Abstract
To walk efficiently and stably on different surfaces under various constrained conditions, humans need to adapt their gait pattern substantially. Although the mechanisms behind locomotor adaptation are still not fully understood, the cerebellum is thought to play an important role. In this study we aimed to address the specific localization of cerebellar involvement in split-belt adaptation by comparing performance in patients with stable focal lesions after cerebellar tumor resection and in healthy controls. We observed that changes in symmetry of those parameters that were most closely related to interlimb coordination (such as step length and relative double stance time) were similar between healthy controls and cerebellar patients during and after split-belt walking. In contrast, relative stance times (proportions of stance in the gait cycle) were more asymmetric for the patient group than for the control group during the early phase of the post-split-belt condition. Patients who walked with more asymmetric relative stance times were more likely to demonstrate lesions in vermal lobules VI and Crus II. These results confirm that deficits in gait adaptation vary with ataxia severity and between patients with different types of cerebellar damage.
Keywords: ataxia, cerebellum, gait, locomotion, step length symmetry
to walk efficiently and stably on different surfaces under various constrained conditions, humans are able to substantially adapt their gait pattern. This locomotor adaptation is an important aspect of human gait and is often studied, both because it sheds light on the neural control of gait (e.g., Choi and Bastian 2007; Dietz et al. 1994) and for its role in motor learning and gait retraining (e.g., Lauzière et al. 2014; Reisman et al. 2007, 2013). Although the mechanisms behind locomotor adaptation are still not fully understood, the cerebellum is thought to play an important role (Hoffland et al. 2014; Ilg et al. 2008; Jayaram et al. 2011, 2012; Morton and Bastian 2006), similar to its involvement in visuomotor and force-field adaptations in the upper limbs (Burciu et al. 2014; Martin et al. 1996; Smith and Shadmehr 2005; Tseng et al. 2007).
Locomotor adaptation is often studied using a split-belt paradigm (for review, see Torres-Oviedo et al. 2011). In such a paradigm, participants walk on a dual-belt treadmill that consists of two parallel belts, one for each leg, which can run at different speeds (split-belt condition). In a classic split-belt adaptation paradigm, participants walk with one of the belts running at twice (or thrice) the speed of the other, for an extended period of time (5–15 min). Initially, participants walk with asymmetric step lengths, taking longer steps with the leg on the slow belt. Over time they adapt their gait pattern toward more symmetric step lengths. This locomotor adaptation results in aftereffects when belts are returned to equal speeds. In this postadaptation condition healthy participants again initially walk with asymmetric step lengths, but now taking longer steps with the leg that has been on the fast belt during the split-belt condition. Within a few minutes these aftereffects disappear and step lengths return to baseline (symmetric) values. Changes in temporal gait parameters and in step length during split-belt walking were first described by Dietz et al. (1994) and Reisman et al. (2005), respectively. Since then, split-belt adaptation has been studied in different conditions and in multiple (patient) populations (for review, see Torres-Oviedo et al. 2011).
In an influential study, Morton and Bastian (2006) were able to show that severely ataxic patients with degenerative cerebellar disease do not display the typical features of split-belt adaptation. Although these patients were able to quickly change intralimb gait parameters when exposed to split-belt walking, similar to healthy controls, they did not adapt interlimb gait parameters, whereas healthy controls did (Morton and Bastian 2006). Specifically, limb excursion and relative stance time (parameters most closely related to intralimb coordination: coordinated within 1 leg, “intralimb parameters”) changed similarly in both groups from baseline to the early phase of the split-belt condition, but no significant aftereffects were observed in either group. Note that limb excursion was referred to as “stride length” by Morton and Bastian (2006). In contrast, gait parameters most closely related to interlimb coordination (coordinated between 2 legs, “interlimb parameters”), such as step length and relative double stance time, differed between groups. In the healthy controls these parameters changed from the early phase to the late phase of the split-belt condition and displayed a significant aftereffect. On the other hand, in the severely ataxic patient group, no changes during the split-belt condition or aftereffects were observed (Morton and Bastian 2006).
In addition to these observations of impaired split-belt adaptation in severely ataxic patients with degenerative cerebellar disease (Morton and Bastian 2006), other studies using a variety of brain stimulation techniques also found relations between the cerebellum and split-belt adaptation (Jayaram et al. 2011, 2012). However, although cerebellar involvement in locomotor adaptation has been observed repeatedly, it is still under debate where exactly this involvement is localized within the cerebellum (Ilg et al. 2008; Morton and Bastian 2006). Morton and Bastian (2006) could not directly address localization due to the diffuse nature of the cerebellar damage in their patient group but suggested that the midline vermis and fastigial nuclei would be most important in split-belt adaptation. Alternatively, Ilg et al. (2008) observed that damage in the intermediate cerebellum, the interposed nuclei, and adjacent dentate nuclei was related to impaired locomotor adaptation. It should be noted, however, that they used a different paradigm (adaptation to added mass at the legs). Hence the question remains whether these results can be extrapolated to split-belt walking.
One part of the cerebellum that has received special attention with respect to locomotion is the vermis, along with the concomitant fastigial nucleus. Mori and colleagues (Mori 1987; Mori et al. 1998, 2004) have underlined that locomotion needs integration of neuronal subsystems involved in posture and locomotion. The fastigial nucleus receives input from a variety of sensory sources and projects to the reticulospinal, vestibulospinal, and fastigiospinal pathways. Stimulation of these pathways (at the midline region of the hook bundle of Russell) evokes a general increase in postural muscle tone in cats standing on a stationary surface (Asanome et al. 1998) and induces locomotion when the cat is placed on the surface of a moving treadmill (Mori et al. 1999). In humans, with the use of functional magnetic resonance imaging, it was shown that the same networks were activated during mental imagery of standing and walking (Jahn et al. 2008). It is also important to point out that there are strong connections between the cerebellar and the mesencephalic locomotor regions (e.g., the pedunculopontine nucleus). In recent years it was found that this connectivity is deficient in patients with Parkinson's disease, especially those with freezing of gait (Fling et al. 2013). This has implications for split-belt walking, since it was observed that patients with freezing of gait and patients in the off dopaminergic state adapt less and slower to split-belt walking (Mohammadi et al. 2015; Nanhoe-Mahabier et al. 2013; Roemmich et al. 2014).
The aim of the present study was to address the localization of the cerebellar involvement in split-belt adaptation by evaluating split-belt adaptation in patients with stable focal lesions following cerebellar tumor resection. On the basis of observations by Morton and Bastian (2006) and Ilg et al. (2008), we hypothesized that these patients would show several impairments during split-belt adaptation and that these impairments would be most pronounced in patients with lesions in the interposed nuclei. Specifically, with respect to interlimb coordination, we expected that patients would walk with a larger asymmetry in step lengths during the early phase of the split-belt condition and that this asymmetry would still be present during the late phase of the split-belt condition. Furthermore, we predicted that because of this reduced adaptation to split-belt walking, these patients would walk with more symmetric step lengths during the early phase of the post condition. Finally, we hypothesized that patients with focal cerebellar lesions would show changes in intralimb gait parameters similar to those in healthy controls during the split-belt paradigm: no changes from the early to the late phase of the split-belt condition and no aftereffects during the post condition.
MATERIALS AND METHODS
Participants.
Fifteen patients with stable focal lesions after cerebellar tumor resection (age 23.0 ± 6.2 yr, mean ± SD; 10 women, 5 men; Table 1) and 13 healthy participants (age: 25.3 ± 4.6 yr; 9 women, 4 men) participated. All patients suffered from cerebellar tumors (pilocytic astrocytoma grade I, n = 6; astrocytoma grade II, n = 2; medulloblastoma, n = 5; Lhermitte-Duclos disease, n = 1; or hemangioblastoma, n = 1; Table 1). Seven patients received adjuvant radiotherapy; four of these patients received adjuvant chemotherapy (overview, Table 1; therapy details, Table 2). Extracerebellar damage, assessed on MRI images, was mainly limited to a residually enlarged supratentorial ventricular system or sequela due to a ventriculoperitoneal shunt in the right frontal lobe in some patients (Table 2). Patients were in a stable condition (>5 years postoperative, range 5.7–30.2 yr; Table 1) and were able to walk independently. All patients were able to walk on the treadmill without holding the hand railing. We rated severity of ataxia using the International Cooperative Ataxia Rating Scale (ICARS) (Trouillas et al. 1997). In this 100-point scale, a score of 0 indicates no deficits and increasing scores indicate more, or more severe, ataxic deficits. ICARS scores in our patient group ranged from 0 to 19, with only 3 patients scoring higher than 10 (Table 1). All participants gave written informed consent. The experiments were conducted in accordance with the Declaration of Helsinki and were approved by the local ethics committee.
