Abstract
Background:
Appropriate diagnosis and treatment of latent tuberculosis infection (LTBI) play the most important role in the control of tuberculosis. This study aimed to determine the prevalence of LTBI among healthy tuberculosis unexposed children vaccinated with BCG using the tuberculin skin test (TST) and QuantiFERON TB Gold In-Tube (QFT-GIT) and comparing the agreement between the two tests.
Methods:
A cross-sectional study was carried out between October 2009 and March 2010 in 24 schools and 11 daycare centers. A total of 967 children were divided into 15 age groups, with a minimum of 64 children per group.
Results:
The prevalence rates of LTBI with TST were 3.8%, and 2.2% with QFT-GIT. One case was positive in TST and QFT-GIT, 20 cases were QFT-GIT positive, but TST negative and 36 cases were TST positive, but QFT-GIT negative, and finally, 910 cases were negative in both. There was poor agreement between TST and QFT-GIT (1.8%, 95%, CI: 0%-5.3%, k=0.007). The specificity of QFT-GIT in the BCG vaccinated, children aged 1-15 years old, was 97.8% (97.8%, 95% CI: 96.8%-98.8%). After three months, 2/17 (11.8%) of those initially QFT-GIT negative converted, and 10/15 (66%) of those initially QFT-GIT positive reverted.
Conclusion:
It seems that TST and QFT-GIT are not appropriate tests for the diagnosis of LTBI among healthy tuberculosis unexposed BCG vaccinated children. There was a low reproducibility rate of QFT-GIT. The cause of the the poor agreement requires further studies.
Keywords: Tuberculin test, QuantiFERON TB gold, Latent tuberculosis, Child, Iran
Introduction
The World Health Organization (WHO) has estimated that approximately one-third of the world’s population is infected with Mycobacterium tuberculosis.1,2 This large population with latent infection poses a major challenge to global tuberculosis (TB) control and elimination campaigns. According to the latest global tuberculosis report, an estimated 8.6 million people developed TB and 1.3 million died from the disease.3 Between eight and nine million people develop TB every year.1,3,4 The trend of tuberculosis is decreasing in Iran and the tuberculosis incidence rate was 13.7 per 100,000 population during 2013 and 3.6% of all cases occurred under 15 years old. The incidence and prevalence of TB varies among provinces in Iran, for example, higher in Golestan province in comparison with other provinces because of its ethnically varied population.5 During latent tuberculosis infection, the M. tuberculosis bacilli that persist in symptom-free individuals can reactivate and cause active disease in about 10% of those infected over their lifetime.6-8 Treatment of latent infection can greatly reduce the likelihood that active tuberculosis will develop. Thus, it has the potential both to preserve the health of an individual person and to protect the health of the public by reducing the number of potential sources of infection.9The tuberculin skin test (TST) remained the only diagnostic tool for LTBI for decades. The results of TST have cross-reactions with the Bacillus Calmette-Guérin (BCG) vaccine and environmental mycobacteria, which lead to many false positives. Some other factors, such as deep injection and viral diseases, result in false negative responses. A new test for the diagnosis of LTBI, which is more specific than TST, and the result of which would not be affected by previous BCG vaccination, would be of more accurate use.10 QFT-GIT detects the in vitro cell-mediated immune response to M. tuberculosis infection by measuring IFN-γ in the whole blood incubated with M. tuberculosis antigen [early secretory antigenic target 6 (ESAT-6), culture filtrate protein 10 (CFP-10) and another peptide from the TB antigen TB7.7 (Rv2654)]. An enzyme-linked immunosorbent assay (ELISA) detects the amount of IFN-γ, produced by the T cells.11 The antigens of ESAT-6 and CFP-10 are absent from all of the BCG vaccine strains and from the commonly encountered non-tuberculous mycobacteria (NTM), except M. kansasii, M. szulgai and M. marinum.12 Data about the performance of QFT-GIT in young children, especially those aged under 5 years, are limited. To the best of our knowledge, there has been no study on the use of QFT-GIT in low-risk children.13
In the present study, we set out to compare the performance of QFT-GIT, used for the detection of LTBI, with TST and to evaluate LTBI prevalence in children aged 1-15 years, who have been vaccinated with BCG at birth. LTBI is determined by positive TST or QFT-GIT in a person with no clinical presentation of active TB disease and no radiographic findings.
