Abstract
Gait adaptation is essential for humans to walk according to the different demands of the environment. Although locomotor adaptation has been studied in different contexts and in various patient populations, the mechanisms behind locomotor adaptation are still not fully understood. The aim of the present study was to test two opposing hypotheses about the control of split-belt walking, one based on avoidance of limping and the other on avoiding limb excursion asymmetry. We assessed how well cerebellar patients with focal lesions and healthy control participants could sense differences between belt speeds during split-belt treadmill walking and correlated this to split-belt adaptation parameters. The ability to perceive differences between belt speeds was similar between the cerebellar patients and the healthy controls. After combining all participants, we observed a significant inverse correlation between stance time symmetry and limb excursion symmetry during the early phase of split-belt walking. Participants who were better able to perceive belt speed differences (e.g., they had a lower threshold and hence were able to detect a smaller speed difference) walked with the smallest asymmetry in stance time and the largest asymmetry in limb excursion. Our data support the hypothesis that humans aim to minimize (temporal) limping rather than (spatial) limb excursion asymmetry when using their perception of belt speed differences in the early phase of adaptation to split-belt walking.
Keywords: ataxia, cerebellum, limping, locomotion, temporal gait symmetry
gait adaptation is a remarkable ability of humans with important implications for meeting the demands of the environment. It has been studied extensively in the last years, either by using a split-belt treadmill paradigm (using different speeds on each side; for review see Torres-Oviedo et al. 2011) or by using force fields (e.g., Barthélemy et al. 2012; Blanchette and Bouyer 2009; Ilg et al. 2008). In the earliest studies on split-belt walking of humans, it was discovered that humans could easily adjust to walking with each leg at a different speed, with speed ratios up to 1:4 (Dietz et al. 1994). Moreover, it was found that when the belts were returned to equal speeds after a period of walking with different speeds (split belt), aftereffects were visible: participants walked with increased step length of the leg that had been on the fast belt compared with the leg that was on the slow belt (Reisman et al. 2005). This step length asymmetry aftereffect was accompanied by a longer double stance time at the end of the stance phase of the leg that had been on the fast belt (Reisman et al. 2005). Furthermore, participants have been shown to be less able to manually adjust belt speeds to equal values after a bout of split-belt walking (Jensen et al. 1998). These aftereffects suggest that gait pattern adaptations made during the split-belt condition were stored, and thus feedforward controlled. Furthermore, these aftereffects de-adapt (or wash out) quickly during walking with both belts on equal speeds (e.g., Reisman et al. 2005), but it has been observed that repeated adaptations can result in a new, stored calibration that can be used immediately (for review see Bastian 2008) and can be retained for at least 3 mo (e.g., Reisman et al. 2013).
The time course of this adaptation process has been studied in detail in recent years (Bruijn et al. 2012; Finley et al. 2014; Malone and Bastian 2010; Tyrell et al. 2014; Vasudevan et al. 2011). Using a split-belt condition, the Bastian group found that intralimb parameters (such as limb excursion; i.e., the distance the foot travels along the belt during the stance phase, previously termed stride length; Hoogkamer et al. 2014a) adjust rapidly and thus can be considered as feedback-controlled parameters (rather than feedforward; Morton and Bastian 2006; Reisman et al. 2005). In contrast, interlimb parameters (such as step length and double support time) adapted slowly and showed marked aftereffects (Reisman et al. 2005). These adaptations have been observed to be reduced or slowed in several populations, such as children (Musselman et al. 2011; Vasudevan et al. 2011), the elderly (Bruijn et al. 2012), and patients with severe cerebellar ataxia (Morton and Bastian 2006), hemispherectomy (Choi et al. 2009), traumatic brain injury (Vasudevan et al. 2014), primary focal dystonia (Hoffland et al. 2014), and Parkinson's disease (Nanhoe-Mahabier et al. 2013; Roemmich et al. 2014).
In the present study we explored whether such impairments in locomotor adaptation could be related to a reduced ability to perceive differences in belt speed. Impaired perception could lead to a poorer detection of errors that normally drive adaptation (Bastian 2011). This is of interest in relation to cerebellar patients. Severely ataxic patients with diffuse cerebellar damage have been observed to demonstrate deficits in locomotor adaptation (Morton and Bastian 2006) and also in proprioception (Bhanpuri et al. 2013; Boisgontier and Swinnen 2014). In a companion article (Hoogkamer et al. 2015), we evaluated split-belt adaptation in mildly ataxic patients with focal lesions in the cerebellum. In the current article we address how split-belt adaptation parameters relate to potential perceptual deficits in these patients and in healthy controls. To evaluate how well participants are able to perceive differences between belt speeds, we used a perception threshold paradigm that was recently introduced by Lauzière et al. (2014). In their study, the speed of one of the two belts was gradually increased and participants had to indicate when they perceived belt speeds to be different. This was regarded to represent their perception threshold of locomotor asymmetry. In the group of elderly that was tested, this perception threshold was at a belt speed ratio of 0.88 (belt speed symmetry value of 0.064). During these trials, belt speed ratio was significantly correlated to the asymmetry in stance times (Lauzière et al. 2014). However, it remains an open question whether one's ability to perceive belt speed differences can also explain motor adaptation during a split-belt adaptation paradigm, where belt speeds are changed instantaneously rather than gradually. Indeed, perceptual deficits may influence several gait parameters when fast adjustments occur, not only in stance time but also in limb excursion or swing speed, since all of these gait parameters are feedback controlled (Hoogkamer et al. 2014b).
