Abstract
Group IV polysaccharide capsules are common in enteric bacteria and have more recently been described in nontyphoidal Salmonella species. Such capsules are known as O-antigen (O-Ag) capsules, due to their high degree of similarity to the O-Ag of the lipopolysaccharide (LPSO-Ag). Capsular polysaccharides are known virulence factors of many bacterial pathogens, facilitating evasion of immune recognition and systemic dissemination within the host. Previous studies on the O-Ag capsule of salmonellae have focused primarily on its role in bacterial surface attachment and chronic infection; however, the potential effects of the O-Ag capsule on acute pathogenesis have yet to be investigated. While much of the in vivo innate immune resistance of Salmonella enterica serovar Typhimurium is attributed to the high-molecular-weight LPS, we hypothesized that the O-Ag capsule may enhance this resistance by diminishing surface expression of pathogen-associated molecular patterns, such as flagella, and increasing resistance to host immune molecules. To test this hypothesis, O-Ag capsule-deficient mutants were constructed, and the loss of O-Ag capsular surface expression was confirmed through microscopy and immunoblotting. Loss of O-Ag capsule production did not alter bacterial growth or production of LPS. Western blot analysis and confocal microscopy revealed that O-Ag capsule-deficient mutants demonstrate reduced resistance to killing by human serum. Furthermore, O-Ag capsule-deficient mutants produced exclusively phase I flagellin (FliC). Although O-Ag capsule-deficient mutants did not exhibit reduced virulence in a murine model of acute infection, in vitro results indicate that the O-Ag capsule may function to modify the antigenic nature of the bacterial surface, warranting additional investigation of a potential role of the structure in pathogenesis.
INTRODUCTION
Salmonella enterica is the most common cause of bacterial gastrointestinal (GI) infection worldwide, causing over 93 million new infections annually (1, 2), the majority of which are caused by nontyphoidal serovars, such as Salmonella enterica serovar Typhimurium and Salmonella enterica serovar Enteritidis (3). Although primarily associated with self-limiting gastroenteritis, nontyphoidal Salmonella species (NTS) are also an important and underrecognized cause of community-acquired bacteremia, particularly in many regions of sub-Saharan Africa (4, 5), where NTS are the most commonly isolated bloodstream pathogens. NTS bacteremia is highly associated with an altered immune status related to advanced HIV, concurrent infection with malaria, malnutrition, and extremes of age (≤2 or ≥60 years) but also occurs in up to 5% of NTS infections in otherwise healthy patients (6–11).
Although extraintestinal infection with NTS is relatively rare, the invasive Salmonella enterica serovar Typhi is particularly adept at systemic dissemination, a characteristic facilitated in part by the production of a surface polysaccharide termed the Vi-antigen (Vi-Ag) capsule. The Vi-Ag capsule is not required for GI colonization but diminishes the local inflammatory response at sites of bacterial invasion and enhances systemic virulence (12) by masking pathogen-associated molecular patterns (PAMPS), such as lipopolysaccharide (LPS); repressing production of highly immunogenic flagellin; increasing resistance to innate immune molecules; and directly interfering with host interleukin-8 (IL-8) inflammatory signaling cascades (13, 14). S. Typhimurium lacks the Vi-Ag capsule but has been reported to produce an alternative capsular polysaccharide (15–17). This structure is a group IV capsule, often referred to as an O-antigen (O-Ag) capsule due to its high degree of similarity to the O-antigen tetrasaccharide repeating unit of lipopolysaccharide (LPSO-Ag). Previous work has identified group IV capsules in Escherichia coli, Vibrio, Shigella sonnei, Francisella tularensis, and Campylobacter jejuni (18–23). Functional analyses of the roles of these capsules during infection have demonstrated that they are able to facilitate serum resistance, aid in systemic dissemination, exhibit shielding of LPS, inhibit immune recognition of the type III secretion system (T3SS) apparatus, reduce host inflammatory response, and delay apoptosis of infected macrophages (21, 24, 25).
Previous studies to understand the role of the O-Ag capsule in Salmonella have reported it to be important for bacterial surface adherence, environmental persistence, and multicellular behavior (15, 26). In S. Enteritidis, the O-Ag capsule operon (yshA-yihU) is regulated by AgfD (CsgD) in concert with extracellular matrix components and thus is maximally expressed in stationary phase at lower temperatures (<30°C) (17). In S. Typhimurium, however, the O-Ag capsule operon is not a target of AgfD regulation (27) and is highly expressed at 37°C in vivo, specifically in the small intestine, liver, and spleen (28), indicating a possible role in systemic virulence. In the present study, we visualized the O-Ag capsule in association with the bacterial surface of S. Typhimurium and observed that the capsule is dependent on the function of a putative transmembrane permease (YihO), shields LPS from recognition by specific antibodies, increases resistance to serum bactericidal activity, and modulates surface expression and phase variation of flagella. The results described here further characterize the O-Ag capsule of S. Typhimurium and support a role for the structure in facilitating pathogenesis and altering immune-mediated clearance through modification of the bacterial surface.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and reagents.
The bacterial strains used in this study are shown in Table 1. Unless otherwise indicated, reagents were purchased from Thermo Fisher Scientific, Rockford, IL. Cultures were grown overnight at 37°C with aeration in Luria-Bertani (LB) broth or on LB agar plates as described below. O-Ag capsule detection and purification were conducted on cultures in stationary phase. For experiments using mid-logarithmic-phase bacteria, overnight cultures were diluted 1:100 in fresh LB broth and grown at 37°C with aeration to an optical density at 600 nm (OD600) of 0.6. When necessary, media were supplemented with chloramphenicol (25 μg ml−1) (LBcam), ampicillin (50 μg ml−1 or 100 μg ml−1) (LBamp), or kanamycin (45 μg ml−1) (LBkan). Capsular production was observed to be similar under all the culture conditions tested (liquid and agar cultures grown in LB, LB without salt, tryptic soy broth [TSB], super optimal broth [SOB], and M9 minimal medium supplemented with glucose or glycerol at incubation temperatures of both 30°C and 37°C), and therefore, subsequent experiments were conducted under standard laboratory culture conditions using LB broth or agar at 37°C.
TABLE 1.
S. enterica serovars used in this study
| Strain | Relevant characteristics | Source |
|---|---|---|
| JSG210 | Wild-type S. Typhimurium; ATCC 14028s (CDC) | ATCC |
| JSG880 | S. Typhimurium ATCC 14028S rough strain lacking LPS O-polysaccharide | 34 |
| JSG1145 | S. Typhimurium 14028S fljB::MudJ; Kan (FljB off, FliC on) | B. Cookson |
| JSG1179 | S. Typhimurium 14028S fliC::Tn10 hin108::Tn10dCm (FljB on) | B. Cookson |
| JSG1190 | S. Typhimurium 14028S hin108::Tn10dCm fliC::Tn10 (FljB off, FliC off) | B. Cookson |
| JSG1221 | S. Typhimurium LT2 galE-zbi812::Tn10 KK151 | K. Klose |
| JSG1676 | S. Typhimurium 14028S rfaD::luc; LPS O-Ag ligase | 66 |
| JSG1727 | S. Typhimurium 14028S carrying pKD46 Lambda red recombinase plasmid | 66 |
| JSG1773 | E. coli DH5a carrying pCP20 Flp recombinase plasmid | D. Provenzano |
| JSG2929 | E. coli DH5a carrying pKD3 plasmid | J. Slaugh |
| JSG3453b | S. Typhimurium 14028S yihO::Camr | This study |
| JSG3672 | S. Typhimurium 14028S ΔyihO | This study |
| JSG3675 | S. Typhimurium 14028S ΔyshA-yihW | This study |
| JSG3675b | S. Typhimurium 14028S ΔyshA-yihW::Camr | This study |
| JSG3676 | S. Typhimurium 14028S JSG3672 ΔyihO pWSK129::yihO | This study |
| JSG3677 | S. Typhimurium 14028S JSG210 pWSK129::yihO | This study |
| JSG3691 | S. Typhimurium 14028S JSG3672 ΔyihO pUC18::yihO | This study |
| JSG3692 | S. Typhimurium 14028S JSG3672 ΔyihO pUC18 | This study |
Construction of mutants.
Unmarked, nonpolar deletions in S. Typhimurium 14028S (wild type) were created according to the phage λ Red-mediated homologous-recombination method as described by Datsenko and Wanner (29). In brief, strain JSG1727 was cultured for 5 h at 30°C to induce expression of the pKD46 λ Red recombinase plasmid. The cells were pelleted and washed in 10% glycerol. Inner primers with homology to the target gene (Table 2, yihO, JG2087/JG2088) were used to PCR amplify the camR cassette of pKD3. The PCR product was gel extracted, transformed into JSG1727, and plated on LBcam at 37°C. Transformants were patched to LBcam and LBamp at 40°C to remove pKD46. Chloramphenicol-resistant, ampicillin-sensitive colonies were selected; screened via colony PCR with outer checking primers (Table 2, yihO, JG2093/JG2094); and sequenced at the Ohio State University (OSU) Plant Microbe Genomics Facility to confirm correct replacement of the target gene with camR. The resultant strains were grown at 37°C and transformed with purified pCP20 encoding Flip recombinase to resolve the camR cassette. Ampicillin-resistant, chloramphenicol-sensitive transformants were selected at 30°C; grown at 37°C to remove pCP20; and screened via colony PCR. Deletion of the target gene(s) was confirmed by sequencing using outer checking primers, and the resultant strain was stored at −80°C in 20% glycerol. For complementation of yihO, plasmids pJM1 and pJM2 were constructed as follows. yihO was amplified and cloned into the HindIII and EcoRI sites of vectors pUC18 and pWSK129 (GenScript Corporation, Piscataway, NJ) (30). Ligation reactions were transformed into One-Shot Top10 chemically competent E. coli (Invitrogen/Life Technologies, Carlsbad, CA), and the transformants were plated on LBamp containing 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) and 40 μg ml−1 X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) to permit blue-white screening. White colonies were selected and screened by colony PCR using M13 Forward (M13F) and the JG2491 primer internal to yihO. Correct clones carrying plasmid pJM1 (yihO in pUC18) and pJM2 (yihO in pWSK129) were isolated. The plasmids were purified, sequenced using M13F, and transformed into JSG3672 ΔyihO.
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Sequence (5′-3′) | Target |
|---|---|---|
| JG1198 | CCCAGTCACGACGTTGTAAAACGACGGCCAGT | pUC/pWSK plasmid primer |
| JG1203 | AAAGACAATAGAAACGCAGACGATGTTCAATATCGCCTG | yihW outer checking primer for verification of yshA-yihW mutation |
| JG1990 | AGCTTTGGCAGATCTTCATC | yshA outer checking primer for verification of yshA-yihW mutation |
| JG2081 | ACGGTCGCGTATGTCCTATC | rpoB |
| JG2082 | GAGTTCGCCTGAGCGATAAC | rpoB |
| JG2087 | CCTGCGGGGCGTTTTGAGAGGCGATTATGTGTGTAGGCTGGAGCTGCTTC | yihO reverse inner primer with pKD3 homology for λ-Red deletion of yihO |
| JG2088 | TAAAGCGGCAAGCGTCGTTTAATTATTTAC CATATGAATATCCTCCTTAG | yihO forward inner primer with pKD3 homology for λ-Red deletion of yihO |
| JG2093 | CTTCACGTTCTTCCGTAAGGTCTC | Forward yihO outer checking primer for verification of yihO mutation |
| JG2094 | GTTTTCTACATAAGCGCCAGCA | Reverse yihO outer checking primer for verification of yihO mutation |
| JG2260 | AAAATAGAGGCAATAATATCAGAAGAAATAGTGTAGGCTGGAGCTGCTTC | Inner primer with pKD3 homology for λ-Red deletion of yshA-yihW |
| JG2261 | CCGGCGCAAGGGCGCTTGTCACGCTGAAACCATATGAATATCCTCCTTAG | Inner primer with pKD3 homology for λ-Red deletion of yshA-yihW |
| JG2490 | GATATGCTCACCGGCGTATT | Primer internal to yihO |
| JG2491 | AAAATGAGCTGACGCAAACC | Primer internal to yihO |
| JG2592 | GTAAAACGACGGCCAGT | M13 Forward |
| JG2593 | AACAGCTATGACCATG | M13 Reverse |
| JG2692 | TGACCAACTCAGCGCCATTA | HinF4 (35) |
| JG2693 | TATCGTGAGCGCGTTACACT | HinR4 (35) |
| JG2694 | AGGTAAACGTACCGACAGCA | HinLR1 (35) |
| JG2695 | AGTGTAACGCGCTCACGATA | HinRF1 (35) |
Purification of bacterial polysaccharides.