Patient and Methods
Study Design
A cross-sectional study was carried out on 1-15 year-old children between October 2009 and March 2010 at the Professor Alborzi Clinical Microbiology Research Center (affiliated with Shiraz University of Medical Sciences, Shiraz, Iran). The sample size was 967 children, divided into 15 age groups each consisting of at least 64 children. All participants were selected by stratified multistage random sampling in sex and age layers, from four different districts in 11 daycare centers, and 24 primary, junior high, and high schools. A minimum of 32 boys and 32 girls were included in each age group, with a BCG vaccination history at birth based on their vaccination documents. Children with acute febrile diseases, immunocompromised, on medications, positive exposure history to active tuberculosis patients, and the immigrants were excluded. Written informed consents from the parents were obtained and approved by the Ethics Committee of Shiraz University of Medical Sciences. In addition, questionnaires were filled out about age, location, nationality, health status, and history of nationwide at birth-BCG vaccination. All patients with positive TST tests, no evidence of active disease, and no radiographic findings were considered as LTBI.
Tuberculin Skin Test
As for TST, 0.1 ml of purified protein derivative (PPD), Razi vaccine and serum research institute, Iran, equivalent to five tuberculin units of PPD solution was injected intradermally into the volar aspect of the forearm, and the mean vertical and transverse induration diameters were measured 72 hours later. The positive interpretation of a TST is an induration of ≥10 mm in individuals.
QuantiFERON TB Gold In-Tube
From each participant, a 3 ml heparinized blood sample was collected by vein puncture for the QFT-GIT assay. IFN-γ responses to ESAT-6, CFP-10 and another peptide from the TB antigen TB7.7 (Rv2654) were measured by the QFT-GIT assay, according to the manufacturer’s instructions (Cellestis Ltd., Carnegie, Victoria, Australia), which involved two stages: (1) incubation of the whole blood with antigens and (2) measurement of IFN-γ production in the harvested plasma by ELISA. One ml of venous blood was added to each of the three heparin-containing tubes. One tube contained only heparin as negative (nil) control, another one contained T-cell mitogen phytohaemagglutinin as positive control, and the third tube had overlapping peptides representing the entire sequences of ESAT-6, CFP-10, and TB7.7. The tubes were incubated at 37°C and after 18-24 hours, they were centrifuged and the plasma was harvested and frozen at -70°C until ELISA was performed. The amount of IFN-γ was quantified using QFT-GIT ELISA. The ELISA readout was analyzed using the QFT-GIT software. IFN-γ ≥0.35 IU/ml for QFT-GIT was considered as positive.
Reproducibility
To study the reproducibility of QFT-GIT, 17 cases of those initially QFT-GIT negative/TST positive and 15 cases of those initially QFT-GIT positive/TST negative, were retested by both tests after 3 months.
Statistical Analysis
Collected data from the questionnaires and the results of TST and QFT-GIT were entered into SPSS, version 16. Agreement between the groups of results was assessed using k coefficients. Accordingly, Kappa values below 0.1 indicate poor agreement, 0.1-0.4 indicates weak correlation, values of 0.41–0.60 indicate good agreement, and values above 0.6 shows strong agreement. Specificity of QFT-GIT was calculated and a P-value of<0.05 was considered significant.
