Abstract
Bacillus anthracis is a pathogenic Gram-positive bacterium that causes a highly lethal infectious disease, anthrax. The poly-γ-d-glutamic acid (PGA) capsule is one of the major virulence factors of B. anthracis, along with exotoxins. PGA enables B. anthracis to escape phagocytosis and immune surveillance. Our previous study showed that PGA activates the human macrophage cell line THP-1 and human dendritic cells, resulting in the production of the proinflammatory cytokine interleukin-1β (IL-1β) (M. H. Cho et al., Infect Immun 78:387–392, 2010, http://dx.doi.org/10.1128/IAI.00956-09). Here, we investigated PGA-induced cytokine responses and related signaling pathways in mouse bone marrow-derived macrophages (BMDMs) using Bacillus licheniformis PGA as a surrogate for B. anthracis PGA. Upon exposure to PGA, BMDMs produced proinflammatory mediators, including tumor necrosis factor alpha (TNF-α), IL-6, IL-12p40, and monocyte chemoattractant protein 1 (MCP-1), in a concentration-dependent manner. PGA stimulated Toll-like receptor 2 (TLR2) but not TLR4 in Chinese hamster ovary cells expressing either TLR2 or TLR4. The ability of PGA to induce TNF-α and IL-6 was retained in TLR4−/− but not TLR2−/− BMDMs. Blocking experiments with specific neutralizing antibodies for TLR1, TLR6, and CD14 showed that TLR6 and CD14 also were necessary for PGA-induced inflammatory responses. Furthermore, PGA enhanced activation of mitogen-activated protein (MAP) kinases and nuclear factor-kappa B (NF-κB), which are responsible for expression of proinflammatory cytokines. Additionally, PGA-induced TNF-α production was abrogated not only in MyD88−/− BMDMs but also in BMDMs pretreated with inhibitors of MAP kinases and NF-κB. These results suggest that immune responses induced by PGA occur via TLR2, TLR6, CD14, and MyD88 through activation of MAP kinase and NF-κB pathways.
INTRODUCTION
Bacillus anthracis, the causative agent of anthrax, is a Gram-positive, spore-forming, facultative anaerobic bacterium and is classified as a tier 1 select agent by the Centers for Disease Control and Prevention (CDC) (1). After infecting a host, anthrax spores are rapidly phagocytosed by antigen-presenting cells, such as macrophages and dendritic cells, and subsequently are transported to regional lymph nodes in which the spores geminate and become toxin-producing vegetative bacteria (2, 3). The bacteria then enter the bloodstream and multiply extracellularly, reaching 107 to 108 organisms per milliliter of blood (4).
Vegetative B. anthracis secretes high levels of exotoxins, which cause edema or cell death (5). In addition to exotoxins, the other virulence factor of B. anthracis is the capsule, which is surrounded by PGA, a homopolymer of d-glutamic acid linked by γ-carboxyl groups (6). The antiphagocytic abilities of the PGA capsule allow B. anthracis to evade host immune surveillance via mechanisms that are similar to those of capsular polysaccharides that protect bacteria such as streptococci, staphylococci, and meningococci from phagocytosis (7, 8). A recent study demonstrated that degradation of PGA by treatment with the PGA depolymerase CapD enhances in vitro macrophage phagocytosis and neutrophil-mediated killing of encapsulated B. anthracis in vitro and in vivo (9, 10). Additionally, overexpression of CapD attenuates B. anthracis virulence (11). PGA capsule released from B. anthracis is associated with lethal toxin (LT) in experimental infection models (12). PGA enhances LT-mediated macrophage death (13), indicating that PGA can intensify the LT-induced toxemia that occurs at the terminal stage of anthrax infection.
The innate immune response is the first line of defense against infection, and recognition of invading pathogens by the host innate immune system is a key event in controlling infection (14). Innate immune cells recognize highly conserved structural motifs of microbial pathogens, called pathogen-associated molecular patterns (PAMPs), using diverse pattern recognition receptors, such as toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD) receptors, and RIG-I-like receptors (RLRs) (15). Among pattern recognition receptors, TLRs play a central role in innate immunity by sensing various PAMPs of infectious agents and by initiating a response to eliminate them (16). To date, 13 TLR members have been identified in mammals, each sensing a different set of bacterial and viral PAMPs (17). TLR2 recognizes diverse bacterial products, including peptidoglycans, lipoteichoic acid (LTA), lipoproteins, and mycobacterial lipoarabinomannan (17, 18). The unique ability of TLR2 to sense various microbial ligands comes from its ability to associate with heterodimerization partners such as TLR1 or TLR6 (19). LTA and diacylated lipoproteins induce activation of the innate immune system through TLR2/TLR6, whereas triacylated lipoproteins require TLR1/TLR2 for activation (19, 20). CD14, a glycophosphatidylinositol-anchored glycoprotein, also is involved in the corecognition of various TLR ligands, including TLR2 ligands LTA and lipoproteins (21, 22). The engagement of TLR2 by a ligand subsequently recruits MyD88, a common adaptor molecule in TLR-mediated signaling, with the exception of TLR3 signaling. The interaction of TLR2 and MyD88 leads to the activation of mitogen-activated protein (MAP) kinases and the transcription factor nuclear factor-kappa B (NF-κB), which are responsible for inducing expression of proinflammatory cytokines (15, 17).
In the present study, we examined how the innate immune system senses PGA and the molecular mechanisms of inflammatory responses induced by PGA using Bacillus licheniformis PGA as a surrogate of B. anthracis PGA. We found that PGA induced the secretion of proinflammatory mediators such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) through TLR2. Furthermore, TLR6 and CD14 were required for PGA-induced inflammatory responses. We also found that MyD88, MAP kinase, and NF-κB pathways were involved in PGA-induced inflammatory responses. These results indicate that PGA is involved in anthrax pathogenesis by inducing proinflammatory cytokine production.
MATERIALS AND METHODS
Reagents and chemicals.
MAP kinase inhibitors, including U0126, SB203580 (SB), and SP600125 (SP), were purchased from Calbiochem (Darmstadt, Germany). The NF-κB inhibitor parthenolide was obtained from Sigma-Aldrich (St. Louis, MO). Antibodies recognizing the phosphorylated and nonphosphorylated forms of MAP kinases, IκB-α, and β-actin were from Cell Signaling Technology (Beverly, MA). The TLR1/TLR2 ligand PAM3CSK4 (P3C), the TLR9 ligand CpG2395, and the TLR4 ligand Escherichia coli lipopolysaccharide (LPS) were purchased from InvivoGen (Cayla SAS, Toulouse, France). The TLR2/TLR6 ligand MALP2 was purchased from Alexis Biochemicals (San Diego, CA). Highly purified Staphylococcus aureus LTA was kindly provided by Seung Hyun Han (Seoul National University, Seoul, South Korea). Blocking antibodies for CD14 and TLR2 were purchased from R&D Systems (Minneapolis, MN), and blocking antibodies for TLR1 and TLR6 were obtained from InvivoGen. HEK 293 cells expressing TLR2, TLR1/2, and TLR2/6 were purchased from InvivoGen. Dulbecco's modified Eagle medium (DMEM), F12 medium, fetal bovine serum (FBS), and antibiotics for cell culture were purchased from Invitrogen (Carlsbad, CA). The plasmid expressing human CD14 and control plasmid were purchased from InvivoGen.
Purification of PGA.