Overnight bacterial cultures (50 ml) were normalized to an OD600 of 1.0 and centrifuged for 30 min at 5,000 × g. The pellets were homogeneously resuspended in TRIzol (Life Technologies, Carlsbad, CA) and incubated at room temperature for 30 min. Chloroform (Sigma-Aldrich, St. Louis, MO) was added at a ratio of 1:5, and the mixture was vigorously vortexed, followed by sitting for 15 min at room temperature to create a phase separation. Samples were centrifuged for 10 min at 11,000 × g, the aqueous phase was removed, and the organic phase was subjected to a secondary aqueous extraction. The aqueous phases were combined, frozen at −80°C, and lyophilized. Lyophilized samples were resuspended in endotoxin-free H2O and dialyzed against double-distilled H2O (ddH2O) in a 7,000 molecular weight cutoff (MWCO) membrane for 18 h, followed by lyophilization in preweighed tubes. The resultant material was weighed and assayed for protein content, which was determined to be less than 0.01% of the sample weight, via bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). The polysaccharide content was estimated using a phenol-sulfuric acid assay (31), which found it to be consistent with the calculated sample weight. The material was resuspended at a concentration of 25 μg ml−1 in endotoxin-free H2O.
Preparation of O-Ag capsule polyclonal antisera.
Rabbit polyclonal antisera directed at extracellular polysaccharides (EPS) of S. Enteritidis (17) (provided by D. L. Gibson and A. P. White) were adsorbed against a ΔyshA-yihW mutant of S. Typhimurium in order to remove potential nonspecific reactivity with other immunogenic components of the cell surface. In brief, 100 ml of stationary-phase culture of JSG3675 (ΔyshA-yihW) was pelleted at 4,800 × g for 20 min at 4°C. The pelleted cells were washed in 10 ml of adsorption buffer (10 mM NaCl, 10 mM KCl) and resuspended in 50 ml ice-cold acetone for 30 min. The acetone was allowed to evaporate in a fume hood until the pellets were completely dry. The dried pellets were then homogenized into a fine powder, and 100 mg was incubated with O-Ag capsular antiserum diluted 1:50 in phosphate-buffered saline (PBS) for 4 h at 4°C with constant agitation. Samples were centrifuged at 14,000 × g for 15 min, and the procedure was repeated 2 more times before the antiserum was passed through a sterile, low-protein-binding filter (Millipore, Billerica, MA); aliquoted; and stored at −20°C until use.
Western blot analysis of isolated polysaccharides.
Six micrograms of isolated polysaccharide was boiled in 2× Laemelli sample buffer with β-mercaptoethanol (BME) (Bio-Rad, Hercules, CA) for 10 min and separated by electrophoresis on a 16-cm SDS-15% PAGE gel. The gels were wet transferred to methanol-activated polyvinylidene difluoride (PVDF) membranes, which were subsequently blocked at 4°C overnight. LPS was detected using murine monoclonal IgG to S. Typhimurium group B LPS (Meridien Life Sciences, Memphis, TN; catalog number C863093M; clone 1E6). The membranes were incubated in anti-LPS (1:2,000) or anti-capsule (1:1,000) diluted in 5% bovine serum albumin (BSA) (Sigma-Aldrich) for 2 h at 22°C, washed in Tris-buffered saline (TBS) plus 0.1% Tween 20 (TBST) 3 times for 15 min each time, and incubated in anti-mouse (for LPS; 1:4,000) or anti-rabbit (for capsule; 1:2,000) horseradish peroxidase-conjugated secondary antibodies (Bio-Rad). All blocking steps were conducted at 4°C overnight in 5% BSA diluted in Tris-buffered saline, pH 7.6 (BSA-TBS). Antibody incubations were conducted in 5% BSA diluted in TBST (BSA-TBST); washes were conducted using TBST alone (3 times for 15 min each time). Western blots were visualized using the Bio-Rad Chemi-Doc XRS system.
Dot blotting to detect LPS and O-Ag capsule.
Dot blotting was conducted to permit detection and quantitation of O-Ag capsule relative to LPS on whole bacterial cells (32). Bacterial cultures were grown overnight, fixed in 4% paraformaldehyde (PFA), and normalized to an OD600 of 0.8 (∼2 × 109 CFU ml−1) in PCMH buffer (1× PBS, 1 mM HEPES, 0.5 mM MgCl2, and 0.15 mM CaCl2, pH 7.3). Tenfold serial dilutions (101 to 10−4) were conducted, and 50 μl of bacterial culture was spotted in duplicate onto activated PVDF membranes and followed with 200 μl PCMH buffer to ensure contact with the membrane. The blots were dried and blocked at 4°C overnight in 5% BSA-TBS, followed by incubation in either anti-LPS (1:2,000) or anti-capsule (1:1,000) antibody diluted in 5% BSA-TBST for 2 h at room temperature. The blots were washed in TBST (3 times for 15 min each time) and incubated with anti-mouse (for LPS; 1:4,000) or anti-rabbit (for capsule; 1:2,000) horseradish peroxidase-conjugated secondary antibodies (Bio-Rad) for 2 h at room temperature prior to visualization using the Bio-Rad Chemi-Doc XRS system. Images were analyzed using Fiji (ImageJ v 1.48f) software to calculate the average pixel intensity of each spot and the adjacent background. The background was subtracted from each value, and the data were graphed as the ratio of O-Ag capsule reactivity to LPS (1:10 dilution) for each strain.
In-gel staining of EPS.
Bacterial polysaccharides were electrophoresed in an SDS-15% PAGE gel (16 cm; 1.5 mm thick). The gel was immersed in 100 ml of alcian blue solution (0.1% alcian blue, 40% ethanol, 5% acetic acid in ddH2O) for 30 min. The gel was rinsed for 1 min in ddH2O, and silver staining was carried out according to the method of Tsai and Frasch (33). The production of LPS was compared to that of negative-control strains of S. Typhimurium with defects in biosynthesis or assembly of the LPSO-Ag. Strain JSG880, previously characterized as a bile-sensitive mutant in a library of MudJ transposon-mutagenized PhoP-constitutive (PhoPc) S. Typhimurium 14028S (34), exhibited no LPSO-Ag production in LB or under any tested conditions and is resistant to phage P22. Therefore, the strain was selected as an LPSO-Ag-deficient control. The results annotated here as “LPS O-Ag deficient” or “LPSO-Ag−” were obtained with strain JSG880 and replicated using JSG1676 ΔrfaD and JSG1221ΔgalE.
Confocal microscopy.
O-Ag capsule was detected using the adsorbed polyclonal capsular antisera described above. Overnight bacterial cultures were normalized in LB medium to an OD600 of 0.8; 100 μl was removed and centrifuged (5 min; 5,000 × g) and resuspended in 50 μl HEPES buffer (0.1 M HEPES, 1× PBS, pH 7.0; filter sterilized). The cells were fixed for 15 to 30 min at room temperature in 2% paraformaldehyde (pH 7.4; sterile filtered in 1× PBS), washed 3 times in HEPES buffer, and blocked in filtered 5% BSA-TBS. Antibody incubations for LPS (1:200) or capsule (1:100) were conducted in 5% BSA-TBST at room temperature for 2 h or at 4°C overnight. Secondary-antibody incubation was conducted simultaneously with DAPI (4′,6-diamidino-2-phenylindole) counterstain (1 μM) in the dark. Antibody incubations in experiments employing multiple fluorophores were conducted simultaneously if unique host and target animal species for the primary and secondary antibodies were compatible. Otherwise, incubations were conducted serially with 1 h blocking in 5% BSA-TBST between consecutive incubation steps. Fluorescent labeling was conducted using Alexa Fluor-conjugated (goat IgG) secondary antibodies (Invitrogen). Alexa Fluor 488 was employed for targets of primary interest or at lower abundance, while Alexa Fluor 594 was employed for targets at greater abundance. Following staining, the cells were mounted on glass slides in ProLong Gold anti-fade (Invitrogen), covered with a coverslip, and left to dry in the dark overnight or stored for up to 2 weeks at 4°C.
Transmission electron microscopy.
Individual bacterial colonies grown at 37°C overnight were gently resuspended in transmission electron microscopy (TEM) sample buffer with 2.5% glutaraldehyde (0.1 M HEPES, 6% sucrose, 1× PBS, pH 7.0; filter sterilized). A 25-μl sample was pipetted onto Formvar-coated 200-mesh nickel grids (Ted Pella Inc., Redding, CA) and allowed to settle for 25 min. The grids were washed with TEM buffer, blocked (30 min; 0.5% BSA; 4°C), and incubated with anti-capsule antibody (1:100; 60 min; 0.5% BSA; 4°C), followed by overnight incubation at 4°C with 10-nm gold-conjugated goat anti-rabbit IgG (catalog number GAF-012-10; EY Laboratories Inc., San Mateo, CA). Prior to viewing, samples were negatively stained with 1% uranyl acetate. All staining steps were performed on a silicon pad in a humidified chamber. Samples were viewed on an FEI Tecnai G2 Spirit Transmission Electron Microscope at the OSU Campus Microscopy Imaging Facility (CMIF).
Serum bactericidal assay.
Blood was collected from healthy adults by venipuncture according to a protocol approved by the Ohio State University Institutional Review Board. The blood was left to clot at room temperature for 1 h in sterile 5-ml round-bottom tubes (BD Falcon, Franklin Lakes, NJ), followed by 1 h at 4°C. Serum was separated by centrifugation at 4°C and frozen in aliquots at −80°C in sterile polypropylene tubes. The serum was thawed on ice immediately prior to use. Heat inactivation of complement was performed, when needed, by incubating serum in a heating block with gentle agitation at 56°C for 30 min. Bacteria from stationary-phase cultures were normalized to an OD600 of 0.8 in LB medium and subsequently diluted in PCMH buffer before the addition of 100 μl (approximately 2 × 106 CFU) to 100 μl 50% serum to yield a 200-μl sample containing approximately 2 × 105 CFU in 25% serum. Ten-microliter aliquots were removed immediately (time zero [T0]) and following 30 (T30), 60, 90, and 180 min of incubation at 37°C with gentle agitation. Aliquots were removed at the indicated time points and serially diluted in ice-cold 1× PBS, placed on ice for 5 min, and plated for viability. Serum killing was calculated compared to the number of CFU recovered at T0 and the numbers of CFU recovered from samples in 56°C heat-inactivated serum and buffer alone at the indicated time points. The graphed data do not include CFU calculations from heat-inactivated samples, all of which exhibited increases of approximately 4 × 105 CFU ml−1 over 180 min, with no significant variance among sample means based on one-way analysis of variance (ANOVA) (P > 0.1).
