Abstract
β-Sitosterol is the most abundant plant sterol in the human diet. It is also the major component of several traditional medicines, including saw palmetto and devil’s claw. Although β-sitosterol is effective against enlarged prostate in human clinical trials and has anti-cancer and anti-inflammatory activities, the mechanisms of action are poorly understood. Here, we report the identification of two new binding proteins for β-sitosterol that may underlie its beneficial effects.
Keywords: β-Sitosterol, 17β-HSD4, E-Syt1, Target identification, Phytosterol
Phytosterols are a group of steroids produced by plants. They are structurally and functionally related to cholesterol and comprise a major component of the human diet.1,2 Among them, β-sitosterol (24-ethylcholesterol) is one of the most abundant dietary phytosterols present in many beans, nuts, and seeds (Figure 1).3–5 It is also an important constituent of saw palmetto, devil’s claw, stinging nettle and several other natural remedies.6–8 β-Sitosterol consumption has been reported to decrease blood cholesterol levels by preventing its intestinal absorption.9,10 It also has been shown to have anti-inflammatory and analgesic properties in various animal models.11–14 Additionally, in both animal models and human clinical trials, β-sitosterol has demonstrated a significant effect on reducing the symptoms of benign prostatic hyperplasia.15,16 β-Sitosterol intake may also be partially responsible for the decreased incidence of prostate, colon and breast cancers among vegetarians and men and women in Asian countries who consume much larger amounts of β-sitosterol than most Westerners.5,17 In support of this hypothesis, β-sitosterol exhibits growth inhibitory and cytotoxic effects against a range of cancer cell lines.7,18–20 However, the precise molecular mechanisms underlying its health promoting effects remain largely uncharacterized. To understand the molecular mechanism(s) by which β-sitosterol exerts its many beneficial health effects, we performed affinity chromatography using biotinylated β-sitosterol to identify its protein targets.
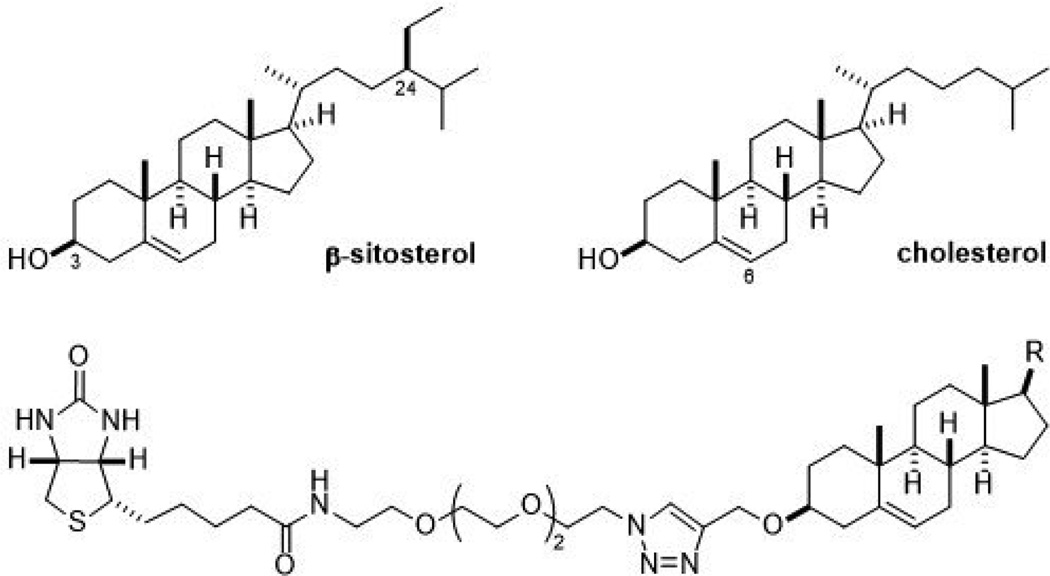
Figure 1.
The structures of β-sitosterol, cholesterol, and the affinity reagents used herein.