During normal walking, humans usually walk with both symmetric stance times and symmetric limb excursions. During split-belt walking, this gait pattern needs to be instantaneously adjusted to asymmetric belt speeds, and symmetry of at least one of those parameters needs to be changed since stance time symmetry and limb excursion symmetry are inversely coupled. Asymmetries in both limb excursion and stance time during split-belt walking have repeatedly been observed (Dietz et al. 1994; Morton and Bastian 2006; Reisman et al. 2005; Zijlstra and Dietz 1995). Therefore, it is expected that during split-belt walking, a complete symmetry in either stance time or limb excursion will not be reached. In this work, we test two opposing hypotheses (Fig. 1). The first hypothesis proposes that participants aim to minimize limping during split-belt walking; hence, they try to walk with symmetric durations of gait phases (stance symmetry hypothesis). According to this hypothesis, participants who are better able to perceive differences between belt speeds will walk with more symmetry in stance time than participants who are less able to perceive belt speed differences. Because stance time is inversely coupled to limb excursion during treadmill walking, the participants with best speed-difference perception will walk with the most asymmetric limb excursions. Alternatively, the control of split-belt walking could depend primarily on spatial input. Such spatial control encompasses the concept that the swing phase is initiated when the hip passes through a particular angle, which comes from animal studies (for review of the evidence, see Duysens et al. 2000). In addition, the swing to stance transition could be largely dependent on passive dynamics and stretch reflexes (for review of the evidence, see Duysens et al. 1998). Limb excursion is the distance the foot travels along the belt during the stance phase, so from the stance phase initiation to the swing phase initiation, this spatial control concept would result in fairly symmetric limb excursions, even during split-belt walking. Therefore, our second hypothesis states that during split-belt walking left-right differences in limb excursion are minimized (excursion symmetry hypothesis). In this case, the participants who are better able to perceive differences between belt speeds will walk with more symmetry in limb excursion than participants who are less able to perceive belt speed differences. Simultaneously, the participants with best speed-difference perception will walk with the most asymmetric stance times.
Fig. 1.
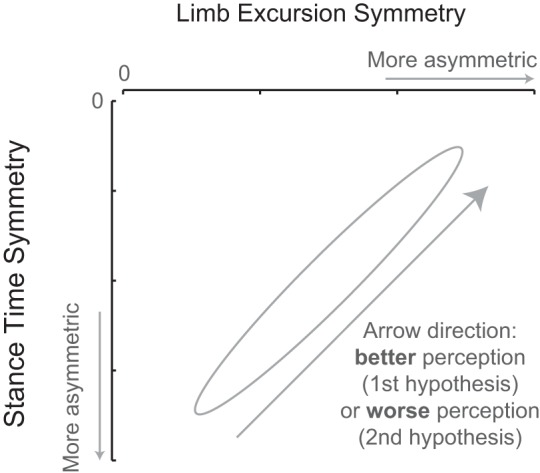
Perception of belt speed differences: two opposing hypotheses. According to the stance symmetry hypothesis, participants who are better able to perceive differences between belt speeds will walk with more symmetry in stance time and more asymmetry in limb excursion than participants who are less able to perceive belt speeds differences. Diagram shows the expected inversed coupling between limb excursion symmetry and stance time symmetry. Data points in top right corner (small asymmetry in stance times, large asymmetry in limb excursions) would be for participants who are well able to perceive differences between belt speeds; data points in bottom left corner (large asymmetry in stance times, small asymmetry in limb excursions) would be for participants who are less able to perceive differences between belt speeds. Alternatively, according to the limb excursion symmetry hypothesis, participants who are better able to perceive differences between belt speeds will walk with more symmetry in limb excursion and less symmetry in stance time than participants who are less able to perceive belt speeds differences.
MATERIALS AND METHODS
Participants.
For this study we performed two experiments in two separate sessions. First, eight patients with stable focal lesions after cerebellar tumor resection (age: 20.6 ± 3.6 yr, mean ± SD; 5 women, 3 men; Table 1) and nine healthy participants (age: 25.9 ± 5.2 yr; 6 women, 3 men) performed a classic split-belt paradigm. The second experiment assessed the perception threshold at which participants could perceive a difference between belt speeds. Patients were in a stable condition (>5 yr postoperative, range 6.5–17.7 yr; Table 1) and were able to walk independently (for lesion and therapy details, see companion article Hoogkamer et al. 2015). We rated severity of ataxia using the International Cooperative Ataxia Rating Scale (ICARS; Table 1) (Trouillas et al. 1997). All participants gave written informed consent. The experiments were conducted in accordance with the Declaration of Helsinki and were approved by the local ethics committee.
Table 1.
Patients were mildly ataxic and in a stable condition (>5 yr postop)
| Adjuvant Therapies |
ICARS |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age, yr | Time Postop, yr | Sex, M/F | Diagnosis | Interposed Nuclei Lesioned | RT | CT | Lesion Volume, cm3 | Total/100 | P&G/34 | Kin Funct/52 |
| 1 | 28.8 | 13.9 | F | Lhermitte-Duclos disease | 58.0 | 3 | 1 | 1 | |||
| 2 | 20.2 | 8.7 | F | Pilocytic astrocytoma | 8.2 | 3 | 0 | 0 | |||
| 3 | 19.6 | 11.8 | M | Pilocytic astrocytoma | Left | 1.7 | 6 | 3 | 2 | ||
| 4 | 18.1 | 6.5 | F | Pilocytic astrocytoma | 4.5 | 1 | 0 | 1 | |||
| 5 | 22.3 | 17.7 | M | Medulloblastoma | Both | Y | 22.0 | 13 | 6 | 2 | |
| 6 | 18.5 | 10.0 | F | Medulloblastoma | Left | Y | Y | 22.6 | 2 | 1 | 1 |
| 7 | 18.6 | 13.7 | M | Medulloblastoma | Right | Y | Y | 6.3 | 19 | 5 | 10 |
| 8 | 18.4 | 15.5 | F | Medulloblastoma | Both | Y | Y | 5.4 | 5 | 1 | 3 |
Postop, postoperative; F, female; M, male; RT, radiotherapy; CT, chemotherapy; Y, yes; ICARS, International Cooperative Ataxia Rating Scale; P&G, posture and gait subscore; Kin Funct, kinetic functions subscore.
