Abstract
Background
Transient ischemic attack (TIA) patients are at risk of recurrent vascular events. The primary objectives were to evaluate among TIA patients: the prevalence of sleep apnea, and among patients with sleep apnea auto-titrating continuous positive airway pressure (auto-CPAP) adherence. The secondary objective was to describe among TIA patients with sleep apnea, the recurrent vascular event rate by auto-CPAP use category.
Methods
All intervention patients received auto-CPAP for two nights, but only intervention patients with evidence of sleep apnea received auto-CPAP for the remainder of the 90-day period. Intervention patients received polysomnography at 90-days post-TIA. Control patients received polysomnography at baseline and at 90-days. Acceptable auto-CPAP adherence was defined as ≥4 hours/night for ≥75% nights. Vascular events included recurrent transient ischemic attack, stroke, hospitalization for congestive heart failure, myocardial infarction or death.
Results
We enrolled 70 acute TIA patients: intervention N=45 and control N=25. The majority of patients had sleep apnea: 57% at baseline and 59% at 90-days. Among the 30 intervention patients with airflow obstruction, 12 (40%) had acceptable auto-CPAP adherence, 18 (60%) had some use, and none had no use. Three intervention patients (12%) had recurrent events compared with 1 (2%, p=0.13) control patient. The vascular event rate was highest among sleep apnea patients with no CPAP use: none, 16%; some, 5%; acceptable adherence 0%; p=0.08.
Conclusions
Sleep apnea is common among acute TIA patients. It appears feasible to provide auto-CPAP in the acute TIA period. Larger studies should evaluate whether a strategy of diagnosing and treating sleep apnea can reduce recurrent vascular events after TIA.
An estimated 300,000 transient ischemic attacks (TIAs) occur annually in the United States.1 TIA patients are at increased risk of recurrent vascular events; 25% of TIA patients will have a cerebrovascular or cardiovascular event or death in the 90-days post-TIA, and over the long-term 11% per year will have a stroke, myocardial infarction or vascular death.2–4 Half of the recurrent events occur in the first two days post-TIA.3 Given that the recurrent event rate is highest early post-TIA, new interventions are needed that can be applied in the acute TIA period.
The treatment of sleep apnea may provide a novel therapeutic target to improve outcomes for patients with TIA. Sleep apnea occurs in one in five adults in the general population5 and in at least 60% of patients with stroke or TIA.6, 7 Auto-titrating continuous positive airway pressure (auto-CPAP) safely and effectively treats sleep apnea.8
The primary objective of this feasibility study was to evaluate: (1) the prevalence of sleep apnea among TIA patients, and (2) the adherence to auto-CPAP among TIA patients with sleep apnea. The secondary objectives were to compare the recurrent vascular event rate among TIA patients: (1) according to an intention-to-treat analysis, and (2) among patients with sleep apnea by auto-CPAP use category. This feasibility trial was designed as a randomized study of patients with acute TIA, comparing an intervention group who received a strategy of diagnosing and treating sleep apnea, and a control group who received diagnostic testing but who did not receive sleep apnea treatment.
METHODS
Sample
TIA patients cared for at three hospitals in Connecticut (December 2004 to February 2008) were included if they had: a TIA and were ≥45 years. Patients were excluded for: a known diagnosis of sleep apnea, respiratory distress requiring mechanical ventilation, oxygen-dependent chronic obstructive pulmonary disease, pregnancy, time from symptom onset to Emergency Department arrival >72 hours, life expectancy less than 6 months, non-English speaking status, or residence outside of Connecticut.
Randomization
Patients were randomly assigned to the intervention or control group in a 1 (control): 1.75 (intervention) ratio to ensure a sufficient number of patients in the intervention group given that a primary outcome was CPAP adherence. A randomization scheme was developed prior to the start of the study. Because patients were often recruited and randomized in the evening hours, our randomization system could not involve a call to the coordinating center (which operated only during regular daytime office hours). For this reason, the randomization system was converted into a package of sealed envelopes which the field staff opened in consecutive order. Randomization was not stratified by any baseline patient characteristic.
