Abstract
In the present study, human atherosclerotic carotid arteries were examined following endarterectomy for the presence of the Gram-positive bacterium Propionibacterium acnes and its potential association with biofilm structures within the arterial wall. The P. acnes 16S rRNA gene was detectable in 4 of 15 carotid artery samples, and viable P. acnes was one among 10 different bacterial species recoverable in culture. Fluorescence in situ hybridization analysis of 5 additional atherosclerotic carotid arteries demonstrated biofilm bacteria within all samples, with P. acnes detectable in 4 samples. We also demonstrated that laboratory-grown cultures of P. acnes biofilms were susceptible to induction of a biofilm dispersion response when challenged with physiologically relevant levels of norepinephrine in the presence of iron-bound transferrin or with free iron. The production and release of lipolytic and proteolytic extracellular enzymes by P. acnes were shown to increase in iron-induced dispersed biofilms, and these dispersion-induced P. acnes VP1 biofilms showed increased expression of mRNAs for the triacylglycerol lipases PPA2105 and PPA1796 and the hyaluronate lyase PPA380 compared to that in untreated biofilms. These results demonstrate that P. acnes can infect the carotid arteries of humans with atherosclerosis as a component of multispecies biofilms and that dispersion is inducible for this organism, at least in vitro, with physiologically relevant levels of norepinephrine resulting in the production and release of degradative enzymes.
INTRODUCTION
Atherosclerosis, or hardening of arteries, is one of the principal predisposing factors for heart attack and stroke in the United States, Europe, and Japan (1, 2). When atherosclerosis develops, the arterial walls thicken and lose elasticity due to the buildup of plaque deposits within the wall. Eventually, this condition may result in rupture of the overlying fibrous cap, which can lead to the development of a thrombosis or blood clot within the circulation (2). The progression of atherosclerosis is widely believed to be driven by increased levels of low-density lipoprotein (LDL) cholesterol in the blood (3), and this disease has been correlated with apolipoprotein E deficiency in mice (4). Over the past 20 years, research has shown that in addition to LDL/cholesterol, arterial plaque deposits typically contain infecting bacteria or signature prokaryotic biomarkers (2, 5–12). Previous work in our laboratory has shown that bacteria within carotid arterial plaques are present as biofilm infections and that at least one species of bacteria, Pseudomonas aeruginosa, which was commonly observed in the carotid arterial plaque samples we analyzed, undergoes a biofilm dispersion response when challenged in vitro with physiologically relevant levels of the catechol hormone norepinephrine in the presence of the iron chelator transferrin (13).
In the present study, we analyzed atherosclerotic human carotid arteries for the presence of Propionibacterium acnes, a non-spore-forming, anaerobic, Gram-positive bacillus that is often found as a member of the cutaneous and conjunctival microbiota of humans (14) and has been associated with pulmonary infections, endocarditis, and other intravascular infections (15–17) as well as with atherosclerotic plaque deposits (18, 19). P. acnes has been shown to form pathogenic biofilms on biological materials, such as joint prostheses (20), catheters (21), and aortic valve implants (22). We screened 15 atherosclerotic carotid arteries for the Propionibacterium 16S rRNA gene and an additional 10 arteries for P. acnes organisms recoverable in culture in reinforced clostridial medium (RCM). Fluorescence in situ hybridization (FISH) was performed on a further 5 atherosclerotic carotid arteries to determine the location of P. acnes within carotid arterial plaque deposits and to assess the degree of involvement of P. acnes within the biofilm infections present.
One of the hallmarks of biofilm growth is the ability of biofilm bacteria to disperse, a bacterial response that requires the release of extracellular lytic enzymes, which may have the potential to damage surrounding tissues in the area of the plaque lesion (23–28). We investigated whether P. acnes would undergo a dispersion response in vitro when norepinephrine was added to biofilms of the organism grown in pure culture in the presence of iron-bound transferrin. To test for the presence of lytic enzymes released as a result of iron-inducible dispersion, we performed tributyrin degradation assays for the detection of lipases and casein degradation assays for the detection of proteolysis following exposure to increased iron in the medium. The transcriptional levels of lipase and hyaluronate lyase genes were assessed using quantitative PCR (qPCR) analysis of iron-treated biofilms. The Propionibacterium acnes strains used in this study were VP1 (ATTC 6919) and the clinical isolate hdn-1 (which was recovered and isolated from a human atherosclerotic carotid artery in this study).
MATERIALS AND METHODS
Bacterial strains and media.
Propionibacterium acnes clinical isolate hdn-1 and strain VP1 (ATTC 6919) were grown directly from carotid artery samples or from frozen stocks in the presence of 5% CO2 at 37°C, in full-strength or 1:5 RCM (Becton, Dickinson & Co., Sparks, MD) containing 0.1% sodium thioglycolate (Sigma), unless otherwise indicated. For RNA analyses, P. acnes strain VP1 was cultured in reinforced clostridium broth (without agar) (HiMedia, Mumbai, India). Biofilm cultures of P. acnes were prepared from frozen stocks in full-strength RCM and precultured for 48 h at 37°C, followed by a 1:20 inoculation into 1:5 RCM under microaerophilic conditions within a CO2 incubator set to 5% CO2.
Carotid artery specimens and sampling.
A total of 30 carotid arteries from patients with advanced atherosclerosis were used in the present study. These were subdivided into three groups: groups A, B, and C. Group A comprised patient samples 1 to 15, which were used for 16S rRNA gene extraction and amplification. Group B comprised patient samples 16 to 25, which were used for separation of bacteria from host tissues and for cultivation of viable bacteria in RCM. Group C comprised patient samples 26 to 30, which were used for FISH. Endarterectomy samples from patients with advanced atherosclerosis were acquired under aseptic conditions at UHS Wilson Hospital (Johnson City, NY), transferred to sterile saline solution upon removal, and stored at 4°C. All samples were transported to Binghamton University within 3 h of removal from patients and processed within 12 h. No patient data were collected for the present study; therefore, the overall health, personal histories, and postoperative outcomes of patients involved in this study are not reported. This project was IRB approved by United Health Services, Binghamton, NY (approval file number IRB00003573).
DNA extraction from atherosclerotic arteries.
