Abstract
Acute retinal necrosis is a viral syndrome characterized by a panuveitis with necrotizing retinitis that may be complicated by retinal detachment, vaso-occlusion, optic neuropathy, and other causes of decreased visual acuity. Polymerase chain reaction testing provides a rapid and sensitive method of identifying the viral etiology of acute retinal necrosis, which is most commonly caused by herpes simplex virus type 1, herpes simplex virus type 2, and varicella zoster virus. Prompt diagnosis and treatment is paramount to prevent further vision loss. We review the management of acute retinal necrosis including systemic, local intravitreal, and combination antiviral medications. We also discuss the appropriate and inappropriate use of corticosteroids, laser retinopexy, surgical therapy, and other adjunctive measures.
Introduction
Acute retinal necrosis (ARN) is a rare infectious viral uveitis syndrome that manifests as a necrotizing retinitis and may result in a devastating visual outcome if not accurately diagnosed and treated.1 The first report of this clinical entity was in 1971, but it was not until 1982 that Culbertson et al. reported the herpetic etiology of ARN2,3 Since this initial finding, further work has shown that ARN is caused by multiple members of the herpes family including varicella zoster virus (VZV), herpes simplex 1 and 2 (HSV-1, HSV-2), cytomegalovirus (CMV), and infrequently, Epstein-Barr virus (EBV).3–6
Clinical Features
ARN commonly causes an acute panuveitis syndrome that may ultimately affect multiple ocular tissues. Findings previously reported include anterior uveitis (granulomatous and non- granulomatous), vitritis (Figure 1), optic disk edema, occlusive vasculitis, necrotizing retinitis, and scleritis. Long-term sight threatening complications include retinal detachment in 50–75% of patients (Figure 2), optic atrophy, cystoid macular edema, retinal atrophy, macular hole, and epiretinal membrane formation.7 A genetic association has been shown in Caucasian patients who have the HLA-DQw7 antigen and the HLA-Bw62, DR4 phenotype, which suggests there may be an immune predisposition to developing ARN.8
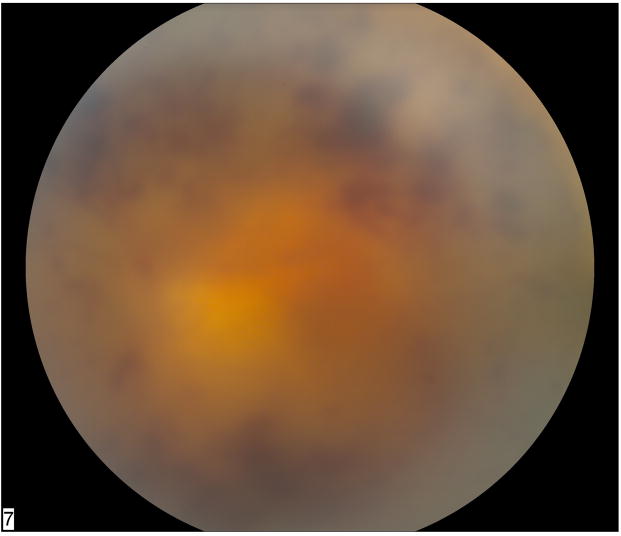
Figure 1.
Fundus photograph of left eye in a patient status post chemotherapy for metastatic prostate carcinoma shows dense vitritis with retinal whitening and hemorrhage consistent with acute retinal necrosis. Polymerase chain reaction of an anterior chamber aspirate was positive for HSV DNA.
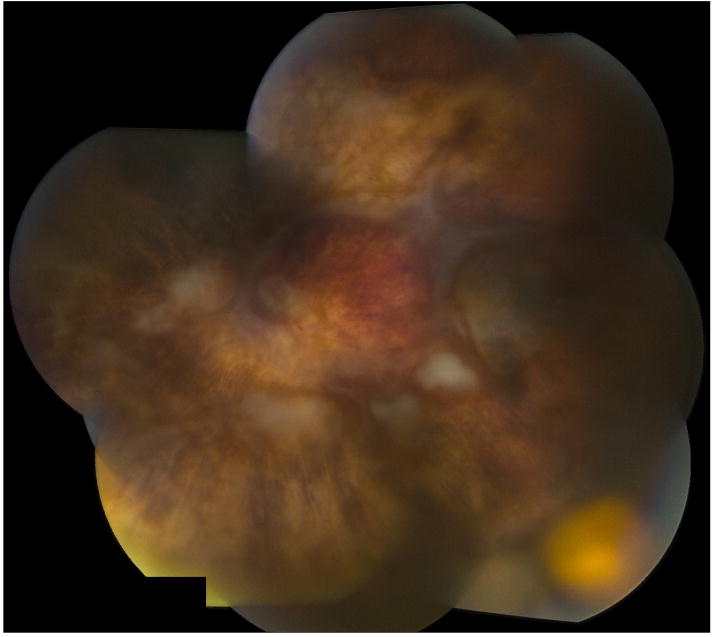
Figure 2.
Fundus photograph montage of left eye of patient with acute retinal necrosis shows that the retina is attached under silicone oil. There is a ganciclovir implant located in the inferotemporal quadrant and some residual areas of patchy retinal whitening.
