Abstract
The relationship between obesity, weight gain and progression of knee osteoarthritis is well supported, suggesting that excessive joint loading may be a mechanism responsible for cartilage deterioration. Examining the influence of weight gain on joint compressive forces is difficult, as both muscles and ground reaction forces can have a significant impact on the forces experienced during gait. While previous studies have examined the relationship between body weight and knee forces, these studies have used models that were not validated using experimental data. Therefore, the objective of this study was to evaluate the relationship between changes in body weight and changes in knee joint contact forces for an individual’s gait pattern using musculoskeletal modeling that is validated against known internal compressive forces. Optimal weighting constants were determined for 3 subjects to generate valid predictions of knee contact forces using in vivo data collection with instrumented total knee arthroplasty. A total of five simulations per walking trial were generated for each subject, from 80–120% body weight in 10% increments, resulting in fifty total simulations. The change in peak knee contact force with respect to body weight was found to be constant and subject-specific, predominantly determined by the peak force during the baseline condition at 100% body weight. This relationship may be further altered by any change in kinematics or body mass distribution that may occur as a result of a change in body weight or exercise program.
Keywords: Osteoarthritis, musculoskeletal simulations, knee contact force, body weight
Introduction
The causal and associative relationship between obesity, weight gain and progression of knee osteoarthritis (OA) is well supported by several large epidemiological studies (Hart & Spector 1993; Hochberg et al. 1995; Felson et al. 1997; Reijman et al. 2007; Niu et al. 2009; Apold et al. 2014). It is likely that mechanical changes, including increased compression force associated with obesity, are the primary factors driving cartilage deterioration. In vivo (Ko et al. 2013) and experimental tissue-level studies (Piscoya et al. 2005) have found that increased compressive forces may initiate or expedite the rate of cartilage deterioration.
Examining the influence of weight gain on joint compressive forces is not a trivial task. In addition to the joint compressive forces arising from external reaction forces, muscle contractions generate a large portion of the forces experienced by a joint. Evaluations that examine the influence of changes in body weight and joint loads must also assess the muscle contribution to the internal forces experienced by the joint. Previous work has identified a linear relationship between change in body weight and change in compressive knee joint force. For every pound increase in body weight there is a 4-fold increase in knee joint compressive forces (Messier et al. 2005). This ratio of weight gain to joint compressive force is especially important as one considers that this additional force is applied with every step. These large changes may have a substantial impact on the integrity of the joint.
Although preliminary work has evaluated change in body weight and change in joint compressive force (Messier et al. 2005), the models and simulations behind the calculations have not been compared to in vivo joint compressive forces because experimental data measuring knee joint loading is limited. However, recently available data from instrumented total knee replacements offers a unique opportunity to validate model predictions using directly measured compressive knee force. Therefore, the objective of this project was to evaluate the relationship between changes in body mass and changes in knee joint contact forces for an individual’s gait pattern using musculoskeletal modeling that is validated against known internal compressive forces. We hypothesize that experimentally induced changes in body mass will have a multiplicative relationship with the compressive forces experienced in the knee.
Methods
Three dimensional simulations were created from the stance phase of gait for 3 subjects with instrumented total knee arthroplasty (TKA)using OpenSim 3.0.1 (Delp et al. 2007). Subject data were obtained as part of the first three years of the Grand Challenge Competition to predict in vivo knee loads (Fregly et al. 2012) (Table 1). Load cell measurements were telemetered using a micro-transmitter and antenna from either four uniaxial force transducers, one each in the four quadrants of the tibial tray, or a six-axis load cell in the stem of the tibial tray, depending on the implant design. The musculoskeletal model included 92 actuators with 23 degrees of freedom and patellae which articulate with the femur. The patellae serve to direct the quadriceps forces along the patellar ligament (Demers et al. 2014).Two to four walking trials were used for each subject, resulting in ten total walking trials. Kinematics and kinetic data were filtered at 6 Hz and inverse kinematics was used to determine the model joint kinematics that best match the experimental data. Simulations were created from heel strike to toe off of the instrumented limb and all data was reported for the instrumented limb. A weighted static optimization technique similar to Steele et al., 2012 (Steele et al. 2012) was used to find the optimal match between experimental (as measured by the instrumented TKA) and simulated knee contact force (KCF). Using this technique, weighting constants for the quadriceps, hamstrings, and plantar flexor muscle groups were included to alter the distribution of muscle activations during the simulation. For each subject, a pattern search optimization was performed to find to an optimal set of weighting constants which minimize the difference between the experimental and simulated knee contact force across multiple subject trials. A cost function of the sum of the root mean squared error across the stance phase of gait and the errors in the first and second peaks of KCF was used for the optimization.
