Abstract
Purpose:
To build Monte Carlo (MC) models of two ultrasound (US) probes and to quantify the effect of beam attenuation due to the US probes for radiation therapy delivered under real-time US image guidance.
Methods:
MC models of two Philips US probes, an X6-1 matrix-array transducer and a C5-2 curved-array transducer, were built based on their megavoltage (MV) CT images acquired in a Tomotherapy machine with a 3.5 MV beam in the EGSnrc, BEAMnrc, and DOSXYZnrc codes. Mass densities in the probes were assigned based on an electron density calibration phantom consisting of cylinders with mass densities between 0.2 and 8.0 g/cm3. Beam attenuation due to the US probes in horizontal (for both probes) and vertical (for the X6-1 probe) orientation was measured in a solid water phantom for 6 and 15 MV (15 × 15) cm2 beams with a 2D ionization chamber array and radiographic films at 5 cm depth. The MC models of the US probes were validated by comparison of the measured dose distributions and dose distributions predicted by MC. Attenuation of depth dose in the (15 × 15) cm2 beams and small circular beams due to the presence of the probes was assessed by means of MC simulations.
Results:
The 3.5 MV CT number to mass density calibration curve was found to be linear with R2 > 0.99. The maximum mass densities in the X6-1 and C5-2 probes were found to be 4.8 and 5.2 g/cm3, respectively. Dose profile differences between MC simulations and measurements of less than 3% for US probes in horizontal orientation were found, with the exception of the penumbra region. The largest 6% dose difference was observed in dose profiles of the X6-1 probe placed in vertical orientation, which was attributed to inadequate modeling of the probe cable. Gamma analysis of the simulated and measured doses showed that over 96% of measurement points passed the 3%/3 mm criteria for both probes placed in horizontal orientation and for the X6-1 probe in vertical orientation. The X6-1 probe in vertical orientation caused the highest attenuation of the 6 and 15 MV beams, which at 10 cm depth accounted for 33% and 43% decrease compared to the respective (15 × 15) cm2 open fields. The C5-2 probe in horizontal orientation, on the other hand, caused a dose increase of 10% and 53% for the 6 and 15 MV beams, respectively, in the buildup region at 0.5 cm depth. For the X6-1 probe in vertical orientation, the dose at 5 cm depth for the 3-cm diameter 6 MV and 5-cm diameter 15 MV beams was attenuated compared to the corresponding open fields to a greater degree by 65% and 43%, respectively.
Conclusions:
MC models of two US probes used for real-time image guidance during radiotherapy have been built. Due to the high beam attenuation of the US probes, the authors generally recommend avoiding delivery of treatment beams that intersect the probe. However, the presented MC models can be effectively integrated into US-guided radiotherapy treatment planning in cases for which beam avoidance is not practical due to anatomy geometry.
Keywords: Monte Carlo, ultrasound imaging, ultrasound probes, MV imaging
1. INTRODUCTION
With the increasing complexity of treatment plans for hypofractionated external beam radiation therapy (EBRT), real-time image guidance assuring accurate treatment beam delivery to the defined target volume is becoming critical. Multiple approaches for real-time IGRT that can be added onto existing clinical linear accelerators (Linacs) have been proposed, such as radiographic tracking of gold markers implanted in the target using a set of x-ray tubes1 or electromagnetic tracking of ferromagnetic transponders.2 Other novel techniques for real-time IGRT incorporating magnetic resonance imaging (MRI) with various EBRT delivery systems have also been considered. The ViewRay™ system (ViewRay, Inc., Oakwood, OH) consists of a MRI scanner and a gantry with three 60Co radiotherapy sources and it has already been introduced to the clinic.3 On the other hand, the MRI-Linac project that aims to combine a MRI scanner with a Linac in various arrangements is still under development by multiple groups.4–6 While the above-mentioned MRI-EBRT systems also provide 3D images of the patient anatomy together with real-time target positioning, which could serve as a useful tool for adaptive radiotherapy, they may be prohibitively expensive for the majority of clinics worldwide.
Our group has developed a minimally interfering robotic ultrasound (US) imaging system for real-time, volumetric, soft tissue imaging that can be integrated with existing Linacs.7–10 The system consists of an US imaging probe attached to a robotic manipulator that autonomously maintains contact of the US probe with the patient while avoiding Linac collisions. An early version of the system has been used for tracking of prostate motion on healthy volunteers,7,8 while a later version is currently being investigated for motion tracking of the liver, the kidneys, and the diaphragm.9,10 Other groups have also investigated robotic US-guided radiotherapy and applied it for imaging of canine prostate, liver, and pancreas using markers,11 for liver tracking due to respiratory motion in healthy volunteers,12 and for imaging of the beating heart.13
Up to date, little attention has been paid to the possible change in dose distributions due to the interference of the US probes with the radiation beam. Hsu et al.14 and Wu et al.,15 demonstrated that prostate dose distributions are not affected by the presence of the US imaging probe when the radiation beam does not intersect the probe. Zhong et al. performed a liver SBRT treatment planning study and showed that, with no or little plan quality degradation, 80% of liver treatment plans can be delivered solely with beams not interfering with an US probe placed at the point on the patient’s skin closest to the internal target.16 Zhong et al. also concluded that real-time US-guided RT might be difficult to perform for large and/or superficial liver tumors.
The aim of this work was to build a full Monte Carlo (MC) model of two commercially available phased-array US probes, respectively, suitable for real-time 2D and 3D intrafractional imaging, and to investigate the effects of beam attenuation should the probes interfere with the treatment beam during radiation delivery. Due to the large metal component of the active volume of the US probes and their associated kilovoltage CT artifacts, the MC models were built based on a megavoltage CT scan, in which metal streaking artifacts are greatly suppressed.17 The MC models were validated with dose attenuation measurements in 6 and 15 MV photon beams. Once the MC models are incorporated into a treatment planning software, the interaction of the probe with the treatment beam can be taken into account, which can help to evaluate the strategy of delivering radiotherapy beams directly through intrafractional US guidance hardware.
