Abstract
Importance
Cortical dysfunction in PD may be caused by disruption to ascending systems or intrinsic cortical neuropathology. We introduce and conduct a joint analysis of metabolism and atrophy capable of identifying whether metabolic disruption occurs in mild PD without cortical atrophy.
Objective
To determine the extent and spatial pattern of cortical involvement in mild Parkinson Disease (PD).
Design
Observational.
Participants
Twenty-three cognitively normal participants with mild PD (mean Hoehn & Yahr stage 2) and 21 healthy controls (HC)
Main Outcome Measures
Cortical thickness (obtained from analysis of structural MRI with FreeSurfer) and cerebral perfusion measures (obtained from ASL) analyzed independently and then together in a joint multiple factorial analysis to identify spatial patterns of perfusion and cortical thickness.
Results
We identify a pattern of changes in perfusion and cortical thickness characterized by symmetric parietal cortical thinning and reduced precuneus perfusion, with relative preservation of thickness and perfusion in the anterior cingulate cortex (ACC), right prefrontal gyrus, and medial frontal gyrus. The expression of this pattern is correlated with motor system symptoms and speed of processing.
Conclusions and Relevance
A spatial pattern of joint parietal cortical thinning and disproportionate reduction in perfusion occurs in our nondemented PD sample. We found no PD-related components of reduced perfusion without cortical thinning. This suggests that PD affects the cortex itself, even when symptoms are relatively mild.
Keywords: Parkinson disease, cortical thickness, cerebral perfusion, multiple factor analysis, joint analysis
1.Introduction
Although Parkinson disease (PD) is characterized by motor deficits caused by degeneration of dopaminergic neurons in the substantia nigra, cognitive impairment and dementia are common non-motor features of the disease. When cognitive impairment appears, it tends to have a profile of more affected and less affected domains (e.g. usually dysexecutive, but sometimes amnesic or visuospatial) that suggests differential regional cortical involvement.1 However, the causes of cognitive symptoms and dementia, and the timing of cortical involvement in PD are unclear.
In PD, cortical dysfunction can be caused by disruption to ascending control systems that affect regional cerebral metabolism and blood flow, or intrinsic cortical pathology (accompanied by α-synuclein [α-Syn] deposition) that causes cortical atrophy. Cerebral metabolism can be assessed in vivo using [18F]-fluorodeoxyglucose/positron emission tomography (18F-FDG/PET). Alternatively, measurements of cerebral blood flow assessed by atrophy-corrected arterial spin labeling (ASL) largely mirror PET findings,2 and can be used as a proxy for cerebral metabolism. Cortical thickness measurements reliably measure regional cortical atrophy in PD.3,4
Changes in perfusion that occur without simultaneous cortical thinning may reflect early neuronal dysfunction, including alterations to network activity caused by disruption of ascending systems5. We would expect such changes to occur early in the disease, before Braak staging6,7 would indicate that α-Syn pathology should affect association cortex and impact cognition.8–10 Conversely, decreases in perfusion that occur with cortical thinning suggest a more advanced neuropathology (e.g., α-Syn pathology, and/or concurrent Alzheimer's disease [AD]) that may result in cell or axonal loss. These two modalities must be examined jointly to determine whether ascending system dysfunction exists without accompanying intrinsic cortical disease at different stages of the disease. For example, some PD subjects could have only reduced perfusion in a specific region and others could have reduced cortical thickness in that region. Such a region might appear lower in perfusion and cortical thickness in a group analyses of each modality, yet these reductions would not necessarily occur together in the same individuals. Joint analyses is a convenient way to identify brain regions where multiple modalities show a pattern of change across individuals, as would be expected from some underlying degenerative process.
An instrumental approach in identifying spatial patterns of metabolic change that are related to symptoms in PD is the scaled subprofile model (SSM), a principal components method that extracts spatial networks with covarying metabolism (a “covariance network”) and allows them to be scored and compared between groups.11,12 SSM has been used to identify a motor dysfunction-related covariance network and a cognitive-dysfunction related covariance network in PD.13
Here we present a novel method for joint analysis of cortical thickness and perfusion (as a proxy for metabolism) to identify covarying components, similar to SSM, but allowing one to identify PD-related spatial patterns of altered cortical thickness and/or metabolism. We separately examine group differences between controls and PD participants in cortical thickness and relative cerebral perfusion (corrected for grey matter density), replicating findings of cortical thinning14 and hypoperfusion15 of higher association areas in early PD without dementia. We then go on to perform the joint analysis described above, hypothesizing that mild PD is characterized by a spatial pattern of joint frontal and parietal decreases in perfusion and in cortical thickness, and that this spatial pattern should be related to motor symptom severity, disease duration, and measures of executive processing (e.g, attention, working memory and fluency).