Table 1.
Subject demographics, subject-specific weighting factors for plantar flexors (PF), hamstrings (H), and quadriceps (Q) and KCF force results.
| Subject 1 | Subject 2 | Subject 3 | ||
|---|---|---|---|---|
| Gender | M | M | F | |
| Height (m) | 1.66 | 1.72 | 1.67 | |
| Weight (kg) | 64.6 | 67 | 78.4 | |
| Knee | R | R | L | |
| Subject-Specific Weights | PF | 2 | 2 | 1 |
| H | 6 | 10 | 1 | |
| Q | 3 | 6 | 3 | |
| Peak Error (BW) | 0.11 | 0.22 | 0.24 | |
| Peak KCF Range (BW) (100% BW Trial) | 2.57–2.75 | 2.24–2.28 | 2.55–2.77 | |
| ΔKCF/ΔBW | 2.67 | 2.26 | 2.66 | |
| Mean GRF contribution to ΔKCF (BW) | 1.03 | 1.1 | 0.93 | |
| % BW Contribution to ΔKCF (BW) | 0.38 | 0.48 | 0.35 |
Once the optimal weighting constants were determined for each subject, a total of five simulations per walking trial were generated for each subject, representing 80–120% body weight (BW) in 10% increments, resulting in fifty total simulations. For simulations with modified body weight, a corresponding change in ground reaction force was applied to maintain dynamic consistency in the model, as ground reaction force is proportional to body weight (Perry & Burnfield 2010). KCF was calculated with the joint reaction force tool in OpenSim, using the ground reaction forces and muscle forces predicted by static optimization with the optimal weighting constants determined through matching instrumented knee data, and expressed along the long axis of the tibia in the tibial reference frame. Finally, we investigated the influence of changes in ground reaction force alone on changes in predicted KCF to determine the relative contribution of muscle forces and external forces on changes in KCF.
Results
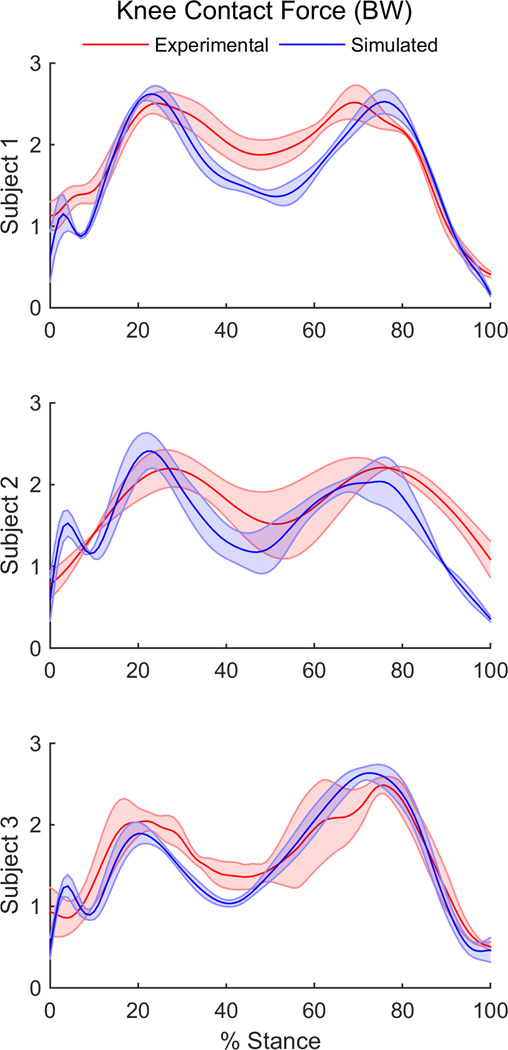
Subject-specific muscle weighting differed for each of the three subjects (Table 1). On average, the first and second peaks of joint contact force predicted by the simulations matched within 0.2 BW of the experimental values when optimal subject-specific weighting constants were used (Figure 1).
Figure 1.
Experimental and simulated knee joint contact force over the stance phase of gait for three subjects included in this study.
