Abstract
Background:
Sleep disturbance is a common complaint of patients undergoing methadone maintenance therapy (MMT). There are limited studies about the effect of different treatments on insomnia due to MMT. In this study, we evaluated the effect of cognitive-behavioral treatment for insomnia (CBTI) on sleep disorders in patients undergoing MMT.
Methods:
Twenty-two patients with insomnia due to MMT (aged 18-60 years) participated in this randomized double-blind clinical trial. The intervention group received CBTI from a clinical psychologist for 8 weeks, whereas the control group received behavioral placebo therapy (BPT). The duration of individual sessions was 45 minutes, which was conducted once a week. The primary outcome was sleep disturbance assessed with Pittsburgh Sleep Quality Index (PSQI). Data were analyzed using SPSS software version 19.
Results:
Eleven patients were assigned to each group. Two groups were matched according to demographic characteristics (age, marital status, education, and daily methadone doses). Although PSQI score was significantly reduced during weeks 5 and 8 after both interventions, there was a significant difference in intervention versus time interaction (P<0.02). The effects of CBTI versus placebo were significantly different (P<0.001). The time course was also significant (P<0.001).
Conclusion:
This study showed that CBTI is more effective than BPT in overall sleep quality. We recommend further studies, with a larger sample, on CBTI in patients undergoing MMT.
Keywords: Methadone, Substance-related disorders, Insomnia, Cognitive therapy, Behavior therapy
Introduction
Insomnia, a common problem in psychiatry, is defined as difficulty in initiating, maintaining sleep, or having non-restorative sleep for one month or more. Based on some studies, 10-15% of patients with chronic sleep disorders are affected by substance abuse problems. According to the DSM-IV-TR, sleep disturbance can be caused by substance intoxication or withdrawal.1 Physicians often underestimate the severity and prevalence of sleep disorders in this population. The high prevalence of sleep disorders (70.2% to 84%) in methadone maintenance therapy (MMT) patient is reported by investigators.2,3 Methadone-treated patients are accompanied with concurrent disorders such as depression and anxiety disorders, which can affect their sleep. Insomnia and other sleep disturbances are exceedingly common during the first months of MMT.4 On the other hand; insomnia is a risk factor for major depressive disorder and suicide. Sleep disorder could be accompanied with overuse of over-the-counter drugs, substance and alcohol abuse, sleepiness-related accidents and absenteeism.
Non-pharmacological treatments are particularly appropriate to this population because of the abuse and overdose potential with some pharmacological agents when mixed with other substances. In addition, when hypnotics are prescribed, the drug use behavior can be reinforced. Moreover, it was shown that psychological and behavioral methods are effective to treat insomnia longer than benzodiazepine receptor agonists.5
Cognitive behavioral therapy for insomnia (CBTI) is a known treatment for sleep disorders.6,7 Arnedt et al. showed the effect of CBTI on insomnia in recovering alcohol dependent participants.8 Also, Yamadera et al. stated that CBTI improved sleep quality.9 Despite the effect of CBTI on insomnia related to alcohol, there is no study about the effect of CBTI on insomnia due to MMT. To our knowledge, this study is the first attempt to compare CBTI with placebo in a methadone-maintained population with insomnia.
Materials and Methods
Participants
Participants were referred to the MMT clinic of the Shafa Hospital in Rasht between January 2011 and January 2012. This study was approved by the Ethics Committee of Guilan University of Medical Sciences, Iran. This research was registered in IRCT (number: 201206011483N3). An informed consent was obtained from all patients.
The inclusion criteria were; (i) PSQI ≥5 and sleep disorder which is confirmed according to DSM-IV-TR criteria, (ii) 18-60 years old, (iii) no psychiatric disorders (bipolar I or II, schizophrenia, MDD during the past month and anxiety disorder), (iv) no chronic medical condition, which is associated with insomnia, and (v) beginning the MMT within the past month. Individuals were not included if they had withdrawal symptoms of drug abuse (except nicotine) or having used hypnotic drugs for a week or longer before starting the study. The exclusion criteria were (i) irregular attendance at the therapy sessions, (ii) hypnotic drug abuse (assessed by weekly urinalysis), (iii) positive urine screening test for drug, and (iv) leaving the MMT. Accordingly, 24 patients were considered as eligible (figure 1).
