Abstract
Background:
Colorectal cancer (CRC) is one of the most common causes of cancer-related death in the world. The expression of N-myc downstream-regulated gene 2 (NDRG2) is down-regulated in CRC. The aim of this study was to investigate the effect of NDRG2 overexpression on cell proliferation and invasive potential of SW48 cells.
Methods:
SW48 cells were transfected with a plasmid overexpressing NDRG2. After stable transfection, the effect of NDRG2 overexpression on cell proliferation was evaluated by MTT assay. The effects of NDRG2 overexpression on cell migration, invasion and cell motility and matrix metalloproteinase 9 (MMP9) activities were also investigated using matrigel transwell assay, wound healing assay and gelatin zymography, respectively.
Results:
MTT assay showed that overexpression of NDRG2 caused attenuation of SW48 cell proliferation. Transwell and wound healing assay revealed that NDRG2 overexpression led to inhibition of migration, invasion, and motility of SW48 cells. The overexpression of NDRG2 also reduced the activity of secreted MMP-9.
Conclusions:
The results of this study suggest that NDRG2 overexpression inhibits proliferation and invasive potential of SW48 cells, which likely occurs via suppression of MMP-9 activity.
Keywords: Colorectal neoplasms, NDRG2 protein, Transfection, Cell movement, Wound healing, Metalloproteinase
Introduction
Colorectal cancer (CRC) is one of the major causes of cancer-related death in the world.1 Currently, chemotherapy, radiotherapy, and surgical removal of primary tumors are available treatments for CRC. Despite improvements in the treatment modalities, side effects of chemotherapeutic agents,2 and metastasis of CRC tumors3 still remain challenges of CRC treatments. Metastasis is a complex and multistep process, which includes the degradation of extracellular matrix, migration of tumor cells into the blood or lymph circulation and invasion of other tissues. Understanding the role of genes or molecules that involve in the CRC metastasis is very crucial because may lead to the development of new and more effective therapeutic strategies.
N-myc downstream-regulated gene 2 (NDRG2, Protein Accession No: Q9UN36) is a 41 kDa cytoplasmic protein of NDRG family, which encodes by human NDRG2 gene (GenBank Accession No. AF159092). It is normally expressed in several tissues and involved in multiple biological processes, including cell growth, differentiation, and apoptosis.4 Increasing evidence suggests that NDRG2 may act as a tumor suppressor gene in several tumors. Decreased expression of NDRG2 has been reported in several malignancies, including breast cancer,5 colon cancer,6 glioblastomas,7 meningiomas,8 and liver cancer.9 In addition, it has been indicated that overexpression of NDRG2 inhibited proliferation of several tumor cell lines such as bladder10 and liver.11
The association of NDRG2 with CRC has been shown in recent studies. The suppression of NDRG2 expression in CRC tumors and its association with the stage, recurrence and outcome of tumors,12 upregulation of cell-cell adhesion protein such as E-cadherin13 and down-regulation of T-cell factor/β-catenin signaling by NDRG214 have been discussed in the aforementioned studies. CRC tumor cells are invasive cells and their metastasis to liver, lung, peritoneum, and lymph nodes has been well indicated. The ability of CRC tumor cells to invade surrounding tissues is highly dependent on the synthesis and release of matrix metalloproteinase (MMPs). MMPs are released from tumor cells and permit the migration of cancer cells by degradation of extracellular matrix.15 MMP-9 is an important member of MMPs family, which is considered to be involved in the metastasis of gastro-intestinal tumors including CRC. Overexpression of MMP-9 and its association with the poor prognosis of CRC patients has been reported in several studies.16 To the best of our knowledge, the effect of NDRG2 on invasion and migration of CRC tumor cells has not been investigated yet. Thus, in the current study, we sought to study the effects of NDRG2 gene overexpression on proliferation, invasion, migration, and motility of SW48 colorectal cancer cells. Furthermore, the effect of NDRG2 overexpression on the activity of matrix MMP9 was also evaluated.
The reason to choose the SW48 for this study was the high invasive and proliferative abilities of this cell line. In a study conducted by Roh et al.,17 the invasiveness and the expression of invasion-associated-genes were compared between 12 commonly used colorectal cancer cell lines. The results indicated that among the cells studied, SW48 cells showed the highest expression of invasion-mediated genes, specially MMP2, MMP9 as well as angiogenic factors such as the vascular endothelial growth factor (VEGF). Furthermore, this cell line exhibited highest invasion and migration activities on a transwell migration and invasion assay, thus it appeared that it was a suitable cellular model for our in vitro study.
Materials and Methods
Cell Culture and Reagents
The human colorectal cancer cell line SW48 was purchased from the cell bank of the Pasteur institute of Iran (ATCC number: CCL-231). The cells were cultured in RPMI medium (Gibco/Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum and 100 units/ml penicillin-streptomycin (Gibco) at 37°C in a humidified 5% CO2 incubator.