We reasoned that the health promoting effects of β-sitosterol not observed with cholesterol originate from the existence of unshared protein targets. β-Sitosterol differs from cholesterol solely by the presence of an ethyl group at the C-24 position, which we hypothesized would be an important moiety for the binding of β-sitosterol specific proteins. Therefore, we prepared affinity reagents for both compounds by attaching a biotin group to each through a polyethylene glycol linker (Figure 1). The C-3 position was chosen as the attaching point because it is furthest away from the C-24 position and thus least likely to interfere with proteins that selectively bind β-sitosterol.
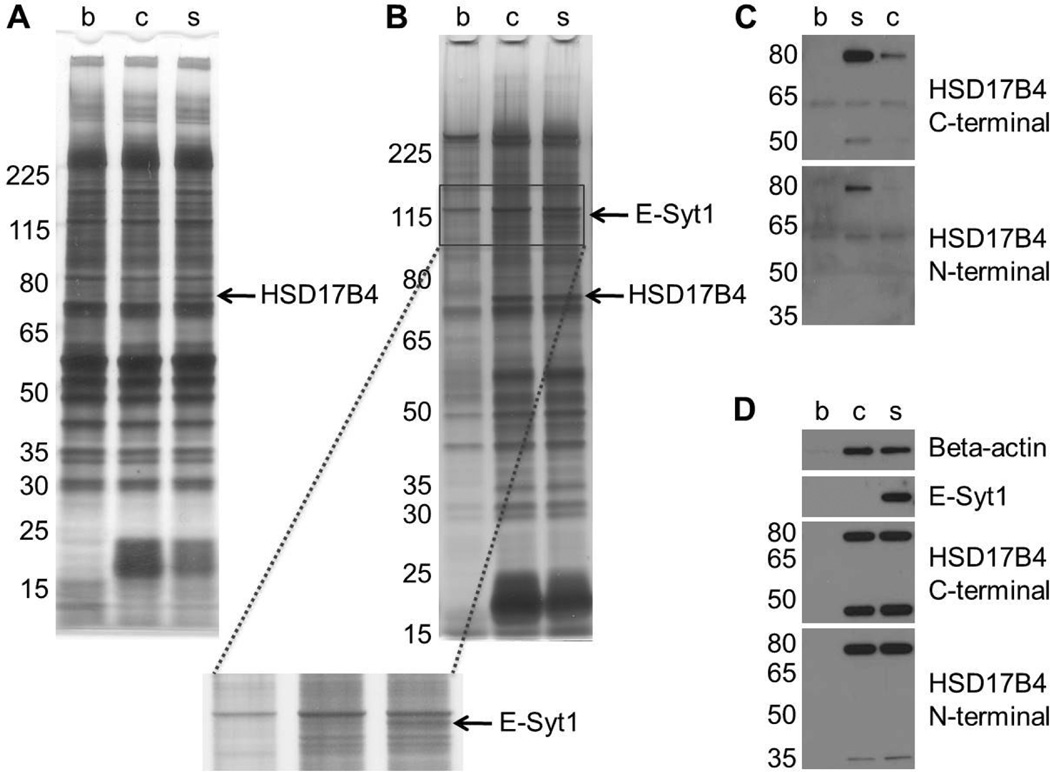
We performed affinity chromatography initially with lipopolysaccharide (LPS)-treated Raw264.7 macrophage cell lysates because many of the anti-inflammatory properties of β-sitosterol may arise from its effects on macrophages.21,22 The lysates were first incubated with the biotinylated compounds or biotin alone as a control at various concentrations for 2 h, followed by overnight incubation of all with streptavidin agarose resin. SDS-PAGE and silver staining analysis revealed two bands that bound specifically to β-sitosterol, a 75-kDa band at 200 nM (Figure 2A) and a 120-kDa band at 600 nM (Figure 2B). MALDI mass spectrometry analysis of the bands identified them as 17-beta-hydroxysteroid dehydrogenase 4 (17β-HSD4) and extended synaptotagmin 1 (E-Syt1).
Figure 2.
β-sitosterol binds to 17β-HSD4 and E-Syt1 in lysates from LPS-treated Raw264.7 mouse macrophages. Affinity chromatography was performed using several concentrations of biotin (b), biotinylated cholesterol (c), and biotinylated β-sitosterol (s). Silver-staining and mass spectrometry analysis discovered two β-sitosterol specific binders: (A) 17β-HSD4 at 200 nM and (B) E-Syt1 at 600 nM. Immunoblotting analysis of the affinity chromatography samples validated the binding of (C) 17β-HSD4 at 200 nM and (D) E-Syt1 at 600 nM.