Figure 1.
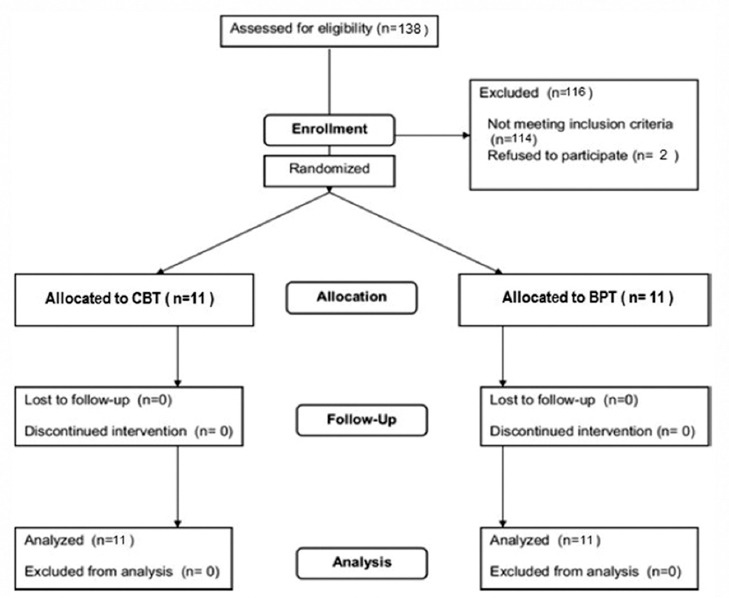
The illustration of patient’s consort flow chart.
Study Design
This study was a randomized double-blind clinical trial. After consent, the participants completed baseline questionnaire. Qualified participants were randomly assigned to CBTI or behavioral placebo therapy (BPT (using a computerized randomization program. Participants and evaluator, but not the study therapist, were blind to treatment condition. CBTI and BPT were conducted in parallel; session duration and schedule were the same for both groups. The CBTI was given by a clinical psychologist who was experienced in this field and the BPT was given by a resident of psychiatry. The 45-minute individual sessions were conducted once a week for 8 weeks. We performed urine screenings (for methamphetamine, morphine, and benzodiazepines) before each session.
Procedures
Cognitive-behavioral therapy for insomnia is a multi-component treatment that incorporates behavioral, cognitive, and educational components, which primarily target factors that perpetuate insomnia over time. The behavioral components included sleep restriction and stimulus control. Sleep restriction curtails the amount of time in bed to the patient’s estimated total sleep time to consolidate sleep and then increases gradually until an optimal sleep time is achieved. Stimulus control provides a set of instructions designed to discourage sleep-incompatible behaviors and reinforce a regular sleep/wake schedule. Cognitive therapy aimed to alter dysfunctional thoughts and beliefs about sleep and help patients develop realistic sleep expectations.10 An overview of the CBTI is listed in table 1.
Table 1.
Treatment Instruments of the cognitive behavioral therapy for insomnia
| Instrument | Description | |
|---|---|---|
| Behavioral components | ||
| Sleep restriction | Participants are instructed to: | |
| (1) Keep strict schedules of sleep and rising times | ||
| (2) Restrict bedtime closer to actual sleeping time, thereby synchronizing the endogenous circadian rhythm and sleep drive | ||
| Stimulus control | Participants learn to: | |
| (1) Interrupt associations between sleep, sleep environment, and wakefulness | ||
| (2) Only stay in bed while asleep or sleepy | ||
| (3) Eliminate activities incompatible with sleep in the bedroom | ||
| Cognitive component | To assist participants in identifying, challenging, and changing misconceptions about sleep requirements, sleep loss, and fears regarding sleep | |
| Educational component | Participants learn about the impact of lifestyle habits (such as dietary habits, regular sleepwake schedule, use of caffeinecontaining beverages) and environmental factors (such as noise, humidity) on sleep quality | |
BPT is based on the concept of desensitization and has been used as a placebo treatment in previous insomnia trials.8,11,12 The goal of BPT is to reduce conditioned arousal due to problems of initiating and maintaining sleep, which is associated with frustration. Each BPT session began by recording diary data. In the first session, the psychiatry resident presented a BPT overview, conceptual model of insomnia, and helped participants develop a 10-item arousal hierarchy of behavioral and cognitive activities that occur during poor sleep (e.g., clock watching, worrying about sleep). Each item was ranked from least to most arousing. A six-item neutral hierarchy was developed (e.g., opening the window, listening to light music). Over the course of BPT, each item in the arousal hierarchy was paired with the neutral hierarchy items. The participants were instructed to practice the exercise once daily at home, but not within 2–3 hours of bedtime to avoid unintended arousal.