B. licheniformis ATCC 9945a was grown in E medium, which contained 20 g l-glutamic acid, 12 g citric acid, 80 g glycerol, 7 g NH4Cl, 0.5 g K2HPO4, 0.5 g MgSO4 · 7H2O, 0.04 g FeCl3 · 6H2O, 0.15 g CaCl2 · 2H2O, and 0.104 g MnSO4 · H2O per liter (23). Usually under these conditions, B. licheniformis 9945a has been known to produce PGA with approximately 80 to 90% d-enantiomer (23, 24). After 4 days of culture, highly viscous bacterial culture was centrifuged at 4°C (6,500 × g, 20 min) to remove bacteria, and collected supernatant was precipitated with 3 volumes of ethanol at 4°C overnight. PGA precipitate was collected by centrifugation and dialyzed against deionized water. Dialyzed PGA was acidified to pH 1.5 with 6 M HCl and immediately precipitated with 3 volumes of 1-propanol at −20°C. PGA was collected by centrifugation and washed twice with acetone and then dissolved in ultrapure deionized water. PGA solution was dialyzed against deionized water and then lyophilized. As the molecular mass of purified PGA was ∼500 kDa, PGA was fragmented to a molecular mass of <50 kDa with 600 mM HCl at 85°C for 0.5 h. The hydrolyzed PGA was neutralized, dialyzed against deionized water, and lyophilized prior to use in experiments. The purity and structure of PGA were verified by UV-visible scanning from 190 to 300 nm and by 1H nuclear magnetic resonance (NMR) spectroscopy. Endotoxin levels in the purified PGA were measured in endotoxin units per milliliter (EU/ml) using a Limulus amebocyte lysate assay kit (Lonza, Walkersville, MN). According to this assay, 100 μg/ml PGA contained <0.1 EU/ml.
Animals.
All animal studies were conducted with the approval of the Institutional Animal Care and Use Committee (IACUC) of the Korea National Institute of Health. C57BL/6 and BALB/c wild-type (WT) mice were purchased from Orient Bio (Seoul, South Korea). TLR2-knockout (KO) C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME), and TLR4-KO C57BL/6 and MyD88-KO BALB/c mice were kindly provided by Shizuo Akira (Osaka University, Osaka, Japan).
Cell culture and stimulation.
Bone marrow-derived macrophages (BMDMs) were prepared as described previously (25). In brief, bone marrow cells were isolated from 6-week-old C57BL/6, BALB/c, TLR2-KO C57BL/6, TLR4-KO C57BL/6, or MyD88-KO BALB/c mice by flushing the marrow space of tibiae and femora with a syringe filled with DMEM supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin. For macrophage differentiation, cells were cultured in the aforementioned medium in the presence of 30% L929 conditioned medium as a source of macrophage colony-stimulating factor (M-CSF) and 50 μM β-mercaptoethanol for 6 to 8 days at 37°C in a humidified incubator with 5% CO2. BMDMs were trypsinized and seeded in 24-well plates at 1 × 106/ml. Cells then were stimulated with various concentrations of PGA (0, 12.5, 25, 50, or 100 μg/ml), P3C (0.1 μg/ml), or CpG2395 (1 μM) for 24 h. Chinese hamster ovary (CHO) cells expressing either TLR2/CD14 (CHO/TLR2/CD14) or TLR4/CD14 (CHO/TLR4/CD14) were cultured in F12 medium in the presence of G418 (1 mg/ml) and hygromycin (400 μg/ml). CHO/TLR2/CD14 or CHO/TLR4/CD14 cells (2 × 105/ml) were stimulated with PGA, P3C, LPS, or LTA for 18 h. HEK 293-TLR2, TLR1/2, and TLR2/6 cells were incubated in DMEM in the presence of blasticidin (10 μg/ml) and stimulated with PGA, P3C, or MALP2 for 24 h.
ELISA.
Concentrations of mouse TNF-α, IL-6, IL-12p40, and monocyte chemoattractant protein 1 (MCP-1) and of human IL-8 were measured by enzyme-linked immunosorbent assay (ELISA) using supernatants from BMDMs or HEK293-TLR2, TLR1/2, and TLR2/6 cells stimulated as described above using commercially available ELISA kits (BioLegend, San Diego, CA) according to the manufacturer's instructions.
Blocking experiments with anti-TLR1, anti-TLR2, anti-TLR6, or CD14.
HEK 293-TLR2 cells were preincubated for 1 h with human anti-TLR1 (5 μg/ml), anti-TLR2 (10 μg/ml), anti-TLR6 (5 μg/ml), or an isotype control mouse IgG1 (10 μg/ml) and then stimulated with PGA, P3C, or MALP2 for an additional 24 h. IL-8 concentrations were measured using an IL-8 ELISA kit (BioLegend). For the CD14 blocking experiment, CHO/TLR2/CD14 cells were preincubated for 1 h with human anti-CD14 (10 μg/ml) or an isotype control antibody, followed by stimulation with PGA or LTA for an additional 18 h.
Flow cytometry analysis.
CHO/TLR2/CD14 and CHO/TLR4/CD14 cells, which are NF-κB reporter cell lines, were used to analyze the ability of PGA to stimulate TLR2 or TLR4 as described previously (26). These cell lines express the human membrane CD25 gene under the control of the E-selectin promoter, which is regulated by NF-κB activation. CHO/TLR2/CD14 or CHO/TLR4/CD14 cells were stimulated with PGA, P3C, LTA, or LPS for 18 h and then stained with fluorescein isothiocyanate (FITC)-conjugated human anti-CD25. TLR2- or TLR4-dependent NF-κB activation was determined by flow cytometry analysis of CD25 on a FACSCalibur (Becton Dickinson, Mountain View, CA) or FC500 (Beckman Coulter, Palo Alto, CA) flow cytometer with FlowJo software (Tree Star, Ashland, OR).
Western blot analysis.
BMDMs were stimulated with 100 μg/ml PGA for 0, 5, 15, 30, or 60 min or 0.1 μg/ml LPS for 15 min. Cells then were lysed with NP-40 cell lysis buffer (Invitrogen) on ice for 30 min. Cell lysates were collected by centrifugation at 13,000 × g for 10 min, and a 30-μg sample of total protein was separated by 10% SDS-PAGE and electrotransferred onto a polyvinylidene fluoride membrane. The membrane was blocked with 5% skim milk in TBS (50 mM Tris-HCl, 150 mM NaCl, pH 7.6) at room temperature for 1 h and then incubated with rabbit anti-MAP kinase (1:1,000), anti-IκB-α (1:1,000), or β-actin (1:3,000) at 4°C overnight. After washing three times with TBST (TBS with 0.05% Tween 20), the membrane was incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:3,000) in blocking buffer at room temperature for 1 h. Finally, the membrane was washed three times with TBST, and the immunoreactive bands were detected using ECL Western blotting reagents (Invitrogen) and analyzed on a ChemiDoc XRS system (Bio-Rad, Hercules, CA).
Immunofluorescence microscopy.
To examine NF-κB activation by PGA, BMDMs (1 × 105/ml) were seeded on a chamber slide (Nalge Nunc) and treated with 100 μg/ml PGA for 1 h. Cells then were washed with cold phosphate-buffered saline (PBS) (Invitrogen) and fixed with 4% paraformaldehyde for 15 min. Cells were permeabilized in PBS containing 0.1% saponin and 5% FBS and then incubated with anti-NF-κB p65 primary antibody (1:50; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at room temperature. Cells then were incubated with Alexa Fluor 488-conjugated anti-mouse secondary antibody (1:100; Invitrogen) for 1 h at room temperature and washed three times with PBS containing 0.1% saponin. Samples were mounted with Prolong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI) and analyzed using an Olympus FV1000 confocal microscope (Tokyo, Japan).
Transfection and luciferase reporter gene assay.
HEK293-TLR2 cells were plated at 5 × 105/ml on 24-well plates. The next day, cells were transfected with 10 ng pNF-κB luciferase plasmid (Clontech, Mountain View, CA) or 1 ng pRL-TK (Promega, Madison, WI) plus the CD14 expression plasmid or vector control (100 ng each) using transfection reagent (TransFectin; Bio-Rad) according to the manufacturer's instructions. After 24 h of transfection, cells were stimulated with PGA (100 μg/ml) or LTA (10 μg/ml) for 8 h. Stimulated cells were lysed with reporter lysis buffer (Promega), and the dual-luciferase assay was performed using the dual-luciferase assay system (Promega) according to the manufacturer's instructions. Firefly luciferase activity was normalized to Renilla luciferase activity to obtain the relative luciferase activity.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism 6 software (GraphPad Software, San Diego, CA). All experiments were performed at least three times. Mean values ± standard deviations (SD) were determined for each treatment group in each individual experiment. Treatment groups were compared with the appropriate control. Two-sample comparisons were conducted with Student's t test, while multiple comparisons were made with a one-way analysis of variance (ANOVA) followed by a Tukey-Kramer's post hoc test. Differences were considered significant when the P value was <0.05.