Complement binding.
Overnight bacterial cultures were normalized and diluted in PCMH as described above, and approximately 2 × 106 CFU was added to 100 μl 20% serum (final concentration, 10% serum) for 30 min. Ten-microliter aliquots were removed for viability plating at T0 and T30. Following a 30-min incubation, samples were placed on ice, washed gently 3 times in PCMH, and fixed in 4% paraformaldehyde for 15 min at room temperature. The fixed samples were blocked and incubated with goat anti-human C3 (1:100; catalog number A205; Quidel Corp., San Diego, CA), followed by donkey anti-goat Alexa Fluor 594 (1:200; Invitrogen). Blocking and antibody incubations were performed in sterile-filtered 5% BSA in TBST for 1 h at room temperature; samples were washed gently 3 times following incubations with cold TBST and mounted with Pro-Long Gold anti-fade. The slides were allowed to dry in the dark at room temperature overnight prior to viewing on an Olympus FV1000 spectral confocal scanning laser microscope.
Flagellin analysis.
Bacteria were grown to mid-log phase (to visualize both FliC and FljB) or stationary phase (with FljB primarily expressed in the wild type) and normalized in LB medium, using wide-bore pipette tips, to an OD600 of 0.8. Confocal microscopy was conducted as described above using anti-FliC diluted 1:200 in BSA-TBST and Alexa Fluor-conjugated secondary antibody (1:400). For SDS-PAGE analysis, bacterial cultures were pelleted (5,000 × g) and lysed by boiling in PBS, and the total protein content was determined by a BCA assay. Samples were resuspended in 2× Laemmli sample buffer with BME and loaded (equivalent to approximately 1 × 109 CFU) onto 8% or 15% SDS-PAGE gels. In addition, trichloroacetic acid (TCA) (Sigma-Aldrich) precipitation was conducted to assay the flagellin contents in whole-cell supernatant proteins. In brief, normalized 50-ml overnight cultures were centrifuged for 1 h at 220,000 × g at 4°C to ensure the separation of whole cells from the supernatant. The supernatant was filtered through a 0.22-μm low-protein-binding filter (Millipore), concentrated at 4°C with 10% TCA, and normalized for total protein content using the BCA assay. Normalized pellet and supernatant samples were diluted in Laemmli sample buffer with BME and electrophoresed as described above. Samples for Western blotting were similarly visualized using Coomassie brilliant blue staining (Pierce/Life Technologies, Carlsbad, CA) to demonstrate the total protein content. For mass spectrometry, protein bands of interest were excised from Coomassie blue-stained gels and submitted to the Ohio State University Mass Spectrometry and Proteomics Facility. Identified peptides were analyzed using Mascot (Boston, MA) Deamon V2.2.1 and compared to the NIH/NCBI database.
Western blotting for flagellin.
Electrophoresed proteins were wet transferred to methanol-activated PVDF membranes, which were subsequently blocked at 4°C overnight in 5% BSA-TBS (pH 7.6). The membranes were probed with monoclonal antibodies detecting either FliC alone (diluted 1:10,000 in BSA-TBST; clone FliC-1; catalog number 629701; Bio-Legend, San Diego, CA) or both FliC and FljB flagellins (diluted 1:8,000 in BSA-TBST; clone 6301; catalog number MAB7071P; Maine Biotechnology, Portland, ME). The membranes were washed, incubated in horseradish peroxidase-conjugated goat anti-mouse IgG (FliC, 1:12,000; flagellin, 1:10,000), and visualized using the Bio-Rad ChemiDoc XRS system.
Motility assay.
Five microliters of bacterial culture containing approximately 1 × 105 CFU was inoculated into the center of a 12-well plate (Corning, Tewksbury, MA) containing either swimming or swarming LB agar (0.35% and 0.7% agar, respectively). The motility plates were supplemented with appropriate antibiotics as needed. Motility inhibition assays were conducted in similarly prepared plates containing anti-FliC antibody (1:100). Prior to inoculation into plates, bacterial cultures were normalized and preincubated in PBS with or without anti-FliC antibody (1:100) for 30 min to ensure antibody binding. The plates were incubated at 37°C and photographed at 3 h, 6 h, 10 h, and 24 h. The area of bacterial motility was measured and subsequently quantitated using Fiji imaging software.
Determination of the fljB-fljA promoter orientation.
Genomic DNA was extracted (GenElute; Sigma) from stationary-phase bacterial cultures normalized to an OD600 of 1.0. Genomic DNA (100 ng) was used as a template for 15 cycles of PCR amplification of the rpoB gene and the promoter region upstream of fljB-fljA. Primer combinations designed to flank the invertible hin region permitted determination of the promoter orientation based on amplification product size (35). The resultant PCR products were as follows: fljB-OFF (1,180-bp product using JG2692/JG2695 and 1,124 bp using JG2693/JG2694) or fljB-ON (1,769-bp product using primers JG2692/JG2693 and 543-bp product using primers JG2694/JG2695). Amplification products were visualized on 1% agarose gels containing ethidium bromide.
In vivo virulence.
Survival studies were conducted in 6- to 8-week-old female BALB/c mice (five animals per group). Food and water were withheld for 4 h prior to inoculation. Overnight bacterial cultures were washed gently in 1× PBS, normalized to an OD600 of 0.8, and plated in duplicate to determine viable CFU. The animals were inoculated via oral gavage with 1.6 × 106 CFU (±3.2 × 105 CFU) of either wild-type, ΔyihO, or ΔyshA-yihW S. Typhimurium. Five animals were infected with each strain, and the experiment was repeated twice (total, 30 mice). Fecal pellets were collected on day 5 and plated on xylose lysine deoxycholate (XLD) agar, and the resultant colonies were screened by PCR to confirm the infecting strain. The surviving animals were sacrificed 15 days postinfection. The animals were monitored for health daily and were euthanized when moribund in accordance with institutional IACUC protocol criteria.
Statistical analysis.
All statistical analysis was performed with Prism 5 software (GraphPad Software, Inc.). The values reported represent data from a minimum of three independent experiments carried out in triplicate. To determine the significance of observed differences between groups, Student's t test or two-way ANOVA was performed. The observed differences were considered statistically significant compared to the wild type at a P value of <0.05, and all reported P values are >0.02.
RESULTS
O-Ag capsule-deficient mutants exhibit full-length, surface-localized LPS.
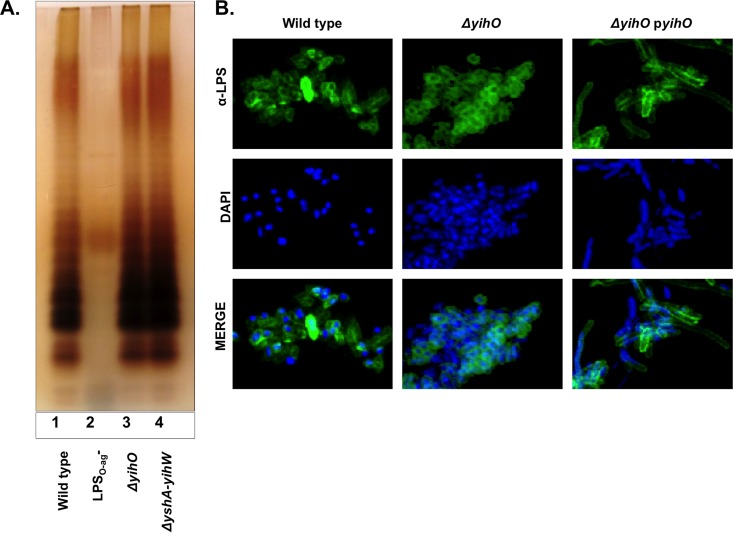
Production of full-length LPS with a variable number of O-Ag repeats, in particular, the production of long LPSO-Ag, is critical for systemic virulence and resistance to host immune molecules (36). Biosynthesis of LPS is a complex and highly sequential process, making it susceptible to disruption by perturbations in enzymatic pathways or biosynthetic precursors. Group IV O-Ag capsules often share common monosaccharide precursors and biosynthetic machinery with LPSO-Ag (37). Therefore, we sought to determine whether O-Ag capsule-deficient mutants exhibit reduced or truncated LPS. LPS production was analyzed by silver staining of electrophoresed bacterial polysaccharides (Fig. 1A). Typical banding patterns were observed in wild-type S. Typhimurium and ΔyihO and ΔyshA-yihW O-Ag capsule-deficient mutants, demonstrating that products of the O-Ag capsule gene cluster are not required for production of full-length LPS. Although ΔyihO and ΔyshA-yihW strains exhibited wild-type LPS, densitometry of the banding intensities indicated that O-Ag capsule-deficient mutants exhibited ∼25 to 45% more short LPS (containing 1 to 8 LPSO-Ag repeating units) than the wild type. Confocal microscopy of whole, fixed bacterial cells labeled for LPSO-Ag and counterstained with DAPI (Fig. 1B) demonstrated that LPS in ΔyihO and ΔyshA-yihW strains is localized to the bacterial surface and distributed in a manner indistinguishable from that observed in wild-type cells. The presence of a wild-type LPSO-Ag was further confirmed by susceptibility to phage P22 (data not shown).
FIG 1.
O-Ag capsule operon mutants produce wild-type lipopolysaccharide. (A) Silver stain analysis of 10 μg TRIzol-extracted exopolysaccharides from S. Typhimurium wild-type (lane 1), LPSO-Ag− (lane 2), and ΔyihO O-Ag capsule-deficient (lane 3) and ΔyshA-yihW (lane 4) strains. (B) Confocal micrographs demonstrating surface localization and distribution of lipopolysaccharide (LPS) (1:200) on fixed, DAPI-counterstained S. Typhimurium.
Detection of O-Ag capsule expression in O-Ag operon and LPSO-Ag mutants.
The LPSO-Ags of S. Enteritidis and S. Typhimurium are structurally similar, sharing identical repeating trisaccharide backbones of α-d-Manp-(1-4)-α-l-Rhap-(1-3)-α-d-Galp-(1-2), with the mannose bearing tyvelose in the former and abequose in the latter. The O-Ag capsular repeating-unit structure in S. Enteritidis was determined to be identical to that of the LPS O-Ag repeating unit, with the exception of glucosylation of the mannose residue and addition of polymeric glucose on the tyvelose residue (17). Due to the similarity of the overall LPS O-Ag content, as well as the O-Ag operon gene sequences in the two organisms, we sought to detect the S. Typhimurium capsule using polyclonal antisera generated against S. Enteritidis EPS. In a previous study (17), the capsular antisera were adsorbed against a ΔgalE mutant; however, such a mutation would result in deficient production of numerous surface-associated polysaccharides, including LPSO-Ag, potentially leaving residual cross-reactivity against alternate surface EPS structures. A defined mutation in the O-Ag capsular operon was employed for antibody adsorption in our study.