Baseline Measurements
A baseline assessment included a medical record review, an interview of patients and/or proxies, and physical measurements. The following data were collected at baseline: demographics; comorbidities; medications; blood pressure; heart rate; neck circumference; height; and weight. The ABCD2 score, a system used to predict short-term recurrent event rates among patients with TIA, was calculated from: age (≥60 years, 1 point); blood pressure (systolic ≥140 mm Hg or diastolic ≥90 mm Hg, 1 point); symptom type (unilateral weakness with or without speech impairment, 2 points; speech impairment without weakness, 1 point); symptom duration (≥60 minutes, 2 points, 10–59 minutes, 1 point), and a history of diabetes (1 point).3 The care the patients received to evaluate the etiology of the TIA and to prevent recurrent vascular events was measured and included the following: brain imaging, evaluation of the carotid arteries, echocardiography use, smoking cessation counseling, antithrombotic medication use, statin medication use, and angiotensin converting enzyme inhibitor or angiotensin receptor blocker medication use. Excessive daytime sleepiness was defined as a score of 10 or higher on the Epworth Sleepiness Scale.9
Control Group Protocol
Control patients received portable unattended polysomnography at baseline and at 90-days post-TIA (Figure 1). The primary care providers of the control patients were notified of the polysomnography results, but control patients were not given CPAP as part of the study. Although it was theoretically possible for control patients with sleep apnea to receive CPAP, none of them actually received CPAP during the three-month study period.
Figure 1.
Research Design
Polysomnography was performed by a trained staff member at the patient’s bedside. The patients were not moved off their unit if they were admitted to the hospital. The polysomnography included: electroencephalogram (EEG); respiratory inductive plethysmography (RIP); body position; electrocardiogram (ECG); blood pressure; and oxygen saturation.10 We used portable unattended polysomonography (LifeShirt, Vivometrics, Ventura, California) because it has been validated against the gold standard of polysomnography in a sleep laboratory, has been successfully used in clinical trials of sleep apnea, and can be used throughout the hospital or at a patients’ residence.11
Diagnosis of Sleep Apnea
Apnea was defined as an airflow cessation of ≥10 seconds and hypopnea was defined as a reduction in airflow of ≥10 seconds or decrease in amplitude of breathing by ≥30%, followed by an oxygen desaturation of ≥4%.12–14 The apnea-hypopnea index (AHI) was calculated from the sum of the number of apneas and hypopneas per hour of sleep. Sleep apnea was diagnosed when the AHI ≥5.13
Intervention Group Protocol
All intervention patients received auto-CPAP. The traditional approach to the treatment of sleep apnea is to use fixed pressure CPAP. This approach requires a two step process. First a sleep study is performed to diagnose sleep apnea. Then CPAP titration is performed by a sleep therapist who increases the fixed pressure CPAP until the apneas and hypopneas are eliminated. This approach is usually conducted on two nights. In cases where patients have many apneas and hypopneas, a “split-night” study can be conducted where both the diagnostic component and the CPAP titration can be performed on the same evening. Whether or not a “split-night” study is performed, the use of fixed-pressure CPAP requires that the patient have a sleep study in the presence of a sleep technician. Auto-CPAP machines eliminate respiratory disturbances by varying the pressure delivered depending on the patients’ respiratory efforts. Auto-CPAP compared with fixed pressure CPAP is equally efficacious, but has improved patient adherence and comfort.15, 16 We used auto-CPAP because it is equally effective as fixed pressure CPAP and because it does not require the presence of a sleep therapist for titration.
The auto-CPAP machine (AutoSet Spirit, ResMed, Poway, California) measured and stored information about: respiratory events (apneas and hypopneas); patient use (minutes used, nights used); mask leak; and pressure delivered. Auto-CPAP adherence was categorized as: no use; any use (more than no use but <4 hours/night for <75% of nights); or acceptable adherence (≥4 hours/night for ≥75% of nights).8, 17
The machine was interrogated after two nights. If there was no evidence of airflow limitation (AHI< 5 or median pressure <6 cmH20), then the auto-CPAP was discontinued. If there was evidence of airflow limitation, then the auto-CPAP was continued for the remainder of the 90-day period (Figure 1). Intervention patients received information about sleep apnea, the benefits of CPAP treatment, and instructions about CPAP machine use daily for two days, weekly for the first month, and then monthly. Research staff made visits to refit masks or to troubleshoot equipment as needed. Because polysomnography, and not auto-CPAP, is the gold standard method of diagnosing sleep apnea, intervention patients received unattended portable polysomnography at 90-days post-TIA (Figure 1).
Outcomes
The primary outcomes were: (1) sleep apnea prevalence at baseline (measured by polysomnography among control patients), and (2) the proportion of TIA patients with sleep apnea with acceptable auto-CPAP adherence. The secondary outcome was the 90-day post-TIA recurrent vascular events rate. Recurrent vascular events included the following: recurrent TIA, stroke, acute myocardial infarction, hospitalization for congestive heart failure, and death.2
Adverse Events
All adverse events were reviewed by a panel that included an internist, a neurologist, and a pulmonologist. The panel classified each adverse event as serious or non-serious and as related or unrelated to the study intervention.
