Abstract
Tactile information processing in the rodent primary somatosensory cortex (S1) is layer specific and involves modulations from both thalamocortical and cortico-cortical loops. However, the extent to which these loops influence the dynamics of the primary somatosensory cortex while animals execute tactile discrimination remains largely unknown. Here, we describe neural dynamics of S1 layers across the multiple epochs defining a tactile discrimination task. We observed that neuronal ensembles within different layers of the S1 cortex exhibited significantly distinct neurophysiological properties, which constantly changed across the behavioral states that defined a tactile discrimination. Neural dynamics present in supragranular and granular layers generally matched the patterns observed in the ventral posterior medial nucleus of the thalamus (VPM), whereas the neural dynamics recorded from infragranular layers generally matched the patterns from the posterior nucleus of the thalamus (POM). Selective inactivation of contralateral S1 specifically switched infragranular neural dynamics from POM-like to those resembling VPM neurons. Meanwhile, ipsilateral M1 inactivation profoundly modulated the firing suppression observed in infragranular layers. This latter effect was counterbalanced by contralateral S1 block. Tactile stimulus encoding was layer specific and selectively affected by M1 or contralateral S1 inactivation. Lastly, causal information transfer occurred between all neurons in all S1 layers but was maximal from infragranular to the granular layer. These results suggest that tactile information processing in the S1 of awake behaving rodents is layer specific and state dependent and that its dynamics depend on the asynchronous convergence of modulations originating from ipsilateral M1 and contralateral S1.
Keywords: barrel cortex, dynamics, thalamocortical loop, cortical loop modulations
rats continuously use their facial whiskers to gather information from their surroundings (Vincent 1912). Anatomical studies have shown that the trigeminal brainstem complex (Ma 1991), the ventral posterior medial (VPM) nucleus of the thalamus (Van Der Loos 1976), and the primary somatosensory cortex (Woolsey and Van der Loos 1970) are characterized by clusters of neurons (termed barrelets, barreloids, and barrels, respectively) that closely mimic the whisker pad organization. In anesthetized animals, neurons recorded from a barrel demonstrate a preferential response to tactile signals resulting from the mechanical displacement of a specific facial whisker (Welker 1971, 1976). These findings have led to the notion that somatosensory processing is strongly dependent on a feed-forward component in which tactile information received through each whisker to the S1 is preserved throughout these pathways (Land et al. 1995; Petersen and Diamond 2000; Simons et al. 1984).
More recently, several groups have shown that, in addition to this feed-forward component, the complexity of tactile information processing is also dependent on modulations provided by multiple cortical loops (Guo et al. 2014; Kinnischtzke et al. 2014; Lee et al. 2008; Pais-Vieira et al. 2013b). For example, in awake animals, somatosensory processing is significantly affected by cortical loop modulations that reflect past experience (Nicolelis and Chapin 1994; Wiest et al. 2010), ongoing motor activity (Fanselow et al. 2001; Hill et al. 2011; Pais-Vieira et al. 2013b), reward expectations (Pantoja et al. 2007), and interhemispheric integration (Shuler et al. 2001).
Additionally, these studies have demonstrated that somatosensory processing is also layer specific (Krupa et al. 2004) and that, in control conditions, neural dynamics may drastically change during the course of a tactile discrimination task (Pais-Vieira et al. 2013b). Although these studies indicate that somatosensory processing is dependent on time and network state as defined by the thalamocortical and cortical loop activity (Ghazanfar and Nicolelis 2001; Nicolelis 2011), there is presently no detailed description of the layer-specific dynamics present during the course of a tactile discrimination task. Here we specifically hypothesized that somatosensory processing dynamics in S1 are layer specific and dependent on the contribution of cortical loops from ipsilateral M1 and contralateral S1. For this, we investigated layer-specific processing of information in S1 while freely behaving rats performed an active tactile discrimination task (Krupa et al. 2001). Populations of individual S1 neurons were simultaneously sampled at different cortical depths along vertical microelectrode penetrations that covered all cortical layers (Krupa et al. 2004; Pais-Vieira et al. 2013b). Selective inactivation of ipsilateral M1 or contralateral S1 was used to address the role of each region in S1 tactile information processing (Pais-Vieira et al. 2013b; Shuler et al. 2001, 2002). Overall, we observed that, during the course of a trial, individual neurons located in different S1 layers exhibited a wide diversity of firing rate modulations, stimulus-driven responses, and patterns of synchronizations with each other that could be modulated by ipsilateral M1 or contralateral S1 inactivation. These physiological disparities encountered across supragranular, granular, and infragranular layers were state and behavioral context dependent and indicate a fundamental role for cortical loops from both ipsilateral M1 and contralateral S1 in tactile information processing.
MATERIALS AND METHODS
Subjects and behavioral task.
Long Evans female rats weighing between 250 and 350 g were used in all experiments. Animals from experiments involving recordings associated with M1 inactivation (control, saline, and muscimol), bilateral S1 recording in saline conditions, and VPM/posterior nucleus of the thalamus (POM) recordings have been used in a previous study (Pais-Vieira et al. 2013b). Hereon, we will refer to the control condition to indicate pooled neurons from both control and saline experiments. The active tactile discrimination task (Krupa et al. 2001) required animals to discriminate between a wide or narrow aperture to receive a water reward. At the beginning of each session, animals were placed in the behavioral box compartment called the outer chamber, where they waited for the central door to open and allow access to the second compartment, the inner chamber. After the animal entered the inner chamber, it had to use its whiskers to touch the edges of an aperture, formed by computer-controlled bars (hereafter referred to as the discrimination bars). The width of this aperture varied from trial to trial. Rats had to judge the aperture diameter and then nose poke the center of the front wall. Animal presence near the discrimination bars and near the front wall was detected by a photobeam. The nose poke in the inner chamber opened two water reward pokes located in the outer chamber from which the animal had to select one. The reward poke on the right corresponded to the wide aperture, whereas the poke on the left corresponded to the narrow aperture. As the animal chose a reward poke, the door separating the inner and outer chambers closed. Correct responses were rewarded by 50-μl water rewards, after which both reward pokes were closed. Incorrect responses were followed by their immediate closing. The aperture was set for a new trial 5–8 s after the reward pokes were closed. The animal's performance was measured by calculating the percentage of trials performed correctly during a session.
Multielectrode implants.
After the animals were trained in the behavioral task, microelectrodes were surgically implanted in multiple cortical areas. The animals were given access to water for a period of at least 24 h before surgery and for at least 7 days after the surgery. Cannula-microelectrode bundles and/or arrays of electrodes were implanted in the M1 and S1. Six animals received unilateral implants in both M1 and S1. Five animals were implanted in S1 bilaterally, and three animals had bilateral implants in M1 and S1. Two additional animals used for control experiments were bilaterally implanted in S1 and unilaterally implanted in M1.
Craniotomies were made and arrays lowered at the following stereotaxic coordinates for each area: S1 (anteroposterior, −1.5 to −3.5 mm; mediolateral, +5.0 to +6.5 mm; dorsoventral, −0.2 mm), M1 (anteroposterior, +2.0 mm; mediolateral, +2.0 mm; dorsoventral, −1.5 mm), according to reference anatomical planes (Paxinos and Watson 1998). To build our recording arrays, microwires were spaced by 250 or 300 μm and lowered to a maximum of −1.8 mm (supragranular: −401.6 ± 14.0 μm; granular: −805.9 ± 15.0 μm; infragranular: −1272.0 ± 39.0 μm).
Histological verification of recording sites was made using Nissl staining, cytochrome oxidase, or immunohistochemical staining for glial fibrillary acidic protein.
Electrophysiological recordings.
A multineuronal acquisition processor (64 channels; Plexon, Dallas, TX) was used to record neuronal spikes, as previously described (Ghazanfar and Nicolelis 1999). Briefly, neural signals were recorded differentially, amplified (20,000–32,000×), filtered (filtering band between 400 Hz and 5 kHz), and digitized at 40 kHz. Up to four single neurons per recording channel were sorted online (Sort Client 2002; Plexon). Online sorting was validated offline using Offline Sorter 2.8.8 (Plexon) according to the following cumulative criteria: 1) signal-to noise ratio >2.5 (as verified on the oscilloscope screen), 2) <0.1% of interspike intervals smaller than 1.0 ms, and 3) stereotypy of waveform shapes, as determined by a waveform template algorithm and principal component analysis. These cumulative criteria were complemented with inspection of metrics of the quality of single-unit isolation in freely behaving animals (J3, Davis-Bouldin, F, and Pseudo-F) (Nicolelis et al. 2003). Local field potentials (LFPs) were acquired from the same electrodes simultaneously by band-pass filtering the raw signal (0.3–400.0 Hz), preamplified (1,000×), and digitized at 1,000 Hz using a digital acquisition card (National Instruments, Austin, TX) and a multineuronal acquisition processor (Plexon).
In S1, microelectrodes were lowered from an initial position of −0.2 mm. Steps of at least 62.5 μm were employed to move the microelectrodes after similar numbers of control, saline, and muscimol sessions were recorded (typically 2) or when a very small number of units was recorded in one session. It is possible that the same units were repeatedly recorded by the same electrode in different sessions. However, we did not assume that the same units were recorded on each channel on different days because muscimol inactivation very often was associated with masking and unmasking of neurons in M1 and the other areas recorded, making it difficult to judge whether a waveform reappearing after a muscimol session belonged to the same or different neuron.
Inactivation with muscimol.
To inactivate M1 or S1, muscimol (500 ng in 500 nl of saline) or saline (500 nl) was slowly injected unilaterally with a microperfusion precision pump (Harvard Apparatus, Holliston, MA) for a period of 4 min under isofluorane anesthesia or in awake, freely behaving animals in an open field (in 4 animals). This dose of muscimol inactivates an injected cortical volume for 6–8 h (Krupa et al. 1999; Martin 1991; Shuler et al. 2001, 2002). The inactivation effect was confirmed by an absence of action potentials on the electrodes surrounding the injected area.
The sequence of control, saline, and muscimol sessions was randomly changed in the same animals to avoid any possible bias.
Data analysis.
Neuronal data obtained from a total of 151 recording sessions were processed and analyzed using NeuroExplorer (version 3.266; NEX Technologies, Madison, AL) and custom scripts written in Matlab (7.9.0; Mathworks, Natick, MA). A trial was defined as the period from −2.0 to 2.0 s relative to the time when the rat broke the photobeam at the discrimination aperture. The beam break was defined as time = 0 s in all analysis and figures presented. Statistical significance of neural responses was evaluated using a method based on cumulative-summed spike counts (Gutierrez et al. 2006; Wiest et al. 2005). The period of −1.5 to −0.5 s was used as a baseline in this test. Depending on the firing rate change, firing rate modulations were classified as facilitated, suppressed, or multiphasic (i.e., a combination of rate increases and decreases). The proportion of single units presenting each type of firing modulation was compared using χ2 tests. For statistical tests with two or more comparisons, we divided the α value by the number of comparisons (up to a maximum of 3 comparisons, corresponding to α = 0.0167); otherwise we used α = 0.05. The magnitude of the firing rate modulations was defined as the average difference in firing rate between the period of interest and the background. The duration of the firing rate modulations was defined as the time interval during which the firing of a unit significantly deviated from the background. Comparisons of characteristics of neuronal firing rate modulations for different conditions were performed using nonparametric tests (Mann-Whitney-Wilcoxon or Kruskal-Wallis).
State maps of significant neuronal modulations dynamics.
To characterize layer-specific neuronal activity patterns across multiple behavioral epochs during a trial, data were pooled from all subjects employed in control studies, including those that received saline injections. Neuronal data from each trial were time aligned when the rats entered the aperture. Increased and decreased significant neuronal discharges were counted in sequential 10-ms time bins and normalized to the maximum value across all bins. A final pair of values, consisting of the fraction of suppressed and the fraction of facilitated firing rate modulations, was then obtained for each time bin. These normalized pairs of values were then plotted in a state space, where the x-axis and y-axis, respectively, represented the fraction of facilitated and suppressed firing rate modulations. From top to bottom and left to right, each of the four quadrants in the state space represented firing rate modulations that were prominently suppressed (Q1), suppressed and facilitated (Q2), had no firing modulations (Q3), and were facilitated (Q4). These pairs of responses were then studied in 10 different windows of 250 ms, corresponding to the different behavioral epochs of a trial. We analyzed responses occurring before (anticipatory: −500-0 ms), during (discrimination: 0–250 ms), and after whisker contact with the tactile stimulus (response: 250–1,000 ms; reward: 1,000–2,000 ms). As a complementary measure for the dynamics observed in the state map, the Euclidean distance between neuronal state maps was compared using a repeated-measures ANOVA followed by Bonferroni corrections for individual trial period comparisons. In panels depicting the Euclidean distance, the values were smoothed with a moving average of 25 bins (corresponding to 250 ms), solely for ease of presentation. All statistical comparisons of Euclidean distances were performed using 10-ms bins within a specific trial period. Bootstrapping of the Euclidean distances was performed to determine a confidence interval for chance in expected distances.
To allow for a better visualization of the continuous changes occurring in the neuronal populations recorded from the different structures, the state maps of facilitated and suppressed activity have been converted to theta angles revolving around the center of the state map (the coordinates 0.5 and 0.5 in abscissa and ordinate axis, respectively). The area occupied by each symbol was proportional to the magnitude of the responses. In this analysis, Q1 (suppressed) in the state map corresponds to the area between 91 and 180°, Q2 (suppressed and facilitated) corresponds to the area between 0 and 90°, Q3 (no modulation) corresponds to the area between 181 and 270°, and Q4 (facilitated) corresponds to the area between 270 and 360°.
Prediction of neuronal state during control conditions.
The prediction of neuronal state maps during control conditions using the values from neuronal state maps from neurons recorded after M1 or S1 inactivation was performed separately for facilitated and for suppressed firing rate modulations. We will only describe here the prediction of suppressed firing rate modulations (the exact same procedure was applied to facilitated firing modulations). The fraction of suppressed firing rate modulations occurring in each 10-ms bin recorded from infragranular layers during the course of a complete trial was correlated to the fraction of suppressed firing rate modulations occurring in infragranular layers after M1 inactivation, and an overall R2 value was determined. The same procedure was then performed for the fraction of suppressed firing rate modulations present in neurons recorded from infragranular layers after S1 inactivation. Lastly, we calculated a third set of values, which corresponded to the average between suppressed firing rate modulations occurring after M1 inactivation and suppressed firing rate modulations occurring after S1 inactivation for each 10-ms bin. To test whether M1 and S1 inactivation had opposite effects in suppressed firing rate modulations, we compared the prediction made by the average of both fractions to the predictions made by either inactivation alone. The average values of each predictor were correlated to the fraction of suppressed firing rate modulations present during control conditions. Thus three different comparisons were made, one using neural state maps recorded after M1 inactivation, one using neural state maps recorded after S1 inactivation, and another one with the average of neural state maps recorded from neurons after M1 and S1 inactivation.
Stimulus-related information.
The presence of stimulus-related information in neuronal activity was studied using a classifier. The input to our classifier was computed as follows: for each trial that a rat discriminated the aperture (wide/narrow) correctly, the time of the discrimination event in the trial was considered time 0, and the spike train of a neuron from time 0 to 2 s was binned with multiresolution time scales (10, 20, and 40 ms). The spike counts at the different resolution scales were concatenated to a long vector as the input of the classifier. An L1-regularized support vector machine (Vapnik 1995) was used as the classifier (Matlab implementation with LIBLINEAR) (Fan 2008). The L1 regularization avoided the overfitting problem in training a classifier when the number of variables (spike count bins) was larger than the number of samples (trials) (Tibshirani 1996). The classifier also balanced the unequal numbers of the correct wide/narrow trials by assigning different weights to misclassified cost. To evaluate whether a neuron encoded information of wide/narrow aperture, we compared the classification accuracies obtained from the correct class labels (narrow/wide) against that obtained from the shuffled class labels (chance level). We performed 10 runs of 10-fold, 2-level cross-validation on the data with both true and permuted class labels with the same fold structure and tested the resulting accuracies by paired t-test with Bonferroni's corrections to determine whether a neuron encoded the information. The two-level cross-validation prevented bias in estimating the classification accuracies. The first level put one fold aside as the testing data, and the remaining nine folds were the training data. In the second level, we performed a ninefold cross-validation on the training data to determine which cost value (100.0, 10.0, 1.0, or 0.1) gave the classifier the best performance on the training data. The cost value was next used to train the classifier on the whole training data and to test on the testing data. The classifier next identified cells that could differentiate the aperture. The overall amount of information encoded after the whiskers contacted with the tactile discriminanda during a trial was studied in all cells and was calculated as the difference between the shuffled class labels (chance level) and the amount of information encoded by the neuron. Kruskal-Wallis tests were used to compare information content between different conditions. The α value was corrected according to the number of comparisons performed.
RESULTS
All experimental procedures were approved by the Duke University Institutional Animal Care and Use Committee. Fourteen female Long-Evans rats were surgically implanted with movable microelectrode arrays and/or infusion cannulae in S1 and M1 in various configurations. All subjects learned to perform a tactile discrimination task as previously described (Krupa et al. 2001). Briefly, animals actively used their facial whiskers to determine whether an aperture, whose diameter could vary between 54 and 78 mm, was wide (78 mm) or narrow (54 mm). After making that discrimination, rats had to move to a reward chamber and nose poke either the left or right ports (for narrow and wide aperture, respectively) to receive a 50-μl water reward (Fig. 1, A–D). Incorrect responses ended the trial.