Measurement Tool
Sleep quality was evaluated based on PSQI. The PSQI is a self-rated questionnaire, which assesses sleep quality during the past 4 weeks. The PSQI, included 7 indexes; namely subjective sleep quality, latency (time needed to fall asleep, converted to a score of zero to 3), duration (actual sleep hours per night, converted to a score of zero to 3), efficiency (total sleep time divided by time in bed, converted to a score of zero to 3), and disturbances (e.g., midnight wake up, pain, and nightmare), use of sleep medication (frequency of use of hypnotics per week), and daytime dysfunction (e.g., daytime sleepiness and loss of enthusiasm). Total score ranged from zero to 21, with the highest scores representing the lower quality of sleep. A global PSQI score more than 5 meant that the person has severe trouble sleeping at least in two areas or moderate difficulty in three areas or more. The PSQI has a sensitivity of 89.6% and specificity of 86.5%.13 Reliability has been proven in numerous studies in Iran (Cronbach’s alpha, 0.78-0.82).14-16
It was administered at baseline and after sessions 5 and 8. This was done by another psychiatry resident, who was unaware of patients’ assignment. A psychiatrist then interviewed patients with PSQI scores ˃5 to confirm insomnia (based on the DSM-IV-TR criteria).
Statistical Analysis
A priori power analyses were based on assessment of psychological intervention efficacy for primary insomnia from 4 randomized controlled trials yielding effect sizes varying from 1.0 on improvement in PSQI global sleep quality index (2±2.0 points) to 1.2 on improvement of sleep efficiency (11%±9%).8,11,17,18 Based on the above studies, the sample size for α=0.05 and β=0.20 was estimated between 20 to 30 patients.
Treatment groups were initially compared on baseline demographic variables with chi-square tests and independent samples t-tests. The one-sample Kolmogorov-Smirnov goodness of fit test was used to test normal distribution of the data. Considering that the patients underwent two different interventions (CBTI and BPT) and evaluations were performed for different time points (baseline, week 5, and week 8), we used a two-way repeated-measures analysis of variance (ANOVA) to test whether there was a main effect of intervention, time, or interaction intervention versus time on the primary outcome. When the ANOVA produced a significant main effect, a Tukey’s honestly significant difference post hoc test was used to detect differences in the measures between the various time points as well as between the different interventions at different time points. All statistical analyses were performed using SPSS software, version 19.0. P values less than 0.05 were considered statistically significant.
Results
Twenty-two male patients with a mean age of 44.1±8.0 years completed the 8-week trial. Baseline demographic characteristics are listed in table 2. Baseline characteristics of participants in both groups were similar (P>0.05). figure 2, illustrates the patterns of change in the outcome over time and by group.
Table 2.
Baseline sample characteristics by BPT and CBTI groups* (n=22)
| Variables | BPT | CBTI | Total | P value |
|---|---|---|---|---|
| Age (year) (mean±SD) | 44.7±7.8 | 43.5±8.3 | 44.1±8.0 | 0.67 |
| Gender (male) | 11 | 11 | 22 | 0.99 |
| Marital status (married) | 9 | 8 | 17 | 0.61 |
| Educational grade (mean±SD) | 10.7±1.3 | 12.2±1.9 | 11.9±1.6 | 0.18 |
| Methadone dosage (mg/day) (mean±SD) | 70.9±6.6 | 64.5±9.1 | 67.7±8.4 | 0.07 |
| Basal PSQI level (mean±SD) | 12.5±2.9 | 11.5±2.5 | 12.0±2.7 | 0.39 |
BPT: Behavioral placebo therapy, CBTI: Cognitive behavioral therapy, The ttest and χ2 were used for quantitative and qualitative variables, respectively
Figure 2.