Carotid artery explants from group A (patients 1 to 15) were processed in our laboratory by grinding subsamples under aseptic conditions in a laminar flow hood, using an autoclave-sterilized mortar and pestle, until no intact tissue remained in 400 μl of extraction buffer (0.2 M NaCl, 50 mM Tris, pH 8.0, 0.2 mM EDTA, 0.5% SDS) for each 0.1 g of tissue. Following extraction, samples were aseptically transferred to sterile microcentrifuge tubes. To protect DNA, 2.0 μl proteinase K (20 mg/ml) was added and mixed well. The solution was incubated at 37°C for 30 min, added to 400 μl of phenol, mixed by inversion for 30 s, and centrifuged for 5 min at 16,000 × g. The upper phase was transferred to a clean tube, reextracted with 400 μl of phenol-chloroform (50:50) followed by 400 μl of chloroform, and mixed 10:1 with 3 M sodium acetate, pH 4 to 5, and an equal volume of isopropanol. The solution was mixed gently and stored at −20°C for 15 h. The solution was centrifuged for 10 min at 4°C and 16,000 × g, and the supernatant was discarded. The DNA pellet was washed in 200 μl of 75% ethanol (EtOH), centrifuged at 10,000 × g for 2 min, and removed. The pellet was allowed to dry for 5 min, dissolved in 30 μl of Tris-EDTA (TE) buffer, and stored at −20°C until further processing.
Propionibacterium PCR and sequencing.
Amplification of the Propionibacterium 16S rRNA gene was carried out using the primers Propionibacterium-F (5′-GTGCTTAACACATGCAAGTCG-3′) and bak4 (5′-AGGAGGTGATCCAACCGCA-3′) (29). DNAs were extracted from 0.1% agarose gels containing samples yielding positive bands for the Propionibacterium 16S rRNA gene by using the Promega Wizard SV gel and PCR clean-up system (Madison, WI). A set of nested primers, Prop-F-Nested (5′-GCCTGAGAGGGTGACCGG-3′) and Prop-R-Nested (5′-GTCAACCCGTATCGAAAGCA-3′), was used to reamplify the DNA. Reamplified DNA was extracted, and the DNA concentration was analyzed using a Tecan Infinite M200 Pro instrument (Mannedorf, Switzerland). Sequencing was performed using 8 pM Prop-Sequencing primer (5′-GATGCAGCAACGCCGCGTG-3′) and sent to the Cornell University sequencing facilities (Ithaca, NY).
Bacterial extraction and growth from atherosclerotic carotid arteries.
Arteries from group B (patients 16 to 25) were screened for viable bacterial cells recoverable in culture. Approximately 1.0-g samples of explanted atherosclerotic carotid arteries were cut into smaller pieces and transferred to a sterile tissue grinder (VWR). A total of 2.5 ml of 1× phosphate-buffered saline (PBS) was added to the tissue grinder. The tissue was ground to a pink paste and transferred to a sterile petri dish. Full-strength and 1:5 RCM broths were inoculated with the paste (1:100 inoculation) and incubated at 37°C and 5% CO2 for 7 days or until visible growth was present. In addition, full-strength RCM agar plates were spread with 150 μl of the paste and incubated under identical conditions. As a control, the saline in which the atherosclerotic arteries were stored during transport to the laboratory was also used as an inoculum in 1:5 RCM and was placed under microaerophilic conditions at 37°C. All tubes inoculated with saline were negative for growth.
DNA sequencing of viable bacteria.
Broth cultures were inoculated onto three full-strength RCM agar plates by using the streak-plate technique and were incubated under anaerobic (saturated atmospheric CO2, using a BD GasPak EZ system), microaerophilic (5% CO2), and aerobic (standard atmospheric) conditions at 37°C. Streak plates were made from individual colonies and incubated under the above-described oxygen conditions, and isolated colonies were then used to inoculate 20 ml of full-strength RCM and incubated at 37°C and 5% CO2. After 2 days of growth, DNA was extracted using an UltraClean microbial DNA isolation kit (MoBio Laboratories Inc., Carlsbad, CA). PCR mixtures to amplify the V2-V3 regions of the 16S rRNA gene were set up using primers 16S-Forward (5′-AGAGTTTGATCCTGGCTCAG-3′) and HDA2 (5′-GTATTACCGCGGCTGCTGGCAC-3′) (30). Reactions were performed in 0.2-ml PCR tubes, using nanopure water (6.2 μl), dimethyl sulfoxide (DMSO) (1.0 μl), 10× PCR buffer (Invitrogen) (1.0 μl), 25× MgSO4 solution (Invitrogen) (0.4 μl), both the 16S-Forward and HDA2 primers (0.1 μl of each), deoxynucleoside triphosphates (dNTPs) (0.2 μl), high-fidelity Taq polymerase (Invitrogen) (0.04 μl), and extracted genomic DNA (1.0 μl). Following electrophoresis, 16S rRNA gene bands were excised and eluted from the gel by using the Promega Wizard SV gel and PCR clean-up system (Madison, WI). The concentration of DNA was analyzed using a Tecan Infinite M200 Pro machine (Mannedorf, Switzerland). Sequencing using 8 pM HDA2 primer was performed at the Cornell University sequencing facilities (Ithaca, NY).
Preparation of arterial plaque samples for fluorescence in situ hybridization.
Sections of carotid arterial plaques from group C (patients 26 to 30) were removed using a sterile razor, embedded in Optimal Cutting Temperature cryo-embedding solution, and maintained at −20°C. Transverse 25-μm sections were obtained using a Leica CM 1850 cryostat (Houston, TX) and were stored on precleaned microscope slides at −20°C.
FISH.
FISH was performed on thin sections of carotid arterial tissue samples by using the following probes: a CAL Fluor Red 590-tagged universal EUB338 probe, a Quasar 570-tagged nonsense EUB338 probe, and a Quasar 670-tagged P. acnes 23S rRNA gene probe (5′-GAGTGTGTGAACCGATCATGTAGTAGGCAA-3′) (31). Probes were purchased from Biosearch Technologies, Novato, CA. Prior to hybridization, tissue samples were incubated in 10% formalin (Macron Chemicals, Phillipsburg, NJ) at 37°C for 2 h in frame-seal incubation chambers (Bio-Rad, Hercules, CA). After incubation, tissues were washed with 1× PBS followed by 10 mM Tris (pH 7.5) and then reincubated in 10 mM Tris with 1 mg/ml lysozyme (Amresco, Solon, OH) and 30 U/ml of achromopeptidase (Sigma, Switzerland) for 25 min at 37°C. Samples were washed for 5 min in 10 mM Tris, pH 7.5, and dehydrated in an ethanol series (30, 70, and 100% ethanol in 10 mM Tris, pH 7.5) prior to hybridization. The probe (final concentration, 125 nM) was added to the hybridization buffer (50% formamide, 2× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 10% dextran sulfate, 0.1% sodium dodecyl sulfate, 1× Denhardt's solution, 40 mM sodium phosphate, pH 7). A total of 70 μl was added to the tissue within the incubation chamber and allowed to incubate for 5 min at 75°C, followed by overnight incubation at 37°C (31).