2. MATERIALS AND METHODS
An X6-1 3D/4D matrix-array transducer and a C5-2 2D curved-array transducer (both Philips, Eidhoven, The Netherlands) were studied for prospective use in real-time US-guided radiotherapy. In order to evaluate treatment beam attenuation through the probes during radiotherapy, MC models of the probes were built. The MC models were validated with dose attenuation measurements for (15 × 15) cm2 6 and 15 MV photon beams delivered by a Trilogy linear accelerator (Varian Medical, Palo Alto, CA). To cover each end of the range of probe orientations feasible during intrafractional US-guided treatment, a horizontal probe orientation (with probe parallel to the patient’s skin) and a vertical probe orientation (probe perpendicular to patient’s skin) were considered. The MC models of the probes can then be used for dose attenuation calculations for intermediate probe orientations.
2.A. Monte Carlo simulations
MC modeling of the X6-1 and C5-2 US probes was performed in the EGSnrc/DOSXYZnrc (V4 2.4.0) code18,19 based on a megavoltage (MV) CT scan of the probes, since manufacturer’s blueprints were not available.
2.A.1. MV CT imaging
MV CT images of the US probes were acquired with the 3.5 MV beam of a Tomotherapy machine (Accuray, Sunnyvale, CA). A CT electron density calibration phantom (model 062M, CIRS, Norfolk, VA) containing tissue-like inserts as well as titanium (mass density ρ = 4.51 g/cm3) and steel rods (ρ = 8.05 g/cm3) was inserted in the phantom [Fig. 1(a)]. The 512 × 512-pixel MV CT images were acquired with (0.76 × 0.76) mm2 in-plane voxel size and 1.0 mm slice thickness. The maximum MV CT number found in the image was 5478 for the steel inserts. The maximum MV CT number for the X6-1 and C5-2 probe was 2663 and 2985, respectively.
FIG. 1.
MC models of the X6-1 and C5-2 US probes was built based on a Tomotherapy 3.5 MV CT scan (a). Maximum-intensity projections of the US probes are shown in (b). MV CT scan of the CIRS electron density calibration phantom with metal inserts (c), from which the electron density calibration curve (d) was generated.
2.A.2. Probe modeling
The MV CT images of the US probes [Fig. 1(b)] were converted into mass density maps calculated by means of a MV CT number-to-relative electron density (ρe) calibration curve [Fig. 1(d)]. The MV CT number-to-relative electron density calibration curve was generated based on the MV CT scan of the CT electron density calibration phantom [Fig. 1(c)] and it was linear with R2 > 0.99. The mass density ρ of each voxel was calculated with ρ = 1.202 × ρe, a relationship found based on NIST data.20 The linear relationship was calculated based on elements with atomic numbers between 1 and 27 spanning mass densities of 8.5 × 10−4–8.9 g/cm3, which was the range of materials found in the US probes. The calibration curve was applied only on voxels corresponding to the US probes; voxels outside of the probes were assigned to air with mass density of 0.001 g/cm3. The maximum mass density for the X6-1 and the C5-2 probe was calculated to be 4.8 and 5.2 g/cm3, respectively.
Due to the predominant Compton interaction and the unknown material composition of the probes, only two material types were considered for EGSnrc MC modeling of the US probes. While air was used for voxels with MV CT numbers below −950, all other voxels were assigned to water. A more detailed material segmentation with air and four metallic materials uniformly distributed over the HU = [ − 950, 3000] range was also performed (Fig. S1 in the supplementary material21). No statistically significant dose differences between the two segmentation schemes were found beyond the buildup region. More specifically, dose differences between the two segmentation schemes were within statistical uncertainties for the 6 MV beam. 15 MV dose at 0.5 cm depth was by 4% higher when water-only segmentation was used compared to the implemented metal segmentation [Fig. S2 (Ref. 21)]. Since the MC model validation simulations were performed at 5 cm depth, well beyond the buildup region, the water material segmentation resulting in 40% shorter simulation time was chosen in this work.
2.A.3. Phase-space file generation and validation
First, 6 and 15 MV phase–space files for the (15 × 15) cm2 fields of the Trilogy accelerator containing 7.5 × 107 and 9.5 × 107 particles, respectively, were calculated at our institution22 in BEAMnrc (V4 2.4.0).23 For validation purposes, MC dose from the 6 and 15 MV beams was calculated in a (30 × 30 × 15) cm3 water phantom with (4 × 4 × 4) mm3 voxels. Normalization factors for conversion of MC dose per particle to dose per delivered MU were determined for both energies based on the open field dose measurements performed with a 2D ionization chamber array, which are described in Sec. 2.B.1. Additionally, in order to investigate small field dose attenuation due to the presence of US probes, phase–space files for a 3-cm diameter 6 MV beam to model lung treatment and for a 5-cm diameter 15 MV beam to model prostate treatment were generated.
2.A.4. Dose calculations
Dose deposited by 6 and 15 MV beams in a (30 × 30 × 15)cm3 water phantom in the presence of the US probes was calculated with the DOSXYZnrc code. The effect of beam attenuation due to the US probes placed in horizontal and vertical orientation on the surface of the phantom was modeled according to the experimental setup described in Sec. 2.B and shown in Fig. 2. A (30 × 30 × 15) cm3 block of water with a varying voxel size of 2–46 mm along the beam direction was modeled underneath the US probes. The water block voxel sizes in the plane perpendicular to the beam direction were derived from the MV CT scan of the US probes and were (0.76 × 1.0) mm2 for the horizontal and (0.76 × 0.76) mm2 for the vertical probe simulations.
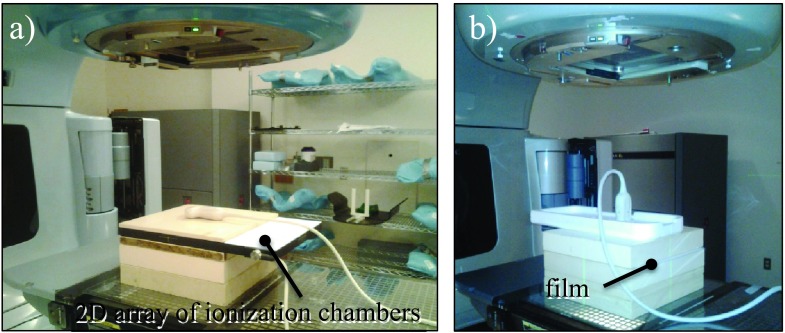
FIG. 2.
The MC models of the US probes were validated with dose measurements with 6 and 15 MV photon beams in the presence of the probe in horizontal (a) and vertical (b) orientations performed with a 2D array of ionization chambers (a) and radiographic films (b).