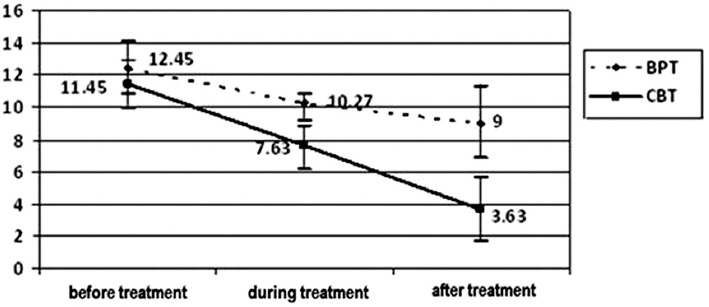
PSQI scores (mean±SD) over time by treatment group (BPT: Behavioral placebo therapy, CBT: Cognitive behavioral therapy).
PSQI score was significantly reduced during weeks 5 and 8 after CBTI or BPT intervention (figure 2). Two-way ANOVA revealed that the effects of CBTI versus BPT were significantly different (P<0.001). The time course was also significant (P<0.001). In addition, there was a significant difference in intervention versus time interaction (P=0.02). According to Tukey’s HSD test, there were no statistically significant differences in PSQI scores between the two baseline measurements. Significant changes in PSQI scores occurred earlier in CBTI group than the BPT group. The PSQI scores were significantly reduced during weeks 5 and 8 after the CBTI intervention (P<0.001). Between the periods of week 5 and week 8, there were no significant changes in PSQI scores in BPT group. BPT intervention did not significantly change PSQI scores after 5 weeks.
Furthermore, we analyzed the effects of interventions on the PSQI sub-scales. We observed a significant intervention versus time interaction on sleep duration, sleep efficiency, sleep disturbance, and daytime dysfunction subscales. table 3 summarizes PSQI sub-scales in both groups and detailed analyses.
Table 3.
Changes in PSQI sub-scales over time by treatment group*
| PSQI sub-scales | Behavioral placebo therapy | Cognitive behavior therapy | Between groups | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 8 weeks | P-value | Baseline | 8 weeks | P value | P value | |
| Quality of sleep | |||||||
| Global PSQI | 12.45 | 9.0 | 0.03 | 11.45 | 3.63 | 0.001 | 0.02 |
| Subjective sleep quality | 2.0 | 1.27 | 0.02 | 2.54 | 0.81 | 0.001 | 0.06 |
| Sleep latency | 2.0 | 1.9 | 0.31 | 2.72 | 1.09 | 0.001 | 0.15 |
| Sleep duration | 1.9 | 1.26 | 0.14 | 0.54 | 0.27 | 0.27 | 0.001 |
| Sleep efficiency | 2.0 | 1.18 | 0.02 | 1.09 | 0 | 0.003 | 0.001 |
| Sleep disturbance | 1.81 | 1.27 | 0.006 | 1.81 | 1.0 | 0.001 | 0.04 |
| Use of sleep medications | 3.0 | 3.0 | 0.99 | 3.0 | 3.0 | 0.99 | 0.99 |
| Daytime dysfunction | 2.27 | 1.63 | 0.002 | 2.45 | 0.45 | 0.001 | 0.002 |
Twoway repeatedmeasures ANOVA with Tukey post hoc test
Discussion
The present study assessed results of an 8-week trial of CBTI for MMT patients with severe insomnia. The main goal was to determine the effects of this psychological treatment on sleep disturbance. The insomnia is one of the most important complaints of patient in MMT and could cause failure to treatment. Then non-pharmacological approaches, such as CBTI, can be a particularly useful option in relieving insomnia.6,7 The etiology of sleep disturbances in the methadone maintenance treatment (MMT) population is often multifactorial and complex. The mechanisms of mu, delta, and kappa receptors in relation to sleep are unknown. However, it has been noted that mu agonist significantly suppressed rapid eye movement sleep.19 In a study by Xiao’s, patients in early MMT had poor sleep quality and abnormal sleep architecture, including lower sleep efficiency, shorter total sleep time, more awakenings and shorter slow wave sleep.20