Split-belt adaptation paradigm.
Methods and results for the first experiment are presented in a companion article (Hoogkamer et al. 2015). In short, the split-belt paradigm started with 3 min of walking with both belts at 1.0 m/s, followed by 10 min of walking with one belt at 1.0 m/s and the other belt at 0.5 m/s (“split”), ending with 5 min with both belts at 1.0 m/s (Bruijn et al. 2012). During split, patients walked with the most affected side on the fast belt and healthy controls walked with their nondominant leg on the fast belt (Morton and Bastian 2006). Between conditions the treadmill was shortly stopped. Throughout all conditions, kinematics of two ankle markers were sampled at 100 samples/s (Vicon Nexus; Oxford Metrics, Oxford, UK). In addition, three-dimensional ground reaction forces and torques were sampled at 1,000 samples/s (instrumented dual-belt treadmill, custom built by ForceLink, Culemborg, The Netherlands).
During all conditions we use “fast” to refer to gait parameters that were calculated for the leg that was on the fast belt during the split condition. First, we determined the instants of heel strike and toe-off based on the center of pressure trajectory (Roerdink et al. 2008). Limb excursion was calculated as the distance traveled by the ankle marker in the anterior-posterior direction from heel contact to toe-off of one limb (following Hoogkamer et al. 2014a). We then calculated symmetry values as (Choi et al. 2009)
Using a similar equation, we calculated stance time symmetry, using the relative stance time of each leg. In other words, the proportion of stance in the gait cycle was compared for both sides. Early split values were calculated as the mean value over the first five strides of the split condition. Furthermore, we calculated symmetry values, stride time, and stride time variability during the baseline condition (both belts at 1.0 m/s, before the split condition) to explore whether differences in perception of belt speed differences could be related to baseline gait parameters or their asymmetries.
Perception threshold paradigm.
The second experiment was based on a recently published protocol to assess the perception threshold of locomotor symmetry (Lauzière et al. 2014). The second session was separated from the first session by at least 3 wk to minimize any transfer from adaptational effects to the second experiment. Participants were fitted with reflective markers on the lateral malleoli and data recording procedures were similar to those of the first session. During the four experimental trials, the belts started at an equal speed of 1.0 m/s, and after a random time interval, one of the belt speeds was increased with 0.00278 m/s (0.01 km/h) each second.
Participants had to indicate when they perceived the belt speeds to be different by pressing a handheld button (Fig. 2). They then had to indicate verbally which of the belts ran faster. If participants did not perceive any difference, the trial was ended after one of the belts had increased speed for 2 min (and ran at 1.33 m/s). To minimize visual and auditory input regarding the speed difference between belts, the participants had to wear industrial ear protection and adapted safety glasses that blocked vision in the lower visual field. In case a participant came close to walking with both feet on the same belt, an experimenter indicated with a pointing gesture that the participant needed to return to the mediolateral center of the treadmill. In two experimental trials the left belt accelerated, and in two other trials the right belt accelerated. In addition, we added a sham trial, in which both belts ran at an equal speed of 1.0 m/s for the complete trial duration of 2 min. Participants were unaware of the total number of trials they had to perform (i.e., 5) but were given feedback on which of the belts ran faster (if any) after each trial. In between trials, participants walked for 2 min with belts at an equal speed of 1.0 m/s to wash out any adaptation effects resulting from the (increasing) differences in belt speeds during the experimental trials.
Fig. 2.
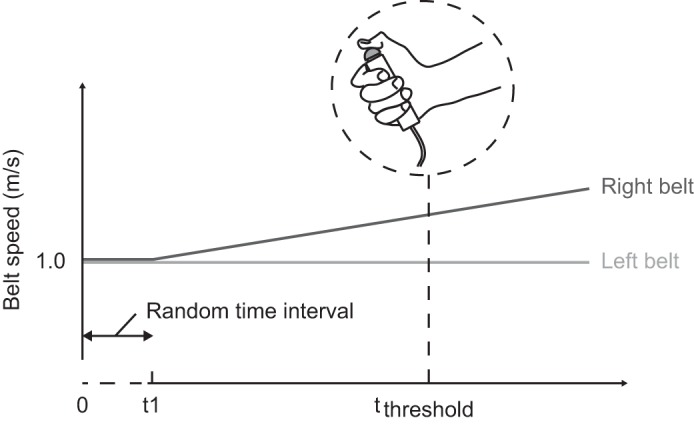
Perception threshold paradigm. The two belts start at the same speed, and then one belt's speed increases (t1). Participants indicate when they perceive the belt speeds to be different (tthreshold) by pressing a hand-held button.
