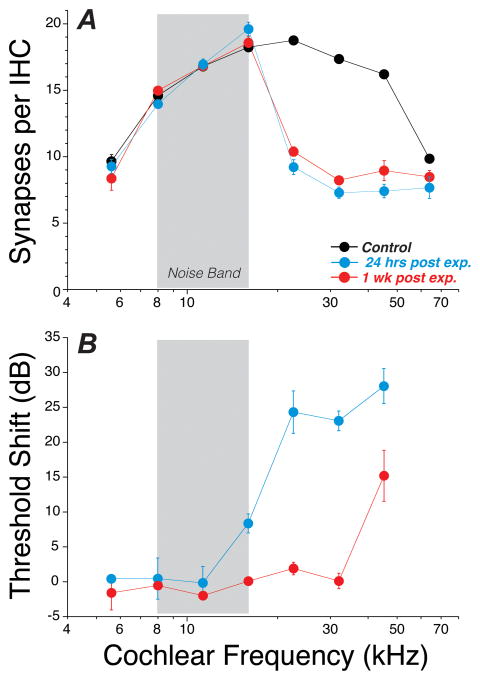
Figure 2. Permanent cochlear synaptopathy after exposure causing largely reversible threshold shift.
A: Synaptic puncta were counted in the IHC area from 8 cochlear locations in control ears (n=16) and noise-exposed ears (n=6 at each post-exposure time), exposed at 8 wks of age and assessed either 24 hrs or 1 wk after exposure to an 8–16 kHz octave band noise at 98 dB for 2 hrs. B: Thresholds in the noise-exposed ears, as measured by DPOAEs, were elevated at 24 hrs post exposure by 25–30 dB in the basal half of the cochlea, but had completely recovered at all but the highest test frequency by 1 wk post exposure. For further details on methodology, see prior studies from our laboratories (Kujawa et al., 2009; Liberman et al., 2015; Liberman et al., 2014).
In this original study, we followed the noise-exposed mice for 8 wks post exposure, and saw no evidence for synaptic regeneration in the IHC area (Kujawa et al., 2009). More recently, we have followed the post-exposure fate of hair cells and cochlear neurons throughout the mouse’s lifespan and observed that IHC synaptic counts in noise-exposed ears only continue to decline with increasing age (Kujawa et al., 2011).
C. Generalizability of Noise-induced Synaptopathy across Species, Age-at-exposure and Exposure Parameters
Our initial work on mouse was carried out with an octave-band noise placed in roughly the middle of the mouse’s hearing range (8 – 16 kHz), and presented for 2 hrs at a level titrated to produce a large, but ultimately completely reversible, threshold elevation. When measured 1-day post-exposure, the TTS peaked at about 40 dB when measured in distortion product otoacoustic emissions (Kujawa et al., 2009). To determine whether the phenomenon was possibly unique to the mouse, we repeated the approach in guinea pig, i.e. placed an octave-band noise near the middle of the animal’s hearing range (4–8 kHz) and presented it for 2 hrs at a level titrated to produce a large, but ultimately reversible, threshold elevation. This exposure also produced a severe synaptopathy, analogous in cochlear position and degree to that seen in the mouse (Furman et al., 2013; Lin et al., 2011).
In our guinea pig studies, post-exposure survival was limited to 2 wks (Furman et al., 2013; Lin et al., 2011). A more recent study of noise-induced synaptopathy in guinea pig has suggested that synapses can regenerate if survival is extended to 4 wks (Shi et al., 2013). The exposure in the latter study was more moderate: 105 dB broadband noise for 2 hrs vs. octave-band noise at 106 or 109 dB for 2 hrs. With more moderate exposures, the apparent post-exposure recovery of synaptic counts could reflect transient down- and up-regulation of pre- and post-synaptic proteins targeted by the immunostains, rather than degeneration and regeneration of the peripheral terminals. Indeed, ribbon counts in mice transiently decrease in the cochlear apex when assessed immediately after exposure to the 98 dB octave band noise, and then recover 24 hrs later. Furthermore, we have recently observed noise-induced cochlear synaptopathy in chinchilla of a similar degree to that seen in the mouse (50% loss) and have seen no signs of synaptic recovery out to 6 wks post-exposure (Liberman, Hickox and Heinz, unpublished).
One of the challenges in understanding the response of the ear to noise, and the overall patterns of damage risk, is the range of possible stimulus parameters. In the study of noise-induced threshold shift, a longstanding idea is that a key predictor of damage is the total energy delivered during the exposure (Ward et al., 1981). Although the equal energy hypothesis is only a rough approximation, it is the basis for the time-intensity trading relation that defines the federal guidelines for daily noise exposure, e.g. those of OSHA which suggest that 90 dB for 8 hrs is as dangerous as 95 dB for 4 hrs or 100 dB for 2 hrs, etc https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=standards&p_id=9735. Given its relevance to human workplace exposures, we wondered if noise-induced synaptopathy also results from much longer exposures at lower SPLs. Thus, in mice, we studied a one-week exposure at 84 dB SPL to the same 8–16 kHz octave-band noise used in earlier studies of 100 dB / 2 hr exposures (Kujawa et al., 2009). We found as much as a 22% loss of synapses in some cochlear regions (Maison et al., 2013). This exposure caused only a very small, and very transient, TTS: < 12 dB when measured immediately after removing the animals from the noise. As in our other studies, there was no PTS and no loss of either inner or outer hair cells, outside of the very basal tip of the cochlear spiral.
Together these results suggest that, in all mammalian ears, presumably including human (see below), the IHC/ANF synapses are the most vulnerable elements in the ear to acoustic overexposures, at least to the type of continuous noise we have studied. It must be noted that the ear’s response to impulse noise may well be different (Henderson et al., 1986) and we do not yet know whether cochlear synaptopathy is also a major component of the inner ear damage after blast injury.
Within a given species, age-at-exposure is another important variable in the study of acoustic injury (Henry, 1984; Ohlemiller et al., 2000). Mouse studies have shown that the vulnerability of hair cells to noise exposure, and thus the degree of noise-induced PTS, varies with age. There is a dramatic change just around the time of puberty, with younger mice (4–8 wks) dramatically more vulnerable than older mice (>16 wks) (Kujawa et al., 2006). Most of our work on noise-induced synaptopathy in the mouse has been carried out on young adult animals, i.e. aged 16 wks out of a total life span of about 144 wks. Recently, we have shown that the same type of noise-induced primary synaptopathy is also achieved in juvenile mice aged 6–8 wks (Liberman et al., 2015): by simply reducing the exposure SPL by a few dB (98 vs 100 dB exposure level), it is also possible to destroy 40–50% of the IHC synapses in an ear in which the noise-induced threshold shift is completely reversible, except at the extreme basal end of the cochlea (Fig. 2).