Table 1.
Patients were mildly ataxic and in a stable condition (>5 yr postoperative)
| Adjuvant Therapies |
ICARS |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age, yr | Time Postop, yr | Sex, M/F | Diagnosis | Interposed Nuclei Lesioned | RT | CT | Lesion Volume, cm3 | Total/100 | P&G/34 | Kin Funct/52 |
| 1 | 28.8 | 13.9 | F | Lhermitte-Duclos disease | 58.0 | 3 | 1 | 1 | |||
| 2 | 20.2 | 8.7 | F | Pilocytic astrocytoma | 8.2 | 3 | 0 | 0 | |||
| 3 | 19.6 | 11.8 | M | Pilocytic astrocytoma | Left | 1.7 | 6 | 3 | 2 | ||
| 4 | 18.1 | 6.5 | F | Pilocytic astrocytoma | 4.5 | 1 | 0 | 1 | |||
| 5 | 22.3 | 17.7 | M | Medulloblastoma | Both | Y | 22.0 | 13 | 6 | 2 | |
| 6 | 18.5 | 10.0 | F | Medulloblastoma | Left | Y | Y | 22.6 | 2 | 1 | 1 |
| 7 | 18.6 | 13.7 | M | Medulloblastoma | Right | Y | Y | 6.3 | 19 | 5 | 10 |
| 8 | 18.4 | 15.5 | F | Medulloblastoma | Both | Y | Y | 5.4 | 5 | 1 | 3 |
| 9 | 39.9 | 30.2 | F | Hemangioblastoma | No MRI | Y | 6 | 4 | 2 | ||
| 10 | 20.5 | 13.1 | F | Pilocytic astrocytoma | 36.3 | 2 | 1 | 0 | |||
| 11 | 19.0 | 5.7 | M | Pilocytic astrocytoma | Right | 15.7 | 7 | 2 | 5 | ||
| 12 | 22.0 | 18.7 | M | Astrocytoma grade II | 2.0 | 1 | 0 | 1 | |||
| 13 | 21.6 | 19.5 | F | Astrocytoma grade II | Right | Y | 7.1 | 0 | 0 | 0 | |
| 14 | 26.9 | 24.9 | F | Pilocytic astrocytoma | Y | 58.4 | 5 | 1 | 1 | ||
| 15 | 31.3 | 18.2 | F | Medulloblastoma | Both | Y | Y | 14.2 | 17 | 10 | 4 |
For patient 9 no MRI data were acquired.
Postop, postoperative; F, female; M, male; RT, radiotherapy; CT, chemotherapy; Y, yes; ICARS, International Cooperative Ataxia Rating Scale; P&G, posture and gait subscore; Kin Funct, kinetic functions subscore.
Table 2.
Treatment details
| Patient | Diagnosis | Time Post-RT, yr | Target Areas Dose RT, Gy | Hypo-pituitarism | Time Post-CT, yr | Total Duration CT, mo | Scheme | VP Shunt | Extracerebellar sequela |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Lhermitte-Duclos disease | Y | |||||||
| 2 | Pilocytic astrocytoma | Y | |||||||
| 3 | Pilocytic astrocytoma | ||||||||
| 4 | Pilocytic astrocytoma | ||||||||
| 5 | Medulloblastoma | 17.6 | 35.2 CSP +10 SP +20 FP | Y | Y | Y* | |||
| 6 | Medulloblastoma | 9.9 | 35.2 CSP +20 FP | 8.7 | 12 | HIT-2000 | Y | Y† | |
| 7 | Medulloblastoma | 13.1 | 35.2 CSP +10 SP +20 FP | Y | 13.0 | 8 | HIT-91 | Y | Y† |
| 8 | Medulloblastoma | 15.2 | 35.2 CSP +10 SP +20 FP | Y | 15.2 | 3 | HIT-91 | Y‡ | |
| 9 | Hemangioblastoma | Y | |||||||
| 10 | Pilocytic astrocytoma | ||||||||
| 11 | Pilocytic astrocytoma | ||||||||
| 12 | Astrocytoma grade II | ||||||||
| 13 | Astrocytoma grade II | 19.4 | 50.4 FP | ||||||
| 14 | Pilocytic astrocytoma | 24.7 | 60 FP | Y† | |||||
| 15 | Medulloblastoma | 18.1 | 35.2 CSP +10 SP +20 FP | Y | 17.5 | 4 | HIT-2000 | Y | Y†,§ |
Target areas: CSP, craniospinal; SP, spinal; FP, fossa posterior. Y, yes; VP, ventriculoperitoneal. CT schemes: HIT-2000, cisplatinum, vincristine, CCNU; HIT-91, ifosfamide, etoposide (VP16), metotrexate, ara-C, cisplatinum. Extracerebellar sequelae:
Thalamic cavernous angioma, asymptomatic.
Hydrocephalus.
Cavernous angioma parietal white matter, asymptomatic; cavernous angioma intramedullary spinal cord, level D12, 1.8 × 2.6 mm, asymptomatic.
Ventriculocisternal shunt.
Experimental setup and protocol.
In general, procedures were similar to those of Bruijn et al. (2012). On arrival of the participant in the lab, reflective markers were placed on the pelvis and lateral malleoli of the participants for movement registration with an optoelectronic system (Vicon Nexus; Oxford Metrics, Oxford, UK). Throughout all conditions, kinematics were sampled at 100 samples/s. In addition, during the walking trials on the treadmill, three-dimensional (3D) ground reaction forces and torques were sampled at 1,000 samples/s (instrumented dual-belt treadmill, custom built by ForceLink, Culemborg, The Netherlands).
Before the split-belt trials, we assessed comfortable overground walking speed. Participants walked a distance of 6 m at their natural pace (Abellan van Kan et al. 2009; Bruijn et al. 2012). This was repeated three times, and all walking trials were performed barefoot. Next, participants were familiarized to treadmill walking. During the treadmill trials, participants wore a safety harness attached to the ceiling and were not holding the hand railing. The split-belt paradigm started with 3-min walking with both belts at 1.0 m/s (baseline). Participants then performed a classic split-belt paradigm, consisting of 10 min of walking with one belt at 1.0 m/s and the other belt at 0.5 m/s, followed by 5 min with both belts at the same speed (e.g., Bruijn et al. 2012; Choi and Bastian 2007; Morton and Bastian 2006). During the split-belt (“split”) condition, patients walked with the most affected side on the fast belt, and healthy controls walked with their nondominant leg on the fast belt (Morton and Bastian 2006). In the subsequent tied-belt (“post”) condition, both belts ran at 1.0 m/s (Bruijn et al. 2012), close to the participants' preferred overground walking speed but different from the study by Morton and Bastian (2006), where aftereffects were assessed at 0.5 m/s. Between conditions the treadmill was stopped for a maximum of 30 s, and during the start of all treadmill conditions the belts had an acceleration of 0.3 m/s2 (Bruijn et al. 2012).
Data analyses.
Generally, data analysis procedures were similar to those of Bruijn et al. (2012). We calculated overground walking speed as the mean forward velocity of the two posterior pelvis markers during the three overground walking trials (Hoogkamer et al. 2015b). We determined the instants of heel strike and toe-off based on the center of pressure trajectory (Roerdink et al. 2008). This method was validated using 3D kinematics. Nevertheless, all trials were visually inspected and manually corrected if needed, also using the 3D kinematic data of the ankle marker. All gait parameters calculated for the leg that was on the fast belt during the split condition are referred to as “fast” parameters, even for the baseline and post conditions (Reisman et al. 2005), and similarly for the “slow” leg. Gait parameters were calculated for each step/stride. Step length was calculated as the anterior-posterior distance between the ankle markers at heel contact (Reisman et al. 2005), with step lengthfast at the heel contact of the fast leg. In other words, step lengthfast is the anterior-posterior distance between the ankle marker of the fast leg and the ankle marker of the slow leg at the time of heel contact of the fast leg. Our main objective was to evaluate adaptation and aftereffects in step length symmetry (Choi et al. 2009):
Similarly to Morton and Bastian (2006), we also evaluated changes in symmetry of double support timing, limb excursion, and stance time. Double stance symmetry was calculated similarly to step length symmetry but based on the relative duration of the double stance phase. The relative duration of the double stance phase occurring at the end of the stance phase of the fast leg was referred to as double stancefast (Reisman et al. 2005).
Limb excursion was calculated as the distance traveled by the ankle marker in the anterior-posterior direction from heel contact to toe-off of one limb (Hoogkamer et al. 2014). It needs to be emphasized that this parameter reflects displacements during the stance phase (and not a step). Limb excursion symmetry was calculated analogous to step length symmetry. Finally, stance time symmetry was also determined in the same way, using the relative stance time of each leg. In other words, the proportion of stance in the gait cycle was compared for both sides.