Analysis Plan
Descriptive statistics (e.g., mean with standard deviations, medians, and proportions) were used to describe the baseline characteristics and outcomes. To compare differences in the outcome rates between control and intervention patients and among sleep apnea patients with varying auto-CPAP use, Chi-square tests or Fisher’s exact tests were used for binary or ordinal variables, and Student’s t-tests or Wilcoxon rank sum tests for dimensional variables. Two-sided p-values were used to evaluate differences between the intervention and control groups. A p-value of 0.05 was used to determine statistical significance. An intention-to-treat analysis was performed comparing the recurrent vascular event rate among intervention patients compared with control patients. A secondary pre-specified analysis of the recurrent vascular event rates was conducted among patients with sleep apnea according to the amount of auto-CPAP use.
This project was designed to enroll a sufficient number of subjects to ensure face validity for our feasibility assessments. It was not powered to identify differences in vascular events between patients in the intervention versus control groups. No imputations were made for missing data. All analyses were conducted using SAS 9.1 (Cary, North Carolina). This study was registered with clinical trials.gov (NCT00251290) and was approved by all of the participating center’s human subjects committees.
RESULTS
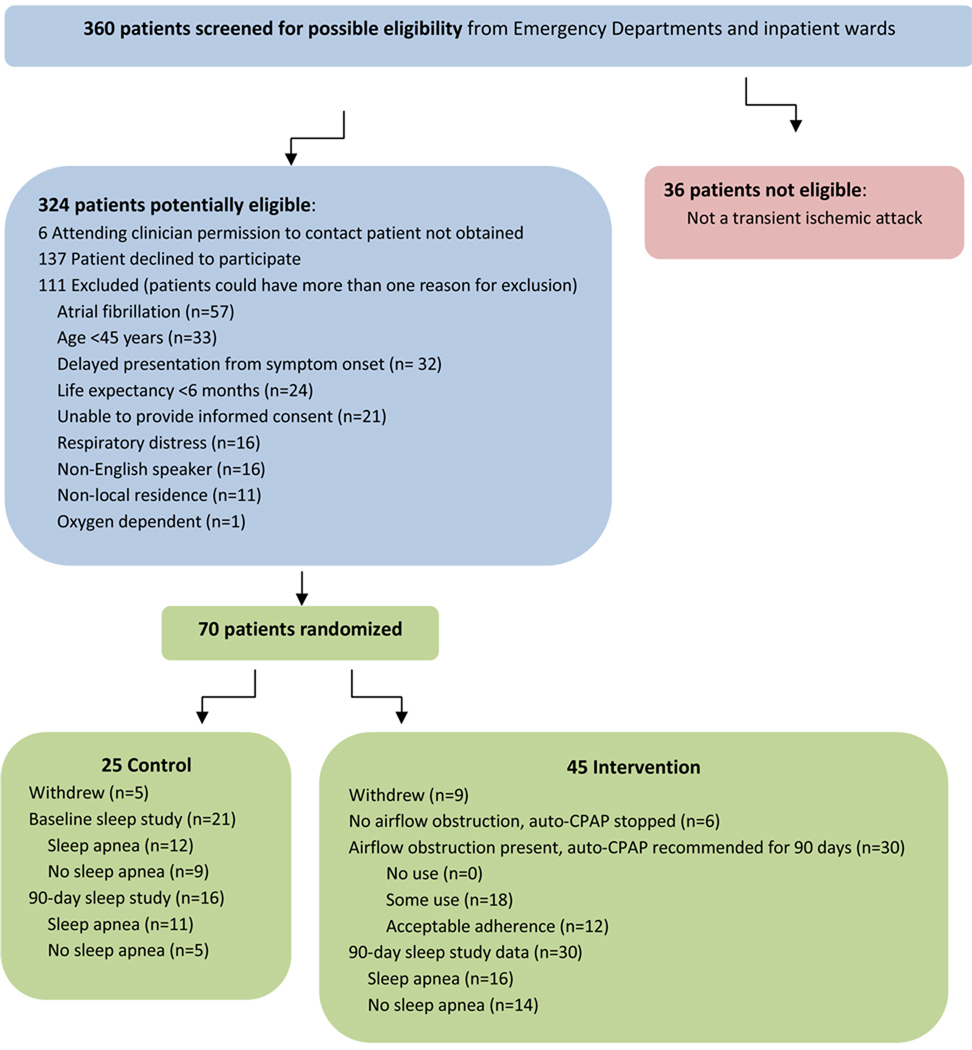
A total of 360 patients were screened for possible eligibility, 36 did not have a TIA, 6 could not be contacted because permission to do so was not received by their attending clinician, 137 patients declined to participate, and 111 met at least one exclusion criterion (Figure 2). A total of 70 patients were enrolled: intervention (N=45) and control (N=25). Among these, 9/45 (20%) were lost to follow-up in the intervention group and 5/25 (20%) were lost in the control group. Among the 45 intervention patients, 36 used the auto-CPAP machine and 6 of these had no evidence of airflow obstruction based on the AHI or the pressure recordings, and therefore, had the CPAP discontinued; leaving 30 intervention patients eligible for ongoing CPAP use. Incomplete sleep study data resulted either from poor quality electroencephalography (EEG) data or from patient refusal to perform or complete the sleep study. Complete unattended polysomnography data were available in 21/25 (84%) control patients at baseline and 16/25 (64%) control patients at 90-days and 30/45 (67%) intervention patients at 90-days (Figure 2).