With respect to the threshold shifts and hair cell damage in acquired SNHL, the basal half of the cochlea is generally more vulnerable than the apical half (Liberman et al., 1978; Schacht et al., 2012). Correspondingly, the acute synaptic loss observed in both the mouse and guinea pig studies was primarily seen in the basal half of the cochlear spiral. For example, in the mouse study, the damage, and the acute threshold shifts, were seen above the 16 kHz region following overexposure to an 8–16 kHz octave band noise (Kujawa et al., 2009). It is more difficult to produce an immediate synaptopathy in the apical regions of the cochlea – it does not occur acutely in mice when the noise band is shifted to lower frequencies until significant basal turn injury has already been produced (Kujawa unpublished). Correspondingly, in the guinea pig, we were unable to produce acute swelling in the IHC area following exposure to low-frequency tones (e.g. 1 kHz), although it was reliably produced by exposure to high-frequency tones, e.g. 10 kHz (Liberman unpublished). However, following a mid-frequency exposure the synaptopathy spreads apically as post-exposure time exceeds 1 yr, and as age-related changes are superimposed on even a single episode of TTS-producing noise exposure early in life (Kujawa et al., 2011).
D. Age-related Cochlear Synaptopathy and the Role of Efferent Feedback
The relationship between noise-induced and age-related hearing loss has been debated for decades (Rosen et al., 1962). The question of the extent to which age-related changes are simply the accumulation of countless noise-induced micro-lesions remains open. However, most prior work on age-related hearing loss focused on hair cell loss and/or threshold shifts (Gates et al., 2005).
Using the same immunostaining techniques developed to study noise-exposed mice, we recently compared hair cell counts, synaptic counts and ganglion cell counts to measures of cochlear function (OAEs and ABRs) in an age-graded series of mice raised in the relative quiet of our sound-monitored room in the animal care facility (Sergeyenko et al., 2013). As with the response to noise exposure, we found that the IHC/ANF synapses in the aging cochlea are the most vulnerable elements, not the hair cells. Synaptic counts in the IHC area decreased monotonically from weaning (4 wks) to death (~144 wks), with mean age-related loss of ~50% across a broad range of cochlear locations. SGN loss was also a monotonically increasing function of age, but with a time course delayed by several months to reach the same degree of loss. Hair cell loss, on the other hand, was minimal until very late in life. For example, at late middle age, i.e. ~ 80 wks in mouse, the OHC loss was < 5% at mid and high cochlear frequencies, and, correspondingly, the OAE threshold shift was < 5dB; in contrast, at the same age, the synaptic loss was 25% (Sergeyenko et al., 2013).
It is well known that feedback from the olivocochlear efferent system can protect hair cells from noise exposure as it reduces the noise-induced threshold shifts, both temporary and permanent (Kujawa et al., 1997; Rajan, 1991). After discovering that the IHC/ANF synapses are actually the most vulnerable elements in the ear, we re-examined the question of a protective role of efferent feedback in two studies: one focused on the noise-exposed ear (Maison et al., 2013) and one on the aging ear (Yin et al., 2014). The noise exposure we studied was the 1-wk exposure at 84 dB SPL. We showed that cutting the efferent bundle, to remove all efferent feedback to the inner ear, greatly exacerbated the synaptopathy: for example, in the de-efferented ears, a doubling of the loss of synapses from 20% to 40% was observed in both basal and apical regions of the cochlea. In the companion study of mice aged in the relative quiet of our animal care facility, the loss of olivocochlear innervation early in life (6 wks of age) almost tripled the age-related loss of IHC synapses, when measured at middle age (i.e. 52 wks): e.g. from ~20% at the cochlear apex in normal aging ears, to ~60% loss in the same cochlear regions of de-efferented aging ears (Liberman et al., 2014).
The olivocochlear system has two components: 1) the medial olivocochlear system, which constitutes a sound-evoked negative feedback loop of myelinated axons projecting to the OHCs and controlling the gain of the cochlear amplifier and 2) the lateral olivocochlear system, consisting of unmyelinated axons projecting to the dendrites of ANFs near the IHC synapses (Guinan, 2006). Analysis of the relative loss of lateral and medial subsystems in our two studies suggested that both subsystems contribute to the anti-synaptopathic protective effect. The medial system does so presumably by continually reducing the sound-evoked discharge rate (and therefore the glutamate challenge) in ANFs to moderate level tones (Guinan et al., 1996) through its cholinergic gain-control function in the OHC area. The lateral system presumably does so via its dopaminergic component (Darrow et al., 2006), given other pharmacologic evidence that dopaminergic agonists can reduce glutamate excitotoxicity in the IHC area (Ruel et al., 2001).
E. Physiological Metrics of Cochlear Synaptopathy – Selective Loss of High-threshold ANFs
In both noise-induced and age-related hearing loss, the degree of noise-induced cochlear synaptopathy is well matched to the decrement in suprathreshold amplitudes of cochlear neural potentials: the reduction in amplitudes of wave 1 of the tone-pip evoked ABR or round-window CAP were reduced by roughly the same percentage as the observed reduction in synaptic counts (Kujawa et al., 2009; Sergeyenko et al., 2013). This parity is observed only if, in the noise-exposure model, OHC function has returned to normal, as evidenced by full recovery of otoacoustic emission thresholds and suprathreshold response amplitudes. In the aging model, it is true only when examined before the OHC damage sets in. Obviously a reduction in OHC amplifier gain will also reduce ABR responses, and an unambiguous resolution of how much reduction is due to synaptopathy vs. OHC dysfunction is likely impossible. Given normal OHC function, parity between the degree of synaptopathy and the degree of neural response reduction is expected 1) if the contribution to the far-field electrical response of each action potential from each ANF is similar in magnitude, as has been empirically demonstrated for the round-window CAP (Kiang et al., 1976; Prijs, 1986), 2) if these unitary contributions add linearly to the far-field response, and 3) if the tone-pip evoked response rates are similar across all ANFs of similar best frequency.
Although decrements in suprathreshold amplitudes are to be expected, it is not immediately obvious how the thresholds for ABR (or CAP) can appear unchanged in the face of 50% loss of ANF synapses. There are several factors that help explain this apparent paradox.