RESULTS
PGA stimulates the secretion of proinflammatory cytokines from BMDMs.
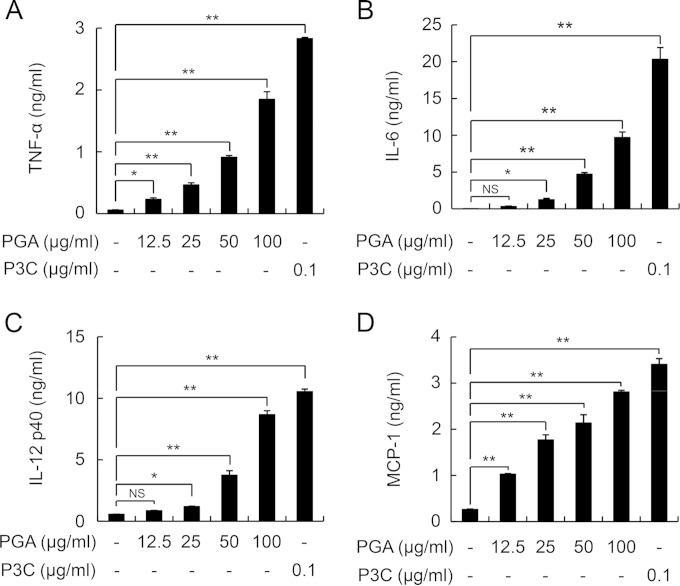
Previously, we demonstrated that PGA induces the release of the proinflammatory cytokine IL-1β from THP-1 macrophages and human monocyte-derived dendritic cells through activation of caspase-1 (27). To further characterize PGA-mediated inflammatory responses, we examined whether PGA can induce inflammatory responses in mouse BMDMs (Fig. 1). Stimulation of cells with PGA significantly augmented the production of the proinflammatory cytokines TNF-α, IL-6, IL-12p40, and MCP-1 in a concentration-dependent manner (Fig. 1A to D).
FIG 1.
PGA induces production of inflammatory mediators in a dose-dependent manner. BMDMs from C57BL/6 mice were incubated with various concentrations of PGA (0, 12.5, 25, 50, and 100 μg/ml) or P3C (0.1 μg/ml) for 24 h. After incubation, levels of TNF-α (A), IL-6 (B), IL-12p40 (C), and MCP-1 (D) in culture supernatants were measured by ELISA. Data are the mean values ± SD from triplicate results. Statistical significance was calculated using one-way ANOVA followed by a Tukey's post hoc test. *, P < 0.05, and **, P < 0.01, compared with the untreated control group; NS, not significant. One of three similar results is shown.
PGA induces TLR2-dependent production of cytokines by BMDMs.
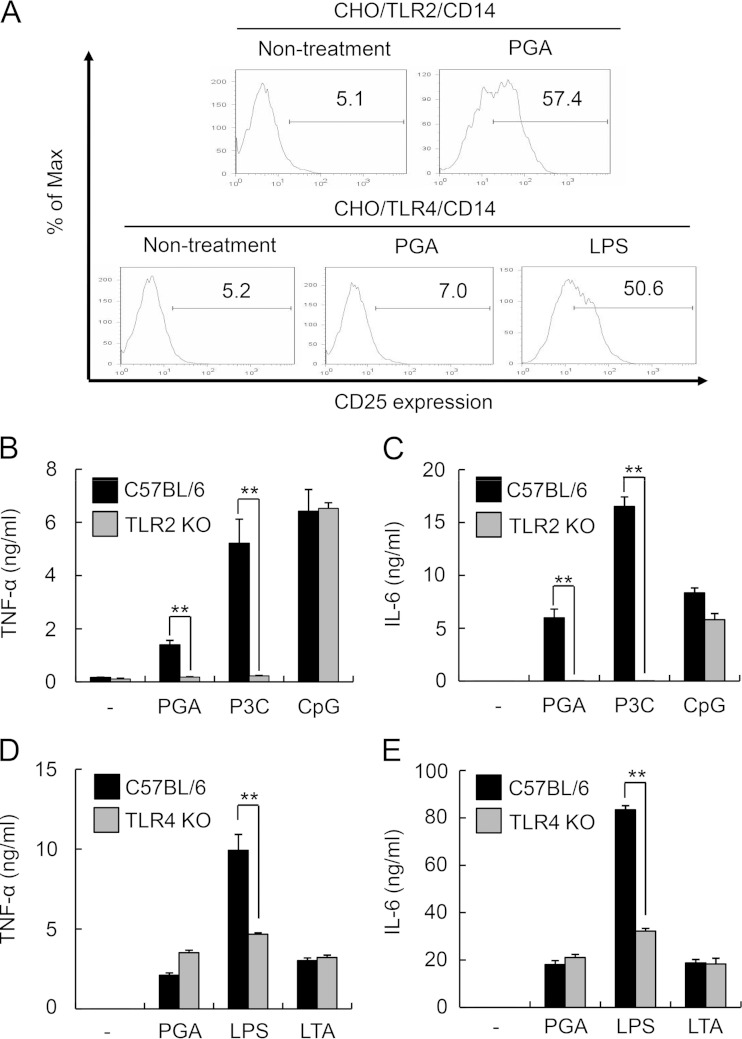
Because TLRs play a pivotal role not only in recognition of PAMPs but also in control of host immune responses against invading pathogens (16), we examined whether immune responses by PGA were mediated through TLR2 or TLR4. CHO/TLR2/CD14 or CHO/TLR4/CD14 cells, which coexpress CD14 and either TLR2 or TLR4, were stimulated with PGA and either P3C or LPS, respectively, for 18 h. CD25 expression, which is regulated by activation of NF-κB, then was analyzed by flow cytometry. In CHO/TLR2/CD14 cells, CD25 expression was augmented by PGA stimulation (Fig. 2A). In contrast, CD25 expression was not enhanced in CHO/TLR4/CD14 cells stimulated with PGA, although the TLR4 ligand LPS did markedly enhance CD25 expression compared to the level in untreated control cells.
FIG 2.
TLR2, but not TLR4, is involved in PGA-mediated inflammatory responses. (A) CHO/TLR2/CD14 or CHO/TLR4/CD14 cells were stimulated with 62.5 μg/ml PGA, 0.1 μg/ml P3C, or 0.1 μg/ml LPS for 18 h and then stained with FITC-conjugated anti-CD25. TLR2- or TLR4-dependent NF-κB activation was determined by flow cytometry analysis of CD25. Values in histograms indicate the percentage of CD25-expressing cells. One of three similar results is shown. (B and C) BMDMs from C57BL/6 and TLR2-KO C57BL/6 mice were stimulated with 100 μg/ml PGA, 0.1 μg/ml P3C, or 1 μΜ CpG2395 for 24 h. Levels of TNF-α and IL-6 were measured by ELISA. (D and E) BMDMs from C57BL/6 or TLR4-KO C57BL/6 mice were stimulated with 100 μg/ml PGA, 0.1 μg/ml LPS, or 10 μg/ml LTA for 24 h. Levels of TNF-α and IL-6 were measured by ELISA. Data are the mean values ± SD from triplicate samples. Statistical significance was calculated using the t test. **, P < 0.01 compared with the appropriate control group. The results shown are representative of three separate experiments.