Figure 2.
Patient Screening and Recruitment Diagram
Table 1 provides a comparison of baseline characteristics among the patients in the intervention versus control groups. There were no statistically significant differences in the baseline characteristics of the two groups although the control patients had higher rates of prior stroke and prior TIA (Table 1). The majority of patients received auto-CPAP within 48 hours after the TIA symptom onset: <24 hours, 8/45 (18%); ≥24<48 hours, 27/45 (60%); and ≥48 hours, 10/45 (22%). The concomitant care rates were similar between the two groups although the control patients received less echocardiography (Table 1).
Table 1.
Baseline Characteristics (N=70)
| Characteristic | Intervention (N=45) |
Control (N=25) |
P-Value* | ||
|---|---|---|---|---|---|
| Age (years): range | 47–88 | 45–88 | - | ||
| Mean ± standard deviation | 66.3 ± 11.9 | 67.4 ± 12.8 | 0.73 | ||
| White race: N (%) | 35 | (77.8) | 16 | (64.0) | 0.21 |
| Female gender: N (%) | 22 | (48.9) | 13 | (52.0) | 0.80 |
| Past Medical History: N (%) | |||||
| Hypertension | 33 | (73.3) | 16 | (64.0) | 0.41 |
| Hyperlipidemia | 26 | (57.8) | 10 | (40.0) | 0.15 |
| Diabetes mellitus | 15 | (33.3) | 6 | (24.0) | 0.41 |
| Stroke | 7 | (15.6) | 5 | (20.0) | 0.74 |
| Transient ischemic attack | 7 | (15.6) | 7 | (28.0) | 0.23 |
| Atrial fibrillation | 6 | (13.3) | 1 | (4.0) | 0.41 |
| Myocardial infarction | 5 | (11.1) | 1 | (4.0) | 0.41 |
| Congestive heart failure | 3 | (6.7) | 1 | (4.0) | 1.00 |
| Chronic obstructive pulmonary disorder | 2 | (4.4) | 0 | (0) | 0.53 |
| Current tobacco smoking: N (%) | 2 | (8.0) | 8 | (17.8) | 0.48 |
| Blood pressure (mmHg): Mean ± standard deviation | |||||
| Systolic | 140.3 ± 21.8 | 142.0 ± 16.9 | 0.75 | ||
| Diastolic | 78.3 ± 12.5 | 82.1 ± 11.5 | 0.21 | ||
| Neck circumference (inches): Mean ± standard deviation | 15.6 ± 1.2 | 15.3 ± 1.4 | 0.40 | ||
| Waist circumference (inches): Mean ± standard deviation | 41.2 ± 3.4 | 40.3 ± 5.5 | 0.48 | ||
| Weight (pounds)): Mean ± standard deviation | 179.5 ± 27.5 | 178.7 ± 42.8 | 0.94 | ||
| Body Mass Index (kg/m2): Mean ± standard deviation | 28.6 ± 4.1 | 27.8 ± 6.5 | 0.59 | ||
| Excessive daytime sleepiness: N (%) | 17 (37.8) | 7 (28.0) | 0.41 | ||
| ABCD2 Score† | 1.67 ± 1.0 | 1.72 ± 0.94 | 0.96 | ||
| Symptom duration: Mean ± standard deviation | 3.9 ± 5.3 | 4.3 ± 7.8 | 0.79 | ||
| Time from symptom onset to CPAP or sleep study (hours): Mean ± standard deviation |
39.4 ± 23.7 | 45.0 ± 37.1 | 0.50 | ||
| <24 hours: N (%) | 8 | (18) | 4 | (16) | - |
| ≥24 hours, <48 hours: N (%) | 27 | (60) | 15 | (60) | - |
| ≥48 hours: N (%) | 10 | (22) | 6 | (24) | - |
| Concomitant Care: N (%) | - | - | - | ||
| Admission brain imaging received | 45 (100) | 25 (100) | 1.00 | ||
| Antithrombotic therapy | 40 (89) | 24 (96) | 0.41 | ||
| Smoking cessation counseling for smokers | 4/5 (80) | 0/2 (0) | 0.14 | ||
| Carotid imaging received | 37 (82) | 17 (68) | 0.17 | ||
| ACEI/ARB medication received* | 23 (51) | 11 (44) | 0.57 | ||
| Statin medication received | 23 (51) | 10 (40) | 0.46 | ||
| Echocardiography received | 42 (93) | 19 (76) | 0.06 | ||
P-values refer to two-sided p-values obtained either from Student’s t-tests, Wilcoxon tests, chi-square tests, or Fisher’s exact tests.