The perception threshold was defined as the symmetry value of the belt speeds at the time that the participant perceived belt speeds to be different (button response), similar to the definition of the symmetry values of the gait parameters (see above):
Belt speeds at the time of the response were assessed by taking the average anterior-posterior velocity of the ankle markers during the preceding stance phase (over the time the heel was on the belt). To minimize effects of learning and differences in initial confidence, only the perception threshold value of the last trial was used. Therefore, each participant had a single perception threshold value, representing their ability to perceive differences between belt speeds, and this value was used for the statistical analyses.
Statistical analyses.
We used Student's t-test to compare perception threshold values between groups. Linear regression analysis was performed to assess potential correlations between gait parameters during the split-belt paradigm and the perception threshold values from the second experiment. We used a traditional level of significance (α = 0.05) for all statistical tests.
RESULTS
The first main finding of the study was that the perception threshold of gait asymmetry was not significantly different between the patient group and the control group (6.5 ± 2.0 vs. 8.8 ± 3.2%; P = 0.089). However, two cerebellar patients appeared to be less able to detect the speed difference between the belts (outside the 95% confidence interval of the values for the healthy controls; Fig. 3A, P7 and P8; Table 1). Next, we explored whether differences in perception threshold were related to potential asymmetries or other gait parameters during the baseline condition. Since no group differences in perception threshold were observed, all subsequent regression analyses were performed for the combined sample consisting of both the patients and the healthy controls. Perception threshold was not significantly correlated to any of the symmetry values, stride time, or stride time variability (all |r| < 0.40, P > 0.10), but participants with a higher perception threshold did walk with longer relative stance times (r = 0.54, P = 0.026; Fig. 3B).
Fig. 3.
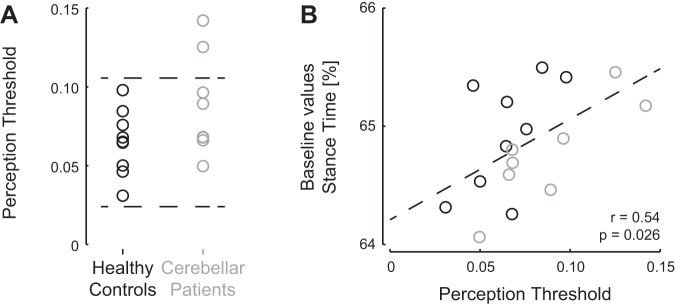
Perception threshold of gait asymmetry is increased in some patients and correlated to baseline relative stance times. A: perception threshold values for healthy controls (black) and cerebellar patients (gray) are similar; however, 2 cerebellar patients appeared to be less able to detect the speed difference between the belts: values above the 95% confidence interval of the values for the healthy controls (indicated by dashed lines). B: perception threshold was correlated to relative stance times during the baseline condition. Values for patients are presented in gray and those for healthy controls in black. Dashed line represents the linear regression line for relative stance times during baseline and at perception threshold (y = 8.5x + 64.2).
The second main findings of the study were the significant correlations between perception threshold on the one hand and stance time symmetry and limb excursion symmetry during split-belt walking on the other hand. The correlations were negative and therefore support the stance symmetry hypothesis: participants who were better able to perceive differences between belt speeds walked with less asymmetry in stance time and more asymmetry in limb excursion than participants who were less able to perceive belt speeds differences. In detail, first, we evaluated the correlation between limb excursion symmetry and stance time symmetry during early split. The expected coupling between stance time symmetry and limb excursion symmetry was confirmed (r = 0.82, P < 0.001; Fig. 4A). In addition the correlations between both symmetry values and the perception threshold were significant. For limb excursion symmetry the relation between perception threshold and symmetry was negative (r = −0.49, P = 0.048; Fig. 4B), indicating that during split, the participants with the highest thresholds initially walked with the smallest asymmetry in limb excursion. Similarly, stance time symmetry was negatively correlated to perception (r = −0.56, P = 0.019; Fig. 4C), but here the negative correlation indicates more asymmetry for higher perception threshold values.
Fig. 4.