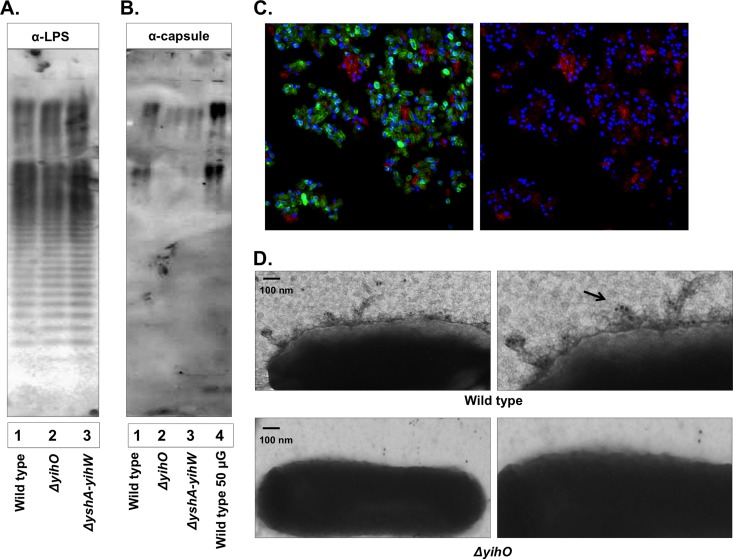
Defined mutations in each of the genes putatively involved in O-Ag capsule synthesis were constructed, growth rates were confirmed to be unaffected, and mutants were preliminarily screened for O-Ag capsule production. Western blotting of whole-cell lysates probed with O-Ag capsule polyclonal antisera (17) demonstrated that all O-Ag operon mutations exhibited reduced O-Ag capsular production but that deletion of yihO, encoding a putative inner membrane permease involved in O-Ag capsular export, resulted in the greatest abrogation of O-Ag capsule production (Fig. 2 and data not shown). In order to minimize potential nonspecific reactivity, O-Ag capsule-directed polyclonal antiserum was triple adsorbed against ΔyshA-yihW cells. Bacterial polysaccharides were isolated using a modified TRIzol method, normalized based on weight and carbohydrate content, and determined to contain less than 0.01% protein. Western blotting of 10 to 50 μg bacterial polysaccharide incubated with anti-LPSO-Ag (Fig. 2A) or adsorbed O-Ag capsule antibody (Fig. 2B) demonstrated O-Ag capsule antibody reactivity with a high-molecular-mass (>150-kDa) polysaccharide of S. Typhimurium that was greatly reduced in both ΔyihO and ΔyshA-yihW mutants. Densitometric analysis revealed relative intensities for ΔyihO and ΔyshA-yihW strains of 3% and 16% that of the wild type, respectively. These data also further supported the presence of wild-type LPS in ΔyihO and ΔyshA-yihW strains.
FIG 2.
Detection of the O-Ag capsule of S. Typhimurium using polyclonal capsule-specific antisera. (A and B) Western blot analysis of 10 μg TRIzol-extracted exopolysaccharides probed for LPS (1:1,500) (A) or O-Ag capsule (1:1,000) (B) production by wild-type S. Typhimurium (lanes 1 and 4) or ΔyihO (lane 2) and ΔyshA-yihW (lane 3) strains. (C) Confocal fluorescence microscopy of whole DAPI-stained wild-type S. Typhimurium cells labeled for LPS (green) and O-Ag capsule (red) (left, merged image of capsule and LPS; right, capsule only) demonstrating heterogeneous O-Ag capsule reactivity. (D) Transmission electron micrographs (enlarged on the right) using O-Ag capsule antisera (1:100) and 10 nM gold-conjugated secondary antibody to detect O-Ag capsule in association with the bacterial surface.
Visualization of the O-Ag capsule on the bacterial surface.
Confocal microscopy of whole, fixed bacterial cells labeled for LPSO-Ag and O-Ag capsule and counterstained with DAPI revealed heterogeneous expression of O-Ag capsule within a wild-type culture (Fig. 2C). This effect was independent of culture media (LB, M9 plus glucose, M9 plus glycerol, TSB, SOB, liquid, and agar), culture temperature (22°C, 30°C, and 37°C), and the order of antibody addition (data not shown). Individual cells exhibited reactivity with either LPSO-Ag or O-Ag capsule, but reactivity with both antibodies was rarely observed, indicating that production of O-Ag capsule may block reactivity with specific antibodies directed at the LPSO-Ag. Immunogold TEM of whole cells labeled with anti-capsule antibody and 10-nm gold-conjugated goat anti-rabbit secondary antibody demonstrated capsular localization at the bacterial cell surface in wild-type cells, which was absent in the ΔyihO strain (Fig. 2D).
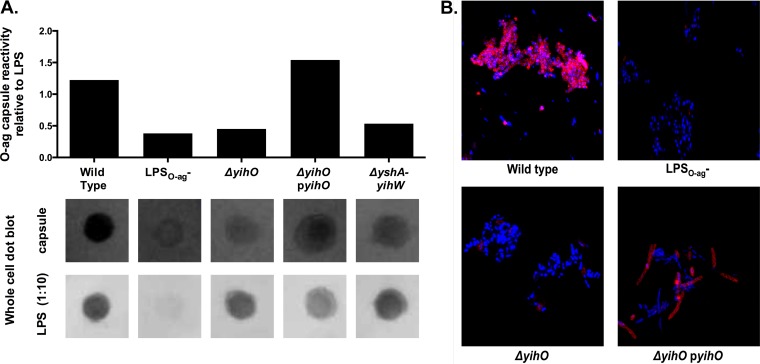
O-Ag capsular production on the surfaces of whole cells was also determined using whole-cell dot blotting (Fig. 3A), as well as confocal microscopy of DAPI- and O-Ag capsule-labeled cells (Fig. 3B). The reactivity of wild-type cells with O-Ag capsule antibody was strongly associated with aggregated cells, although minimal reactivity was observed with individual cells, as shown in Fig. 2. Both dot blotting and confocal microscopy demonstrated minimal O-Ag capsular production in LPSO-Ag− and ΔyihO strains. Complementation of the ΔyihO mutant with the high-copy-number pUC18-derived plasmid pJM1 (ΔyihO pyihO) resulted in restoration of capsular production to levels approaching those observed in wild-type cells. ΔyihO pJM1-complemented cells exhibited an elongated morphology and small, mucoid colony morphology on agar plates; however, there was no difference in the growth rate (as measured by OD or CFU per milliliter [data not shown]). The low-copy-number pWSK129-derived plasmid pJM2 was insufficient to provide functional complementation of observed capsular or flagellar phenotypes (data not shown).
FIG 3.
O-Ag capsule is not expressed by strains harboring mutations in O-Ag capsule operon or LPS-biosynthetic genes. (A) Dot immunoblot of normalized S. Typhimurium cultures probed for either LPS (bottom) or O-Ag capsule (top) indicating capsular expression relative to LPS in a whole-cell dot blot of S. Typhimurium. (B) Confocal fluorescence microscopy of whole DAPI-stained S. Typhimurium cells labeled for O-Ag capsule (red), demonstrating the absence of O-Ag capsule in ΔyihO, ΔyshA-yihW, and LPSO-Ag− strains and restoration of O-Ag capsule production by complementation of yihO.
Loss of O-Ag capsule results in increased serum sensitivity.
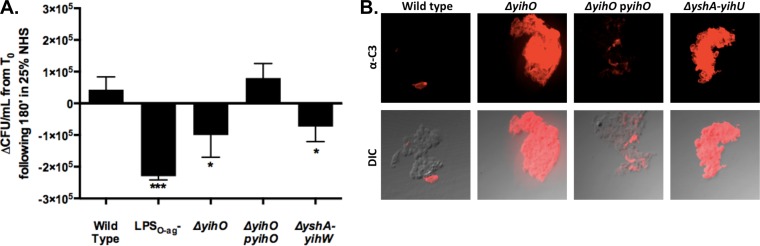
Production of high-molecular-weight surface polysaccharides, such as LPSO-Ag and capsular polysaccharide, is frequently associated with increased resistance to killing by human serum complement (38). To determine the impact of O-Ag capsule production on bacterial survival in normal human serum (NHS) from healthy donors, ΔyihO and ΔyshA-yihW O-Ag capsule-deficient mutants were tested for viability following incubation in 25% fresh or heat-inactivated NHS for 180 min (Fig. 4A) and compared to viable bacteria in wild-type and LPSO-Ag− control strains. As previously reported, wild-type S. Typhimurium was highly resistant to killing by NHS (increase from T0, 4 × 104 CFU ml−1), while LPSO-Ag− strains were effectively killed within 30 min (decrease from T0, −2 × 105 CFU ml−1). ΔyihO and ΔyshA-yihW O-Ag capsule-deficient mutants exhibited increased serum sensitivity relative to the wild type (decrease from T0, −1 × 105 CFU ml−1 and −7 × 104 CFU ml−1, respectively), and complementation of yihO restored serum resistance to wild-type levels (increase from T0, 8 × 104 CFU ml−1). Heat-inactivated serum did not result in bactericidal activity for any strain (average increase from T0, 4 × 105 CFU ml−1 among all samples at 180 min; no significant differences among strains [P > 0.5; one-way ANOVA]), demonstrating that the observed bactericidal activity was due to the activity of functional complement.
FIG 4.
O-Ag capsule-deficient strains exhibit reduced resistance to human serum and increased C3 associated with the bacterial surface. (A) Serum bactericidal assay depicting the change in numbers of viable CFU per milliliter from T0 following incubation of ∼2 × 105 CFU S. Typhimurium in 25% normal human serum for 180 min (P < 0.01; n = 3). Changes in numbers of viable CFU ml−1 for each strain were as follows: wild type, +4.33 × 104; LPSO-Ag−, −2.30 × 105; ΔyihO, −1.00 × 105; ΔyshA-yihW, −7.33 × 104; ΔyihO pyihO, +8.00 × 104. Equivalent incubations of strains in heat-inactivated sera resulted in increases of approximately 4 × 105 CFU ml−1 over the same period, with no significant variance among sample means based on one-way ANOVA (P > 0.1). *, P < 0.05; ***, P < 0.001. The error bars represent means and standard deviations. (B) Confocal micrographs of 2 × 106 bacterial CFU incubated in 10% human serum for 30 min and labeled to visualize complement C3 deposition on the bacterial membrane. The data are representative of the results of experiments conducted in 10 individual donor sera. DIC, differential inference contrast.
Cleavage of complement component C3 and deposition of C3b on the bacterial membrane represents the point of convergence for all three pathways of complement activation; therefore, confocal microscopy was employed to qualitatively visualize C3 associated with the bacterial surface following 30 min of incubation in 10% donor sera. The increased serum sensitivity of ΔyihO and ΔyshA-yihW O-Ag capsule-deficient mutants was accompanied by a qualitative increase in deposition of complement component C3 on the surface relative to C3 levels observed in wild-type and ΔyihO pyihO strains (Fig. 3B). Following longer incubation periods (>90 min), however, differences in C3 deposition on the wild type and capsule-deficient mutants were less remarkable, although there was a clear difference in viability at these times, indicating that complement binding to wild-type cells may occur without resulting in lysis. Immunoblotting of electrophoresed bacterial polysaccharides incubated in pooled donor sera revealed that bacterial targets of IgG binding in the tested sera corresponded to molecular masses of ≤25 kDa, with no reactivity observed in regions corresponding to high-molecular-mass polysaccharides (data not shown).
O-Ag capsule-deficient mutants exhibit alterations in production of phase I flagellin.