2. Methods
2.1 Participants
Subjects are matched on age from a larger study (see Supplemental Materials). The sample included 23 PD (Mage =64, SD=8 years) and 21 controls (Mage =62; SD=10 years) (Table 1, Cortical Thickness sample). The ASL sequence was added after the study began, resulting in 5 fewer participants in each group of the ASL Subsample (Table 1, ASL Subsample). Males were over-represented in the PD group, consistent with higher incidence rates of PD in men.16
Table 1.
Demographics of Sample
| Cortical Thickness Sample | ASL Subsample | |||||
|---|---|---|---|---|---|---|
| PD | Control | Total | PD | Control | Total | |
| N | 23 | 21 | 44 | 18 | 16 | 34 |
| Age at Scan | 63.96(8.12) | 61.90(10.00) | 62.98(9.02) | 63.28(8.71) | 61.94(10.47) | 62.65( 9.45) |
| Sex(number males) | 16(70%) | 9 (43%) | 25 (57%) | 12(67%) | 6 (38%) | 18 (53%) |
| Education (years) | 16.41(2.28) | 15.90(2.39) | 16.16(2.32) | 16.18(2.07) | 16.06(2.64) | 16.12(2.33) |
| Hoen & Yahr | 2.04(1–2.5) | 2.06(1–2.5) | ||||
| Handedness (Right) | 19 | 19 | 38 | 17 | 14 | 31 |
| Dominant side of motor symptoms | 5 Left | 5 Left | ||||
| 16 Right | 12 Right | |||||
| 1 Symmetric | 1 Symmetric | |||||
| 1 Unknown | ||||||
| UPDRS Part I | 10.17(5.81) | 9.79(5.99) | 11.33(5.82) | 11.33(5.82) | ||
| UPDRS Part II | 8.48(4.79) | 7.80(5.15) | 8.72(4.76) | 8.26(5.04) | ||
| UPDRS Part III | 22.52(8.20) | 0.81(1.40) | 12.16(12.48) | 22.61(8.62) | 0.88(1.59) | 12.38(12.68) |
| UPDRS Part IV | 1.96(3.78) | 1.88(3.72) | 2.50(4.13) | 2.50(4.13) | ||
| Levodopa (current) | 14 | 12 | ||||
| Dopamine agonist (current) | 9 | 9 | ||||
| Years since symptom onset | 8.64(5.24) | 8.50(5.13) | ||||
| Years since diagnosis | 6.55(4.88) | 6.54(5.05) | ||||
| MOCA | 26.52(2.17) | 27.29(1.95) | 26.89(2.08) | 26.06(2.04) | 27.44(1.93) | 26.71(2.08) |
| Hopkins Verbal Learning Test | 25.39(5.50) | 25.61(5.51) | ||||
| Golden Stroop (total correct) | 188.52(24.75) | 188.89(25.77) | ||||
| Trails B (seconds) | 71.59(29.79) | 76.94(31.46) | ||||
Note: PD patients do not differ significantly from controls on age (p=.457 for CT sample, p=.686 for ASL sample) or years of education (p=.483 for CT sample, p=.891 for ASL sample).
Although lumbar punctures were not part of this experiment, CSF biomarkers (including α-synuclein, total tau, phosphorylated tau (p-tau) and amyloid beta peptide 1-42 [Aβ]), measured with Luminex assay17 were available for 15 PD participants only as part of the Pacific Northwest Udall Center clinical core (obtained a mean of 10.6 months prior to imaging). Compared to subjects with AD measured using the same assay, total tau and p-tau levels were not increased (total tau: median 45 [21-72] pg/ml compared to median 70 [32-299] pg/ml; p-tau: median 27 [16-41]pg/ml compared to median 51 pg/ml) nor were Aβ levels decreased (median 434 [189-535] pg/ml compared to median 188 [38-1021] pg/ml).
Most PD participants were at H&Y stage 2 (N=18) and had been experiencing symptoms for an average of 8.6 years and diagnosed for an average of 6.5 years. Most were taking dopaminergic medications (Table 1). This study was approved by the University of Washington Institutional Review Board. All participants provided written informed consent.
2.2 Neuropsychological evaluation
All participants were assessed using the MoCA and the motor subscale of the Unified Parkinson's Disease Rating Scale (UPDRS-III) prior to scanning. Although within the range of normative values for controls,18 11 PD participants were classified via consensus rating as having mild cognitive impairment.19 PD participants in the cortical thickness group did not differ significantly from controls on the MoCA (p=.228), however, the PD participants in the ASL subsample had slightly lower MoCA scores than controls in the ASL subsample (p=.052, Mpd= 26.06 (23-30), Mcontrol=27.44 (23-30)). PD participants had significantly higher scores on the UPDRS-III (cortical thickness sample: t(42)=11.96, p < .001; ASLsubsample: t(32)=9.91, p < .001).