The ABCD2 score is a score used to predict short-term recurrent event rates among patients with TIA.
ACEI/ARB refers to an angiotensin converting enzyme inhibitor or receptor blocking medication.
Sleep Apnea Prevalence
The majority of TIA patients had sleep apnea, both at baseline (12/21 [57%] control patients, and at 90-days, 27/46 (59%) control and intervention patients (Table 2). The control and intervention groups had similar rates of sleep apnea based on polysomnography at 90-days: control, 11/16 (69%) and intervention 16/30 (53%; p=0.31).
Table 2.
Prevalence of Sleep Apnea*
| Characteristic | Baseline (N=21) |
90-Day (N=46) |
|---|---|---|
| Apnea Hypopnea Index (AHI): range | 0–36.7 | 0–61.6 |
| Mean ± standard deviation | 11.1 ± 11.7 | 11.0 ± 13.2 |
| Sleep Apnea Prevalence: N (%) | 12/21 (57) | 27/46 (59) |
| Central Event AHI: range | 0–1.6 | 0–35.3† |
| Mean ± standard deviation | 0.41 ± 0.59 | 1.88 ± 7.36 |
| Total Sleep Time (hours:minutes): range | 1:10–9:16 | 0:46–10:48 |
| Mean ± standard deviation | 5:51 ± 2:33 | 5:60 ± 1:54 |
| Mean Oxygenation (%): range | 92–97 | 90–98 |
| Mean ± standard deviation | 94.4 ± 1.6 | 94.7 ± 1.6 |
| Oxygen Desaturation Index ≥4% | 0.2–16.8 | 0–54.8 |
| Mean ± standard deviation | 3.4 ± 4.8 | 3.5 ± 8.1 |
The prevalence of sleep apnea is based on polysomnography data. The diagnosis of sleep apnea was made if the apnea hypopnea index was ≥5.
We observed frequent central events in only one intervention patient with severe sleep apnea (AHI of 61.6) who also had frequent obstructive events.
Among control patients who had complete polysomnography data at both baseline and 90-days: 6/14 (43%) had sleep apnea at both time points, 2/14 (14%) did not have sleep apnea at both time points, 4/14 (29%) had no sleep apnea in the acute TIA period but sleep apnea present after 90-days, and 2/14 (14%) had sleep apnea in the acute TIA period but not after 90-days. Therefore, in 8/14 (57%) patients their diagnostic categorization was stable but in 6/14 (43%) patients their diagnostic categorization changed from the acute TIA period to 90-days post-TIA. The AHI from the acute TIA period ranged from 0.5 to 36.7 among patients with stable diagnoses and ranged from 0.0 to 7.5 among patients with a change in diagnosis. Therefore, an AHI of >7.5 could be used to identify patients who are unlikely to change their diagnostic category.
Among the 30 intervention patients with complete polysomnography and auto-CPAP data, the diagnostic agreement was: agreement in 15/27 (55%) patients, with a positive predictive value of 55% and a negative predictive value of 57%.
Auto-CPAP Use
Among the 30 intervention patients who had evidence of airflow obstruction based on data from the auto-CPAP machine, acceptable adherence was observed in 12 (40%), some use in 18 (60%), and none with no use.
Recurrent Vascular Events
When examining the effect of auto-CPAP on the recurrent vascular event rate according to an intention-to-treat analysis (including both patients with and without sleep apnea and including patients in the intervention group who did not use CPAP), we observed that 3/25 (12%) of control patients had a recurrent vascular event compared with 1/45 (2%) of intervention patients (p=0.13; Table 4). Among patients with sleep apnea at baseline, the recurrent vascular event rate was highest among patients with no CPAP use: no use, 1/12 (16%); some use, 1/18 (6%); acceptable adherence, 0/12 (0%); p=0.34; Table 4). Among patients with any evidence of sleep apnea, either from the auto-CPAP machine or from unattended polysomnography either at baseline or at 90-days post-TIA (this approach allows for the classification of the most number of patients because it uses all possible data), the recurrent vascular event rate was highest among patients with no CPAP use: no use, 3/19 (16%); some use, 1/21 (5%); acceptable adherence, 0/13 (0%); p=0.08.