To validate these proteins we performed immunoblotting using separate aliquots of the affinity chromatography samples. As shown in Figure 2C, western blotting with two different 17β-HSD4 antibodies confirms that it is bound much more strongly by β-sitosterol than cholesterol. While the full length 17β-HSD4 polypeptide is 79 kDa, a portion of the cellular pool of 17β-HSD4 is proteolytically cleaved into two polypeptides, a 34-kDa N-terminal fragment and a 45-kDa C-terminal fragment.23 These two polypeptides are stable within the cell and are thought to retain their enzymatic functions, either alone or as homodimers or heterodimers.24–26 Although both fragments are present in the macrophage lysates, only the C-terminal fragment was bound by β-sitosterol (Figure 2C). Interestingly, the C-terminal fragment contains a sterol carrier protein type 2 (SCP-2) domain, which may be the site of β-sitosterol and cholesterol binding.27 Immunoblotting of the 600 nM affinity chromatography samples likewise validated that E-Syt1 bound specifically to β-sitosterol (Figure 2D). At this concentration, 17β-HSD4 appears to be bound equally well by β-sitosterol and cholesterol, suggesting that the ethyl group at C-24 in β-sitosterol increases its affinity to 17β-HSD4 but is not necessary for binding.
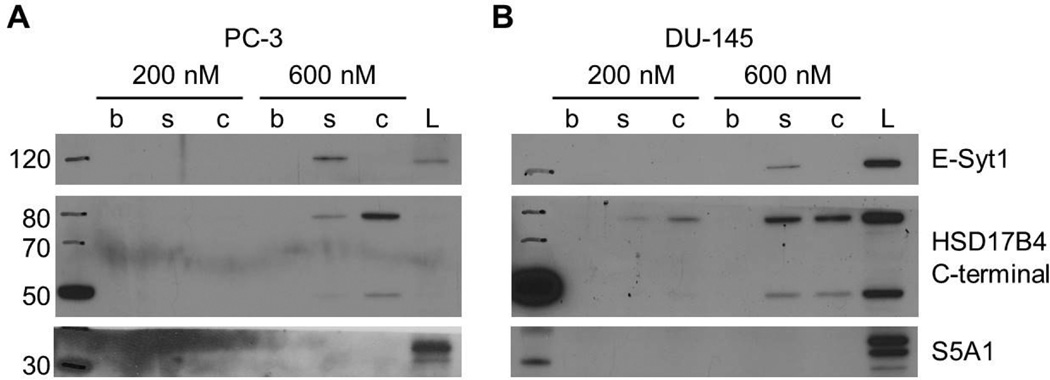
Next, we extended our affinity chromatography studies to two prostate cancer cell lines, PC-3 and DU-145, because β-sitosterol has been reported to inhibit the growth, migration, and invasion of prostate cancer cells and is used to treat enlarged prostate.7,15,28 As shown in Figure 3, E-Syt1 bound specifically to biotinylated β-sitosterol at 600 nM, just as in macrophage lysates. In contrast, 17β-HSD4 bound more strongly to biotinylated cholesterol than biotinylated β-sitosterol in both prostate cancer cell lines (Figure 3), which is opposite to what we observed in macrophage lysates. β-Sitosterol has been reported to inhibit 5α-reductase at micromolar concentrations, and this inhibition has been hypothesized to be responsible for its activities in prostate cancer and enlarged prostate.29 However, we did not detect binding of 5α-reductase (S5A1) to biotinylated β-sitosterol or biotinylated cholesterol at our nanomolar test concentrations (Figure 3). While this could indicate that the affinity of β-sitosterol for 5α-reductase is lower than for both 17β-HSD4 and E-Syt1, we cannot rule out the possibility that the biotin tag interferes with binding to 5α-reductase.
Figure 3.