Diagnostic Criteria
In 1994, the clinical diagnostic criteria of ARN was defined by the American Uveitis Society and includes anterior and posterior uveitis, peripheral retinal necrosis, occlusive vasculopathy, and disease progression without therapeutic intervention.9 Histological analysis of the retina in ARN shows inflammatory cell (lymphocytes and plasma cells) infiltration of the retina and around vascular networks with eosinophilic intranuclear inclusions suggestive of herpes virus particles.10
Diagnosis
Polymerase chain reaction (PCR) analysis or local antibody analysis (i.e. calculation of Goldmann-Witmer coefficient) from anterior chamber fluid or vitreous fluid can identify the particular virus causing ARN. Prior studies support the utility of PCR analysis with a sensitivity and specificity of greater than 90% in detecting VZV, HSV, and CMV (Figure 3). Given the developments in PCR and its usefulness in the diagnosis of ARN, PCR is currently the preferred method of viral diagnosis.11–15 It has been proposed to include laboratory data in the diagnostic criteria for ARN.16 Both qualitative and quantitative real-time PCR testing may be used both to ascertain the etiology of ARN and potentially to assess the response of ARN to therapy.17,18 The differential diagnosis for ARN includes other infectious and inflammatory processes such as syphilis, toxoplasmosis, cytomegalovirus retinitis, Behcet’s disease, pars planitis, sarcoidosis, and intraocular lymphoma.19
Figure 3.
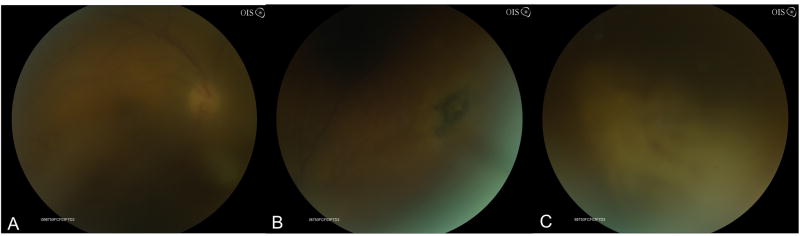
Fundus photograph of patient with HSV-2-ARN with moderate vitreous haze (A). Superonasally there was a patch of retinal pigment epithelial hyperpigmentation suggestive of toxoplasmosis or HSV-2 ARN, which characteristically shows pigmented chorioretinal scars (B). Inferonasally and nasally there are patches of confluent retinal whitening with hemorrhages and vasculitis (C).
Epidemiology
The exact incidence of ARN is unknown, but in 2007 the United Kingdom estimated that there is 1 case per 1.6 to 2.0 million population per year.20 In the United States, no data exists on the incidence of ARN. A report in 1988 predicted that there are 48,000 new cases of ocular HSV disease although the proportion of patients with retinal involvement was not defined.21 With the aging population and the increased number of individuals that are immunosuppressed this disease burden may increase. It is important to diagnose and treat ARN promptly and appropriately.22 Herein, we review the major advances in therapy for the management of ARN.
Treatment
Historically, treatment was supportive therapy with or without corticosteroids, which commonly led to progression of the retinitis, fellow eye involvement, and poor visual outcomes. With the discovery of the herpes family causing the retinitis, antiviral therapy emerged as well as other adjunct therapies including corticosteroids, aspirin, warfarin, prophylactic laser, and vitrectomy.
Systemic Therapy
The goals of systemic therapy are to inhibit viral replication and halt disease progression in the affected eye and prevent involvement of the unaffected eye. Systemic therapy for ARN includes intravenous and oral acyclovir, oral valacyclovir, famciclovir, and valganciclovir, and intravenous foscarnet and ganciclovir. Historically, ARN was treated initially with intravenous acyclovir (1500 mg/m2/day) for 5–10 days and transitioned to oral acyclovir (800 mg 5 times/day) for 4–6 weeks. Acyclovir therapy has been shown to decrease viral progression and prevent bilateral eye involvement.
Specifically, Palay et al. showed in his retrospective review that paraenteral acyclovir followed by oral acyclovir significantly decreased second eye involvement and that second eye disease was more likely to occur in the first 14 weeks after the initial infection. Patients treated with acyclovir developed bilateral disease in 12.9% of patients and patients that were acyclovir naive developed fellow eye disease in 69.6% of individuals.23,24,25 Given the development of more cost-effective drugs and improved pharmacokinetics of other oral agents with regards to their improved vitreous penetration, other therapies, particularly the val- esters may be preferable as first-line therapy in ARN.