After incubation, 1 ml of wash buffer (1× SSC, 10% formamide in nuclease-free water) was added to the slides and incubated at 37°C for 15 min. After 15 min, an additional 1.0 ml of wash buffer was added and allowed to incubate for 15 min at 37°C. Glox antifade mounting solution was added to the slide (Biosearch Technologies, Novato, CA), followed by a clean coverslip. The slides were stored at 4°C until used for analysis. A Leica TCS SP5 confocal laser scanning microscope (CLSM; Leica, Solms, Germany) was used to detect all three probes, using the HeNe 1-mW (543 nm), DPSS 20-mW (561 nm), and HeNe 10-mW (633 nm) lasers.
Biofilm dispersion assay.
Sterile 24-well plates (Nalge Nunc International, Rochester, NY) were inoculated with 500 μl of 1:20 P. acnes VP1 in 1:5 RCM and incubated at 37°C for 48 h under microaerophilic conditions. Medium in the wells was exchanged with 250 μl of fresh sterile medium at 48, 72, 96, 120, 132, 144, and 156 h. The assay was performed at 159 h. At the time of the assay, medium containing planktonic bacterial cells was removed from the wells, and the wells were washed with 0.85% saline. The wash medium was exchanged with fresh medium supplemented with 0.1 or 0.5 mM Fe2+ and incubated under microaerophilic conditions at 37°C for 1 h. Dispersion was determined by recording the optical density at 595 nm (OD595) of released bacteria following transfer of culture supernatants to optically clear microtiter plates, using a Fisher Scientific multiscan MCC instrument (Thermo Electron Corporation, Vantaa, Finland).
Transferrin/norepinephrine dispersion assays were carried out with P. acnes VP1 and hdn-1, using the same protocol, under microaerophilic conditions at 37°C. All media used for transferrin/norepinephrine dispersion assays contained 0.5 g/liter bovine holo-transferrin (MP Biomedicals, Solon, OH). Instead of being treated with increasing levels of Fe2+, the medium was supplemented with 0.4 mM norepinephrine at 159 h (Sigma, St. Louis, MO). All subsequent steps were performed as described above. In addition to obtaining the optical densities of the culture supernatants, culture techniques on RCM agar were used to obtain the numbers of CFU per milliliter of dispersed cells for treated and untreated biofilms.
Biofilm harvesting from tube reactors for qPCR and enzyme activity assays.
Continuous-flow biofilm reactors were configured with 4 oxygen-impermeable Norprene tubes in parallel, with an internal diameter of 1.6 mm and a length of 32 in. (size 14; Masterflex). RCM was pumped through the tubing via a Masterflex 8-roller-head pump to a closed effluent medium reservoir. The assembled system was sterilized by autoclaving prior to inoculation. Tubes were inoculated with 3 ml of planktonic P. acnes VP1 culture grown in full-strength RCM, injected by syringe through rubber septa into the lumen of the tubing. Cells were allowed to attach for 24 h before the flow of 1:5 RCM (2.5 ml/h) was initiated. The residence time in the tubing was 38.7 min, allowing only attached organisms to be retained in the tubing (32). Biofilms were cultured on the interior surfaces of the Norprene tubing in a 5% CO2 incubator at 37°C prior to harvesting. P. acnes dispersion within tube reactors was carried out by the addition of free iron (through ferrous sulfate), as we had previously established that an increase in free iron (resulting from the interaction of norepinephrine and iron-bound transferrin) induces a dispersion response in P. aeruginosa (13).
Extracellular protein harvesting and extracellular enzyme activity assays.
The absorbance values for the effluents from iron-treated and untreated biofilm tube reactors were obtained in order to calculate the numbers of CFU per milliliter. Protein extracts of these samples were prepared by centrifugation at 10,800 × g for 5 min at 4°C (Sorvall Legend XTR centrifuge; Thermo Scientific, Asheville, NC), followed by syringe filtration (0.2-μm Supor membrane; Pall Life Sciences, Port Washington, NY) to remove cell material. Volumes were adjusted to equalize the numbers of CFU per milliliter for both samples. Samples were frozen at −20°C and lyophilized (Freezone 2.5-liter freeze-dry system; Labconco, Kansas City, MO).
Protein extracts were resuspended in 1 ml of ice-cold 1× TE buffer and split into two 500-μl samples. Lipase activity was determined using a protein extract to which 56 μl of phenylmethylsulfonyl fluoride (PMSF; 3 mg in 1 ml of EtOH) was added per 500 μl of sample in order to inhibit protease activity. A total volume of 160 μl of protein extract containing PMSF was added to wells in tributyrin agar (23 g tributyrin HiVeg agar base [HiMedia, Mumbai, India] and 10 ml tributyrin [Acros Organics, Belgium] in 990 ml water). Additionally, 160 μl of the protein extract not containing PMSF was added to wells in milk agar (50 g skim milk [Becton Dickinson, NJ] and 15 g Bacto agar [Becton Dickinson, NJ] in 1.0 liter of water). The plates were sealed with Parafilm and allowed to incubate for 24 h at 37°C. After 24 h, wells were inspected for the volume of agar cleared. Images were acquired and analyzed using ImageJ software. All assays were performed in triplicate.
qPCR analysis of virulence factors.
After 7 days of biofilm growth in tube reactors under continuous-flow conditions, 1:5 RCM was supplemented with 0.5 mM iron by addition of ferrous sulfate (FeSO4·7H2O). Iron-supplemented RCM was allowed to flow through the system for 38 min, and then the continuous flow was turned off and tubes were clamped off upstream from the inoculation septa and maintained under static conditions for 12 min. Biofilm reactor effluent and biofilm mass attached to the walls were rolled out into 50-ml Falcon tubes containing 7.5 ml of RNAprotect bacterial reagent (Qiagen, Limburg, Netherlands) and placed immediately on ice.