The first is that neural activity spreads very rapidly along the cochlear spiral as sound pressure increases, such that only a small increase in SPL (i.e. 2–3 dB) is required near threshold to double the ensemble discharge rate to a particular tone-pip frequency across the ANF population, and thus to compensate for the loss of 50% of ANF synapses (Bourien et al., 2014). Since ABR near-threshold responses are noisy, and since thresholds are usually measured with 5 dB step size, such a small change in threshold would appear as insignificant without enormous group sizes and very long averaging times.
The second is that the loss of synapses appears to be selective for (or at least highly biased towards) the subset of ANFs with high thresholds (Furman et al., 2013). In the normal mammalian ear, there is a range of sensitivity among ANFs from the same cochlear region: fibers with similar best frequency can differ by up to 60 dB in pure-tone thresholds (Liberman, 1978). This threshold difference is tightly correlated with the spontaneous discharge rate (SR), the spike rate in the absence of controlled acoustic stimulation (Liberman, 1978). SR varies in different fibers from essentially 0 sp/sec to over 100 sp/sec, and the distribution of SRs is bimodal, at least in cat and guinea pig, with a low-rate peak (SRs < 20 sp/sec) comprising 40% of the population and high-rate peak (SRs > 20 sp/sec) comprising 60% of the sample (Liberman, 1978; Tsuji et al., 1997). It appears that both high- and low-SR fibers can contact the same IHC, however, the two fiber types tend to synapse on opposite sides of the IHC (Liberman, 1982). High-SR, low-threshold fibers also tend to have larger diameters, more mitochondria in their peripheral terminals and more AMPA-type glutamate receptors expressed at their synapses with IHCs (Liberman et al., 2011; Liberman, 1980; Liberman et al., 1990). There may also be pre-synaptic differences including differences in the size of the pre-synaptic ribbon (Liberman et al., 2011; Merchan-Perez et al., 1996) and possibly also in the number of voltage gated calcium channels (Frank et al., 2009).
In both the noise-exposed guinea pig (Furman et al., 2013) and the aging gerbil (Schmiedt et al., 1996), single-fiber recordings from ANFs show a reduced percentage of low-SR fibers and an increased percentage of high-SR fibers, suggesting that the synaptopathy has been selective for the high-threshold, low-SR fiber population. Clearly, if the synaptopathy has selectively removed only those fibers with higher thresholds, this would explain how the cochlea can lose so many fibers without a change in the observed thresholds for an ABR or a CAP response.
In the noise-exposed guinea pig, the response properties of remaining high-SR fibers were normal in all respects, consistent with the full recovery of OHC function as well as complete recovery of IHC synaptic function in the surviving neurons (Furman et al., 2013). In an earlier study of ANF responses in noise-exposed cats, electric shocks were used as a search stimulus while advancing the microelectrode through the nerve bundle. The shocks, delivered to the round window, revealed numerous silent fibers, with no spontaneous activity and no response to sound, but otherwise normal action potential waveforms and conduction velocity for the shock-evoked spikes (Liberman et al., 1978). We assumed, at the time, that such silent fibers belonged to ANFs that had lost their peripheral terminals subsequent to loss of the contacted IHCs. In light of recent work, we believe that they also represent low-SR fibers that have lost their peripheral terminals despite the survival of the IHC they formerly contacted. The normality of their conduction velocities is consistent with the normal histological appearance of the SGNs in noise-exposed ears prior to their slow degeneration after loss of IHC synapses.
There is no direct evidence as to why the low-SR population might be more vulnerable to noise-induced neuropathy than the high-SR population. However, it is interesting to consider two hypotheses. The first arises from the observation that one of the primary morphological differences between the two fiber groups is in the density of mitochondria (Liberman, 1980). High-threshold fibers with low SR have many fewer mitochondria in their peripheral terminals than the low-threshold high-SR fibers, consistent with the assumed increase in metabolic load produced in high SR fibers by the need to counteract the ongoing ionic fluxes associated with the continually high spike rates, even in quiet. This difference in mitochondrial content is interesting, because, in the central nervous system where it has been best studied, the cell death cascade initiated by glutamate excitotoxicity is thought to involve Ca++ overload, and one of the most important intracellular Ca++ buffering systems is the mitochondria (Szydlowska et al., 2010). A second view is suggested by the differences in glutamate transporter levels in the support cells on the two sides of the IHC (Furness et al., 2003). Control of excitotoxicity at the IHC/ANF synapse requires re-uptake of glutamate via the GLAST transporter, and an immunostaining study at the electron microscopic level suggests that GLAST expression is weaker on the side of the IHC where low-SR synapses predominate (Furness et al., 2003).
F. Cochlear Synaptopathy and Hidden Hearing Loss - the Behavioral Consequences
A number of lines of evidence make it clear that the pure-tone audiogram is remarkably insensitive to diffuse neural degeneration, as are the far-field neural potentials like ABR and CAP. Even if noise- and age-induced neuropathy were randomly distributed among ANFs, without regard to threshold/SR group, effects on behavioral thresholds would be minimal until the loss exceeds 80%. We know this from classic behavioral studies of cats with partial surgical section of the ANFs (Schuknecht et al., 1955) and from more recent behavioral studies of chinchilla with selective loss of IHCs subsequent to administration of the chemotherapeutic drug carboplatin, which is also ototoxic (Lobarinas et al., 2013). Similarly, some patients with severe auditory neuropathy, as defined by normal otoacoustic emissions in the absence of any measureable ABR response, also have normal audiometric thresholds, but extremely poor speech discrimination scores (Starr et al., 2000; Starr et al., 1996). Apparently, only a very small fraction of ANFs along the cochlear spiral is necessary for the detection of a pure tone stimulus in a quiet environment. Nevertheless, it seems reasonable to assume that cochlear synaptopathies on the order of the 50% loss we observed in noise-exposed and aging mice would have an impact on some measure of hearing ability. Based on this assumption, and given that this putative dysfunction “hides” behind a normal audiogram, just as the cochlear histopathology is initially “hidden” within the complex neuropil of the IHC area, the phenomenon has been termed “hidden hearing loss” (Schaette et al., 2011).