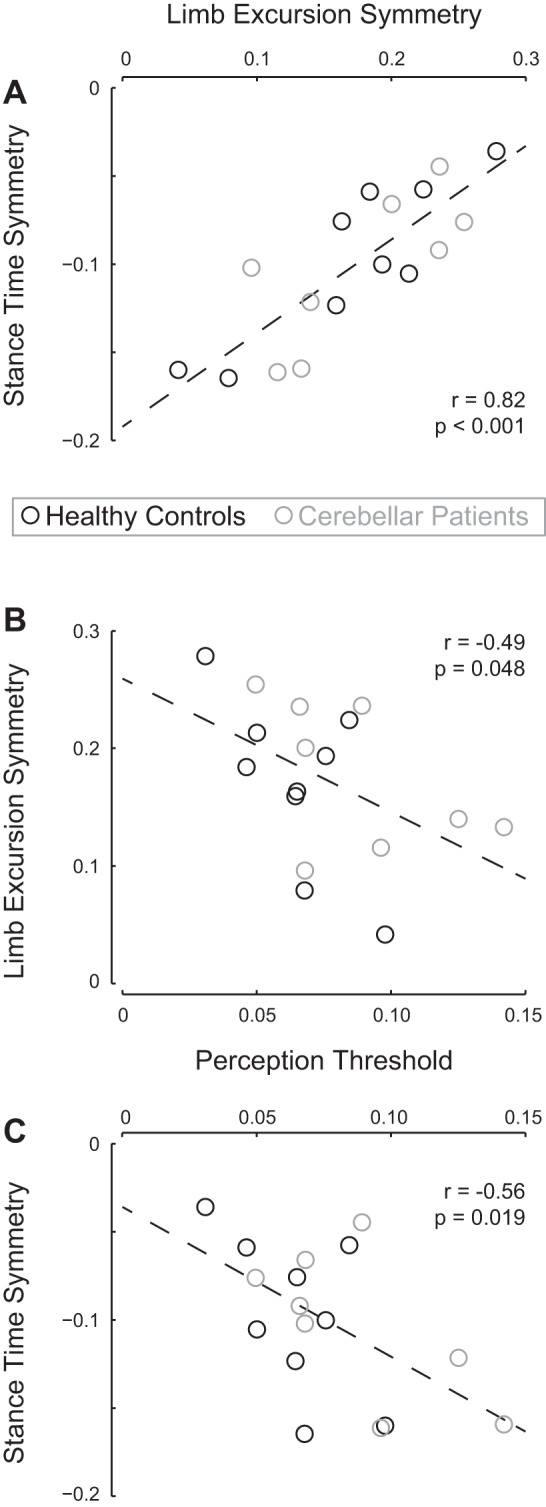
Observed relationships between speed-difference perception, stance time symmetry, and limb excursion symmetry during split-belt walking. A: stance time symmetry and limb excursion symmetry were significantly correlated, as was to be expected based on their mutual coupling to belt speed. Dashed line represents the linear regression line (y = 0.53x − 0.19). B: limb excursion symmetry was negatively correlated to perception threshold. Participants better able to perceive belt speed differences (low threshold) walk with less symmetric limb excursions. Dashed line represents the linear regression line (y = −1.33x + 0.26). C: stance time symmetry was negatively correlated to perception threshold. Participants better able to perceive belt speed differences (low threshold) walk with more symmetric relative stance times. Dashed line represents the linear regression line (y = −0.85x − 0.04). Values for patients are presented in gray and those for healthy controls in black.
Furthermore, perception threshold was significantly related to the relative stance time of the slow leg (r = 0.64, P = 0.006; Fig. 5B) but not to that of the fast leg (|r| < 0.40, P > 0.10; Fig. 5A). This indicates that the more asymmetric relative stances times in participants with a higher perception threshold were driven by an initial increase in the relative stance time of the slow leg.
Fig. 5.
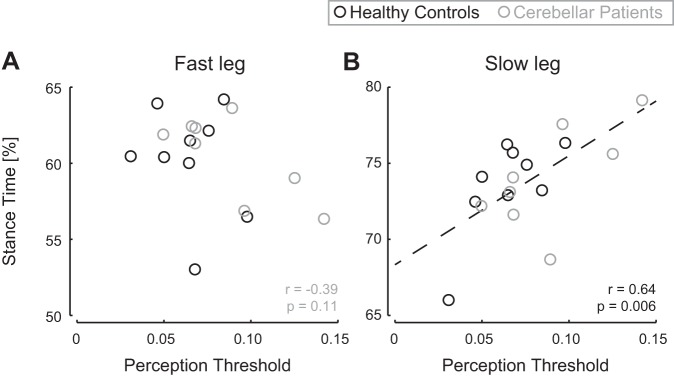
Correlations between perception threshold values and relative stance times of each leg. A: relative stance time of the fast leg was not correlated to perception threshold. B: relative stance time of the slow leg was correlated to perception threshold. Dashed line represents the linear regression line (y = 71.6x + 68.3). Values for patients are presented in gray and those for healthy controls in black.
DISCUSSION
The present study showed that during split-belt walking, some participants initially walk with small asymmetry in relative stance times and large asymmetry in limb excursion, whereas other participants walk with large asymmetry in relative stance times and small asymmetry in limb excursion. In addition, it was observed that the participants who initially walked with smaller asymmetry in relative stance times (and simultaneously with larger asymmetry in limb excursion) were better able to perceive differences between belts speeds during the perception threshold paradigm. Interestingly, no group differences between mildly ataxic patients with focal lesions in the cerebellum and healthy control participants were observed in ability to perceive differences between belts speeds. This lack of difference is in line with the relatively mild changes described in the companion article (Hoogkamer et al. 2015), underlining the fact that these mildly affected patients recovered quite well. Furthermore, it should be noted that the perception threshold was significantly correlated to the relative stance time of the slow leg for all participants. Participants with worse speed-difference perception initially walked with a disproportionally long stance phase of the slow leg. In contrast, the best participants (in terms of perception) shortened this stance time already adequately during the first five strides of split-belt walking to achieve higher temporal symmetry.
The decrease in stance phase on the slow side was more prominent than the increase in stance phase on the fast side. This difference between the effects on slow and fast leg can be easily explained by assuming that consciously perceived differences in speed are more easily translated into shortening of a relatively long stance phase on the slow side than into lengthening of a short stance phase on the fast side. This may be related to the size of the time window over which the perception is made. Contact with the belt is a powerful sensory stimulus, and it will be present for a longer time when the relative stance duration is longer (such as occurs on the slow side). This suggests that a shortening of a long stance phase is more easily perceived than a lengthening of a short stance phase. Overall, the present data are in good agreement with Lauzière et al. (2014), who claimed that stance time symmetry was the main criterion used by their participants to identify the perception threshold. In addition, here the relation with limb excursion is highlighted.
Limb excursion.