The binding of β-sitosterol and cholesterol to 17β-HSD4 and E-Syt1 in prostate cancer cell lysates. Affinity chromatography was performed using 200 nM and 600 nM concentrations of biotin (b), biotinylated β-sitosterol (s), and biotinylated cholesterol (c) in (A) PC-3 and (B) DU-145 prostate cancer cell lysates (L). Immunoblotting determined that E-Syt1 binds specifically to β-sitosterol, whereas 17β-HSD4 binds more strongly to cholesterol. S5A1 does not bind either compound at the tested concentrations.
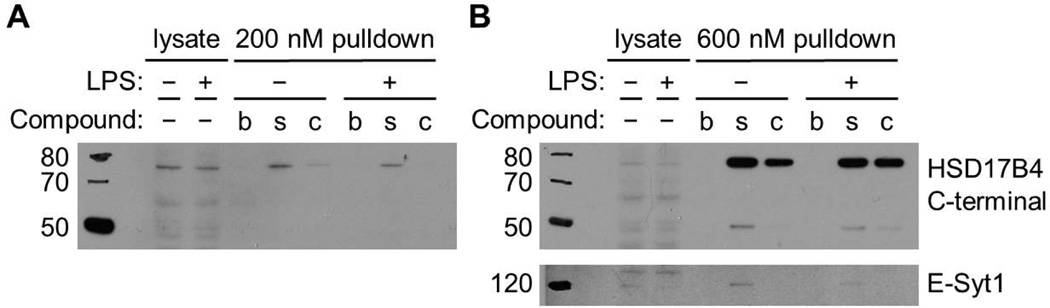
Given the surprising finding that 17β-HSD4 binds to β-sitosterol more strongly in LPS-treated macrophage lysates but to cholesterol more strongly in prostate cancer cell lysates, we tested whether LPS stimulation was responsible for this effect. We observed that 17β-HSD4 preferentially bound to β-sitosterol over cholesterol in macrophage lysates both with and without LPS treatment (Figure 4). These results suggest that 17β-HSD4 has different affinities to β-sitosterol and cholesterol in different cell lines or cell types, which could be due to differential splicing, post-translation modification, protein complex composition, or other factors in each cell line. Alternatively, there could be a species-specific difference between the mouse and human 17β-HSD4 homologs, which are 86% identical.
Figure 4.
Lipopolysaccharide treatment does not affect the binding of 17β-HSD4 or E-Syt1 to β-sitosterol. Affinity chromatography was performed using (A) 200 nM and (B) 600 nM concentrations of biotin (b), biotinylated β-sitosterol (s), and biotinylated cholesterol (c) in lysates from LPS-treated and non-treated Raw264.7 mouse macrophages. Immunoblotting analysis revealed that the binding of 17β-HSD4 and E-Syt1 to cholesterol and β-sitosterol is unchanged by LPS treatment.
E-Syt1, along with its homolog E-Syt2, were recently found to bind cholesterol in a chemoproteomic screen using clickable, photoreactive sterol probes and quantitative mass spectrometry.30 Although we did not detect binding of E-Syt1 to biotinylated cholesterol, this could be due to the low concentration (200 and 600 nM) of the probe used in our experiment (compared to 10 µM in the chemoproteomic screen). Moreover, in this chemoproteomic study, the photoreactive sterol probes were added to live cells, which were subsequently treated with UV light to covalently crosslink the probes to target proteins. If the interaction between cholesterol and E-Syt1 is weak or transient it may not be maintained during the affinity chromatography wash steps, but it would not be lost by washing the photoreactive probe since it is covalently bound.
17β-HSD4, on the other hand, was not detected as a cholesterol binder in the chemoproteomic study despite the much higher concentration. Although this could be due to the use of a different cell line, it is more likely that the nature of the probes is responsible. Whereas our sterols were modified at the C-3 position for biotin labeling, the photoreactive sterol probes were modified on their alkyl side chains to incorporate alkynes via an ester linkage at the C-24 position, as well as contain a photoactivatable diazirine group at the C-6 position. However, since the ethyl group in β-sitosterol that is absent in cholesterol is at the C-24 position, it is likely that the addition of an ester linker at C-24 disrupts binding to 17β-HSD4 in the chemoproteomic experiments. Although the steroid core of cholesterol was unmodified in the photoreactive probes with the exception of the diazirine group, which was hypothesized to maintain binding to most sterol interactors, there may be a significant number of cholesterol binding proteins that require the side chain to be unmodified for the interaction to occur.