Samples were centrifuged at 10,000 × g at 4°C for 5 min (Sorvall Legend XTR centrifuge; Thermo Scientific, Asheville, NC), supernatant liquid was removed, and the bacterial pellet was resuspended in TRIzol reagent (Life Technologies, Carlsbad, CA). Both samples were sonicated at 5 W for 10 s on and 15 s off, for 4 total cycles. Following sonication, the TRIzol reagent protocol was followed for RNA isolation and precipitation.
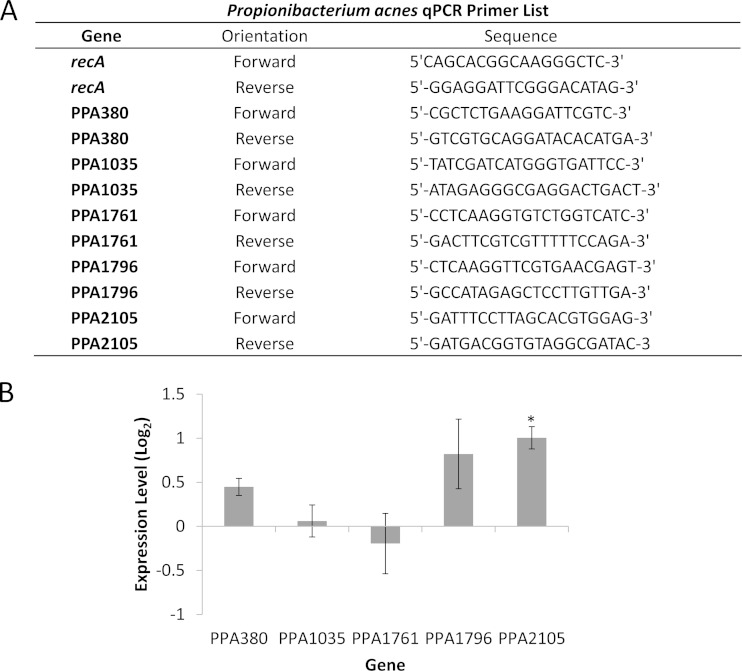
DNA was removed from the sample according to the manufacturer's specifications, using Turbo DNA-free (Life Technologies, Carlsbad, CA), and total RNA was measured using a Tecan Infinite M200 Pro instrument (Mannedorf, Switzerland). Generation of cDNA was performed with an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA), using 2 μg of RNA, according to the manufacturer's specifications. The synthesized cDNA was used for qPCR following the protocol from a Kapa SYBR Fast Universal qPCR kit (Kapa Biosystems, Wilmington, MA). qPCR was performed using an Eppendorf Realplex2 Mastercycler machine (Eppendorf, Hamburg, Germany). Primer sequences used for specific genes are provided in Fig. 6A. The RecA gene was used as the housekeeping gene for standardization.
FIG 6.
qPCR primers and mRNA levels of virulence factors produced by P. acnes biofilms challenged with an elevated concentration of free iron following 8 days of growth. (A) Primers used for qPCR analysis of P. acnes virulence factors. (B) mRNA levels in 8-day-old biofilms of P. acnes VP1 challenged with 0.5 mM iron for 50 min. Expression levels are compared to those in untreated 8-day-old P. acnes biofilms. Error bars represent ±1 standard deviation. *, statistically significant. All experiments were performed in triplicate.
RESULTS
Involvement of P. acnes in carotid arteries of patients with advanced atherosclerosis.
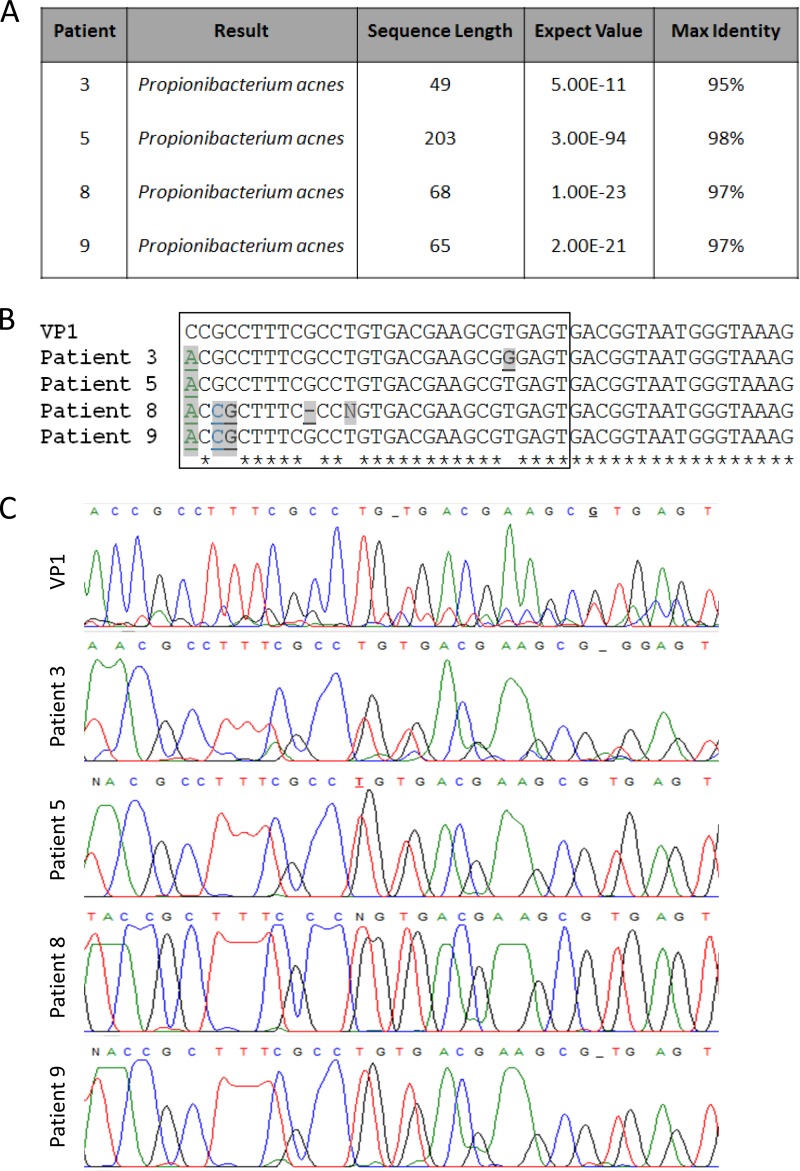
Of 15 atherosclerotic carotid arteries probed for the Propionibacterium sp. 16S rRNA gene sequence from group A (patients 1 to 15), 4 were shown to be positive (from patients 3, 5, 8, and 9). The PCR products from these positive samples were sequenced and analyzed for alignment to known bacterial 16S rRNA gene sequences by using BLAST (Basic Local Alignment Search Tool), and all 4 products aligned with the 16S rRNA gene sequence from P. acnes (Fig. 1A). We also performed a comparison of these sequences with our laboratory strain, P. acnes VP1, to ensure that we did not have contamination of our sample DNA from this source. Comparative sequence alignments are shown in Fig. 1B, and chromatograms for these sequences are shown in Fig. 1C. The first nucleotide in the alignment is the single nucleotide polymorphism (SNP) we used to establish that the recovered strains differed from the lab strain. As shown in Fig. 1C, this region of the chromatogram has low background noise, as analyzed using the software FinchTV.