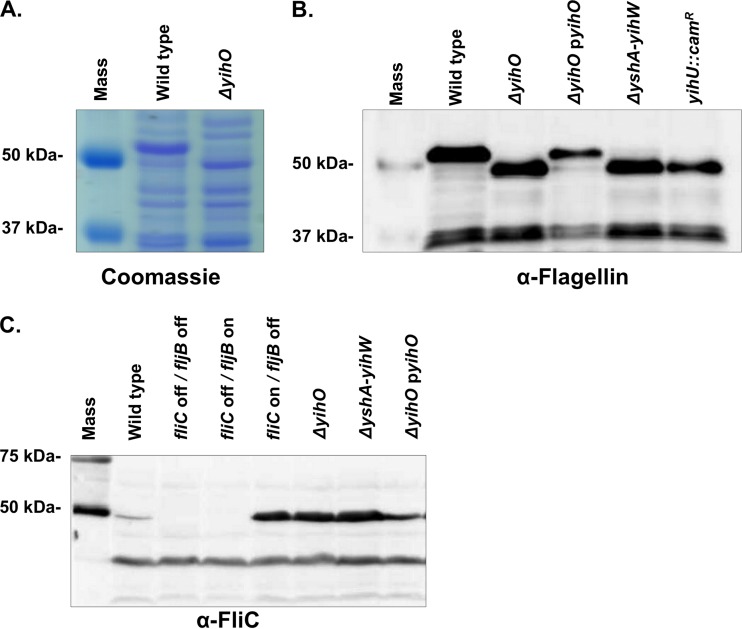
Synthesis of surface polysaccharides has been linked to flagellar regulation in a number of bacterial species (39, 40). S. Typhimurium expresses two antigenically distinct forms of flagellin whose expression is partly controlled by a Hin-invertible cassette containing a single promoter. Inversion of this cassette results in individual cells expressing flagella composed of either FliC (phase 1) or FljB (phase 2) flagellin on their surfaces (41, 42). Although the precise role of flagellar phase variation in vivo remains incompletely understood, it is thought that alternate expression of such highly immunogenic epitopes may facilitate immune evasion (43). Analysis of total protein profiles of the wild type or the ΔyihO mutant revealed a clear alteration in production of proteins at approximately 50 kDa (Fig. 5A), which were excised and determined by mass spectrometry to be flagellin. Western blotting using an antibody capable of detecting FliC and FljB (Fig. 5B) or FliC alone (Fig. 5C) revealed that stationary-phase cultures of wild-type S. Typhimurium and the ΔyihO complemented strain expressed primarily FljB (44, 45), while ΔyihO and ΔyshA-yihW capsule-deficient mutants expressed primarily FliC with no detectible expression of FljB. The flagellar phase was also shifted in the yihU::cam strain. The observation that flagellar alterations were similar in ΔyihO, ΔyshA-yihW, and yihU::cam O-Ag capsule-deficient strains indicates that the effect is not specifically attributable to the loss of yihO but is instead associated with loss of the O-Ag capsule, as the yihU::cam strain is also O-Ag capsule deficient. Densitometry of total flagella detected by Western blotting indicated that while the phase of flagellin monomer composition is shifted from FljB to FliC in O-Ag capsule-deficient mutants, the overall amounts of flagella produced are similar to that of wild-type cells.
FIG 5.
Altered production of phase I and phase II flagellin in O-Ag capsule-deficient strains. (A) Visualization of total proteins on Coomassie blue-stained SDS-PAGE gel of bacterial lysates. The molecular mass marker (lane 1) indicates altered production of an ∼50-kDa protein in the wild-type (lane 2) and ΔyihO (lane 3) strains. The bands were excised, analyzed by mass spectrometry, and determined to correspond to FljB (phase II) and FliC (phase I) flagellins in the wild-type and ΔyihO strains, respectively. (B) Western blot detection of both FliC and FljB production in whole-cell lysates of S. Typhimurium. (C) Western blot detection of FliC flagellin only.
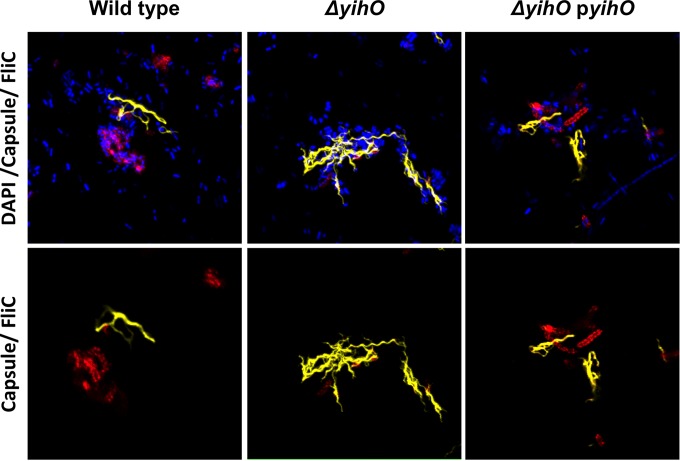
Confocal microscopy (Fig. 6) and motility assays (Fig. 7) were performed in order to assess whether FliC monomers detected in supernatants and whole-cell lysates of O-Ag capsule-deficient mutants were assembled into surface-associated and functional flagella. For microscopic visualization of flagella, whole bacterial cells were fixed and labeled with anti-FliC and anti-O-Ag capsule antibodies (Fig. 6). The micrographs revealed that flagella, like O-Ag capsule, are most clearly visible in association with aggregative cells. In ΔyihO cells, there is a marked increase in FliC flagella and no reactivity with O-Ag capsular antibodies, while wild-type and ΔyihO complemented strains exhibit similar levels of both O-Ag capsule and FliC.
FIG 6.
Increased cell-associated phase I flagella are visible on ΔyihO S. Typhimurium. Shown are confocal micrographs of fixed, whole bacteria labeled for phase I (FliC) flagella and O-Ag capsule on wild-type, ΔyihO, or ΔyihO pyihO complemented strains. The top row includes DAPI counterstain, while the bottom row depicts capsule (red) and flagella (yellow) only.
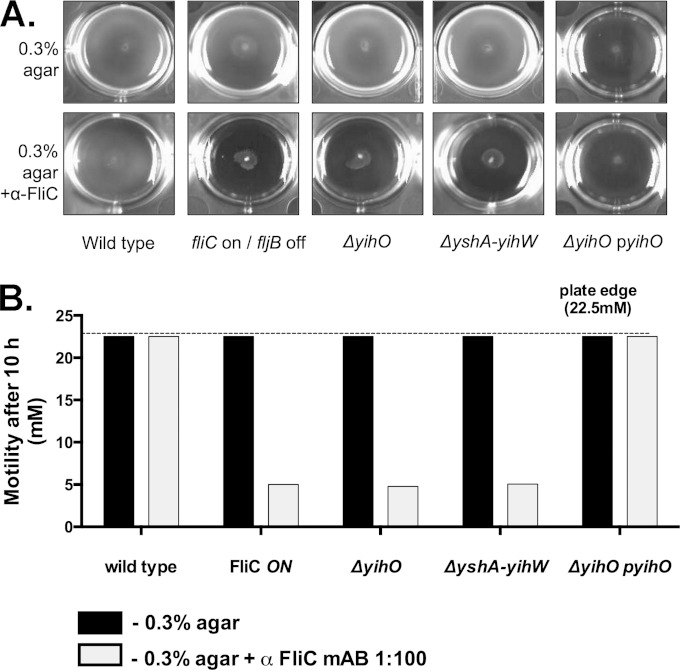
FIG 7.
Motility of O-Ag capsule-deficient mutants is inhibited by FliC-specific antisera. (A) Motility assay to determine swimming in 0.3% LB agar (top row) or 0.3% LB agar containing anti-FliC antibodies (bottom row). (B) Quantitation of the zones of motility in the presence of anti-FliC relative to 0.3% agar alone. Inoculated plates were imaged after 10 h of incubation. Swimming motility was calculated for O-Ag capsule-deficient strains and is depicted relative to the motility of the biphasic wild-type or FliC-ON monophasic control strain. mAB, monoclonal antibody.
Swarming and swimming motilities were assessed on 0.3% and 0.7% LB agar, respectively. Although no swimming defect was observed in O-Ag capsule-deficient cells, swarming motility in the ΔyihO strain was reduced to 44% that of the wild type (data not shown), a defect that was restored in the ΔyihO complemented strain. To determine the capacity of O-Ag capsule mutants to switch from FliC to FljB flagellin, bacterial motility was monitored in the presence or absence of anti-FliC antibodies (43). Under these conditions, the motility of phase-locked cells expressing only FliC flagellin is inhibited, while biphasic strains capable of expressing FljB flagellin are able to exhibit normal swimming motility. Swimming motility in agar with or without anti-FliC antibodies was compared to that of a fliC-ON phase-locked strain (JSG1145). In the absence of anti-FliC antibodies, there was no detectible difference in the swimming motilities of any tested strains (Fig. 7A and B). No inhibition of motility by anti-FliC was observed in the wild-type or complemented ΔyihO strains, which exhibited the same level of motility regardless of the presence of anti-FliC antibodies. Although there was a decrease in overall bacterial density of the ΔyihO complemented strain, the diameter and rate of motility were no different than those observed in the wild type. While no defect in swimming motility was observed for the ΔyihO or ΔyshA-yihW strain incubated in 0.3% agar alone, incubation in the presence of anti-FliC resulted in motility inhibition similar to that of a fliC-ON phase-locked strain (Fig. 7A and B).
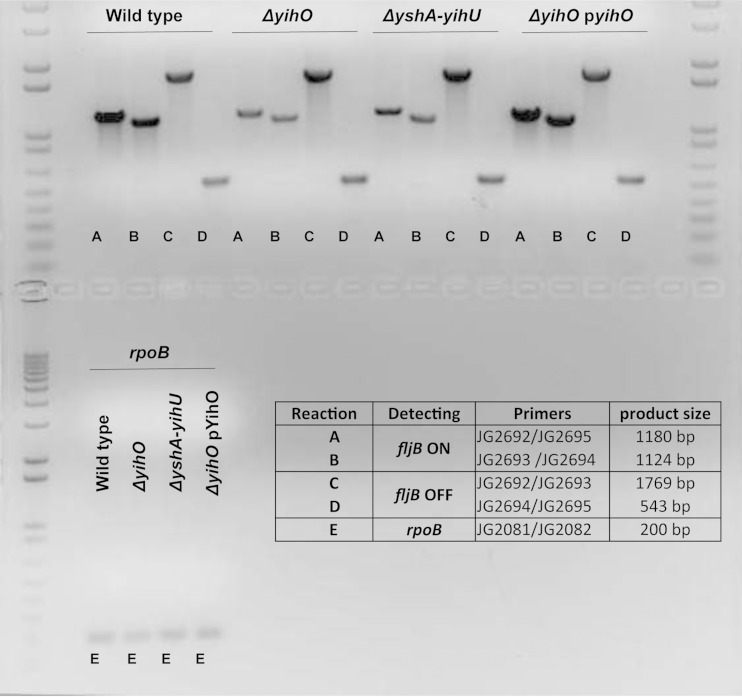
Because the alternate expression of either FljB or FliC is controlled by the inversion of the fljB-fljA promoter region, primers were designed to detect H-segment promoter orientation. In spite of the absence of detectible FljB protein in ΔyihO and ΔyshA-yihW O-Ag capsule-deficient mutants, both ON and OFF promoter orientations were detected (Fig. 8). Quantitation of the fljB-ON versus fljB-OFF products relative to rpoB indicated an increased proportion of fljB-ON promoter orientation in wild-type cells relative to O-Ag capsule-deficient strains. Thus, while there may be a shift toward the fljB-OFF promoter orientation in O-Ag capsule-deficient strains, all strains exhibited both promoter orientations, indicating that the lack of FljB protein is likely to due to posttranscriptional regulation and not to an absence of promoter inversion.