Oral acyclovir has poor bioavailability in plasma when compared to valacyclovir (acyclovir pro-drug). Valacyclovir is capable of achieving plasma levels comparable to intravenous acyclovir making valacyclovir an excellent oral option for the treatment of ARN and avoid the need for hospitalization or home therapy for intravenous acyclovir.26,27 Huynh et al. demonstrated that oral valacyclovir (1 gram TID) can reach concentrations in the vitreous and achieve inhibitory ranges of HSV-1, HVS-2, and VZV.28 Furthermore, multiple case reports support valacyclovir’s use in ARN and it has been shown to be effective in halting disease progression and preventing second eye involvement.26,29–31 Aslanides et al. treated 3 patients with ARN with valacyclovir and prophylactic laser photocoagulation. Regression of the retinitis was seen in 4 days to 2 weeks with no contralateral eye involvement or mention of retinal detachment.30 Some case reports use valacyclovir 1 gram twice daily dosing while other use it three times daily. Valacyclovir at twice daily dosing increases the acyclovir bioavailability to 54% compared to 20% with acyclovir 800mg 5 times/day. In addition, treatment with valacyclovir reduces overall medical cost and decreases the loss of productivity cost in cost-consequence analysis.32
Another oral antiviral famciclovir (pro-drug for penciclovr) has been used to successfully treat ARN. In one case report famciclovir 500mg TID was effective in the treatment of a patient with VZV-ARN resistant to traditional acyclovir therapy.33 Oral famciclovir 500 mg TID can achieve vitreous concentrations to inhibit VZV, HSV-1, and HSV-2.33 Aizman et al. reported their treatment of four patients with valacyclovir and four with famciclovir. Patients in their series showed clinical improvement and regression of the retinitis in all patients as early as 4 days after initiation of treatment, no contralateral eye involvement with 30.7% of patients (3 patients) developing retinal detachment. He also used prophylactic laser retinopexy and oral steroids in select patients.26
Intravenous ganciclovir and valganciclovir (pro-drug of ganciclovir) have been used less frequently in the treatment of ARN and are the first line therapy in CMV retinitis.34,35 Valganciclovir has excellent bioavailability and has been shown to be as efficacious as intravenous ganciclovir for the treatment of CMV retinitis.36 Savant et al. reported improvement of vision from 6/12 to 6/6 with regression of the VZV-ARN within one week of treatment with valganciclovir 900 mg BID.37 Both intravenous ganciclovir and valganciclovir are used more commonly in CMV retinitis, CMV-associated ARN, and cases of ARN refractory to therapy. However, renal function and bone marrow suppression are considerations in patients treated with ganciclovir.
Intravenous foscarnet is another antiviral medication that may be used for the treatment of ARN. Typically foscarnet is reserved for patients who have failed traditional antiviral therapy. Foscarnet is a DNA polymerase inhibitor and does not depend on viral thymidine kinase for activation making it more effective in treating resistant strains. Rabbit models of intravenous foscarnet at 120 mg/kg did prove to achieve the 50% inhibitory concentration in the vitreous for CMV.38 Antiviral resistant strains of HSV in immunocompetent individuals is extremely rare (0.1–0.7%), but increases in immunocompromised patients (3.5–10%).39 Intravenous foscarnet is effective in treating viral retinitis resistant to traditional therapy. In one case report of a 17-year-old male with HSV-2 associated ARN resistant to acyclovir and ganciclovir, intravenous foscarnet was initiated and successfully halted the progression of retinitis.40,41
Intravitreal Therapy
Intravitreal therapy options for ARN include intravitreal foscarnet and ganciclovir. This provides direct and immediate therapy to the area of active infection, which may be necessary given the aggressive nature of ARN. Berthe et al. showed that no retinal toxicity was observed with single and repeat intravitreal foscarnet (2.4mg/0.1cc) injections by ophthalmic exam, histological analysis of the retina, and by electroretinogram in rabbit eyes.42 In addition, intravitreal pharmacokinetics of intravitreal foscarnet and ganciclovir have been studied in rabbit eyes. The mean inhibitory concentration for herpes viruses was reached up until 60 hours and 36 hours after treatment with intravitreal ganciclovir and intravitreal foscarnet respectively.43 A number of case reports support the use intravitreal foscarnet and ganciclovir in individuals resistant to initial systemic therapy or in patients with side effects leading to termination of antiviral agents.40,44–47
Combination systemic and intravitreal antiviral therapy
The combination of systemic and intravitreal therapy has increasingly reported as a treatment paradigm for patients with ARN. Flaxel et al. recently reported their single-center, interventional, comparative case series of 24 patients with ARN treated over a twenty-year period. Twelve patients received combination systemic and intravitreal antiviral therapy while 12 patients received systemic therapy alone. Patients receiving combination therapy showed a higher incidence of two-line-or-greater visual acuity and decreased incidence of retinal detachment and severe visual acuity loss to 20/200 or poorer when compared to patients who received systemic antiviral alone.1,48 Using a Cox regression model, factors contributing to the development of RD were also assessed in this work. Interestingly, an increased risk of RD was observed in patients receiving prednisone. Although this finding may seem counterintuitive, selection bias could be a factor as patients with greater degrees of inflammation and likely increased risk of RD would be more likely to require oral prednisone. Nonetheless, the 29% rate of RD observed in their series compared favorably to prior series that showed a RD rate varying from 17% to 85%.
Several other authors have evaluated patients who were treated with combination systemic and intravitreal antiviral therapy. Wong et al. demonstrated a decrease in the risk of retinal detachment by 67% in patients treated with systemic antivirals and intravitreal foscarnet injections.49 Meghpara et al followed 20 patients with ARN over a ten-year period and intravitreal injections were administered in 11 of 25 eyes. They observed that when stratified by quadrantic involvement (i.e. < 25%, 25–50%, or > 50% of retina affected), patients with moderate disease (i.e. 25–50% retina involved) did well with intravitreal therapy. Specifically, this cohort of patients benefited from stable or improved visual acuity. While these series showed improved functional and anatomic outcomes, Tibbetts et al. found that there was no statistically significant difference in the visual acuity and prevalence of retinal detachment in patients treated in the acyclovir-only era compared to the newer antiviral era. In addition 75% of patients had a visual acuity of 20/200 at 5-year follow with 50% developing retinal detachment.50 However, the minority of the patients in their series received intravitreal injections and further studies are needed to define whether intravitreal antiviral therapies are a primary therapy for all patients with ARN and the proper dosing regimen to optimize clinical outcomes.