PD participants additionally were assessed using a neuropsychological battery designed to cover all major cognitive domains.19,20 Measures included the Mini Mental State Examination (MMSE),21 tests of attention and working memory (Trail-Making Test,22 Tower of London,23 Stroop24 and Digit Span24), declarative memory (Wechsler Memory Scale-R,25 Benton Visual Retention Test,26 Hopkins Verbal Learning Test-Revised27), and visuospatial ability (Judgement of Line Orientation28). (For an overview, see Cholerton et al.19).
2.3 MRI acquisition and processing
Magnetic resonance imaging was performed on a Philips 3.0 T Achieva scanner using a 32-channel head coil. Two baseline scan sessions were acquired between 1 and 3 weeks apart. A 1mm isotropic MPRAGE was obtained using the following parameters: inversion time (TI) =1100ms, repetition time (TR) = 7.46ms, echo time (TE) = 3.49ms, flip angle = 7°. Arterial spin labeling (ASL) was performed using a Pseudo Continuous ASL (pCASL) sequence with the following parameters: single-shot gradient-echo EPI, 16 ascending slices, slice thickness=5mm, slice gap=1mm, labeling duration=1650 ms, post spin labeling delay=1600ms, TR= 4000ms, TE=15ms, SENSE factor 2.3, number of controls/labels=30 pairs, bandwidth/pixel=2.077kHz, echo train length=39.
2.4 MRI processing
Cortical reconstruction and volumetric segmentation were automatically performed with FreeSurfer version 5.329 using the longitudinal processing pipeline. Data were smoothed using a typical 15mm full width at half maximum (FWHM) Gaussian kernel. Vertex-wise group analysis was performed using a multilevel model, implemented in Revolutions R (www.revolutionanalytics.com) with the nlme library.30 This model determines the predictive effect of group (PD or control) on the cortical thickness of each vertex, modeling correlated random error within subjects at each occasion. Because groups were balanced on age, age was not included as a covariate in the model. Using the same model, we conducted exploratory analysis using the log of tau/Aβ to predict cortical thickness across the cortical surface, controlling for time between CSF collection and scanning. See Supplemental Materials for analyses controlling for gender. Cluster-wise correction for multiple comparisons was performed at a corrected p value of .05 using mri_surfcluster. Data were visually inspected and no manual editing was performed.
PCASL data were motion corrected using FSL.31 We calculated the difference image between averaged control and labeled images, performed brain extraction,32 registration to Montreal Neurological Institute (MNI) space, smoothing using a 10mm FWHM Gaussian kernel, and masking to include only voxels present in all subjects at each session. Each subject's data were normalized by the sum of the subject's values that correspond to the voxels in the relevant session mask. Voxelwise group analysis was performed using a multilevel model as for cortical thickness. However, we corrected for individual grey matter loss or partial volume effects by using grey matter partial volume maps obtained from FSL FAST. We corrected for multiple comparisons by simulating the null distribution of the maximum cluster size across the masked voxels that are at least 40% likely to be grey matter (p < .05, α=.05) at the given level of smoothing using 3dClusterSim.33
2.5 Multiple Factorial Analysis
We combined cortical thickness and cerebral perfusion in a joint analysis using multiple factorial analysis (MFA)34 which we implemented in R. MFA is designed to analyze several data tables (e.g., ASL and cortical thickness) measured on the same individuals, generating a set of scores to describe the common components across modalities.
Abdi et al34 provide a comprehensive review of the MFA algorithm. To summarize, there are three main steps to MFA. In the first step, one performs a PCA of each data table individually. In the second step, each data table is normalized by its first singular value. This step is conceptually similar to Z-score normalization that allows one to compare variables with different variance. Dividing by the first singular value ensures that the first principal component of each data table is one, and therefore no data table dominates the analysis. These normalized tables are then concatenated and subjected to a PCA. Because a PCA attempts to model all the variance in the data, we limited our analysis to relevant data (voxels more than 40% likely to be grey matter by the standard grey matter template in FSL, and vertices within FreeSurfer cortical parcels). Because the spatial pattern of cortical thickness group differences is symmetric, we first used MFA to model components describing whole brain perfusion data and the left hemisphere cortical thickness, using subjects with perfusion and cortical thickness data from the first session. This allowed us to examine the stability of the joint components by replicating the analysis using the same perfusion data and the right hemisphere cortical thickness data. Separation of hemispheres also allowed us to avoid considering components that reflect normal hemispheric asymmetry, for clarity of interpretation.