Baseline values were calculated over all steps/strides of the baseline condition. For statistical analyses we calculated values over the early and late phases of the split and post conditions (summing up to 5 “episodes” in total: baseline, early split, late split, early post, and late post). early split and early post values were calculated as the mean value over the first five strides of the respective conditions. Late split and late post values were calculated as the mean value over the last 50 strides of the respective conditions, to obtain a more accurate plateau value.
MRI data acquisition and processing.
Image acquisition and processing procedures were similar to those in Hoogkamer et al. (2015c). A Philips 3T Achieva MRI scanner (Philips, Best, The Netherlands) with a 32-channel matrix head coil was used for image acquisition. A 3D MPRAGE (magnetization-prepared rapid acquisition gradient echo) high-resolution T1-weighted image (repetition time 970 ms, echo time 4.60 ms, flip angle 8°, 230 1-mm slices, in-plane resolution 0.97 × 0.98, 384 × 384 matrix) was acquired for all patients except P9 (see Table 1).
MRIcron software (http://www.mccauslandcenter.sc.edu/mricro/mricron/index.html) was used to manually trace the lesions on the MPRAGE images. Lesion traces were spatially normalized to the atlas of the cerebellum (Diedrichsen et al. 2009, 2011) using the SUIT toolbox (http://www.icn.ucl.ac.uk/motorcontrol/imaging/suit.htm; Diedrichsen 2006) in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). In some cases (large lesions at the outer border of the cerebellum) spatial normalization with the SUIT toolbox was inaccurate. In those cases lesions were spatially normalized, based on the whole brain image, and the normalized lesions were manually corrected in atlas space when needed, based on the original image (Ilg et al. 2008). Lobules and nuclei with lesions were listed (Table 3), and patients with lesions in the interposed nuclei were identified (Table 1). For further analysis, all left-sided normalized lesions were flipped to the right along the midline (Ilg et al. 2013).
Table 3.
Overview of lesioned lobules and nuclei
| Vermis |
Paravermis |
Hemispheres |
Nuclei |
|||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | VI | CI | CII | VIIb | VIIIa | VIIIb | IX | X | I–IV | V | VI | CI | CII | VIIb | VIIIa | VIIIb | IX | X | I–IV | V | VI | CI | CII | VIIb | VIIIa | VIIIb | X | F | I | D |
| 1 | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | ||||||||||||||
| 2 | R | R | R | R | R | |||||||||||||||||||||||||
| 3 | Y | Y | Y | Y | Y | L | L | L | L | L | L | |||||||||||||||||||
| 4 | R | R | R | R | R | R | ||||||||||||||||||||||||
| 5 | Y | Y | Y | Y | L | B | B | B | B | B | B | L | L | B | B | B | ||||||||||||||
| 6 | Y | Y | Y | Y | Y | Y | Y | B | B | L | L | B | B | B | B | B | L | L | B | L | B | |||||||||
| 7 | Y | Y | Y | Y | R | R | R | R | B | R | B | |||||||||||||||||||
| 8 | Y | Y | Y | Y | Y | Y | Y | B | B | B | R | B | B | |||||||||||||||||
| 9* | ||||||||||||||||||||||||||||||
| 10 | L | L | L | L | L | |||||||||||||||||||||||||
| 11 | R | R | R | R | R | R | R | R | R | R | R | R | R | R | ||||||||||||||||
| 12 | Y | Y | Y | Y | Y | Y | B | R | ||||||||||||||||||||||
| 13 | Y | Y | Y | Y | Y | Y | R | R | R | B | R | R | R | R | B | |||||||||||||||
| 14 | L | L | L | L | L | L | L | L | L | L | ||||||||||||||||||||
| 15 | Y | Y | Y | Y | Y | Y | Y | B | B | B | B | B | B | B | B | B | B | |||||||||||||
For patient 9, no MRI data were acquired.
Y, yes; L, left; R, right; B, both; F, fastigial nuclei; I, interposed nuclei; D, dentate nuclei.
We hypothesized that patients with lesions in the interposed nuclei would show several impairments during split-belt adaptation: a reduced step length symmetry during early split and late split and a more symmetric step length during early post compared with the healthy controls. To test this, we performed statistics on these outcome measures for the patient subgroups with and without lesions in the interposed nuclei (lesion-based approach; see Statistical analyses of behavioral data).
In addition, for voxel-based lesion-symptom mapping the patients were classified as “affected” or “unaffected” based on behavioral outcome measures, and then lesion locations between these subgroups were compared (symptom-based approach). This classification is commonly done with a cutoff threshold based on the behavioral data of the healthy controls, and this is not always straightforward (Hoogkamer and Meyns 2014). We applied a cutoff based on the 95% confidence interval of the values for the healthy controls. This was done for step length symmetry and in addition for stance time symmetry during early post. We used nonparametric mapping software within MRIcron (http://www.mccauslandcenter.sc.edu/mricro/npm/; Rorden et al. 2007) to perform the voxel-based lesion-symptom mapping analysis. We applied both statistical Liebermeister tests and subtraction analysis to identify lesion areas associated with deviant behavior (Christensen et al. 2014). For the Liebermeister test, significance threshold was set to z = 1.65 (α = 0.05), and only voxels damaged in at least two patients were considered (Ilg et al. 2013). Subtraction analysis was performed by subtracting the percentage of normally performing patients with a lesion in a specific voxel from the percentage of patients with deviant behavior with a lesion in that voxel (Christensen et al. 2014; Karnath et al. 2002). This was done for each lesioned voxel. We considered voxels that were at least 25% more likely to be lesioned in patients with deviant behavior (Christensen et al. 2014).
Statistical analyses of behavioral data.
Student's t-tests were used to compare overground walking speed and global gait parameters during baseline between healthy controls and the cerebellar lesion patient group. To evaluate changes over time and differences between groups in gait parameters during the split-belt paradigm, we performed two-factor repeated-measures ANOVAs with group and episode as factors. For each gait parameter we performed an ANOVA to compare early split and late split with baseline values. An additional ANOVA was performed to compare early post and late post with baseline values. Additionally, to compare gait parameters between the subgroups with and without lesions in the interposed nuclei (lesion-based approach, see above) and the healthy control group, we performed a one-way ANOVA with three groups. For significant main and interaction effects we performed Tukey's honestly significant difference post hoc analyses to identify significant differences between groups and/or episodes. Alongside significance, we report t values of t-tests, F values of ANOVAs, and q values of Tukey's post hoc tests, along with the corresponding degrees of freedom for each test. We used a traditional level of significance (α = 0.05) for all statistical tests; when appropriate, this value was corrected for the number of analyses.
RESULTS
Self-selected overground walking speed was reduced in patients with cerebellar lesions compared with healthy controls [1.13 ± 0.13 vs. 1.35 ± 0.13 m/s, respectively; t(26) = 4.47; P < 0.001]. During treadmill walking in the baseline condition (1.0 m/s, tied belts), gait parameters were similar between groups: stride time was 1.12 ± 0.06 vs. 1.12 ± 0.06 s [t(26) = 0.13; P = 0.90], stride time variability (SD) was 28 ± 8 vs. 28 ± 14 ms [t(26) = 0.01; P = 0.99], step length was 0.51 ± 0.03 vs. 0.51 ± 0.02 m [t(26) = 0.62; P = 0.54], and the relative stance time was 64.9 ± 0.7 vs. 65.0 ± 0.5% [t(26) = 0.58; P = 0.57] for the cerebellar lesion patients and the healthy controls, respectively. As such, these gait parameters confirmed that this group of patients with focal lesions in the cerebellum had only mild gait ataxia (see Table 1 for results of clinical evaluation).
Interlimb parameters.
First, we evaluated the parameters that were expected to differ, namely, the interlimb gait parameters: step length symmetry and double stance symmetry. However, in both groups, these parameters increased similarly during the split-belt paradigm (Fig. 1). There was asymmetry during the early phase of the split condition, and values approached symmetry in the late phase of the split condition. the curve for step length symmetry suggests that the patients return to more symmetric values in late split; however, there was no significant main effect for group [F(1,26) = 0.58; P = 0.45; Table 4] or group × episode interaction effect [Fig. 1A; F(2,52) = 0.21; P = 0.81]. Double stance symmetry had not completely returned to baseline values during late split [q(52) = 3.83; P = 0.024]. During the post condition, both step length symmetry and double stance symmetry initially showed an overshoot in asymmetry and gradually returned to symmetric values.