Plasmid Amplification and Purification
pCMV6-AC-GFP plasmid encoding C-terminal green fluorescent protein (GFP)-tagged NDRG2 (NDRG2 plasmid) and negative control pCMV6-AC-GFP plasmid without NDRG2 (Mock plasmid) were purchased from Origene (Origene Technologies Inc., USA). The competent Escherichia coli strains DH5α were used for the proliferation of plasmid constructs. For each transformation, 100 ng of DNA was added to 25 μl of competent cells, and incubated on ice for 30 minutes, followed by heat shock at 42°C for 2 minutes and incubation on ice for 2 minutes. The cells were allowed to recover in 1 ml Luria-Bertani (LB) broth and then incubated for 60 minutes at 37°C with shaking. The cells were plated on LB-agar plate containing 100 μg/ml ampicillin (plasmids encoded ampicillin resistance) and incubated at 37°C overnight to select the transformants. After overnight culture, one colony of each plasmid was transferred to 3 ml of LB broth supplemented with ampicillin (50 μg/ml) for 5 hours of pre-culture at 37°C before transfer to 500 ml LB broth for a further overnight of incubation in a rotating incubator. The overnight culture was centrifuged at 5000 g for 10 minutes, and the resulting pellet was used to extract plasmid DNA using PureLink™ HiPure plasmid filter purification kit (Invitrogen, UK) as per manufacturer’s instructions. The concentration of the extracted DNA was measured using the NanoDrop ND-100 Spectrophotometer.
Stable Overexpression of the NDRG2 Gene in SW48 Colorectal Cancer Cells
SW48 cells were transfected with NDRG2 plasmid or mock plasmid using Lipofectamine 2000 and plus reagents (Invitrogen, Carlsbad, CA) according to the manufacture’s instruction. The transfection efficiency was tested using counting of GFP expression cells under fluorescence microscope with filter sets designed for GFP. For the selection of stable transfected SW48 cells, medium containing 300 µg/ml of G418 (Gibco) was applied 48 hours after transfection. After 2 weeks, the colonized cells were selected and then cultured for further selection. The established cell lines were maintained with normal growth medium containing 300 µg/ml of G418.18
Cell Proliferation Assay
The effect of NDRG2 overexpression on SW48 cell proliferation was evaluated by MTT assay. The normal non-transfected (WT group), pCMV6-AC-GFP (mock group), and pCMV6-AC-GFP-NDRG2 transfected cells (5×103 cells/ml) were cultured into 96 well plates for different durations (1, 2, 3, 4, 5, 6 and 7 days after stable transfection) and then MTT assay was performed. Briefly, MTT reagent (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide, 5 mg/ml dissolved in PBS) was added to each well and incubated at 37ºc for 4 hours. The reaction was stopped by the addition of 150 µl dimethyl sulfoxide (DMSO) followed by shaking for 10 minutes. Absorbance values were then measured at 570 nm.19
Migration and Invasion Assays
Invasion and migration assay were performed using a 24 well transwell insert (8 µm pore filters, BD Bioscience, Bedford, MA) with and without matrigel-coated membrane, respectively. Briefly, for migration assays, after filling the lower part of the transwell with RPMI plus 10% FBS, A549 cells (5×103) suspended in serum-free RPMI were added to the upper part of the transwell, and incubated for 6 hours at 37°C. The cells were allowed to migrate to the bottom of the well through a porous membrane. After incubation, the cells migrating to the lower surface of the membrane were fixed with methanol for 5 minutes and stained with 0.1% crystal violet. For the invasion assays, the membrane was coated with 100 µl (1 mg/ml) matrigel (BD Bioscience, Bedford, MA). SW48 cells (5×103) were plated onto the upper part of the matrigel-coated transwell chamber and incubated for 24 hours. The invaded cells were then fixed with methanol, stained and counted.20
Wound Healing Assay
SW48 cells were cultured at a density of 1.0×106 cells/well in 6 well plates. After the cells had grown to a full confluent monolayer, the cell monolayer was carefully scraped using a sterile tip to create a wound (scratch) and washed twice with fresh medium to remove any debris. The cells were incubated for 24 hours. Photographs of the wound were taken immediately (0 hour) and 24 hours after scraping and the area, the scratch was measured using Image software.21
Determination of MMP-9 Activity by Gelatin Zymography
The enzymatic activity of MMP-9 was analyzed by gelatin zymography. Serum-free culture supernatant fractions were collected and mixed at a 1:1 ratio with non-reducing sample buffer (2% sodium dodecyl sulphate, 50 mM Tris-HCl (pH 6.8), 10% glycerol, 0.001% bromophenol blue) for preparing samples. The samples were electrophoresed on a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) containing 0.1% gelatin. The gels were washed twice with washing buffer (50 mM Tris HCL, PH7.5, 100 mM Nacl, 2.5% triton x100) and then treated with incubation buffer (50 mM Tris-HCL, PH7.5, 150 mM Nacl,10 mM Cacl 2, 0.02% NaN3) at 37°C for 24-48 hours. After incubation, the gels were stained (0.05% coomassie blue, 10% isopropanol, 10% acetic acid) and then destained (20% methanol, 10% acetic acid). MMPs were detected as a clear zone on the blue background. The gels were scanned using a densitometer (Bio-Rad GS-800) and the density of bands were measured using Image J software (National Institutes of Health, Bethesda, MD, USA).22
Statistical Analysis
All statistical analysis was performed using SPSS software. Comparisons of the experimental results between the groups were made using a one-way ANOVA analysis, followed by Tukey post-hoc for multiple comparisons. A two-way repeated measures ANOVA, followed by the Bonferroni post-hoc test, varying treatment [between-subjects factor (3 levels)]×time[within-subjects repeated measure (7 levels)] was performed on the MTT assay data to test the effects of different treatments at different time points on cell viability. All data are represented by the mean±standard deviation of at least three independent experiments. P values<0.05 were considered as significant difference between groups.