The primary function of 17β-HSD4 is catalysis of the second and third steps of peroxisomal β-oxidation, although it can also dehydrogenate Δ5-androstene-3β,17β-diol and estradiol to the less potent 17-keto compounds dihydroepiandrosterone and estrone.23 Almost all human tissues possess detectable 17β-HSD activity, and 17β-HSD4 is thought to be an important housekeeping enzyme responsible for inactivating the most potent estrogen, 17β-estradiol, in all tissues.27,31,32 While 17β-HSD4 is not known to play a role in inflammation or enlarged prostate, elevated 17β-HSD4 expression and activity as well as increased peroxisomal β-oxidation pathway activity have been found in prostate cancer tissues compared to normal prostate tissue, and may be indicative of a poor prognosis.33–35 Loss of 17β-HSD4 activity also leads to a severe d-bifunctional protein deficiency that is usually lethal by the age of one.32 β-Sitosterol may therefore derive its activity against prostate cancer through modulation of 17β-HSD4 activity. However, since β-sitosterol does not bind to the N-terminal domain of 17β-HSD4, it is unlikely to affect its dehydrogenase activity. It is therefore more likely to affect the activity of the central dehydratase domain or the C-terminal SCP-2 domain, whose function is not clear but may be involved in lipid transfer.23
Less is known about the cellular function of E-Syt1. It is localized to the endoplasmic reticulum (ER) where it directly tethers the ER membrane and the plasma membrane (PM) in response to elevated cytosolic calcium.36 E-Syt1 is a two-pass transmembrane protein that extends into the cytoplasm and binds PI(4,5)P2 in the PM via its C2 domains.36 Phosphorylation of E-Syt1 by the oncogenic tyrosine kinase CD74-ROS has been linked to invasiveness in non-small cell lung cancer, and RNAi knockdown of E-Syt1 attenuated the invasive properties of CD74-ROS expressing cells in vitro and in vivo.37 As β-sitosterol has been reported to inhibit prostate cancer cell migration and invasion in vitro, it is possible this may occur through modulation of E-Syt1 function.28 Interestingly, recent evidence suggests that the synaptotagmin-like mitochondrial-lipid-binding protein (SMP) domain of E-Syt2 functions in lipid transfer between the ER and PM, although binding of the SMP domain of E-Syt2 to cholesterol was not detected.38 If cholesterol and β-sitosterol both bind to the SMP domain of E-Syt1, it is possible that β-sitosterol interferes with the lipid transfer activity of E-Syt1, and perhaps the other E-Syt proteins as well. Hypercholesterolemia is a risk factor for estrogen receptorpositive breast cancer, the pathology of which is mediated via its conversion to the estrogen receptor and liver X receptor ligand 27-hydroxycholesterol.39 It will be interesting to determine if β-sitosterol inhibits the transfer or conversion of cholesterol and its metabolites in pre-malignant and transformed breast cancer cells.
In summary, we report the identification of two new binding proteins for β-sitosterol that has been shown to be effective against enlarged prostate in human clinical trials. β-Sitosterol also has anti-cancer and anti-inflammatory activities in cell culture (including prostate, breast, colon, leukemia, T-cells, and macrophages). Previously, only one enzyme (5α-reductase) was known to be inhibited by β-sitosterol. It remains unclear if inhibition of 5α-reductase is responsible for the effects of β-sitosterol on benign prostatic hyperplasia and prostate cancer, and modulation of additional targets may be essential. Moreover, inhibition of 5α-reductase is unlikely to play a role in the antiinflammatory activity of β-sitosterol or in its effects on other cancers. Using an affinity chromatography approach, we provide evidence that β-sitosterol directly binds to 17β-HSD4 and E-Syt1. These novel targets may be responsible for many of the beneficial effects of β-sitosterol. The two biotinylated affinity probes may also serve as useful tools for further comprehensive analysis of the sterol binding subset of the proteome.
Supplementary Material
Acknowledgments
Support for this research is provided by the NIH National Center for Complementary and Alternative Medicine (R01-AT006889 to C.C. and J.H.). B.L. is supported by the NIHUCLA Tumor Immunology Training Program (T32-CA009120). We thank Yi-Pei Chen and Tremylla Johnson for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Methods for biological and biochemical assays, MALDI mass spectrometry data, procedures for chemical synthesis, and compound characterization data.