Fig. 1.
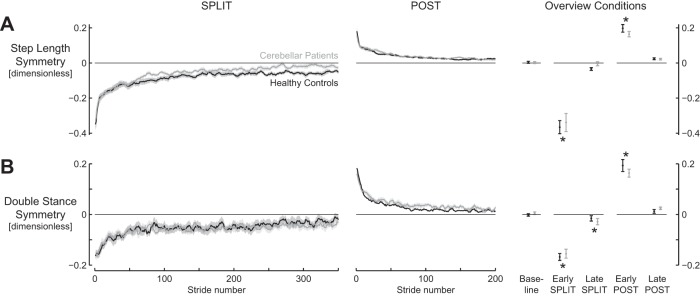
Interlimb gait parameters show similar changes for cerebellar lesion patients and healthy controls during split-belt walking. A: step length symmetry. B: double stance symmetry. Traces for cerebellar patients (gray) and healthy controls (black) during split (left) and post (middle) conditions. “Overview conditions” (right) summarizes values during the different phases and conditions. Shaded areas and error bars represent SE. Asterisks indicate values significantly different from baseline.
Table 4.
Summary of statistics
| SPLIT |
POST |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ANOVA |
Post hoc |
ANOVA |
Post hoc |
||||||||
| Factor | P value | Condition | Values | Comparison | P value | Factor | P value | Condition | Values | Comparison | P value |
| Step length symmetry | |||||||||||
| Group | 0.45 | Group | 0.27 | ||||||||
| Episode | <0.001 | BL | 0.00 ± 0.02 | BL vs. ES | <0.001 | Episode | <0.001 | BL | 0.00 ± 0.02 | BL vs. EP | <0.001 |
| ES | −0.35 ± 0.17 | ES vs. LS | <0.001 | ES | 0.18 ± 0.07 | EP vs. LP | <0.001 | ||||
| LS | −0.02 ± 0.04 | BL vs. LS | 0.70 | LS | 0.02 ± 0.02 | BL vs. LP | 0.14 | ||||
| Interaction | 0.81 | Interaction | 0.22 | ||||||||
| Double stance symmetry | |||||||||||
| Group | 0.87 | Group | 0.75 | ||||||||
| Episode | <0.001 | BL | 0.00 ± 0.02 | BL vs. ES | <0.001 | Episode | <0.001 | BL | 0.00 ± 0.02 | BL vs. EP | <0.001 |
| ES | −0.16 ± 0.06 | ES vs. LS | <0.001 | ES | 0.18 ± 0.07 | EP vs. LP | <0.001 | ||||
| LS | −0.02 ± 0.04 | BL vs. LS | 0.024 | LS | 0.02 ± 0.02 | BL vs. LP | 0.27 | ||||
| Interaction | 0.32 | Interaction | 0.11 | ||||||||
| Limb excurstion symmetry | |||||||||||
| Group | 0.93 | Group | 0.33 | ||||||||
| Episode | <0.001 | BL | −0.00 ± 0.01 | BL vs. ES | <0.001 | Episode | <0.001 | BL | −0.00 ± 0.01 | BL vs. EP | <0.001 |
| ES | 0.16 ± 0.06 | ES vs. LS | <0.001 | ES | 0.03 ± 0.03 | EP vs. LP | <0.001 | ||||
| LS | 0.23 ± 0.03 | BL vs. LS | <0.001 | LS | 0.00 ± 0.01 | BL vs. LP | 0.74 | ||||
| Interaction | 0.99 | Interaction | 0.07 | ||||||||
| Stance time symmetry | |||||||||||
| Group | 0.43 | Group | 0.009 | HC | 0.00 ± 0.00 | ||||||
| P | 0.02 ± 0.00 | ||||||||||
| Episode | <0.001 | BL | 0.00 ± 0.01 | BL vs. ES | <0.001 | Episode | <0.001 | BL | 0.00 ± 0.01 | BL vs. EP | <0.001 |
| ES | −0.11 ± 0.04 | ES vs. LS | <0.001 | ES | 0.02 ± 0.03 | EP vs. LP | <0.001 | ||||
| LS | −0.07 ± 0.04 | BL vs. LS | <0.001 | LS | 0.00 ± 0.01 | BL vs. LP | 0.59 | ||||
| Interaction | 0.99 | Interaction | 0.012 | HC | −0.00 ± 0.01 | HC vs. P | 0.98 | ||||
| P | 0.00 ± 0.01 | ||||||||||
| HC | 0.00 ± 0.02 | HC vs. P | 0.001 | ||||||||
| P | 0.04 ± 0.03 | ||||||||||
| HC | 0.00 ± 0.00 | HC vs. P | 0.96 | ||||||||
| P | 0.01 ± 0.01 | ||||||||||
| Stride time | |||||||||||
| Group | 0.45 | Group | 0.23 | ||||||||
| Episode | <0.001 | BL | 1.12 ± 0.06 | BL vs. ES | 1 | Episode | <0.001 | BL | 1.12 ± 0.06 | BL vs. EP | <0.001 |
| ES | 1.12 ± 0.17 | ES vs. LS | <0.001 | ES | 1.03 ± 0.12 | EP vs. LP | <0.001 | ||||
| LS | 1.30 ± 0.15 | BL vs. LS | <0.001 | LS | 1.13 ± 0.07 | BL vs. LP | 0.62 | ||||
| Interaction | 0.39 | Interaction | 0.001 | HC | 1.12 ± 0.06 | HC vs. P | 1 | ||||
| P | 1.12 ± 0.06 | ||||||||||
| HC | 0.98 ± 0.14 | HC vs. P | 0.041 | ||||||||
| P | 1.07 ± 0.09 | ||||||||||
| HC | 1.13 ± 0.04 | HC vs. P | 1 | ||||||||
| P | 1.13 ± 0.07 | ||||||||||
Values are means ± SE.
BL, baseline; ES, early split; LS, late split. P values in bold indicate significance.
Intralimb parameters.
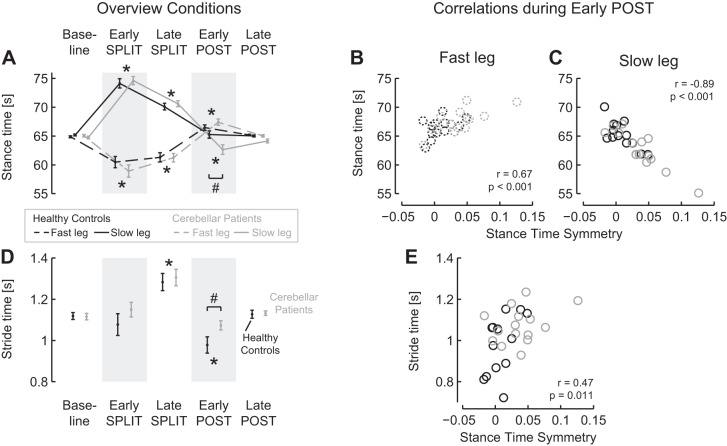
Second, we evaluated the parameters that were not expected to differ between groups, namely, the intralimb gait parameters: limb excursion symmetry and stance time symmetry. Both these parameters increased significantly during the split condition in both groups (Fig. 2; Table 4). Furthermore, during the post condition a larger overshoot in relative stance times was observed for the patient group. Positive limb excursion symmetry values indicate that limb excursion of the fast leg was higher than that of the slow leg. Negative stance time symmetry values indicate that relative stance times of the fast leg were shorter than those of the slow leg. No significant main effects for group or group × episode interaction effect was observed for limb excursion symmetry and stance time symmetry during split. During post, a significant asymmetric overshoot was observed during early post, returning to baseline values in late post, for both parameters. The asymmetry in relative stance times during early post was larger for the patient group (0.04 ± 0.03) than for the control group [0.00 ± 0.02; q(73) = 8.41; P = 0.001].
Fig. 2.
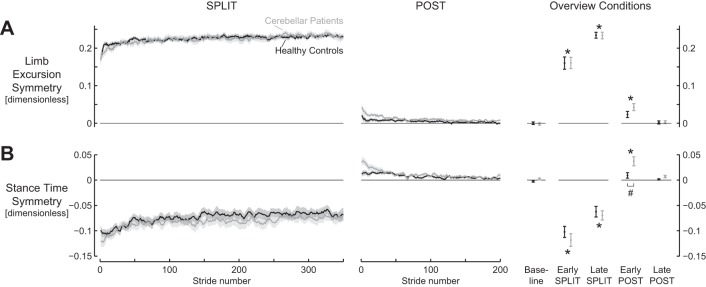
Intralimb parameters displayed adaptation and aftereffects for both cerebellar lesion patients and healthy controls during split-belt walking. A: limb excursion symmetry. B: stance time symmetry. Traces for cerebellar patients (gray) and healthy controls (black) during split (left) and post (middle) conditions. “Overview conditions” (right) summarizes values during the different phases and conditions. Shaded areas and error bars represent SE. Asterisks indicate values significantly different from baseline. Pound sign (#) indicates values significantly different between groups.