Dose deposited in the water phantom with the US probes in place was calculated with the 6 and 15 MV (15 × 15) cm2 phase-space files. Dose was also calculated for the 6 and 15 MV small circular beams that were incident on the distal tip of the probes in horizontal orientation and on the center of the X6-1 probe in vertical orientation. All photon and electron interactions relevant to megavoltage beam simulations were modeled, including triplet production. The cutoff energy for electron and photon generation and transport was 10 keV. In order to increase simulation efficiency, each particle was recycled 20 times and photon splitting number of 5 was used, which resulted in a statistical uncertainty in high-dose regions of 1.5% for the simulated 1.6 × 109 histories. The simulations were split into 20 batches and run on a cluster with 16 × 3 GHz Quad-Core Intel Xeon processors. The simulations took approximately 9 h on 20 CPUs.
2.B. Dose measurements
MC models of the US probes were validated by means of dose measurements in a solid water phantom using a 2D ionization chamber array and radiographic films on a Trilogy clinical linear accelerator. First, dose for the 6 and 15 MV (15 × 15) cm2 open fields was measured at 5 cm depth in a (30 × 30 × 15) cm3 solid water block in order to determine the MC-to-MU normalization factor. Next, the X6-1 and C5-2 probes were placed in horizontal orientation and the X6-1 probe was placed in vertical orientation on top of the solid water phantom and the dose from 6 and 15 MV beams was measured. Unless stated otherwise, dose measurements were performed with (15 × 15) cm2 fields at 5 cm depth with gantry and collimator at 0°. An overview of the measurements and MC simulations presented in this work is summarized in Table I.
TABLE I.
Overview of MC simulations and experimental measurements for the calculation of MC-to-MU normalization factor (open field) and for evaluation of dose attenuation in the presence of the X6-1 and C5-2 US probes placed in a horizontal and vertical orientation on top of the phantom (Fig. 2).
| X6-1 | C5-2 | |||
|---|---|---|---|---|
| Open field | Horizontal | Vertical | Horizontal | |
| 6 MV | MC, 2D array | MC, film, 2D array | MC, film | MC, film, 2D array |
| 15 MV | MC, 2D array | MC, film, 2D array | MC, film | MC, film, 2D array |
2.B.1. 2D ionization chamber array measurements
First, dose was measured with the 2D-ARRAY seven29 (PTW, Freiburg, Germany) for 6 and 15 MV open fields. Second, the dose for both US probes placed in horizontal orientation was measured for 6 and 15 MV beams [Fig. 2(a)]. The 2D array seven29 consists of 27 × 27 ionization chambers of (5 × 5 × 5) mm3 in size separated by 10 mm and covering an area of (27 × 27) cm2. The 2D array was placed at 5 cm depth in the solid water phantom and the US probes were positioned on top of the phantom with the center of the probe aligned with the center of the radiation field. The 6 and 15 MV beams were delivered with 100 MU. Additionally, the 2D array was used to evaluate dose attenuation of the 6 MV beam at 1.5 cm depth for both probes.
2.B.2. Film measurements
Attenuation of the X6-1 US probe in horizontal orientation was also measured with Kodak EDR2 radiographic films (Eastman Kodak Company Rochester, NY) with the setup used for the 2D array measurements at 5 cm depth. Additionally, 6 and 15 MV beam attenuation through the X6-1 probe placed in vertical orientation was measured with films. We focused our investigation on the X6-1 probe, the transducer providing real-time 3D images, as we expect it to be used in experiments for US-guided radiotherapy. The X6-1 probe was secured in vertical orientation using a 1-cm thick styrofoam slab with a density of less than 0.1 g/cm3, thus producing negligible attenuation of the 6 and 15 MV beams [Fig. 2(b)]. The cable, not fully included in the MC model, was put to the side of the probe. Films were chosen for the vertical X6-1 probe measurements due to the small (3 × 4) cm2 attenuated area of the probe that would cover only 12 ionization chambers in the 2D array.
The EDR2 films were calibrated for both 6 and 15 MV beams using 14 dose levels in the 0–3 Gy range with the step-wedge method. The films were scanned on a flatbed Epson Expression 10000 XL scanner (Epson, Long Beach, CA) with 72 dpi and converted into absorbed dose using their respective step-wedge calibration curves.
2.C. Comparison of Monte Carlo simulations and dose measurements
MC simulations were compared to dose measurements in three ways. First, calculated and measured beam profiles along the two major axes through the center of the probes were plotted. Second, gamma analysis with a 3 mm/3% criterion was performed for comparison of MC and film dose distributions in the RIT 113 analysis software (Radiological Imaging Technology, Inc., CO). To restrict the gamma analysis results to the probe attenuation, a region of interest (ROI) of (8.5 × 13) cm2 and (8 × 5) cm2 centered on the probe was selected for the horizontal and vertical probe dose comparison, respectively. Third, MC dose distributions were compared to 2D array dose measurements using local percent dose differences. The statistics of the comparison, also restricted to the ROI centered on the probe, was summarized.
3. RESULTS
The open field 2D array measurements and MC simulations revealed that the 6 and 15 MV beam MC-to-MU normalization factors were 8.15 × 1017 particles/(100 MU) and 1.92 × 1017 particles/(100 MU), respectively.
3.A. Comparison of MC simulations and dose measurements
Figure 3 demonstrates the differences in spatial resolution of the three dose evaluation techniques. The film dose measurements had the highest spatial resolution with pixel size of (0.35 × 0.35) mm2. The pixel size of the MC simulations was (0.76 × 1.00) mm2 and it was (10 × 10) mm2 for the 2D array. Dose distributions calculated by MC and measured by films and the 2D array are compared by means of dose profiles along the center of the probes in the in-plane and cross-plane directions and gamma analyses. Note that the X6-1 probe was measured and simulated in the horizontal and vertical orientation and the C5-2 probe was measured only in the horizontal orientation.
FIG. 3.

Dose distributions for the X6-1 probe and 6 MV beam calculated with MC (a) and measured with films (b) and 2D array (c). The difference in spatial resolution of the three dose distributions is evident.