FIG 1.
P. acnes sequence data from carotid artery explants from study patients. (A) BLAST results for 16S rRNA gene sequences from patients 3, 5, 8, and 9, indicating species identities, sequence lengths, expect values (numbers of hits by chance in searches of a database of a particular size), and maximum identities (percentages of similarity between the query and subject sequences over the length of the coverage area). (B) Alignment of the P. acnes VP1 16S rRNA gene sequence from our laboratory with 16S rRNA gene sequences from carotid arterial plaque samples obtained from patients with atherosclerosis. Nucleotide differences between the 4 plaque-derived samples and our laboratory strain are indicated in bold, underlined, and shaded, while nucleotides consistent among all 5 sequences are highlighted with asterisks below the alignment. (C) Chromatograms for lab strain VP1 and the 4 clinical isolates. The nucleotides included in the chromatogram are boxed in panel B. Locations where nucleotides are replaced with a dash denote a location where the software called for a base but no evidence of a base to be called was present on the chromatogram. Underlined nucleotides denote locations where the software replaced the nucleotide with “N” and the nucleotide was assigned after chromatogram analysis.
Recovery and identification of viable bacteria from atherosclerotic carotid arteries.
While recovery of the 16S rRNA genes of P. acnes and other bacteria demonstrated that this nucleic acid was present within carotid arterial plaque deposits, it did not prove the presence of viable infecting bacteria. We wished to demonstrate that cultivable bacteria, including P. acnes cells, were present within carotid arterial plaque by recovery of these cells in culture on artificial medium. From 9 of 10 carotid arteries in group B (patients 16 to 25), obtained from patients showing advanced atherosclerosis, we were able to recover 10 species of bacteria in culture on RCM. Among these 9 arterial samples, single species were recoverable from 5 arteries and two or more species were recoverable from 4 arteries. The recovered species included Rothia mucilaginosa, Rothia sp. oral clone BP1-71, Streptococcus parasanguinis, Propionibacterium acnes (4 strains), Staphylococcus sciuri, Staphylococcus caprae, Staphylococcus warneri, Staphylococcus epidermidis (2 strains), Enterococcus faecalis, and uncultured bacterial clone ncd2696d05c1. A complete list of bacteria recovered from each atherosclerotic carotid artery is shown in Table 1.
TABLE 1.
16S rRNA gene sequence data for viable bacteria recovered from carotid arterial explants from 10 study patientsa
| Patient no. | Organism | Accession no. | Sequence length (bp) | E value |
|---|---|---|---|---|
| 16 | Rothia mucilaginosa strain DY-18 | NR_074690.1 | 130 | 3.00E−47 |
| Streptococcus parasanguinis strain M143 | JQ511682.1 | 398 | 0.00 | |
| Rothia sp. oral clone BP1-71 | AB121855.1 | 465 | 0.00 | |
| 17 | Staphylococcus sciuri strain MRI493 | JQ511539.1 | 79 | 2.00E−19 |
| Propionibacterium acnes HL096PA1 | CP003293.1 | 470 | 0.00 | |
| Propionibacterium acnes clone A24-2 | GU413917.1 | 314 | 1.00E−153 | |
| 18 | Propionibacterium acnes clone A24-2 | KM099464.1 | 405 | 0.00 |
| Uncultured bacterium clone nck342d06c1 | KF110262.1 | 344 | 8.00E−177 | |
| 19 | Propionibacterium acnes hdn-1 | CP006032.1 | 473 | 0.00 |
| 20 | Staphylococcus caprae strain S3 | HG421011.1 | 509 | 0.00 |
| Staphylococcus epidermidis strain Se-C108 | KR149342.1 | 302 | 1.00E−138 | |
| 21 | Staphylococcus warneri strain S407 | AY126245.1 | 503 | 0.00 |
| 22 | Staphylococcus caprae strain S3 | HG421011.1 | 504 | 0.00 |
| 23 | Staphylococcus epidermidis strain ECNU-He1 | JN175380.1 | 435 | 0.00 |
| 25 | Enterococcus faecalis strain BalI 19 | FJ619724.1 | 397 | 0.00 |
The data give BLAST results for the V2-V3 region of the 16S rRNA genes, showing sample number, species, sequence length, and expect value (number of hits by chance in searches of a database of a particular size).
Eubacterial 16S rRNA gene and P. acnes 23S rRNA gene FISH-probed samples.