Individual limbs.
To further address the group difference in stance time symmetry, we evaluated the relative stance times of the individual limbs (Fig. 3A). In general, stance time symmetry changed simultaneously with the relative stance times of both the slow and the fast limb. However, in the overshoot in stance time symmetry during the early post phase, the changes in the relative stance time of the slow leg were more important than those of the fast leg.
Fig. 3.
Changes in relative stance times and stride time during split-belt walking (A and D) and correlations with stance time symmetry during the early phase of the post condition (B, C, and E). A: relative stance times of the fast and the slow leg changed in opposite directions during split-belt walking. Aftereffects (early post) were significantly different from baseline, and for the slow leg this aftereffect was larger in the patient group. B and C: relative stances times of both legs were correlated with stance time symmetry during the early phase of the post condition. D: stride time was increased during late split and reduced during early post; this reduction was most pronounced for the healthy controls. E: stride time and stance time symmetry were correlated during the early phase of the post condition. Data for cerebellar patients are presented in gray and for healthy controls in black; data for the fast leg are presented with dashed lines and for the slow leg with solid lines. Error bars represent SE. Asterisks indicate values significantly different from baseline. Pound signs indicate values significantly different between groups.
During split, the between-limb difference in relative stance times became smaller, similarly in both groups. This occurred simultaneously with both an increase in the relative stance time of the fast leg [q(52) = 3.54; P = 0.040] and a decrease in the relative stance time of the slow leg [q(52) = 10.61; P < 0.001] from early to late split. During early post, the increased asymmetry in relative stance times was related to both an increased relative stance time of the fast leg compared with baseline [q(52) = 9.21; P < 0.001] and a decreased relative stance time of the slow leg compared with baseline [q(52) = 3.98; P = 0.019]. During early post, the relative stance times were more asymmetric for the patient group than for the control group. When the fast and slow leg were evaluated separately during post, the group × episode interaction effect was only significant for the slow leg [F(2,52) = 4.41; P = 0.017], not for the fast leg [F(2,52) = 1.66; P = 0.20]. Post hoc analysis revealed that during early post, the relative stance time of the slow leg was lower in the patient group (62.7 ± 3.2%) than in the control group [65.3 ± 2.3%; q(63) = 7.73; P = 0.004]. Furthermore, during early post, the relative stance times of both legs were significantly correlated to the stance time symmetry (fast leg: r = 0.67; P < 0.001; Fig. 3B; slow leg: r = −0.89; P < 0.001; Fig. 3C). Multiple regression analysis revealed that the relative stance time of the slow leg explained a larger part (55%) of the variance in stance time symmetry than the relative stance time of the fast leg (21%).
Stride times.
Along with the changes in the interlimb and intralimb gait parameters, changes in the stride time were observed (Fig. 3D; Table 4). In general, stride time changed similarly between groups, except during early post, when healthy controls walked with shorter stride times than the patient group and stride times were significantly correlated to stance time symmetry. Stride time increased from early split to late split and was reduced during early post compared with during baseline. Whereas stride time was similar between groups at baseline and during late post, during early post healthy controls walked with shorter stride times (0.98 ± 0.14 s) than the patient group [1.07 ± 0.09 s; q(46) = 6.12; P = 0.041]. To evaluate whether the group difference in stance time symmetry during early post was related to the group difference in stride time, we performed a regression analysis. This showed indeed a significant correlation between stride time and stance time symmetry (r = 0.47; P = 0.011; Fig. 3B).
Lesion mapping of locomotor adaptation.
Eight patients had lesions in the interposed nuclei, six patients had no lesions in the interposed nuclei, and one patient was not included in the lesion-symptom mapping analyses because no MRI data were available (Table 1). In summary, we observed that lesion-based lesion-symptom mapping did not reveal any important group differences. Alternatively, symptom-based lesion-symptom mapping could identify regions important for stance time symmetry, whereas lesion-symptom mapping based on step length symmetry was not feasible.
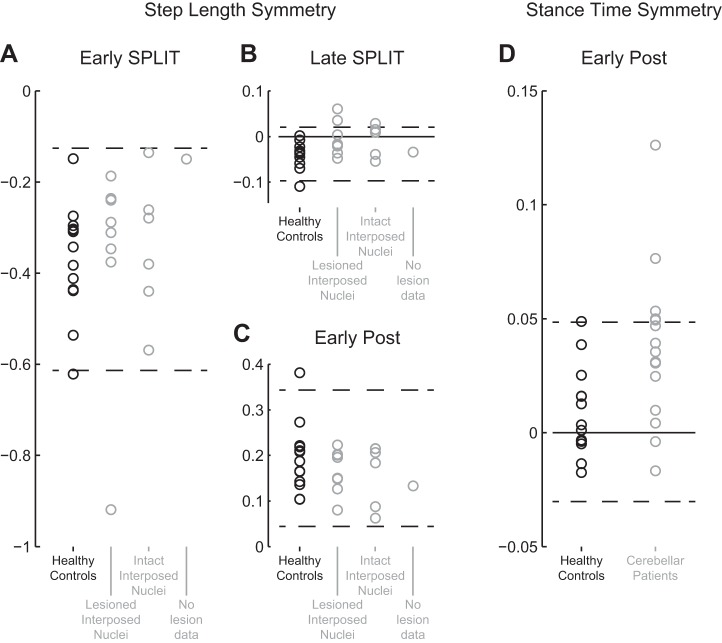
In the lesion-based approach, subgroups based on damage in the interposed nuclei were compared. During early split, no significant group effect on step length symmetry was observed [F(2,24) = 0.05; P = 0.95; Fig. 4A]. During late split, a significant group effect was observed [F(2,24) = 3.50; P = 0.046, Fig. 4B]. Post hoc intergroup comparisons suggest that the healthy controls had less symmetric step length than both patients with and patients without lesions in the interposed nuclei, but these differences were not significant [q(24) = 5.38; P = 0.081 and q(24) = 0.02; P = 0.118, respectively]. Finally, during early post, no significant group effect was observed [F(2,24) = 0.67; P = 0.52; Fig. 4C].
Fig. 4.
Lesions in the interposed nuclei were not related to differences in step length symmetry; several patients walked with asymmetric stance times during the early phase of the post condition. A: step length symmetry was similar between groups during early split. One patient walked with more asymmetric step lengths: value below 95% confidence interval of the values for the healthy controls (indicated by dashed lines). B: step length symmetry was similar between groups during late split. None of the patients walked with more asymmetric step lengths than the healthy controls. C: step length symmetry was similar between groups during early post. All patient values are within the 95% confidence interval of the values for the healthy controls. D: 5 patients walked with increased asymmetry in relative stance times during early post: values above 95% confidence interval of the values for the healthy controls. Values for cerebellar patients are presented in gray and for healthy controls in black; dashed lines indicate 95% confidence interval of the values for the healthy controls.
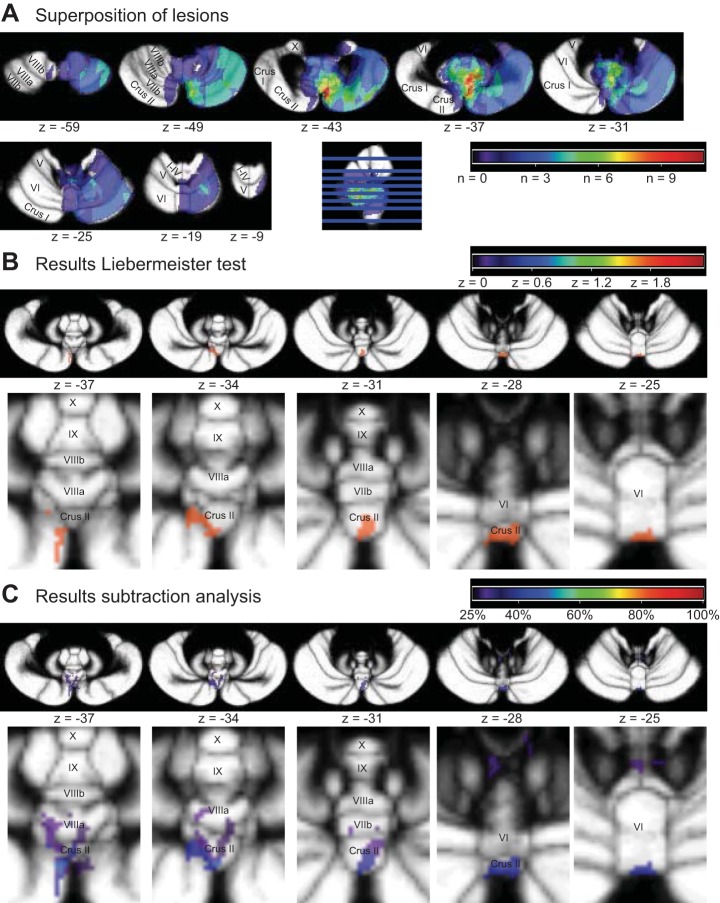
To further explore potential relations between focal cerebellar damage and split-belt walking behavior, we applied voxel-based lesion-symptom mapping (see lesion overlap in Fig. 5A; Table 3). We evaluated both the step length symmetry during the episodes mentioned above and the stance time symmetry, which was observed to be different between groups during early post.