Results
The Prevalence of LTBI with TST
Both the TST and QFT-GIT were administered to 967 individuals. Analysis of the collected data revealed that the prevalence of LTBI with TST among all the cases was 3.8% (37 positive/967 with TST≥10 mm). All participants returned for TST interpretation. The highest rate was 7.8% in 15-year old and the lowest rate (1.6%) was in the 1-year old group. Using the Chi-square test, the LTBI prevalence rates of 3.7% in the 1-5 year old group, 3.4% in the 6-10 year old group and 4.4% in the 11-15 year old group by TST, indicate a direct correlation between age and LTBI prevalence among the children (figure 1, table 1). The prevalence rates by TST in males and females were 3.7% and 4%, respectively.
Figure 1.
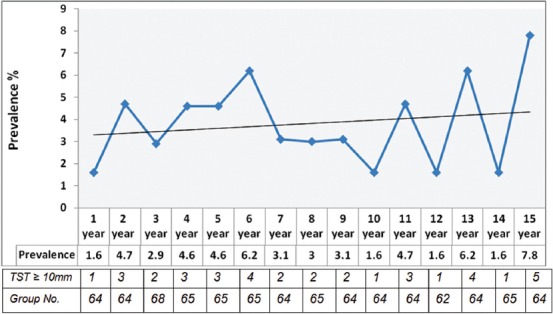
There is a relationship between age and prevalence of LTBI ( using Chi-square test ).
Table 1.
Results of TST and QFT-GIT tests in 1-15 years old BCG vaccinated children, Shiraz, Iran, 2010 (No=967)
| TST results (n (%)) |
QFTGIT results (%(n)) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0-4 mm | 5-9 mm | 10-14 mm | ≥15 mm | Total | Negative | Positive | Total | |
| Age groups | ||||||||
| 1-5 yr | 270 (82.8) | 44 (13.5) | 10 (3.1) | 2 (0.6) | 326 (100) | 315 (97.2) | 9 (2.8) | 326 (100) |
| 6-10 yr | 257 (79.8) | 54 (16.8) | 7 (2.2) | 4 (1.2) | 322 (100) | 317 (98.3) | 5 (1.5) | 322 (100) |
| 11-15 yr | 222 (69.6) | 83 (26) | 7 (2.2) | 7 (2.2) | 319 (100) | 312 (97.8) | 7 (2.2) | 319 (100) |
| Total | 749 (77.5) | 181 (18.7) | 24 (2.5) | 13 (1.3) | 967 (100) | 946 (97.8) | 21 (2.2) | 967 (100) |
| Gender | ||||||||
| Male | 362 (74.8) | 104 (21.5) | 12 (2.5) | 6 (1.2) | 484 (100) | 468 (96.7) | 16 (3.3) | 484 (100) |
| Female | 387 (80) | 77 (16) | 12 (2.5) | 7 (1.5) | 483 (100) | 478 (99) | 5 (1) | 483 (100) |
| Total | 749 (77.5) | 181 (18.7) | 24 (2.5) | 13 (1.3) | 967 (100) | 946 (97.8) | 21 (2.2) | 967 (100) |
| Location in Shiraz | ||||||||
| Region 1 | 193 (81.4) | 36 (15.2) | 5 (2.1) | 3 (1.3) | 237 (100) | 232 (97.9) | 5 (2.1) | 237 (100) |
| Region 2 | 200 (86.2) | 24 (10.3) | 6 (2.6) | 2 (0.9) | 232 (100) | 221 (95.3) | 11 (4.7) | 232 (100) |
| Region 3 | 195 (78.6) | 44 (17.7) | 6 (2.4) | 3 (1.2) | 248 (100) | 248 (100) | 0 | 248 (100) |
| Region 4 | 161 (64.4) | 77 (30.8) | 7 (2.8) | 5 (2) | 250 (100) | 245 (98) | 5 (2) | 250 (100) |
| Total | 749 (77.5) | 181 (18.7) | 24 (2.5) | 13 (1.3) | 967 (100) | 946 (97.8) | 21 (2.2) | 967 (100) |
The Prevalence of LTBI with QFT-GIT
The prevalence of LTBI by QFT-GIT was 2.2% (21 positive/967) table 1. The prevalence rates of LTBI by QFT-GIT were 3.3% in males and 1% in females, thus the difference was significant (P≤0.05).