The present data show that spatial asymmetry (limb excursion symmetry) is relatively more tolerated than temporal asymmetry (relative stance duration) if participants are well able to perceive speed differences. This suggests that during split-belt walking the swing phase is not initiated at a certain hip angle, opposite to expectations based on observations of hip signals being important in the automatic switching of gait phases in animal studies (for review of the evidence, see Duysens et al. 2000). In cat studies it was shown that input from hip afferents to the central pattern generators can entrain the locomotor rhythm (Andersson and Grillner 1983). Such data supported the idea that the swing phase would be initiated when the hip passes through a particular angle (Shik and Orlovsky 1965). However, experiments on hip joint denervation showed that there is very little effect on gait cycle parameters, thereby supporting the idea that the important hip signal is unlikely to be derived from hip joint afferents (Duysens and Pearson 1998; Kriellaars et al. 1994). Instead, there is good evidence pointing out that spindle afferent from hip flexors provide the important source of the hip position signal (Hiebert et al. 1996). Whereas these findings were almost all obtained in surgically reduced cat models, it is unknown to what extent this hip signal is important during normal gait. One would expect that hip position is a more tightly controlled variable than the position of other joints. This was investigated in intact cats by measuring these angles under conditions of constrained gait (crouch; Duysens and Pearson 1998). It was found that cats indeed kept the maximum excursions of hip flexion and extension within stricter limits than the corresponding angles at other joints. However, these data clearly showed that for cats, walking is still quite possible even when hip angles deviate strongly from the normal hip threshold angle. This indicates that in cats the hip signal for phase switching can be easily overridden. Although it should be noted that cats walk with a flexed limb pattern and humans do not, the same may apply to the present work, as well. Although we did not directly evaluate hip angles, our data on limb excursion symmetry do not support the hypothesis that during split-belt walking the swing phase is initiated when the hip passes through a particular angle and that therefore participants try to reach that angle on both sides of the body.
All of this suggests that the hip signal for phase switching can be easily overridden. For the cat there is evidence that load receptor input both from extensor muscles and from cutaneous receptors in the foot is able to reinforce the ongoing extensor activity in the stance phase and delay the ensuing swing phase (Duysens and Pearson 1980; for review, see Duysens et al. 2000). This load feedback may be more important than hip signals for split-belt walking. In this respect it is worth noting that load feedback has been invoked in some of the earliest work in this field to explain the muscle activation patterns in human split-belt walking (Dietz et al. 1994). Later, it was also shown that sensory aftereffects of split-belt walking are load dependent (Jensen et al. 1998). One can expect that a sudden speed difference between the two sides introduces an imbalance in limb loading. This was indeed observed in kinetic studies. For example, propulsive ground reaction force impulses have been observed to become asymmetric instantaneously during split-belt walking (Ogawa et al. 2012; Roemmich et al. 2014).
In general, changes in stance time and limb excursion symmetry during walking are subject to several additional biomechanical constraints. Retaining a walking gait pattern with double stance phases and without flight phases limits the asymmetry in stance time. Furthermore, the limb excursion of the slow limb (relative to the stance time of the fast leg) is limited by the maximum swing speed of the slow leg (Bruijn et al. 2012).
Why do adaptations during split-belt walking take place?
The present data support the idea that at least some of the early adaptation can be driven by perceptual processes. This does not exclude the possibility that other factors also may be important. For example, it has been argued that energy consumption is an important element, as well. The question of energy efficiency was already explored by Finley et al. (2013). They observed that improvements in step length symmetry went hand in hand with reductions in metabolic power. In addition, the improvements in step length predicted the size of the reduction in metabolic power, thereby indicating that increasing economy could be a key element driving locomotor adaptation. This process is not mutually exclusive with the presently proposed mechanism.
Role of the cerebellum.
As indicated, the regression results were obtained on the grouped data of patients and controls since we observed no group differences between mildly ataxic patients with focal lesions in the cerebellum and healthy control participants in ability to perceive differences between belts speeds. Furthermore, stance time symmetry and limb excursion symmetry during early split were not significantly different between patients with cerebellar lesions and healthy controls (as described in the companion article, Hoogkamer et al. 2015). Taken together, these data suggest that deficits can be mild despite cerebellar lesions, particularly if these lesions are chronic and allow compensatory mechanisms (conform Barash et al. 1999; Martin et al. 1996; Synofzik et al. 2008). However, it should be noted that in some cases there were clear signs of cerebellar deficits. In particular, the two patients who were least able to detect the speed difference between the belts (Fig. 3A, P7 and P8) also had the highest kinetic function ICARS subscores (Table 1). A role in the (predictive) integration of feedback inputs could explain the increased perception threshold we observed in these patients. This is supported by observations of impaired proprioception in severely ataxic cerebellar patients (Bhanpuri et al. 2013; Maschke et al. 2003). It is also supported by data from pointing and reaching experiments in less affected patients (Izawa et al. 2012; Synofzik et al. 2008). In the study of Synofzik et al. (2008), for example, it was shown that cerebellar patients could adapt (to a shifted target), but they failed as soon as visual feedback was absent and they had to rely on predictions of the sensory consequences of their actions. Likewise in the present experiments, with each step the participants made a prediction of the speed of the belt, and this prediction was contradicted by the real speed, causing a conflict that normally is detected (e.g., by the cerebellum). The early landing phase of the step has been shown to be the important part of the step cycle, and it was proposed that predictive control of ankle stiffness at heel contact is a key element of locomotor adaptation during split-belt walking in humans (Ogawa et al. 2014). Because participants could not see their feet in the present experiments, they had to rely on the discrepancy between expected and real proprioceptive feedback.