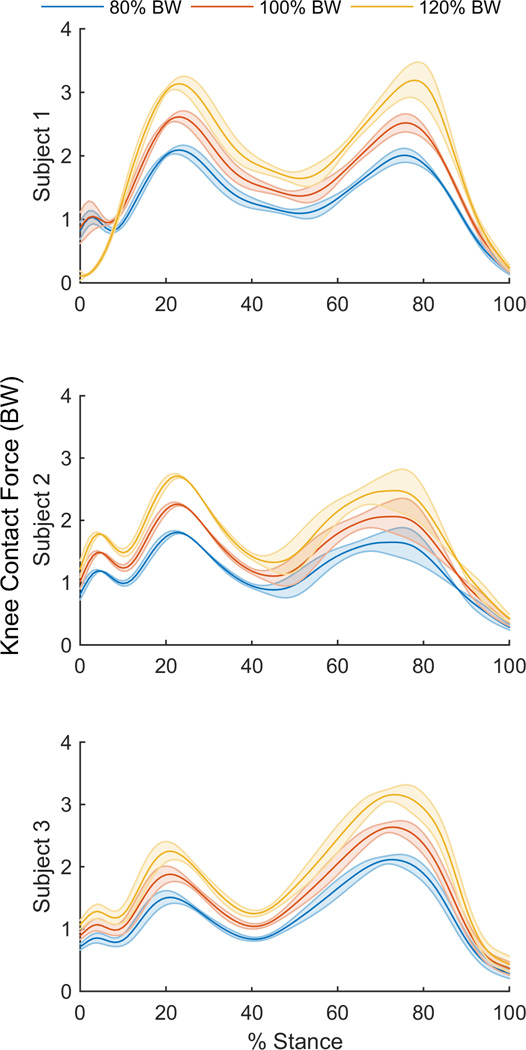
Peak KCF ranged from 2.24 – 2.77 BWs across the subject trials at 100% body weight. Simulations showed a constant relationship between peak KCF and body mass for each of the three subjects. On average, KCF increased by a factor of2.26–2.67 per each unit (e.g. N or kg) increase in BW for each subject (Table 2). A linear relationship was also seen between the contribution of peak GRF to peak KCF and body weight for each of the three subjects, with a 0.93–1.1 increase per unit increase in BW. Across all trials, contributions from ground reaction force amounted to only 35–48% of the joint compressive force experienced.
Discussion
For each of the three subjects, the change in peak KCF with respect to body weight is constant and subject-specific. In fact, the rate of peak KCF change is predominantly determined by the peak KCF for the baseline simulation with 100% body weight. For example, if a subject’s peak KCF is 2.5 BWs, then the results of this study suggest that there will be a 2.5 fold increase in KCF for every increase in unit BW when walking with the same gait pattern.
The contribution of GRF to the total KCF was also constant and subject-specific, changing less than one percent across the range of body weights. Overall, our simulations suggest that less than half of the change in KCF due to change in body weight can be attributed to the associated ground reaction forces. This suggests that the change in muscle forces required to accelerate segments with increased or decreased mass is a predominant contributor to the change in KCF due to changes in body mass.
The ratio of change in compression forces for each unit of weight gained is slightly lower than previously reported values. Messier et al. found a 4-fold decrease in knee joint compressive force for every pound of weight lost (Messier et al. 2005). It is important to note that these authors estimated changes in compression forces based on subjects who actually lost weight as part of a diet and exercise program; subjects underwent motion analysis testing at baseline and follow-up. The results from our study are based on computationally altered weight and are independent of any change in kinematics that may occur as a result of a change in body weight or exercise program. It is not unreasonable to assume that individuals may adopt a new overall gait pattern after gaining or losing weight. This new gait pattern (and corresponding muscle coordination strategy) would likely cause additional changes in the peak KCF experienced, however exactly what kinematic and kinetic changes may occur as a result of weight gain or loss is not well studied. Furthermore, the specific importance of segment mass on resulting KCF suggests that the distribution of weight loss or gain may be relevant to changes in KCF. It was assumed in this study that distribution of weight loss/gain was proportional across all segments, however it is commonly seen that changes in weight can be focused to specific segments in some individuals (Walker et al. 1999; Singh et al. 2012), and further investigation is warranted on the effect of segment mass distribution on resultant KCF.