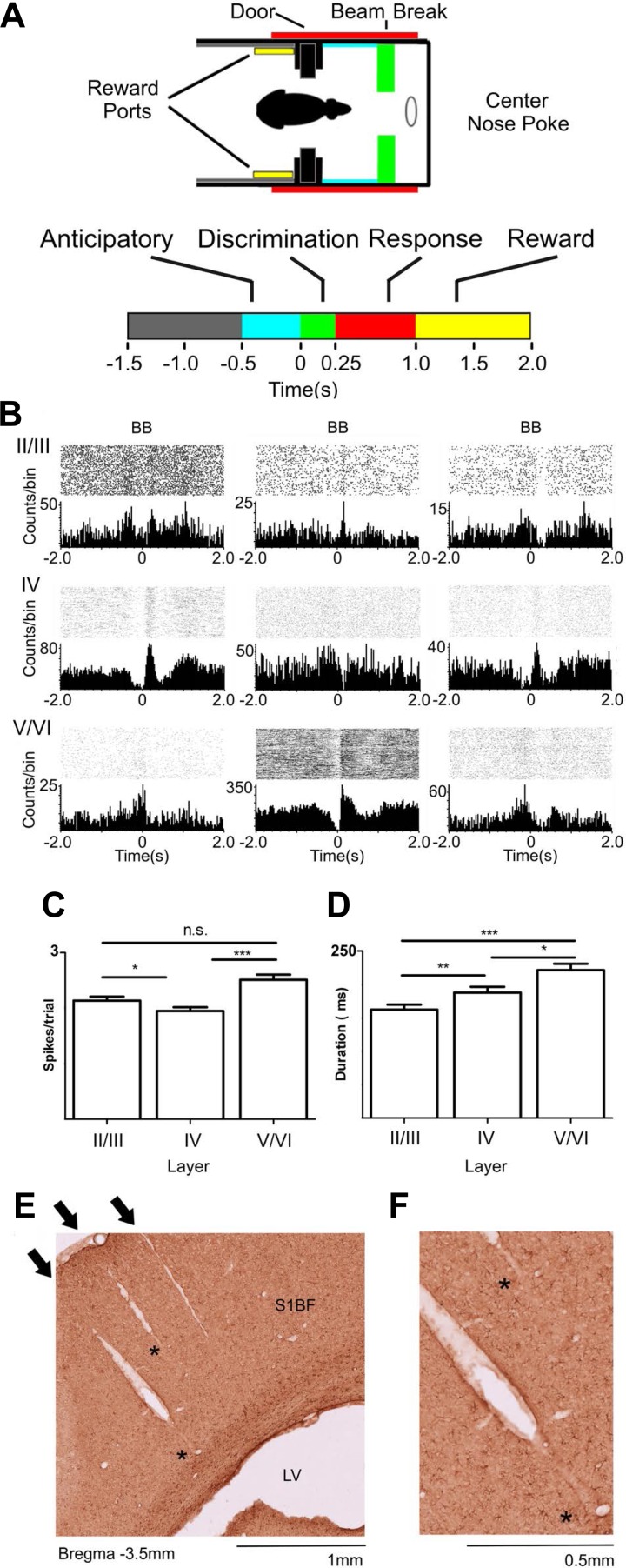
Fig. 1.
Layer-specific activity during tactile discrimination. A: active tactile discrimination task with different behavioral epochs, respectively: baseline (gray), anticipatory (cyan: −500-0 ms), discrimination (green: 0–250 ms), response (red: 250–1,000 ms), and reward (yellow: 1,000–2,000 ms) periods. B: perievent raster histograms from representative cells recorded from different depths in S1. BB, beam break in the discrimination bars (time = 0 s). Multiple different types of neuronal firing rate modulations were found. These neuronal firing modulations could be facilitated or suppressed and were present in all layers at all different periods of the task. C: layer-specific differences in magnitude of facilitated firing modulations during a trial. Neurons recorded from layers V/VI presented the largest neuronal firing rate modulations, followed by firing modulations recorded from layers II/III, and lastly by the ones recorded from layer IV. D: duration of neuronal firing modulations was layer specific during tactile discrimination. Neurons recorded from layers V/VI presented the longest facilitated firing modulations, followed by neurons recorded from layer IV, and lastly by neurons recorded from layers II/III. Error bars represent SE. *P < 0.05, **P <0.01, ***P < 0.001. E: example of 3 electrode tracks in the S1 cortex barrel field (S1BF). Note that the electrode tracks are orthogonal to the cortical surface and extend throughout multiple layers. The arrows indicate the point of entry in the cortical surface. The asterisks indicate places where the electrode tracks are present but not as clearly visible. LV, lateral ventricle. F: same histological preparation as E magnified to show the electrode tracks indicated by asterisks in E.
Layer-specific patterns.
Single-unit activity was recorded with arrays of microelectrodes implanted in S1. A total of 1,246 single S1 neurons were sampled in 151 recording sessions. Of these, 404 neurons were recorded in the supragranular layers, 506 in the granular layer, and 301 in the infragranular layers (see Figs. 1, E and F, and 2, A and B, for histological preparation).
Fig. 2.
Histological verification of electrode traces. A: example of 2 electrode tracks, each 1 inside a different barrel in S1 cortex. B: example of a single electrode track ending in the infragranular layers of the primary somatosensory cortex.
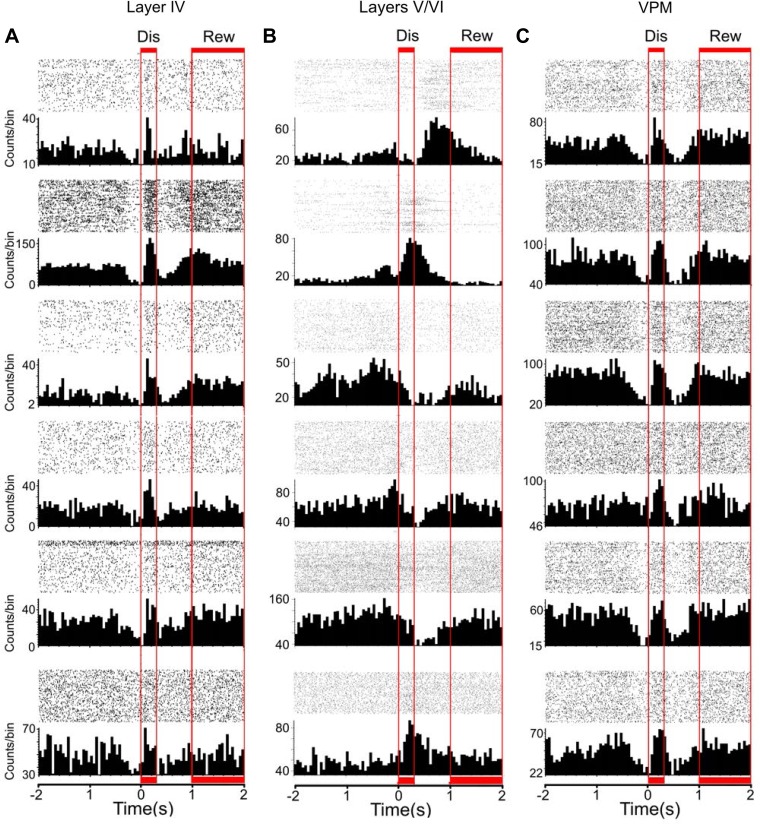
Multielectrode recordings were obtained while rats performed a tactile discrimination task (Fig. 1A). Initially, we noticed that task-related firing rate modulations (Fig. 1B), which were found in 79% of neurons (Table 1), were fundamentally different across the cortical layers. Cortical layers differed in the proportion of neurons with firing rate increases, decreases, and multiphasic firing modulations (composed of increases and decreases) observed during the different epochs that define a single task trial (Table 1). For example, whereas neuronal firing rate modulations recorded from the granular layer were frequently characterized by a sequence of suppression/facilitation/suppression (Fig. 1B, middle), neurons located in the infragranular and supragranular layers exhibited distinct patterns of firing rate increases and decreases (Fig. 1B and Table 1). Moreover, the overall magnitude of firing rate modulations was stronger (P < 0.001; Kruskal-Wallis with post hoc Dunn's test) in the infragranular layers (2.7 ± 0.1 spikes/trial) than in the granular (2.2 ± 0.1 spikes/trial) and supragranular layers (2.3 ± 0.1 spikes/trial) (Fig. 1C). Additionally, the duration of firing rate modulations depended on the layer (P < 0.0001), with the longest modulations observed in the infragranular layers (215.3 ± 9.0 ms) followed by granular (191.5 ± 7.0 ms) and supragranular (162 ± 8 ms) layers (Fig. 1D). Notice that the duration of these firing rate modulations is much longer than those observed in anesthetized animals (Krupa et al. 2004). Altogether, these initial results indicated that neuronal firing rate magnitude, duration, and temporal modulation pattern were all layer specific.
Table 1.
Layer-specific neuronal firing rate modulations
| Modulated, % | Facilitated, % | Suppressed, % | Multiphasic, % | |
|---|---|---|---|---|
| Layers II/III | 69.80 | 41.49 | 26.60 | 31.91 |
| Layer IV | 82.81 | 44.63 | 20.76 | 34.61 |
| Layers V/VI | 85.42 | 34.49 | 20.91 | 44.60 |
| χ2 | df | P | Significance | |
| 14.46 | 4 | 0.006 | * |
The leftmost column of the table shows the fraction of cells with firing rate modulations during a trial for each layer (% modulated). In every layer, a very large proportion of cells presented facilitated, suppressed, or multiphasic (a combination of both types) firing rate modulations during the task. The three columns in the right show the different proportions of neurons that presented each type of firing modulation. Multiphasic modulations were more likely to occur in layers V/VI (also see χ2 test for statistical significance).
P < 0.01.
Modulations across trial epochs.
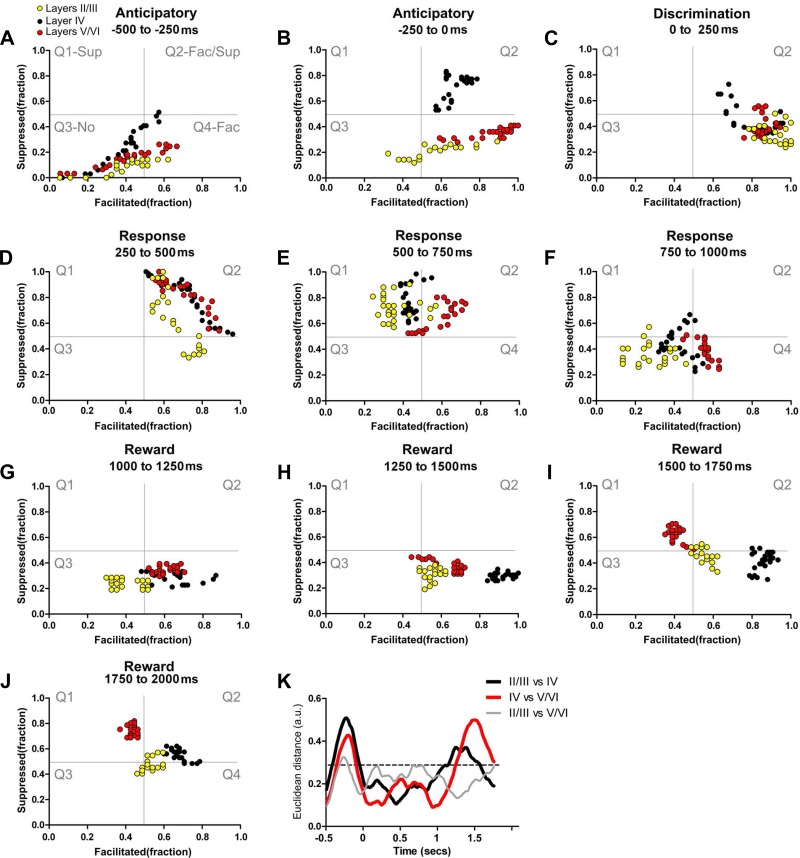
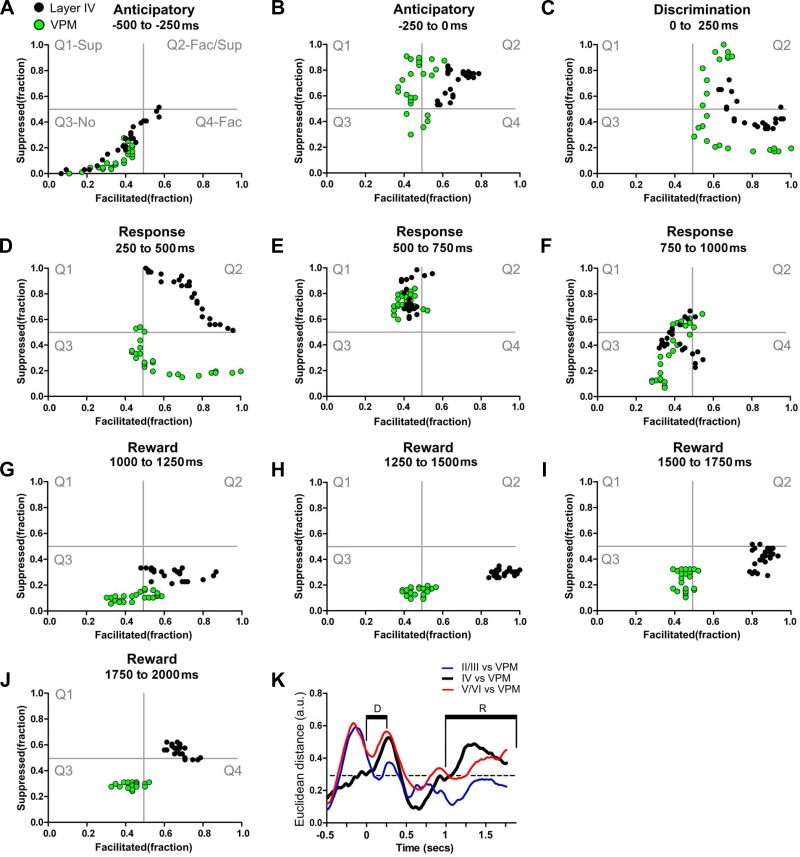
We also measured how neuronal firing pattern modulations varied across different S1 layers during the different time epochs (anticipatory, tactile discrimination, animal behavioral response, and reward periods; Fig. 1A) that define a single task trial. State maps (see materials and methods for details) were employed to characterize the differences in neuronal firing rates observed in supragranular, granular, and infragranular layers (Figs. 3, A–J, and 4, and Supplemental Video S1; supplemental material for this article is available online at the Journal of Neurophysiology website) across these sequential trial epochs. The background of each plate in Fig. 3 depicts (in gray lines) the space occupied by four different quadrants representing the fraction of neuronal firing modulations along a trial epoch. These include prominently suppressed firing modulation (Q1, top left), suppressed + facilitated (Q2, top right), no firing modulation (Q3, lower left), and predominantly facilitated (Q4, lower right).
Fig. 3.
Layer-specific dynamical neuronal modulations. Each colored circle depicts simultaneously the fraction of facilitated (Fac) and suppressed (Sup) neuronal firing rate modulations in a 10-ms bin. Neuronal state maps calculated for layers II/III (yellow circles), layer IV (black circles), and layers V/VI (red circles) are presented. As depicted in gray in A, from top to bottom and left to right, the 4 quadrants indicate firing rate modulations that are prominently: Q1, suppressed; Q2, suppressed and facilitated; Q3, no firing modulations; and Q4, facilitated. A–J: different trajectories of neuronal state maps of granular, supragranular, and infragranular layers are clear in A and B. Additionally, note that in G–J (which are associated with intense whisker stimulation) a large fraction of neuronal firing modulations recorded from layer IV are in the lower right quadrant (facilitated responses), whereas very different neuronal firing modulations occur in layers V/VI (which initially are in the facilitated responses quadrant but gradually shift toward the suppressed quadrant; Q4 to Q1). K: Euclidean distances between neuronal state maps of different S1 layers. As suggested by the different trajectories in the state maps, the largest Euclidean distances between neuronal state maps of layer IV and neuronal state maps of layers V/VI (highlighted red line) were found during the anticipatory (−0.5-0 s) and the reward period (1.0–2.0 s), where the firing rate modulations concentrated in different quadrants for long periods. Euclidean distances between neuronal state maps of layers II/III and layer IV (black line) were maximal during the anticipatory period. Euclidean distances between neuronal state maps of layers II/III and layers V/VI (gray line) were maximal during the anticipatory period. Neuronal state maps of all layers presented minimal Euclidean distances during the discrimination, animal behavioral response, and at the beginning of the reward period.