Results
Stable Expression of NDRG2 in SW48 Cells
For stable overexpression of NDRG2 in SW48 cells, the cells were transfected with a plasmid encoding C-terminal GFP-tagged NDRG2. The expression of GFP-tagged NDRG2 was confirmed by fluorescence microscopy (figure 1). NDRG2 was localized in the cytoplasm and nucleus of the SW48 cells. Efficiency of transfection was more than 98% after stable transfection using G418 (figure 1).
Figure 1.
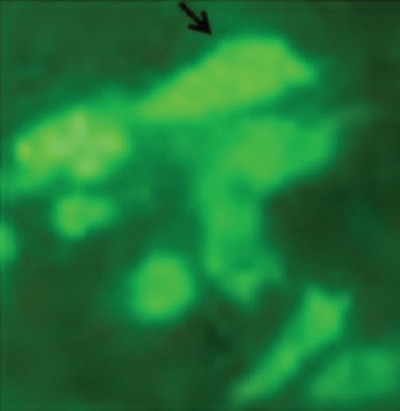
GFP-tagged NDRG2 overexpressed in the SW48 cells. Green fluorescence represented the successful transfection and overexpression of GFP-tagged NDRG2. The exogenously introduced NDRG2 was localized in the cytoplasm of the SW48 cells.
NDRG2 Overexpression Inhibits Viability of SW48 Cells
MTT assay was used to investigate the effect of NDRG2 overexpression on SW48 cells viability. Two-way repeated measures ANOVA analysis on MTT data showed a significant effect of treatment [F(2)=619.99, P<0.001], time [F(4,330)=2799.8, P<0.001] and “time×treatment” interaction [F(8,97)=163.69; P<0.001].The cell viability increased over time in all the three experimental groups. However, Bonferroni post-hoc test indicated that this increase occurred more gradually in the NDRG2 group compared with wild type or mock-transfected cells (figure 2). The cell viability of NDRG2-overexpressed cells were lowered compared to both wild type and mock-transfected cells at days 4, 5, 6 and 7 (P<0.001, two-way repeated-measures ANOVA and Tukey tests). The same statistical analysis showed no significant difference between the cell viability of control and mock group at all the time tested (P=0.231).
Figure 2.
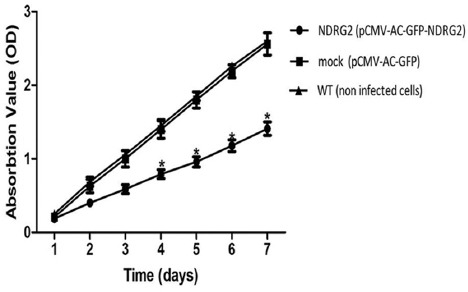
NDRG2 overexpression reduced SW48 cell viability. Cell viability was determined by MTT assay. Compared with the control and mock group, overexpression of NDRG2 significantly inhibited the proliferation of SW48 cells at 4 to 7 days after stable transfection. The results are the mean±SD of at least three independent experiments (*P<0.001 compare to WT group, by two-way repeated measures ANOVA).
NDRG2 Gene Transfer Inhibits the Migration and Invasion of SW48 Colorectal Cancer Cells
To explore the effect of NDRG2 overexpression on metastatic activity of SW48 cells, in vitro transwell migration assay/invasion assay was performed. The representative micrographs in figures 3A and 4A reveal the migrated and invaded SW48 cells at the lower surface of the transwell filter, respectively. As shown in the micrographs, overexpression of NDRG2 in SW48 cells (NDRG2 group) significantly suppressed their migration and invasion through the transwell compared to mock and WT group. The number of migrated cells (figure 3B) (P<0.001) and invaded cells (figure 4B) (P<0.001) were significantly lower in NDRG2 group compared to mock group and WT group. No significant differences were observed between mock group and the WT group with respect to the number of migrated cells (P=0.60) and invaded cells (P=0.20).
Figure 3.