To further verify whether PGA induces inflammatory responses through TLR2, we examined cytokine production induced by PGA in BMDMs from TLR2- and TLR4-KO mice. BMDMs from C57BL/6 and either TLR2- or TLR4-KO mice were stimulated with PGA, P3C, or CpG DNA or with PGA, LPS, or LTA, respectively, for 24 h. As shown in Fig. 2B and C, PGA-induced secretion of TNF-α and IL-6 was significantly reduced in TLR2-KO BMDMs. A similar effect was seen for P3C-induced secretion but not for CpG-induced secretion. Furthermore, no changes in PGA-mediated or LTA-mediated TNF-α and IL-6 secretion were observed in TLR4-KO BMDMs, whereas secretion of LPS-induced TNF-α and IL-6 was significantly reduced in TLR4-KO BMDMs (Fig. 2D and E). Taken together, these results indicate that PGA induced inflammatory responses through TLR2 but not TLR4.
We next examined whether the stimulatory effect of PGA on TLR2 activation is due to contaminants such as lipoproteins, LTA, or peptidoglycan. As shown in Fig. S1A in the supplemental material, NMR analysis of our purified PGA demonstrated a conventional peak pattern of γ-PGA with no additional peak, which is consistent with previously published results (23, 28). Pretreatment of PGA with hydrofluoric acid (HF), which has been known to deactivate lipoproteins and LTA (29), did not significantly affect PGA-induced TNF-α production in RAW264.7 cells (data not shown). Additionally, preincubation of PGA with lipase did not significantly affect PGA-induced TNF-α production, while pretreatment of P3C and LTA with lipase attenuated their stimulatory effects on TNF-α production (see Fig. S1B). Furthermore, pretreatment of proteinase K caused significant reduction in IFN-γ-induced TNF-α production, whereas it did not influence PGA-induced TNF-α production (data not shown). Taken together, these data demonstrate that our purified PGA does not contain the minimum amounts of LTA or lipoproteins required to induce cytokine production. Peptidoglycan, which contains a carbohydrate backbone of alternating units of N-acetyl glucosamine and N-acetyl muramic acid, has been known to be a potent inducer of proinflammatory cytokines in immune cells, and it is recognized by the intracellular receptor NOD2 (30, 31). We examined whether our purified PGA contains peptidoglycan by measuring total carbohydrate quantity. The assay result showed that the quantity of carbohydrate in the purified PGA is almost similar to that of the water control (data not shown). To further verify whether the purified PGA contains peptidoglycan, we examined TNF-α production by PGA in BMDMs from C57BL/6 and NOD2-KO mice. As shown in Fig. S1C, no difference in TNF-α production between C57BL/6 and NOD2-KO BMDMs was found, indicating that purified PGA does not contain peptidoglycan.
TLR6 and CD14 also are required for PGA-induced inflammatory responses.
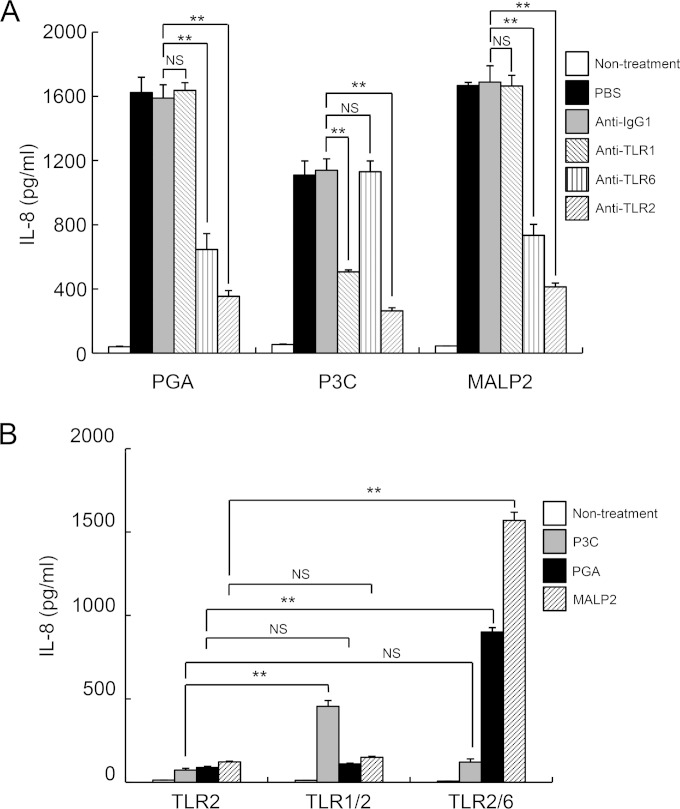
Unlike other TLRs, TLR2 functions as a heterodimer with either TLR1 or TLR6, which mediates inflammatory responses depending on the ligand (19, 32). Thus, we examined whether PGA is a ligand for TLR1/TLR2 or TLR2/TLR6. Because HEK293 cells endogenously express TLR1 and TLR6, HEK293-TLR2 cells were pretreated with blocking antibodies against TLR1, TLR2, or TLR6 or isotype-matched control antibody for 1 h and then stimulated with PGA, P3C (a TLR2/TLR1 ligand), or MALP2 (a TLR2/TLR6 ligand) for 24 h (Fig. 3A). As expected, pretreatment of cells with TLR2 blocking antibody markedly reduced PGA-, P3C-, and MALP2-induced IL-8 secretion (Fig. 3A). Furthermore, addition of TLR6 blocking antibody significantly attenuated IL-8 secretion from PGA- and MALP2-stimulated cells, whereas TLR1 blocking antibody decreased IL-8 secretion only in P3C-treated cells (Fig. 3A). To further verify the role of TLR6 in PGA-induced inflammatory responses, HEK293 cells expressing TLR2, TLR1/TLR2, or TLR2/TLR6 were stimulated with P3C, PGA, or MALP2 for 24 h. As expected, P3C and MALP2 specifically augmented IL-8 secretion from HEK293-TLR1/TLR2 cells and TLR2/TLR6 cells, respectively, compared to secretion from HEK293-TLR2 cells. PGA significantly enhanced IL-8 production by HEK293-TLR2/TLR6 cells but not TLR1/TLR2 cells (Fig. 3B). These results indicate that PGA induces inflammatory responses through the TLR2/TLR6 heterodimer.
FIG 3.
TLR6 is required for inflammatory responses by PGA. (A) HEK293-TLR2 cells were pretreated with control IgG1 (10 μg/ml), anti-TLR1 (5 μg/ml), anti-TLR6 (5 μg/ml), or anti-TLR2 (10 μg/ml) for 1 h and then stimulated with 60 μg/ml PGA, 10 ng/ml P3C, or 10 ng/ml MALP2 for an additional 24 h. Cell culture medium was collected and IL-8 production was measured by ELISA. (B) HEK293-TLR2, TLR1/TLR2, and TLR2/TLR6 cells were treated with 10 ng/ml P3C, 60 μg/ml PGA, or 10 ng/ml MALP2 for 24 h, and the level of IL-8 was measured by ELISA. Data are the mean values ± SD from triplicate results. Statistical significance was calculated using one-way ANOVA followed by a Tukey's post hoc test. **, P < 0.01 compared with the appropriate control group. One of three similar results is shown.