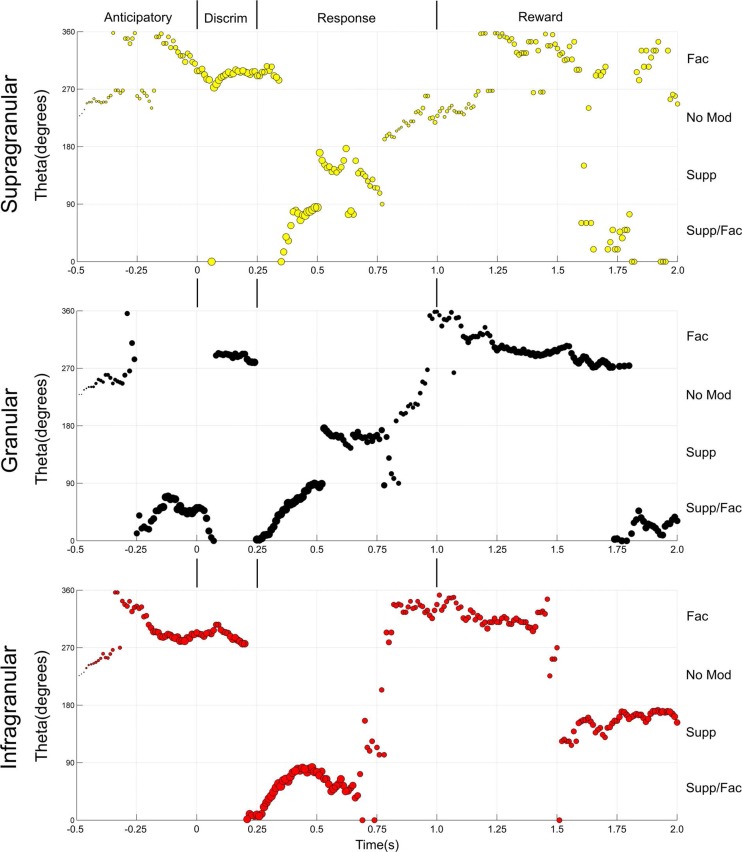
Fig. 4.
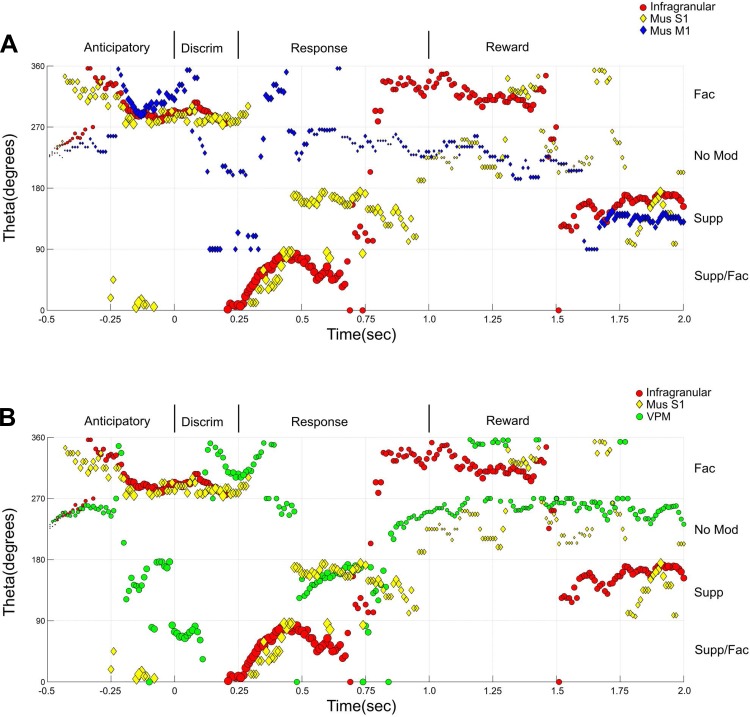
Theta angle analysis of S1 layer state maps. The x-axis represents time (−0.5–2.0 s). The y-axis corresponds to the change in degrees (measured as a theta angle) for the markers presented in the state maps, revolving around the center of the 4 quadrants (coordinates facilitated: 0.5 and suppressed: 0.5). Here, Q2, Supp/Fac, corresponds to the angles between 0 and 90° (bottom of panel); Q1, Supp, corresponds to the angles between 90 and 180° (2nd from the bottom); Q3, No, corresponds to the angles between 180 and 270° (3rd from the bottom); and Q4, Fac, corresponds to the angles between 270 and 360° (upper part of the panel). The size of the markers corresponds to the magnitude of the response. Major differences between the theta angles of the S1 layers can be observed in the anticipatory and reward periods.
Detailed analysis of Fig. 3 traces revealed a clear difference in dynamics for each specific cortical layer of the rat S1 cortex. For example, during the anticipatory period (−500-0 ms, Fig. 3A), an epoch in which rats approached the tactile discriminanda, the state map trajectory for the granular layer (black dots) stretched from Q3 to Q2, indicating an elevation in both facilitated and suppressed firing modulations (Fig. 3, A and B). At the same time, the trajectory for supragranular (yellow dots) and infragranular neuron (red dots) layers stretched from Q3 to the lower right quadrant (Q4), indicating a larger fraction of anticipatory facilitated firing modulations. Thus, during the anticipatory epoch, supragranular (II/III) and infragranular layer (V/VI) neuronal firing modulations did not follow those of granular layer neurons.
During the discrimination period (Fig. 3C, 0–250 ms), an epoch in which rats sampled the aperture width, granular layer neurons (black dots) made a transition to the quadrant representing predominantly facilitated firing modulations (a shift from Q2 to Q4). Meanwhile, neurons in the supragranular (yellow dots) and infragranular (red dots) layers remained mostly in the facilitated firing modulation quadrant. Again, supra and infragranular neurons exhibited firing patterns that were distinct from cells located in the granular layer.
As rats began to produce a behavioral response (250–500 ms), granular neuron-firing modulations extended from the border of the purely facilitated quadrant Q4 to the border of the solely suppressed quadrant Q1 (Fig. 3D and Supplemental Video S1). Infragranular neurons very closely matched those in the granular layer, crossing Q2 (the facilitated and suppressed quadrant), but stopped short of reaching the border of Q1. Meanwhile, supragranular neurons defined a distribution of firing patterns that spread from Q4 (purely facilitated) to Q2 (mix of suppressed and facilitated quadrant) and also avoided crossing the border to Q1. This specific supragranular pattern was characterized by an overall reduction in the number of facilitated neuronal firing modulations (see x-axis left displacement in Fig. 3D) compared with other layers.
During the subsequent behavioral response epoch (Fig. 3E, 500–750 ms), the firing modulation patterns of granular and supragranular neurons stretched from the top border between Q1 and Q2 (close to the maximum suppressed modulation value) toward the Q1 center (the suppressed quadrant) and occasionally crossed to Q2. During the same period, infragranular neurons stayed mostly within the Q2 or moved from Q2 toward Q1, close to the center of all four quadrants.
The last epoch (750–1,000 ms) of the animal behavioral response (Fig. 3F) was characterized by granular layer neurons spreading from the border of Q1 and Q2 (close to the center of all 4 quadrants) toward Q4 (the facilitated quadrant), via the Q3. Conversely, the firing modulation of supragranular neurons spread from Q1 to Q3 (no modulations quadrant) and remained there close to the border of Q4. Meanwhile, infragranular neurons stretched from Q1 (close to the center of all 4 quadrants) to Q4 (the facilitated quadrant) and remained there for most of this period. When we analyzed the initial 750 ms of the reward period (Fig. 3, G–I, 1,000–1,750 ms), we noticed that the firing modulations of granular neurons (black dots) initially moved from the Q3 to Q4, remained mostly in Q4, and then slowly stretched toward the border between Q4 and Q2 (mixed suppressed/facilitated quadrant). Meanwhile, infragranular neurons (red dots) gradually moved from Q4 to Q1 (suppressed quadrant) and remained there until the end of the trial. In contrast, supragranular neurons remained in the Q3–Q4 border (initially more in Q3 and then more in Q4) during the first half of the reward period. For the next 250 ms, supragranular neurons remained close to the center of all four quadrants, occupying mainly Q2 and Q4.
By the end of the reward period (Fig. 3J, 1,750–2,000 ms), granular layer neurons remained mostly in Q2 (the facilitated/suppressed quadrant), close to the border with Q4. Meanwhile, supragranular layer neurons stayed located close to the center of all four quadrants, primarily at the intersection of Q2-Q3-Q4. In total opposition, infragranular layer neurons clustered primarily in Q1.
To further quantify the differences observed in the state space, we next measured the distance between clusters formed by the neuronal firing patterns in different cortical layers (Fig. 3K, the dashed black line indicates the confidence interval). Multiple significant differences in Euclidean distances were found between state maps in all epochs (black line: layers II/III vs. layer IV; red line: layers V/VI vs. layer IV; gray line: layers II/III vs. layers V/VI; P < 0.0001). No significant differences in Euclidean distances were found during the first half of the anticipatory epoch (Fig. 3A, −500 to −250 ms; P > 0.05, NS). However, large and significant Euclidean distances were identified during the late anticipatory period (Fig. 3B, −250-0 ms; P < 0.0001), indicating that supragranular and infragranular S1 neurons behaved significantly differently from those located in the granular layer during the late part of the anticipatory epoch.
During the discrimination epoch, the Euclidian distances between clusters from granular, infragranular, and supragranular neurons were similar (P > 0.05, NS). However, significant differences were found in the first two-thirds of the animal's response epoch (Fig. 3, D and E, 250–750 ms, P < 0.01 and P < 0.001, respectively). The opposite pattern was observed when the Euclidean distance between granular and supragranular layer neurons (black line) was analyzed, i.e., they initially diverged but later became very close. Meanwhile, no significant differences in Euclidean distances between the three groups were found during the last part of the response period (Fig. 3F, 750–1,000 ms, P > 0.05, NS).
Large Euclidean distances were observed when the reward epoch was analyzed (1,000–2,000 ms). During the early part of this epoch, the Euclidean distance between granular and infragranular neurons (red line) was smaller than the distance between granular and supragranular neurons (black line) (Fig. 3, G, H, and K; 1,000–1,500 ms, P < 0.0001 and P < 0.05, respectively; black line). As the trial progressed, the Euclidean distance between infragranular and granular neurons became larger than the one obtained for granular and supragranular neurons (Fig. 3, I–K; 1,500–2,000 ms, P < 0.0001 and P < 0.001, respectively; red line).
Overall, this detailed state map analysis revealed that the rat S1 cortex exhibited layer-specific dynamics that changed during the course of a tactile discrimination task trial.
S1 neurons reflect VPM or POM dynamics.
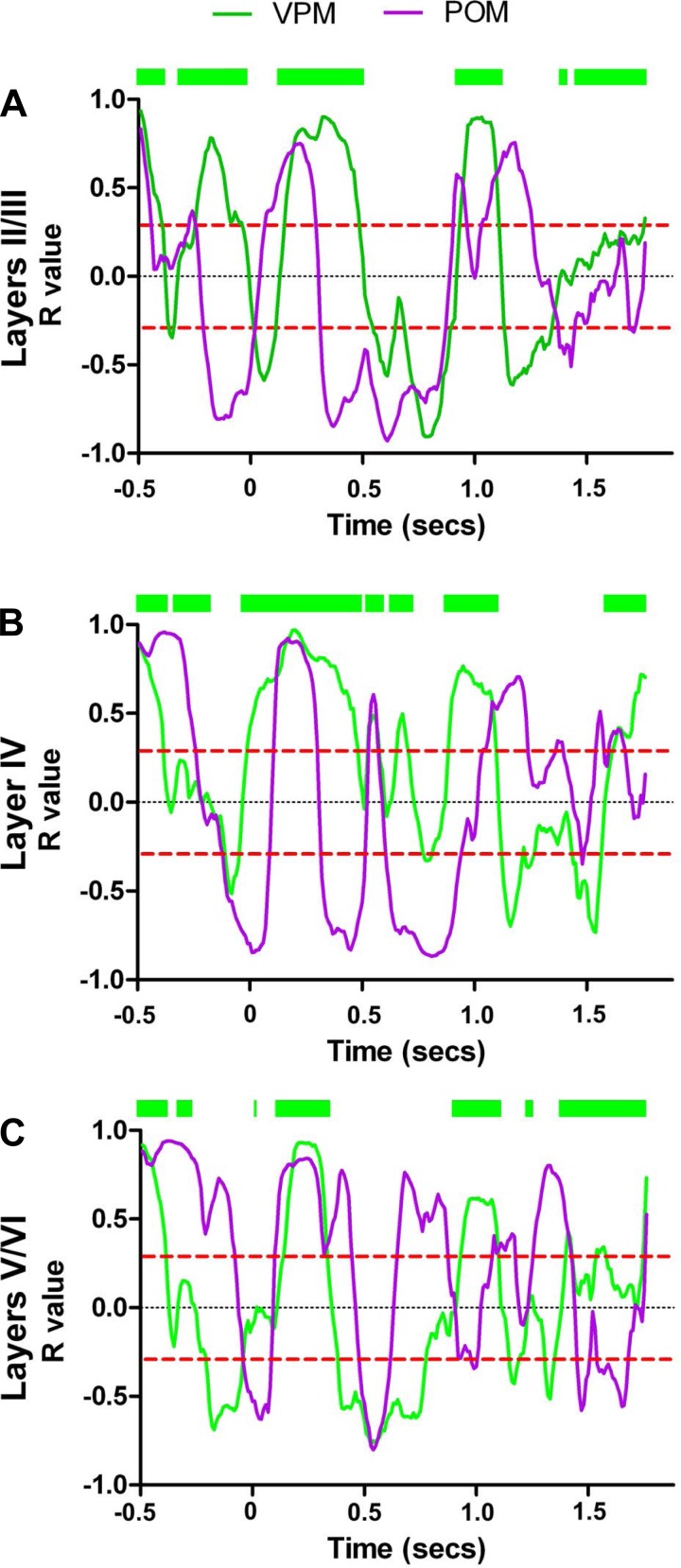
To determine to what extent the lemniscal and paralemniscal pathways may have contributed to neuronal firing modulations in the rat S1 during the task epochs, we compared the dynamics of granular layer S1 neuron-firing modulations with those of 394 neurons recorded from the VPM (Pais-Vieira et al. 2013b) in 10 additional rats. Overall granular layer neurons (black dots) and VPM (green dots) behaved very similarly although some differences could be found in specific trial periods (see Supplemental Video S2, Figs. 5, A–K, and 6, A–C). For example, during the second half of the anticipatory period (Fig. 5, −250–0 ms), while granular neurons occupied mostly Q2 (mixed facilitated/suppressed quadrant), the firing modulations of VPM neurons were found in all four quadrants. The discrimination period (Fig. 5C, 0–250 ms) was characterized by a quick displacement of both VPM and granular neurons from Q2 (mixed facilitated/suppressed modulations), toward a maximum fraction of facilitated sensory-evoked responses in Q4. This firing rate increase matched the moment whiskers made contact with the tactile discriminanda.
Fig. 5.
Ventral posterior medial nucleus of the thalamus (VPM) and granular layer share many dynamical neuronal modulations. Each colored circle depicts simultaneously the fraction of facilitated and suppressed neuronal firing rate modulations in a 10-ms bin. Neuronal state maps of layer IV (black dots) and VPM (green dots) are shown. A–J: neuronal state maps calculated from neurons recorded in VPM and layer IV of S1 were similar during several periods of the active tactile discrimination task. Major differences were found during the 1st part of the response period (250–500 ms) and during most of the reward period (1,250–2,000 ms). K: Euclidean distance between neuronal state maps of different S1 layers and neuronal state maps of VPM. As suggested by the trajectories in the state map, neuronal state maps of layer IV are closer to neuronal state maps of VPM (black line) during the anticipatory and part of the animal behavioral response period. Euclidean distances between neuronal state maps from layers II/III and VPM (blue line) were maximal during the anticipatory period. Euclidean distances between neuronal state maps from layers V/VI and VPM (red line) were maximal during anticipatory, discrimination, and part of the animal behavioral response periods.
Fig. 6.
Discrimination (Dis) and reward (Rew) periods are associated with facilitated firing rate modulations in the lemniscal pathway. The figure depicts peristimulus time histograms (PSTHs) of single neurons recorded from layer IV (A), layers V/VI (B), and VPM (C). The red boxes indicate the discrimination (0–250 ms) and reward periods (1,000–2,000 ms). A: PSTHs of the neurons presented in this column were all recorded in the same session. Increases in the firing rate that correspond to intense whisker stimulation are generally present during both discrimination and reward periods. B: PSTHs of the neurons presented in this column were all recorded in the same session. Note that increases and decreases in firing rate occur at multiple time periods that may or may not match the discrimination and reward periods. C: PSTHs of the neurons presented in this column were all recorded in the same session. As observed in layer IV of S1, discrimination and reward periods were characterized by intense whisker stimulation, which was associated with increases in the firing rate of a large fraction of neurons in VPM.
During the first third of the animal behavioral response period (Fig. 5D, 250–500 ms), VPM and granular layer S1 neurons behaved very differently. Whereas the firing patterns of granular layer neurons stretched from the border of Q2–Q4, via Q2, toward the border of Q1–Q2, VPM neurons spread from Q4 to Q1, via the border of Q3–Q4, until they reached the vicinity of the center of all four quadrants. During the second third of the animal behavioral response period (Fig. 5E, 500–750 ms), both structures shared similar firing patterns, whereas, in the final third (Fig. 5F, 750–1,000 ms), VPM and granular neurons exhibited a large reduction in the fraction of suppressed modulations.
The reward period (Fig. 5, G–J, 1,000–1,250 ms) revealed the larger differences in firing behavior between VPM and granular neurons. This reached a maximum during the final half of this epoch (Fig. 5, H and I, 1,250-1,750 ms).