The single-fiber work in noise-exposed and aging guinea pigs or gerbils, respectively, suggests that this primary neural degeneration is biased towards fibers that normally have high thresholds and low SRs. Based on the literature on single-fiber responses in the AN, what can we infer about the nature of the perceptual dysfunction in hidden hearing loss? In addition to their higher pure-tone thresholds, low-SR ANFs tend to have larger dynamic ranges (Schalk et al., 1980) and reduced susceptibility to excitatory masking by continuous noise stimuli (Costalupes et al., 1984). This should make them particularly important to understanding of complex stimuli in a noisy environment. The reduced masking is intimately related to their high threshold and large dynamic range. In quiet, any ANF shows prominent post-onset adaptation to a tone burst, with a maximum onset rate that is many times higher than the steady-state rate to which it asymptotically falls as the tone burst continues (Smith, 1979). This post-onset adaptation arises from depletion of a readily releasable pool of synaptic vesicles at the IHC/ANF synapse (Spassova et al., 2004). As the level of a continuous background noise increases, it will eventually elicit a response equal to the maximum steady-state rate that a fiber can achieve, at which point an additional tone-burst stimulus will not elicit any increase in firing rate (Costalupes et al., 1984) regardless of tone-burst intensity. At this point, the fiber’s tone-burst response is completely eliminated via a phenomenon known as excitatory masking (Delgutte,1990). High-SR fibers, by virtue of their lower thresholds and smaller dynamic ranges, reach full excitatory masking at a much lower SPL of background noise than low-SR fibers. Thus, to some extent, we must rely increasingly on our low-SR fibers to hear as the levels of background noise increase.
A major complaint of people with age- or noise-induced SNHL is the inability to understand speech in a noisy environment, and people with normal or near-normal audiograms can have widely differing abilities to understand speech in a noisy environment (Frisina et al., 1997). We hypothesize that both of these longstanding observations may be largely explained by the phenomenon of hidden hearing loss.
It is also well known that noise-exposure often leads to poorly understood perceptual phenomena like tinnitus, the sensation of phantom tones, and hyperacusis, a reduced tolerance to moderate-level sounds. Furthermore, these perceptual anomalies can be permanent results of an exposure that causes only a temporary threshold elevation (Roberts et al., 2010). It has been hypothesized that both these phenomena may arise from a type of homeostatic plasticity, i.e. the upregulation of synaptic gain in central auditory neurons subsequent to the loss of ascending inputs from ANFs to central circuits (Schaette et al., 2011). Indeed, we have observed that, despite the reduction in wave 1 of the ABR in both noise-induced and age-related IHC synaptopathy, there is no reduction in the amplitude of wave 5, which is thought to arise mainly from responses in the inferior colliculus (Hickox et al., 2014; Sergeyenko et al., 2013). We also observed that noise-induced synaptopathy enhances the auditory startle responses, a phenomenon which might reflect something akin to the phenomenon of hyperacusis in humans (Hickox et al., 2014). Control animals exposed to noise at a lower level, which created neither PTS nor synaptopathy, showed normal startle responses. Studies from noise-exposed rats have suggested that auditory behavior consistent with tinnitus is produced when there is primary neural degeneration after exposure to a TTS-producing noise band (Bauer et al., 2007). Two recent human studies have shown that the ratio of Wave I to Wave V is reduced in tinnitus sufferers with normal audiometric thresholds, compared to non-tinnitus sufferers with carefully matched audiometric profiles (Gu et al., 2012; Schaette et al., 2011). Such a pattern is consistent with the idea that primary degeneration of the ANFs, coupled with upregulation of central gain, is one way to elicit tinnitus in the absence of threshold elevations: see (Knipper et al., 2013) for a recent review.
Apart from such speculations, there is also direct histological evidence for widespread primary neurodegeneration and/or synaptopathy in the human cochlea. We counted SGNs in an age-graded series of human temporal bones (from birth to 100 yrs of age), selected from the collection at the Massachusetts Eye and Ear to include only those ears with no significant loss of hair cells (Makary et al., 2011). We observed a steady decline in the mean SGN counts with age, which showed a mean 30% loss by the 9th decade. Interestingly, cases with clear noise-exposure history had individual SGN counts well below the mean and up to 50% below the mean value from the youngest ears. Given that the loss of SGNs is greatly delayed re the initial synaptic loss, and speculating that the time course of SGN death in human ears may be much slower than that in mouse (otherwise cochlear implants would not continue to work for decades and there would be no SGNs remaining in ears with longstanding profound deafness due to loss of the sensory epithelium), we speculate that the observed SGN counts are greatly underestimating the degree of synaptopathy in the aging and noise-exposed human ear.
G. Strategies for Neural Regeneration in Hidden Hearing Loss
In the adult cochlea, the survival of ANFs depends on release of neurotrophins from the supporting cells in the IHC area (Stankovic et al., 2004). These supporting cells, which surround the unmyelinated portions of the AN terminals, act in a manner analogous to central glia, where neuregulin released by neurons binds to ErbB receptors on glia that, in turn, elicits the release of NT-3 or BDNF, which then binds to Trk receptors on the neurons and promotes survival. Based on experiments in transgenic mice with genetically induced overexpression or deletion of either NT-3 or BDNF, we conclude that NT-3 is more important in the cochlear epithelium, whereas BDNF is more important in the vestibular epithelia (Gomez-Casati et al., 2010; Stankovic et al., 2004; Wan et al., 2014). Supporting cells appear to be sufficient for ANF survival, even in the absence of IHCs, if IHCs are killed by targeted deletion of a gene expressed only in IHCs, rather than by noise or ototoxic drugs (Zilberstein et al., 2012). We hypothesize that the slow death of ANFs after noise arises because these cochlear insults directly damage the nerve terminals themselves (Robertson, 1983; Wang et al., 2003). Based on our genetic studies of neurotrophin signaling (Wan et al., 2014), we hypothesize that the slow death of SGNs is a consequence of the immediate post-noise retraction of the unmyelinated dendrite. Because this unmyelinated dendrite is in the region of intimate contact with IHC supporting cell, it is this region in which the NT-3 / TrkC signaling takes place, and loss of this contact zone leads to ultimate death of the entire SGN. The observation that ANFs degenerate in mice constitutively lacking vesicular glutamate release (Ruel et al., 2008) suggests that IHCs may be more important for neural survival during development than in the adult.
Animal studies of profound SNHL induced by ototoxic drugs have shown that cochlear perfusion of a neurotrophin cocktail, including NT-3, BDNF and/or GDNF, can prolong SGN survival for up to six months (Miller et al., 1997; Ylikoski et al., 1998). Most intriguingly, these neurotrophin treatments also elicit extension of peripheral processes from the SGNs through the osseous spiral lamina to the basilar membrane, where they spiral along the basilar membrane among the undifferentiated epithelial cells that have replaced the degenerated organ of Corti (Wise et al., 2005). Such results inspired us to ask, in the noise-induced synaptopathy model, whether NT-3 treatments can elicit regeneration of ANF peripheral terminals and reformation of synapses with the IHCs, which remain intact. Indeed, we recently showed that genetically mediated overexpression of NT-3 in supporting cells, elicited after a noise exposure, partially rescued the synaptopathy phenotype, as it partially restored the amplitudes of the suprathreshold ABRs (Wan et al., 2014).