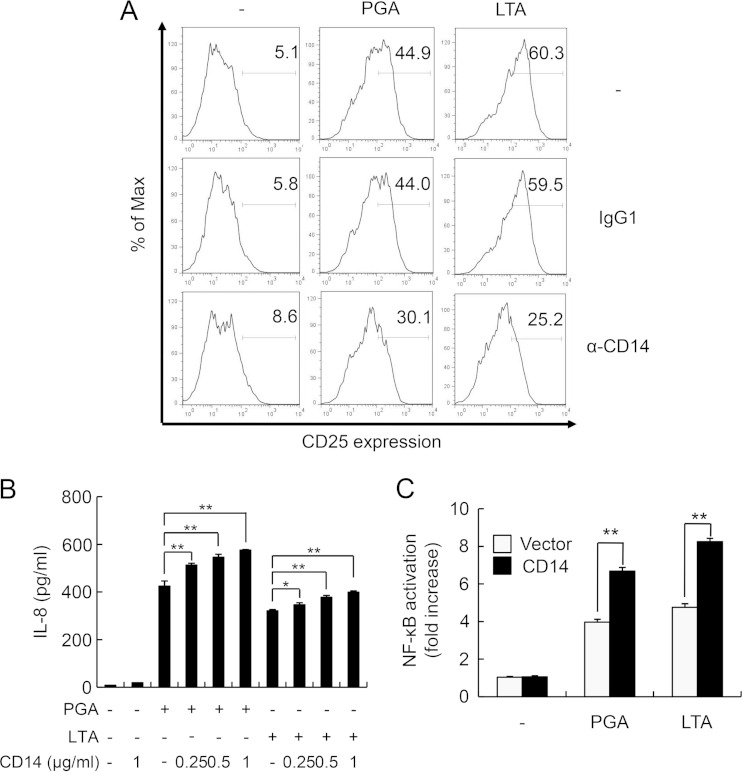
Because CD14 plays an important role in TLR2-mediated inflammatory responses (33), we determined the involvement of CD14 in PGA-induced immune responses. CHO/TLR2/CD14 cells were preincubated with CD14 blocking antibody or isotype-matched control antibody for 1 h and then stimulated with either PGA or LTA for an additional 18 h (Fig. 4A). Stimulation of CHO/TLR2/CD14 cells with PGA or LTA markedly enhanced CD25 expression compared with that of untreated control cells, and this enhancement was attenuated by pretreatment with CD14 neutralizing antibody. Moreover, addition of soluble CD14 augmented the PGA- and LTA-induced IL-8 secretion from HEK293-TLR2 cells in a concentration-dependent manner (Fig. 4B).
FIG 4.
CD14 is involved in PGA-induced inflammatory responses. (A) CHO/TLR2/CD14 cells were preincubated with 10 μg/ml control IgG1 or anti-CD14 for 1 h and then stimulated with 100 μg/ml PGA or 10 μg/ml LTA for an additional 18 h. Cells were stained with FITC-conjugated anti-CD25 and subjected to flow cytometry. Values in histograms represent the percentage of CD25-expressing cells. The results shown are representative of three separate experiments. (B) HEK293-TLR2 cells were treated with increasing concentrations (0, 0.25, 0.5, or 1 μg/ml) of soluble CD14 in the presence or absence of PGA or LTA for 24 h. IL-8 concentrations were measured by ELISA. Data are the mean values ± SD from triplicate results. Statistical significance was calculated using one-way ANOVA followed by a Tukey's post hoc test. P < 0.05 (*) and P < 0.01 (**) compared with the appropriate control group. One of three similar results is shown. (C) HEK293-TLR2 cells were transiently transfected with an NF-κB luciferase reporter plasmid together with a Renilla luciferase plasmid in the presence of a CD14 expression plasmid or control vector for 24 h. Cells then were stimulated with PGA (100 μg/ml) or LTA (10 μg/ml) for an additional 24 h. Culture supernatants were used for analysis of IL-8 secretion by ELISA. Data are the mean values ± SD from triplicate samples. Statistical significance was examined using a t test. **, P < 0.01 compared with the appropriate control group. One of three similar results is shown.
Since soluble CD14 augmented PGA-induced IL-8 production in HEK293-TLR2 cells, we next examined whether enforced CD14 expression can enhance PGA-induced inflammatory responses. HEK293-TLR2 cells were transiently transfected with human CD14 expression vector or control vector with an NF-κB luciferase reporter plasmid. The cells then were stimulated with PGA or LTA for 24 h. Stimulation with either PGA or LTA increased NF-κB luciferase reporter activity in control vector-transfected HEK293-TLR2 cells, and this effect was further enhanced by overexpression of CD14 (Fig. 4C). These results indicate that CD14 also is involved in PGA-mediated inflammatory responses.
MyD88, MAP kinase, and NF-κB signaling pathways are involved in the PGA-induced inflammatory response.
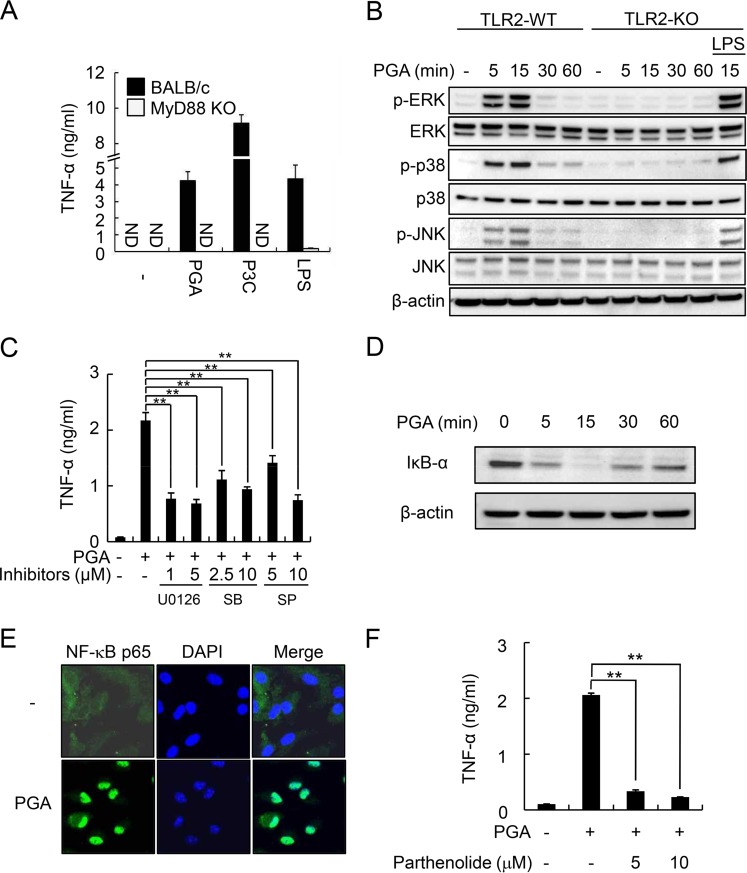
Because TLR2 requires the intracellular adaptor molecule MyD88 to induce inflammatory responses and activation of downstream signaling pathways involving MAP kinases and NF-κB (16, 17), we investigated the effect of MyD88 deficiency on PGA-induced TNF-α production (Fig. 5A). TNF-α secretion by PGA as well as P3C and LPS was completely abolished in MyD88-KO BMDMs, indicating that MyD88 is required for PGA-induced cytokine production.
FIG 5.
MyD88, MAP kinase, and NF-κB signaling pathways are involved in PGA-induced cytokine production. (A) BMDMs from BALB/c and MyD88-KO BALB/c mice were stimulated with 100 μg/ml PGA, 100 ng/ml P3C, or 100 ng/ml LPS for 24 h. TNF-α concentrations were measured by ELISA. (B) BMDMs from C57BL/6 and TLR2-KO C57BL/6 mice were stimulated with 100 μg/ml PGA for the indicated times (0, 5, 15, 30, or 60 min) or with 0.1 μg/ml LPS for 15 min. Cells were lysed, and equal amounts of lysates were subjected to Western blot analysis using antibodies specific to MAP kinases in their nonphosphorylated (ERK, p38, and JNK) and phosphorylated (p-ERK, p-p38, and p-JNK) forms. (C) BMDMs were pretreated with the MAP kinase inhibitor U0126 (ERK inhibitor; 1 or 5 μM), SB (p38 inhibitor; 2.5 or 10 μM), or SP (JNK inhibitor; 5 or 10 μM) for 1 h and then stimulated with 100 μg/ml PGA for an additional 24 h. TNF-α concentrations were measured by ELISA. (D) BMDMs were stimulated with 100 μg/ml PGA for the indicated times (0, 5, 15, 30, or 60 min). Cells then were lysed, and lysates were subjected to Western blot analysis to determine levels of IκB-α protein. (E) BMDMs were left untreated or were treated with 100 μg/ml PGA for 1 h and then stained with anti-p65- and Alexa Fluor 488-conjugated secondary antibody. Fluorescence images were obtained by confocal microscopy. (F) BMDMs were pretreated with parthenolide (5 or 10 μM) for 1 h and then stimulated with 100 μg/ml PGA for an additional 24 h. TNF-α concentrations were measured by ELISA. Data are the mean values ± SD from triplicate samples. Statistical significance was examined using one-way ANOVA followed by a Tukey's post hoc test. **, P < 0.01 compared with PGA alone. The results shown are representative of three separate experiments. ND, not detected.