By measuring Euclidean distances, we were able to quantify how similar VPM and S1 granular neurons behaved over the course of a task trial (Fig. 5K, black lines, also see Fig. 7, A–C; note that the same data are presented in Figs. 5K and Fig. 7, to allow comparison of multiple structures and time epochs; these plots are segmented differently only for ease of presentation, and error bars are not shown in cases where variability was too small for depiction). Thus, during the anticipatory period (−500–0 ms), the distance between VPM and granular layer neurons was initially very small but later became larger (−250–0 ms). This distance kept increasing during the discrimination period (0–250 ms) and reached its maximum value during the first third of the animal behavioral response period (250–500 ms). During the rest of the behavioral response period, the Euclidean distance remained at its minimum values (500–1,000 ms). The first half of the reward period (1,000–1,500 ms) was characterized by a large increase in the distance of the two clusters. Although this distance decreased during the second half of the reward period (1,500–2,000 ms), it remained significantly large.
Fig. 7.
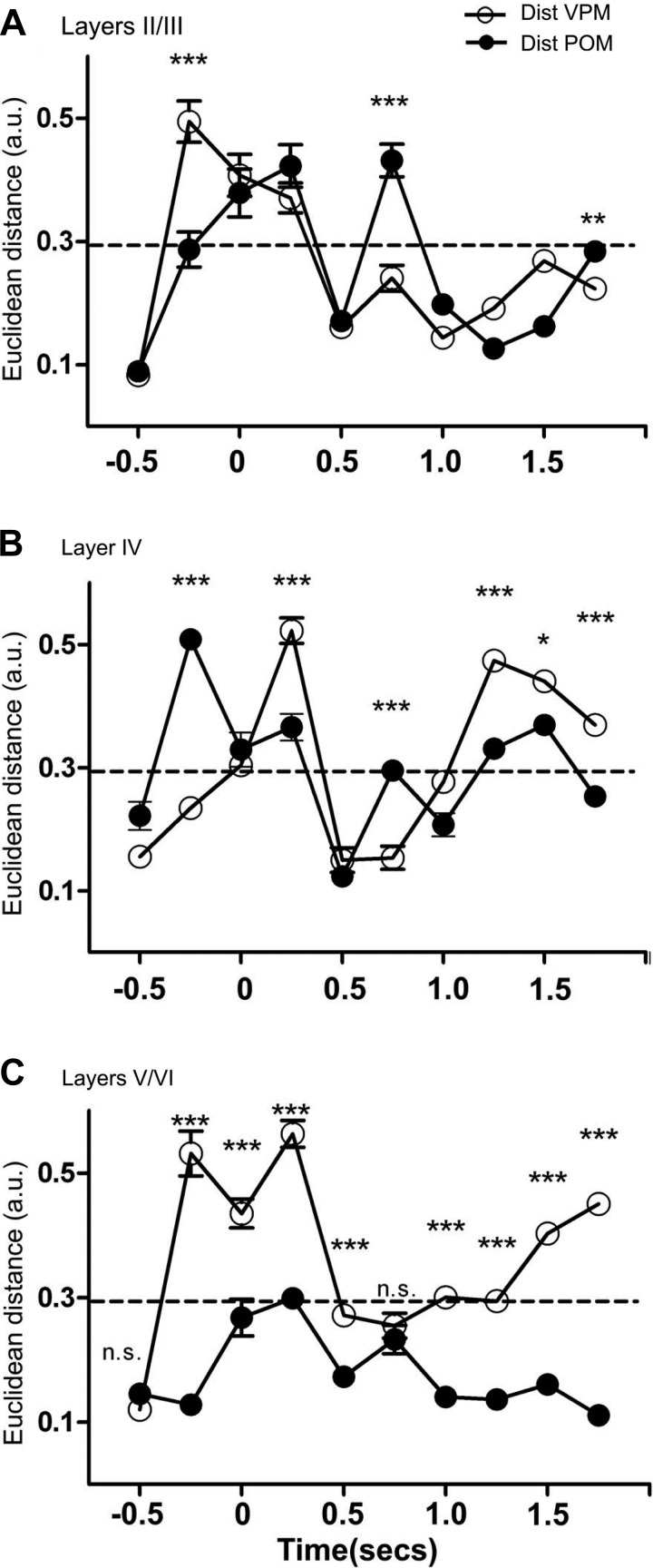
Euclidean distances between neural state maps of S1, VPM, and the posterior nucleus of the thalamus (POM). Each panel depicts the Euclidean distance between neural state maps of a different structure and the neural state maps of VPM and POM. Error bars were not shown in cases where variability was too small for depiction. Each bin corresponds to a 250-ms interval. A: supragranular layers presented neural state maps that were closer to neural state maps of POM (i.e., smaller Euclidean distances) during the late anticipatory period (−250–0 ms) and during the third part of the reward period (1,500–1,750 ms). Neural state maps from supragranular layers were closer to those of VPM during the 3rd part of the animal behavioral response period (750–1,000 ms). Meanwhile, no significant differences could be found in the remaining periods. B: neural state maps for layer IV were closer to neural state maps for VPM during the late anticipatory period (−250–0 ms) and during the 3rd part of the animal behavioral response period (750–1,000 ms). Meanwhile, neural state maps for layer IV presented smaller Euclidean distances to neural state maps for POM during the 1st part of the animal behavioral response period (250–500 ms) and during most of the reward period (1,250–2,000 ms). C: neural state maps for the infragranular layers were closer to neural state maps for POM during the late anticipatory period (−250–0 ms), during the discrimination period (0–250 ms), during most of the animal behavioral response period (250–750 ms), and during the whole reward period (1,000–2,000 ms). Although the Euclidean distance between the neural state maps for layers V/VI and POM was closer during the discrimination period, the dynamical stretches between quadrants suggest that neuronal state maps from layers V/VI more accurately reflect the overall dynamics of VPM during this specific epoch (see text for details). *P < 0.05, **P <0.01, ***P < 0.001.
The Euclidean distance between the VPM and S1 supragranular layers (II/III) neuronal clusters (Fig. 5K, blue line; the dashed black line depicts the confidence interval, also see Fig. 7A) also exhibited multiple differences across the task trial. Whereas the distance was small during the first half of the anticipatory period (−500 to −250 ms), it quickly increased and reached its maximum during the second half of this period (−250–0 ms), remaining very large during the discrimination period (0–250 ms) and the first third of the behavioral response period (250–500 ms). This VPM-II/III distance was small during the second and final third of this epoch (500–1,000 ms) and during the reward period.
The Euclidean distance between VPM and S1 infragranular layer (V/VI) neurons (Fig. 5K, red line; also see Fig. 7C) was also small during the first half of the anticipatory period (−500 to −250 ms), but it quickly increased during its second half (−250–0 ms). Although a reduction was found during the discrimination period (0–250 ms), the distance between these two clusters remained large, again increasing during the first part of the behavioral response period (250–500 ms). Then, a quick reduction in distance was observed during the second part of the behavioral response period (500–750 ms). Thereafter, the Euclidean distance increased slowly during both the remaining behavioral response period (750–1,000 ms) and the reward period (1,000–2,000 ms).
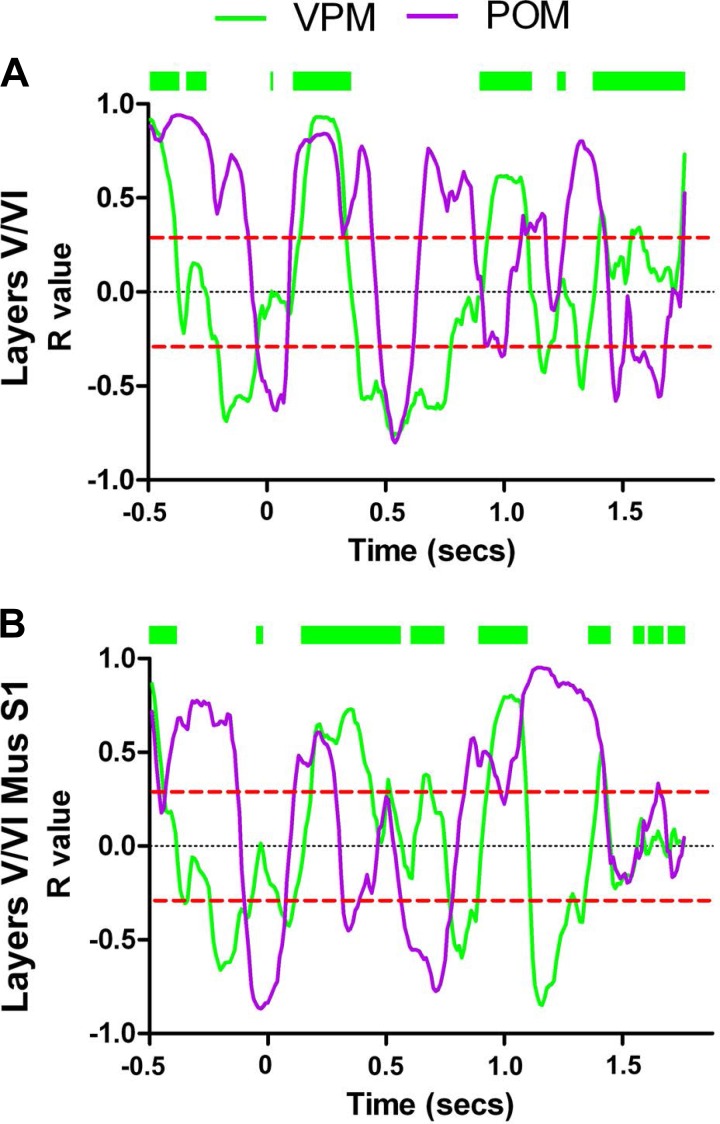
Comparison of the Euclidean distance between neuronal response clusters from different S1 layers and POM (Fig. 7, A–C) (n = 398 neurons in 10 rats), also from a previous study (Pais-Vieira et al. 2013b), showed that the neuronal cluster from S1 infragranular layers had the smallest Euclidean distance to the POM cluster during most of the temporal structure of the trial. The largest difference was observed during the discrimination period (also see Fig. 8, A–K and Supplemental Video S3 for comparison of state map).
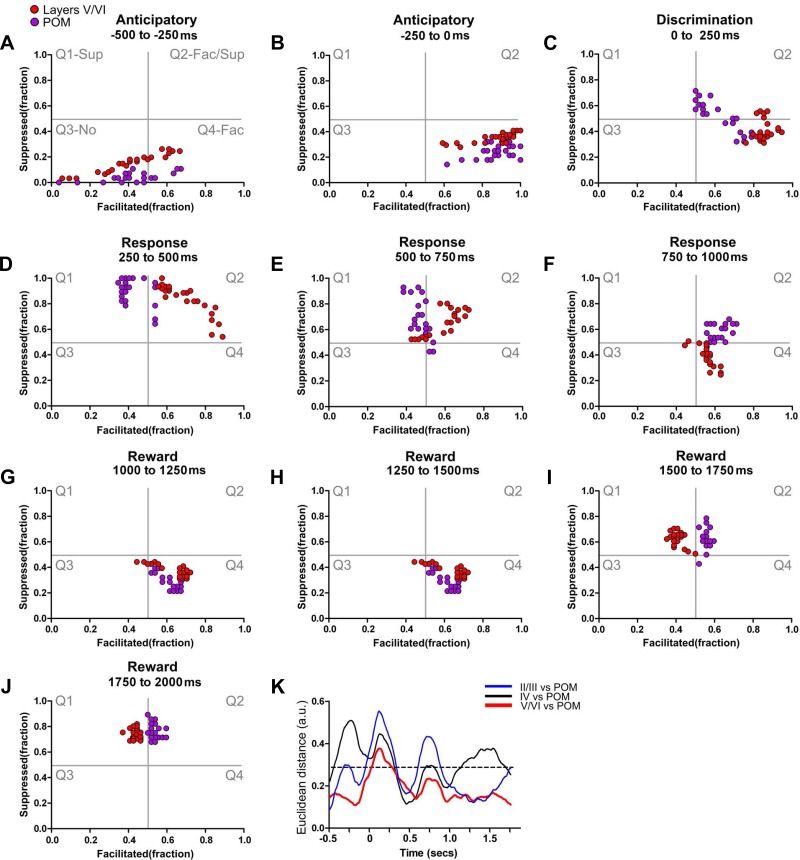
Fig. 8.
POM and infragranular layers share many dynamical neuronal modulations. Each colored circle depicts simultaneously the fraction of facilitated and suppressed neuronal firing rate modulations in a 10-ms bin. Red circles indicate neuronal state maps of layers V/VI, and magenta circles indicate neuronal state maps of POM. A–J: neuronal state maps of layers V/VI and POM were fundamentally similar during active tactile discrimination. The largest difference between neuronal state maps of layers V/VI and POM was observed during the discrimination period (0–250 ms). K: Euclidean distance between neuronal state maps of different S1 layers and neuronal state maps of POM. As suggested by the trajectories in the state map, neuronal state maps of layers V/VI of S1 are closer to neuronal state maps of POM (red line) during most of the trial. Meanwhile, neuronal state maps of layer IV (black line) and layers II/III (blue line) present multiple periods of large Euclidean distances to neuronal state maps of POM. The dashed line indicates the overall chance level for the Euclidean distances.
It is noteworthy that, despite the anatomical proximity between POM and VPM, we found that each of these thalamic nuclei exhibited very distinct patterns of neurophysiological activity, supporting our previous histological and neurophysiological differentiation of these two nuclei for the same data (Pais-Vieira et al. 2013b). As VPM and POM presented neural state maps that more closely resembled layer IV or layers V/VI, respectively, we then compared the neural state maps of supragranular layers with neural state maps of VPM and POM (see Supplemental Video S4). For this comparison we did not assume a priori that state maps from each layer were closer to the state map of any specific thalamic nucleus. Analysis of the variation of Euclidean distances between layers II/III, VPM, and POM clusters across the trial epochs (Fig. 7A) revealed that the firing modulations of layer II/III neurons more closely resembled those of POM neurons during the anticipatory period, whereas they overlapped more with the VPM cluster during the animal behavioral response period (Supplemental Video S4; also see Fig. 7A).
To further confirm these findings, we also correlated the fraction of facilitated modulations occurring in VPM or POM with each of the S1 layers. Although all S1 layers displayed multiple time periods in which the facilitated firing modulations of their neurons were positively or negatively correlated to either thalamic nucleus, facilitated modulations from layer II/III and layer IV neurons were generally correlated to their counterparts in the VPM (layers II/III: P < 0.0001; layer IV: P < 0.0001; Table 2 and Fig. 9, A and B). On the other hand, facilitated firing modulations from layer V/VI neurons were generally correlated to equivalent modulations in the POM (layers V/VI: P < 0.0001, Table 2 and Fig. 9C).
Table 2.
Facilitated firing rate modulations are correlated among multiple structures
| Layer | R Value VPM | R Value POM | P Value | More Correlated To |
|---|---|---|---|---|
| II/III | 0.1156 ± 0.034 | −0.1050 ± 0.034 | P < 0.0001 | VPM |
| IV | 0.2217 ± 0.030 | 0.02 ± 0.034 | P < 0.0001 | VPM |
| V/VI | −0.001 ± 0.031 | 0.2529 ± 0.034 | P < 0.0001 | POM |
| V/VI Muscimol | 0.006 ± 0.028 | 0.01873 ± 0.034 | P < 0.0001 | POM |
Facilitated firing modulations occurring in layers II/III and layer IV were overall more correlated to facilitated firing modulations occurring in the ventral posterior medial nucleus of the thalamus (VPM), whereas facilitated firing modulations recorded from layers V/VI were generally more correlated to facilitated firing modulations recorded from the posterior nucleus of the thalamus (POM). The inactivation of contralateral S1 drastically changed the periods that facilitated firing rate modulations and were correlated between layers V/VI and VPM (compare V/VI muscimol to control condition). Despite this change, overall facilitated firing rate modulations still remained more correlated to POM.
Fig. 9.
Facilitated firing rate modulations are correlated among multiple structures. The green lines indicate R values for the correlation between the fraction of facilitated firing modulations in each S1 layer and the fraction of facilitated firing modulations occurring in neurons recorded from VPM. The magenta lines indicate R values for the correlation between the fraction of facilitated responses occurring in each S1 layer and the fraction of facilitated firing rate modulations occurring in POM. The green bars above each panel indicate periods where R values of cross correlation between facilitated responses from S1 layers and VPM were above or equal to 0. All layers of S1 presented multiple periods where the fraction of facilitated firing modulations were positively or negatively correlated with each thalamic nucleus; however, differences were found for each specific layer. Facilitated firing modulations occurring in layers II/III (A) and layer IV (B) were overall more correlated to excitatory firing modulations occurring in VPM, whereas facilitated firing modulations recorded from layers V/VI (C) were generally more correlated to facilitated firing modulations recorded from POM. The red dashed lines indicate the overall chance level for the R values.
Modulations from cortical loops affect dynamic layer processing in the rat S1.
To probe the potential cortico-cortical inputs responsible for S1 layer dynamics during tactile discrimination (Krupa et al. 2004; Nicolelis 2005; Pais-Vieira et al. 2013b), we further analyzed layer-specific neurophysiological properties during sessions in which either the ipsilateral M1 or the contralateral S1 were inactivated with muscimol injections (Pais-Vieira et al. 2013b). M1 or S1 inactivation induced physiological changes in neuronal firing rates that were more pronounced at the S1 infragranular layers (Tables 3 and 4). Following M1 inactivation, an increase in firing rate magnitude occurred in the ipsilateral S1 infragranular layers (control: 2.40 ± 0.10 spikes/trial; saline: 2.32 ± 0.20 spikes/trial; muscimol: 3.18 ± 0.30 spikes/trial; Mann-Whitney U = 5,103; P = 0.0113). In addition, the duration of the facilitated responses of these ipsilateral S1 neurons decreased (control: 202.9 ± 18.8 ms spikes/trial; saline: 201.6 ± 20.2 ms; muscimol: 152.0 ± 18.7 ms; Mann-Whitney U = 4,895; P = 0.0031).