Fig. 5.
Lesion overlap and overview of areas related to an increased asymmetry in relative stance times during the early phase of the post condition. A: superposition of the regions of cerebellar lesions of all patients. Note that lesions were flipped to the right for analysis. Maximum overlap (10 patients) was within vermal lobule VIIIa and paravermal lobules VIIb and Crus II (color coding according to the heat index above cerebellar slices). B: vermal lobules VI and Crus II were significantly correlated to an increased asymmetry in relative stance times during early post. Regions with z values >1.65 (P < 0.05), resulting from the Liebermeister test are indicated (color coding according to the heat index above cerebellar slices). C: subtraction analysis identified the same regions; regions that were at least 25% more likely to be lesioned in patients with increased asymmetry in relative stance times are indicated (color coding according heat index above cerebellar slices).
With regard to step length symmetry during split and post, patients were classified affected when symmetry values were below the 95% confidence interval of the values for the healthy controls (Fig. 4, A–C). Only one patient (P5; Table 1) could be classified to have a reduced step length symmetry during early split (Fig. 4A); during the other episodes, none of the patients displayed values lower than the cutoff threshold (Fig. 4, B and C). Therefore, voxel-based lesion-symptom mapping based on step length symmetry was not feasible. The disproportionately reduced step length symmetry during early split for P5 (Fig. 4A) was related to a negative step length during one of the first strides, where the foot of fast leg was placed posterior from the slow leg's foot. This also occurred for the single healthy control whose step length symmetry was lower than the cutoff threshold (Fig. 4A).
Stance time symmetry during early post was higher in the patient group than in the control group (see above), and therefore patients were classified affected with values above the 95% confidence interval of the values for the healthy controls (Fig. 4D). Five patients had larger differences in relative stance times than the cutoff threshold (P4, P6, P8, P10, P12; Table 1). Subtraction analysis and the statistical Liebermeister test revealed importance for similar regions (Fig. 5, B and C). These were primarily in the posterior vermis; vermal lobules VI and Crus II, with, according to the subtraction analysis, extensions into vermal lobules VIIb and VIIIa. Interestingly, these affected patients walking with larger asymmetry in relative stance times during early post also showed increased values for step length symmetry during late split (0.01 ± 0.05) compared with the control group [−0.03 ± 0.03; t(16) = 2.65; P = 0.017]. Four of the 5 affected patients walked with positive step length symmetry values (range 0.010–0.089), overcompensating their asymmetric step lengths, as opposed to only 1 of the 13 controls (0.004).
DISCUSSION
The aim of the present study was to address the localization of the cerebellar involvement in split-belt adaptation. For this reason we included relatively mildly affected patients having reasonably well-localized lesions. A major finding of the present study was that even mildly affected cerebellar patients show some changes in split-belt adaptation. Group differences were observed in stance time symmetry during early post: relative stance times were more asymmetric for the patient group than for the control group (Fig. 6). Specifically, affected cerebellar patients walked with shorter relative stance times of the slow leg during early post, placing the slow leg on the belt later than during baseline, whereas healthy controls placed their slow leg on the belt earlier than during baseline. Patients who walked with more asymmetric relative stance times were more likely to have lesions in vermal lobules VI and Crus II.
Fig. 6.
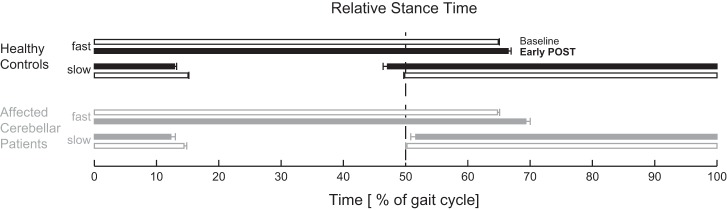
Differences in relative stance phases during the early phase of the post condition between healthy controls and “affected” cerebellar patients. Relative stance phases for healthy controls (black) and affected cerebellar patients (gray) during baseline (open bars) and during the early phase of the post condition (solid bars). Affected cerebellar patients walked with shorter relative stance times of the slow leg during early post, placing the slow leg on the belt later than during baseline, whereas healthy controls placed their slow leg on the belt earlier than during baseline. Error bars represent SE.
In general, the differences between patients and controls were small, in line with the mild degree of the deficits in the patient group. The present observation that patients with focal cerebellar lesions did not show any deficits in adaptation of step length symmetry may appear to be in contrast with observations of such deficits in patients with diffuse cerebellar damage (Morton and Bastian 2006). However, an important, often overlooked element is that Morton and Bastian (2006) only included severely ataxic patients (ICARS > 30) in their main study. In additional analyses, they showed that adaptation impairments were related to severity of (posture and gait) ataxia. Patients in our study had less severe ataxia, with ICARS scores ranging from 0 to 19 and only three patients scoring higher than 10. We did not observe a significant correlation between ICARS posture and gait (P&G) subscore and step length symmetry during early post, but the patient with the highest subscore (P&G = 10; P15; Table 1) did show the smallest asymmetry in step length of all participants. Furthermore, the patient that showed the lowest step length symmetry during early split (P5; Fig. 4A) also had a rather high P&G subscore (P&G = 6; Table 1) compared with the other patients.
The mild degree of ataxia may also explain some of the differences observed with other studies. For example, we observed no differences in split-belt adaptation between patients with and patients without lesions in the interposed nuclei. This observation may appear in contrast with the observation that the interposed nuclei are important in adaptation of limb coordination, when walking with added mass at the shanks (Ilg et al. 2008). Again, one could argue that such differences were related to differences between patients. However, interestingly, ataxia severity of the patients with focal lesions in the latter study was similar to that of our patients (Ilg et al. 2008). This could suggest that adaptation to split-belt walking and adaptation to added-mass walking are rather different processes. The latter is impaired in mildly ataxic patients with focal lesions, specifically in patients with lesions in the interposed nuclei. In contrast, adaptation of interlimb parameters in split-belt walking was not observed to be impaired in mildly ataxic patients or to be dependent of the interposed nuclei. Split-belt adaptation appears to be more related to the control of posture and gait (Morton and Bastian 2006), whereas added-mass adaptation appears to be more related to the control of multi-joint movements (Ilg et al. 2008).
Intralimb parameters.
Whereas most recent split-belt studies have focused mainly on changes in interlimb parameters such as step length, we observed significant aftereffects in intralimb parameters, as well. These aftereffects were most prominent in the patient group (Fig. 2) and were closely related to the relative stance time of the slow leg specifically. During the split condition, the relative stance times of the slow leg were higher than those of the fast leg. During the split trial, the participants reduced the relative stance time of the slow leg from the early to the late phase. In the patients, a larger part of this reduction was still present in the early phase of the post condition (storage and transfer), because they walked with shorter relative stance times in the slow leg than the controls. Although the larger aftereffects in intralimb parameters are not necessarily a deficit in adaptation, their presence suggests that the patients used a slightly different strategy to adapt their gait pattern to split-belt walking (see companion article, Hoogkamer et al. 2015a), reminiscent of the faster adjustments in swing times in elderly (Bruijn et al. 2012). This was confirmed when we compared the symmetry values of different gait parameters between the group of affected patients and the control group during split. Specifically, we observed that step length symmetry during the late phase of the split-belt condition was higher in the affected patients than in the control group. Four of these five patients showed overcompensation: during split, they first walked with smaller steps with the fast leg than with the slow leg; during split-belt walking, this asymmetry was not only reduced but overcompensated, resulting in asymmetry of opposite sign (smaller steps with the slow leg).