3.A.1. X6-1 US probe
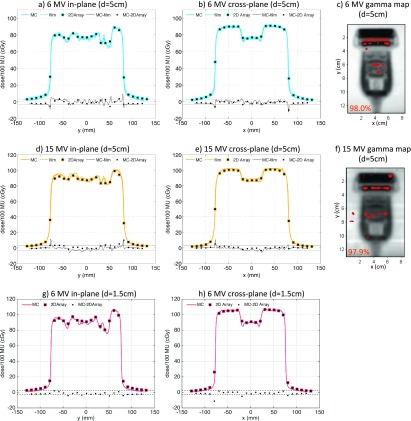
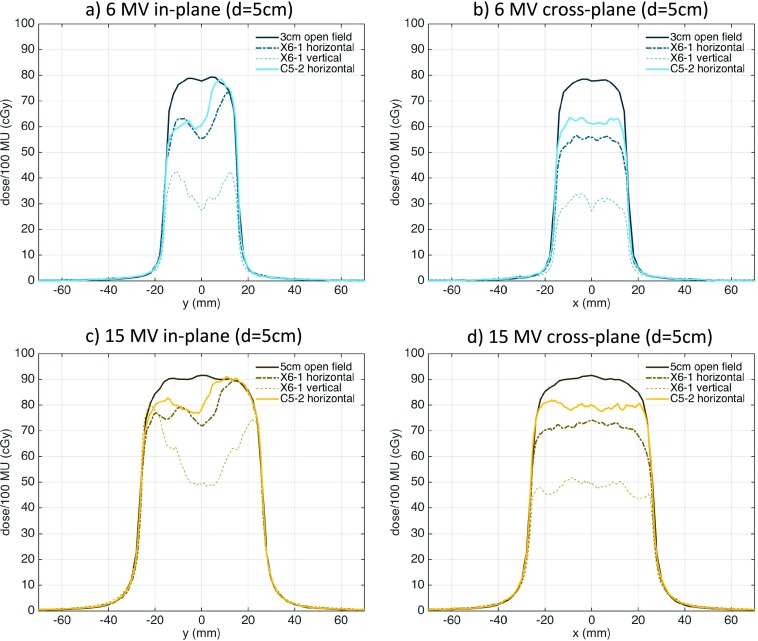
The horizontal orientation X6-1 probe dose profiles as calculated with MC and measured with films and the 2D array as well as gamma maps comparing MC simulations to film measurements are shown in Fig. 4. The differences between MC simulations and film and 2D array dose profiles presented in Fig. 4 indicated that most MC-simulated points were within 3% from measured points. Larger dose differences between MC simulations and measurements of up to 6% were observed in the penumbra region characterized by a large dose gradient.
FIG. 4.
Comparison of MC simulations and dose measurements for the X6-1 probe in horizontal orientation for 6 MV (top row—5 cm depth, bottom row—1.5 cm depth) and 15 MV (middle row—5 cm depth) beams. The in-plane [(a), (d), and (g)] and cross-plane [(b), (e), and (h)] dose profiles and dose differences are shown with the ±3% difference line plotted for reference. Gamma maps comparing MC simulations and film measurements with 3%/3 mm passing criteria (failing pixels with gamma >1 are shown) and the pixel percentage passing rates are shown for the 5-cm depth 6 MV (c) and 15 MV (f) beams.
The gamma maps presented in Fig. 4 demonstrate that more than 97% of pixels in the probe region of interest passed the 3%/3 mm criterion for both 6 and 15 MV beams. Failing pixels were mainly found in the regions with abrupt changes of high and low mass density.
Comparison of MC simulations and 2D array dose measurements are summarized in Table II. The 6 MV dose difference between MC and 2D array was (0.3% ± 1.3%), the maximum dose difference was 5.3%, and 95.7% of measurement points had a local dose difference of less than 3% for the 6 MV beam. The 15 MV dose difference between MC and 2D array was (−0.3% ± 1.4%), the maximum dose difference was 3.6%, and 96.6% of measurement points had a local dose difference of less than 3%.
TABLE II.
Statistics of local dose difference comparison at 5 cm depth between MC simulations and 2D array measurements for US probes in horizontal orientation.
| X6-1 | C5-2 | |||
|---|---|---|---|---|
| 6 MV | 15 MV | 6 MV | 15 MV | |
| Mean (%) | 0.3 | −0.3 | 0.1 | 0.0 |
| Standard deviation (%) | 1.3 | 1.4 | 1.2 | 1.2 |
| Max (%) | 5.3 | 3.6 | 5.9 | 4.3 |
| Voxels with <3% local difference | 95.7 | 96.6 | 97.4 | 99.1 |
The vertical orientation X6-1 probe dose profiles as calculated by MC and measured with films, as well as gamma maps comparing MC simulations to film measurements are shown in Fig. 5. The comparisons suffered from artifacts due to the incorrect MC modeling of the probe cable. This was mainly pronounced in the center of the probe and particularly in the cross-plane profiles, since the probe cable was moved to the side. The dose profiles in Fig. 5 indicate that dose differences up to 5% and 6% were observed in the 6 and 15 MV beam profiles, respectively. The gamma maps show that a large number of failing pixels were in the center of the probe or to the left of the probe due to the cable for both the 6 and 15 MV beams, as expected. The agreement between MC simulations and dose measurements was acceptable with 3%/3 mm gamma passing rate of >96%. The discrepancy between MC modeling and dose measurements was also investigated by shifting the probe cable to the other side of the probe. 2D-Array dose measurements at 5 cm depth for the X6-1 probe with its cable placed to the right and to the left showed dose differences of 4%–6% in the shadow of the cable [Fig. S3 (Ref. 21)]. This was consistent with the differences observed between film measurements and MC modeling, in which the cable was taken into account.
FIG. 5.
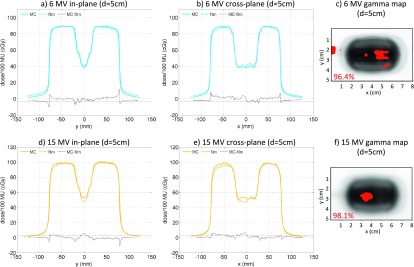
Comparison of MC simulations and dose measurements for the X6-1 probe in vertical orientation for 6 MV (top row) and 15 MV (bottom row) beams. The in-plane [(a) and (d)] and cross-plane [(b) and (e)] dose profiles and dose differences are shown with the ±3% difference line plotted for reference. Gamma maps comparing MC simulations and film measurements with 3%/3 mm passing criteria (failing pixels with gamma >1 are shown) and the pixel percentage passing rates are shown for the 6 MV (c) and 15 MV (f) beams. The effect of the incorrectly modeled cable probe is seen outside of the probe in (c) and it is seen towards the center in (f).