Table 3.
Magnitude of facilitated firing rate modulations
| Region Manipulated | Region Recorded | Condition | Magnitude, spikes/trial | MWU | P Value | |
|---|---|---|---|---|---|---|
| M1 | Layers II/III | Control | 2.66 ± 0.14 | |||
| Saline | 2.51 ± 0.17 | 5807 | 0.0841 | n.s. | ||
| Muscimol | 2.03 ± 0.17 | |||||
| Layer IV | Control | 1.91 ± 0.09 | ||||
| Saline | 2.21 ± 0.22 | 2632 | 0.3673 | n.s. | ||
| Muscimol | 2.24 ± 0.19 | |||||
| Layers V/VI | Control | 2.40 ± 0.14 | ||||
| Saline | 2.32 ± 0.18 | 5103 | 0.0113 | * | ||
| Muscimol | 3.18 ± 0.31 | |||||
| S1 | Layers II/III | Saline | 1.70 ± 0.13 | 889 | 0.6492 | n.s. |
| Muscimol | 1.72 ± 0.12 | |||||
| Layer IV | Saline | 2.14 ± 0.16 | 2259 | 0.8758 | n.s. | |
| Muscimol | 2.07 ± 0.19 | |||||
| Layers V/VI | Saline | 3.14 ± 0.21 | 13640 | 0.0036 | † | |
| Muscimol | 2.41 ± 0.15 |
Values are means ± SE. Inactivation of ipsilateral M1 or S1 contralateral to the recording site selectively modulated infragranular layers of S1. After M1 inactivation, an increase in the magnitude of facilitated firing rate modulations was observed, and a nonsignificant trend was observed in neurons recorded from the supragranular layers. After contralateral S1 inactivation, a reduction in the magnitude of facilitated firing rate modulations was observed in neurons recorded from the infragranular layers of S1.
P < 0.05,
P < 0.01.
MWU, Mann-Whitney U-test.
Table 4.
Duration of facilitated firing rate modulations
| Region Manipulated | Region Recorded | Condition | Duration, ms | MWU | P Value | |
|---|---|---|---|---|---|---|
| M1 | Layers II/III | Control | 180.3 ± 12.3 | |||
| Saline | 168.5 ± 14.2 | 6707 | 0.9955 | n.s. | ||
| Muscimol | 151.3 ± 14.6 | |||||
| Layer IV | Control | 179.4 ± 11.7 | ||||
| Saline | 141.7 ± 22.5 | 2450 | 0.1166 | n.s. | ||
| Muscimol | 156.7 ± 33.1 | |||||
| Layers V/VI | Control | 202.9 ± 18.8 | ||||
| Saline | 201.6 ± 20.2 | 4895 | 0.0031 | * | ||
| Muscimol | 152.0 ± 18.7 | |||||
| S1 | Layers II/III | Saline | 109.8 ± 12.9 | 839.5 | 0.4872 | n.s. |
| Muscimol | 82.0 ± 6.90 | |||||
| Layer IV | Saline | 153.2 ± 15.0 | 2362 | 0.3133 | n.s. | |
| Muscimol | 113.4 ± 12.6 | |||||
| Layers V/VI | Saline | 208.1 ± 15.4 | 16100 | 0.5866 | n.s. | |
| Muscimol | 204.2 ± 17.1 |
Values are means ± SE. Inactivation of M1 selectively decreased the duration of firing rate modulations infragranular layers of S1. No clear effect was detected after S1 inactivation.
P < 0.01.
S1 inactivation decreased the magnitude of facilitated sensory-evoked responses in neurons located in the infragranular layers of the contralateral S1 (saline: 3.136 ± 0.200 spikes/trial; muscimol: 2.409 ± 0.200 spikes/trial; Mann-Whitney U = 13,640; P = 0.0036).
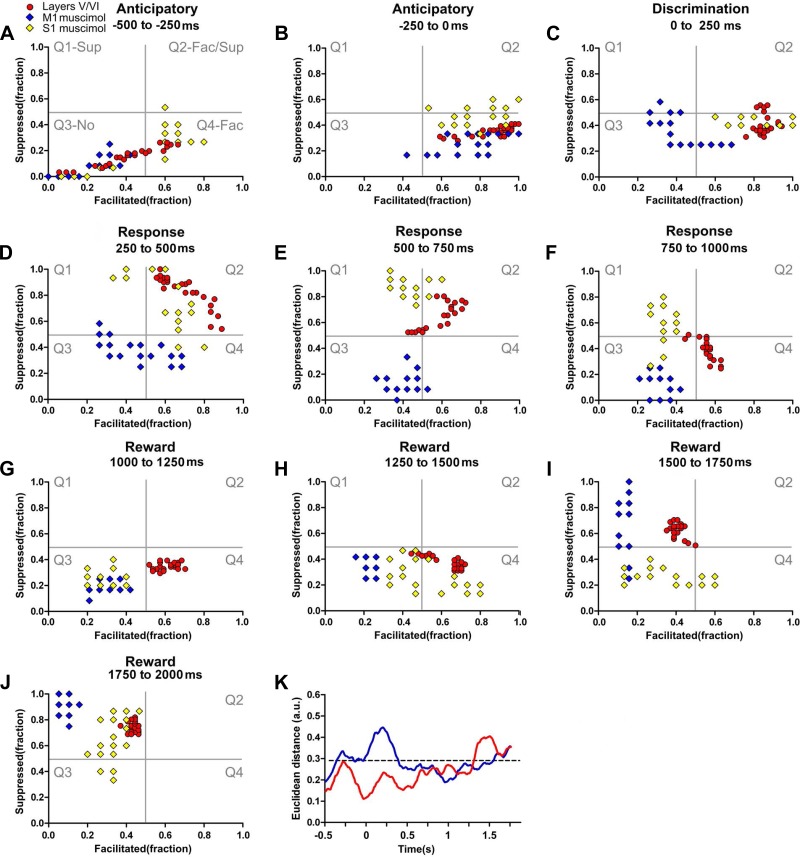
Following M1 or S1 inactivation, the dynamics of the neural state maps calculated for S1 infragranular layers presented multiple changes across different task epochs (see Figs. 10, A–J, 11, A–L, and 12, A and B and Supplemental Video S5 for details). Whereas M1 inactivation (blue diamonds, Figs. 10, A–J, and 12A and Supplemental Video S5) induced considerable changes in all periods, the effect of S1 inactivation (yellow diamonds, Figs. 10, A–J, and 12B and Supplemental Video S5) was particularly powerful for the animal behavioral response and for the reward epochs. Comparison of Euclidean distances between distinct cortical layer clusters (Fig. 10K, the dashed black line depicts the confidence interval) revealed that M1 inactivation (blue line) induced its largest effect during both the discrimination epoch and the first two parts of the animal behavioral response period (discrimination, P < 0.001; animal behavioral response, P < 0.001, P < 0.05, and P > 0.05, n.s., respectively). S1 inactivation (red line) produced the largest impact during the third part of the reward period (reward period: P > 0.05, n.s.; P > 0.05, n.s.; P < 0.001; and P > 0.05, n.s.; respectively).
Fig. 10.
Cortical loop modulation of infragranular neuronal dynamics. Each colored circle or diamond depicts simultaneously the fraction of facilitated and suppressed neuronal firing rate modulations in a 10-ms bin. A–J: both M1 inactivation (blue diamonds) and S1 inactivation (yellow diamonds) were associated with multiple changes in infragranular neuronal state maps in multiple task periods. M1 inactivation induced an overall reduction in facilitated firing rate modulations (shift toward left quadrants) that was present during all periods of the task and a variable effect in the fraction of suppressed firing rate modulations (compare E to J). Contralateral S1 inactivation induced multiple changes in the neural state maps that could include fewer facilitated firing rate modulations (G) or multiple variations in the fraction of suppressed firing modulations (compare F to I and J). K: comparison of Euclidean distances between neuronal state maps of infragranular layers of S1 after contralateral S1 inactivation (red line) or ipsilateral M1 inactivation (blue line). M1 inactivation was associated with larger Euclidean distances in the neuronal state maps of infragranular layers. This distance was maximal during the discrimination and animal behavioral response periods (0–500 ms). S1 inactivation induced the largest Euclidean distance during the reward period (1,250–2,000 ms) and the smallest during the discrimination period (0–250 ms).
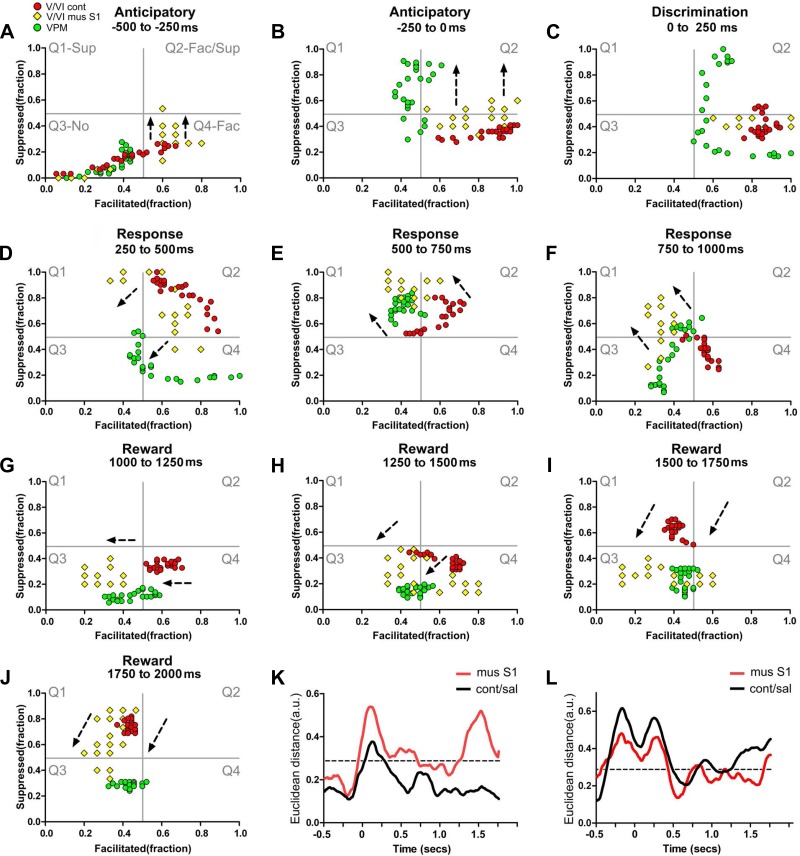
Fig. 11.
Bilateral integration is associated with paralemniscal neuronal dynamics in infragranular layers. Each colored circle or diamond depicts simultaneously the fraction of facilitated and suppressed neuronal firing rate modulations in a 10-ms bin. Red circles represent neuronal state maps of layers V/VI in control conditions, yellow diamonds represent neuronal state maps of layers V/VI after contralateral S1 inactivation, and green circles indicate neuronal state maps of VPM. The black intermittent lines indicate the overall translational shift observed in the neuronal state maps after S1 contralateral inactivation. A–J: neuronal state maps of layers V/VI after contralateral S1 inactivation presented multiple shifts that were now closer to the neuronal state maps of VPM, suggesting that bilateral S1 integration may function as a switch between preferential lemniscal or paralemniscal modes of processing. K: during control conditions (black line), the Euclidean distance between neuronal state maps of layers V/VI and POM was smaller than after S1 inactivation (compare black to red line). L: after S1 inactivation, the Euclidean distance between the neuronal state maps of layers V/VI of S1 and VPM became closer (compare black to red line). Overall the inactivation of S1 increased the Euclidean distance between neural state maps of infragranular layers and POM and reduced the Euclidean distance between the neural state maps of infragranular layers and VPM. Mus, muscimol.
Fig. 12.
Theta angle analysis of S1 layers after M1 or S1 inactivation. The x-axis represents time (−0.5 to 2.0 s). The y-axis corresponds to the change in degrees (measured as a theta angle) for the markers presented in the state maps, revolving around the center of the 4 quadrants (coordinates facilitated: 0.5 and suppressed: 0.5). Here, Q2, Supp/Fac, corresponds to the angles between 0 and 90° (bottom of panel); Q1, Supp, corresponds to the angles between 90 and 180° (2nd from the bottom); Q3, No, corresponds to the angles between 180 and 270° (3rd from the bottom); and Q4, Fac, corresponds to the angles between 270 and 360° (upper part of the panel). The size of the markers corresponds to the magnitude of the response. A: M1 inactivation (blue diamonds) induced multiple changes across the trial, of which the most clear is a reduction in the magnitude of modulations, that were more pronounced during the response period. Meanwhile, S1 inactivation (yellow diamonds) also induced multiple changes that were more pronounced during the response and the reward periods. B: overall effects of S1 inactivation suggest that the theta angles measured in the state maps of layers V/VI (yellow dots) became closer to VPM (green dots).
Directly comparing the neural state maps during the two periods characterized by intense tactile stimulation (i.e., discrimination and reward) revealed that, after M1 inactivation, the fraction of facilitated neuronal firing rate modulations observed in the S1 infragranular layer decreased (shift toward the left side in the x-axis during both periods), while the fraction of suppressed firing rate modulations increased only during the reward period. This finding indicates that M1 activity differentially affected S1 neuronal firing modulations during the discrimination (i.e., fewer facilitated sensory-evoked responses) and the reward (i.e., fewer facilitated and more suppressed sensory-evoked responses) periods. Meanwhile, S1 inactivation did not induce clear changes in neuronal firing rate modulations during discrimination, but, instead, it was associated with reductions in facilitated and suppressed neuronal sensory evoked responses during the reward period.
Overall, these results suggest the existence of a differential role for M1 and S1 cortical loop modulations during two periods of intense tactile stimulation (discrimination and reward). They also strongly indicate that a significant component of the neuronal firing modulations observed at the S1 infragranular layers during active whisker stimulation can be better accounted by a joint balance between modulations from M1 and bilateral S1 cortical loop modulations rather than by any of these afferents alone.
S1 inactivation induces preferential lemniscal processing in infragranular layers.
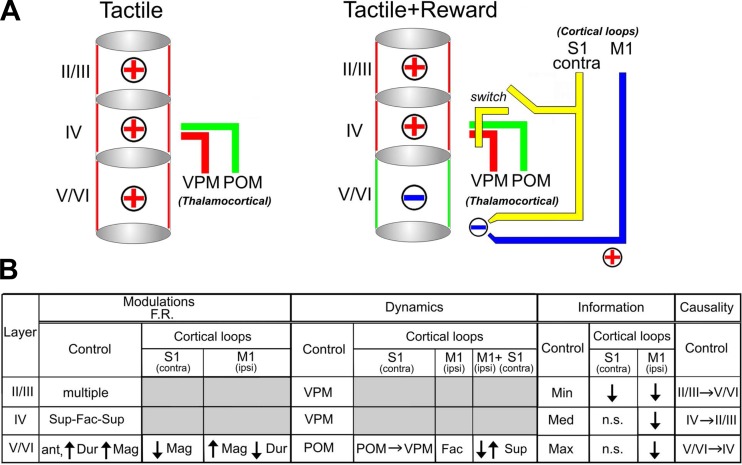
During regular control conditions (i.e., no inactivation), the neuronal sensory-evoked response clusters obtained from the S1 infragranular layers resembled mainly the dynamics observed at the POM. This suggested the existence of a preferential influence of paralemniscal pathways on processing of information in infragranular S1 layers. However, during the discrimination period, the overall S1 pattern of layer activation was closer to the one observed in the VPM. Interestingly, the S1 supragranular layers constantly changed their activation pattern, matching the dynamic behavior of either the VPM or POM. These observations suggested to us that S1 processing in behaving rats can dynamically switch between preferential lemniscal and paralemniscal modes of operation throughout the time epochs that define a task trial.
We next measured the effects on S1 infragranular layers following contralateral S1 inactivation and compared them to the dynamics observed in the VPM and infragranular layers of S1 during the control condition (Figs. 11, A–J, and 12B; also see Supplemental Video S6). Although S1 inactivation induced multiple sparse effects in the contralateral S1 infragranular layers, detailed analysis revealed a single major shift toward the dynamics observed in VPM (Fig. 11, translational effects shown by black intermittent arrows; also see Supplemental Video S6). This POM-to-VPM shift occurred in 8 out of 10 behavioral periods analyzed (the only exceptions were the first anticipatory period and the discrimination period). Accordingly, Euclidean distances between S1 infragranular layers and POM clusters increased after S1 inactivation, whereas those between the S1 infragranular layers and VPM decreased (Fig. 11, K and L, compare red line with black line). Notice that, during the tactile discrimination epoch, a period in which neuronal modulations recorded from all S1 layers were already suggestive of preferential lemniscal processing, contralateral S1 inactivation produced no clear effects.
We also compared how the fraction of increased neuronal responses from infragranular layers correlated to VPM and POM, before and after S1 inactivation. After S1 inactivation, facilitated sensory-evoked responses in contralateral S1 infragranular layers remained more correlated to POM than VPM, albeit with a much smaller value (Table 2 and Fig. 13, A and B).
Fig. 13.