The study demonstrates the multiplicative relationship between body weight and compressive forces experienced at the knee during gait using an experimentally validated simulation technique. This relationship was found to be subject-specific in nature and is likely influenced by additional factors, such as body mass distribution and kinematic changes, which should be examined in association with KCF in the future.
Figure 2.
Average KCF for across walking trials for the stance phase of gait for 80%, 100%, and 120% body weight simulations.
Acknowledgements
This work was supported by the NIH under Grants P30GM103333 and K12 HD055931 Comprehensive Opportunities in Rehabilitation Research Training.
REFERENCES
- Apold H, Meyer HE, Nordsletten L, Furnes O, Baste V, Flugsrud GB. Weight Gain and the Risk of Knee Replacement Due to Primary Osteoarthritis A Population Based, Prospective Cohort Study of 225,908 Individuals. Osteoarthritis Cartilage. 2014 doi: 10.1016/j.joca.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Delp SL, Anderson FC, Arnold AS, Loan P, Habib A, John CT, Guendelman E, Thelen DG. OpenSim: open-source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng. 2007;54:1940–1950. doi: 10.1109/TBME.2007.901024. [DOI] [PubMed] [Google Scholar]
- Demers MS, Pal S, Delp SL. Changes in tibiofemoral forces due to variations in muscle activity during walking. J Orthop Res. 2014;32:769–776. doi: 10.1002/jor.22601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman B, Aliabadi P, Levy D. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40:728–733. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- Fregly BJ, Besier TF, Lloyd DG, Delp SL, Banks SA, Pandy MG, D’Lima DD. Grand challenge competition to predict in vivo knee loads. J Orthop Res. 2012;30:503–513. doi: 10.1002/jor.22023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart DJ, Spector TD. The relationship of obesity, fat distribution and osteoarthritis in women in the general population: the Chingford Study. J Rheumatol. 1993;20:331–335. [PubMed] [Google Scholar]
- Hochberg MC, Lethbridge-Cejku M, Scott WW, Reichle R, Plato CC, Tobin JD. The association of body weight, body fatness and body fat distribution with osteoarthritis of the knee: data from the Baltimore Longitudinal Study of Aging. J Rheumatol. 1995;22:488–493. [PubMed] [Google Scholar]
- Ko FC, Dragomir C, Plumb DA, Goldring SR, Wright TM, Goldring MB, van der Meulen MCH. In vivo cyclic compression causes cartilage degeneration and subchondral bone changes in mouse tibiae. Arthritis Rheum. 2013;65:1569–1578. doi: 10.1002/art.37906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier SP, Gutekunst DJ, Davis C, DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52:2026–2032. doi: 10.1002/art.21139. [DOI] [PubMed] [Google Scholar]
- Niu J, Zhang YQ, Torner J, Nevitt M, Lewis CE, Aliabadi P, Sack B, Clancy M, Sharma L, Felson DT. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61:329–335. doi: 10.1002/art.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J, Burnfield JM. Gait Analysis: Normal and Pathological Function. 2nd Editio. Thorofare, NJ: SLACK Incorporated; 2010. [Google Scholar]
- Piscoya JL, Fermor B, Kraus VB, Stabler TV, Guilak F. The influence of mechanical compression on the induction of osteoarthritis-related biomarkers in articular cartilage explants. Osteoarthr Cartil. 2005;13:1092–1099. doi: 10.1016/j.joca.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Reijman M, Pols HAP, Bergink AP, Hazes JMW, Belo JN, Lievense AM, Bierma-Zeinstra SMA. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam Study. Ann Rheum Dis. 2007;66:158–162. doi: 10.1136/ard.2006.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Somers VK, Romero-Corral A, Sert-Kuniyoshi FH, Pusalavidyasagar S, Davison DE, Jensen MD. Effects of weight gain and weight loss on regional fat distribution. Am J Clin Nutr. 2012;96:229–233. doi: 10.3945/ajcn.111.033829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele KM, DeMers MS, Schwartz MH, Delp SL. Compressive tibiofemoral force during crouch gait. Gait Posture. 2012;35:556–560. doi: 10.1016/j.gaitpost.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KZ, O’Dea K, Nicholson GC. Dietary composition affects regional body fat distribution and levels of dehydroepiandrosterone sulphate (DHEAS) in post-menopausal women with Type 2 diabetes. Eur J Clin Nutr. 1999;53:700–705. doi: 10.1038/sj.ejcn.1600835. [DOI] [PubMed] [Google Scholar]