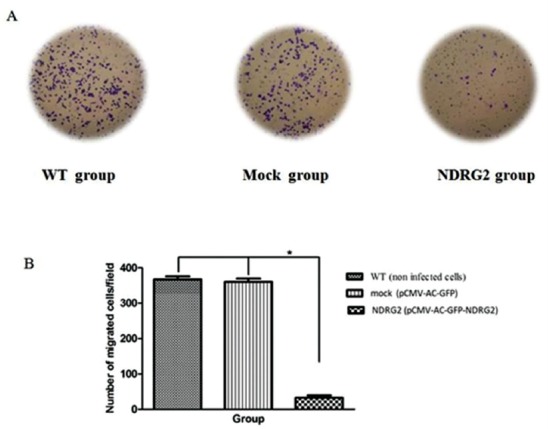
NDRG2 overexpression reduced migration of SW48 cell. Cell migration was determined by transwell assay. Compared with the control and mock group, overexpression of NDRG2 significantly decreased the number of migrated SW48 cells. The results are the mean±SD of at least three independent experiments (*P<0.001 compare to WT group).
Figure 4.
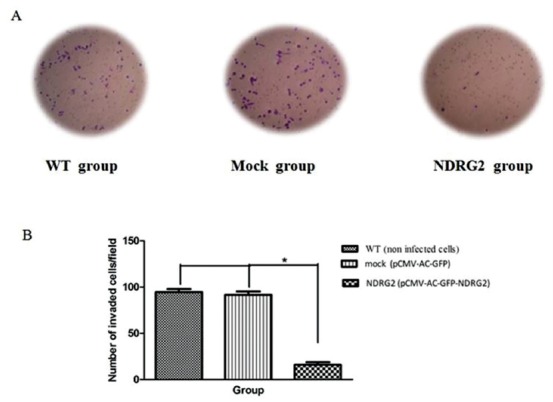
NDRG2 overexpression reduced invasion of SW48 cell. Cell invasion was determined by matrigel-transwell assay. Compared with the control and mock group, overexpression of NDRG2 significantly decreased the number of invaded SW48 cells. The results are the mean±SD of at least three independent experiments (*P<0.001 compare to WT group).
The Effects of NDRG2 Overexpression on Cell Motility of SW48 Cells
The effect of NDRG2 overexpression on SW48 cell motility was measured at 24 hours after stable transfection, using wound healing assay. NDRG2 expressing cells spread along the wound edges significantly moved slower to area of scratch than vector-transfect cells (mock group) indicating NDRG2 inhibit cell motility (figure 5).
Figure 5.
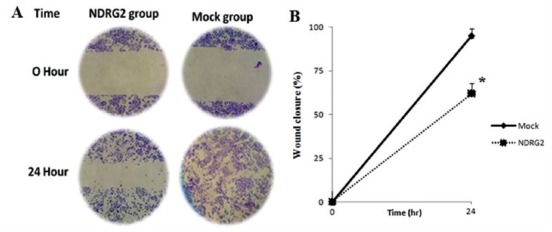
NDRG2 overexpression reduced SW48 cells cell motility. The cell motility of stably transfected pCMV6-AC-GFP-NDRG2 cells (NDRG2 group) and pCMV6-AC-GFP transfected cells (mock group) was determined by wound migration assay. A: Representative photographs taken at 0 h and 24 h after scratch creating (40×). B: Percent of wound closure was measured at 0 h and 24 h after scratch creating using image J software. The experiments were performed in triplicate (*P<0.01).
NDRG2 Overexpression Decreases Activity of MMP-9 of SW48 Cells
Gelatin zymography technique was used to examine the effect of NDRG2 on the secretion of MMP9 from the cells into the surrounding medium. The activity of MMP-9 is appeared as clear band at 82 kd. As shown in figure 6, NDRG2 caused a significant decrease in the activity of secreted MMP-9 in the surrounding medium of NDRG2 group compared to WT and mock group (*P<0.001).
Figure 6.
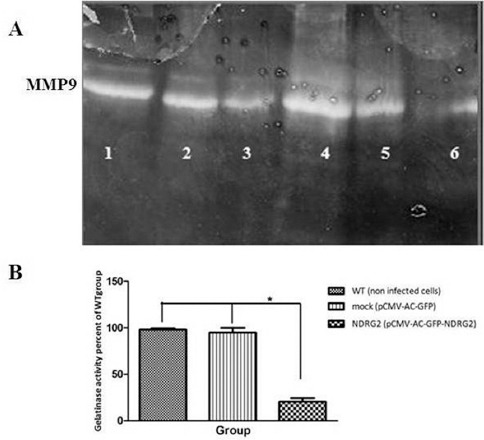
Gelatinase activity of MMP-9 in non-infected cells (WT group, Lane 1, 4), pCMV-Ac-GFP (mock group, Lane 2, 5), and pCMV-Ac-GFP-NDRG2 (NDRG2 group, Lane 3, 6) was measured. Active MMP-9 (82 Kd) degraded gelatin in gelatin-containing SDS-PAGE and left a band that was not stained by Coomassie Blue (A). NDRG2 expression reduced MMP-9 activity compared to the other groups (B). The experiment was performed in triplicate. (*P<0.001).