The mean PSQI in our patients was 12.0±2.7. Stein et al. reported a mean PSQI of 10.6±4.9.2 The mean PSQI in patients in Hsu’s study was 9.1±5.4.4 In Pele’s study, a mean PSQI of 9±4.8 was shown.21 Our patients had more severity of sleep disorder than those studies. Previous studies have shown that there is a linear correlation between PSQI scores and methadone dosage.2,21 Patients with a higher methadone daily dosage had a higher PSQI score. The average daily dosage of methadone in the participants was 67.7±8.4 mg, which is higher than that in other studies. Clinical studies have shown the effects of opioid medications and methadone on sleep quality. Dimsdale et al. found that a single dose of oral opioid medications (morphine sulfate or methadone) could significantly affect sleep architecture in healthy adults (decreased deep sleep and increased stage two sleep).22 Paying more attention to this issue may yield a better sleep quality.
Although the PSQI score of our patients improved at the end of the fifth week of CBTI, the score of 73% of patients was above 5. However, PSQI score of all patients was less than 5 at the end of the eighth week. In the CBTI group, perceived PSQI declined by 7.83 points at post-intervention, which mean insomnia severity scores dropped to “no clinically significant insomnia”. In BPT group, the percent of patients with the PSQI score of less than 5, at the end of the fifth and eighth week, were 0% and 10%, respectively. In the BPT group, we observed a 3.45-point reduction in PSQI scores post-intervention, therefore remaining at or above subthreshold insomnia throughout the study. Considering the PSQI of more than 5 is used to distinguish clinical insomnia from normal sleep, such finding is clinically meaningful. This finding is consistent with results of previous studies.11,18
It is known that BPT can reduce arousal from repeated frustration about not sleeping and, therefore, may be most helpful to those with high anxiety or annoyance related to sleep disturbances. In studies by Arnedt and colleagues, the CBTI utility in the treatment of insomnia and prevention of relapse risk of alcohol dependent patients was confirmed.8,23 Improvement in daytime dysfunction shows that CBTI may reconstruct social relationship functions and self-esteem in this population.24 In spite of previous investigations,11,25 improvement in subjective sleep quality and sleep latency between both groups (CBTI and BPT) was not significantly different in our patients. These are mainly caused by the limited number of participants in this study.
The exact mechanism of CBTI is unknown. Only proposed physiological effect of CBTI is on anxiety and depression.18 Many studies have shown that IL-1 has a pivotal role in sleep-wake behavior.26 IL-1β not only increases non–rapid eye movement sleep in vertebrates, but also fatigue and daytime sleepiness.27 Chen et al. observed an average 54% decrease in IL-1β levels in plasma of participants after CBTI.28
No serious side effects were observed during CBTI, and all patients completed the study. Edinger et al. indicated that CBTI was an acceptable method in the treatment of insomnia associated with non-psychotic psychiatric problems.29 Also, the preliminary study on the effect of telephone-delivered CBTI for patients with chronic insomnia has been published.30
This study has several limitations. The first is relatively small and short observation period (8 weeks), which limited the statistical significance of results. Secondly, our samples were only male. The third is the lack of polysomnography to provide objective sleep evaluation. Sharkey et al. reported objective sleep measures confirmed subjective measures in MMT patients with disturbed sleep. And, both objective and subjective measures were useful in research and clinical settings to assess sleep in opioid-dependent patients.31 Future investigations of insomnia in MMT should be conducted with larger samples in randomized and controlled group studies using both subjective and objective (such as polysomnography) measures of sleep quality.