Adjunctive Therapies
Aggressive antiviral therapy is the mainstay treatment for ARN. Corticosteroids, aspirin, warfarin, prophylactic laser, and vitrectomy are proposed adjuvant therapies for this condition.
Appropriate and Inappropriate Use of Corticosteroids
ARN characteristically features an intense inflammatory response manifesting as anterior uveitis and vitritis, as well as a necrotizing, obliterative vasculitis. Corticosteroids in the form of topical drops, oral preparations, and periocular and intravitreal injections are modalities for delivery of corticosteroids in ARN. Careful consideration must be given to the timing of corticosteroid administration. If initiated too early or without co-administration of systemic antiviral treatment it may potentiate viral replication and cause rapid progression of the retinitis.10,51–53
Oral corticosteroids are typically added to the treatment regimen at 24–48 hours after initiation of antiviral therapy. An interventional case series of 4 patients treated initially with valacyclovir (2 gram TID) and oral corticosteroids (1 kg/mg/day) with supplemental intravitreal triamcinolone acetonide (4mg/.1) after one week of treatment showed there was a decrease in the vitritis and improved vision with a final visual acuity of 20/40 in 3 of 4 patients.46,54 Other reports are unable to conclusively show an improvement in outcomes and the inflammatory response with the use of corticosteroids.50,55
Both the development of ARN de novo and the severe exacerbation of ARN following systemic and local corticosteroid administration are reminders of the importance of judicious use of corticosteroid-based therapies whenever an infectious viral uveitis is a diagnostic consideration. Weissman et al recently described a patient who developed bilateral central retinal artery occlusions associated with HSV-ARN. Following a suspected diagnosis of optic neuritis based on optic disc edema and headaches, the patient received three pulse doses of intravenous solumedrol. The patient developed a fulminant, bilateral acute retinal necrosis, vitreous hemorrhage, and combined tractional and rhegmatogenous retinal detachments. Despite aggressive therapy including hospitalization for intravenous antivirals and repeated intravitreal foscarnet and ganciclovir injections, bilateral severe vision loss to hand motions level developed at final follow-up.56 Other reports of severe cases of herpetic retinitis following corticosteroids include patients with presumptive optic neuritis, optic neuropathy, and seventh nerve palsy of unclear etiology.57,58,59 ARN has also been described in patients treated with intravitreal triamcinolone for choroidal neovascular membranes.60,61
Aspirin, Heparin, and Warfarin
The pathogenesis of ARN involves vascular occlusion manifesting as retinal ischemia.62 Hyperaggregation of platelets is reported in ARN and has been treated successful with corticosteroids and aspirin.63 Other therapies for anticoagulation include heparin and warfarin. Strong evidence does not currently exist, however, for the use of anticoagulation. In addition, the safety of these drugs in the context of other systemic diseases in each patient is an important consideration10,25
Surgical Considerations in Acute Retinal Necrosis
One of the most common complications in ARN is the development of atrophic retinal holes and rhegmatogenous retinal detachments (RRD) that develop secondary to retinal necrosis leading to retinal atrophy and vitreoretinal traction. Because of the resultant visual loss that may ensue following this complication, measures to prevent RD including laser retinopexy and early vitrectomy prior to RD development have been discussed as therapeutic options.
Prophylactic laser photocoagulation
Prophylactic laser photocoagulation has been used to prevent retinal detachment by creating strong chorioretinal adhesions posterior to the area of the involved retina.64 Meghpara et al. noted in their retrospective chart review that the 6 patients treated with prophylactic laser did not develop retinal detachments.65 Lau et al. demonstrated in their cohort of 22 patients that prophylactic laser retinopexy decreased the incidence of retinal detachment from 80% to 35.3% in the treatment group.55 While some cases series note decreased reports of retinal detachment others report minimal benefit.50,66,67 Factors that prevent laser photocoagulation in ARN patients are vitreous inflammation and the view of the posterior pole. This may bias the results of retrospective reviews where patients receiving laser therapy have less retinal involvement and vitreous inflammation which would favor better outcomes.10,64
Pars plana vitrectomy
Prophylactic vitrectomy has also been described for the prevention of RRD. Cases reports show variable outcomes with prevention in some series but of the failure to prevent RRD in others. In one retrospective review of 104 patients in which 48 eyes received a prophylactic vitrectomy at final follow up 52% of the retinas were attached in the vitrectomy group and 75% of the retinas were attached in the non-vitrectomy group.68–72
Conclusion
While diagnostic criteria for ARN have been defined previously, these criteria were largely based on clinical findings. PCR diagnostics have rapidly improved our ability to identify the precise herpetic etiology of ARN and initiate prompt therapy. A number of systemic and local antiviral therapies are available for ARN and include intravenous, oral, and intravitreal options. The val- esters of acyclovir and ganciclvoir achieve high vitreous levels when administered orally and have been increasingly used in clinical practice.