References and notes
- 1.Ostlund RE., Jr Annu. Rev. Nutr. 2002;22:533–549. doi: 10.1146/annurev.nutr.22.020702.075220. [DOI] [PubMed] [Google Scholar]
- 2.Moreau RA, Whitaker BD, Hicks KB. Pro. Lipid Res. 2002;41:457–500. doi: 10.1016/s0163-7827(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 3.Weihrauch JL, Gardner JM. J. Am. Diet Assoc. 1978;73:39–47. [PubMed] [Google Scholar]
- 4.Ovesná Z, Vachálková A, Horváthová K. Neoplasma. 2004;51:407–414. [PubMed] [Google Scholar]
- 5.Awad AB, Fink CS. J. Nutr. 2000;130:2127–2130. doi: 10.1093/jn/130.9.2127. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig-Müller J, Georgiev M, Bley T. Process Biochem. 2008;43:15–23. [Google Scholar]
- 7.Scholtysek C, Krukiewicz AA, Alonso J-L, Sharma KP, Sharma PC, Goldmann WH. Biochem. Biophys. Res. Commun. 2009;379:795–798. doi: 10.1016/j.bbrc.2008.11.114. [DOI] [PubMed] [Google Scholar]
- 8.Nahata A, Dixit VK. Andrologia. 2012;44:396–409. doi: 10.1111/j.1439-0272.2011.01197.x. [DOI] [PubMed] [Google Scholar]
- 9.Vahouny GV, Connor WE, Subramaniam S, Lin DS, Gallo LL. Am. J. Clin. Nutr. 1983;37:805–809. doi: 10.1093/ajcn/37.5.805. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda I, Tanaka K, Sugano M, Vahouny GV, Gallo LL. J. Lipid Res. 1988;29:1573–1582. [PubMed] [Google Scholar]
- 11.Gupta MB, Nath R, Srivastava N, Shanker K, Kishor K, Bhargava KP. Planta Med. 1980;39:157–163. doi: 10.1055/s-2008-1074919. [DOI] [PubMed] [Google Scholar]
- 12.Villaseñor IM, Angelada J, Canlas AP, Echegoyen D. Phytother. Res. 2002;16:417–421. doi: 10.1002/ptr.910. [DOI] [PubMed] [Google Scholar]
- 13.Mahajan SG, Mehta AA. Eur. J. Pharmacol. 2011;650:458–464. doi: 10.1016/j.ejphar.2010.09.075. [DOI] [PubMed] [Google Scholar]
- 14.Lee I-A, Kim E-J, Kim D-H. Planta Med. 2012;78:896–898. doi: 10.1055/s-0031-1298486. [DOI] [PubMed] [Google Scholar]
- 15.Berges RR, Windeler J, Trampisch HJ, Senge T. Lancet. 1995;345:1529–1532. doi: 10.1016/s0140-6736(95)91085-9. [DOI] [PubMed] [Google Scholar]
- 16.Klippel KF, Hiltl DM, Schipp B. Br. J. Urol. 1997;80:427–432. [PubMed] [Google Scholar]
- 17.Woyengo TA, Ramprasath VR, Jones PJ. Eur. J. Clin. Nutr. 2009;63:813–820. doi: 10.1038/ejcn.2009.29. [DOI] [PubMed] [Google Scholar]
- 18.Moon DO, Lee KJ, Choi YH, Kim GY. Int. Immunopharmacol. 2007;7:1044–1053. doi: 10.1016/j.intimp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Park C, Moon DO, Rhu CH, Choi BT, Lee WH, Kim GY, Choi YH. Biol. Pharm. Bull. 2007;30:1317–1323. doi: 10.1248/bpb.30.1317. [DOI] [PubMed] [Google Scholar]
- 20.Park C, Moon DO, Ryu CH, Choi Bt, Lee Wh, Kim GY, Choi Yh. Acta Pharmacol. Sin. 2008;29:341–348. doi: 10.1111/j.1745-7254.2008.00761.x. [DOI] [PubMed] [Google Scholar]
- 21.Ding Y, Nguyen HT, Kim SI, Kim HW, Kim YH. Bioorg. Med. Chem. Lett. 2009;19:3607–3610. doi: 10.1016/j.bmcl.2009.04.129. [DOI] [PubMed] [Google Scholar]