Furthermore, these data revealed some lesion site dependencies. We identified five patients with larger differences in relative stance times than the healthy controls (Fig. 4D), and these patients were more likely to have lesions in the posterior vermis: vermal lobules VI and Crus II (Fig. 5, B and C). However, it should be noted that the identified regions were small and that accompanying z values (z = 1.8) and subtracted percentages were low (<50%). Traditionally these regions have not been endowed with an important motor control function, but several observations from lesion and functional MRI studies suggest otherwise. From structural and functional connectivity studies, these regions appear to be mainly related to the limbic system (Stoodley and Schmahman 2010) and frontoparietal and dorsal attention networks (Buckner et al. 2011). In lesion studies, vermal lobule Crus II has been related to visuomotor adaptation, both in cerebellar stroke patients (chronic) and in patients with cerebellar degeneration (Donchin et al. 2012). In patients with acute and subacute stroke lesions, this relation was not observed (Burciu et al. 2014). Studies on force-field adaptation and added-mass walking did not observe a significant role for vermal lobules VI and Crus II (Burciu et al. 2014; Donchin et al. 2012; Ilg et al. 2008). It should be noted, however, that lesion overlap images from those studies suggest that very few patients had lesions in these regions. In animal studies, the vermis (and specifically the fastigial nucleus) has often been related to the control of posture and gait (for review, see Mori et al. 2004). In humans, the posterior vermis has been related to performance on tandem walking (Bastian et al. 1998), but all lesions in that patient sample included vermal lobule X, which can be expected to be more important in relation to balance (Stoodley and Schmahman 2010). In functional MRI studies, vermal lobule CII has been related to eye-hand coordination motor learning (Miall and Jenkinson 2005). Functional imaging studies from our group using bimanual coordination tasks have repeatedly observed involvement of cerebellar lobule VI, most often in the paravermal and hemispheric regions (Debaere et al. 2003; Heuninckx et al. 2005; Swinnen et al. 2010; Wenderoth et al. 2004, 2005) but also in vermal lobule VI (Beets et al. 2015; Debaere et al. 2004). Furthermore, both vermal lobules VI and Crus II have been observed to be enlarged in well-trained basketball players, based on MRI volumetric analyses (Park et al. 2009). In studies on cats, the importance of the vermis and associated nuclei (especially the fastigial nucleus) for posture and locomotion has been highlighted by Mori and colleagues (see Introduction). In addition, detailed studies are available on registration from the vermis and paravermis during cat locomotion, mostly from lobule V (Andersson and Armstrong 1987; Armstrong et al. 1982; Udo et al. 1981). A striking finding of one of these studies (on the lateral part of the vermis of lobule V) was that complex spikes were selectively generated when unexpected events occurred (such as during ladder stepping when a rung underwent an unexpected descent when stepped upon; Andersson and Armstrong 1987). This part of the vermis projects to the lateral vestibular nucleus.
Limitations.
First, since our aim was to address the localization of the cerebellar involvement in split-belt adaptation, we included patients with focal cerebellar lesions. These patients are commonly only mildly ataxic, which turned out to be a limitation for our study, as argued above. Second, even though we aimed to include mainly patients who did not receive adjuvant radio- or chemotherapy, several patients displayed extracerebellar damage (Table 2). This is suboptimal for lesion symptom mapping analysis (Timmann et al. 2009), but it did not bias our results: subtraction analysis within the subset of patients who did not receive adjuvant therapies indicated importance for similar regions to those identified in our main analysis (vermal lobules VI and Crus II) and in addition for paravermal lobule IX. Another limitation of lesion analysis is that whereas it gives indications of how behavior changes when a specific region is dysfunctional, it does not prove that this region is involved under healthy conditions. Furthermore, regions that are important for specific functions might not be identified, either because few, if any, of the patients have lesions in these regions or because other regions compensate for the deficits.
To further address the functional localization of locomotor adaptation, future studies could include patients with more severe forms of ataxia. Voxel-based morphometry could be used to localize deficits in cerebellar degeneration patients. Studies on severely ataxic patients with focal lesions (e.g., subacute or chronic stroke) could also provide useful insights. It should be mentioned, however, that this group is not easy to obtain because stroke lesions are seldom limited to the cerebellum. Furthermore, stroke patients are often at advanced age, which could confound behavioral outcomes (Bruijn et al. 2012). Finally, it should be mentioned that when studying severely ataxic patients, there is also the possibility that behavioral outcomes are confounded by other deficits (in balance or muscle coordination) or by compensation strategies to cope with those.
In summary, we observed that changes in symmetry of interlimb parameters, such as step length and relative double stance time during the split and post conditions, were similar between healthy controls and mildly ataxic patients with focal cerebellar lesions. Relative stance times were more asymmetric for the patient group than for the control group during the early phase of the post condition. Patients who walked with more asymmetric relative stance times were more likely to have lesions in vermal lobules VI and Crus II.
GRANTS
This work was supported by Research Foundation-Flanders (FWO Grants G.0756.10 and G.0901.11). S. M. Bruijn was supported by The Netherlands Organisation for Scientific Research (NWO Grant 451-12-041). J. Duysens was supported by a visiting professorship from the National Research Council of Brazil (CNPq 478863/2010-1).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.H., S.M.B., S.S., S.P.S., F.V.C., and J.D. conception and design of research; W.H. performed experiments; W.H., S.S., and F.V.C. analyzed data; W.H., S.M.B., S.P.S., F.V.C., and J.D. interpreted results of experiments; W.H. prepared figures; W.H. drafted manuscript; W.H., S.M.B., S.S., S.P.S., F.V.C., and J.D. edited and revised manuscript; W.H., S.M.B., S.S., S.P.S., F.V.C., and J.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Katrien Bastenie and Iris Degrande for assistance during the data collections and Inge Leunissen for fruitful discussions.
REFERENCES
- Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, Nourhashemi F, Onder G, Ritz P, Salva A, Visser M, Vellas B. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 13: 881–889, 2009. [DOI] [PubMed] [Google Scholar]
- Andersson G, Armstrong DM. Complex spikes in Purkinje cells in the lateral vermis (b zone) of the cat cerebellum during locomotion. J Physiol 385: 107–134, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Campbell NC, Edgley SA, Schild RF, Trott JR. Investigations of the olivocerebellar and spino-olivary pathways. Exp Brain Res Suppl 6: 195–232, 1982. [Google Scholar]
- Asanome M, Matsuyama K, Mori S. Augmentation of postural muscle tone induced by the stimulation of the descending fibers in the midline area of the cerebellar white matter in the acute decerebrate cat. Neurosci Res 30: 257–269, 1998. [DOI] [PubMed] [Google Scholar]
- Bastian AJ, Mink JW, Kaufman BA, Thach WT. Posterior vermal split syndrome. Ann Neurol 44: 601–610, 1998. [DOI] [PubMed] [Google Scholar]
- Beets IA, Gooijers J, Boisgontier MP, Pauwels L, Coxon JP, Wittenberg G, Swinnen SP. Reduced neural differentiation between feedback conditions after bimanual coordination training with and without augmented visual feedback. Cereb Cortex 25: 1958–1969, 2015. [DOI] [PubMed] [Google Scholar]
- Bruijn SM, Van Impe A, Duysens J, Swinnen SP. Split-belt walking: adaptation differences between young and older adults. J Neurophysiol 108: 1149–1157, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106: 2322–2345, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burciu RG, Reinold J, Rabe K, Wondzinski E, Siebler M, Müller O, Theysohn N, Gerwig M, Donchin O, Timmann D. Structural correlates of motor adaptation deficits in patients with acute focal lesions of the cerebellum. Exp Brain Res 232: 2847–2857, 2014. [DOI] [PubMed] [Google Scholar]
- Choi JT, Bastian AJ. Adaptation reveals independent control networks for human walking. Nat Neurosci 10: 1055–1062, 2007. [DOI] [PubMed] [Google Scholar]