In contrast to the cerebellar patient studies mentioned above (Bhanpuri et al. 2013; Maschke et al. 2003), we included patients with focal cerebellar lesions who were only mildly ataxic. This had a purpose because, in general, more localized lesions will result in more specific deficits but also less prominent ones. It can be expected that more or stronger correlations could have been observed when patients were included with more severe ataxic symptoms. For future studies it would be interesting to evaluate how the perception threshold relates to changes in the gait pattern during and after split-belt walking in patients with more severely affected ataxia (Morton and Bastian 2006) or stroke (Reisman et al. 2013). Specifically, in the latter patient group, it would be of interest to evaluate whether the perception threshold paradigm (Lauzière et al. 2014) could be used as an assessment tool within split-belt gait retraining programs (Hoogkamer et al. 2014b; Reisman et al. 2013).
To summarize, the present data indicate that participants choose rhythm comfort above spatial symmetry. Presumably, rhythm can be regulated more simply by the central pattern generators for gait when relative stance durations are more equal on both sides. More generally, the tendency of participants to try to obtain symmetry after walking for some time in split-belt conditions has been noted by others as well (Reisman et al. 2005), but not in relation to data on speed-difference perceiving ability. Our data on relative stance phase support the hypothesis that humans aim to minimize (temporal) limping by using their ability to perceive differences in belt speeds during split-belt walking. The cerebellum may be important in this respect, because the deficit in sensory perception of speed differences was typically seen in the most affected cerebellar patients. The present data also suggest that, at least in specific conditions such as split-belt walking, symmetry of limb angles is less tightly regulated than walking rhythm.
GRANTS
This work was supported by Research Foundation-Flanders (FWO Grants G.0756.10 and G.0901.11).
S. M. Bruijn was supported by The Netherlands Organisation for Scientific Research (NWO Grant 451-12-041). J. Duysens received a Visiting Professor Grant (CNPq 400819/2013-9). Z. Potocanac was funded by the European Commission through MOVE-AGE, an Erasmus Mundus Joint Doctorate program (2011-0015).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.H., S.M.B., Z.P., F.V.C., S.P.S., and J.D. conception and design of research; W.H. and Z.P. performed experiments; W.H. analyzed data; W.H., S.M.B., Z.P., F.V.C., S.P.S., and J.D. interpreted results of experiments; W.H. prepared figures; W.H. and J.D. drafted manuscript; W.H., S.M.B., Z.P., F.V.C., S.P.S., and J.D. edited and revised manuscript; W.H., S.M.B., Z.P., F.V.C., S.P.S., and J.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Katrien Bastenie and Iris Degrande for assistance during the data collections.
REFERENCES
- Andersson O, Grillner S. Peripheral control of the cat's step cycle. II. Entrainment of the central pattern generators for locomotion by sinusoidal hip movements during “fictive locomotion.” Acta Physiol Scand 118: 229–239, 1983. [DOI] [PubMed] [Google Scholar]
- Barash S, Melikyan A, Sivakov A, Zhang M, Glickstein M, Thier P. Saccadic dysmetria and adaptation after lesions of the cerebellar cortex. J Neurosci 19: 10931–10939, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthélemy D, Alain S, Grey MJ, Nielsen JB, Bouyer LJ. Rapid changes in corticospinal excitability during force field adaptation of human walking. Exp Brain Res 217: 99–115, 2012. [DOI] [PubMed] [Google Scholar]
- Bastian AJ. Moving, sensing and learning with cerebellar damage. Curr Opin Neurobiol 21: 596–601, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian AJ. Understanding sensorimotor adaptation and learning for rehabilitation. Curr Opin Neurobiol 21: 628–633, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanpuri NH, Okamura AM, Bastian AJ. Predictive modeling by the cerebellum improves proprioception. J Neurosci 33: 14301–14306, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette A, Bouyer LJ. Timing-specific transfer of adapted muscle activity after walking in an elastic force field. J Neurophysiol 102: 568–577, 2009. [DOI] [PubMed] [Google Scholar]
- Boisgontier MP, Swinnen SP. Proprioception in the cerebellum. Front Hum Neurosci 8: 212, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn SM, Van Impe A, Duysens J, Swinnen SP. Split-belt walking: adaptation differences between young and older adults. J Neurophysiol 108: 1149–1157, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JT, Vining EP, Reisman DS, Bastian AJ. Walking flexibility after hemispherectomy: split-belt treadmill adaptation and feedback control. Brain 132: 722–733, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Zijlstra W, Duysens J. Human neuronal interlimb coordination during split-belt locomotion. Exp Brain Res 101: 513–520, 1994. [DOI] [PubMed] [Google Scholar]
- Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev 80: 83–133, 2000. [DOI] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res 187: 321–332, 1980. [DOI] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. From cat to man: basic aspects of locomotion relevant to motor rehabilitation of SCI. Neurorehabilitation 10: 107–118, 1998. [DOI] [PubMed] [Google Scholar]
- Duysens J, van Wezel BM, van de Crommert HW, Faist M, Kooloos JG. The role of afferent feedback in the control of hamstrings activity during human gait. Eur J Morphol 36: 293–299, 1998. [DOI] [PubMed] [Google Scholar]