The Specificity and Agreement of QFT-GIT with TST
It is revealed that, one case was positive for TST and QFT-GIT, 20 cases were QFT-GIT positive but TST negative, and 36 cases were TST positive but QFT-GIT negative, and finally, 910 cases were both QFT-GIT and TST negative table 2. Comparison of the two tests by Kappa coefficient indicated a poor agreement between TST and QFT-GIT in the diagnosis of LTBI among low-risk children (1.8%, 95%, CI: 0%-5.3%) and (k=0.007). The specificity of QFT-GIT in the diagnosis of LTBI among the low-risk BCG vaccinated children aged 1-15 years was 97.8% (97.8%, 95%, CI: 96.8%-98.8%). Since there is no gold standard test for LTBI, we were not able to estimate the sensitivity of QFT-GIT.
Table 2.
Correlation of TST and QFT-GIT tests in 1-15 years old BCG vaccinated children, Shiraz, Iran, 2010
| QFTGIT results | No. of cases with TST results |
||
|---|---|---|---|
| ≥10 mm | <10 mm | Total | |
| Positive (≥0.35 IU/ml) | 1 | 20 | 21 |
| Negative (<0.35 IU/ml) | 36 | 910 | 946 |
| Total | 37 | 930 | 967 |
Reproducibility of QFT-GIT
To evaluate the reproducibility of QFT-GIT, 32 Individuals (17 negative and 15 positive at first visit) were retested after 3 months. The results showed that 2/17 (11.8%) of those initially QFT-GIT negative converted, and 10/15 (66%) of those initially QFT-GIT positive reverted. Among the 10 individuals with reverted QFT-GIT results, the maximum and minimum IFN-γ responses at first visit were 1.081 IU/ml and 0.42 IU/ml, respectively. There was no significant difference between the IFN-γ mean values for the remaining 5 persistent positive and the 10 reverted cases (0.71 vs. 0.81, P=0.983). Evaluation of the TST conversion rate revealed that 1/15 (6.7%) of those initially TST negative were converted.
One-year Follow-up of Children with LTBI
One year follow-up of children who considered as LTBI with TST of ≥10 mm, no evidence of active disease and no radiographic findings revealed no change to active TB disease.
Discussion
This study compared the TST and QFT-GIT in 967 BCG-vaccinated children for the diagnosis of LTBI that selected among the general population. Most previous studies have been performed either in countries with low incidence tuberculosis, in adults, or in high-risk groups. The performance of QFT-GIT in children for the diagnosis of LTBI is not clear and remains challenging. There is limited data about the performance of QFT-GIT in children under 17 years, especially in low-risk groups in regions where tuberculosis is endemic.13
In spite of the good agreement between TST and QFT-G in a few limited studies on high-risk children for the diagnosis of LTBI, in this study we found poor agreement in low-risk children (1.8%, 95%, CI: 0%-5.3%, k=0.007). In a study, among hospitalized Indian children, the agreement between the two tests was 100% (k=1.0) in BCG scar-negative children, as compared to 94% (k=0.63) in scar positive children.14Harada et al. and Carvalho et al. suggest that QFT-G may be used as a confirmatory test for TST.15,16 A meta-analysis indicated that the agreement between the two tests was 95.6% in German-born individuals younger than 40 years and not BCG-vaccinated. They concluded that the agreement of QFT-G and TST is excellent, with little potential that the TST is more likely to detect old infection than the QFT-G.17 Kariminia et al. also showed acceptable agreement between the two tests.18