Because MAP kinase pathways play a pivotal role in TLR2-mediated inflammatory responses (17, 34), we examined whether PGA can induce activation of the MAP kinases ERK, p38, and JNK in TLR2-WT and TLR2-KO BMDMs by Western blot analysis using LPS as a control. PGA induced phosphorylation of all three MAP kinase isoforms with maximal activation after 15 min of stimulation in TLR2-WT BMDMs, but these enhancements were not seen in TLR2-KO BMDMs (Fig. 5B). Unlike PGA, LPS induced phosphorylation of these three MAP kinases after 15 min of stimulation in TLR2-KO BMDMs (Fig. 5B). To further confirm the involvement of MAP kinase subtypes on PGA-induced inflammatory responses, BMDMs were pretreated with inhibitors of ERK (U0126), p38 (SB), or JNK kinases (SP) for 1 h and then stimulated with PGA for an additional 24 h. As shown in Fig. 5C, all three MAP kinase inhibitors significantly attenuated PGA-induced TNF-α secretion in BMDMs, indicating that MAP kinases play an important role in PGA-induced cytokine production.
We next examined the effect of PGA on NF-κB activation by Western blotting and confocal microscopy analysis in BMDMs. Stimulation of cells with PGA enhanced maximal degradation of I-κB-α, which inhibits nuclear translocation of the NF-κB transcription factor, after stimulation for 15 min (Fig. 5D) and subsequently induced nuclear translocation of NF-κB p65 after 1 h of stimulation (Fig. 5E). Additionally, PGA-induced TNF-α production was abrogated by pretreatment with the NF-κB inhibitor parthenolide (Fig. 5F). Taken together, these results indicate that MAP kinase and NF-κB signaling pathways are crucially involved in PGA-induced inflammatory responses.
DISCUSSION
The PGA capsule is considered a major virulence factor of B. anthracis because of its antiphagocytic activity (7, 8). However, the mechanisms underlying the immune stimulatory activity of PGA have remained largely unknown. Using TLR2-WT and TLR2-KO BMDMs, we demonstrated that PGA induced inflammatory responses through TLR2, a pattern recognition receptor that plays a pivotal role in the host immune response to invading pathogens. In addition, we showed that PGA required TLR6, but not TLR1, to initiate inflammatory cytokine production. Furthermore, CD14, an accessory molecule required for TLR-mediated signaling, appeared to be involved in the PGA-mediated inflammatory response. Among various signaling molecules, MyD88, MAP kinase, and NF-κB signaling pathways were involved in PGA-mediated immune responses. These results suggest that PGA stimulates the innate immune system through TLR2/6/CD14/MyD88-dependent signaling pathways.
TLR2 appears to be required for PGA-induced innate immune responses, because PGA directly stimulated TLR2 followed by production of TNF-α and IL-6, which did not occur in TLR2-deficient macrophages. TLR2 plays an important role in B. anthracis-mediated innate immunity. Hughes et al. reported that TLR2 plays a pivotal role in recognition of B. anthracis and the subsequent cytokine responses (35). TLR2 is involved in CD14-dependent Mac-1 activation and spore internalization by macrophages (36). In addition, anthrax cell wall components trigger inflammatory responses via TLR2/TLR6 heterodimers (37). In accordance with our results, Broos et al. showed that PGA nanoparticles induce dendritic cell maturation, and this response is attenuated by preincubation with TLR2 blocking antibody (38). Furthermore, Weiss et al. showed that PGA induces TLR2-dependent cytokine production using macrophages from hamster strains with or without functional TLR2 (39). These results suggest that PGA is a functional TLR2 ligand.
TLR2 recognizes lipidated antigens, including lipoproteins, LTA, lipomannans, and certain lipoarabinomannans (18). Here, we showed that PGA, which is a nonlipidated molecule, stimulated TLR2. Several lines of evidence have shown that nonlipidated antigens also can stimulate TLR2. Drage et al. showed that the Mycobacterium tuberculosis lipoprotein LprG retains TLR2-stimulating activity regardless of N-terminal lipidation (40). Wang et al. demonstrated that the zwitterionic charge motif of Bacteroides fragilis capsular polysaccharides is important for TLR2-agonist activity (41). Similarly, peptide sequences also affect TLR2-stimulating activity (42). Furthermore, nonlipidated intracellular proteins such as heat shock proteins 60 and 70 stimulate TLR2, although heat shock protein 70 also activates TLR4 (43, 44).
Immunopathological studies with a particular cellular component of pathogens need special care for purity of the tested components, because many different cellular components can elicit the same biological effects. The immunostimulatory activity of PGA observed in this study is unlikely to be due to contamination of other bacterial cellular components, such as LPS, lipoproteins, LTA, and peptidoglycan. When we tested for LPS contamination in PGA preparations using an endotoxin assay kit, the levels of endotoxin, a well-known TLR4 ligand, were very low (0.1 EU/ml). Furthermore, cytokine production and MAP kinase activation by PGA were almost completely abolished in TLR2-KO BMDMs but not in TLR4-KO BMDMs. Additionally, PGA specifically enhanced NF-κB activation in a TLR2-dependent manner, indicating that purified PGA did not contain LPS. In addition, NMR analysis of purified PGA showed a conventional peak pattern of γ-PGA without additional peaks. Treatment of HF and lipase, which are known to deactivate lipoproteins and LTA, did not significantly affect PGA-induced cytokine production. Furthermore, digestion of the purified PGA with proteinase K did not influence PGA-induced TNF-α production, implying that purified PGA did not contain lipoproteins. Moreover, stimulation of NOD2-WT and NOD2-KO macrophages with purified PGA did not induce any significant change in TNF-α production, demonstrating that PGA-mediated cytokine production is not due to peptidoglycan contamination.
We conducted experiments with B. licheniformis PGA as a surrogate for B. anthracis PGA. It has been known that the B. anthracis capsule is composed of only d-glutamate, while the B. licheniformis capsule contains both d and l enantiomers on the surface (45). Thus, we maximized the production of the d-glutamate capsule by B. licheniformis using E medium. The PGA from B. licheniformis, which is a nonpathogenic bacterium treated in a biosafety level 1 facility, has been used as a surrogate for PGA from B. anthracis in several studies, including ours (13, 27, 46, 47). In addition, it has been known that B. anthracis PGA capsule was first polymerized on the cell surface to the high-molecular-mass form (>100 kDa) and then degraded by capsule depolymerase to the lower-molecular-mass capsule, concomitantly releasing from the cell surface (48). Thus, we fragmented the purified B. licheniformis PGA (∼500 kDa) to the molecular mass of <50 kDa using HCl. Although PGA from B. licheniformis was used in this study, further study using highly purified B. anthracis PGA will be required to confirm our conclusions.
Although we used high concentrations of PGA (60 to 100 μg/ml) to examine the immunostimulatory activity of PGA compared with that of other PAMP, these concentrations could be relevant, because high levels of serum PGA approaching 1 mg/ml were observed in B. anthracis infection models (49). Moreover, local concentrations may be much higher than those in serum, because specific tissues such as liver appear to be primary tissue depots for PGA (49).