S1 inactivation reduces correlation between facilitated firing rate modulations occurring in layers V/VI and POM. The green lines indicate R values for the cross correlation of facilitated firing rate modulations in each structure and VPM. The magenta lines indicate R values for the cross correlation between facilitated firing rate modulations in each structure and POM. The large green bar above each panel indicates periods where R values of cross correlation were above or equal to 0. Facilitated firing rate modulations recorded from layers V/VI in control conditions (A) were more correlated to facilitated firing rate modulations recorded from POM. B: inactivation of contralateral S1 drastically changed the periods where facilitated firing rate modulations were correlated between layers V/VI and VPM or POM. The red dashed lines indicate the overall chance level for the R values.
Overall, these results indicate that contralateral S1 afferents may provide a switch between preferential lemniscal or paralemniscal processing modes and further enhance the role of cortical loop modulations in layer-specific processing.
M1 and S1 inactivation induced opposite effects on S1.
Comparison of the effects of M1 inactivation across the whole trial demonstrated an overall reduction in the facilitated firing modulations of infragranular S1 neurons (see x-axis displacements of blue diamonds vs. red dots in Fig. 10 and Supplemental Video S5). Additionally, comparison of the fraction of suppressed firing modulations in S1 infragranular layers during M1 or contralateral S1 inactivation suggested that these two cortical areas counterbalanced the effects of the other. This interaction was particularly marked during the animal's behavioral response period (250–1,000 ms, see Fig. 10 and Supplemental Video S5).
To test the hypothesis that M1 and S1 controlled the magnitude of suppression in the S1 infragranular layers across different trial periods, we first determined whether there was any correlation between the amounts of inhibition exhibited by these cortical neurons during the control period and when M1 or S1 were inactivated (see Fig. 14, A–D, also see materials and methods for details). As depicted in Fig. 14A, after either M1 (F1,248 = 105, P < 0.0001, R2 = 0.2974, open bar) or S1 inactivation (F1,248 = 136, P < 0.0001, R2 = 0.3010, shaded bar), suppressed firing modulation in S1 infragranular layers could only modestly predict the firing pattern of the same neurons during control conditions. That meant that M1 or S1 inactivation significantly disrupted inhibition exhibited by these S1 infragranular neurons.
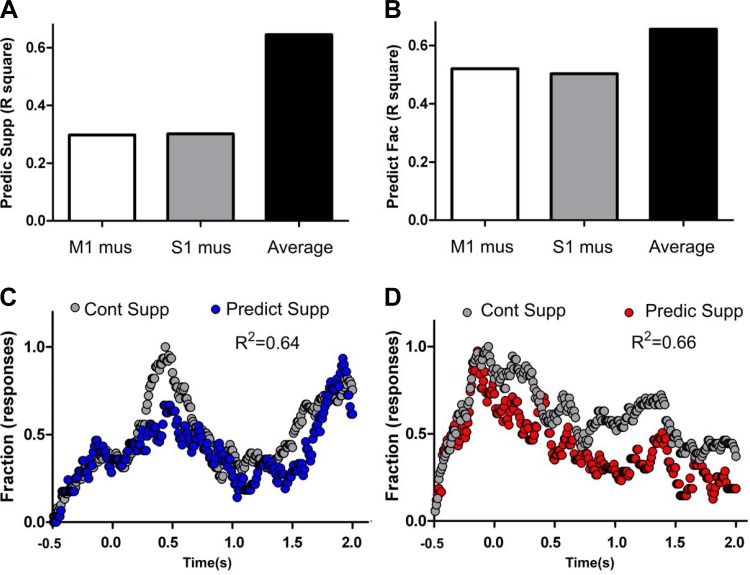
Fig. 14.
M1 and S1 control the gain of suppressed firing rate modulations in infragranular layers. A: prediction of neural state maps for suppressed firing rate modulations in control conditions using the neural state maps from M1 inactivation (open), S1 inactivation (shaded), or from both combined (solid) indicated that a large increase in prediction can be achieved by combining both conditions. B: prediction of neural state maps for facilitated firing rate modulations in control conditions using the neural state maps from M1 inactivation (open), S1 inactivation (shaded), or from both combined (solid) indicated that only a small increase in prediction can be achieved by combining both conditions (because each condition alone was already a good predictor of neural state maps in control conditions). C: control (gray) and predicted values (blue) for suppressed firing rate modulations during the task. Note that by averaging the effects of M1 and S1 inactivation an overall very good prediction of the fraction of suppressed firing rate modulations in control conditions can be obtained. This suggests that ipsilateral M1 and contralateral S1 control the gain of suppressed firing rate modulations during a trial. D: control (gray) and predicted values (red) for facilitated firing rate modulations during the task. Note that facilitated firing rate modulations from M1 or S1 inactivation conditions alone were already good predictors of neuronal firing rate modulations occurring in control conditions, and the average of the 2 sets of data did not drastically increase the overall prediction.
We next examined whether the average suppression generated by S1 infragranular neurons after M1 or S1 inactivation could better predict the fraction of suppressed firing of the same cortical neurons during control conditions. As depicted in Fig. 14A (solid bar), the overall variation of suppressed neuronal modulations in the S1 infragranular layers was much better captured by the combination of M1 and S1 inactivation (F1,248 = 450.3, P < 0.0001, R2 = 0.6448). Thus infragranular S1 neurons that exhibited mainly suppression were more significantly influenced by the combined afferents from S1 and M1.
Meanwhile, when we examined the subpopulation of S1 infragranular neurons that exhibited mainly facilitated firing modulations during control conditions (Fig. 14B), we noticed that the activity of these neurons provided a much better predictor of their own facilitated firing after the inactivation of M1 (F1,248 = 268.6, P < 0.0001, R2 = 0.5199, open bar) or S1 (F1,248 = 247.2, P < 0.0001, R2 = 0.5028, shaded bar). These higher predictive values indicated that state map-facilitated firing modulations in the S1 infragranular layers were not affected by the individual inactivation of either M1 or S1. However, when we performed the same analysis using a combined S1 and M1 inactivation, the prediction of these neurons for control-facilitated firing modulations in infragranular layer state maps also improved (F1,248 = 472.8, P < 0.0001, R2 = 0.6560; Fig. 14B, solid bar). This latter improvement, however, was much smaller than what was observed in the case of infragranular suppression (Fig. 14, A and B, compare open and shaded to solid bars). These results support the notion that a combined influence from ipsilateral M1 and contralateral S1 primarily controls the gain of suppressed firing modulations of infragranular S1 neurons and, secondarily, may also influence the gain of facilitated firing modulations in the same cells.
Sensorimotor information content is layer and epoch specific.
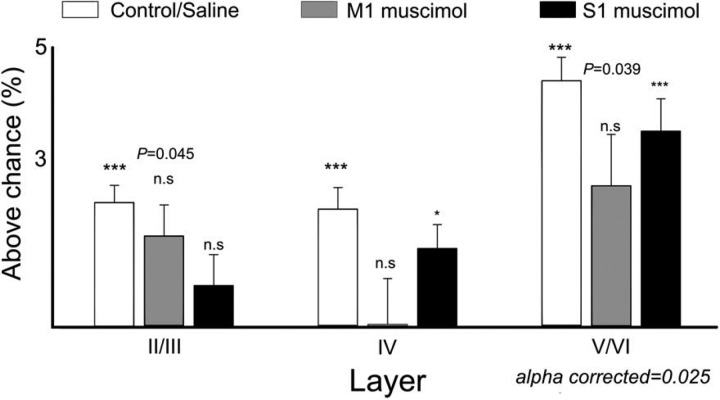
Although the behavioral performance was affected by M1 inactivation (control: 83.06 ± 1.00% correct; saline M1: 84.04 ± 2.20% correct; muscimol M1: 75.41 ± 3.80% correct; F4,139 = 3.756, P = 0.0062) as well as S1 inactivation (saline S1: 83.82 ± 1.40% correct; muscimol S1: 75.95 ± 1.90% correct; t = 3.356, df = 41, P = 0.0017), all animals were able to perform the task above chance levels. Additionally, as neuronal firing rate modulations were fundamentally different across S1 layers and were dependent on afferents from cortical loops, we then asked whether aperture width, the main parameter rats had to discriminate during execution of our behavioral task, was differently encoded across cortical layers (Krupa et al. 2004). For this, we used a classifier and tested the amount of information above chance level that was present in sessions with neural activity recorded from supragranular, granular, or infragranular layers. As previously reported (Krupa et al. 2004; Pantoja et al. 2007; Wiest et al. 2010), neural activity in all different layers contained sufficient information to decode the aperture width significantly above chance (see Fig. 15, open bars). The largest amount of trial-related information was found in infragranular layers (infragranular layers: 4.41 ± 0.40% above chance; P < 0.0001) followed by supragranular (supragranular layers: 2.23 ± 0.30% above chance, P < 0.0001) and granular layer (granular layer: 2.12 ± 0.40% above chance, P < 0.0001).
Fig. 15.
Trial-related information processed is layer specific and modulated by cortical loop afferents. The figure shows the average percentage of information above chance decoded by single neurons in each layer. Trial information could be decoded from all S1 layers significantly above chance (open bars). The largest amount of information decoded was from neurons recorded from the infragranular layers. Additionally, inactivation of M1 (shaded) or S1 (solid) differentially affected the amount of information decoded from each layer. M1 inactivation reduced the amount of trial-related information decoded in all layers, and this effect was more pronounced in layer IV. S1 inactivation contralateral to the recording site reduced the amount of trial-related information only in supragranular layers. *P <0.05, ***P < 0.001.
Moreover, we found that, after inactivation of contralateral S1, neuronal information in layers II/III (P = 0.4314, n.s., Fig. 15, solid bars) was not sufficient to decode the tactile stimulus above chance, whereas layer IV (P = 0.0072, α corrected = 0.025) and layer V/VI neurons (P < 0.0001, α corrected = 0.025) could still be used to discriminate the tactile stimulus.
Inactivation of ipsilateral M1, however, affected information content in all S1 layers (supragranular layers: P = 0.0445, n.s.; granular layer: P = 0.21115, n.s.; infragranular layers: P = 0.0393, n.s., α corrected = 0.025; Fig. 15, shaded bars).
These results demonstrated that infragranular layers presented the largest amount of trial-related information processing during the tactile discrimination task and that cortical loop modulations from M1 or contralateral S1 could reduce the amount of information processed in each layer.
Infragranular layers of S1 transfer information to granular layer.
We next asked whether S1 infragranular layers played a causal role in information transfer. For this, LFPs were recorded simultaneously from all S1 layers in five rats. Granger causality (Granger 1969) was then calculated for all possible layer pairs in each trial. A large proportion of trials presented significant Granger causality, indicating that all layers exchanged information with one other (Table 5). By comparing the proportion of trials in which statistically significant amounts of information were exchanged between layers, we observed that infragranular layer neurons transmitted significant amounts of information in almost every trial (layers V/VI to layer IV; 762/765 = 99.61% trials). Meanwhile, granular layer neurons transmitted statistically significant amounts of information to infragranular layer neurons in approximately half of the trials (layer IV to layers V/VI: 365/765 = 47.71% trials; χ2 = 530.94, P < 0.0001; α corrected = 0.008). Even less information was transferred from neurons in layer IV to neurons in layers V/VI during all task periods (χ2 test: anticipatory, P < 0.0001; discrimination, P <0.0001; animal behavioral response, P <0.0001; reward, P < 0.0001), and even during tactile stimulation in anesthetized conditions (n = 2 rats) (P < 0.0001).
Table 5.
Proportion of trials with Granger causality across layers
| Target/Trigger | Supragranular | Granular | Infragranular |
|---|---|---|---|
| Supragranular | ———————— | 0.980 | 0.78 |
| Granular | 0.669 | ———————– | 0.996 |
| Infragranular | 0.908 | 0.477 | ———————— |
Overall, neuronal activity recorded from all layers presented significant values of Granger causality, indicating that neurons from all layers causally affected the activity of neurons present in other layers. Neurons recorded from layers V/VI presented a significantly larger proportion of trials where they causally affected neural activity of neurons recorded from layer IV, compared with the proportion of trials where neurons from layer IV causally affected neuronal activity in layers V/VI. Bonferroni corrected P values were used to determine the significance of Granger causality for each trial (see text for details).
To exclude the possibility that one single source could be responsible for all the significant transfers of information, we further tested whether Granger causality remained stable across the multiple periods of the task. For that, we employed a bootstrap confidence interval followed by a one-way ANOVA with repeated measures. We found that the proportion of trials with significant Granger causality changed across time (P < 0.0001 for all layers), suggesting that multiple sources were responsible for the differences found in information transfer.
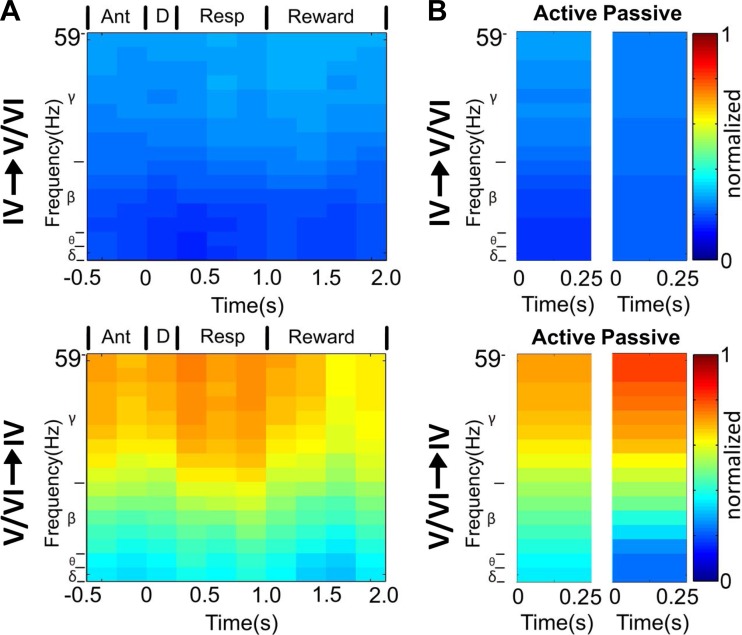
To identify which specific frequencies were involved in intracortical information transfer within S1, we also calculated partial directed coherence (PDC) for the LFPs recorded from all layers across different behavioral periods (Sameshima and Baccala 1999). Considering transmission between layer V/VI to layer IV neurons, we observed that information flowed more frequently through the gamma band (χ2 test, P < 0.0001), particularly during the response period (see Fig. 16A). Additionally, to determine potential differences in the amount of information transferred intracortically during awake and anesthetized states, we compared the number of trials with significant PDC between layers. Neuronal activity recorded from layer IV presented a similar proportion of trials with significant PDC in both awake and anesthetized states (δ: P = 0.058; θ: P = 0.05; β: P = 0.057; γ: P = 0.049; corrected α value = 0.0125, Fig. 16, A and B, top). Meanwhile, layers V/VI exhibited very different proportions of trials with significant PDC in awake or anesthetized conditions (δ: P < 0.0001; θ: P < 0.0001; β: P < 0.0001; γ: P < 0.0001; corrected α value = 0.0125; see Fig. 16, A and B, bottom).
Fig. 16.
Causal information transfer is layer specific. A: partial directed coherence, a measure of information transfer, calculated from local field potential activity, as rats performed the tactile discrimination task, by frequency bands and for specific time periods of the task. Dark red indicates maximum transfer of information, whereas deep blue indicates minimum information transfer. Top: information transfer from neurons in layer IV to neurons in layers V/VI. Bottom: information transfer from neurons in layers V/VI to neurons in layer IV. Although neurons in all layers causally transferred information during the trial (see text for details), neurons in layers V/VI transferred significantly more information to neurons in layer IV than neurons in layer IV transferred to neurons in layers V/VI during all periods of the task (as noted by the overall yellow and red colors of bottom). This information transfer from neurons in layers V/VI to neurons in layer IV peaked during the animal behavioral response period and was more intense in the gamma band. B, top: amount of information transferred between neurons in layer IV and neurons in layers V/VI during the task (active, the same as depicted in the discrimination period in A) or during passive tactile stimulation in anesthetized conditions (passive). No differences were found in the amount of trials with significant information transfer for any of the 4 frequency bands (see text for details). Bottom: amount of information transferred between neurons in layers V/VI and layer IV during tactile discrimination (active, the same as depicted in the discrimination period in A) or during passive tactile stimulation in anesthetized conditions (passive). During anesthetized conditions (passive), neurons in layers V/VI transferred information to neurons in layer IV in a much smaller number of trials compared with the active state. These results suggest that overall information processing in layers V/VI is more affected by active vs. passive conditions than information processing in layer IV.
DISCUSSION
In this study, we recorded 2,038 units from all S1 layers and the two main thalamic nuclei (VPM and POM) of the rat trigeminal system to investigate the role of cortical loop modulations in S1 layer-specific neuronal ensemble dynamics as rats executed an active tactile discrimination task. To our knowledge, the findings reported here derive from the largest recording sample ever of thalamocortical neurons in awake behaving rats performing a tactile discrimination task. Our results demonstrated multiple dynamic modulations across S1 layers. Overall, neuronal ensembles recorded from supragranular and granular layers mostly reflected lemniscal (VPM) neural dynamics, whereas ensembles recorded from infragranular layers mostly reflected paralemniscal (POM) neural dynamics. In infragranular layers, M1 inactivation greatly reduced the fraction of facilitated firing rate modulations, whereas selective inactivation of contralateral S1 switched the patterns from POM- to VPM-like neural dynamics. Additionally, the effects of ipsilateral M1 inactivation counterbalanced the periods where suppressed firing rate modulations were affected by S1 inactivation. Analysis of tactile stimulus encoding revealed that neural ensemble activity in all S1 layers encoded significant amounts of information with maximum values present in the infragranular layers. The information content in each layer was affected by M1 or S1 inactivation. Also, all layers transferred information to other layers, but infragranular layers transferred the largest amount of information to layer IV. Altogether, these results reveal fundamental differences in the dynamics of neural ensembles recorded across different S1 layers during the different epochs of a tactile task and suggest a fundamental role for both ipsilateral M1 and contralateral S1 in neuronal dynamics of S1 during tactile information processing.