In the present study, 20 cases were QFT-GIT positive but TST negative, 36 cases were TST positive but QFT-GIT negative and 910 cases were both QFT-GIT and TST negative. A few explanation can be found in literature for the disagreement and discordance between TST and GFT-GIT. In contrast to the TST, which remains positive for a protracted period after past- or clearance MTB infection, recent evidence suggests that QFT-GIT and T-SPOT TB assays detect more recent or ongoing infection. This may be because the IGRAs predominantly detects effector MTB-specific T cells in an overnight stimulation assay, whereas TST induration is measured at 48-72 hours, allowing for the expansion of memory T cell population.19 Therefore, these assays would be important for outbreak and contact investigations, where it is important not to miss recently infected individuals. Moreover, negative IGRA results might be seen after eradication of TB infection. Lastly, it is possible that fluctuations in responses are due to concomitant infections such as NTM or helminthes and other host factors that may be influential.20,21
Since there is no gold standard test for LTBI, we could not estimate the sensitivity of QFT-GIT, but the estimation of specificity in low-risk children with TST negative results was found to be 97.8% (97.8%, 95%, CI: 96.8%-98.8%). The findings indicate that QFT-GIT in BCG-vaccinated, low-risk children has an excellent specificity similar to that in other reported studies. Menzies et al. and Pai et al. demonstrated by two meta-analysis studies that the interferon gamma release assay, especially QFT-G and QFT-GIT, have excellent specificity unaffected by BCG vaccination.22,23 Generally speaking, negative TST is taken as indicative of non-infected cases, except for special conditions. In the present study, 18 cases of those with a TST of 15 mm or greater were QFT-GIT negative, which can suggest the possible lack of sensitivity for QFT-GIT, as has been reported in several previous studies. 24-26
In the present study, 2/17 (11.8%) of those initially QFT-GIT negative converted, and 10/15 (66%) of those initially QFT-GIT positive reverted, were retested after 3 months. As reported, transient response to QFT-GIT is common, 6.3% conversion and 33% reversion for QFT-GIT, respectively, in their second visits after 3 months.27 The present findings, are consistent with the previous reports22,23 and indicate the transient response of QFT-GIT. A high proportion of the participants with discordant IGRA+/TST-reactions had spontaneous QFT-GIT reversion to negativity (66%). However, a higher reversion rate among children may also reflect the variability of IFN-γ responses during the adaptation of the cellular immune system.28 It also suggests that QFT-GIT may provide a more quantitative and dynamic measurement of cellular immune response than TST, which would be important for serial-testing studies.29 Because of no change in active TB disease after one year follow up, the TST reversion (6.7%) could be due to boosting effect, therefor boosting effect can play a confusing role in interpretation of TST results. A recent study in Guilan province of Iran demonstrated that non hospital employees had a higher age-associated booster reaction to the second tuberculin skin test than health care workers.30
Conclusion
According to the current study, it is concluded that, (i) there was poor agreement between QFT-GIT and TST, (ii) there was a low reproducibility rate of QFT-GIT, and (iii) considering the low incidence of tuberculosis in the community under study, it seems that TST and QFT-GIT are not appropriate tests for the diagnosis of LTBI among healthy tuberculosis unexposed BCG vaccinated children. The cause of a poor agreement requires further studies.
Acknowledgment
This study was supported by the Prof. Alborzi Clinical Microbiology Research Center and the Deputy of Research Affairs of Shiraz University of Medical Sciences. Also, we would like to extend our sincere thanks to Hassan Khajehei, PhD, for his linguistic editing and Dr Payman Hemmati for editing this manuscript.
Conflict of Interest: None declared.