Discussion
Tumor suppressor genes inhibit the proliferation and invasion of cancer cells. A growing body of evidence has suggested the role of human NDRG2 as a tumor suppressor gene. In order to investigate the effects of NDRG2 on the proliferation and invasion of CRC cells, we established an invasive CRC cell line, SW48-NDRG2 cell, which stably expressed human NDRG2. Our findings demonstrated that the stable expression of NDRG2 attenuated the proliferation, invasion, and cell motility of the SW48-NDRG2 cells. The results also revealed reduction of MMP9 activity upon expression of NDRG2. These findings suggested that NDRG2 may function as an anti-proliferative and anti-metastasis factor in the CRC cells.
The first finding of our study was the inhibitory effects of NDRG2 on the proliferation of SW48-NDRG2 cells. The results of MTT assay demonstrated that the proliferative capacity of SW48-NDRG2 cells was continuously reduced in the days after overexpression of NDRG2 protein, suggesting a role of NDRG2 in inhibiting SW48 cell proliferation. Similar results have been shown in normal and tumor cells. Yang et al. has reported that overexpression of NDRG2 inhibited proliferation of normal liver cell through p53- and p21-mediated suppression of cell cycle.23 In a study conducted by Kim et al.,24 they have shown that the stable expression of NDRG2 arrested the SW620 cells in the G1/S phase of the cell cycle through inhibiting cyclin D and increasing p21 expression. The other possible mechanism for anti-proliferative effects of NDRG2 may be induction of cell apoptosis. It has been demonstrated that NDRG2 reduced proliferation of prostate25 and breast cancer cells26 through stimulation of cell apoptosis. Furthermore, it has been recently shown that NDRG2 inhibited the proliferation of renal tumor cells upon expression in the mitochondria. Thus, inhibiting ATP synthesis through interfering in the enzymatic activity of ATP synthase or through the inhibiting respiratory chain in the inner membrane of mitochondria is another possible explanation for the anti-proliferative effects of NDRG2.27
Another finding of the present study was the inhibition of SW48 cells migration and invasion by NDRG2. Our results revealed that the numbers of migrated and invaded cells were significantly fewer in SW48-NDRG2 cells compared to control cells. These results also confirmed by the findings of wound healing motility assay, which showed a lower migration rate of SW48-NDRG2 cells compared to the control cells. Similar results have been reported in prostate,28 breast,29 and hepatocarcinoma cancer30 cell lines. Taken together, these data implied the role of NDRG2 as general anti-metastatic factor in these tumors. The exact molecular mechanisms that are behind the NDRG2 effects in SW48 cell remain unknown at present. Down-regulation of cyclin D,24 up-regulation of P53,11 induction of bone morphogenetic protein-4,31 attenuation of TCF/catenin signaling pathway18 are among the mechanisms that previously suggested for the effects of NDRG2 on other cell line. Further investigations are needed to explore the role of these pathways in the mechanism of NDRG2 effects in SW48 cells.
MMP9 (gelatinase B) is known to play important roles in tumor invasion via degradation of extracellular matrix proteins and activation of latent transforming growth factor beta (TGF-β).32 The role of MMP-9 in the invasion and metastasis of CRC has been implicated.33 In a recent study, it has been demonstrated that overexpression of MMP-9 increased metastasis of CRC tumors to the lung.34 In the patients with CRC, high levels of serum MMP-9 and its positive correlation with the stage of the tumor have been shown.35 Furthermore, it has been indicated that the inhibitory effects of several natural substances36,37 or drugs38 against invasion of CRC cells is mediated by inhibition of MMP9 activity. SW48 cell is among highly invasive and proliferative CRC cell lines, which display a high expression of invasion-associated genes, including MMP9.17 In this study, we used a well-known technique of gelatin zymography to investigate the effects of NDRG2 overexpression on secreted MMP-9 activity. This technique is based on the degradation of gelatin by MMP-9, a repeatable and sensitive method that can detect picogram levels of MMP-9. Furthermore, it can discriminate between MMP-2 and MMP-9 and their respective pro-enzymes (pro-MMP-2 and pro-MMP-9). Consistent with the findings of a previous study, our data showed a high level of MMP-9 activity in the medium of SW48 cells.17 The results of gelatin zymography also demonstrated a reduction of MMP9 activity in NDRG2 overexpressing cells compared to control and to mock cells. These findings suggest that NDRG2 overexpression, probably affects cell invasion via inhibition of MMP-9. Similar results have been shown in other tumor cells, including breast cancer cells,31 bladder cancer cell line,10 and prostate cancer cells.28 The activities of MMP9 can be influenced by several factors, including the rate of its expression. We did not evaluate the effect of NDRG2 on MMP9 gene expression. It is a limitation of our study, thus our results need to be reconfirmed in studies employing real time PCR in order to define the effect of NDRG2 on MMP9 gene expression. However, even with this limitation, our results indicated that NDRG2 inhibited invasion and reduced MMP 9 activity.