The first evidence supporting our main conclusion is the observation that, during most of the behavioral epochs in which the tactile discrimination task was divided (Krupa et al. 2004; Pais-Vieira et al. 2013b), neuronal ensembles, located in different S1 layers, modulated their firing rates in an independent way. In other words, analysis of dynamical state maps, which separated different types of neural ensemble firing rate modulations, revealed that the S1 layers did not uniformly and sequentially process trial-related information (Nicolelis 2011). Furthermore, comparison of the neural state maps obtained for the S1 layers and two thalamic nuclei further revealed that, although layer IV neural dynamics resembled those of VPM neurons and S1 infragranular layer neural dynamics mimicked those of POM, thalamic neural state maps alone could not predict the full sequence of dynamic changes taking place in the S1 cortex. Instead, horizontal and cortico-cortical afferents played an important role in shaping the distinct dynamic behavior observed among supragranular, granular, and infragranular S1 layers in freely behaving rats (Fig. 17, A and B). Accordingly, our data demonstrate that a simple feed-forward model of tactile information processing cannot account for the exuberant dynamic behavior of S1 neuronal ensembles in freely behaving rats engaged in performing an active tactile discrimination task.
Fig. 17.
Summary of physiological findings supporting layer-specific dynamics in tactile discrimination. A: schematics of physiological findings present in state maps from neurons recorded across S1 layers in awake behaving rats performing a tactile discrimination task. Left: when rats were sampling an aperture, all layers presented mostly excited firing rates (+), similar to what was observed in neurons recorded from VPM (red encasing in all layers). Right: during the reward period (which was also characterized by intense whisker stimulation), neurons recorded from layers V/VI alone presented suppressed firing rates (−), as observed in neurons recorded from POM (green encasing only in layers V/VI). Inactivation of M1 or contralateral S1 demonstrated that cortical loop modulations accounted for multiple firing rate dynamics across all periods of the task. M1 inactivation affected facilitated firing rate modulations, contralateral S1 served as a switch between lemniscal and paralemniscal modes of processing, and M1 and contralateral S1 jointly controlled suppressed modulations. B: table with the main differences found in firing rate modulations (F.R.), neuronal dynamics (i.e., state maps), amount of information encoded across structures, and causal transfer of information between structures. Ant, anticipatory firing rate modulations; Sup, suppressed; Fac, facilitated; Mag, magnitude; Dur, duration.
In addition to the analysis of state maps, a series of complementary experiments further supported our central conclusion. For instance, following selective inactivation of the contralateral S1 or ipsilateral M1, we documented multiple changes in S1 neuronal firing, which were more prominent in the infragranular layers. There, inactivation of the contralateral S1 induced a shift from POM neural dynamics toward patterns that more closely resemble VPM neural state maps. Meanwhile, M1 inactivation induced an overall reduction in the amount of facilitated firing modulations observed in infragranular neurons (i.e., an overall leftward shift in the space maps). This effect was observed during almost all task trial epochs.
Interestingly, we also observed that the effects of M1 inactivation on the ipsilateral S1 infragranular layer were counterbalanced by a concurrent contralateral S1 block. For instance, in periods where the inactivation of M1 increased the amount of suppressed firing modulations, the inactivation of the contralateral S1 typically reduced this effect. Conversely, when M1 inactivation decreased the S1-suppressed firing modulations, a contralateral S1 typically increased it (i.e., upward shifts in the space maps were counterbalanced by downward shifts, and vice versa).
Other physiological measurements allowed us to show that S1 layers behave in very distinct ways from one another in freely behaving animals. For example, the amount of tactile stimulus-related information one could extract from neuronal ensembles varied from layer to layer in the S1 cortex. Not only was this information layer specific, but also it was affected by selective inactivation of M1 or S1. Moreover, PDC analysis (Sameshima and Baccala 1999) revealed that much more information was transmitted from layer V/VI to layer IV neurons than in the opposite direction. This information transfer peaked during the animal behavioral response period.
Technical details.
We have grouped S1 layers as supragranular, granular, and infragranular layers, as in previous studies of the same task (Krupa et al. 2004; Pais-Vieira et al. 2013a, 2013b; Pantoja et al. 2007; Shuler et al. 2001; Wiest et al. 2005). However, it should be noted that physiological differences in our set of data could occur attributable to the existence of further anatomical subdivisions currently not surveyed in the present study. Moreover, it is also possible that additional variability may be introduced because of some of our recording microwires being located within or between barrels in the S1 barrel field. Future studies, where state map response accounts for these refinements, may shed some light on the role of these variables. Despite the possibility that all these factors could influence our results, the overall patterns of neuronal activity observed here were mostly constant for each of the different cortical depths and spatial locations, demonstrating the high level of consistency of our findings.
Layer-specific processing depends on behavioral context.
As a rule, neural activity recorded from infragranular and supragranular layers of the rat S1 exhibited complex firing rate modulation patterns that could not be accounted for by simply taking into account the physiological profiles of layer IV neurons. Indeed, the behavior of supragranular, granular, and infragranular layer neurons varied according to the distinct epochs that defined a tactile discrimination task trial. In previous studies, we have shown that, during the anticipatory period, the firing modulations of S1 infragranular layer neurons were fundamentally different from the ones observed in layers II/III and IV (Krupa et al. 2004; Pais-Vieira et al. 2013b; Pantoja et al. 2007; Wiest et al. 2010). We have also shown that M1 was partially responsible for these specific infragranular neuron-firing patterns (Pais-Vieira et al. 2013b). Here, we have reproduced and extended these previous findings by demonstrating that neural firing modulations in the infragranular layers of S1 were larger in magnitude, presented longer durations, and encoded more trial-related information than other layers (Krupa et al. 2004; Pais-Vieira et al. 2013b).
Because the anticipatory period was not associated with tactile input (Krupa et al. 2004; Pais-Vieira et al. 2013b; Wiest et al. 2010) and differences in somatosensory processing have been shown to depend on both behavioral state and context determined by cortical loop modulations from M1 or S1 (Hill et al. 2011; Lee et al. 2013; Pais-Vieira et al. 2013b; Petreanu et al. 2012; Zagha et al. 2013), it could be argued that the presence of tactile input from the periphery defines a completely different mode of processing in the S1 cortex. To demonstrate that the behavioral context can determine layer processing (Nicolelis 2011), we have described here neuronal firing modulations across S1 layers during a complete sequence of behaviors that included multiple periods with and without tactile stimulation (Krupa et al. 2001; Pais-Vieira et al. 2013b; Pantoja et al. 2007; Wiest et al. 2010).
As expected, during two periods associated with intense whisker stimulation (i.e., the discrimination and reward periods), layer IV neural state maps depicted primarily facilitated firing rate modulations. Conversely, layer V/VI neurons only exhibited facilitated firing rate modulations during the tactile discrimination period; during the reward period, when facial whiskers were also intensely stimulated by the rat nose poking, infragranular neurons displayed primarily suppressed firing rate modulations. Although it is possible that this suppressed response could simply originate from neurons that stop firing because the animals were immobile, we have not specifically tested this hypothesis. This discrepancy between different types of neural firing modulations in granular vs. infragranular layers during two epochs of intense tactile input suggests that the layer V/VI suppressed firing modulations arise as a result of multiple afferents and not exclusively as a result of layer IV afferents. These findings further suggest that S1 processing depends on cortical loop afferents as much as on exclusive thalamocortical signals from layer IV neurons (Nicolelis 2011). Additionally, it is important to note that behavioral context may also influence the dynamical interactions occurring between the different layers of S1. We have not addressed this specific possibility in this study.
Layer-specific processing reveals preferential lemniscal or paralemniscal processing.
We also observed that VPM and POM neuronal populations generated neural state maps that accounted for most of the firing modulations observed in different S1 layers during all epochs of the task trial. As expected, layer IV neural state maps were more closely related to the VPM, whereas V/VI state maps more closely followed the profile of POM ensembles. Anatomical studies have shown that VPM sends multiple projections to the barrels in layer IV and to layer VI of S1 (Bernardo and Woolsey 1987; Killackey 1973; Lorente de No 1922; Woolsey and Van der Loos 1970), whereas POM projections to layer IV terminate mostly in the septa (Chmielowska et al. 1989; Fabri and Burton 1991; Koralek et al. 1988; Lu and Lin 1993). As the area occupied by barrels in layer IV is considerably larger than the area occupied by septa (Meyer et al. 2013; Wimmer et al. 2010; Woolsey and Van der Loos 1970), the observation that layer IV matches VPM neural state maps is in line with this anatomical data. However, the existence of clear differences between layer IV and VPM neural state maps during the animal behavioral response and reward epochs also suggests that other afferents influence S1 granular layer dynamics. This notion that cortical loop modulations play a fundamental role in preferential lemniscal or paralemniscal modes of processing is further corroborated by the observation that supragranular or infragranular layer neuronal dynamics cannot be predicted solely from the behavior of VPM or POM neural ensembles. If there was no role for cortical loop modulations, one would expect to see layer II/III and V/VI state maps reproducing the overall physiological pattern defined by the lemniscal afferents to layer IV. However, this was not the case. Instead, we observed that, in freely behaving rats, layer II/III neural state maps differed considerably from those of layer IV during the anticipatory and reward periods. Moreover, during most of the trial epochs (anticipatory, animal behavioral response, and reward periods), layer V/VI neural state maps more closely resembled the neural dynamics determined by a paralemniscal processing mode. Conversely, infragranular layers only approached a preferential lemniscal processing mode during the discrimination period.
In essence, our findings suggest that neurons in each S1 layer are constantly modulated by the asynchronous convergence of multiple thalamocortical and cortical loop afferents and that the preferential mode of processing can dynamically change according to many parameters, like animal behavioral state and expectation, task context, task epoch, reward amount, etc. (Ghazanfar and Nicolelis 2001).
Lemniscal and paralemniscal pathways have traditionally been considered parallel pathways converging on different S1 targets (Shepherd and Svoboda 2005; Welker 1976; Woolsey and Van der Loos 1970). However, several lines of evidence support the opposite contention, i.e., that both such systems communicate extensively while receiving information from multiple afferents. Indeed, a recent detailed anatomical study has shown that VPM and POM afferents overlap considerably in the S1 infragranular layers (Wimmer et al. 2010). In mouse brain slices, it has been proposed that the paralemniscal projection from POM modulates S1, rather than working as a parallel pathway to the lemniscal system (Viaene et al. 2011). Lastly, we have previously demonstrated that sensorimotor information is distributed widely across the rodent trigeminal system (Nicolelis et al. 1995) and that it can be modulated by M1 (Pais-Vieira et al. 2013b). These previous observations are in line with our present findings that revealed major physiological differences between granular and infragranular neural state maps.
As a whole, the present study provides support for the hypothesis that the typical lemniscal and paralemniscal modes of processing reflect only the two extremes of a continuous range of physiological states that the entire rat somatosensory system is capable of exhibiting. According to this view, typical lemniscal or paralemniscal processing can predominate at a given moment in time, but, in general, both systems seem to work in collaboration during most of the period in which rats are engaged in a tactile discrimination task. In this context, we propose that, at the level of the somatosensory cortex, neuronal firing modulations are primarily determined by the asynchronous convergence of multiple thalamocortical and cortical loop afferents that generate layer-specific dynamics, rather than simply by thalamocortical processing (Fanselow et al. 2001; Krupa et al. 2004; Nicolelis 2005, 2011; Pais-Vieira et al. 2013b).
Bilateral integration controls preferential processing mode.
Contralateral S1 inactivation induced multiple effects that were more pronounced at the level of infragranular layers. Bilateral anatomical connections between S1 barrel cortex are most prominent in layers II/III, layer V, and in the septa of layer IV (Welker et al. 1988; White and DeAmicis 1977). These afferents are thought to be excitatory (Cipolloni and Peters 1983; Giuffrida and Rustioni 1989). We and others have previously shown in anesthetized rats that S1 neurons in layers V/VI exhibit bilateral whisker responses that are dependent on the integrity of the contralateral S1 cortex (Li et al. 2005; Rema and Ebner 2003; Shuler et al. 2001). In awake immobilized rats, we have also demonstrated that bilateral multi-whisker stimulation induces a high level of heterogeneity of bilateral neuronal responses and a strong interaction between synchronous bilateral stimuli (Wiest et al. 2005). Therefore, our current observation that S1 inactivation reduces facilitated firing rate modulations in the infragranular layers of the contralateral S1 is in line with previous studies demonstrating the importance of bilateral integration in the S1 (Miyashita and Feldman 2013; Shuler et al. 2002). However, the present study goes further by showing that contralateral S1 has a fundamental role in shaping ipsilateral S1 neuronal dynamics during different epochs of an active tactile discrimination task, something that had not been appreciated before.
In previous studies, we have demonstrated that bilateral S1 integration plays fundamental roles related to tactile processing, sensorimotor integration, learning, and reward (Krupa et al. 2004; Nicolelis et al. 1995; Pais-Vieira et al. 2013a, 2013b; Thomson et al. 2013; Wiest et al. 2010). Here, we are proposing that bilateral integration in S1 may work as a physiological switch between preferential paralemniscal and lemniscal modes of processing at the level of the infragranular layers. This new finding was derived by showing that POM and infragranular layer neural state maps matched each other during anticipatory, animal behavioral response, and reward periods. However, during the tactile discrimination epoch, all S1 layers more closely reflected the VPM neural state map. Further support for this new role for callosal S1 projections was obtained by showing that, after contralateral S1 inactivation, infragranular layer neural state maps exhibited an overall shift toward VPM neural state maps.
The present findings are in line with a previous study in anesthetized rats, where inactivation of S1 contralateral to the recording site more profoundly depressed neural activity in POM than VPM neurons (Li and Ebner 2006). Because it is known that callosal and thalamocortical projections are distributed in a complementary pattern in S1 (Olavarria et al. 1984), a possible anatomical substrate exists for the new “switching” physiological mechanism described here. As such, our results suggest that, whereas S1 continuously processes information under both lemniscal and paralemniscal modes, afferents from multiple cortical loops can bias processing from one to another thalamocortical input at any given moment in time. Lastly, note that our present results were analyzed in general patterns that reflect large time scales. These large time scales do not allow identification of whether small millisecond interactions were generated in the cortex or in the thalamus. Further studies, with a more refined time scale analysis, will be required to determine the specific origin (i.e., cortico-thalamic or thalamocortical) of each neural modulation present during the multiple periods of the task.
M1 and S1 influence on infragranular neuronal firing modulations.
Inactivation experiments revealed that M1 afferents mediate multiple types of neuronal firing modulations in the infragranular layers of the S1 cortex during each trial epoch. As M1 sends massive inputs to S1 layers V/VI (Miyashita et al. 1994) and has been shown to modulate neurons in these layers in awake and anesthetized animals (Kinnischtzke et al. 2014; Lee et al. 2008; Pais-Vieira et al. 2013b), our results are in line with these previous observations. Detailed analysis revealed that M1 inactivation was associated with changes in suppressed firing rate modulations that were epoch and layer specific (Pais-Vieira et al. 2013b). For example, the inactivation of M1 specifically induced opposite effects in suppressed firing rate modulations during the behavioral response or reward periods. Thus, although M1 activity prevented suppressed modulations in infragranular layers during the reward period, the same M1 neurons seem to mediate an increase in layer V/VI suppression during the behavioral response epoch.
Interestingly, the same cortical loop effect mediated by M1 afferents was not observed in the S1 granular layer; during control conditions, M1 was only associated with a larger fraction of layer IV suppression, no matter which trial epoch was analyzed (results not shown). Our data indicate that the level of infragranular layer suppression in the S1 is determined by the interplay of both ipsilateral M1 and contralateral S1 afferents. Thus, despite the fact that ipsilateral M1 and contralateral S1 send excitatory afferents to the infragranular layers (Miyashita et al. 1994; Olavarria et al. 1984), we observed that M1 or S1 inactivation induces opposite shifts in infragranular layer suppression. In this context, by sharing the control of layer V/VI suppression, the interplay of ipsilateral M1 and contralateral S1 afferents may underlie the dynamic shift between lemniscal and paralemniscal processing modes at the infragranular layers of the rat barrel cortex.
Comparison of the tactile discrimination and reward epochs showed that M1 did not induce the same type of neuronal firing modulations in epochs characterized by the generation of intense tactile-evoked responses in layer IV. During the tactile discrimination epoch, the inactivation of M1 reduced the fraction of neurons with facilitated firing modulations in layers V/VI. This supports the notion that M1 afferents contribute to the emergence of representations of the tactile stimulus and body movements in S1 infragranular layers, generated during active exploration tasks (Pais-Vieira et al. 2013b).
During the reward period, M1 inactivation on one side reduced the fraction of neurons with facilitated firing modulations in layers V/VI, while raising the fraction of cells with suppressed firing modulations in this same layer. This suggests that M1 afferents normally prevent some form of suppression from being generated during the reward period.