References
- 1.Chiang CY, Van Weezenbeek C, Mori T, Enarson DA. Challenges to the global control of tuberculosis. Respirology. 2013;18:596–604. doi: 10.1111/resp.12067. [DOI] [PubMed] [Google Scholar]
- 2.Dye C, Bassili A, Bierrenbach AL, Broekmans JF, Chadha VK, Glaziou P, et al. Measuring tuberculosis burden, trends, and the impact of control programmes. Lancet Infect Dis. 2008;8:233–43. doi: 10.1016/S1473-3099(07)70291-8. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Geneva: WHO; 2013. Global tuberculosis Report; pp. 1–289. [Google Scholar]
- 4.Pai M, Joshi R, Dogra S, Mendiratta DK, Narang P, Dheda K, et al. Persistently elevated T cell interferon-gamma responses after treatment for latent tuberculosis infection among health care workers in India:a preliminary report. JOccup Med Toxicol. 2006;23:1–7. doi: 10.1186/1745-6673-1-7. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rafiee S, Besharat S, Jabbari A, Golalipour F, Nasermoaadeli A. Epidemiology of Tuberculosis in Northeast of Iran:APopulation-Based Study. Iran J Med Sci. 2009;34:193–7. [Google Scholar]
- 6.Stewart GR, Robertson BD, Young DB. Tuberculosis:a problem with persistence. Nat Rev Microbiol. 2003;1:97–105. doi: 10.1038/nrmicro749. [DOI] [PubMed] [Google Scholar]
- 7.Jasmer RM, Nahid P, Hopewell PC. Clinical practice Latent tuberculosis infection. N Engl J Med. 2002;347:860–6. doi: 10.1056/NEJMcp021045. [DOI] [PubMed] [Google Scholar]
- 8.Tufariello JM, Chan J, Flynn JL. Latent tuberculosis:mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect Dis. 2003;3:578–90. doi: 10.1016/S1473-3099(03)00741-2. [DOI] [PubMed] [Google Scholar]
- 9.Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999 This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000;161:S221–S247. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 10.Arend SM, van Meijgaarden KE, de Boer K, de Palou EC, van Soolingen D, Ottenhoff TH, et al. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii. J Infect Dis. 2002;186:1089–96. doi: 10.1086/345760. [DOI] [PubMed] [Google Scholar]
- 11.Taggart EW, Hill HR, Ruegner RG, Martins TB, Litwin CM. Evaluation of an in vitro assay for gamma interferon production in response to Mycobacterium tuberculosis infections. Clin Diagn Lab Immunol. 2004;11:1089–93. doi: 10.1128/CDLI.11.6.1089-1093.2004. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356:1099–104. doi: 10.1016/S0140-6736(00)02742-2. [DOI] [PubMed] [Google Scholar]
- 13.National Tuberculosis Controllers Association. Centers for Disease Control and Prevention (CDC) Guidelines for the investigation of contacts of persons with infectious tuberculosis Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep. 2005;54:1–47. [PubMed] [Google Scholar]
- 14.Dogra S, Narang P, Mendiratta DK, Chaturvedi P, Reingold AL, Colford JM, Jr, et al. Comparison of a whole blood interferon-gamma assay with tuberculin skin testing for the detection of tuberculosis infection in hospitalized children in rural India. J Infect. 2007;54:267–76. doi: 10.1016/j.jinf.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Harada N, Nakajima Y, Higuchi K, Sekiva Y, Tothel J, Mori T. Screening for tuberculosis infection using whole- blood interferon-gamma and Mantoux testing among Japanese healthcare workers. Infect Control Hosp Epidemiol. 2006;27:442–8. doi: 10.1086/504358. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho AC, Pezzoli MC, El-Hamad I, Arce P, Bigoni S, Scarcella C, et al. Quanti FERON–TB Gold test in the identification of latent tuberculosis infection in immigrants. J Infect. 2007;55:164–8. doi: 10.1016/j.jinf.