Thus, there is reason to believe that, in humans, round-window delivery of neurotrophins in a slow-release gel, as well as possible systemic delivery of a neurotrophin agonist, could rescue the synaptopathic phenotype, if delivered within some yet unknown therapeutic window before the degeneration of the SGN had proceeded too far along its path.
H. Implications for Public Health and the Epidemiology of Acquired Sensorineural Hearing Loss
Over the last few decades, numerous studies have asked whether overexposure to loud sound in the course of leisure activities, including the listening to music through earphones or insert earbuds, is leading to an epidemic of noise-induced hearing loss: see (Rabinowitz et al., 2012) for a review. Until recently, such studies focused on changes to the behavioral audiogram (Niskar et al., 2001; Rabinowitz et al., 2006) or otoacoustic emissions (Serra et al., 2014), and therefore on the condition of the hair cells. If our new view of hidden hearing loss is correct, then neither the audiogram nor otoacoustic emissions is the appropriate test to reveal the first signs of accumulating noise-induced hearing loss. What is required is a non-invasive test for cochlear synaptopathy that is applicable to human populations.
Based on the animal work, we hypothesize that some measure of the amplitudes of suprathreshold auditory evoked potentials should be useful in the non-invasive diagnosis of cochlear synapthopathy: see for example the recent work on the ABR Wave V:I ratios and Wave I amplitudes in the studies of auditory function in aging and noise-exposed humans (Konrad-Martin et al., 2012; Stamper et al., 2014). Although the ABR is the more commonly applied clinical evoked potential, the Envelope Following Response (EFR), also known as the Auditory Steady State Response, may provide even greater sensitivity to cochlear synaptopathy. This is because the tone-pip evoked ABR relies on the onset responses of ANFs, and low-SR ANFs have a relatively lower onset to steady-state response ratio than high-SR fibers (Rhode et al., 1985), and thus contribute relatively less to ABR amplitudes (Bourien et al., 2014). For the EFR, on the other hand, the low-SR contribution is relatively higher than the high-SR contribution (Joris et al., 1992). Indeed, preliminary results from an ongoing study of college-age students in the UK with normal audiometric thresholds has revealed a significantly lower EFR amplitude in the group self-identified as having a history of high-level sound exposure (Plack et al., 2014).
The emerging data suggest that risk of hidden hearing loss from both recreational and occupational sources is underappreciated, and that the cochlear synaptopathy that is its cause may contribute to the common experience of increasing hearing difficulty through middle and old age (e.g. (Snell et al., 2000)). Existing federal guidelines governing allowable daily workplace exposures are all based on the fundamental assumption that full threshold recovery indicates full cochlear recovery. The emerging work on hidden hearing loss makes it quite clear that this fundamental assumption is severely flawed and thus that noise is much more dangerous than we have previously thought.
Acknowledgments
Research supported by grants from the NIH including R01 DC0188, R01 DC08577 and P30 DC 05209.
List of Abbreviations
- ABR
Auditory Brainstem Response
- AMPA
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid
- ANF
Auditory Nerve Fiber
- CAP
Compound Action Potential
- BDNF
Brain-Derived Neurotrophic Factor
- CtBP2
C-terminal Binding Protein 2
- (DP)OAE
(Distortion Product) Otoacoustic Emission
- EFR
Envelope Following Response
- GLAST
Glutamate Aspartate Transporter
- IHC
Inner Hair Cell
- NT-3
Neurotrophin-3
- OHC
Outer Hair Cell
- PTS
Permanent Threshold Shift
- SGN
Spiral Ganglion Neuron
- SNHL
Sensorineural Hearing Loss
- SPL
Sound Pressure Level
- TTS
Temporary Threshold Shift
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Bauer CA, Brozoski TJ, Myers K. Primary afferent dendrite degeneration as a cause of tinnitus. Journal of neuroscience research. 2007;85:1489–98. doi: 10.1002/jnr.21259. [DOI] [PubMed] [Google Scholar]
- Beurg M, Michalski N, Safieddine S, Bouleau Y, Schneggenburger R, Chapman ER, Petit C, Dulon D. Control of exocytosis by synaptotagmins and otoferlin in auditory hair cells. J Neurosci. 2010;30:13281–90. doi: 10.1523/JNEUROSCI.2528-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne BA, Harding GW. Degeneration in the cochlea after noise damage: primary versus secondary events. Am J Otol. 2000;21:505–9. [PubMed] [Google Scholar]
- Bourien J, Tang Y, Batrel C, Huet A, Lenoir M, Ladrech S, Desmadryl G, Nouvian R, Puel JL, Wang J. Contribution of auditory nerve fibers to compound action potential of the auditory nerve. J Neurophysiol. 2014;112:1025–39. doi: 10.1152/jn.00738.2013. [DOI] [PubMed] [Google Scholar]
- Costalupes JA, Young ED, Gibson DJ. Effects of continuous noise backgrounds on rate response of auditory nerve fibers in cat. J Neurophysiol. 1984;51:1326–44. doi: 10.1152/jn.1984.51.6.1326. [DOI] [PubMed] [Google Scholar]
- Darrow KN, Maison SF, Liberman MC. Selective removal of lateral olivocochlear efferents increases vulnerability to acute acoustic injury. J Neurophysiol. 2007;97:1775–85. doi: 10.1152/jn.00955.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow KN, Simons EJ, Dodds L, Liberman MC. Dopaminergic innervation of the mouse inner ear: evidence for a separate cytochemical group of cochlear efferent fibers. J Comp Neurol. 2006;498:403–14. doi: 10.1002/cne.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgutte B. Physiological mechanisms of psychophysical masking: observations from auditory-nerve fibers. J Acoust Soc Am. 1990;87:791–809. doi: 10.1121/1.398891. [DOI] [PubMed] [Google Scholar]