We further examined components describing > 5% of the variance in the data that differed between PD participants and controls. For each component, each voxel or vertex has a weight that can be multiplied by the scaled individual's values for each voxel/vertex and summed to compute an individual score, or the “expression” of this component. We computed individual component scores, and then tested for group differences, age relationships, and relationships to cognitive performance, motor symptoms, and duration of disease (among the PD subset). Specifically, we examined selected measures of attention and working memory (Tower of London, Trail Making A and B, Digit Span total, and Stroop total correct), semantic fluency (animals and vegetables), phonemic fluency (a words, f words, s words), and UPDRS-III, controlling for age.
3. Results
3.1 Cortical thickness
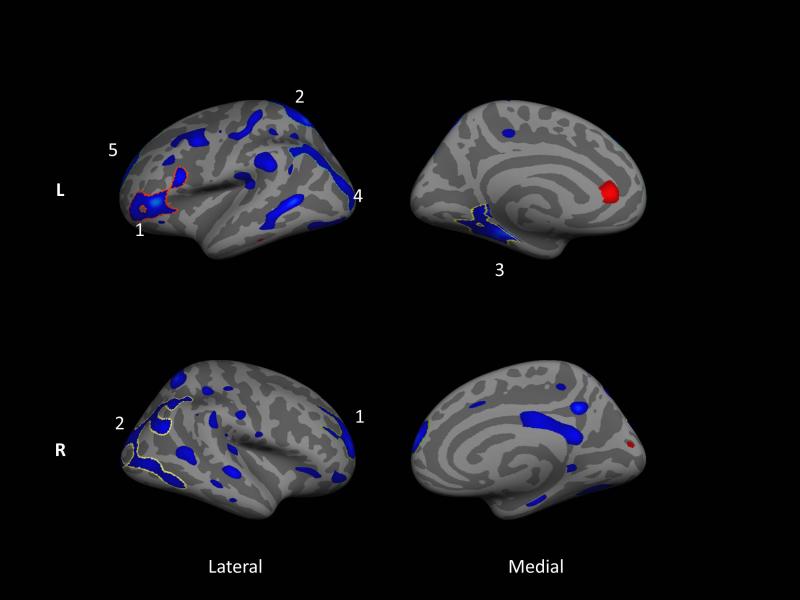
Overall cortical thickness was significantly lower in PD than in controls in both hemispheres (Left: t(41)=−2.02, p=.049, Right: t(41)=−2.05, p=.047)). Figure 1 shows the whole-brain vertex-wise group differences in cortical thickness between PD and controls. Significant clusters are outlined and numbered (see Table e-1 for details); PD participants have bilaterally thinner cortex than controls in superior parietal, rostral middle frontal, and inferior temporal regions. Differences were slightly more pronounced in the left hemisphere.
Figure 1.
Whole brain vertex-wise group differences in cortical thickness between PD subjects and controls based on structural images taken at two baseline sessions. Blue shows areas where PD subjects have thinner cortex. Colored regions are significant at p < .05; outlined and numbered regions further survive Monte Carlo null-Z correction for multiple comparisons at p < .05. Note that this statistical map represents data from both sessions as analyzed in a multilevel model. Although there was a region of thicker cortex in the anterior cingulate cortex in the left hemisphere, it did not reach significance after correction for multiple comparisons.
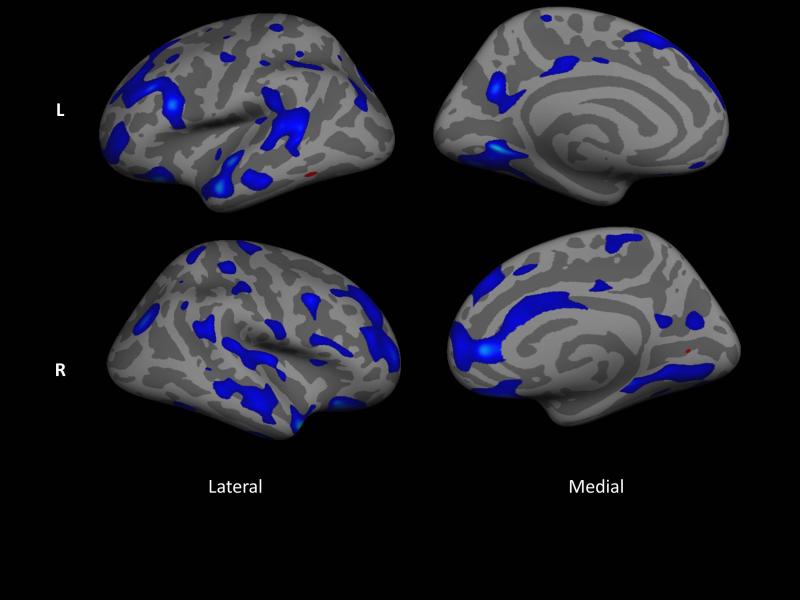
To investigate the potential contribution of AD pathology to cortical thinning in PD (see Discussion), we conducted an exploratory analysis to examine the relationship of log(tau/Aβ) to cortical thickness . Figure 2 shows that prominent lateral temporal and temporal pole thinning is related to a higher ratio of log(tau/Aβ).