Adverse Events
Serious adverse events were observed among 4/25 (16.0%) control patients and 7/45 (15.6%) intervention patients (p=1.0). The four serious events among the control group included a new stroke in two patients and recurrent TIAs in two patients. The serious events among the seven intervention patients included: a new stroke in two patients; a hospitalization for atrial fibrillation complicated by hypotension in one patient; a hospitalization for hyperglycemia in one patient; a hospitalization for hypoglycemic in one patient; one patient who was hospitalized once for congestive heart failure and once with dehydration; and one patient who was hospitalized for acute renal failure and also for radiotherapy for cancer. No serious adverse events were related to the intervention.
Non-serious adverse events were observed among 1/25 (4.0%) control patient and 5/45 (11.1%) intervention patients (p=0.41). The non-serious adverse events that were related to the study intervention included skin irritation due to the mask (n=2) and sneezing/nasal irritation due to the CPAP (n=2).
DISCUSSION
Our findings indicate that sleep apnea is common among this selected group of acute TIA patients, and that it is feasible to implement a strategy of using auto-CPAP in the acute TIA period to diagnose and treat sleep apnea. The recurrent vascular event rate seen in this cohort of TIA patients (12% among the controls) was lower than the expected 25% event rate. 2–4 This study was designed to assess the feasibility of diagnosing and treating sleep apnea among patients with acute TIA. The study was designed to have adequate sample size, and therefore, adequate statistical power to address the feasibility aims; it was not designed to have adequate power to evaluate the efficacy of auto-CPAP in the prevention of recurrent vascular events among post-TIA patients. Although the recurrent event rate was lower among intervention patients in this study, this difference did not reach statistical significance, perhaps because this study was not powered to detect this difference.
The prevalence of sleep apnea that was observed in this study is similar to rates observed in prior studies. 6, 7 For example, Parra, et al. studied 39 TIA patients and found that 62% had sleep apnea based on an AHI of >10.6 Moreover, we found that although the overall prevalence remains fairly stable over the first three months post-TIA (acute, 57% versus 90-days, 59%), the diagnostic categorization of an individual patient may shift from the acute TIA period to the 90-day period. Our results indicate that an AHI of >7.5 can identify patients with sleep apnea in the acute TIA period who will continue to have sleep apnea after 90 days.
The auto-CPAP use observed in this study was similar to some prior reports in non-TIA patients, but higher than those reported several studies of CPAP use among patients with stroke.7, 18, 19 It may be that TIA patients have higher adherence than stroke patients because TIA patients do not have neurological impairments that could interfere with regular CPAP use. We might also have observed a higher adherence because our study staff visited with patients to encourage auto-CPAP use and to help patients overcome any difficulties they may have experienced with the equipment. The auto-CPAP use analysis excluded patients who did not have sleep apnea and patients who withdrew from the study; this approach increases the relative adherence rate, but is the standard analytic practice.7 The 20% withdrawal rate among intervention patients serves to potentially bias our results in favor of reporting a relatively higher auto-CPAP adherence. Despite this limitation, the adherence that was observed was better than expected given that a small minority of patients were symptomatic with excessive daytime sleepiness.
Despite a lower than expected recurrent vascular event rate, our finding that increasing auto-CPAP use was associated with a lowered recurrent vascular event rate (albeit not statistically significantly so) is consistent with the findings of Martinez-Garcia, and colleagues who found among patients distant from a stroke or TIA, that the recurrent vascular event rate was 7% among CPAP users (n=15) and 36% among patients without CPAP use (N=36; p=0.03).19 The mechanisms by which CPAP may reduce recurrent vascular events post-TIA are unknown, but may include lowering blood pressure, improving oxygenation, decreasing platelet activation, improved left-ventricular ejection fraction which may contribute to improve cerebral perfusion, or lowering fibrinogen.20–22 Future studies should confirm the effectiveness of CPAP in the acute TIA period and should also be directed at examining the mechanism by which CPAP may improve outcomes. Future trials that evaluate post-TIA interventions should design their sample size with the 12% event rate that we observed (as opposed to the 25% event rate described in cohort studies). 2–4
One noteworthy aspect of this research design is the application of an intervention not based on patient symptoms but rather based on estimated disease prevalence within a group. Specifically, we did not provide the intervention strategy only to patients with daytime sleepiness or other symptoms consistent with sleep apnea, but rather provided the intervention to all stroke patients randomized to the intervention group. The rationale for this approach was based on (1) the prior literature6, 7 that has demonstrated that sleep apnea is common among TIA patients, (2) the safety profile of the intervention in routine clinical practice, and (3) the need to provide treatment as early as possible in the acute TIA period to achieve maximal benefit. Our findings suggest that this approach is feasible and may improve patient outcomes. An alternative research design would have been to use sham CPAP for patients randomized to the control arm. Sham CPAP was not used in this study because the “usual care” control arm approach provided a scientifically sound comparison while enhancing the feasibility of conducting the study and ensuring patient safety.