FIG 8.
Phase-variable expression of either FljB or FliC is controlled in part by the inversion of the fljB-fljA promoter region. Endpoint PCR using primers designed to flank the Hin-invertible promoter region upstream of fljB-fljA was conducted, and the resultant product sizes (lower right) enabled determination of fljB-fljA-ON (fliC-OFF) or fljB-fljA-OFF (fliC-ON) promoter orientation. The genomic DNA template was normalized prior to 15 cycles of PCR amplification of the fljB-fljA promoter region or rpoB (bottom).
O-Ag-deficient strains do not exhibit reduced virulence in mice.
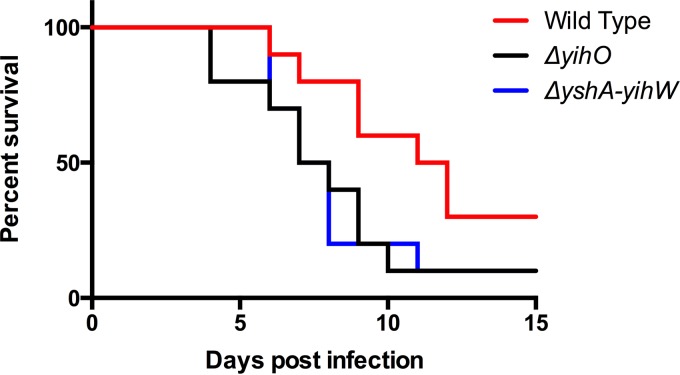
To determine whether the observed alterations in flagella and serum resistance corresponded to a reduction in systemic virulence, the ability of O-Ag capsule-deficient mutants to cause lethal disease was compared to that of wild-type S. Typhimurium in a murine model. BALB/c mice were inoculated individually with 1.6 × 106 CFU of wild-type, ΔyihO, or ΔyshA-yihW bacteria and monitored for 15 days. Three animals infected with the wild type survived to the study endpoint at day 15, while only one animal infected with either ΔyihO or ΔyshA-yihW bacteria survived to the study endpoint. The overall survival and time to death with the O-Ag capsule-deficient strains was less than that observed for animals infected with the wild type, though it was not found to be statistically significant (Fig. 9).
FIG 9.
The virulence of the wild type was compared to that of O-Ag capsule-deficient (ΔyihO and ΔyshA-yihU) strains in a murine model of systemic infection. BALB/c mice were orally inoculated with 1.6 × 106 CFU (±3.2 × 105 CFU) of either wild-type S. Typhimurium or the ΔyihO or ΔyshA-yihW mutant, The animals were monitored for health daily and were euthanized when moribund. The survival curves were analyzed using a log-rank (Mantel-Cox) test and were found not to reach statistical significance (P = 0.093).
DISCUSSION
Bacterial surface antigens play a key role in determining the interaction between the pathogen and the host. Studies of the Vi-Ag capsule have greatly increased our understanding of the pathogenic mechanisms of S. Typhi and continue to yield promising options for vaccine candidates. The Vi-Ag capsule is important for systemic dissemination of S. Typhi (46), and although the polysaccharide is absent from the surface of nontyphoidal Salmonella, NTS are known to result in extraintestinal infection in up to 5% of cases (47). Previous work has demonstrated that invasive NTS (iNTS) is most commonly observed in immunocompromised individuals; however, the preponderance of invasive Salmonella relative to other bacterial infections (48) may imply bacterial factors facilitating invasive disease.
The O-Ag capsule is one exopolysaccharide produced by S. Typhimurium and S. Enteritidis, the two serovars most frequently isolated in patients with iNTS, and is dependent upon a highly conserved operon (yshA-yihU). Previous studies have focused on potential roles for the O-Ag capsule in bacterial aggregation related to environmental colonization and chronic infection; however, the potential for this structure to facilitate immune evasion during acute disease has not yet been investigated. This study has demonstrated that the O-Ag capsule operon genes in S. Typhimurium function downstream of the LPSO-Ag-biosynthetic pathway to produce a surface-associated polysaccharide capable of shielding LPSO-Ag, increasing resistance to human serum, and modulating expression of phase I flagellin monomer (FliC).
Detection of the O-Ag capsule in this study relied on polyclonal antisera generated against purified S. Enteritidis extracellular polysaccharides and subsequently adsorbed against a mutant of S. Typhimurium lacking all 10 genes in the O-Ag capsule-encoding gene cluster (ΔyshA-yihW). Preliminary screening of capsular production by bacterial strains harboring individual mutations in each gene of the O-Ag capsule operon revealed that a yihO::cam mutant exhibited the greatest decrease in capsular production, consistent with previous reports of YihO playing an important role in O-Ag capsular production (15, 17). Western blotting of purified bacterial polysaccharides indicated the presence of a high-molecular-mass (>150-kDa) structure exhibiting reactivity with O-Ag capsule-specific antiserum. This region of capsular-antibody reactivity was absent in bacterial strains lacking the complete O-Ag gene cluster (ΔyshA-yihW), as well as those lacking only yihO. Previous work by Gibson et al. (17) demonstrated that O-Ag capsule was absent on the surfaces of ΔyihO mutants of S. Enteritidis but could be detected in whole-cell lysates. The authors thus hypothesized that YihO was involved in translocation of the completed capsule to the surface of the bacterial cell (17). However, in the work presented here, O-Ag capsule was not detectible on whole cells or in lysates of a ΔyihO mutant of S. Typhimurium, possibly indicating that S. Typhimurium relies on YihO for capsular assembly, as well as translocation. It is also possible that differing adsorption of the O-Ag capsular antibody in these two studies resulted in altered reactivity. In the Gibson et al. study, the antiserum was adsorbed against a ΔgalE mutant, while in this study, it was adsorbed against the ΔyshA-yihW strain. Adsorption against the ΔgalE mutant may have resulted in residual or altered reactivity to noncapsular surface polysaccharides or O-Ag capsule precursors, leading to the differences in capsule detection between the two studies.
The O-Ag capsule could be clearly visualized in association with the bacterial cell surface via confocal and electron microscopy and, while evident in wild-type S. Typhimurium, was absent from the surfaces of ΔyihO and ΔyshA-yihW mutants. Complementation in trans of yihO on the high-copy-number plasmid pJM1 was sufficient to restore O-Ag capsular production in a ΔyihO mutant. The O-Ag capsule was undetectable in all tested LPSO-Ag− strains (JSG1221ΔgalE, JSG1676ΔrfaD, and JSG880 LPSRe), implying that production of the O-Ag capsule required functional LPS biosynthesis. This association appeared to be unidirectional, as production of LPS was not disrupted in O-Ag capsule mutants, implying that synthesis of O-Ag capsule occurs downstream of, and is dependent upon, LPS biosynthesis. The O-Ag capsule was observed to be heterogeneously expressed on wild-type cells under all tested conditions, and although the majority of cells in a culture population exhibited reactivity with antisera directed at the LPSO-Ag, confocal microscopy demonstrated that cells reacting with O-Ag capsule-specific antibodies failed to react with antibodies directed at the LPSO-Ag. Although shielding of LPS is a well-documented function of capsular polysaccharides, the phenomenon has not previously been reported in nontyphoidal Salmonella. Invasive infections with NTS are becoming increasingly recognized as an important cause of mortality in immunocompromised individuals. The presence of a subpopulation of cells expressing a unique surface polysaccharide that is not recognized by LPSO-Ag antibodies could potentially facilitate immune escape in the context of invasive infection.
In S. Typhi, the Vi-Ag capsule is thought to facilitate bacterial survival and extracellular dissemination through the bloodstream by reducing complement deposition on the bacterial surface and facilitating evasion of innate immune detection and bacteriolysis (49, 50). Therefore, we hypothesized that the O-Ag capsule may similarly function to increase bacterial resistance to complement-mediated killing, critical to host control of iNTS (51). Determination of bacterial viability following incubation in normal human serum revealed that O-Ag capsule-producing strains exhibited an increase of >104 CFU ml−1 while ΔyihO and ΔyshA-yihW mutants lacking the O-Ag capsule decreased by >103 CFU ml−1, indicating that the O-Ag capsule affords additional resistance to serum killing. Microscopic visualization indicated increased C3 deposition on the surfaces of O-Ag capsule-deficient mutants following incubation in human sera. Although the ability to survive incubation in human sera was increased in the presence of the O-Ag capsule, bacterial strains deficient in both LPSO-Ag and O-Ag capsule were more completely and rapidly susceptible to serum bactericidal activity than those lacking the O-Ag capsule alone, indicating that the majority of bacterial serum resistance under laboratory culture conditions is likely attributable to LPSO-Ag production. The observation that O-Ag capsule-producing strains exhibited a significant but modest increase in overall serum resistance is consistent with the observed heterogeneous expression of O-Ag capsule, which indicated that only a portion of the culture population would be afforded the increased serum resistance associated with capsular production. The current study was limited by the lack of identifiable mutations resulting in loss of LPSO-Ag alone without also disrupting the O-Ag capsule, which would have enabled better estimation of the precise effects of the O-Ag capsule without the effects of the LPSO-Ag.
The data presented here suggest a link between O-Ag capsule and flagellar phase variation in S. Typhimurium, as has previously been reported with other polysaccharides in Pseudomonas and S. Typhi (52, 53). Transcriptional regulation of S. Typhimurium flagellar phase variation is controlled through inversion of the H-segment promoter region located 1 kb upstream of fljB. Inversion of the promoter into the fljB-fljA-ON orientation results in production of the phase 2 flagellin monomer FljB, as well as FljA, which binds to and degrades fliC mRNA, resulting in phenotypically FljB-expressing cells (54). In the absence of promoter inversion (the fljB-fljA-OFF orientation), FljB is not produced and FljA repression of FliC translation does not occur, resulting in phenotypically FliC-expressing cells. Laboratory cultures of S. Typhimurium consist of a mixed population of FliC- and FljB-expressing cells.
Determination of the flagellin monomeric composition in O-Ag capsule-deficient mutants versus wild-type S. Typhimurium revealed a clear difference in the levels of FliC and FljB flagellins. Wild-type and complemented strains exhibited both FliC and FljB flagellins, with stationary-phase cultures producing primarily FljB, consistent with previous reports indicating that S. Typhimurium 14028S is capable of phase variation and expresses both FliC and FljB in laboratory culture but tends to express more FljB in stationary phase (44). O-Ag capsule-deficient mutants exhibited an increase in cell-associated and secreted FliC and produced no detectible FljB. Complementation of the ΔyihO strain resulted in restoration of wild-type FljB production. The flagellar phenotype was similarly observed in all tested mutations in the O-Ag operon gene cluster (ΔyihO, ΔyshA-yihW, and yihU::cam) and thus was not attributable to the inactivation of any single gene in the O-Ag operon. This phenotype was not observed in LPSO-Ag− strains and has not previously been reported to be associated with LPS or EPS mutations. Thus, the observed flagellar alteration appears to indicate a link between O-Ag capsule biosynthesis and a switch to expression of phase II flagellin but is not a pleiotropic effect associated with alterations in EPS production.