Although the destructive nature of the disease process may limit visual acuity outcomes and predispose patients to RD if they are not rapidly treated, recent advances in combination systemic and intravitreal antiviral therapy suggest therapeutic benefit. Outcome measures including visual acuity gain, avoidance of severe visual loss, and avoidance of RD should be measured in future clinical trials of antiviral therapy for ARN. When choosing a specific therapeutic regimen, multiple factors including the precise herpetic viral etiology, the immune status of the patient, concomitant medical morbidities, and response to therapy are essential to guide the management of each patient.
Acknowledgments
Sources of Funding
Supported in part by an unrestricted departmental grant (Department of Ophthalmology, Emory University School of Medicine) from Research to Prevent Blindness, Inc., New York, and by NIH/NEI core grant P30-EY06360 (Department of Ophthalmology, Emory University School of Medicine). Dr. Yeh is supported by an unrestricted Alcon Young Investigator Research Grant.
References
- 1.Flaxel CJ, Yeh S, Lauer AK. Combination systemic and intravitreal antiviral therapy in the management of acute retinal necrosis syndrome (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2013;111:133–144. [PMC free article] [PubMed] [Google Scholar]
- 2.Urayama AYN, Sasaki T. Unilateral acute uveitis with retinal periarteritis and detachment. Jpn J Clin Ophthalmol. 1971;25:607. [Google Scholar]
- 3.Culbertson WW, Blumenkranz MS, Haines H, Gass DM, Mitchell KB, Norton EW. The acute retinal necrosis syndrome. Part 2: Histopathology and etiology. Ophthalmology. 1982;89(12):1317–1325. doi: 10.1016/s0161-6420(82)34638-2. [DOI] [PubMed] [Google Scholar]
- 4.Schaal S, Kagan A, Wang Y, Chan CC, Kaplan HJ. Acute retinal necrosis associated with Epstein-Barr virus: immunohistopathologic confirmation. JAMA ophthalmology. 2014;132(7):881–882. doi: 10.1001/jamaophthalmol.2014.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverstein BE, Conrad D, Margolis TP, Wong IG. Cytomegalovirus-associated acute retinal necrosis syndrome. Am J Ophthalmol. 1997;123(2):257–258. doi: 10.1016/s0002-9394(14)71046-3. [DOI] [PubMed] [Google Scholar]
- 6.Walters G, James TE. Viral causes of the acute retinal necrosis syndrome. Curr Opin Ophthalmol. 2001;12(3):191–195. doi: 10.1097/00055735-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Wong RW, Jumper JM, McDonald HR, et al. Emerging concepts in the management of acute retinal necrosis. Br J Ophthalmol. 2013;97(5):545–552. doi: 10.1136/bjophthalmol-2012-301983. [DOI] [PubMed] [Google Scholar]
- 8.Holland GN, Cornell PJ, Park MS, et al. An association between acute retinal necrosis syndrome and HLA-DQw7 and phenotype Bw62, DR4. Am J Ophthalmol. 1989;108(4):370–374. doi: 10.1016/s0002-9394(14)73303-3. [DOI] [PubMed] [Google Scholar]
- 9.Holland GN. Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. Am J Ophthalmol. 1994;117(5):663–667. doi: 10.1016/s0002-9394(14)70075-3. [DOI] [PubMed] [Google Scholar]
- 10.Duker JS, Blumenkranz MS. Diagnosis and management of the acute retinal necrosis (ARN) syndrome. Surv Ophthalmol. 1991;35(5):327–343. doi: 10.1016/0039-6257(91)90183-g. [DOI] [PubMed] [Google Scholar]
- 11.McCann JD, Margolis TP, Wong MG, et al. A sensitive and specific polymerase chain reaction-based assay for the diagnosis of cytomegalovirus retinitis. Am J Ophthalmol. 1995;120(2):219–226. doi: 10.1016/s0002-9394(14)72610-8. [DOI] [PubMed] [Google Scholar]
- 12.Short GA, Margolis TP, Kuppermann BD, Irvine AR, Martin DF, Chandler D. A polymerase chain reaction-based assay for diagnosing varicella-zoster virus retinitis in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1997;123(2):157–164. doi: 10.1016/s0002-9394(14)71031-1. [DOI] [PubMed] [Google Scholar]
- 13.Knox CM, Chandler D, Short GA, Margolis TP. Polymerase chain reaction-based assays of vitreous samples for the diagnosis of viral retinitis. Use in diagnostic dilemmas. Ophthalmology. 1998;105(1):37–44. doi: 10.1016/s0161-6420(98)71127-2. discussion 44–35. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham ET, Jr, Short GA, Irvine AR, Duker JS, Margolis TP. Acquired immunodeficiency syndrome--associated herpes simplex virus retinitis. Clinical description and use of a polymerase chain reaction--based assay as a diagnostic tool. Arch Ophthalmol. 1996;114(7):834–840. doi: 10.1001/archopht.1996.01100140048006. [DOI] [PubMed] [Google Scholar]