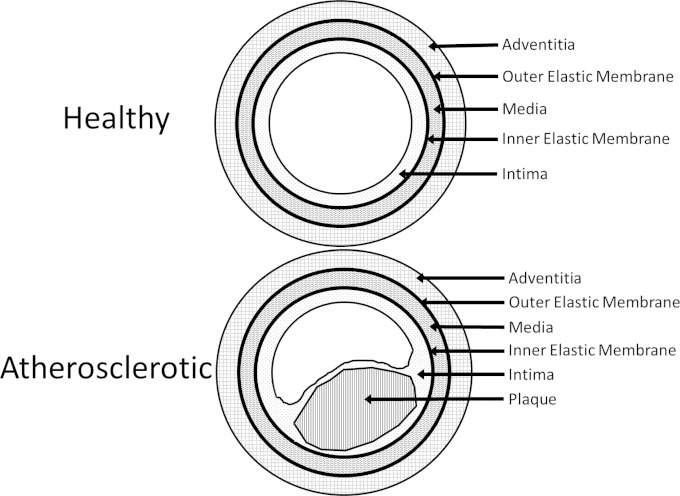
The common carotid artery, a prominent location for the development of atherosclerosis, is comprised of three major anatomical structures, each separated by an elastic membrane. The adventitia is the carotid artery's outermost tissue, distal from the blood flow and overlying the media, which contains smooth muscle. Beneath the media lies the intima, the innermost layer of the artery, which includes the endothelium that forms the wall of the arterial lumen. The external elastic membrane lies between the adventitia and the media, while the inner elastic membrane lies between the media and the intima (33). Arteriosclerosis results in distortion of the anatomy of the artery, causing the intima, which is concave in cross section, to become convex. In addition, a fibrous cap typically forms over the plaque lesion buildup and is associated with the development of an atheroma or body within the tunica intima. The anatomy of healthy and atherosclerotic arteries is shown in Fig. 2. In a previous study on human atherosclerotic carotid arteries, we demonstrated the presence of bacteria growing as biofilms, as determined by criteria established by Parsek and Singh (34). In the present study, the 5 human carotid arterial samples with advanced atherosclerosis that made up group C (patients 26 to 30) were examined. From each sample, 2 transverse slices with a 25-μm thickness were probed by FISH and observed by fluorescence microscopy. Each of the carotid arterial samples was found to be negative for the presence of the bound nonsense EUB338 16S rRNA gene probe and positive for the presence of the bound eubacterial probe (n = 5/5), and 4 of these 5 samples were observed to bind the P. acnes probe (n = 4/5), as shown in Fig. 3A. Bound probe was present throughout the 25-μm thickness of the tissue for all five samples analyzed, not solely on the surface, indicating that the bacterial biofilms were attached and embedded throughout the tissue.
FIG 2.
Anatomy of healthy and atherosclerotic carotid arteries. The three tissue layers (adventitia, media, and intima) and the elastic membranes are pointed out in both healthy and diseased arteries. Within the atherosclerotic artery, plaque buildup is also pointed out, and the consequences of this buildup on the anatomy of the artery are depicted.
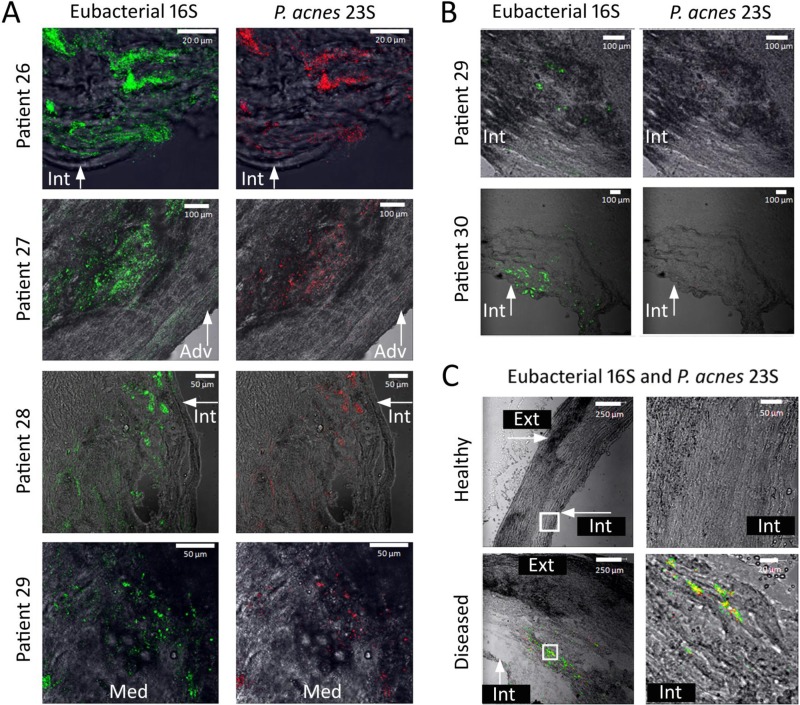
FIG 3.
Confocal laser scanning photomicrographs of FISH-probed atherosclerotic carotid arteries. (A) Locations and presence of eubacterial 16S rRNA gene- and P. acnes 23S rRNA gene-bound probes throughout atherosclerotic carotid arteries of 4 different patients. Green fluorescence indicates the presence of the bound eubacterial 16S rRNA gene probe, while red fluorescence indicates the presence of the bound P. acnes 23S rRNA gene probe. These samples contained both eubacterial 16S rRNA gene- and P. acnes 23S rRNA gene-bound probes. (B) Locations and presence of eubacterial 16S rRNA gene- and P. acnes 23S rRNA gene-bound probes throughout atherosclerotic carotid arteries of 2 different patients. Green fluorescence indicates the presence of the bound eubacterial 16S rRNA gene probe, while red fluorescence indicates the presence of the bound P. acnes 23S rRNA gene probe. These samples contained minimal to no detectable bound P. acnes 23S rRNA gene probe. (C) FISH-probed sample from patient 27, comparing a location of healthy tissue to a location where the atheroma was present. Locations examined under a higher magnification are indicated by white boxes. Green fluorescence indicates the presence of the bound eubacterial 16S rRNA gene probe, while red fluorescence indicates the presence of the bound P. acnes 23S rRNA gene probe. Healthy tissue contains no bound probe, while diseased tissue contains bound eubacterial 16S rRNA and P. acnes 23S rRNA gene probes. For all three panels, the anatomical location is indicated as follows: Int, intima; Med, media; and Adv, adventitia.
The sample from patient 26 contained extensive green fluorescence from the eubacterial 16S rRNA gene probe bound within the intima, located less than 5 μm distal from the blood-artery interface, as shown in Fig. 3A. We observed red fluorescence from the P. acnes 23S rRNA gene probe associated with all of the microcolonies. P. acnes represented approximately 45% of the total amount of detected probe, indicating that other, non-P. acnes bacteria were also present. The sample from patient 27 (Fig. 3A) contained both green fluorescence from the eubacterial 16S rRNA gene probe and red fluorescence from the P. acnes 23S rRNA gene probe and revealed the presence of microcolonies within a thickened area of the media proximal to the external elastic lamina and adventitia. The green fluorescent probe was observed throughout the external elastic lamina and into the adventitia, less than 30 μm distal from the outer surface of the artery. The P. acnes 23S rRNA gene probe was associated with microcolonies within the media in an area of damaged tissue. Red fluorescence from the P. acnes 23S rRNA gene probe appeared to comprise approximately 35% of the total amount of probe present. The sample from patient 28 (Fig. 3A) demonstrated extensive binding of the eubacterial 16S rRNA gene probe and the P. acnes 23S rRNA gene probe within an area of damaged intima and media. We observed the P. acnes 23S rRNA gene probe associated with all of the microcolonies and representing approximately 65% of the total amount of probe detected. Both the eubacterial and P. acnes probes were present less than 5 μm from the blood-artery interface, and the biofilm infection continued for more than 300 μm into the arterial wall. The sample from patient 29 (Fig. 3A) contained the eubacterial 16S rRNA gene probe and the P. acnes 23S rRNA gene probe associated with the media. The bound P. acnes 23S rRNA gene probe represented approximately 30% of the total amount of probe present.