Mutations in the O-Ag operon resulted in reduced swarming motility but no reduction in swimming motility, indicating a possible loss of surface wetting in the absence of the O-Ag capsule (55) but no loss of flagellar functionality. The presence of flagellin-specific antibodies can inhibit flagellar functionality; however, these effects may be overcome through flagellar phase variation, resulting in expression of antigenically distinct flagella. Incubation of the biphasic wild-type S. Typhimurium in motility agar containing FliC-specific antibody resulted in no loss of swimming motility, while the motility of a FliC-ON mutant was fully inhibited. The swimming motility of ΔyihO and ΔyshA-yihW strains was reduced and was similar to that of the FliC-ON mutant, indicating that these strains may be unable to undergo phase variation. Analysis of the Hin-invertible promoter region (H segment) demonstrated that wild-type and O-Ag capsule-deficient strains had both fljB-fljA-ON and fljB-fljA-OFF promoter orientations, suggesting that observed alterations in flagellar monomer composition occur subsequent to transcription.
Over 50 genes are involved in flagellar synthesis and regulation, and S. Typhimurium 14028S, like most NTS, is biphasic. Although phase variation is thought to facilitate immune evasion, monophasic variants of nontyphoidal Salmonella are a frequently reported cause of invasive disease (4). FliC is known to be a critical target of protective immunity against S. Typhimurium (43, 56, 57), and yet 90% of monophasic isolates have retained fliC and lost fljB (58), and modeling of invasive disease has identified loss of FljB expression as a pathoadaptive mutation in S. Typhimurium (59).
S. Typhi is monophasic and exclusively expresses FliC. In S. Typhi, both flagellin and Vi-Ag capsule are important for full pathogenesis; however, their production at the level of individual cells is inversely regulated through TviA-mediated repression of the master flagellar regulator, flhD-flhC. Although flagella are important for motility and invasion (60), bacterial flagellin is a highly immunostimulatory PAMP (61). It has been proposed that in vivo infections involve heterogeneous bacterial populations exhibiting variable invasive and inflammatory properties (62, 63) and that up to 40% of the invading bacterial population in the gut lumen express no flagellin at all (56). Thus, appropriate coordination of capsular production and repression of flagellar expression could facilitate innate immune evasion while interfering with the generation of a protective adaptive immune response (64).
It was observed that the loss of the O-Ag capsule resulted in increased lethality in a murine model of invasive disease, though it failed to reach statistical significance. This suggests an effect of O-antigen capsule in the systemic phase of disease. Based on the affect of the O-Ag capsule on complement resistance, it might have been predicted that the mutants would demonstrate reduced virulence. The lack of observation of an avirulent phenotype may be explained in part by the reduced classical and alternative bactericidal activities of the complement pathway in mice (65). Additionally, increased numbers of cell-associated phase I flagella on the O-Ag capsule-deficient mutants may result in enhanced invasiveness or intestinal necrosis of these strains. Additional studies employing various models of infection, inoculation methods, or background strains would enable further understanding of possible roles of the O-Ag capsule during infection.
During In vivo infection with S. Typhimurium, O-Ag capsule operon genes are maximally expressed in the small intestine, liver, and spleen (28). The data presented here indicate that increased expression of the O-Ag capsule operon genes would result in reduced production of phase I flagellin and production of the O-Ag capsule. In biphasic isolates, this results in a shift to phase II flagellin. The effects of O-Ag capsular production in monophasic isolates may result in an absence of surface-associated flagella and should be investigated further in subsequent work. It is possible that bistable capsular expression and/or rapid transition between distinct phenotypic variants permits population diversity. Expression of the O-Ag capsule at such locations key to systemic dissemination may be clinically relevant, as cells expressing the O-Ag capsule would be afforded additional serum resistance in concert with reduced exposure of two major targets of host immunity, LPS and FliC flagellin. This may prove advantageous at key steps of infection—particularly in monophasic NTS isolates, where a virulence phenotype associated with the O-Ag capsule may be more clearly discernible—by affording efficient motility and invasion during initial stages of colonization, as well as increased membrane resistance to innate immune mediators should host conditions provide an opportunity for systemic dissemination. Further studies will be required to determine the clinical relevance of the O-Ag capsule, particularly in the context of monophasic isolates of nontyphoidal Salmonella.
ACKNOWLEDGMENTS
This work was sponsored by a grant from the U.S. National Institutes of Health (NIH/NIAID), award numbers AI066208, AI116917, and R56AI109002 (to J.S.G.), and T32-AI-065411 (an NRSA training grant administered by the Center for Microbial Interface Biology [CMIB] at The Ohio State University).
We thank members of the OSU MII for their technical and scientific input, as well as R. Monitone, B. Kimmenoe, C. S. Justice, and M. Carruthers for assistance with microscopy and A. S. Daigle for assistance with animal inoculation. We thank D. L. Gibson and A. P. White for capsular antisera, B. T. Cookson for flagellar phase-locked strains, and the Ohio State Mass Spectrometry and Proteomics Facility for assistance in protein identification and data interpretation (NIH P30 CA016058).
REFERENCES
- 1.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O'Brien SJ, Jones TF, Fazil A, Hoekstra RM. 2010. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis 50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 2.Chen HM, Wang Y, Su LH, Chiu CH. 2013. Nontyphoid salmonella infection: microbiology, clinical features, and antimicrobial therapy. Pediatr Neonatol 54:147–152. doi: 10.1016/j.pedneo.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Crump JA, Medalla FM, Joyce KW, Krueger AL, Hoekstra RM, Whichard JM, Barzilay EJ. 2011. Antimicrobial resistance among invasive nontyphoidal Salmonella enterica isolates in the United States: National Antimicrobial Resistance Monitoring System, 1996 to 2007. Antimicrob Agents Chemother 55:1148–1154. doi: 10.1128/AAC.01333-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tennant SM, Diallo S, Levy H, Livio S, Sow SO, Tapia M, Fields PI, Mikoleit M, Tamboura B, Kotloff KL, Nataro JP, Galen JE, Levine MM. 2010. Identification by PCR of non-typhoidal Salmonella enterica serovars associated with invasive infections among febrile patients in Mali. PLoS Negl Trop Dis 4:e621. doi: 10.1371/journal.pntd.0000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacLennan CA, Levine MM. 2013. Invasive nontyphoidal Salmonella disease in Africa: current status. Expert Rev Anti Infect Ther 11:443–446. doi: 10.1586/eri.13.27. [DOI] [PubMed] [Google Scholar]
- 6.Parry CM, Thomas S, Aspinall EJ, Cooke RP, Rogerson SJ, Harries AD, Beeching NJ. 2013. A retrospective study of secondary bacteraemia in hospitalised adults with community acquired non-typhoidal Salmonella gastroenteritis. BMC Infect Dis 13:107. doi: 10.1186/1471-2334-13-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendriksen RS, Hyytia-Trees E, Pulsrikarn C, Pornruangwong S, Chaichana P, Svendsen CA, Ahmed R, Mikoleit M. 2012. Characterization of Salmonella enterica serovar Enteritidis isolates recovered from blood and stool specimens in Thailand. BMC Microbiol 12:92. doi: 10.1186/1471-2180-12-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandal BK, Brennand J. 1988. Bacteraemia in salmonellosis: a 15 year retrospective study from a regional infectious diseases unit. BMJ 297:1242–1243. doi: 10.1136/bmj.297.6658.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thuluvath PJ, McKendrick MW. 1988. Salmonella and complications related to age—Sheffield experience. Q J Med 67:497–503. [PubMed] [Google Scholar]
- 10.McCarron B. 1998. A 3-year retrospective review of 132 patients with Salmonella enterocolitis admitted to a regional infectious diseases unit. J Infect 37:136–139. doi: 10.1016/S0163-4453(98)80167-9. [DOI] [PubMed] [Google Scholar]
- 11.Shimoni Z, Pitlik S, Leibovici L, Samra Z, Konigsberger H, Drucker M, Agmon V, Ashkenazi S, Weinberger M. 1999. Nontyphoid Salmonella bacteremia: age-related differences in clinical presentation, bacteriology, and outcome. Clin Infect Dis 28:822–827. doi: 10.1086/515186. [DOI] [PubMed] [Google Scholar]
- 12.Hornick RB, Greisman SE, Woodward TE, DuPont HL, Dawkins AT, Snyder MJ. 1970. Typhoid fever: pathogenesis and immunologic control. N Engl J Med 283:686–691. doi: 10.1056/NEJM197009242831306. [DOI] [PubMed] [Google Scholar]
- 13.Raffatellu M, Chessa D, Wilson RP, Dusold R, Rubino S, Baumler AJ. 2005. The Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent interleukin-8 expression in the intestinal mucosa. Infect Immun 73:3367–3374. doi: 10.1128/IAI.73.6.3367-3374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma A, Qadri A. 2004. Vi polysaccharide of Salmonella typhi targets the prohibitin family of molecules in intestinal epithelial cells and suppresses early inflammatory responses. Proc Natl Acad Sci U S A 101:17492–17497. doi: 10.1073/pnas.0407536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crawford RW, Gibson DL, Kay WW, Gunn JS. 2008. Identification of a bile-induced exopolysaccharide required for Salmonella biofilm formation on gallstone surfaces. Infect Immun 76:5341–5349. doi: 10.1128/IAI.00786-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder DS, Gibson D, Heiss C, Kay W, Azadi P. 2006. Structure of a capsular polysaccharide isolated from Salmonella enteritidis. Carbohydr Res 341:2388–2397. doi: 10.1016/j.carres.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Gibson DL, White AP, Snyder SD, Martin S, Heiss C, Azadi P, Surette M, Kay WW. 2006. Salmonella produces an O-antigen capsule regulated by AgfD and important for environmental persistence. J Bacteriol 188:7722–7730. doi: 10.1128/JB.00809-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patrick S, Houston S, Thacker Z, Blakely GW. 2009. Mutational analysis of genes implicated in LPS and capsular polysaccharide biosynthesis in the opportunistic pathogen Bacteroides fragilis. Microbiology 155:1039–1049. doi: 10.1099/mic.0.025361-0. [DOI] [PubMed] [Google Scholar]
- 19.Lindemann SR, Peng K, Long ME, Hunt JR, Apicella MA, Monack DM, Allen LA, Jones BD. 2011. Francisella tularensis Schu S4 O-antigen and capsule biosynthesis gene mutants induce early cell death in human macrophages. Infect Immun 79:581–594. doi: 10.1128/IAI.00863-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldman RC, White D, Orskov F, Orskov I, Rick PD, Lewis MS, Bhattacharjee AK, Leive L. 1982. A surface polysaccharide of Escherichia coli O111 contains O-antigen and inhibits agglutination of cells by O-antiserum. J Bacteriol 151:1210–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caboni M, Pédron T, Rossi O, Goulding D, Pickard D, Citiulo F, MacLennan CA, Dougan G, Thomson NR, Saul A, Sansonetti PJ, Gerke C. 2015. An O antigen capsule modulates bacterial pathogenesis in Shigella sonnei. PLoS Pathog 11:e1004749. doi: 10.1371/journal.ppat.1004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakhamchik A, Wilde C, Rowe-Magnus DA. 2007. Identification of a Wzy polymerase required for group IV capsular polysaccharide and lipopolysaccharide biosynthesis in Vibrio vulnificus. Infect Immun 75:5550–5558. doi: 10.1128/IAI.00932-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlyshev AV, Linton D, Gregson NA, Lastovica AJ, Wren BW. 2000. Genetic and biochemical evidence of a Campylobacter jejuni capsular polysaccharide that accounts for Penner serotype specificity. Mol Microbiol 35:529–541. [DOI] [PubMed] [Google Scholar]