- Finley JM, Bastian AJ, Gottschall JS. Learning to be economical: the energy cost of walking tracks motor adaptation. J Physiol 591: 1081–1095, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JM, Statton MA, Bastian AJ. A novel optic flow pattern speeds split-belt locomotor adaptation. J Neurophysiol 111: 969–976, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert GW, Whelan PJ, Prochazka A, Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol 75: 1126–1137, 1996. [DOI] [PubMed] [Google Scholar]
- Hoffland BS, Veugen LC, Janssen MM, Pasman JW, Weerdesteyn V, van de Warrenburg BP. A gait paradigm reveals different patterns of abnormal cerebellar motor learning in primary focal dystonias. Cerebellum 13: 760–766, 2014. [DOI] [PubMed] [Google Scholar]
- Hoogkamer W, Bruijn SM, Duysens J. Stride length asymmetry in split-belt locomotion. Gait Posture 39: 652–654, 2014a. [DOI] [PubMed] [Google Scholar]
- Hoogkamer W, Bruijn SM, Duysens J. Gait parameters affecting the perception threshold of locomotor symmetry: comment on Lauzière et al. (2014). Percept Mot Skills 119: 474–477, 2014b. [DOI] [PubMed] [Google Scholar]
- Hoogkamer W, Bruijn SM, Sunaert S, Swinnen SP, Van Calenbergh F, Duysens J. Adaptation and after-effects of split-belt walking in cerebellar lesion patients. J Neurophysiol (July 22, 2015). doi: 10.1152/jn.00936.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg W, Giese MA, Gizewski ER, Schoch B, Timmann D. The influence of focal cerebellar lesions on the control and adaptation of gait. Brain 131: 2913–2927, 2008. [DOI] [PubMed] [Google Scholar]
- Izawa J, Criscimagna-Hemminger SE, Shadmehr R. Cerebellar contributions to reach adaptation and learning sensory consequences of action. J Neurosci 32: 4230–4239, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen L, Prokop T, Dietz V. Adaptational effects during human split-belt walking: influence of afferent input. Exp Brain Res 118: 126–130, 1998. [DOI] [PubMed] [Google Scholar]
- Kriellaars DJ, Brownstone RM, Noga BR, Jordan LM. Mechanical entrainment of fictive locomotion in the decerebrate cat. J Neurophysiol 71: 2074–2086, 1994. [DOI] [PubMed] [Google Scholar]
- Lauzière S, Miéville C, Duclos C, Aissaoui R, Nadeau S. Perception threshold of locomotor symmetry while walking on a split-belt treadmill in healthy elderly individuals. Percept Mot Skills 118: 475–490, 2014. [DOI] [PubMed] [Google Scholar]
- Malone LA, Bastian AJ. Thinking about walking: effects of conscious correction versus distraction on locomotor adaptation. J Neurophysiol 103: 1954–1962, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschke M, Gomez CM, Tuite PJ, Konczak J. Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain 126: 2312–2322, 2003. [DOI] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain 119: 1199–1211, 1996. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci 26: 9107–9116, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman KE, Patrick SK, Vasudevan EVL, Bastian AJ, Yang JF. Unique characteristics of motor adaptation during walking in young children. J Neurophysiol 105: 2195–2203, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanhoe-Mahabier W, Snijders AH, Delval A, Weerdesteyn V, Duysens J, Overeem S, Bloem BR. Split-belt locomotion in Parkinson's disease with and without freezing of gait. Neuroscience 236: 110–116, 2013. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Kawashima N, Ogata T, Nakazawa K. Limited transfer of newly acquired movement patterns across walking and running in humans. PLoS One 7: e46349, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Kawashima N, Ogata T, Nakazawa K. Predictive control of ankle stiffness at heel contact is a key element of locomotor adaptation during split-belt treadmill walking in humans. J Neurophysiol 111: 722–732, 2014. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol 94: 2403–2415, 2005. [DOI] [PubMed] [Google Scholar]
- Reisman DS, McLean H, Keller J, Danks KA, Bastian AJ. Repeated split-belt treadmill training improves poststroke step length asymmetry. Neurorehabil Neural Repair 27: 460–468, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemmich RT, Hack N, Akbar U, Hass CJ. Effects of dopaminergic therapy on locomotor adaptation and adaptive learning in persons with Parkinson's disease. Behav Brain Res 268: 31–39, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerdink M, Coolen BH, Clairbois BH, Lamoth CJ, Beek PJ. Online gait event detection using a large force platform embedded in a treadmill. J Biomech 41: 2628–2632, 2008. [DOI] [PubMed] [Google Scholar]
- Shik ML, Orlovsky GN. Coordination of the limbs during running of the dog. Biophysics 10: 1048–1059, 1965. [Google Scholar]
- Synofzik M, Lindner A, Thier P. The cerebellum updates predictions about the visual consequences of one's behavior. Curr Biol 18: 814–818, 2008. [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Vasudevan E, Malone L, Bastian AJ. Locomotor adaptation. Prog Brain Res 191: 65–74, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci 145: 205–211, 1997. [DOI] [PubMed] [Google Scholar]
- Tyrell CM, Helm E, Reisman DS. Learning the spatial features of a locomotor task is slowed after stroke. J Neurophysiol 112: 480–489, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan EV, Glass RN, Packel AT. Effects of traumatic brain injury on locomotor adaptation. J Neurol Phys Ther 38: 172–182, 2014. [DOI] [PubMed] [Google Scholar]
- Vasudevan EV, Torres-Oviedo G, Morton SM, Yang JF, Bastian AJ. Younger is not always better: development of locomotor adaptation from childhood to adulthood. J Neurosci 31: 3055–3065, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra W, Dietz V. Adaptability of the human stride cycle during split-belt walking. Gait Posture 3: 250–257, 1995. [Google Scholar]