- 15.Dabil H, Boley ML, Schmitz TM, Van Gelder RN. Validation of a diagnostic multiplex polymerase chain reaction assay for infectious posterior uveitis. Arch Ophthalmol. 2001;119(9):1315–1322. doi: 10.1001/archopht.119.9.1315. [DOI] [PubMed] [Google Scholar]
- 16.Takase H, Okada AA, Goto H, et al. Development and validation of new diagnostic criteria for acute retinal necrosis. Jpn J Ophthalmol. 2015;59(1):14–20. doi: 10.1007/s10384-014-0362-0. [DOI] [PubMed] [Google Scholar]
- 17.Bernheim D, Germi R, Labetoulle M, Romanet JP, Morand P, Chiquet C. Time profile of viral DNA in aqueous humor samples of patients treated for varicella-zoster virus acute retinal necrosis by use of quantitative real-time PCR. J Clin Microbiol. 2013;51(7):2160–2166. doi: 10.1128/JCM.00294-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugita S, Shimizu N, Watanabe K, et al. Use of multiplex PCR and real-time PCR to detect human herpes virus genome in ocular fluids of patients with uveitis. Br J Ophthalmol. 2008;92(7):928–932. doi: 10.1136/bjo.2007.133967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.S. NRaW. Uveitis Fundamentals and Clinical Practice. 4. 2010. p. 179. [Google Scholar]
- 20.Muthiah MN, Michaelides M, Child CS, Mitchell SM. Acute retinal necrosis: a national population-based study to assess the incidence, methods of diagnosis, treatment strategies and outcomes in the UK. Br J Ophthalmol. 2007;91(11):1452–1455. doi: 10.1136/bjo.2007.114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20(1):1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Pepose JS. The potential impact of the varicella vaccine and new antivirals on ocular disease related to varicella-zoster virus. Am J Ophthalmol. 1997;123(2):243–251. doi: 10.1016/s0002-9394(14)71042-6. [DOI] [PubMed] [Google Scholar]
- 23.Jeon S, Kakizaki H, Lee WK, Jee D. Effect of prolonged oral acyclovir treatment in acute retinal necrosis. Ocul Immunol Inflamm. 2012;20(4):288–292. doi: 10.3109/09273948.2012.689073. [DOI] [PubMed] [Google Scholar]
- 24.Palay DA, Sternberg P, Jr, Davis J, et al. Decrease in the risk of bilateral acute retinal necrosis by acyclovir therapy. Am J Ophthalmol. 1991;112(3):250–255. doi: 10.1016/s0002-9394(14)76725-x. [DOI] [PubMed] [Google Scholar]
- 25.Blumenkranz MS, Culbertson WW, Clarkson JG, Dix R. Treatment of the acute retinal necrosis syndrome with intravenous acyclovir. Ophthalmology. 1986;93(3):296–300. doi: 10.1016/s0161-6420(86)33740-0. [DOI] [PubMed] [Google Scholar]
- 26.Aizman A, Johnson MW, Elner SG. Treatment of acute retinal necrosis syndrome with oral antiviral medications. Ophthalmology. 2007;114(2):307–312. doi: 10.1016/j.ophtha.2006.06.058. [DOI] [PubMed] [Google Scholar]
- 27.Beutner KR. Valacyclovir: a review of its antiviral activity, pharmacokinetic properties, and clinical efficacy. Antiviral Res. 1995;28(4):281–290. doi: 10.1016/0166-3542(95)00066-6. [DOI] [PubMed] [Google Scholar]
- 28.Huynh TH, Johnson MW, Comer GM, Fish DN. Vitreous penetration of orally administered valacyclovir. Am J Ophthalmol. 2008;145(4):682–686. doi: 10.1016/j.ajo.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 29.Taylor SR, Hamilton R, Hooper CY, et al. Valacyclovir in the treatment of acute retinal necrosis. BMC Ophthalmol. 2012;12:48. doi: 10.1186/1471-2415-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aslanides IM, De Souza S, Wong DT, et al. Oral valacyclovir in the treatment of acute retinal necrosis syndrome. Retina. 2002;22(3):352–354. doi: 10.1097/00006982-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Emerson GG, Smith JR, Wilson DJ, Rosenbaum JT, Flaxel CJ. Primary treatment of acute retinal necrosis with oral antiviral therapy. Ophthalmology. 2006;113(12):2259–2261. doi: 10.1016/j.ophtha.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 32.Grant DM, Mauskopf JA, Bell L, Austin R. Comparison of valaciclovir and acyclovir for the treatment of herpes zoster in immunocompetent patients over 50 years of age: a cost-consequence model. Pharmacotherapy. 1997;17(2):333–341. [PubMed] [Google Scholar]
- 33.Figueroa MS, Garabito I, Gutierrez C, Fortun J. Famciclovir for the treatment of acute retinal necrosis (ARN) syndrome. Am J Ophthalmol. 1997;123(2):255–257. doi: 10.1016/s0002-9394(14)71045-1. [DOI] [PubMed] [Google Scholar]
- 34.Kanoff J, Sobrin L. New diagnosis and treatment paradigms in acute retinal necrosis. Int Ophthalmol Clin. 2011;51(4):25–31. doi: 10.1097/IIO.0b013e31822d6864. [DOI] [PubMed] [Google Scholar]