3.A.2. C5-2 US probe
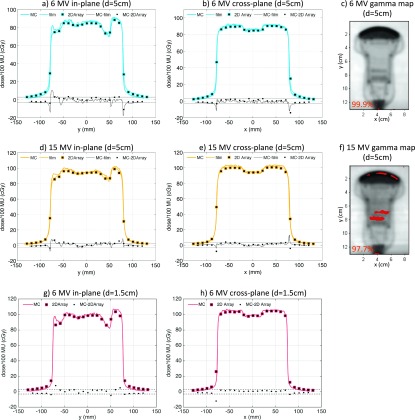
The horizontal orientation C5-2 probe dose profiles as calculated with MC and measured with films and the 2D array as well as gamma maps comparing MC simulations to film measurements are shown in Fig. 6. As demonstrated by the dose profiles and gamma maps, the agreement between MC simulations and film and 2D array measurements was good, with >97% of pixels passing the 3%/3 mm gamma criterion. The largest discrepancy of 5% was observed in the highly attenuating region of the probe for the 6 MV beam. Dose differences between MC simulations and film measurements were also observed at the end of the cable that was not fully modeled by MC.
FIG. 6.
Comparison of MC simulations and dose measurements for the C5-2 probe in horizontal orientation for 6 MV (top row—5 cm depth, bottom row—1.5 cm depth) and 15 MV (middle row—5 cm depth) beams. The in-plane [(a), (d), and (g)] and cross-plane [(b), (e), and (h)] dose profiles and dose differences are shown with the ±3% difference line plotted for reference. Gamma maps comparing MC simulations and film measurements with 3%/3 mm passing criteria (failing pixels with gamma >1 are shown) and the pixel percentage passing rates are shown for the 5-cm depth 6 MV (c) and 15 MV (f) beams.
The comparison of MC simulations and 2D array measurements is summarized in Table II. The 6 MV dose difference between MC and 2D array was (0.1% ± 1.2%), the maximum dose difference was 5.9%, and 97.4% of measurement points had a local dose difference of less than 3% for the 6 MV beam. The 15 MV dose difference between MC and 2D array was (0.0% ± 1.2%), the maximum dose difference was 4.3%, and 99.1% of measurement points had a local dose difference of less than 3%.
3.B. Depth dose attenuation due to US probes
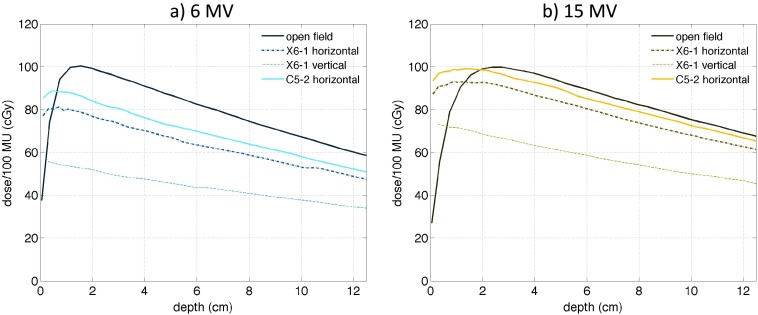
In order to evaluate photon beam attenuation during radiotherapy, MC depth dose curves calculated in the water phantom were also examined. Depth dose curves for open field 6 and 15 MV beams and depth dose curves in the presence of the X6-1 and C5-2 US probes are shown in Fig. 7. To demonstrate the highest possible beam attenuation for probes in horizontal orientation, depth dose curves corresponding to the highly attenuating parts of the probe were plotted.
FIG. 7.
MC-simulated depth dose curves for (15 × 15) cm2 6 MV (a) and 15 MV (b) beams for open field (solid thin line), the X6-1 probe in horizontal (dashed-dotted line), and vertical (dashed line) orientation, and for the C5-2 probe in horizontal orientation (thick solid line). Central-axis depth dose curves were plotted for the X6-1 probe in vertical orientation. In horizontal orientation, depth dose curves most attenuated by the probes at the probe distal tip were plotted.
Dose attenuation due to the presence of US probes at depths beyond the buildup region is evident for both 6 and 15 MV beams. As expected, the attenuation of the 6 MV beam was more pronounced with respect to the open field compared to the 15 MV beam. The open field 6 MV dose at 10 cm depth was decreased by 13%, 21%, and 43% due to the presence of the C5-2 probe in horizontal orientation and the X6-1 probe in horizontal and vertical orientation, respectively. The open field 15 MV dose at 10 cm depth was decreased by 3%, 9%, and 33% due to the presence of the C5-2 probe in horizontal orientation and the X6-1 probe in horizontal and vertical orientation, respectively.
Interestingly, the dose at shallow depths was higher in the presence of the US probes compared to open field due to the secondary electrons generated in the probes. The open field 6 MV dose at shallow depth of 0.5 cm was increased by 10% due to the presence of the C5-2 probe in horizontal orientation. The dose at 0.5 cm depth was decreased by 1% and 32% due to the presence of the X6-1 probe in horizontal and vertical orientation, respectively. The open field 15 MV surface dose increase was more pronounced. Dose at 0.5 cm depth was increased by 53%, 42%, and 13% due to the presence of the C5-2 probe in horizontal orientation and the X6-1 probe in horizontal and vertical orientation, respectively. The depth dose data for 0.5 and 10-cm depth for all studied scenarios are summarized in Table III.
TABLE III.