Specific recognition of bacterial components through TLR2 occurs via heterodimerization with either TLR1 or TLR6. Triacylated lipoproteins, lipomannan, and meningococcal PorB are recognized by TLR1/TLR2 (50), whereas diacylated lipoproteins, LTA, and Shigella dysenteriae porin are recognized by TLR2/TLR6 (51). Here, we showed that TLR6 in conjunction with TLR2 is critically involved in the PGA-induced immune response. TLR6 plays an important role in the host immune response to invading pathogens or PAMPs. Blocking of TLR6 with a transmembrane domain-derived peptide significantly attenuates S. aureus LTA-induced cytokine production but not LPS-induced cytokine production (52). In addition, TLR6 plays an important role in host innate resistance to Brucella abortus infection (53) and a protective role in Aspergillus fumigatus or house dust mite antigen-induced chronic asthma (54). Further studies are needed to clarify the role of TLR6-mediated recognition of PGA in anthrax pathogenesis.
In conclusion, the current study demonstrates that the PGA capsule is recognized by TLR2/6/CD14, and that recognition stimulates cytokine production via MyD88, MAP kinase, and NF-κB signaling pathways in murine macrophages. To the best of our knowledge, our current study is the first to demonstrate that the PGA-induced cytokine response is mediated by TLR2/6/CD14, and our findings may contribute to a better understanding of the pathogenesis of anthrax.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Han for helpful discussions.
This research was supported by the Korea National Institute of Health (grants 2007-N00357-00, 2009-N45001-00, and 2011-N45001-00 to G.R.).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00888-15.
REFERENCES
- 1.Morse SA. 2015. Pathogen security-help or hindrance? Front Bioeng Biotechnol 2:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brittingham KC, Ruthel G, Panchal RG, Fuller CL, Ribot WJ, Hoover TA, Young HA, Anderson AO, Bavari S. 2005. Dendritic cells endocytose Bacillus anthracis spores: implications for anthrax pathogenesis. J Immunol 174:5545–5552. doi: 10.4049/jimmunol.174.9.5545. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Moayeri M, Leppla SH. 2014. Anthrax lethal and edema toxins in anthrax pathogenesis. Trends Microbiol 22:317–325. doi: 10.1016/j.tim.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeon J, Kim Y, Choi M, Kim K, Lee HR, Jang J, Kim YR, Chun JH, Eo S, Kim T, Rhie GE. 2014. Bacillus anthracis genomic DNA enhances lethal toxin-induced cytotoxicity through TNF-α production. BMC Microbiol 14:300. doi: 10.1186/s12866-014-0300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guichard A, Nizet V, Bier E. 2012. New insights into the biological effects of anthrax toxins: linking cellular to organismal responses. Microbes Infect 14:97–118. doi: 10.1016/j.micinf.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welkos SL, Vietri NJ, Gibbs PH. 1993. Non-toxigenic derivatives of the Ames strain of Bacillus anthracis are fully virulent for mice: role of plasmid pXO2 and chromosome in strain-dependent virulence. Microb Pathog 14:381–388. doi: 10.1006/mpat.1993.1037. [DOI] [PubMed] [Google Scholar]
- 7.Chabot DJ, Scorpio A, Tobery SA, Little SF, Norris SL, Friedlander AM. 2004. Anthrax capsule vaccine protects against experimental infection. Vaccine 23:43–47. doi: 10.1016/j.vaccine.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Makino S, Uchida I, Terakado N, Sasakawa C, Yoshikawa M. 1989. Molecular characterization and protein analysis of the cap region, which is essential for encapsulation in Bacillus anthracis. J Bacteriol 171:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scorpio A, Chabot DJ, Day WA, O'Brien DK, Vietri NJ, Itoh Y, Mohamadzadeh M, Friedlander AM. 2007. Poly-gamma-glutamate capsule-degrading enzyme treatment enhances phagocytosis and killing of encapsulated Bacillus anthracis. Antimicrob Agents Chemother 51:215–222. doi: 10.1128/AAC.00706-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scorpio A, Tobery SA, Ribot WJ, Friedlander AM. 2008. Treatment of experimental anthrax with recombinant capsule depolymerase. Antimicrob Agents Chemother 52:1014–1020. doi: 10.1128/AAC.00741-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scorpio A, Chabot DJ, Day WA, Hoover TA, Friedlander AM. 2010. Capsule depolymerase overexpression reduces Bacillus anthracis virulence. Microbiology 156:1459–1467. doi: 10.1099/mic.0.035857-0. [DOI] [PubMed] [Google Scholar]
- 12.Ezzell JW, Abshire TG, Panchal R, Chabot D, Bavari S, Leffel EK, Purcell B, Friedlander AM, Ribot WJ. 2009. Association of Bacillus anthracis capsule with lethal toxin during experimental infection. Infect Immun 77:749–755. doi: 10.1128/IAI.00764-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang J, Cho M, Chun JH, Cho MH, Park J, Oh HB, Yoo CK, Rhie GE. 2011. The poly-gamma-d-glutamic acid capsule of Bacillus anthracis enhances lethal toxin activity. Infect Immun 79:3846–3854. doi: 10.1128/IAI.01145-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medzhitov R, Janeway C Jr. 2000. Innate immunity. N Engl J Med 343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 15.Mogensen TH. 2009. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aderem A, Ulevitch RJ. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 17.Moresco EM, LaVine D, Beutler B. 2011. Toll-like receptors. Curr Biol 21:R488–R493. doi: 10.1016/j.cub.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 18.Zahringer U, Lindner B, Inamura S, Heine H, Alexander C. 2008. TLR2–promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology 213:205–224. [DOI] [PubMed] [Google Scholar]
- 19.Farhat K, Riekenberg S, Heine H, Debarry J, Lang R, Mages J, Buwitt-Beckmann U, Roschmann K, Jung G, Wiesmuller KH, Ulmer AJ. 2008. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J Leukoc Biol 83:692–701. [DOI] [PubMed] [Google Scholar]
- 20.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci U S A 97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellingsen E, Morath S, Flo T, Schromm A, Hartung T, Thiemermann C, Espevik T, Golenbock D, Foster D, Solberg R, Aasen A, Wang J. 2002. Induction of cytokine production in human T cells and monocytes by highly purified lipoteichoic acid: involvement of Toll-like receptors and CD14. Med Sci Monit 8:BR149–BR156. [PubMed] [Google Scholar]
- 22.Triantafilou M, Gamper FG, Haston RM, Mouratis MA, Morath S, Hartung T, Triantafilou K. 2006. Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J Biol Chem 281:31002–31011. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- 23.Birrer GA, Cromwick AM, Gross RA. 1994. Gamma-poly(glutamic acid) formation by Bacillus licheniformis 9945a: physiological and biochemical studies. Int J Biol Macromol 16:265–275. doi: 10.1016/0141-8130(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 24.Leonard CG, Housewright RD, Thorne CB. 1958. Effects of some metallic ions on glutamyl polypeptide synthesis by Bacillus subtilis. J Bacteriol 76:499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park OJ, Han JY, Baik JE, Jeon JH, Kang SS, Yun CH, Oh JW, Seo HS, Han SH. 2013. Lipoteichoic acid of Enterococcus faecalis induces the expression of chemokines via TLR2 and PAFR signaling pathways. J Leukoc Biol 94:1275–1284. doi: 10.1189/jlb.1012522. [DOI] [PubMed] [Google Scholar]