- Choi JT, Vining EPG, Reisman DS, Bastian AJ. Walking flexibility after hemispherectomy: split-belt treadmill adaptation and feedback control. Brain 132: 722–733, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A, Giese MA, Sultan F, Mueller OM, Goericke SL, Ilg W, Timmann D. An intact action-perception coupling depends on the integrity of the cerebellum. J Neurosci 34: 6707–6716, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Cerebellar and premotor function in bimanual coordination: parametric neural responses to spatiotemporal complexity and cycling frequency. Neuroimage 21: 1416–1427, 2004. [DOI] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Internal vs external generation of movements: differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. Neuroimage 19: 764–776, 2003. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage 33: 127–138, 2006. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage 46: 39–46, 2009. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Maderwald S, Küper M, Thürling M, Rabe K, Gizewski ER, Ladd ME, Timmann D. Imaging the deep cerebellar nuclei: a probabilistic atlas and normalization procedure. Neuroimage 54: 1786–1794, 2011. [DOI] [PubMed] [Google Scholar]
- Dietz V, Zijlstra W, Duysens J. Human neuronal interlimb coordination during split-belt locomotion. Exp Brain Res 101: 513–520, 1994. [DOI] [PubMed] [Google Scholar]
- Donchin O, Rabe K, Diedrichsen J, Lally N, Schoch B, Gizewski ER, Timmann D. Cerebellar regions involved in adaptation to force field and visuomotor perturbation. J Neurophysiol 107: 134–147, 2012. [DOI] [PubMed] [Google Scholar]
- Fling BW, Cohen RG, Mancini M, Nutt JG, Fair DA, Horak FB. Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain 136: 2405–2418, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP. Neural basis of aging: the penetration of cognition into action control. J Neurosci 25: 6787–6796, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffland BS, Veugen LC, Janssen MMHP, Pasman JW, Weerdesteyn V, van de Warrenburg BP. A gait paradigm reveals different patterns of abnormal cerebellar motor learning in primary focal dystonias. Cerebellum 13: 760–766, 2014. [DOI] [PubMed] [Google Scholar]
- Hoogkamer W, Bruijn SM, Duysens J. Stride length asymmetry in split-belt locomotion. Gait Posture 39: 652–654, 2014. [DOI] [PubMed] [Google Scholar]
- Hoogkamer W, Bruijn SM, Potocanac Z, Van Calenbergh F, Swinnen SP, Duysens J. Gait asymmetry during early split-belt walking is related to perception of belt speed difference. J Neurophysiol (July 22, 2015a). doi: 10.1152/jn.00937.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogkamer W, Bruijn SM, Sunaert S, Swinnen SP, Van Calenbergh F, Duysens J. Toward new sensitive measures to evaluate gait stability in focal cerebellar lesion patients. Gait Posture 41: 592–596, 2015b. [DOI] [PubMed] [Google Scholar]
- Hoogkamer W, Meyns P. Is action-perception coupling improved with delay in patients with focal cerebellar lesions? J Neurosci 34: 11175–11176, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogkamer W, Van Calenbergh F, Swinnen SP, Duysens J. Cutaneous reflex modulation and self-induced reflex attenuation in cerebellar patients. J Neurophysiol 113: 915–924, 2015c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg W, Christensen A, Mueller OM, Goericke SL, Giese MA, Timmann D. Effects of cerebellar lesions on working memory interacting with motor tasks of different complexities. J Neurophysiol 110: 2337–2349, 2013. [DOI] [PubMed] [Google Scholar]
- Ilg W, Giese MA, Gizewski ER, Schoch B, Timmann D. The influence of focal cerebellar lesions on the control and adaptation of gait. Brain 131: 2913–2927, 2008. [DOI] [PubMed] [Google Scholar]
- Jahn K, Deutschländer A, Stephan T, Kalla R, Wiesmann M, Strupp M, Brandt T. Imaging human supraspinal locomotor centers in brainstem and cerebellum. Neuroimage 39: 786–792, 2008. [DOI] [PubMed] [Google Scholar]
- Jayaram G, Galea JM, Bastian AJ, Celnik P. Human locomotor adaptive learning is proportional to depression of cerebellar excitability. Cereb Cortex 21: 1901–1909, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram G, Tang B, Pallegadda R, Vasudevan EV, Celnik P, Bastian A. Modulating locomotor adaptation with cerebellar stimulation. J Neurophysiol 107: 2950–2957, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath HO, Himmelbach M, Rorden C. The subcortical anatomy of human spatial neglect: putamen, caudate nucleus and pulvinar. Brain 125: 350–360, 2002. [DOI] [PubMed] [Google Scholar]
- Lauzière S, Miéville C, Betschart M, Duclos C, Aissaoui R, Nadeau S. Plantarflexion moment is a contributor to step length after-effect following walking on a split-belt treadmill in individuals with stroke and healthy individuals. J Rehabil Med 46: 849–857, 2014. [DOI] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain 119: 1199–1211, 1996. [DOI] [PubMed] [Google Scholar]
- Miall RC, Jenkinson EW. Functional imaging of changes in cerebellar activity related to learning during a novel eye-hand tracking task. Exp Brain Res 166: 170–183, 2005. [DOI] [PubMed] [Google Scholar]
- Mohammadi F, Bruijn SM, Vervoort G, van Wegen EE, Kwakkel G, Verschueren S, Nieuwboer A. Motor switching and motor adaptation deficits contribute to freezing of gait in Parkinson's disease. Neurorehabil Neural Repair 29: 132–142, 2015. [DOI] [PubMed] [Google Scholar]
- Mori S. Integration of posture and locomotion in acute decerebrate cats and in awake, freely moving cats. Prog Neurobiol 28: 161–195, 1987. [DOI] [PubMed] [Google Scholar]
- Mori S, Matsui T, Kuze B, Asanome M, Nakajima K, Matsuyama K. Cerebellar-induced locomotion: reticulospinal control of spinal rhythm generating mechanism in cats. Ann NY Acad Sci 860: 94–105, 1998. [DOI] [PubMed] [Google Scholar]
- Mori S, Matsui T, Kuze B, Asanome M, Nakajima K, Matsuyama K. Stimulation of a restricted region in the midline cerebellar white matter evokes coordinated quadrupedal locomotion in the decerebrate cat. J Neurophysiol 82: 290–300, 1999. [DOI] [PubMed] [Google Scholar]
- Mori S, Nakajima K, Mori F, Matsuyama K. Integration of multiple motor segments for the elaboration of locomotion: role of the fastigial nucleus of the cerebellum. Prog Brain Res 143: 341–351, 2004. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci 26: 9107–9116, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanhoe-Mahabier W, Snijders AH, Delval A, Weerdesteyn V, Duysens J, Overeem S, Bloem BR. Split-belt locomotion in Parkinson's disease with and without freezing of gait. Neuroscience 236: 110–116, 2013. [DOI] [PubMed] [Google Scholar]
- Park IS, Lee KJ, Han JW, Lee NJ, Lee WT, Park KA, Rhyu IJ. Experience-dependent plasticity of cerebellar vermis in basketball players. Cerebellum 8: 334–339, 2009. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol 94: 2403–2415, 2005. [DOI] [PubMed] [Google Scholar]
- Reisman DS, McLean H, Keller J, Danks KA, Bastian AJ. Repeated split-belt treadmill training improves poststroke step length asymmetry. Neurorehabil Neural Repair 27: 460–468, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain 130: 1861–1872, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemmich RT, Hack N, Akbar U, Hass CJ. Effects of dopaminergic therapy on locomotor adaptation and adaptive learning in persons with Parkinson's disease. Behav Brain Res 268: 31–39, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerdink M, Coolen BH, Clairbois BH, Lamoth CJ, Beek PJ. Online gait event detection using a large force platform embedded in a treadmill. J Biomech 41: 2628–2632, 2008. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci 19: 1081–1088, 2007. [DOI] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington's disease but not cerebellar degeneration. J Neurophysiol 93: 2809–2821, 2005. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46: 831–844, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen SP, Vangheluwe S, Wagemans J, Coxon JP, Goble DJ, Van Impe A, Sunaert S, Peeters R, Wenderoth N. Shared neural resources between left and right interlimb coordination skills: the neural substrate of abstract motor representations. Neuroimage 49: 2570–2580, 2010. [DOI] [PubMed] [Google Scholar]
- Timmann D, Konczak J, Ilg W, Donchin O, Hermsdörfer J, Gizewski ER, Schoch B. Current advances in lesion-symptom mapping of the human cerebellum. Neuroscience 162: 836–851, 2009. [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Vasudevan E, Malone L, Bastian AJ. Locomotor adaptation. Prog Brain Res 191: 65–74, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci 145: 205–211, 1997. [DOI] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98: 54–62, 2007. [DOI] [PubMed] [Google Scholar]
- Udo M, Matsukawa K, Kamei H, Minoda K, Oda Y. Simple and complex spike activities of Purkinje cells during locomotion in the cerebellar vermal zones of decerebrate cats. Exp Brain Res 41: 292–300, 1981. [DOI] [PubMed] [Google Scholar]
- Wenderoth N, Debaere F, Sunaert S, Swinnen SP. Spatial interference during bimanual coordination: differential brain networks associated with control of movement amplitude and direction. Hum Brain Mapp 26: 286–300, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenderoth N, Debaere F, Sunaert S, van Hecke P, Swinnen SP. Parieto-premotor areas mediate directional interference during bimanual movements. Cereb Cortex 14: 1153–1163, 2004. [DOI] [PubMed] [Google Scholar]