Conclusion
This study represents the first attempt to show, in several participants with MMT, that CBTI is a valuable therapeutic option with significant benefits. This treatment involves simple and straightforward procedures that could easily be implemented during MMT as a therapeutic tool. Improving sleep quality after CBTI may attenuate the relapse as well as reduce the need for hypnotic medication through improved sleep quality. Therefore, CBTI may be considered as an effective treatment for insomnia in patients undergoing MMT. Further large-scale investigations are necessary to validate our results.
Acknowledgments
This project was supported by the Vice-Chancellor for Research of Guilan University of Medical Sciences.
Conflict of Interest: None declared.
References
- 1.Sadock BJ, Sadock VA. 10th ed. Philadelphia: Lippincott Williams &Wilkins; 2007. Kaplan Sadock's synopsis of psychiatry; p. 1470. [Google Scholar]
- 2.Stein MD, Herman DS, Bishop S, Lassor JA, Weinstock M, Anthony J, et al. Sleep disturbances among methadone maintained patients. JSubst Abuse Treat. 2004;26:175–80. doi: 10.1016/s0740-5472(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 3.Hsu WY, Chiu NY, Liu JT, Wang CH, Chang TG, Liao YC, et al. Sleep quality in heroin addicts under methadone maintenance treatment. Acta Neuropsychiatr. 2012;24:356–60. doi: 10.1111/j.1601-5215.2011.00628.x. [DOI] [PubMed] [Google Scholar]
- 4.Strain EC, Lofwall MR, Jaffe JH. Opioid –related disorder. In: Sadock BJ, Sadock VA, Ruiz P, Kaplan HI, editors. Kaplan &Sadock's comprehensive text book of psychiatry. 9th ed. Philadelphia: Lippincott Williams &Wilkins; 2009. pp. 1360–86. [Google Scholar]
- 5.Riemann D, Perlis ML. The treatments of chronic insomnia:a review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Med Rev. 2009;13:205–14. doi: 10.1016/j.smrv.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin Psychol Rev. 2005;25:559–92. doi: 10.1016/j.cpr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Currie SR, Clark S, Hodgins DC, El-Guebaly N. Randomized controlled trial of brief cognitive-behavioural interventions for insomnia in recovering alcoholics. Addiction. 2004;99:1121–32. doi: 10.1111/j.1360-0443.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- 8.Arnedt JT, Conroy DA, Armitage R, Brower KJ. Cognitive-behavioral therapy for insomnia in alcohol dependent patients:a randomized controlled pilot trial. Behav Res Ther. 2011;49:227–33. doi: 10.1016/j.brat.2011.02.003. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamadera W, Sato M, Harada D, Iwashita M, Aoki R, Obuchi K, et al. Comparisons of short-term efficacy between individual and group cognitive behavioral therapy for primary insomnia. Sleep Biol Rhythms. 2013;11:176–84. doi: 10.1111/sbr.12019. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morin CM, Espie CA. 1st ed. New York: Kluwer Academic/Plenum Publishers; 2003. Insomnia:A clinical guide to assessment and treatment; p. 190. [Google Scholar]
- 11.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia:a randomized controlled trial. JAMA. 2001;285:1856–64. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- 12.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31:489–95. doi: 10.1093/sleep/31.4.489. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index:a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 14.Farrahi Moghaddam J, Nakhaee N, Sheibani V, Garrusi B, Amirkafi A. Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P) Sleep Breath. 2012;16:79–82. doi: 10.1007/s11325-010-0478-5. [DOI] [PubMed] [Google Scholar]
- 15.Rowshani S, Moghadasi A, Dareh Bidi Abbasi M, Abdolmohammadi A, Ahanjan Sh. The effect of 4-weeks rehabilitation program on range of motion and shoulder pain in men with frozen shoulder. Salmand. 2010;5:7–15. Persian. [Google Scholar]