Dose (cGy) at 0.5 and 10 cm depths for open field and in the presence of US probes for 6 and 15 MV (15 × 15) cm2 fields and 100 MU.
| 6 MV | 15 MV | |||
|---|---|---|---|---|
| 0.5 cm depth | 10 cm depth | 0.5 cm depth | 10 cm depth | |
| Open field | 81 | 67 | 64 | 75 |
| C5-2 horizontal | 89 | 58 | 98 | 73 |
| X6-1 horizontal | 80 | 53 | 91 | 68 |
| X6-1 vertical | 55 | 38 | 72 | 50 |
3.C. Small field dose attenuation due to US probes
MC-simulated dose profiles at 5 cm depth for a 3-cm diameter 6 MV beam and a 5-cm diameter 15 MV beams for the X6-1 and C5-2 probes in horizontal orientation and the X6-1 probe in vertical orientation are plotted in Fig. 8. For the 3-cm diameter 6 MV beam, the presence of the C5-2 and X6-1 probes in horizontal orientation and the X6-1 probe in vertical orientation caused dose attenuation relative to open field of 22%, 28%, and 65%, respectively. For the 5-cm diameter 15 MV beam, the dose was attenuated by 13%, 20%, and 46% due to the C5-2 and X6-1 probe in horizontal orientation and the X6-1 probe in vertical orientation, respectively.
FIG. 8.
MC-simulated beam profiles for a 3-cm diameter 6 MV [(a) and (b)] and a 5-cm diamter 15 MV [(c) and (d)] beams for open field (solid thin line), the X6-1 probe in horizontal (dashed-dotted line) and vertical (dashed line) orientation, and for the C5-2 probe in horizontal orientation (thick solid line). In horizontal orientation, the beam was incident on the most attenuating part of the probe at the distal tip.
4. DISCUSSION
Two US probes, X6-1 and C5-2, were modeled with MC in the EGSnrc code and compared to 6 and 15 MV photon beam dose attenuation measurements performed with a 2D ionization chamber array and radiographic films. To evaluate the two extreme cases of dose attenuation due to the US probes, both probes were validated with dose measurements in horizontal orientation and the X6-1 probe was also validated in vertical orientation. While MC dose profiles through the center of the probes were generally within 3% from the measured profiles, the passing rates for gamma analysis with 3%/3 mm criteria comparing MC and film doses did not pass with 100%. Potential reasons for the observed dose differences, aside from the inaccurate US probe cable modeling, are discussed in the following paragraphs.
The models of the probes in horizontal orientation were derived directly from the MV CT images, since the probes were lying flat during both the MV CT imaging and the validation dose measurements. The model of the X6-1 probe in the vertical orientation could not be built by simply rotating the probe by 90°, since the axis of the probe is at a ∼2° angle with respect to the surface when the probe is lying flat. Some discrepancy between dose attenuation MC simulations and measurements of the X6-1 probe in vertical orientation could therefore be attributed to the potentially inaccurate rotation of the probe in the MC model as well as inaccurate positioning of the probe in vertical orientation during film dose measurements.
Discrepancies between MC simulations and films seen in the gamma analyses can be further attributed to the uncertainties in the MC modeling and film measurements. Note that EDR2 films have been reported to measure IMRT dose with 3% accuracy.24 Additionally, the accuracy of the MC models derived from Tomotherapy MV CT scans was limited by the spatial resolution of the scans. The modulation transfer function (MTF) of Tomotherapy MV CT imaging with the same protocol as used for US probe scanning was measured with a high-density ball-bearing [Figure S4 (Ref. 21)]. The in-plane spatial resolution was calculated to be 1.3 mm, which was in agreement with published data.25 The spatial resolution in the cross-plane direction was 3.6 mm, which was significantly lower than the 0.35-mm film resolution. We estimated that volume averaging effects in regions with detailed probe geometry containing alternating high-density and low-density parts could cause dose calculation differences between MC and films of ∼5%. The combined gamma analysis uncertainty between MC and measurements was therefore approximately 6%. However, the spatial resolution of the MC models of the probes will have a negligible effect on patient dose calculations, as commonly used patient dose calculation grids are on the order of 2–3 mm.26
Based on the film measurement and simulation uncertainty discussion, gamma analysis passing rates for a 5%/1 mm criteria comparing MC simulations and film measurements were evaluated and were presented together with the 3%/3 mm passing rates in Table S1.21 It can be seen that the passing rates improved significantly. For example, 100% of points pass the 5%/1 mm criteria for the C5-2 probe in horizontal orientation. Additionally, over 99% of points pass the 5%/1 mm criteria for the more complex and detailed X6-1 probe in horizontal orientation. Gamma function did not pass in the areas of steep dose gradients, as demonstrated in Figs. 4(c) and 4(e). The lowest 5%/1 mm passing rates of 98% and 99% were observed for the X6-1 vertical probe that can be partially explained by inaccurate probe positioning as described above.
It is important to note that the US probes do not only attenuate dose at depth, but they also serve as a “bolus” and increase the surface dose. This could potentially cause high dose to the skin and at shallow depths in tissue and must be accounted for in treatment planning should the treatment beam intersect the probe. We would like to remind the reader that water material segmentation of the US probes was performed in this work. We noted a <4% increase in dose at 0.5 cm depth when a more detailed material segmentation based on metals was performed, which could potentially result in underestimation of the 0.5 cm depth dose.
Since neither the blueprints nor the elemental composition of the probes were available, one could only assume what the probe elemental composition was. Two material segmentation schemes were investigated in this work: water-only segmentation and 4-metallic material segmentation. We demonstrated that beyond 3-cm depth, the dose differences between metal and water-only material assignment were within statistical uncertainties. Note that water-only geometry used water as the voxel material; however, the mass density of each voxel was assigned based on its MVCT number. Due to the differences in atomic numbers, the cross section of photoelectric effect differs significantly for a metal material and water. However, photoelectric effect becomes a significant interaction type only for low-energy x-rays and results in low-energy electrons and low-energy characteristic x-rays. The low-energy electrons have a short range in water in the order of 5 mm in the extreme and improbable case of a 1 MeV photoelectron released in a lead atom. As a result, dose discrepancies between the two segmentation schemes are seen only at shallow depths.
In this work, dose attenuation due to the presence of US probes was evaluated for large open fields and for smaller circular fields. We determined that dose attenuation due to the presence of US probes was more significant for smaller fields. For the X6-1 probe in vertical orientation, the 3-cm diameter 6 MV beam was attenuated by 65%, and it was attenuated by 43% in the (15 × 15) cm2 6 MV beam. On the other hand, the 5-cm diameter 15 MV beam was attenuated by 46%, and it was attenuated by 33% in the (15 × 15) cm2 15 MV beam. More complex plans with small segment apertures used for treatments will likely create different dose attenuation patterns compared to the presented large fields and small circular fields. The effects of the interaction of small segment apertures on dose attenuation will be investigated in future work.