While the images in Fig. 3A demonstrate extensive P. acnes involvement and biofilm colonization within human atherosclerotic carotid arteries, Fig. 3B demonstrates biofilm colonization with virtually no P. acnes involvement. Figure 3B depicts a micrograph for patient 29 containing the bound eubacterial 16S rRNA gene probe but no detectable P. acnes 23S rRNA gene probe within the media adjacent to the intima, approximately 400 μm distal from the blood-artery interface. This observation demonstrated that involvement of P. acnes can vary with location in the artery, as this carotid arterial sample contained extensive P. acnes involvement at other locations (Fig. 3A, patient 29). Figure 3B shows a micrograph of an arterial sample from patient 30, containing green fluorescence from the eubacterial 16S rRNA gene probe near and adjacent to the endothelial layer at the blood-artery interface. No bound red fluorescence from the P. acnes 23S rRNA gene probe was detectable in this thin section. These findings showed that P. acnes was present and extensively involved within infecting biofilms in 4 of 5 atherosclerotic carotid arteries, that its involvement was typically associated with damaged tissue, and that it could be located throughout the media and into the adventitia. Our observations also demonstrated that P. acnes is often associated with other, unidentified eubacteria in polymicrobial biofilms.
Iron-induced P. acnes hdn-1 and VP1 biofilm dispersion.
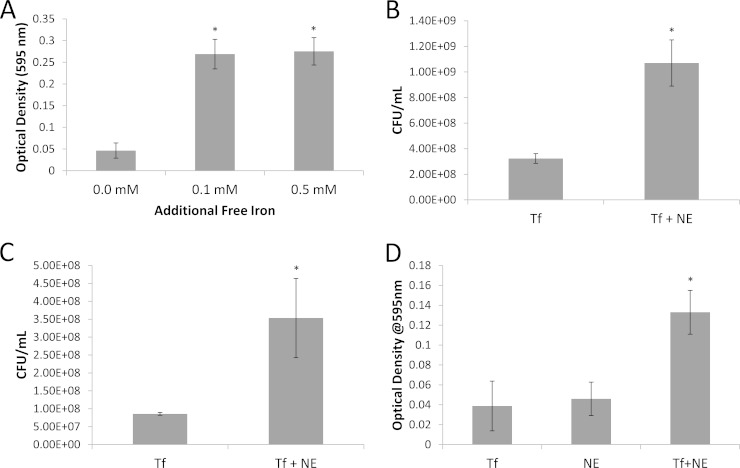
One of the principal concerns regarding the presence of biofilm bacteria within the walls of diseased atherosclerotic carotid arteries is whether these bacteria have the potential to play a role in the stability of the arterial lesion. Biofilm dispersion events are crucial to the biofilm life cycle, because dispersion allows bacteria to escape overcrowding and to colonize new locations within a host. In order for bacteria to become released from their attachments within a biofilm matrix, dispersion events must presumably be coordinated with the release of extracellular degradative enzymes (23–28). We postulate that these enzymes have the potential to cause collateral damage to the surrounding host tissues and may possibly contribute to arterial rupture. Our previous work showed P. aeruginosa biofilms to be involved in carotid arterial plaque deposits, which could be induced to disperse when challenged with free iron in laboratory-grown cultures (13). In the current work, we observed 4 of 5 atheromas to bind the P. acnes 23S rRNA gene probe in a manner characteristic of biofilm colonization, and we wished to determine whether biofilms formed by P. acnes could also undergo a dispersion response when exposed in vitro to a sudden increase in Fe2+. To test if this phenomenon was possible, we grew mature biofilms of P. acnes VP1, added FeSO4 to the medium on the 7th day of growth, incubated the biofilms for 1 h, and measured the bacteria released from the biofilms via the OD595. As shown in Fig. 4A, statistically significant biofilm dispersion was induced in P. acnes strain VP1 when 0.1 mM or 0.5 mM Fe was added to the medium (n = 3; P < 0.01). In control experiments with VP1, addition of 1.0 mM free iron did not result in detectable population growth over a period of 1.5 h, indicating that the increased OD measured in the dispersion experiments was not the result of population growth over the time interval of the experiment due to additional iron.
FIG 4.
P. acnes VP1 and hdn-1 biofilm dispersion assays. Bars on all graphs represent cells released from biofilms following treatment. (A) P. acnes VP1 biofilms grown in 12-well plates challenged with 0.1 and 0.5 mM iron following 7 days of growth (n = 3; P < 0.01). (B) P. acnes VP1 biofilms challenged with 0.4 mM norepinephrine (NE) following 7 days of growth in the presence of 0.5 g/liter of holo-transferrin (Tf) (n = 4; P < 0.01). (C) P. acnes hdn-1 biofilms challenged with 0.4 mM NE in the presence of 0.5 g/liter of Tf after 7 days of growth (n = 3; P < 0.05). (D) P. acnes VP1 biofilms challenged with either 0.5 g/liter of Tf, 0.4 mM NE, or 0.4 mM NE in the presence of 0.5 g/liter Tf after 7 days of growth (n = 48; P < 0.01). For all graphs, error bars represent ±1 standard deviation. *, statistically significant.