Figure 2.
Areas of cortex that are significantly related (p < .05) to the log of the ratio of total tau to aβ, correcting for time between CSF collection and scan, for 15 PD participants with CSF biomarkers. Data are uncorrected for multiple comparisons. Blue regions have decreasing thickness with increasing ratio. Note that this statistical map represents data from both sessions as analyzed in a multilevel model.
3.2 Cerebral perfusion
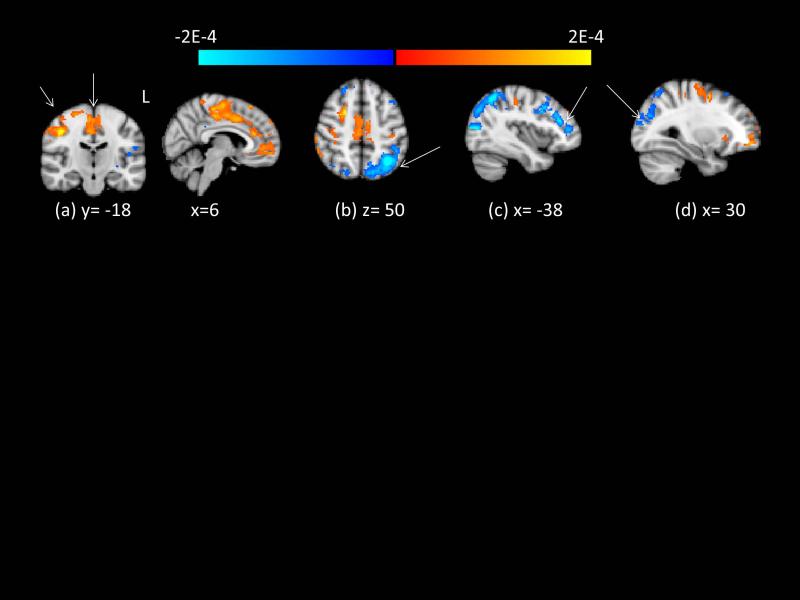
Figure 3 shows lower relative perfusion in PD compared to controls (beyond that expected by reduced grey matter volume) in the left and right lateral occipital gyrus and left superior parietal lobule (b, d) and the left middle frontal gyrus (c). Regions with higher relative perfusion in PD relative to controls are the right postcentral gyrus (a). See Table e-2 for cluster information.
Figure 3.
Group differences in relative cerebral perfusion, computed across both sessions, correcting for grey matter partial volume. Clusters indicated by arrows survive Monte Carlo null- Z correction for multiple comparisons at p < .05. Red/yellow regions indicate greater relative perfusion in PD. Blue/light blue regions indicate lower relative perfusion in PD. Intensity of color indicates log of p-statistic. (a) R pre/postcentral gyrus, shown in coronal and sagittal views (b) L lateral occipital gyrus/superior parietal lobule (c) L middle frontal gyrus (d) R lateral occipital gyrus/angular gyrus.
3.3 Joint cortical thickness and perfusion components
MFA of left-hemisphere cortical thickness and perfusion data in grey matter resulted in four components that each accounted for > 5% of the variance. Of these, only scores on the second component (8.9% of the variance) differed between the PD and control group, with lower expression of this component in PD (t(35)=−2.65, p=.011). Figure 4a shows the spatial map of this PD-related component. Lower scores characterize a pattern of symmetric parietal cortical thinning with relative anterior cingulate cortex preservation, which is associated with reduced precuneus perfusion and relatively preserved perfusion in the ACC, right prefrontal gyrus, and right medial frontal gyrus. See Supplemental for remaining components.
Figure 4.
Joint MFA analysis of (a) left hemisphere cortical thickness and cerebral perfusion (x=0,y=42,z=4), and (b) right hemisphere cortical thickness and cerebral perfusion (x=0,y=42,z=4) for the second component. Loadings with magnitude > 1 SD above mean load ing for each vertex/voxel are shown for display purposes. Color (blue is positive, red is negative) indicates the weight of the loading. PD have lower scores of these components than controls. Loading weights are multiplied by the value of cortical thickness or perfusion to obtain a score; therefore, PD subjects have relatively smaller values of cortical thickness or perfusion than con trols in blue areas, and relatively larger values in the red areas.
The pattern of perfusion loadings was asymmetric. This was not an artifact of including only the left hemisphere cortical thickness in the analysis. Results were virtually identical using right hemisphere thickness and perfusion data (Figure 4b) for the second component, and the group difference was also significant (t(35)=−2.92, p=.006). Expression of these scores did not differ between participants with left versus right dominant motor impairment. The apparent asymmetry must be interpreted in the context of our relative perfusion measures (see Discussion).