Future studies are needed to answer several key questions that relate to the implementation of this strategy within routine clinical practice: is there a group of patients for whom the auto-CPAP intervention is most beneficial and a group for whom it is unlikely to be helpful; how long should the auto-CPAP be continued; at what point, if ever, should a formal polysomnography be performed; and for TIA patients who nap during the day and sleep at night, should the auto-CPAP be used both during the day and at night?
The primary strength of this study’s design was that it was a randomized controlled comparison of usual care versus a strategy of diagnosing and treating sleep apnea in the acute TIA period. We sought to include a broad range of patients to enhance the generalizability of these findings. Despite these strengths, the following limitations require attention. First, although the patients were recruited from a Department of Veterans Affairs medical center, a large tertiary care center, a moderately large community hospital, and physician offices in one county; these findings should be replicated in a larger multi-site study that is powered to detect differences in the recurrent vascular event rate.
Second, we employed an approach using auto-CPAP early post-TIA without first using diagnostic polysomnography. We found relatively poor diagnostic agreement between the auto-CPAP machine and the polysomnography (55% agreement). This strategy provides for the earliest possible administration of auto-CPAP therapy. Given the safety of auto-CPAP and the relatively high prevalence of sleep apnea, this approach is likely to provide the earliest treatment to the largest number of patients with low risk of harm. Future studies will have to address the issue of whether auto-CPAP is beneficial to patients despite this diagnostic inaccuracy.
Third, although the losses to follow-up were equal in both groups, 20% losses in each arm was higher than we expected. Any conclusions drawn from this study must be made in the context of this withdrawal rate.
Despite these limitations, these research findings suggest that the strategy of diagnosis and treating sleep apnea among selected acute TIA patients may be a novel therapeutic strategy to improve patient outcomes. Future studies are needed to confirm these findings. Given that no specialized systems are needed to implement CPAP therapy, this intervention may have the potential to be applicable to most TIA patients regardless of the size or complexity of the facility at which they receive their care.
Table 3.
Auto-titrating Continuous Positive Airway Pressure Use
| Auto-titrating Continuous Positive Airway Pressure (auto-CPAP) Use Category |
Eligible Intervention Patients* (N=30) |
|---|---|
| Proportion of nights used: range | 0.02–0.88 |
| Mean ± standard deviation | 0.47 ± 0.33 |
| Number of hours/night CPAP used: range (hours) | 1.5–8.5 |
| Mean ± standard deviation | 5.6 ± 1.9 |
| CPAP Use: N (%) | - |
| None: 0 nights or 0 hours/night | 0 (0) |
| Some: <70% nights for <4 hours/night | 18 (60) |
| Excellent: ≥70% nights for ≥4 hours/night | 12 (40) |
Eligible intervention patients included the (N=30) patients who had evidence of sleep apnea from the auto-titrating continuous positive airway pressure (auto-CPAP) machine.
Table 5.
Recurrent Vascular Events
| Outcome | Overall (Intention-To-Treat) N=70 |
Sleep Apnea† (By Auto-CPAP Use Category) N=42 |
||||
|---|---|---|---|---|---|---|
| Control (N=25) |
Intervention (N=45) |
P-value | None (N=12) |
Some (N=18) |
Acceptable (N=12) |
|
| Vascular Events* | 3 (12) | 1 (2) | 0.13 | 1 (8) | 1 (6) | 0 (0) |
Vascular events included the following: recurrent transient ischemic attacks; stroke; myocardial infarction; hospitalization for congestive heart failure; or any death.
The adherence analysis only included sleep apnea patients, including 12 control patients in the “none” group.