Our findings demonstrated the expression of NDRG2 in the cytoplasm and nucleus of stably transfected SW48-NDRG2 cells. A similar result has been reported in astrocytes.39 The presence of nuclear localization signal (NLS) sequence is required for translocation of many proteins to the nucleus of the cells. However, NLS sequence has not been shown in the primary structure of NDRG2. Hwang et al.40 showed that a part of NDRG2 structure between 101 to 178 amino acid sequences of NDRG2 protein is responsible for translocation of NDRG2 to the nucleus. It has been demonstrated that under basal condition when the expression of NDRG2 is low, it is mainly localized in the cytoplasm of the cells. When the cells are activated by stimulation factors, such as ischemia or hypoxia, NDRG2 is overexpressed and translocated to the nucleus.40 Thus, it appears that nucleolar localization of NDRG2, which seen in our study, may be related to the overexpression of NDRG2. Further studies using confocal microscopic studies are necessary to confirm these findings.
Conclusions
In conclusion, the present data support the view that overexpression of NDRG2 inhibits proliferation, migration, invasion, and cell motility of SW48 cells. Although further studies are needed to explore the potential mechanisms by which NDRG2 modulates viability and invasive characteristics of SW48 cells, our data suggests that inhibition of MMP9 activity could be a molecular mechanism for anti-metastatic effects of NDRG2. Confirmation of these in vitro data by further in vivo studies may provide a basis for use of NDRG2 as a molecular target in inhibition of metastasis of colorectal cancer.
Acknowledgment
This manuscript was extracted from the MSc thesis of Ali Golestan and was supported by grant number 92-6530 from the Vice-Chancellor for Research Affairs of Shiraz University of Medical Sciences. We are also grateful to all staff of Diagnostic Laboratory Sciences and Technology Research Center and Institute for Cancer Research Center for technical assistance in this work.
Conflict of Interest: None declared.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide:sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2014;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Mols F, Beijers T, Vreugdenhil G, van de Poll-Franse L. Chemotherapy-induced peripheral neuropathy and its association with quality of life:a systematic review. Support Care Cancer. 2014;22:2261–9. doi: 10.1007/s00520-014-2255-7. [DOI] [PubMed] [Google Scholar]
- 3.Worni M, Shah KN, Clary BM. Colorectal cancer with potentially resectable hepatic metastases:optimizing treatment. Curr Oncol Rep. 2014;16:407. doi: 10.1007/s11912-014-0407-z. [DOI] [PubMed] [Google Scholar]
- 4.Melotte V, Qu X, Ongenaert M, van Criekinge W, de Bruïne AP, Baldwin HS, et al. The N-myc downstream regulated gene (NDRG) family:diverse functions, multiple applications. FASEB J. 2010;24:4153–66. doi: 10.1096/fj.09-151464. [DOI] [PubMed] [Google Scholar]
- 5.Lorentzen A, Lewinsky RH, Bornholdt J, Vogel LK, Mitchelmore C. Expression profile of the N-myc Downstream Regulated Gene 2 (NDRG2) in human cancers with focus on breast cancer. BMC Cancer. 2011;11:14. doi: 10.1186/1471-2407-11-14. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dandoy-Dron F, Monthioux E, Jami J, Bucchini D. Regulatory regions of rat insulin I gene necessary for expression in transgenic mice. Nucleic Acids Res. 1991;19:4925–30. doi: 10.1093/nar/19.18.4925. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou B, Tang Z, Deng Y, Hou S, Liu N, Lin W, et al. Tumor suppressor candidate gene, NDRG2 is frequently inactivated in human glioblastoma multiforme. Mol Med Rep. 2014;10:891–6. doi: 10.3892/mmr.2014.2237. [DOI] [PubMed] [Google Scholar]
- 8.Skiriute D, Tamasauskas S, Asmoniene V, Saferis V, Skauminas K, Deltuva V, et al. Tumor grade-related NDRG2 gene expression in primary and recurrent intracranial meningiomas. J Neurooncol. 2011;102:89–94. doi: 10.1007/s11060-010-0291-9. [DOI] [PubMed] [Google Scholar]