In summary, M1 influenced infragranular layers of the S1 by increasing neuronal activity during both the discrimination and reward periods, while preventing suppression during the reward period. In comparison, inactivation of the contralateral S1 did not induce any significant difference during tactile discrimination but reduced facilitated firing rate modulations during the reward period. Thus, unlike what was observed in the case of suppressed firing modulations, where ipsilateral M1 and contralateral S1 afferents shared the control of gain, ipsilateral M1 afferents seem to affect facilitated firing rate modulations in infragranular layers of S1 during the whole trial, whereas contralateral S1 inputs exert only partial control during the behavioral response and reward periods. Additionally, note that these effects cannot be explained solely by a motor-gating mechanism (Chapin and Woodward 1981). Although motor gating involves blocking neuronal firing modulations to tactile inputs in the trigeminal nuclei and across S1 layers (Chapin and Woodward 1981, 1982; Lee et al. 2008), the mechanism described here is associated with facilitated firing modulations in layer IV and suppressed firing modulations in layers V/VI.
These results further support the notion that ipsilateral M1 and contralateral S1 do not operate independently but instead interact with each other in different degrees during each trial period.
Trial-related information is layer specific.
We found that all layers of S1 encode trial-related information (Krupa et al. 2004; Pantoja et al. 2007; Pereira et al. 2007; Wiest et al. 2010) and that, after M1 or S1 inactivation, the amount of information that could be decoded varied according to each layer. Inactivation of M1 reduced the amount of information decoded from all layers, whereas the inactivation of S1 reduced the amount of trial information decoded from supragranular layers alone. Although it has been shown that M1 sends few direct inputs to layer IV of S1 (Kinnischtzke et al. 2014), we have found that, after M1 inactivation, neurons recorded from all S1 layers no longer presented relevant information about the tactile stimulus presented on a single trial basis. Moreover, our analysis of causal information transfer further revealed that, although neurons from all layers exchanged information among themselves, neurons from layers V/VI transferred the largest amounts of information to layer IV. This information transfer, which peaked during the behavioral response epoch (Lapray et al. 2009), was carried primarily through the gamma band (Hamada et al. 1999; Jones and Barth 1997; Zagha et al. 2013). Our results are supported in part by previous studies that showed that propagation of neural activity in the somatosensory (Constantinople and Bruno 2013) and the auditory cortices (Sakata and Harris 2009) starts in the deeper layers.
Conclusions.
In conclusion, we propose that cortical loop modulations resulting from the interplay between M1 and bilateral S1 integration significantly contribute to the overall state of the trigeminal system during tactile stimulus information processing. This state is characterized by complex dynamics that are shaped by the behavioral context and can range from an overall lemniscal to an overall paralemniscal processing mode.
GRANTS
This work was supported by the Program for National Institutes of Science and Technology and the National Council for Scientific and Technological Development CNPq/MCTI/INCT-INCEMAQ 610009/2009-5, FINEP 01.06.1092.00, FAPERN 01/2011, and CAPES, the Hartwell Foundation, National Institutes of Health grants R01DE011451, R01NS073125, RC1HD063390, and a National Institute of Mental Health award DP1MH099903 (M. A. L. Nicolelis).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.P.-V. and M.A.L.N. conception and design of research; M.P.-V., C.K., and J.M. performed experiments; M.P.-V., C.K., P.-H.T., M.L., and M.A.L.N. analyzed data; M.P.-V., C.K., P.-H.T., and M.A.L.N. interpreted results of experiments; M.P.-V. and P.-H.T. prepared figures; M.P.-V., M.L., and M.A.L.N. drafted manuscript; M.P.-V., M.L., and M.A.L.N. edited and revised manuscript; M.P.-V., C.K., P.-H.T., J.M., M.L., and M.A.L.N. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank James Meloy for microelectrode array manufacturing, Laura Oliveira, Terry Jones, and Susan Halkiotis for miscellaneous assistance.
REFERENCES
- Bernardo KL, Woolsey TA. Axonal trajectories between mouse somatosensory thalamus and cortex. J Comp Neurol 258: 542–564, 1987. [DOI] [PubMed] [Google Scholar]
- Chapin JK, Woodward DJ. Modulation of sensory responsiveness of single somatosensory cortical cells during movement and arousal behaviors. Exp Neurol 72: 164–178, 1981. [DOI] [PubMed] [Google Scholar]
- Chapin JK, Woodward DJ. Somatic sensory transmission to the cortex during movement: phasic modulation over the locomotor step cycle. Exp Neurol 78: 670–684, 1982. [DOI] [PubMed] [Google Scholar]
- Chmielowska J, Carvell GE, Simons DJ. Spatial organization of thalamocortical and corticothalamic projection systems in the rat SmI barrel cortex. J Comp Neurol 285: 325–338, 1989. [DOI] [PubMed] [Google Scholar]
- Cipolloni PB, Peters A. The termination of callosal fibres in the auditory cortex of the rat. A combined Golgi-electron microscope and degeneration study. J Neurocytol 12: 713–726, 1983. [DOI] [PubMed] [Google Scholar]
- Constantinople CM, Bruno RM. Deep cortical layers are activated directly by thalamus. Science 340: 1591–1594, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabri M, Burton H. Ipsilateral cortical connections of primary somatic sensory cortex in rats. J Comp Neurol 311: 405–424, 1991. [DOI] [PubMed] [Google Scholar]
- Fan RC, Hsieh C. LIBLINEAR: A library for large linear classification. J Mach Learn Res 9: 1871–1874, 2008. [Google Scholar]
- Fanselow EE, Sameshima K, Baccala LA, Nicolelis MA. Thalamic bursting in rats during different awake behavioral states. Proc Natl Acad Sci USA 98: 15330–15335, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazanfar AA, Nicolelis MA. Spatiotemporal properties of layer V neurons of the rat primary somatosensory cortex. Cereb Cortex 9: 348–361, 1999. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Nicolelis MA. Feature article: the structure and function of dynamic cortical and thalamic receptive fields. Cereb Cortex 11: 183–193, 2001. [DOI] [PubMed] [Google Scholar]
- Giuffrida R, Rustioni A. Glutamate and aspartate immunoreactivity in cortico-cortical neurons of the sensorimotor cortex of rats. Exp Brain Res 74: 41–46, 1989. [DOI] [PubMed] [Google Scholar]
- Granger C. Investigating causal relations by econometric models and cross-spectral methods. Econometrica 37: 424–438, 1969. [Google Scholar]
- Guo ZV, Li N, Huber D, Ophir E, Gutnisky D, Ting JT, Feng G, Svoboda K. Flow of cortical activity underlying a tactile decision in mice. Neuron 81: 179–194, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez R, Carmena JM, Nicolelis MA, Simon SA. Orbitofrontal ensemble activity monitors licking and distinguishes among natural rewards. J Neurophysiol 95: 119–133, 2006. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Miyashita E, Tanaka H. Gamma-band oscillations in the “barrel cortex” precede rat's exploratory whisking. Neuroscience 88: 667–671, 1999. [DOI] [PubMed] [Google Scholar]
- Hill DN, Curtis JC, Moore JD, Kleinfeld D. Primary motor cortex reports efferent control of vibrissa motion on multiple timescales. Neuron 72: 344–356, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MS, Barth DS. Sensory-evoked high-frequency (gamma-band) oscillating potentials in somatosensory cortex of the unanesthetized rat. Brain Res 768: 167–176, 1997. [DOI] [PubMed] [Google Scholar]
- Killackey HP. Anatomical evidence for cortical subdivisions based on vertically discrete thalamic projections from the ventral posterior nucleus to cortical barrels in the rat. Brain Res 51: 326–331, 1973. [DOI] [PubMed] [Google Scholar]
- Kinnischtzke AK, Simons DJ, Fanselow EE. Motor cortex broadly engages excitatory and inhibitory neurons in somatosensory barrel cortex. Cereb Cortex 24: 2237–2248, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralek KA, Jensen KF, Killackey HP. Evidence for two complementary patterns of thalamic input to the rat somatosensory cortex. Brain Res 463: 346–351, 1988. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Ghazanfar AA, Nicolelis MA. Immediate thalamic sensory plasticity depends on corticothalamic feedback. Proc Natl Acad Sci USA 96: 8200–8205, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa DJ, Matell MS, Brisben AJ, Oliveira LM, Nicolelis MA. Behavioral properties of the trigeminal somatosensory system in rats performing whisker-dependent tactile discriminations. J Neurosci 21: 5752–5763, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa DJ, Wiest MC, Shuler MG, Laubach M, Nicolelis MA. Layer-specific somatosensory cortical activation during active tactile discrimination. Science 304: 1989–1992, 2004. [DOI] [PubMed] [Google Scholar]
- Land PW, Buffer SA Jr, Yaskosky JD. Barreloids in adult rat thalamus: three-dimensional architecture and relationship to somatosensory cortical barrels. J Comp Neurol 355: 573–588, 1995. [DOI] [PubMed] [Google Scholar]
- Lapray D, Bergeler J, Luhmann HJ. Stimulus-induced gamma activity in the electrocorticogram of freely moving rats: the neuronal signature of novelty detection. Behav Brain Res 199: 350–354, 2009. [DOI] [PubMed] [Google Scholar]
- Lee S, Carvell GE, Simons DJ. Motor modulation of afferent somatosensory circuits. Nat Neurosci 11: 1430–1438, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci 16: 1662–1670, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Ebner FF. Balancing bilateral sensory activity: callosal processing modulates sensory transmission through the contralateral thalamus by altering the response threshold. Exp Brain Res 172: 397–415, 2006. [DOI] [PubMed] [Google Scholar]
- Li L, Rema V, Ebner FF. Chronic suppression of activity in barrel field cortex downregulates sensory responses in contralateral barrel field cortex. J Neurophysiol 94: 3342–3356, 2005. [DOI] [PubMed] [Google Scholar]
- Lorente de No R. The cerebral cortex of the mouse. Trabajos del Laboratorio de Investigaciones Biologicas de la Universidad de Madrid 20: 41–78, 1922. [Google Scholar]
- Lu SM, Lin RC. Thalamic afferents of the rat barrel cortex: a light- and electron-microscopic study using Phaseolus vulgaris leucoagglutinin as an anterograde tracer. Somatosens Mot Res 10: 1–16, 1993. [DOI] [PubMed] [Google Scholar]
- Ma PM. The barrelettes—architectonic vibrissal representations in the brainstem trigeminal complex of the mouse. I. Normal structural organization. J Comp Neurol 309: 161–199, 1991. [DOI] [PubMed] [Google Scholar]
- Martin JH. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett 127: 160–164, 1991. [DOI] [PubMed] [Google Scholar]
- Meyer HS, Egger R, Guest JM, Foerster R, Reissl S, Oberlaender M. Cellular organization of cortical barrel columns is whisker-specific. Proc Natl Acad Sci USA 110: 19113–19118, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Feldman DE. Behavioral detection of passive whisker stimuli requires somatosensory cortex. Cereb Cortex 23: 1655–1662, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita E, Keller A, Asanuma H. Input-output organization of the rat vibrissal motor cortex. Exp Brain Res 99: 223–232, 1994. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA. Computing with thalamocortical ensembles during different behavioural states. J Physiol 566: 37–47, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis M. Beyond Boundaries:The New Neuroscience Of Connecting Brains With Machines—And How It Will Change Our Lives. New York: Times Books/Henry Holt, 2011, p. 353. [Google Scholar]
- Nicolelis MA, Chapin JK. Spatiotemporal structure of somatosensory responses of many-neuron ensembles in the rat ventral posterior medial nucleus of the thalamus. J Neurosci 14: 3511–3532, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MA, Baccala LA, Lin RC, Chapin JK. Sensorimotor encoding by synchronous neural ensemble activity at multiple levels of the somatosensory system. Science 268: 1353–1358, 1995. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Dimitrov D, Carmena JM, Crist R, Lehew G, Kralik JD, Wise SP. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc Natl Acad Sci USA 100: 11041–11046, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olavarria J, Van Sluyters RC, Killackey HP. Evidence for the complementary organization of callosal and thalamic connections within rat somatosensory cortex. Brain Res 291: 364–368, 1984. [DOI] [PubMed] [Google Scholar]
- Pais-Vieira M, Lebedev M, Kunicki C, Wang J, Nicolelis MA. A brain-to-brain interface for real-time sharing of sensorimotor information. Sci Rep 3: 1319, 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais-Vieira M, Lebedev MA, Wiest MC, Nicolelis MA. Simultaneous top-down modulation of the primary somatosensory cortex and thalamic nuclei during active tactile discrimination. J Neurosci 33: 4076–4093, 2013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoja J, Ribeiro S, Wiest M, Soares E, Gervasoni D, Lemos NA, Nicolelis MA. Neuronal activity in the primary somatosensory thalamocortical loop is modulated by reward contingency during tactile discrimination. J Neurosci 27: 10608–10620, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 1998. [DOI] [PubMed] [Google Scholar]
- Pereira A, Ribeiro S, Wiest M, Moore LC, Pantoja J, Lin SC, Nicolelis MA. Processing of tactile information by the hippocampus. Proc Natl Acad Sci USA 104: 18286–18291, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RS, Diamond ME. Spatial-temporal distribution of whisker-evoked activity in rat somatosensory cortex and the coding of stimulus location. J Neurosci 20: 6135–6143, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Gutnisky DA, Huber D, Xu NL, O'Connor DH, Tian L, Looger L, Svoboda K. Activity in motor-sensory projections reveals distributed coding in somatosensation. Nature 489: 299–303, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rema V, Ebner FF. Lesions of mature barrel field cortex interfere with sensory processing and plasticity in connected areas of the contralateral hemisphere. J Neurosci 23: 10378–10387, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata S, Harris KD. Laminar structure of spontaneous and sensory-evoked population activity in auditory cortex. Neuron 64: 404–418, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sameshima K, Baccala LA. Using partial directed coherence to describe neuronal ensemble interactions. J Neurosci Methods 94: 93–103, 1999. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Svoboda K. Laminar and columnar organization of ascending excitatory projections to layer 2/3 pyramidal neurons in rat barrel cortex. J Neurosci 25: 5670–5679, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuler MG, Krupa DJ, Nicolelis MA. Bilateral integration of whisker information in the primary somatosensory cortex of rats. J Neurosci 21: 5251–5261, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuler MG, Krupa DJ, Nicolelis MA. Integration of bilateral whisker stimuli in rats: role of the whisker barrel cortices. Cereb Cortex 12: 86–97, 2002. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Durham D, Woolsey TA. Functional organization of mouse and rat SmI barrel cortex following vibrissal damage on different postnatal days. Somatosens Res 1: 207–245, 1984. [DOI] [PubMed] [Google Scholar]
- Thomson EE, Carra R, Nicolelis MA. Perceiving invisible light through a somatosensory cortical prosthesis. Nat Commun 4: 1482, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R. Regression shrinkage and selection via the lasso. J Royal Stat Soc B 58: 267–288, 1996. [Google Scholar]
- Van Der Loos H. Barreloids in mouse somatosensory thalamus. Neurosci Lett 2: 1–6, 1976. [DOI] [PubMed] [Google Scholar]
- Vapnik V. The Nature of Statistical Learning Theory. New York: Springer-Verlag, 1995. [Google Scholar]
- Viaene AN, Petrof I, Sherman SM. Properties of the thalamic projection from the posterior medial nucleus to primary and secondary somatosensory cortices in the mouse. Proc Natl Acad Sci USA 108: 18156–18161, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SB. The Function of the Vibrissae in the Behavior of the White Rat. Cambridge, MA: Holt, 1912, pp. 81–84. [Google Scholar]
- Welker C. Microelectrode delineation of fine grain somatotopic organization of (SmI) cerebral neocortex in albino rat. Brain Res 26: 259–275, 1971. [PubMed] [Google Scholar]
- Welker C. Receptive fields of barrels in the somatosensory neocortex of the rat. J Comp Neurol 166: 173–189, 1976. [DOI] [PubMed] [Google Scholar]
- Welker E, Hoogland PV, Van der Loos H. Organization of feedback and feedforward projections of the barrel cortex: a PHA-L study in the mouse. Exp Brain Res 73: 411–435, 1988. [DOI] [PubMed] [Google Scholar]
- White EL, DeAmicis RA. Afferent and efferent projections of the region in mouse SmL cortex which contains the posteromedial barrel subfield. J Comp Neurol 175: 455–482, 1977. [DOI] [PubMed] [Google Scholar]
- Wiest MC, Bentley N, Nicolelis MA. Heterogeneous integration of bilateral whisker signals by neurons in primary somatosensory cortex of awake rats. J Neurophysiol 93: 2966–2973, 2005. [DOI] [PubMed] [Google Scholar]
- Wiest MC, Thomson E, Pantoja J, Nicolelis MA. Changes in S1 neural responses during tactile discrimination learning. J Neurophysiol 104: 300–312, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer VC, Bruno RM, de Kock CP, Kuner T, Sakmann B. Dimensions of a projection column and architecture of VPM and POM axons in rat vibrissal cortex. Cereb Cortex 20: 2265–2276, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res 17: 205–242, 1970. [DOI] [PubMed] [Google Scholar]
- Zagha E, Casale AE, Sachdev RN, McGinley MJ, McCormick DA. Motor cortex feedback influences sensory processing by modulating network state. Neuron 79: 567–578, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]