- 24.Attridge SR, Holmgren J. 2009. Vibrio cholerae O139 capsular polysaccharide confers complement resistance in the absence or presence of antibody yet presents a productive target for cell lysis: implications for detection of bactericidal antibodies. Microb Pathog 47:314–320. doi: 10.1016/j.micpath.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Caboni M, Pedron T, Rossi O, Goulding D, Pickard D, Citiulo F, MacLennan CA, Dougan G, Thomson NR, Saul A, Sansonetti PJ, Gerke C. 2015. An O antigen capsule modulates bacterial pathogenesis in Shigella sonnei. PLoS Pathog 11:e1004749. doi: 10.1371/journal.ppat.1004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barak JD, Jahn CE, Gibson DL, Charkowski AO. 2007. The role of cellulose and O-antigen capsule in the colonization of plants by Salmonella enterica. Mol Plant Microbe Interact 20:1083–1091. doi: 10.1094/MPMI-20-9-1083. [DOI] [PubMed] [Google Scholar]
- 27.Zakikhany K, Harrington CR, Nimtz M, Hinton JC, Romling U. 2010. Unphosphorylated CsgD controls biofilm formation in Salmonella enterica serovar Typhimurium. Mol Microbiol 77:771–786. doi: 10.1111/j.1365-2958.2010.07247.x. [DOI] [PubMed] [Google Scholar]
- 28.White AP, Gibson DL, Grassl GA, Kay WW, Finlay BB, Vallance BA, Surette MG. 2008. Aggregation via the red, dry, and rough morphotype is not a virulence adaptation in Salmonella enterica serovar Typhimurium. Infect Immun 76:1048–1058. doi: 10.1128/IAI.01383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang RF, Kushner SR. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195–199. doi: 10.1016/0378-1119(91)90366-J. [DOI] [PubMed] [Google Scholar]
- 31.DuBois M, Gilles K, Hamilton J, Rebers P, Smith F. 1956. Colorimetric method for determination of sugars and related substances. Anal Chem 28:6. [DOI] [PubMed] [Google Scholar]
- 32.Irie Y, Starkey M, Edwards AN, Wozniak DJ, Romeo T, Parsek MR. 2010. Pseudomonas aeruginosa biofilm matrix polysaccharide Psl is regulated transcriptionally by RpoS and post-transcriptionally by RsmA. Mol Microbiol 78:158–172. doi: 10.1111/j.1365-2958.2010.07320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai CM, Frasch CE. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem 119:115–119. doi: 10.1016/0003-2697(82)90673-X. [DOI] [PubMed] [Google Scholar]
- 34.Prouty AM, Van Velkinburgh JC, Gunn JS. 2002. Salmonella enterica serovar Typhimurium resistance to bile: identification and characterization of the tolQRA cluster. J Bacteriol 184:1270–1276. doi: 10.1128/JB.184.5.1270-1276.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kutsukake K, Nakashima H, Tominaga A, Abo T. 2006. Two DNA invertases contribute to flagellar phase variation in Salmonella enterica serovar Typhimurium strain LT2. J Bacteriol 188:950–957. doi: 10.1128/JB.188.3.950-957.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cross AS, Kim KS, Wright DC, Sadoff JC, Gemski P. 1986. Role of lipopolysaccharide and capsule in the serum resistance of bacteremic strains of Escherichia coli. J Infect Dis 154:497–503. doi: 10.1093/infdis/154.3.497. [DOI] [PubMed] [Google Scholar]
- 37.Whitfield C. 2006. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem 75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 38.Kong Q, Yang J, Liu Q, Alamuri P, Roland KL, Curtiss R III. 2011. Effect of deletion of genes involved in lipopolysaccharide core and O-antigen synthesis on virulence and immunogenicity of Salmonella enterica serovar Typhimurium. Infect Immun 79:4227–4239. doi: 10.1128/IAI.05398-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castelli ME, Fedrigo GV, Clementin AL, Ielmini MV, Feldman MF, Garcia Vescovi E. 2008. Enterobacterial common antigen integrity is a checkpoint for flagellar biogenesis in Serratia marcescens. J Bacteriol 190:213–220. doi: 10.1128/JB.01348-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cota I, Blanc-Potard AB, Casadesus J. 2012. STM2209-STM2208 (opvAB): a phase variation locus of Salmonella enterica involved in control of O-antigen chain length. PLoS One 7:e36863. doi: 10.1371/journal.pone.0036863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nilsson AI, Kugelberg E, Berg OG, Andersson DI. 2004. Experimental adaptation of Salmonella typhimurium to mice. Genetics 168:1119–1130. doi: 10.1534/genetics.104.030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lucchini S, McDermott P, Thompson A, Hinton JC. 2009. The H-NS-like protein StpA represses the RpoS (sigma 38) regulon during exponential growth of Salmonella Typhimurium. Mol Microbiol 74:1169–1186. doi: 10.1111/j.1365-2958.2009.06929.x. [DOI] [PubMed] [Google Scholar]
- 43.Ikeda JS, Schmitt CK, Darnell SC, Watson PR, Bispham J, Wallis TS, Weinstein DL, Metcalf ES, Adams P, O'Connor CD, O'Brien AD. 2001. Flagellar phase variation of Salmonella enterica serovar Typhimurium contributes to virulence in the murine typhoid infection model but does not influence Salmonella-induced enteropathogenesis. Infect Immun 69:3021–3030. doi: 10.1128/IAI.69.5.3021-3030.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon R, Samuel CE. 2007. Activation of NF-kappaB-dependent gene expression by Salmonella flagellins FliC and FljB. Biochem Biophys Res Commun 355:280–285. doi: 10.1016/j.bbrc.2007.01.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gewirtz AT, Simon PO Jr, Schmitt CK, Taylor LJ, Hagedorn CH, O'Brien AD, Neish AS, Madara JL. 2001. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J Clin Invest 107:99–109. doi: 10.1172/JCI10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wain J, House D, Zafar A, Baker S, Nair S, Kidgell C, Bhutta Z, Dougan G, Hasan R. 2005. Vi antigen expression in Salmonella enterica serovar Typhi clinical isolates from Pakistan. J Clin Microbiol 43:1158–1165. doi: 10.1128/JCM.43.3.1158-1165.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hohmann EL. 2001. Nontyphoidal salmonellosis. Clin Infect Dis 32:263–269. doi: 10.1086/318457. [DOI] [PubMed] [Google Scholar]
- 48.Gordon MA. 2008. Salmonella infections in immunocompromised adults. J Infect 56:413–422. doi: 10.1016/j.jinf.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 49.Jansen AM, Hall LJ, Clare S, Goulding D, Holt KE, Grant AJ, Mastroeni P, Dougan G, Kingsley RA. 2011. A Salmonella Typhimurium-Typhi genomic chimera: a model to study Vi polysaccharide capsule function in vivo. PLoS Pathog 7:e1002131. doi: 10.1371/journal.ppat.1002131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crawford RW, Wangdi T, Spees AM, Xavier MN, Tsolis RM, Baumler AJ. 2013. Loss of very-long O-antigen chains optimizes capsule-mediated immune evasion by Salmonella enterica serovar Typhi. mBio 4:e00232–13. doi: 10.1128/mBio.00232-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gondwe EN, Molyneux ME, Goodall M, Graham SM, Mastroeni P, Drayson MT, MacLennan CA. 2010. Importance of antibody and complement for oxidative burst and killing of invasive nontyphoidal Salmonella by blood cells in Africans. Proc Natl Acad Sci U S A 107:3070–3075. doi: 10.1073/pnas.0910497107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garrett ES, Perlegas D, Wozniak DJ. 1999. Negative control of flagellum synthesis in Pseudomonas aeruginosa is modulated by the alternative sigma factor AlgT (AlgU). J Bacteriol 181:7401–7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tran QT, Gomez G, Khare S, Lawhon SD, Raffatellu M, Baumler AJ, Ajithdoss D, Dhavala S, Adams LG. 2010. The Salmonella enterica serotype Typhi Vi capsular antigen is expressed after the bacterium enters the ileal mucosa. Infect Immun 78:527–535. doi: 10.1128/IAI.00972-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chevance FF, Hughes KT. 2008. Coordinating assembly of a bacterial macromolecular machine. Nat Rev Microbiol 6:455–465. doi: 10.1038/nrmicro1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Partridge JD, Harshey RM. 2013. More than motility: Salmonella flagella contribute to overriding friction and facilitating colony hydration during swarming. J Bacteriol 195:919–929. doi: 10.1128/JB.02064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cummings LA, Wilkerson WD, Bergsbaken T, Cookson BT. 2006. In vivo, fliC expression by Salmonella enterica serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Mol Microbiol 61:795–809. doi: 10.1111/j.1365-2958.2006.05271.x. [DOI] [PubMed] [Google Scholar]
- 57.Bergman MA, Cummings LA, Alaniz RC, Mayeda L, Fellnerova I, Cookson BT. 2005. CD4+-T-cell responses generated during murine Salmonella enterica serovar Typhimurium infection are directed towards multiple epitopes within the natural antigen FliC. Infect Immun 73:7226–7235. doi: 10.1128/IAI.73.11.7226-7235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McQuiston JR, Parrenas R, Ortiz-Rivera M, Gheesling L, Brenner F, Fields PI. 2004. Sequencing and comparative analysis of flagellin genes fliC, fljB, and flpA from Salmonella. J Clin Microbiol 42:1923–1932. doi: 10.1128/JCM.42.5.1923-1932.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koskiniemi S, Gibbons HS, Sandegren L, Anwar N, Ouellette G, Broomall S, Karavis M, McGregor P, Liem A, Fochler E, McNew L, Rosenzweig CN, Rhen M, Skowronski EW, Andersson DI. 2013. Pathoadaptive mutations in Salmonella enterica isolated after serial passage in mice. PLoS One 8:e70147. doi: 10.1371/journal.pone.0070147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmitt CK, Ikeda JS, Darnell SC, Watson PR, Bispham J, Wallis TS, Weinstein DL, Metcalf ES, O'Brien AD. 2001. Absence of all components of the flagellar export and synthesis machinery differentially alters virulence of Salmonella enterica serovar Typhimurium in models of typhoid fever, survival in macrophages, tissue culture invasiveness, and calf enterocolitis. Infect Immun 69:5619–5625. doi: 10.1128/IAI.69.9.5619-5625.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Nunez G. 2006. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol 7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 62.Ackermann M, Stecher B, Freed NE, Songhet P, Hardt WD, Doebeli M. 2008. Self-destructive cooperation mediated by phenotypic noise. Nature 454:987–990. doi: 10.1038/nature07067. [DOI] [PubMed] [Google Scholar]
- 63.Knodler LA, Vallance BA, Celli J, Winfree S, Hansen B, Montero M, Steele-Mortimer O. 2010. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc Natl Acad Sci U S A 107:17733–17738. doi: 10.1073/pnas.1006098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cummings LA, Barrett SL, Wilkerson WD, Fellnerova I, Cookson BT. 2005. FliC-specific CD4+ T cell responses are restricted by bacterial regulation of antigen expression. J Immunol 174:7929–7938. doi: 10.4049/jimmunol.174.12.7929. [DOI] [PubMed] [Google Scholar]
- 65.Siggins MK, Cunningham AF, Marshall JL, Chamberlain JL, Henderson IR, MacLennan CA. 2011. Absent bactericidal activity of mouse serum against invasive African nontyphoidal Salmonella results from impaired complement function but not a lack of antibody. J Immunol 186:2365–2371. doi: 10.4049/jimmunol.1000284. [DOI] [PubMed] [Google Scholar]
- 66.Prouty AM, Gunn JS. 2003. Comparative analysis of Salmonella enterica serovar Typhimurium biofilm formation on gallstones and on glass. Infect Immun 71:7154–7158. doi: 10.1128/IAI.71.12.7154-7158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]