Expression of the PD-related component was uncorrelated with age. We tested its relationship to cognitive and motor scores, controlling for age. Lower expression of the left PD component was related to lower scores on the Tower of London test score (t(13)= 3.24, p=.007) and to higher UPDRS-III symptom total (t(34)=−3.28, p=.002). Lower expression of the left PD component was marginally correlated with worse performance on Trail Making B (t(15)=−1.82, p=.089) and Trail Making A (t(15)=−2.045, p=.059). Expression of the right PD component showed a similar pattern of relationships (see Supplemental). PD component scores were not related to measures of digit span, fluency, or disease duration.
4. Discussion
Replicating findings of others,3,35 we identified a pattern of thinner cortex in parietal, rostral frontal, lateral temporal and occipital regions among PD participants. We found group differences in cerebral perfusion that included relative decreases and increases in PD compared to controls, corrected for grey matter volume. These differences must be interpreted carefully considering that our cerebral perfusion measure is relative, normalized by the sum of the voxels in a common subject template. Relative perfusion is a more sensitive index of small changes in regional cerebral blood flow, as opposed to quantified cerebral perfusion, because of the large inter-subject variability in global cerebral blood flow.36 However, when assessed by absolute measures, global cerebral perfusion in PD is lower than in controls.15 Normalizing the patient group by a smaller mean value inflates voxel values relative to the control group. This causes areas of relatively preserved perfusion to appear increased in the patient population relative to controls, and inflates areas of relatively decreased perfusion. We thus interpret the areas of relative perfusion increase in the superior frontal gyrus and medial frontal cortex as areas where per-fusion is relatively preserved, though not probably actually increased.
Linking relative perfusion to cortical thickness in a joint MFA analysis grounds the interpretation of both modalities. We identify a joint PD-related component that reflects symmetric parietal cortical thinning with relative anterior cingulate cortex preservation. This pattern of cor tical thickness is associated with reduced precuneus perfusion and relatively preserved perfusion in the ACC, right prefrontal gyrus, and right medial frontal gyrus. This component showed no decreased perfusion without cortical thinning, suggesting that reductions in perfusion in PD are associated with cortical pathology and not solely caused by disruption to ascending control systems.
The cause of parietal cortical thinning in PD is unknown, but parietal cortical thinning is also part of a cortical signature of thinning that occurs with concurrent AD.37 Progressively lower CSF Aβ concentrations in PD patients with increasing levels of cognitive impairment suggest that AD may underlie cognitive decline in PD.38 To explore this confound we examined CSF biomarker data available from a subsample of study participants. Tau and Aβ CSF levels did not have an AD-like profile. Exploratory analysis of the cortical thickness correlates of the ratio of total tau to Aβ showed that parietal cortex thickness was uncorrelated with this marker of AD pathology. Although not conclusive, this evidence supports the idea that parietal thinning is a characteristic of PD itself.
The asymmetry in both the perfusion and joint analyses indicates relatively preserved perfusion in the right hemisphere. Our subjects exhibited primarily right-sided dominant motor impairment, which could explain this asymmetry; we did not have enough subjects with left-dominant motor impairment to fully evaluate this possibility.
Our groups are not balanced for gender and more men have PD. Because of this, in theory and in analysis group differences cannot be formally attributed to gender vs. PD. However, regions that show group differences do not align with reported gender differences in cortical thickness39 and our single modality findings replicate those by others found in groups balanced for gender. Finally, we show in Supplemental Materials that results are diminished in significance but their topography is generally unaffected by controlling for gender.
One limitation of PCA-based approaches is that the components depend upon the variation included in the sample by design. Therefore, joint components of cortical thickness and per-fusion may be different in another population with greater or lesser variability in disease progression and/or cognitive symptoms.
Our novel joint analysis links changes in perfusion and cortical thickness in relatively mild PD, demonstrating an informative pattern of relative differences in both modalities. We observe a spatial consistency between the modalities, consistent with neuropathology that results in decreases in perfusion and cortical thickness. This PD-related pattern is negatively associated with motor symptoms and measures of processing speed. This is further evidence that PD affects the cortex itself in mild PD, to a degree that cannot be explained solely by loss of ascending influences. The approach holds promise for quantifying independent pathological trajectories, such as concurrent AD pathology or atypical progression of α-Syn pathology.
Supplementary Material
Acknowledgements
This research was supported by grants from the National Institutes of Health 1RC4NS073008-01 and P50NS062684, Veterans Affairs, and the Jane and Lee Seidman Fund. The funding organizations had no role in the design and conduct of the study; collection, management, and analysis of the data; or preparation, review, and approval of the manuscript.