ACKNOWLEDGMENTS
Drs. Bravata, Yaggi, Lo and Agostini received support from career development awards from the Department of Veterans Affairs (VA) Health Services Research and Development Service, Rehabilitation Research and Development Service, and Clinical Science Service. Dr. Fried was supported by the NIH (K24 AG028443). Dr. Concato was supported by the VA Cooperative Studies Program. This work was funded by the Claude D. Pepper Older Americans Independence Center at Yale (P30AG21342 NIH/NIA), the Robert Wood Johnson Generalist Physician Faculty Scholars Award Program, the ResMed Foundation, a pilot grant from the VA Cooperative Studies Program Clinical Epidemiology Research Center, the Max Patterson Stroke Research Fund at Yale, and a grant from VA HSR&D (IIR-06-233). The authors had full access to all of the data and take responsibility for the integrity and accuracy of the analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Johnston S. Transient Ischemic Attack. NEJM. 2002;347:1687–1692. doi: 10.1056/NEJMcp020891. [DOI] [PubMed] [Google Scholar]
- 2.Johnston S, Gress D, Browner W, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284:2901–2906. doi: 10.1001/jama.284.22.2901. [DOI] [PubMed] [Google Scholar]
- 3.Johnston S. Short-term prognosis after a TIA: a simple score predicts risk. Cleveland Clinic Journal of Medicine. 2007;74(10):729–736. doi: 10.3949/ccjm.74.10.729. [DOI] [PubMed] [Google Scholar]
- 4.Rothwell P, Johnston S. Transient ischemic attacks: stratifying risk. Stroke. 2006;37(2):320–322. doi: 10.1161/01.STR.0000200555.89117.d2. [DOI] [PubMed] [Google Scholar]
- 5.Young T, Peppard P, D G. Epidemiology of obstructive sleep apnea: a population health perspective. American Journal of Respiratory & Critical Care Medicine. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 6.Parra O, Arboix A, Bechich S, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. American Journal of Respiratory & Critical Care Medicine. 2000;161:375–380. doi: 10.1164/ajrccm.161.2.9903139. [DOI] [PubMed] [Google Scholar]
- 7.Bassetti C, Aldrich M. Sleep Apnea in Acute Cerebrovascular Diseases: Final Report on 128 Patients. Sleep. 1999;22:217–223. doi: 10.1093/sleep/22.2.217. [DOI] [PubMed] [Google Scholar]
- 8.Giles T, Lasserson T, Smith B, White J, Wright J, Cates C. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD001106.pub2. CD001106. [DOI] [PubMed] [Google Scholar]
- 9.Johns M. Daytime Sleepiness, Snoring, and Obstructive Sleep Apnea: The Epworth Sleepiness Scale. Chest. 1993;103:30–36. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 11.Goodrich S, Orr W. An investigation of the validity of the Lifeshirt in comparison to standard polysomnography in the detection of obstructive sleep apnea. Sleep Medicine. 2009;10(1):118–122. doi: 10.1016/j.sleep.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Sleep Disorders Atlas Task Force. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep Medicine. 1992;15(2):173–184. [PubMed] [Google Scholar]
- 13.Loube D, Gay P, Strohl K, Pack A, White D, Collop N. Indications for positive airway pressure treatment of adult obstructive sleep apnea patients: a consensus statement. Chest. 1999;115:863–866. doi: 10.1378/chest.115.3.863. [DOI] [PubMed] [Google Scholar]
- 14.Meoli A, Casey K, Clark R, Coleman J, Fayle R, Troell R, Iber C. Hypopnea in sleep-disordered breathing in adults. Sleep Medicine. 2001;24(4):469–470. [PubMed] [Google Scholar]
- 15.Massie C, McArdle N, Hart R, Schmidt-Nowara W, Lankford A, Hudgel D, Gordon N, Douglas N. Comparison between automatic and fixed positive airway pressure therapy in the home. American Journal of Respiratory & Critical Care Medicine. 2003;167(1):20–23. doi: 10.1164/rccm.200201-022OC. [DOI] [PubMed] [Google Scholar]
- 16.Fitzpatrick M, Alloway C, Wakeford T, MacLean A, Munt P, Day A. Can patients with obstructive sleep apnea titrate their own continuous positive airway pressure? American Journal of Respiratory & Critical Care Medicine. 2003;167(5):716–722. doi: 10.1164/rccm.200204-360OC. [DOI] [PubMed] [Google Scholar]
- 17.Marin J, Carrizo S, Vicente E, Agusti A. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. 365. 2005;9464:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 18.Hsu C, Vennelle M, Li H, Engleman H, Dennis M, Douglas N. Sleep-disordered breathing after stroke: a randomised controlled trial of continuous positive airway pressure. J Neurol Neurosurg Psychiatry. 2006;77:1143–1149. doi: 10.1136/jnnp.2005.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Garcia M, et al. Continuous positive airway pressure treatment in sleep apnea prevents new vascular events after ischemic stroke. Chest. 2005;128(4):2123–2129. doi: 10.1378/chest.128.4.2123. [DOI] [PubMed] [Google Scholar]
- 20.White J, Cates C, Wright J. Continuous positive airways pressure for obstructive sleep apnoea. Cochrane Database of Systematic Reviews. 2002;4 doi: 10.1002/14651858.CD001106. [DOI] [PubMed] [Google Scholar]
- 21.Bradley T, Floras J. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 22.Yasuma F. Cheyne-Stokes respiration in congestive heart failure: continuous positive airway pressure of 5–8 cm H2O for 1 year in five cases. Respiration. 2005;72:198–201. doi: 10.1159/000084053. [DOI] [PubMed] [Google Scholar]