- Frank T, Khimich D, Neef A, Moser T. Mechanisms contributing to synaptic Ca2+ signals and their heterogeneity in hair cells. Proc Natl Acad Sci U S A. 2009;106:4483–8. doi: 10.1073/pnas.0813213106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisina DR, Frisina RD. Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hear Res. 1997;106:95–104. doi: 10.1016/s0378-5955(97)00006-3. [DOI] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. Journal of neurophysiology. 2013;110:577–86. doi: 10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness DN, Lawton DM. Comparative distribution of glutamate transporters and receptors in relation to afferent innervation density in the mammalian cochlea. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:11296–304. doi: 10.1523/JNEUROSCI.23-36-11296.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbycusis. Lancet. 2005;366:1111–20. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- Gomez-Casati ME, Murtie JC, Rio C, Stankovic K, Liberman MC, Corfas G. Nonneuronal cells regulate synapse formation in the vestibular sensory epithelium via erbB-dependent BDNF expression. Proc Natl Acad Sci U S A. 2010;107:17005–10. doi: 10.1073/pnas.1008938107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JW, Herrmann BS, Levine RA, Melcher JR. Brainstem auditory evoked potentials suggest a role for the ventral cochlear nucleus in tinnitus. Journal of the Association for Research in Otolaryngology : JARO. 2012;13:819–33. doi: 10.1007/s10162-012-0344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ., Jr Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear and hearing. 2006;27:589–607. doi: 10.1097/01.aud.0000240507.83072.e7. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Jr, Stankovic KM. Medial efferent inhibition produces the largest equivalent attenuations at moderate to high sound levels in cat auditory-nerve fibers. J Acoust Soc Am. 1996;100:1680–90. doi: 10.1121/1.416066. [DOI] [PubMed] [Google Scholar]
- Henderson D, Hamernik RP. Impulse noise: critical review. J Acoust Soc Am. 1986;80:569–84. doi: 10.1121/1.394052. [DOI] [PubMed] [Google Scholar]
- Henry KR. Noise and the young mouse: genotype modifies the sensitive period for effects on cochlear physiology and audiogenic seizures. Behavioral neuroscience. 1984;98:1073–82. doi: 10.1037//0735-7044.98.6.1073. [DOI] [PubMed] [Google Scholar]
- Hickox AE, Liberman MC. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J Neurophysiol. 2014;111:552–64. doi: 10.1152/jn.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson LG. Sequence of degeneration of Corti’s organ and its first-order neurons. Ann Otol Rhinol Laryngol. 1974;83:294–303. doi: 10.1177/000348947408300303. [DOI] [PubMed] [Google Scholar]
- Joris PX, Yin TC. Responses to amplitude-modulated tones in the auditory nerve of the cat. J Acoust Soc Am. 1992;91:215–32. doi: 10.1121/1.402757. [DOI] [PubMed] [Google Scholar]
- Khimich D, Nouvian R, Pujol R, Tom Dieck S, Egner A, Gundelfinger ED, Moser T. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature. 2005;434:889–94. doi: 10.1038/nature03418. [DOI] [PubMed] [Google Scholar]
- Kiang NYS, Moxon EC, Kahn AR. The relationship of gross potentials recorded from the cochlea to single unit activity in the auditory nerve. In: Ruben RJ, Eberling C, Solomon G, editors. Electrocochleography. University Park; Baltimore: 1976. [Google Scholar]
- Knipper M, Van Dijk P, Nunes I, Ruttiger L, Zimmermann U. Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Prog Neurobiol. 2013;111:17–33. doi: 10.1016/j.pneurobio.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Konrad-Martin D, Dille MF, McMillan G, Griest S, McDermott D, Fausti SA, Austin DF. Age-related changes in the auditory brainstem response. Journal of the American Academy of Audiology. 2012;23:18–35. doi: 10.3766/jaaa.23.1.3. quiz 74–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Conditioning-related protection from acoustic injury: effects of chronic deefferentation and sham surgery. J Neurophysiol. 1997;78:3095–106. doi: 10.1152/jn.1997.78.6.3095. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci. 2006;26:2115–23. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–85. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Micucci S, Liberman MC. Noise-induced primary neural degeneration: Effects of spectrum, duration, intensity and survival. Midwinter Meeting of the Association for Research in Otolaryngology; 2011. p. 56. [Google Scholar]
- Liberman LD, Wang H, Liberman MC. Opposing gradients of ribbon size and AMPA receptor expression underlie sensitivity differences among cochlear-nerve/hair-cell synapses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:801–8. doi: 10.1523/JNEUROSCI.3389-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman LD, Suzuki J, Liberman MC. Dynamics of cochlear synaptopathy after acoustic overexposure. Journal of the Association for Research in Otolaryngology. 2015 doi: 10.1007/s10162-015-0510-3. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC. Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am. 1978;63:442–455. doi: 10.1121/1.381736. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Morphological differences among radial afferent fibers in the cat cochlea: An electron-microscopic study of serial sections. Hear Res. 1980;3:45–63. doi: 10.1016/0378-5955(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Single-neuron labeling in the cat auditory nerve. Science. 1982;216:1239–1241. doi: 10.1126/science.7079757. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Kiang NY. Acoustic trauma in cats. Cochlear pathology and auditory-nerve activity. Acta oto-laryngologica. 1978;358:1–63. [PubMed] [Google Scholar]
- Liberman MC, Mulroy MJ. Acute and chronic effects of acoustic trauma: Cochlear pathology and auditory nerve pathophysiology. In: Hamernik RP, Henderson D, Salvi R, editors. New Perspectives on Noise-Induced Hearing Loss. 1982. pp. 105–136. [Google Scholar]
- Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear Res. 1984;16:55–74. doi: 10.1016/0378-5955(84)90025-x. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW, Pierce S. Afferent and efferent innervation of the cat cochlea: quantitative analysis with light and electron microscopy. J Comp Neurol. 1990;301:443–60. doi: 10.1002/cne.903010309. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Chesney CP, Kujawa SG. Effects of selective inner hair cell loss on DPOAE and CAP in carboplatin-treated chinchillas. Auditory Neuroscience. 1997;3:255–268. [Google Scholar]