- 9.Lee DC, Kang YK, Kim WH, Jang YJ, Kim DJ, Park IY, et al. Functional and clinical evidence for NDRG2 as a candidate suppressor of liver cancer metastasis. Cancer Res. 2008;68:4210–20. doi: 10.1158/0008-5472.CAN-07-5040. [DOI] [PubMed] [Google Scholar]
- 10.Li R, Yu C, Jiang F, Gao L, Li J, Wang Y, et al. Overexpression of N-Myc downstream-regulated gene 2 (NDRG2) regulates the proliferation and invasion of bladder cancer cells in vitro and in vivo. PLoS One. 2013;8:e76689. doi: 10.1371/journal.pone.0076689. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao W, Zhang JL, Feng DY, Liu XW, Li Y, Wang LF, et al. The effect of adenovirus-conjugated NDRG2 on p53-mediated apoptosis of hepatocarcinoma cells through attenuation of nucleotide excision repair capacity. Biomaterials. 2014;35:993–1003. doi: 10.1016/j.biomaterials.2013.09.096. [DOI] [PubMed] [Google Scholar]
- 12.Feng L, Xie Y, Zhang H, Wu Y. Down-regulation of NDRG2 gene expression in human colorectal cancer involves promoter methylation and microRNA-650. Biochem Biophys Res Commun. 2011;406:534–8. doi: 10.1016/j.bbrc.2011.02.081. [DOI] [PubMed] [Google Scholar]
- 13.Kim YJ, Kang HB, Yim HS, Kim JH, Kim JW. NDRG2 positively regulates E-cadherin expression and prolongs overall survival in colon cancer patients. Oncol Rep. 2013;30:1890–8. doi: 10.3892/or.2013.2642. [DOI] [PubMed] [Google Scholar]
- 14.Kim JT, Kim JW, Kang YH, Kim KD, Lee SJ, Choi SC, et al. NDRG2 and PRA1 interact and synergistically inhibit T-cell factor/β-catenin signaling. FEBS Lett. 2012;586:3962–8. doi: 10.1016/j.febslet.2012.09.045. [DOI] [PubMed] [Google Scholar]
- 15.Chu D, Zhao Z, Zhou Y, Li Y, Li J, Zheng J, et al. Matrix metalloproteinase-9 is associated with relapse and prognosis of patients with colorectal cancer. Ann Surg Oncol. 2012;19:318–25. doi: 10.1245/s10434-011-1686-3. [DOI] [PubMed] [Google Scholar]
- 16.Li CY, Yuan P, Lin SS, Song CF, Guan WY, Yuan L, et al. Matrix metalloproteinase 9 expression and prognosis in colorectal cancer:a meta-analysis. Tumour Biol. 2013;34:735–41. doi: 10.1007/s13277-012-0601-2. [DOI] [PubMed] [Google Scholar]
- 17.Roh SA, Choi EY, Cho DH, Jang SJ, Kim SY, Kim YS, et al. Growth and invasion of sporadic colorectal adenocarcinomas in terms of genetic change. J Korean Med Sci. 2010;25:353–60. doi: 10.3346/jkms.2010.25.3.353. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim YJ, Yoon SY, Kim JT, Song EY, Lee HG, Son HJ, et al. NDRG2 expression decreases with tumor stages and regulates TCF/beta-catenin signaling in human colon carcinoma. Carcinogenesis. 2009;30:598–605. doi: 10.1093/carcin/bgp047. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flis S, Gnyszka A, Flis K. DNA methyltransferase inhibitors improve the effect of chemotherapeutic agents in SW48 and HT-29 colorectal cancer cells. PLoS One. 2014;9:e92305. doi: 10.1371/journal.pone.0092305. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen HX, Wang S, Wang Z, Zhang ZP, Shi SS. Overexpression of RUNX3 inhibits malignant behaviour of Eca109 cells in vitro and vivo. Asian Pac J Cancer Prev. 2014;15:1531–7. doi: 10.7314/APJCP.2014.15.4.1531. [DOI] [PubMed] [Google Scholar]
- 21.Zhao S, Sun HZ, Zhu ST, Lu H, Niu ZF, Guo WF, et al. Effects of parafibromin expression on the phenotypes and relevant mechanisms in the DLD-1 colon carcinoma cell line. Asian Pac J Cancer Prev. 2013;14:4249–54. doi: 10.7314/APJCP.2013.14.7.4249. [DOI] [PubMed] [Google Scholar]
- 22.Hwang BM, Chae HS, Jeong YJ, Lee YR, Noh EM, Youn HZ, et al. Protein tyrosine phosphatase controls breast cancer invasion through the expression of matrix metalloproteinase-9. BMB Rep. 2013;46:533–8. doi: 10.5483/BMBRep.2013.46.11.053. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Li Y, Wu L, Zhang Z, Han T, Guo H, et al. NDRG2 in rat liver regeneration:role in proliferation and apoptosis. Wound Repair Regen. 2010;18:524–31. doi: 10.1111/j.1524-475X.2010.00614.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim YJ, Yoon SY, Kim JT, Choi SC, Lim JS, Kim JH, et al. NDRG2 suppresses cell proliferation through down-regulation of AP-1 activity in human colon carcinoma cells. Int J Cancer. 2009;124:7–15. doi: 10.1002/ijc.23945. [DOI] [PubMed] [Google Scholar]