List of Acronyms
- AD
Alzheimer's disease
- ASL
arterial spin labeling
- MRI
magnetic resonance imaging
- MNI
Montreal Neurological Institute
- PCA
Principal components analysis
- PD
Parkinson disease
- MFA
multiple factorial analysis
- PCASL
pseudo-continuous arterial spin labeling
- FWHM
Full-width half-maximum
Footnotes
Contributions to Manuscript:
Dr. Madhyastha, Dr. Grabowski, and Dr. Leverenz participated in design and conceptualization of the study. Dr. Askren and Dr. Zhang participated in acquisition of data. Dr. Madhyastha and Dr. Grabowski analysed the data and drafted the manuscript. All participated in interpretation of the results and critical revision of the manuscript.
Disclosures:
Dr. Madhyastha, Dr. Askren, Dr. Boord, Dr. Grabowski and Dr. Zhang report no disclosures. Dr. Leverenz has been a paid consultant for Boerhinger-Ingelheim, Navidea Biopharmaceuticals, and Piramal Healthcare.
Author Contributions: Research project conception, organization and execution (T.M., T.G., J.L.), statistical analysis design (T.M. and T.G.) execution (T.M.) and review and technique (all), and manuscript preparation (all).
Additional Contributions:
Sarah Heniges (Research Coordinator) and Gretchen Todd (Physician Assistant) recruited subjects. Desiree Gulliford (Research Assistant), Elliot Collins (Research Scientist) and Zoé Mestre (Research Scientist) assisted with data collection. Chris Gatenby (PhD) programmed the MRI sequences.
References
- 1.Watson GS, Leverenz JB. Profile of Cognitive Impairment in Parkinson's Disease. Brain Pathology. 2010;20(3):640–645. doi: 10.1111/j.1750-3639.2010.00373.x. doi:10.1111/j.1750-3639.2010.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma Y, Huang C, Dyke JP, et al. Parkinson's disease spatial covariance pattern: noninvasive quantification with perfusion MRI. J Cereb Blood Flow Metab. 2010;30(3):505–509. doi: 10.1038/jcbfm.2009.256. doi:10.1038/jcbfm.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jubault T, Gagnon J-F, Karama S, et al. Patterns of cortical thickness and surface area in early Parkinson's disease. NeuroImage. 2011;55(2):462–467. doi: 10.1016/j.neuroimage.2010.12.043. doi:10.1016/j.neuroimage.2010.12.043. [DOI] [PubMed] [Google Scholar]
- 4.Pereira JB, Ibarretxe-Bilbao N, Marti M-J, et al. Assessment of cortical degeneration in patients with Parkinson's disease by voxel-based morphometry, cortical folding, and cortical thickness. Human Brain Mapping. 2012;33(11):2521–2534. doi: 10.1002/hbm.21378. doi:10.1002/hbm.21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee PH, Yong SW, An Y-S. Changes in Cerebral Glucose Metabolism in Patients with Parkinson Disease with Dementia After Cholinesterase Inhibitor Therapy. J Nucl Med. 2008;49(12):2006–2011. doi: 10.2967/jnumed.108.054668. doi:10.2967/jnumed.108.054668. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Tredici KD, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiology of Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. doi:10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 7.Braak H, Ghebremedhin E, Rüb U, Bratzke H, Tredici KD. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318(1):121–134. doi: 10.1007/s00441-004-0956-9. doi:10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 8.Irwin DJ, White MT, Toledo JB, et al. Neuropathologic substrates of Parkinson disease dementia. Ann. Neurol. 2012;72(4):587–598. doi: 10.1002/ana.23659. doi:10.1002/ana.23659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irwin DJ, Lee VM-Y, Trojanowski JQ. Parkinson's disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat Rev Neurosci. 2013;14(9):626–636. doi: 10.1038/nrn3549. doi:10.1038/nrn3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braak H, Rüb U, Del Tredici K. Cognitive decline correlates with neuropathological stage in Parkinson's disease. Journal of the Neurological Sciences. 2006;248(1–2):255–258. doi: 10.1016/j.jns.2006.05.011. doi:10.1016/j.jns.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Eckert T, Tang C, Eidelberg D. Assessment of the progression of Parkinson's disease: a metabolic network approach. The Lancet Neurology. 2007;6(10):926–932. doi: 10.1016/S1474-4422(07)70245-4. doi:10.1016/S1474-4422(07)70245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eidelberg D, Moeller JR, Dhawan V, et al. The metabolic topography of parkinsonism. J. Cereb. Blood Flow Metab. 1994;14(5):783–801. doi: 10.1038/jcbfm.1994.99. doi:10.1038/jcbfm.1994.99. [DOI] [PubMed] [Google Scholar]