- 35.Patil AJ, Sharma A, Kenney MC, Kuppermann BD. Valganciclovir in the treatment of cytomegalovirus retinitis in HIV-infected patients. Clin Ophthalmol. 2010;4:111–119. doi: 10.2147/opth.s3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin DF, Sierra-Madero J, Walmsley S, et al. A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis. N Engl J Med. 2002;346(15):1119–1126. doi: 10.1056/NEJMoa011759. [DOI] [PubMed] [Google Scholar]
- 37.Savant V, Saeed T, Denniston A, Murray PI. Oral valganciclovir treatment of varicella zoster virus acute retinal necrosis. Eye (London, England) 2004;18(5):544–545. doi: 10.1038/sj.eye.6700703. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Cortes LF, Ruiz-Valderas R, Lucero-Munoz MJ, Cordero E, Pastor-Ramos MT, Marquez J. Intravitreal, retinal, and central nervous system foscarnet concentrations after rapid intravenous administration to rabbits. Antimicrob Agents Chemother. 2000;44(3):756–759. doi: 10.1128/aac.44.3.756-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55(2):459–472. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dokey AT, Haug SJ, McDonald HR, et al. Acute retinal necrosis secondary to multidrug-resistant herpes simplex virus 2 in an immunocompetent adolescent. Retin Cases Brief Rep. 2014;8(4):260–264. doi: 10.1097/ICB.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 41.Khurana RN, Charonis A, Samuel MA, Gupta A, Tawansy KA. Intravenous foscarnet in the management of acyclovir-resistant herpes simplex virus type 2 in acute retinal necrosis in children. Med Sci Monit. 2005;11(12):Cs75–78. [PubMed] [Google Scholar]
- 42.Berthe P, Baudouin C, Garraffo R, Hofmann P, Taburet AM, Lapalus P. Toxicologic and pharmacokinetic analysis of intravitreal injections of foscarnet, either alone or in combination with ganciclovir. Invest Ophthalmol Vis Sci. 1994;35(3):1038–1045. [PubMed] [Google Scholar]
- 43.Lopez-Cortes LF, Pastor-Ramos MT, Ruiz-Valderas R, et al. Intravitreal pharmacokinetics and retinal concentrations of ganciclovir and foscarnet after intravitreal administration in rabbits. Invest Ophthalmol Vis Sci. 2001;42(5):1024–1028. [PubMed] [Google Scholar]
- 44.Lee MY, Kim KS, Lee WK. Intravitreal foscarnet for the treatment of acyclovir-resistant acute retinal necrosis caused by varicella zoster virus. Ocul Immunol Inflamm. 2011;19(3):212–213. doi: 10.3109/09273948.2010.544857. [DOI] [PubMed] [Google Scholar]
- 45.Guo LB, Sun D, Ye JJ, Geng S, Xu HY, Zhang MF. Intravitreal injection of Ganciclovir in the treatment of acute retinal necrosis. [Zhonghua yan ke za zhi] Chinese journal of ophthalmology. 2007;43(7):631–637. [PubMed] [Google Scholar]
- 46.Kishore K, Jain S, Zarbin MA. Intravitreal ganciclovir and dexamethasone as adjunctive therapy in the management of acute retinal necrosis caused by varicella zoster virus. Ophthalmic Surg Lasers Imaging. 2011;42:e87–90. doi: 10.3928/15428877-20110901-06. Online. [DOI] [PubMed] [Google Scholar]
- 47.Luu KK, Scott IU, Chaudhry NA, Verm A, Davis JL. Intravitreal antiviral injections as adjunctive therapy in the management of immunocompetent patients with necrotizing herpetic retinopathy. Am J Ophthalmol. 2000;129(6):811–813. doi: 10.1016/s0002-9394(00)00462-1. [DOI] [PubMed] [Google Scholar]
- 48.Yeh S, Suhler EB, Smith JR, et al. Combination systemic and intravitreal antiviral therapy in the management of acute retinal necrosis syndrome. Ophthalmic surgery, lasers & imaging retina. 2014;45(5):399–407. doi: 10.3928/23258160-20140908-02. [DOI] [PubMed] [Google Scholar]
- 49.Wong R, Pavesio CE, Laidlaw DA, Williamson TH, Graham EM, Stanford MR. Acute retinal necrosis: the effects of intravitreal foscarnet and virus type on outcome. Ophthalmology. 2010;117(3):556–560. doi: 10.1016/j.ophtha.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 50.Tibbetts MD, Shah CP, Young LH, Duker JS, Maguire JI, Morley MG. Treatment of acute retinal necrosis. Ophthalmology. 2010;117(4):818–824. doi: 10.1016/j.ophtha.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Satoh N, Abe T, Nakajima A, Sakuragi S. Recurrent varicella-zoster virus retinitis in a patient treated with systemic corticosteroids. Ocul Immunol Inflamm. 1998;6(3):185–188. doi: 10.1076/ocii.6.3.185.4040. [DOI] [PubMed] [Google Scholar]
- 52.Young NJ, Bird AC. Bilateral acute retinal necrosis. Br J Ophthalmol. 1978;62(9):581–590. doi: 10.1136/bjo.62.9.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeh S, Fahle G, Flaxel CJ, Francis PJ. Central retinal vascular occlusion associated with acute retinal necrosis. Arch Ophthalmol. 2012;130(4):514–517. doi: 10.1001/archophthalmol.2011.1735. [DOI] [PubMed] [Google Scholar]