We have determined that material composition of the US probes had no effect on MC dose calculations at depths beyond the buildup region. This offers an interesting possibility to include modeling of US probes in commercial treatment planning systems by adding boluses and over-riding their densities to the US probe densities. While this is a compelling idea, further research is needed due to the high number of US probe voxels of approximately 3 × 105. In order to achieve similar dose calculation accuracy, all voxels would have to be modeled by individual boluses, which might not be feasible.
US imaging probes used for image guided radiotherapy might potentially be receiving high doses of radiation. Our MC dose calculations showed that the maximum dose to the probes per 100 MU was 125 and 140 cGy for the probes in horizontal and vertical orientations, respectively, irrespective of photon beam energy and US probe type. The increased dose to the probes is due to combination of inverse square law and the increased attenuation in the high-density material of the probe.
Dose deposition in the ultrasound probes poses a question regarding the impact of radiation damage on probe performance, an important and complex issue since radiation damage to the probe depends on cumulative dose, dose rate, fractionation, as well as the metrics for damage. Our preliminary investigations indicate that for the purpose of tumor tracking, ultrasound image quality is not affected by the dose that a probe will receive over a representative 5 × 10 Gy SBRT course. However, the long term damage from such repeated courses remains to be evaluated.
Due to the 33%–43% beam attenuation due to the presence of the US probes and the potential difficulty to deliver treatment dose to deep-seated targets, we recommend that treatment beams not intersecting the probe be chosen during the treatment planning process. However, since dose discrepancies with the proposed MC model were generally under 3%, radiation can be delivered directly through the probe if (a) avoidance of the US probe is not clinically feasible due to anatomy geometry; (b) the probe position can be accurately reproduced during treatment (e.g., using robotics or tracking); (c) there is a better way to reproduce cable position. The probe cable generated 7% and 5% attenuation for the 6 and 15 MV beam, respectively, and should be kept outside of the treatment beam if possible. Treatment beam attenuation due to the probe cable could be modeled by MC, if the cable is attached to a fixture or made rigid and its position is reproducible.
The strategy of MC probe modeling as a way of accounting for the probe presence during beam delivery relies on the assumption that the planned imaging configuration (probe on the body) can be reproduced during treatment. For image guidance, the US probe will need to be tracked both during simulation and delivery and therefore its absolute position and orientation with regard to the delivery device should be reproducible. As to the patient body, high reproducibility of its position with respect to the device is routinely achieved, as this is a major requirement to assert that the delivered dose distribution matches the planned one.
We note that patient physiological motion such as breathing could cause US probe motion, which could result in large dose differences between the planned and delivered dose. If the US probe does move during treatment delivery, we could either interrupt the treatment or we could use MC to calculate the actual delivered dose with the new probe position and adapt the plan for future fractions. In principle, we could also apply 4D MC dose calculation techniques, for which framework already exists.27 However, 4D MC dose calculations are more complex and require further investigation.
MC models of two US probes were built and dose attenuation through the probes was evaluated for 6 and 15 MV large open fields and two smaller circular fields delivered by a Varian Trilogy Linac. The MC model can be used to assess the attenuation for other Linacs, for which the magnitude of the US probe effects might differ.
5. CONCLUSIONS
We have built Monte Carlo models of two ultrasound probes, the X6-1 matrix-array transducer and a C5-2 curved-array transducer, considered for the use in real-time US image guided radiotherapy. The models were built based on a MV CT scan performed on a Tomotherapy machine. The models were validated with dose attenuation measurements using 6 and 15 MV photon beams for both probes in horizontal orientation as well as for the X6-1 probe in vertical orientation. A good agreement with dose profile differences of less than 3% between dose measurements performed with a 2D array of ionization chambers and radiographic films and MC simulations was found for measurements of probes in horizontal orientation. Disagreement of less than <6% was observed for dose profile measurements for the X6-1 probe in vertical orientation due to inadequate modeling of the probe cable.
Depth dose attenuation due to the presence of the probes was investigated by means of MC simulations. The (15 × 15) cm2 open field dose at 10 cm depth was decreased by 43% and 33% for the 6 and 15 MV beams, respectively, due to the X6-1 probe in vertical orientation. The (15 × 15) cm2 open field dose at 0.5 cm depth was on the other hand increased by 10% and 53% for the 6 and 15 MV beams, respectively, due to the C5-2 probe in horizontal orientation.
The presented MC models of the US probes can now be incorporated into the treatment planning process of radiation therapy delivered under real-time US image guidance. This will enable us to account for the potentially significant alterations of patient dose distributions due to the interaction of the treatment beam with the US probe during the novel US image guided radiotherapy technique.
ACKNOWLEDGMENTS
The authors would like to thank Xin Chen for generating the Monte Carlo phase–space files. The authors wish to acknowledge the support from the NCI (No. R41CA174089).