- 13.Huang C, Mattis P, Tang C, Perrine K, Carbon M, Eidelberg D. Metabolic brain networks associated with cognitive function in Parkinson's disease. Neuroimage. 2007;34(2):714–723. doi: 10.1016/j.neuroimage.2006.09.003. doi:10.1016/j.neuroimage.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zarei M, Ibarretxe-Bilbao N, Compta Y, et al. Cortical thinning is associated with disease stages and dementia in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2013:jnnp–2012-304126. doi: 10.1136/jnnp-2012-304126. doi:10.1136/jnnp-2012-304126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández-Seara MA, Mengual E, Vidorreta M, et al. Cortical hypoperfusion in Parkinson's disease assessed using arterial spin labeled perfusion MRI. Neuroimage. 2012;59(3):2743–2750. doi: 10.1016/j.neuroimage.2011.10.033. doi:10.1016/j.neuroimage.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 16.Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson's disease than women? J. Neurol. Neurosurg. Psychiatr. 2004;75(4):637–639. doi: 10.1136/jnnp.2003.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi M, Bradner J, Hancock AM, et al. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol. 2011;69(3):570–580. doi: 10.1002/ana.22311. doi:10.1002/ana.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasreddine ZSP,NA, Bedirian VCS, Whitehead VCI, Cummings JLCH. The Montreal Cognitive Assessment, MoCA : A brief screening tool for mild cognitive impairment. Journal American Geriatric Society. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 19.Cholerton B, Zabetian C, Quinn J, et al. Pacific northwest udall center of excellence clinical consortium: study design and baseline cohort characteristics. Journal of Parkinson's disease. 2013;3(2):205–14. doi: 10.3233/JPD-130189. doi:10.3233/JPD-130189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson GS, Cholerton BA, Gross RG, et al. Neuropsychologic assessment in collaborative Parkinson's disease research: A proposal from the National Institute of Neurological Disorders and Stroke Morris K. Udall Centers of Excellence for Parkinson's Disease Research at the University of Pennsylvania and the University of Washington. Alzheimer's & Dementia. 2013;9(5):609–614. doi: 10.1016/j.jalz.2012.07.006. doi:10.1016/j.jalz.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Army Individual Test Battery: Manual of Directions and Scoring. War Department, Adjutant General's Office; Washington, DC: 1944. [Google Scholar]
- 23.Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci. 1982;298(1089):199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- 24.Strauss ES,EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press; Oxford, New York: 2006. [Google Scholar]
- 25.Wechsler D. Wechsler Memory Scale-Revised Manual. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- 26.Benton AL. Revised Visual Retention Test. 4th edition Psychological Corporation; New York, NY: 1974. [Google Scholar]
- 27.Benedict RHBSD, Groninger LBJ. The Hopkins Verbal Learning Test-Revised: Normative data and analysis of inter-form and inter-rater reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- 28.Benton ALS,AB, Hamsher KVN, Spreen O. Contributions to Neuropsychological Assessment-A Clinical Manual. Psychological Assessment Resources; Lutz, FL: 1994. [Google Scholar]
- 29.Dale AM, Fischl B, Sereno MI. Cortical Surface-Based Analysis: I. Segmentation and Surface Reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. doi:10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 30.Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-PLUS. Springer; New York, NY: 2000. [u.a.] [Google Scholar]
- 31.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.09.015. doi:10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 34.Abdi H, Williams LJ, Valentin D. Multiple factor analysis: principal component analysis for multitable and multiblock data sets. Wiley Interdisciplinary Reviews: Computational Statistics. 2013;5(2):149–179. doi:10.1002/wics.1246. [Google Scholar]
- 35.Ibarretxe-Bilbao N, Junque C, Segura B, et al. Progression of cortical thinning in early Parkinson's disease. Movement Disorders. 2012;27(14):1746–1753. doi: 10.1002/mds.25240. doi:10.1002/mds.25240. [DOI] [PubMed] [Google Scholar]
- 36.Aslan S, Lu H. On the sensitivity of ASL MRI in detecting regional differences in cerebral blood flow. Magnetic Resonance Imaging. 2010;28(7):928–935. doi: 10.1016/j.mri.2010.03.037. doi:10.1016/j.mri.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickerson BC, Bakkour A, Salat DH, et al. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb. Cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. doi:10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montine TJ, Shi M, Quinn JF, et al. CSF Aβ42 and tau in Parkinson's disease with cognitive impairment. Mov. Disord. 2010;25(15):2682–2685. doi: 10.1002/mds.23287. doi:10.1002/mds.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sowell ER, Peterson BS, Kan E, et al. Sex Differences in Cortical Thickness Mapped in 176 Healthy Individuals between 7 and 87 Years of Age. Cereb. Cortex. 2007;17(7):1550–1560. doi: 10.1093/cercor/bhl066. doi:10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.