- 16.Afkham Ebrahimi A, Ghale Bandi MF, Salehi M, Kafian Tafti AR, Vakili Y, Akhlaghi Farsi E. Sleep Parameters and the Factors Affecting the Quality of Sleep in Patients Attending Selected Clinics of Rasoul-e-Akram Hospital. Razi J Med Sci. 2008;15:311–8. Persian. [Google Scholar]
- 17.Li F, Fisher KJ, Harmer P, Irbe D, Tearse RG, Weimer C. Tai chi and self-rated quality of sleep and daytime sleepiness in older adults:a randomized controlled trial. J Am Geriatr Soc. 2004;52:892–900. doi: 10.1111/j.1532-5415.2004.52255.x. [DOI] [PubMed] [Google Scholar]
- 18.Sivertsen B, Omvik S, Pallesen S, Bjorvatn B, Havik OE, Kvale G, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults:a randomized controlled trial. JAMA. 2006;295:2851–8. doi: 10.1001/jama.295.24.2851. [DOI] [PubMed] [Google Scholar]
- 19.Cronin A, Keifer JC, Baghdoyan HA, Lydic R. Opioid inhibition of rapid eye movement sleep by a specific mu receptor agonist. Br J Anaesth. 1995;74:188–92. doi: 10.1093/bja/74.2.188. [DOI] [PubMed] [Google Scholar]
- 20.Xiao L, Tang YL, Smith AK, Xiang YT, Sheng LX, Chi Y, et al. Nocturnal sleep architecture disturbances in early methadone treatment patients. Psychiatry Res. 2010;179:91–5. doi: 10.1016/j.psychres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Peles E, Schreiber S, Adelson M. Variables associated with perceived sleep disorders in methadone maintenance treatment (MMT) patients. Drug Alcohol Depend. 2006;82:103–10. doi: 10.1016/j.drugalcdep.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Dimsdale JE, Norman D, DeJardin D, Wallace MS. The effect of opioids on sleep architecture. J Clin Sleep Med. 2007;3:33–6. [PubMed] [Google Scholar]
- 23.Arnedt JT, Conroy DA, Brower KJ. Treatment options for sleep disturbances during alcohol recovery. J Addict Dis. 2007;26:41–54. doi: 10.1300/J069v26n04_06. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman EM, Hayney MS, Love GD, Urry HL, Rosenkranz MA, Davidson RJ, et al. Social relationships, sleep quality, and interleukin-6 in aging women. Proc Natl Acad Sci USA. 2005;102:1–62. doi: 10.1073/pnas.0509281102. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobs GD, Pace-Schott EF, Stickgold R, Otto MW. Cognitive behavior therapy and pharmacotherapy for insomnia:a randomized controlled trial and direct comparison. Arch Intern Med. 2004;164:1888–96. doi: 10.1001/archinte.164.17.1888. [DOI] [PubMed] [Google Scholar]
- 26.Opp MR. Cytokines and sleep. Sleep Med Rev. 2005;9:355–64. doi: 10.1016/j.smrv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida H, Peterfi Z, Garcia-Garcia F, Kirkpatrick R, Yasuda T, Krueger JM. State-specific asymmetries in EEG slow wave activity induced by local application of TNFalpha. Brain Res. 2004;1009:129–36. doi: 10.1016/j.brainres.2004.02.055. [DOI] [PubMed] [Google Scholar]
- 28.Chen HY, Chiang CK, Wang HH, Hung KY, Lee YJ, Peng YS, et al. Cognitive-behavioral therapy for sleep disturbance in patients undergoing peritoneal dialysis:a pilot randomized controlled trial. Am J Kidney Dis. 2008;52:314–23. doi: 10.1053/j.ajkd.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Edinger JD, Olsen MK, Stechuchak KM, Means MK, Lineberger MD, Kirby A, et al. Cognitive behavioral therapy for patients with primary insomnia or insomnia associated predominantly with mixed psychiatric disorders:a randomized clinical trial. Sleep. 2009;32:499–510. doi: 10.1093/sleep/32.4.499. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnedt JT, Cuddihy L, Swanson LM, Pickett S, Aikens J, Chervin RD. Randomized controlled trial of telephone-delivered cognitive behavioral therapy for chronic insomnia. Sleep. 2013;36:353–62. doi: 10.5665/sleep.2448. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, Stein MD. Assessing sleep in opioid dependence:a comparison of subjective ratings, sleep diaries, and home polysomnography in methadone maintenance patients. Drug Alcohol Depend. 2011;113:245–8. doi: 10.1016/j.drugalcdep.2010.08.007. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]