- Liberman MC, Liberman LD, Maison SF. Efferent feedback slows cochlear aging. J Neurosci. 2014;34:4599–607. doi: 10.1523/JNEUROSCI.4923-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC. Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol. 2011;12:605–16. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Salvi R, Ding D. Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hearing research. 2013 doi: 10.1016/j.heares.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Usubuchi H, Liberman MC. Efferent feedback minimizes cochlear neuropathy from moderate noise exposure. J Neurosci. 2013;33:5542–52. doi: 10.1523/JNEUROSCI.5027-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makary CA, Shin J, Kujawa SG, Liberman MC, Merchant SN. Age-related primary cochlear neuronal degeneration in human temporal bones. J Assoc Res Otolaryngol. 2011;12:711–7. doi: 10.1007/s10162-011-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara A, Laake JH, Davanger S, Usami S, Ottersen OP. Organization of AMPA receptor subunits at a glutamate synapse: a quantitative immunogold analysis of hair cell synapses in the rat organ of Corti. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:4457–67. doi: 10.1523/JNEUROSCI.16-14-04457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean WJ, Smith KA, Glowatzki E, Pyott SJ. Distribution of the Na, K-ATPase alpha subunit in the rat spiral ganglion and organ of corti. J Assoc Res Otolaryngol. 2009;10:37–49. doi: 10.1007/s10162-008-0152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchan-Perez A, Liberman MC. Ultrastructural differences among afferent synapses on cochlear hair cells: correlations with spontaneous discharge rate. J Comp Neurol. 1996;371:208–21. doi: 10.1002/(SICI)1096-9861(19960722)371:2<208::AID-CNE2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Miller JM, Chi DH, O’Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15:631–43. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- Mills DM. Differential responses to acoustic damage and furosemide in auditory brainstem and otoacoustic emission measures. J Acoust Soc Am. 2003;113:914–24. doi: 10.1121/1.1535942. [DOI] [PubMed] [Google Scholar]
- Niskar AS, Kieszak SM, Holmes AE, Esteban E, Rubin C, Brody DJ. Estimated prevalence of noise-induced hearing threshold shifts among children 6 to 19 years of age: the Third National Health and Nutrition Examination Survey, 1988–1994, United States. Pediatrics. 2001;108:40–3. doi: 10.1542/peds.108.1.40. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Wright JS, Heidbreder AF. Vulnerability to noise-induced hearing loss in ‘middle-aged’ and young adult mice: a dose-response approach in CBA, C57BL, and BALB inbred strains. Hear Res. 2000;149:239–47. doi: 10.1016/s0378-5955(00)00191-x. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Takumi Y, Matsubara A, Landsend AS, Laake JH, Usami S. Molecular organization of a type of peripheral glutamate synapse: the afferent synapses of hair cells in the inner ear. Prog Neurobiol. 1998;54:127–48. doi: 10.1016/s0301-0082(97)00054-3. [DOI] [PubMed] [Google Scholar]
- Plack CJ, Barker D, Prendergast G. Perceptual consequences of “hidden” hearing loss. Trends in hearing 18. 2014 doi: 10.1177/2331216514550621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prijs VF. Single-unit response at the round window of the guinea pig. Hear Res. 1986;21:127–133. doi: 10.1016/0378-5955(86)90034-1. [DOI] [PubMed] [Google Scholar]
- Puel JL, Ruel J, Gervais d’Aldin C, Pujol R. Excitotoxicity and repair of cochlear synapses after noise-trauma induced hearing loss. Neuroreport. 1998;9:2109–14. doi: 10.1097/00001756-199806220-00037. [DOI] [PubMed] [Google Scholar]
- Pujol R, Puel JL. Excitotoxicity, synaptic repair, and functional recovery in the mammalian cochlea: a review of recent findings. Ann N Y Acad Sci. 1999;884:249–54. doi: 10.1111/j.1749-6632.1999.tb08646.x. [DOI] [PubMed] [Google Scholar]
- Pujol R, Lenoir M, Robertson D, Eybalin M, Johnstone BM. Kainic acid selectively alters auditory dendrites connected with cochlear inner hair cells. Hear Res. 1985;18:145–51. doi: 10.1016/0378-5955(85)90006-1. [DOI] [PubMed] [Google Scholar]
- Rabinowitz PM, Slade MD, Galusha D, Dixon-Ernst C, Cullen MR. Trends in the prevalence of hearing loss among young adults entering an industrial workforce 1985 to 2004. Ear and hearing. 2006;27:369–75. doi: 10.1097/01.aud.0000224125.12338.9a. [DOI] [PubMed] [Google Scholar]
- Rabinowitz PM, Slade MD, Galusha D, Dixon-Ernst C, Cullen R. The public health significance of noise induced hearing loss. In: Le Prell CD, Henderson D, Fay RR, Popper AN, editors. Noise-Induced Hearing Loss: Scientific Advances: Springer Handbook of Auditory Research. Springer Science and Business Media, LLC; 2012. pp. 13–25. [Google Scholar]
- Rajan R. Protective functions of the efferent pathways to the mammalian cochlea: A review. Mosby Year Book; St. Louis: 1991. [Google Scholar]
- Rhode WS, Smith PH. Characteristics of tone-pip response patterns in relationship to spontaneous rate in cat auditory nerve fibers. Hear Res. 1985;18:159–68. doi: 10.1016/0378-5955(85)90008-5. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: the neuroscience of tinnitus. J Neurosci. 2010;30:14972–9. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. Functional significance of dendritic swelling after loud sounds in the guinea pig cochlea. Hearing Res. 1983;9:263–278. doi: 10.1016/0378-5955(83)90031-x. [DOI] [PubMed] [Google Scholar]
- Rosen S, Bergman M, Plester D, El-Mofty A, Satti MH. Presbycusis study of a relatively noise-free population in the Sudan. Ann Otol Rhinol Laryngol. 1962;71:727–43. doi: 10.1177/000348946207100313. [DOI] [PubMed] [Google Scholar]
- Ruel J, Nouvian R, Gervais d’Aldin C, Pujol R, Eybalin M, Puel JL. Dopamine inhibition of auditory nerve activity in the adult mammalian cochlea. Eur J Neurosci. 2001;14:977–86. doi: 10.1046/j.0953-816x.2001.01721.x. [DOI] [PubMed] [Google Scholar]
- Ruel J, Wang J, Rebillard G, Eybalin M, Lloyd R, Pujol R, Puel JL. Physiology, pharmacology and plasticity at the inner hair cell synaptic complex. Hear Res. 2007;227:19–27. doi: 10.1016/j.heares.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Ruel J, Emery S, Nouvian R, Bersot T, Amilhon B, Van Rybroek JM, Rebillard G, Lenoir M, Eybalin M, Delprat B, Sivakumaran TA, Giros B, El Mestikawy S, Moser T, Smith RJ, Lesperance MM, Puel JL. Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice. American journal of human genetics. 2008;83:278–92. doi: 10.1016/j.ajhg.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli R, Del Castillo I, Rodriguez-Ballesteros M, Scimemi P, Cama E, Arslan E, Starr A. Abnormal cochlear potentials from deaf patients with mutations in the otoferlin gene. Journal of the Association for Research in Otolaryngology : JARO. 2009;10:545–56. doi: 10.1007/s10162-009-0181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht J, Talaska AE, Rybak LP. Cisplatin and aminoglycoside antibiotics: hearing loss and its prevention. Anatomical record. 2012;295:1837–50. doi: 10.1002/ar.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:13452–7. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalk TB, Sachs MB. Nonlinearities in auditory-nerve fiber responses to bandlimited noise. J Acoust Soc Am. 1980;67:903–13. doi: 10.1121/1.383970. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA, Mills JH, Boettcher FA. Age-related loss of activity of auditory-nerve fibers. J Neurophysiol. 1996;76:2799–803. doi: 10.1152/jn.1996.76.4.2799. [DOI] [PubMed] [Google Scholar]