- 54.Choudhury H, Jindal A, Mithal K, Bawdekar AC, Pathengay A. Intravitreal triamcinolone acetonide as an adjuvant in the management of acute retinal necrosis. Can J Ophthalmol. 2014;49(3):279–282. doi: 10.1016/j.jcjo.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 55.Lau CH, Missotten T, Salzmann J, Lightman SL. Acute retinal necrosis features, management, and outcomes. Ophthalmology. 2007;114(4):756–762. doi: 10.1016/j.ophtha.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 56.Weissman HM, BV, Coutinho-Schechter M, Del Rio C, Yeh S. Bilateral central retinal artery occlusion associated with herpes simplex virus-associated acute retinal necrosis and meningitis: case report and literature review. Ophthalmic surgery, lasers & imaging retina. 2015 doi: 10.3928/23258160-20150213-24. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saatci AO, Ayhan Z, Arikan G, Sayiner A, Ada E. Unilateral acute retinal necrosis in a multiple sclerosis patient treated with high-dose systemic steroids. Int Ophthalmol. 2010;30(5):629–632. doi: 10.1007/s10792-010-9380-1. [DOI] [PubMed] [Google Scholar]
- 58.Sims JL, Zamir E. Acute retinal necrosis following steroid treatment for unrecognized Ramsay-Hunt syndrome. Clin Experiment Ophthalmol. 2008;36(9):894–895. doi: 10.1111/j.1442-9071.2009.01923.x. [DOI] [PubMed] [Google Scholar]
- 59.Nakamoto BK, Dorotheo EU, Biousse V, Tang RA, Schiffman JS, Newman NJ. Progressive outer retinal necrosis presenting with isolated optic neuropathy. Neurology. 2004;63(12):2423–2425. doi: 10.1212/01.wnl.0000147263.89255.b8. [DOI] [PubMed] [Google Scholar]
- 60.Toh T, Borthwick JH. Acute retinal necrosis post intravitreal injection of triamcinolone acetonide. Clin Experiment Ophthalmol. 2006;34(4):380–382. doi: 10.1111/j.1442-9071.2006.01229.x. [DOI] [PubMed] [Google Scholar]
- 61.Shah AM, Oster SF, Freeman WR. Viral retinitis after intravitreal triamcinolone injection in patients with predisposing medical comorbidities. Am J Ophthalmol. 2010;149(3):433–440. e431. doi: 10.1016/j.ajo.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawaguchi T, Spencer DB, Mochizuki M. Therapy for acute retinal necrosis. Semin Ophthalmol. 2008;23(4):285–290. doi: 10.1080/08820530802111192. [DOI] [PubMed] [Google Scholar]
- 63.Ando F, Kato M, Goto S, Kobayashi K, Ichikawa H, Kamiya T. Platelet function in bilateral acute retinal necrosis. Am J Ophthalmol. 1983;96(1):27–32. doi: 10.1016/0002-9394(83)90451-8. [DOI] [PubMed] [Google Scholar]
- 64.Park JJ, Pavesio C. Prophylactic laser photocoagulation for acute retinal necrosis. Does it raise more questions than answers? Br J Ophthalmol. 2008;92(9):1161–1162. doi: 10.1136/bjo.2008.147181. [DOI] [PubMed] [Google Scholar]
- 65.Meghpara B, Sulkowski G, Kesen MR, Tessler HH, Goldstein DA. Long-term follow-up of acute retinal necrosis. Retina. 2010;30(5):795–800. doi: 10.1097/IAE.0b013e3181c7013c. [DOI] [PubMed] [Google Scholar]
- 66.Sternberg P, Jr, Han DP, Yeo JH, et al. Photocoagulation to prevent retinal detachment in acute retinal necrosis. Ophthalmology. 1988;95(10):1389–1393. doi: 10.1016/s0161-6420(88)32999-4. [DOI] [PubMed] [Google Scholar]
- 67.McDonald HR, Lewis H, Kreiger AE, Sidikaro Y, Heckenlively J. Surgical management of retinal detachment associated with the acute retinal necrosis syndrome. Br J Ophthalmol. 1991;75(8):455–458. doi: 10.1136/bjo.75.8.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsuo T. Timing of prophylactic and early vitrectomy for first-presenting or recurrent acute retinal necrosis syndrome. Acta Med Okayama. 2012;66(6):493–497. doi: 10.18926/AMO/49046. [DOI] [PubMed] [Google Scholar]
- 69.Luo YH, Duan XC, Chen BH, Tang LS, Guo XJ. Efficacy and necessity of prophylactic vitrectomy for acute retinal necrosis syndrome. International journal of ophthalmology. 2012;5(4):482–487. doi: 10.3980/j.issn.2222-3959.2012.04.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ishida T, Sugamoto Y, Sugita S, Mochizuki M. Prophylactic vitrectomy for acute retinal necrosis. Jpn J Ophthalmol. 2009;53(5):486–489. doi: 10.1007/s10384-009-0698-z. [DOI] [PubMed] [Google Scholar]
- 71.Hillenkamp J, Nolle B, Bruns C, Rautenberg P, Fickenscher H, Roider J. Acute retinal necrosis: clinical features, early vitrectomy, and outcomes. Ophthalmology. 2009;116(10):1971–1975. e1972. doi: 10.1016/j.ophtha.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 72.Iwahashi-Shima C, Azumi A, Ohguro N, et al. Acute retinal necrosis: factors associated with anatomic and visual outcomes. Jpn J Ophthalmol. 2013;57(1):98–103. doi: 10.1007/s10384-012-0211-y. [DOI] [PubMed] [Google Scholar]