- 22.Alappat L, Valerio M, Awad AB. Int. Immunopharmacol. 2010;10:1390–1396. doi: 10.1016/j.intimp.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Huyghe S, Mannaerts GP, Baes M, Van Veldhoven PP. Biochim. Biophys. Acta. 2006;1761:973–994. doi: 10.1016/j.bbalip.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Adamski J, Leenders F, Carstensen JF, Kaufmann M, Markus MM, Husen B, Tesdorpf JG, Seedorf U, de Launoit Y, Jakob F. Steroids. 1997;62:159–163. doi: 10.1016/s0039-128x(96)00175-4. [DOI] [PubMed] [Google Scholar]
- 25.de Launoit Y, Adamski J. J. Mol. Endocrinol. 1999;22:227–240. doi: 10.1677/jme.0.0220227. [DOI] [PubMed] [Google Scholar]
- 26.Jiang LL, Miyazawa S, Souri M, Hashimoto T. J. Biochem. 1997;121:364–369. doi: 10.1093/oxfordjournals.jbchem.a021596. [DOI] [PubMed] [Google Scholar]
- 27.Adamski J, Normand T, Leenders F, Monté D, Begue A, Stéhelin D, Jungblut PW, de Launoit Y. Biochem. J. 1995;311:437–443. doi: 10.1042/bj3110437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Awad AB, Fink CS, Williams H, Kim U. Eur. J. Cancer Prev. 2001;10:507–513. doi: 10.1097/00008469-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Cabeza M, Bratoeff E, Heuze I, Ramírez E, Sánchez M, Flores E. Proc. West. Pharmacol. Soc. 2003;46:153–155. [PubMed] [Google Scholar]
- 30.Hulce JJ, Cognetta AB, Niphakis MJ, Tully SE, Cravatt BF. Nat. Methods. 2013;10:259–264. doi: 10.1038/nmeth.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martel C, Rhéaume E, Takahashi M, Trudel C, Couët J, Luu-The V, Simard J, Labrie F. Steroid Biochem. Mol. Biol. 1992;41:597–603. doi: 10.1016/0960-0760(92)90390-5. [DOI] [PubMed] [Google Scholar]
- 32.Möller G, Adamski J. Mol. Cell. Endorinol. 2006;248:47–55. doi: 10.1016/j.mce.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 33.Zha S, Ferdinandusse S, Denis S, Wanders RJ, Ewing CM, Luo J, De Marzo AM, Isaacs WB. Cancer Res. 2003;63:7365–7376. [PubMed] [Google Scholar]
- 34.Zha S, Ferdinandusse S, Hicks JL, Denis S, Dunn TA, Wanders RJ, Luo J, De Marzo AM, Isaacs WB. Prostate. 2005;63:316–323. doi: 10.1002/pros.20177. [DOI] [PubMed] [Google Scholar]
- 35.Rasiah KK, Gardiner-Garden M, Padilla EJ, Möller G, Kench JG, Alles MC, Eggleton SA, Stricker PD, Adamski J, Sutherland RL, Henshall SM, Hayes VM. Mol. Cell. Endocrinol. 2009;301:89–96. doi: 10.1016/j.mce.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 36.Giordano F, Saheki Y, Idevall-Hagren O, Colombo SF, Pirruccello M, Milosevic I, Gracheva EO, Bagriantsev SN, Borgese N, De Camilli P. Cell. 2013;153:1494–1509. doi: 10.1016/j.cell.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jun HJ, Johnson H, Bronson RT, de Feraudy S, White F, Charest A. Cancer Res. 2012;72:3764–3774. doi: 10.1158/0008-5472.CAN-11-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schauder CM, Wu X, Saheki Y, Narayanaswamy P, Torta F, Wenk MR, De Camilli P, Reinisch KM. Nature. 2014;510:552–555. doi: 10.1038/nature13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V, Umetani M, Geradts J, McDonnell DP. Science. 2013;342:1094–1098. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.