REFERENCES
- 1.Shirato H., Shimizu S., Kitamura K., Nishioka T., Kagei K., Hashimoto S., Aoyama H., Kunieda T., Shinohara N., and Dosaka-Akita H., “Four-dimensional treatment planning and fluoroscopic real-time tumor tracking radiotherapy for moving tumor,” Int. J. Radiat. Oncol., Biol., Phys. 48, 435–442 (2000). 10.1016/S0360-3016(00)00625-8 [DOI] [PubMed] [Google Scholar]
- 2.Willoughby T. R., Kupelian P. A., Pouliot J., Shinohara K., Aubin M., Roach M., Skrumeda L. L., Balter J. M., Litzenberg D. W., and Hadley S. W., “Target localization and real-time tracking using the Calypso 4D localization system in patients with localized prostate cancer,” Int. J. Radiat. Oncol., Biol., Phys. 65, 528–534 (2006). 10.1016/j.ijrobp.2006.01.050 [DOI] [PubMed] [Google Scholar]
- 3.Mutic S. and Dempsey J. F., presented at the Seminars in Radiation Oncology, 2014. [DOI] [PubMed]
- 4.Lagendijk J. J., Raaymakers B. W., Raaijmakers A. J., Overweg J., Brown K. J., Kerkhof E. M., van der Put R. W., Hårdemark B., van Vulpen M., and van der Heide U. A., “MRI/Linac integration,” Radiother. Oncol. 86, 25–29 (2008). 10.1016/j.radonc.2007.10.034 [DOI] [PubMed] [Google Scholar]
- 5.Fallone B., Carlone M., Murray B., Rathee S., Stanescu T., Steciw S., Wachowicz K., and Kirkby C., “TU-C-M100F-01: Development of a Linac-MRI system for real-time ART,” Med. Phys. 34, 2547 (2007). 10.1118/1.2761342 [DOI] [Google Scholar]
- 6.Keall P. J., Barton M., and Crozier S., presented at the Seminars in Radiation Oncology, 2014. [DOI] [PubMed]
- 7.Schlosser J., Salisbury K., and Hristov D., “Telerobotic system concept for real-time soft-tissue imaging during radiotherapy beam delivery,” Med. Phys. 37, 6357–6367 (2010). 10.1118/1.3515457 [DOI] [PubMed] [Google Scholar]
- 8.Schlosser J., Salisbury K., and Hristov D., “Online image-based monitoring of soft-tissue displacements for radiation therapy of the prostate,” Int. J. Radiat. Oncol., Biol., Phys. 83, 1633–1640 (2012). 10.1016/j.ijrobp.2011.10.049 [DOI] [PubMed] [Google Scholar]
- 9.Schlosser J., “Robotic ultrasound image guidance for radiation therapy,” Ph.D. thesis,Stanford University, Stanford, CA, 2013. [Google Scholar]
- 10.Western C., Hristov D., and Schlosser J., “Ultrasound imaging in radiation therapy: From interfractional to intrafractional guidance” (unpublished). [DOI] [PMC free article] [PubMed]
- 11.Bell M. A. L., Sen H. T., Iordachita I., Kazanzides P., and Wong J., “In vivo reproducibility of robotic probe placement for a novel ultrasound-guided radiation therapy system,” J. Med. Imaging 1, 025001 (2014). 10.1117/1.JMI.1.2.025001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris E. J., Miller N. R., Bamber J. C., Symonds-Tayler J. R. N., and Evans P. M., “Speckle tracking in a phantom and feature-based tracking in liver in the presence of respiratory motion using 4D ultrasound,” Phys. Med. Biol. 55, 3363–3380 (2010). 10.1088/0031-9155/55/12/007 [DOI] [PubMed] [Google Scholar]
- 13.Bruder R., Ernst F., Schlaefer A., and Schweikard A., “Real-time PV tracking in 3D ultrasound of the beating heart,” Med. Phys. 36, 2804 (2009). 10.1118/1.3182643 [DOI] [Google Scholar]
- 14.Hsu A., Miller N., Evans P., Bamber J., and Webb S., “Feasibility of using ultrasound for real-time tracking during radiotherapy,” Med. Phys. 32, 1500–1512 (2005). 10.1118/1.1915934 [DOI] [PubMed] [Google Scholar]
- 15.Wu J., Dandekar O., Nazareth D., Lei P., D’Souza W., and Shekhar R., presented at the 28th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS’06, 2006. [DOI] [PubMed]
- 16.Zhong Y., Stephans K., Qi P., Yu N., Wong J., and Xia P., “Assessing feasibility of real-time ultrasound monitoring in stereotactic body radiotherapy of liver tumors,” Technol. Cancer Res. Treat. 12, 243–250 (2013). 10.7785/tcrt.2012.500323 [DOI] [PubMed] [Google Scholar]
- 17.Paudel M., Mackenzie M., Fallone B., and Rathee S., “Evaluation of metal artifacts in MVCT systems using a model based correction method,” Med. Phys. 39, 6297–6308 (2012). 10.1118/1.4754647 [DOI] [PubMed] [Google Scholar]
- 18.Kawrakow I. and Rogers D. W. O., “The EGSnrc code system: Monte Carlo simulation of electron and photon transport,” NRCC Report PIRS-701, 2006.
- 19.Walters B. R. B., Kawrakow I., and Rogers D. W. O., “DOSXYZnrc users manual,” NRCC Report PIRS-794 (rev C), 2007.
- 20.Hubbell J. H. and Seltzer S. M., Tables of X-Ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients (version 1.4). [Published online]. Available: http://physics.nist.gov/xaamdi. National Institute of Standards and Technology, Gaithersburg, MD (2004). [Google Scholar]
- 21.See supplementary material at http://dx.doi.org/10.1118/1.4929978 E-MPHYA6-42-023510 for the effect of material segmentation of US probes on Monte Carlo dose calculations.
- 22.Chen X., Bush K., Ding A., and Xing L., “Independent calculation of monitor units for VMAT and SPORT,” Med. Phys. 42, 918–924 (2015). 10.1118/1.4906185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers D. W. O., Walters B., and Kawrakow I., “BEAMnrc users manual,” NRCC Report PIRS-0509(A)RevK, 2006.
- 24.Shi C. and Papanikolaou N., “Analysis of the sources of uncertainty for EDR2 film-based IMRT quality assurance,” J. Appl. Clin. Med. Phys. 7, 1–8 (2006). 10.1120/jacmp.v7i2.2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meeks S. L., J. F. Harmon, Jr., Langen K. M., Willoughby T. R., Wagner T. H., and Kupelian P. A., “Performance characterization of megavoltage computed tomography imaging on a helical tomotherapy unit,” Med. Phys. 32, 2673–2681 (2005). 10.1118/1.1990289 [DOI] [PubMed] [Google Scholar]
- 26.Chetty I. J., Curran B., Cygler J. E., DeMarco J. J., Ezzell G., Faddegon B. A., Kawrakow I., Keall P. J., Liu H., Ma C. M. C., Rogers D. W. O., Seuntjens J., Sheikh-Bagheri D., and Siebers J. V., “Report of the AAPM Task Group No. 105: Issues associated with clinical implementation of Monte Carlo-based photon and electron external beam treatment planning,” Med. Phys. 34, 4818–4853 (2007). 10.1118/1.2795842 [DOI] [PubMed] [Google Scholar]
- 27.Keall P., Siebers J., Joshi S., and Mohan R., “Monte Carlo as a four-dimensional radiotherapy treatment-planning tool to account for respiratory motion,” Phys. Med. Biol. 49, 3639–3648 (2004). 10.1088/0031-9155/49/16/011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- See supplementary material at http://dx.doi.org/10.1118/1.4929978 E-MPHYA6-42-023510 for the effect of material segmentation of US probes on Monte Carlo dose calculations.