- 25.Deng Y, Yao L, Chau L, Ng SS, Peng Y, Liu X, et al. N-Myc downstream-regulated gene 2 (NDRG2) inhibits glioblastoma cell proliferation. Int J Cancer. 2003;106:342–7. doi: 10.1002/ijc.11228. [DOI] [PubMed] [Google Scholar]
- 26.Ma J, Liu W, Yan X, Wang Q, Zhao Q, Xue Y, et al. Inhibition of endothelial cell proliferation and tumor angiogenesis by up-regulating NDRG2 expression in breast cancer cells. PLoS One. 2012;7:e32368. doi: 10.1371/journal.pone.0032368. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Qiang S, Du ZF, Huang M. Adenovirus-mediated NDRG2 inhibits the proliferation of human renal cell carcinoma cell line OS-RC-2 in vitro. Asian Pac J Trop Med. 2014;7:873–8. doi: 10.1016/S1995-7645(14)60152-8. [DOI] [PubMed] [Google Scholar]
- 28.Gao L, Wu GJ, Liu XW, Zhang R, Yu L, Zhang G, et al. Suppression of invasion and metastasis of prostate cancer cells by overexpression of NDRG2 gene. Cancer Lett. 2011;310:94–100. doi: 10.1016/j.canlet.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Zheng J, Liu Q, Li Y, Yang J, Ma J, Yu F, et al. NDRG2 expression regulates CD24 and metastatic potential of breast cancer cells. Asian Pac J Cancer Prev. 2010;11:1817–21. [PubMed] [Google Scholar]
- 30.Zheng J, Li Y, Yang J, Liu Q, Shi M, Zhang R, et al. NDRG2 inhibits hepatocellular carcinoma adhesion, migration and invasion by regulating CD24 expression. BMC Cancer. 2011;11:251:1–9. doi: 10.1186/1471-2407-11-251. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shon SK, Kim A, Kim JY, Kim KI, Yang Y, Lim JS. Bone morphogenetic protein-4 induced by NDRG2 expression inhibits MMP-9 activity in breast cancer cells. Biochem Biophys Res Commun. 2009;385:198–203. doi: 10.1016/j.bbrc.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 32.Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9):the next decade. Crit Rev Biochem Mol Biol. 2013;48:222–72. doi: 10.3109/10409238.2013.770819. [DOI] [PubMed] [Google Scholar]
- 33.Chen JS, Wang Q, Fu XH, Huang XH, Chen XL, Cao LQ, et al. Involvement of PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in hepatocellular carcinoma:Association with MMP-9. Hepatol Res. 2009;39:177–86. doi: 10.1111/j.1872-034X.2008.00449.x. [DOI] [PubMed] [Google Scholar]
- 34.Chen X, Su Y, Fingleton B, Acuff H, Matrisian LM, Zent R, et al. Increased plasma MMP9 in integrin alpha1-null mice enhances lung metastasis of colon carcinoma cells. Int J Cancer. 2005;116:52–61. doi: 10.1002/ijc.20997. [DOI] [PubMed] [Google Scholar]
- 35.Dragutinović VV, Radonjić NV, Petronijević ND, Tatić SB, Dimitrijević IB, Radovanović NS, et al. Matrix metalloproteinase-2 (MMP-2) and -9 (MMP-9) in preoperative serum as independent prognostic markers in patients with colorectal cancer. Mol Cell Biochem. 2011;355:173–8. doi: 10.1007/s11010-011-0851-0. [DOI] [PubMed] [Google Scholar]
- 36.Auyeung KK, Law PC, Ko JK. Combined therapeutic effects of vinblastine and Astragalus saponins in human colon cancer cells and tumor xenograft via inhibition of tumor growth and proangiogenic factors. Nutr Cancer. 2014;66:662–74. doi: 10.1080/01635581.2014.894093. [DOI] [PubMed] [Google Scholar]
- 37.Umesalma S, Nagendraprabhu P, Sudhandiran G. Antiproliferative and apoptotic-inducing potential of ellagic acid against 1,2-dimethyl hydrazine-induced colon tumorigenesis in Wistar rats. Mol Cell Biochem. 2014;388:157–72. doi: 10.1007/s11010-013-1907-0. [DOI] [PubMed] [Google Scholar]
- 38.Papi A, Ferreri AM, Guerra F, Orlandi M. Anti-invasive effects and proapoptotic activity induction by the rexinoid IIF and valproic acid in combination on colon cancer cell lines. Anticancer Res. 2012;32:2855–62. [PubMed] [Google Scholar]
- 39.Okuda T, Kokame K, Miyata T. Differential expression patterns of NDRG family proteins in the central nervous system. J Histochem Cytochem. 2008;56:175–82. doi: 10.1369/jhc.7A7323.2007. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hwang J, Kim Y, Kang HB, Jaroszewski L, Deacon AM, Lee H, et al. Crystal structure of the human N-Myc downstream-regulated gene 2 protein provides insight into its role as a tumor suppressor. J Biol Chem. 2011;286:12450–60. doi: 10.1074/jbc.M110.170803. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]