- Schmitz F, Konigstorfer A, Sudhof TC. RIBEYE, a component of synaptic ribbons: a protein’s journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28:857–72. doi: 10.1016/s0896-6273(00)00159-8. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Woellner RC. An experimental and clinical study of deafness from lesions of the cochlear nerve. The Journal of laryngology and otology. 1955;69:75–97. doi: 10.1017/s0022215100050465. [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:13686–94. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra MR, Biassoni EC, Hinalaf M, Abraham M, Pavlik M, Villalobo JP, Curet C, Joekes S, Yacci MR, Righetti A. Hearing and loud music exposure in 14–15 years old adolescents. Noise & health. 2014;16:320–30. doi: 10.4103/1463-1741.140512. [DOI] [PubMed] [Google Scholar]
- Shi L, Liu L, He T, Guo X, Yu Z, Yin S, Wang J. Ribbon synapse plasticity in the cochleae of Guinea pigs after noise-induced silent damage. PloS one. 2013;8:e81566. doi: 10.1371/journal.pone.0081566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RL. Adaptation, saturation, and physiological masking in single auditory-nerve fibers. J Acoust Soc Am. 1979;65:166–78. doi: 10.1121/1.382260. [DOI] [PubMed] [Google Scholar]
- Snell KB, Frisina DR. Relationships among age-related differences in gap detection and word recognition. J Acoust Soc Am. 2000;107:1615–26. doi: 10.1121/1.428446. [DOI] [PubMed] [Google Scholar]
- Spassova MA, Avissar M, Furman AC, Crumling MA, Saunders JC, Parsons TD. Evidence that rapid vesicle replenishment of the synaptic ribbon mediates recovery from short-term adaptation at the hair cell afferent synapse. J Assoc Res Otolaryngol. 2004;5:376–90. doi: 10.1007/s10162-004-5003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoendlin H. Primary structural changes in the organ of Corti after acoustic overstimulation. Acta Otolaryng. 1971;71:166–176. doi: 10.3109/00016487109125346. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Retrograde degeneration of the cochlear nerve. Acta Otolaryngologica. 1975;79:266–275. doi: 10.3109/00016487509124683. [DOI] [PubMed] [Google Scholar]
- Spoendlin HH. Innervation densities of the cochlea. Acta Otolaryng. 1972;73:235–248. doi: 10.3109/00016487209138937. [DOI] [PubMed] [Google Scholar]
- Stamataki S, Francis HW, Lehar M, May BJ, Ryugo DK. Synaptic alterations at inner hair cells precede spiral ganglion cell loss in aging C57BL/6J mice. Hear Res. 2006;221:104–18. doi: 10.1016/j.heares.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Stamper GC, Johnson TA. Auditory Function in Normal-Hearing, Noise-Exposed Human Ears. Ear and hearing. 2014 doi: 10.1097/AUD.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankovic K, Rio C, Xia A, Sugawara M, Adams JC, Liberman MC, Corfas G. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci. 2004;24:8651–61. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr A, Sininger YS, Pratt H. The varieties of auditory neuropathy. J Basic Clin Physiol Pharmacol. 2000;11:215–30. doi: 10.1515/jbcpp.2000.11.3.215. [DOI] [PubMed] [Google Scholar]
- Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain. 1996;119 (Pt 3):741–53. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Corfas G, Liberman MC. Influence of supporting cells on neuronal degeneration after hair cell loss. J Assoc Res Otolaryngol. 2005;6:136–47. doi: 10.1007/s10162-004-5050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuka Y, Schuknecht HF. Retrograde cochlear neuronal degeneration in human subjects. Acta oto-laryngologica. 1988;450:1–20. doi: 10.3109/00016488809098973. [DOI] [PubMed] [Google Scholar]
- Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47:122–9. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Tsuji J, Liberman MC. Intracellular labeling of auditory nerve fibers in guinea pig: Central and peripheral projections. J Comp Neurol. 1997;381:188–202. [PubMed] [Google Scholar]
- Wan G, Gomez-Casati ME, Gigliello AR, Liberman MC, Corfas G. Neurotrophin-3 regulates ribbon synapse density in the cochlea and induces synapse regeneration after acoustic trauma. eLife. 2014:3. doi: 10.7554/eLife.03564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ding D, Salvi RJ. Carboplatin-induced early cochlear lesion in chinchillas. Hear Res. 2003;181:65–72. doi: 10.1016/s0378-5955(03)00176-x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;3:248–68. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward WD, Santi PA, Duvall AJ, 3rd, Turner CW. Total energy and critical intensity concepts in noise damage. Ann Otol Rhinol Laryngol. 1981;90:584–90. doi: 10.1177/000348948109000615. [DOI] [PubMed] [Google Scholar]
- Wise AK, Richardson R, Hardman J, Clark G, O’Leary S. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol. 2005;487:147–65. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- Yin Y, Liberman LD, Maison SF, Liberman MC. Olivocochlear innervation maintains the normal modiolar-pillar and habenular-cuticular gradients in cochlear synaptic morphology. J Assoc Res Otolaryngol. 2014;15:571–83. doi: 10.1007/s10162-014-0462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Virkkala J, Suvanto P, Liang XQ, Magal E, Altschuler R, Miller JM, Saarma M. Guinea pig auditory neurons are protected by glial cell line-derived growth factor from degeneration after noise trauma. Hear Res. 1998;124:17–26. doi: 10.1016/s0378-5955(98)00095-1. [DOI] [PubMed] [Google Scholar]
- Zilberstein Y, Liberman MC, Corfas G. Inner hair cells are not required for survival of spiral ganglion neurons in the adult cochlea. J Neurosci. 2012;32:405–10. doi: 10.1523/JNEUROSCI.4678-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]