- 26.Medvedev AE, Henneke P, Schromm A, Lien E, Ingalls R, Fenton MJ, Golenbock DT, Vogel SN. 2001. Induction of tolerance to lipopolysaccharide and mycobacterial components in Chinese hamster ovary/CD14 cells is not affected by overexpression of Toll-like receptors 2 or 4. J Immunol 167:2257–2267. doi: 10.4049/jimmunol.167.4.2257. [DOI] [PubMed] [Google Scholar]
- 27.Cho MH, Ahn HJ, Ha HJ, Park J, Chun JH, Kim BS, Oh HB, Rhie GE. 2010. Bacillus anthracis capsule activates caspase-1 and induces interleukin-1beta release from differentiated THP-1 and human monocyte-derived dendritic cells. Infect Immun 78:387–392. doi: 10.1128/IAI.00956-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez-Camero G, Congregado F, Bou JJ, Munoz-Guerra S. 1999. Biosynthesis and ultrasonic degradation of bacterial poly(gamma-glutamic acid). Biotechnol Bioeng 63:110–115. [PubMed] [Google Scholar]
- 29.Seo HS, Nahm MH. 2009. Lipoprotein lipase and hydrofluoric acid deactivate both bacterial lipoproteins and lipoteichoic acids, but platelet-activating factor-acetylhydrolase degrades only lipoteichoic acids. Clin Vaccine Immunol 16:1187–1195. doi: 10.1128/CVI.00115-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 31.Volz T, Nega M, Buschmann J, Kaesler S, Guenova E, Peschel A, Rocken M, Gotz F, Biedermann T. 2010. Natural Staphylococcus aureus-derived peptidoglycan fragments activate NOD2 and act as potent costimulators of the innate immune system exclusively in the presence of TLR signals. FASEB J 24:4089–4102. doi: 10.1096/fj.09-151001. [DOI] [PubMed] [Google Scholar]
- 32.Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, Lee JO. 2007. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. 1999. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol 163:1–5. [PubMed] [Google Scholar]
- 34.Kawai T, Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 35.Hughes MA, Green CS, Lowchyj L, Lee GM, Grippe VK, Smith MF Jr, Huang LY, Harvill ET, Merkel TJ. 2005. MyD88-dependent signaling contributes to protection following Bacillus anthracis spore challenge of mice: implications for Toll-like receptor signaling. Infect Immun 73:7535–7540. doi: 10.1128/IAI.73.11.7535-7540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliva C, Turnbough CL Jr, Kearney JF. 2009. CD14-Mac-1 interactions in Bacillus anthracis spore internalization by macrophages. Proc Natl Acad Sci U S A 106:13957–13962. doi: 10.1073/pnas.0902392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Triantafilou M, Uddin A, Maher S, Charalambous N, Hamm TS, Alsumaiti A, Triantafilou K. 2007. Anthrax toxin evades Toll-like receptor recognition, whereas its cell wall components trigger activation via TLR2/6 heterodimers. Cell Microbiol 9:2880–2892. doi: 10.1111/j.1462-5822.2007.01003.x. [DOI] [PubMed] [Google Scholar]
- 38.Broos S, Lundberg K, Akagi T, Kadowaki K, Akashi M, Greiff L, Borrebaeck CA, Lindstedt M. 2010. Immunomodulatory nanoparticles as adjuvants and allergen-delivery system to human dendritic cells: implications for specific immunotherapy. Vaccine 28:5075–5085. doi: 10.1016/j.vaccine.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Weiss S, Levy H, Fisher M, Kobiler D, Altboum Z. 2009. Involvement of TLR2 in innate response to Bacillus anthracis infection. Innate Immun 15:43–51. doi: 10.1177/1753425908100379. [DOI] [PubMed] [Google Scholar]
- 40.Drage MG, Tsai HC, Pecora ND, Cheng TY, Arida AR, Shukla S, Rojas RE, Seshadri C, Moody DB, Boom WH, Sacchettini JC, Harding CV. 2010. Mycobacterium tuberculosis lipoprotein LprG (Rv1411c) binds triacylated glycolipid agonists of Toll-like receptor 2. Nat Struct Mol Biol 17:1088–1095. doi: 10.1038/nsmb.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Q, McLoughlin RM, Cobb BA, Charrel-Dennis M, Zaleski KJ, Golenbock D, Tzianabos AO, Kasper DL. 2006. A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J Exp Med 203:2853–2863. doi: 10.1084/jem.20062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Epelman S, Stack D, Bell C, Wong E, Neely GG, Krutzik S, Miyake K, Kubes P, Zbytnuik LD, Ma LL, Xie X, Woods DE, Mody CH. 2004. Different domains of Pseudomonas aeruginosa exoenzyme S activate distinct TLRs. J Immunol 173:2031–2040. doi: 10.4049/jimmunol.173.3.2031. [DOI] [PubMed] [Google Scholar]
- 43.Ohashi K, Burkart V, Flohe S, Kolb H. 2000. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol 164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 44.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. 2002. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem 277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 45.Candela T, Fouet A. 2006. Poly-gamma-glutamate in bacteria. Mol Microbiol 60:1091–1098. doi: 10.1111/j.1365-2958.2006.05179.x. [DOI] [PubMed] [Google Scholar]
- 46.Aulinger BA, Roehrl MH, Mekalanos JJ, Collier RJ, Wang JY. 2005. Combining anthrax vaccine and therapy: a dominant-negative inhibitor of anthrax toxin is also a potent and safe immunogen for vaccines. Infect Immun 73:3408–3414. doi: 10.1128/IAI.73.6.3408-3414.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang J, Cho M, Lee HR, Cha K, Chun JH, Hong KJ, Park J, Rhie GE. 2013. Monoclonal antibody against the poly-gamma-d-glutamic acid capsule of Bacillus anthracis protects mice from enhanced lethal toxin activity due to capsule and anthrax spore challenge. Biochim Biophys Acta 1830:2804–2812. doi: 10.1016/j.bbagen.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Makino S, Watarai M, Cheun HI, Shirahata T, Uchida I. 2002. Effect of the lower molecular capsule released from the cell surface of Bacillus anthracis on the pathogenesis of anthrax. J Infect Dis 186:227–233. doi: 10.1086/341299. [DOI] [PubMed] [Google Scholar]
- 49.Kozel TR, Murphy WJ, Brandt S, Blazar BR, Lovchik JA, Thorkildson P, Percival A, Lyons CR. 2004. mAbs to Bacillus anthracis capsular antigen for immunoprotection in anthrax and detection of antigenemia. Proc Natl Acad Sci U S A 101:5042–5047. doi: 10.1073/pnas.0401351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Massari P, Visintin A, Gunawardana J, Halmen KA, King CA, Golenbock DT, Wetzler LM. 2006. Meningococcal porin PorB binds to TLR2 and requires TLR1 for signaling. J Immunol 176:2373–2380. doi: 10.4049/jimmunol.176.4.2373. [DOI] [PubMed] [Google Scholar]
- 51.Biswas A, Banerjee P, Mukherjee G, Biswas T. 2007. Porin of Shigella dysenteriae activates mouse peritoneal macrophage through Toll-like receptors 2 and 6 to induce polarized type I response. Mol Immunol 44:812–820. doi: 10.1016/j.molimm.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 52.Fink A, Reuven EM, Arnusch CJ, Shmuel-Galia L, Antonovsky N, Shai Y. 2013. Assembly of the TLR2/6 transmembrane domains is essential for activation and is a target for prevention of sepsis. J Immunol 190:6410–6422. doi: 10.4049/jimmunol.1202033. [DOI] [PubMed] [Google Scholar]
- 53.de Almeida LA, Macedo GC, Marinho FA, Gomes MT, Corsetti PP, Silva AM, Cassataro J, Giambartolomei GH, Oliveira SC. 2013. Toll-like receptor 6 plays an important role in host innate resistance to Brucella abortus infection in mice. Infect Immun 81:1654–1662. doi: 10.1128/IAI.01356-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moreira AP, Cavassani KA, Ismailoglu UB, Hullinger R, Dunleavy MP, Knight DA, Kunkel SL, Uematsu S, Akira S, Hogaboam CM. 2011. The protective role of TLR6 in a mouse model of asthma is mediated by IL-23 and IL-17A. J Clin Investig 121:4420–4432. doi: 10.1172/JCI44999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.