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Nienhaus A, Schablon A, Diel R. Interferon-Gamma Assay for the Diagnosis of Latent TB Infection –Analysis of Discordant Results, When Compared to the Tuberculin Skin Test. PloS One. 2008;3:e2665. doi: 10.1371/journal.pone.0002665. doi: 10.1371/journal.pone.0002665. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kariminia A, Sharifnia Z, Aghakhani A, Banifazl M, Eslamifar A, Hazrati M, et al. Comparison of QuantiFERON TB-G test to TST for detecting latent tuberculosis infection in a high-incidence area containing BCG-vaccinated population. JEval Clin Pract. 2009;15:148–51. doi: 10.1111/j.1365-2753.2008.00970.x. [DOI] [PubMed] [Google Scholar]
- 19.Connell TG, Ritz N, Paxton GA, Buttery JP, Curtis N, Ranganathan SC. AThree-Way Comparison of Tuberculin Skin Testing, QuantiFERON-TB Gold and T-SPOT.TB in Children. PloS One. 2008;3:e2624. doi: 10.1371/journal.pone.0002624. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Black GF, Weir RE, Floyd S, Bliss L, Warndorff DK, Crampin AC, et al. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK:two randomized controlled studies. Lancet. 2002;359:1393–401. doi: 10.1016/S0140-6736(02)08353-8. [DOI] [PubMed] [Google Scholar]
- 21.Elias D, Wolday D, Akuffo H, Petros B, Bronner U, Britton S. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination. Clin Exp Immunol. 2001;123:219–25. doi: 10.1046/j.1365-2249.2001.01446.x. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menzies D, Pai M, Comstock G. Meta-analysis:new tests for the diagnosis of latent tuberculosis infection:areas of uncertainty and recommendations for research. Ann Intern Med. 2007;146:340–54. doi: 10.7326/0003-4819-146-5-200703060-00006. [DOI] [PubMed] [Google Scholar]
- 23.Pai M, Zwerling A, Menzies D. Systematic Review:T-Cell–based Assays for the Diagnosis of Latent Tuberculosis Infection:An Update. Ann Intern Med. 2008;149:177–84. doi: 10.7326/0003-4819-149-3-200808050-00241. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrara G, Losi M, D'Amico R, Roversi P, Piro R, Meacci M, et al. Use in routine clinical practice of two commercial blood tests foe diagnosis of infection with Mycobacterium tuberculosis:a prospective study. Lancet. 2006;367:1328–34. doi: 10.1016/S0140-6736(06)68579-6. [DOI] [PubMed] [Google Scholar]
- 25.Pai M, Gokhale K, Joshi R, Dogra S, Kalantri S, Mendiratta DK, et al. Mycobacterium tuberculosis infection in health care workers in rural India:Comparison of a whole-blood interferon gamma assay with tuberculin skin testing. JAMA. 2005;293:2746–55. doi: 10.1001/jama.293.22.2746. [DOI] [PubMed] [Google Scholar]
- 26.Mahomed H, Hughes EJ, Hawkridge T, Minnies D, Simon E, Little F, et al. Comparison of Mantoux skin test with three generations of a whole blood IFN-gamma assay for tuberculosis infection. Int J Tuberc Lung Dis. 2006;10:310–6. [PubMed] [Google Scholar]
- 27.Perry S, Sanchez L, Yang S, Agarwal Z, Hurst P, Parsonnet J. Reproducibility of QuantiFERON- TB Gold IN-Tube Assay. Clin Vaccine Immunol. 2008;15:425–32. doi: 10.1128/CVI.00398-07. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewinsohn DA, Gennaro ML, Scholvinck L, Lewinsohn DM. Tuberculosis immunology in children:diagnostic and therapeutic challenges and opportunities. Int J tuberc Lung Dis. 2004;8:658–74. [PubMed] [Google Scholar]
- 29.Pai M, Menzies D. The New IGRA and the Old TST:making good use of disagreement. Am J Respir Crit Care Med. 2007;15:529–31. doi: 10.1164/rccm.200701-024ED. 175. [DOI] [PubMed] [Google Scholar]
- 30.Nikokar I, Dadgran A, Mafozei L. A Comparison of Two-Step Tuberculin Skin Test between Health-Care Workers and Nonhospital Employees. Iran J Med Sci. 2010;35:201–4. [Google Scholar]