We next wished to determine whether P. acnes VP1 and hdn-1 biofilms could undergo a dispersion response through the interaction of norepinephrine with holo-transferrin at physiologically relevant concentrations, as shown in our previous work (13). Mature P. acnes VP1 biofilms were grown in the presence of holo-transferrin (0.5 g/liter) and were challenged with a spike of norepinephrine (0.4 mM) on the 7th day of growth. Bacteria released from the biofilms were measured via the OD595 and via serial dilution followed by plating to determine the number of CFU per milliliter of released bacteria. Both measurement methods demonstrated that a significant dispersion event resulting in the release of viable cells had occurred (n = 4; P < 0.01) (Fig. 4B). This dispersion assay was repeated for mature P. acnes hdn-1 biofilms, which were demonstrated to undergo a significant dispersion event when challenged with 0.4 mM norepinephrine in the presence of iron-bound transferrin as well (n = 3; P < 0.05) (Fig. 4C). As a control, mature P. acnes VP1 biofilms were subjected to an increase in holo-transferrin and norepinephrine alone. As shown in Fig. 4D, either agent alone did not induce a dispersion event; however, when the agents were combined, a significant increase in bacterial cells (measured via the OD) was recorded (n = 38; P < 0.01) (Fig. 4D).
Increased lipase and protease activities after iron-induced P. acnes dispersion.
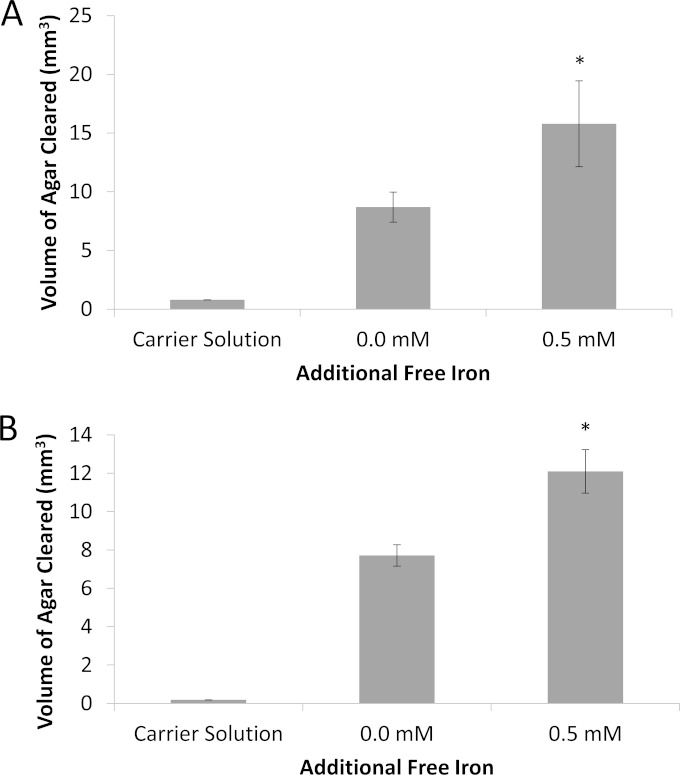
Once it was established that dispersion was inducible due to an increase in the concentration of free iron, we wanted to assess what was occurring during this dispersion event in relation to the enzymes released from P. acnes biofilm cells. Due to the findings of Gribbon et al., who demonstrated in vivo that the production of free fatty acids from lipases released by P. acnes assisted in the bacterium's adherence to and colonization of the skin (35), we wished to determine if lipase activity was affected by the dispersion response. Additionally, protease activity was examined to assess any extracellular proteolytic activity changes due to iron-induced biofilm dispersion. In order to test this, supernatant liquids from iron-induced and uninduced 8-day-old P. acnes biofilms were collected, and activity was assessed. The volume of agar cleared of the appropriate substrate was measured from the areas cleared by bacterial cell-free supernatant liquids diffusing from wells punched into tributyrin or milk agar (see Fig. S1 in the supplemental material). As shown in Fig. 5A, the volume of clearing in tributyrin agar for iron-induced P. acnes biofilm dispersion was 7.0947 mm3 larger than the volume of clearing for uninduced P. acnes biofilms, indicating an increased level of lipase activity for dispersed P. acnes biofilms (n = 3; P < 0.05). Additionally, as shown in Fig. 5B, the volume of clearing in milk agar was 4.3849 mm3 larger than the volume of clearing for uninduced P. acnes biofilms, demonstrating a difference in protease activity for iron-induced dispersed P. acnes biofilms (n = 3; P < 0.01). For both lipase and protease assays, the wells contained 160 μl of resuspended supernatant from biofilm cultures of 4.4 × 107 CFU/ml, and data were analyzed using ImageJ software.
FIG 5.
Lipase and protease activities in cultures of P. acnes biofilms induced to disperse by addition of free iron. (A) Average volume of clearing in tributyrin agar exposed to protein extracts of 8-day-old P. acnes biofilms induced to disperse by challenge with free iron compared to that in tributyrin agar exposed to protein extracts from uninduced biofilms. (B) Average volume of clearing in milk agar exposed to protein extracts of 8-day-old P. acnes biofilms induced to disperse by challenge with free iron compared to that in milk agar exposed to protein extracts from uninduced biofilms. Error bars represent ±1 standard deviation. *, statistically significant (n = 3; P < 0.05).
mRNA levels of lipases and hyaluronate lyase after iron-induced P. acnes dispersion.
Once an increase in lipase activity was observed, we wanted to see if we could measure an upregulation of mRNAs for lipase genes common to P. acnes. We additionally wanted to look at the mRNA levels of hyaluronate lyase (36) because of the potential harmful effects of this enzyme within the atherosclerotic arterial environment. In order to determine whether mRNA levels for certain lipases (PPA1035, PPA1761, PPA1796, and PPA2105) and hyaluronate lyase (PPA380) were affected by iron-induced dispersion, 8-day-old P. acnes biofilms were challenged with 0.5 mM iron and compared to control biofilms that were challenged with carrier alone. The primers used for qPCR are described in Fig. 6A. Following dispersion, we observed a <2-fold increase in mRNA expression for PPA380 (log2 expression level = 0.449) and PPA1796 (log2 expression level = 0.821) (Fig. 6B), while the PPA2105 lipase showed a >2-fold increase in mRNA expression level (1.005 after log2 transformation). Both PPA1035 and PPA1761 were unaffected by iron-induced P. acnes dispersion.
DISCUSSION
A common concern regarding the use of molecular approaches to provide evidence of bacteria in arterial plaque samples has been that these techniques do not prove the existence of viable microorganisms. To date, few studies have been able to link the presence of bacterial 16S rRNA gene biomarkers to recoverable infecting bacteria within an atherosclerotic lesion (37–39). In the current study, we used both molecular approaches and cultivation on artificial medium to demonstrate the presence of viable bacteria within carotid arterial plaque deposits. Previously, we showed that the bacterial 16S rRNA gene demonstrated the presence of microcolonies within arterial plaque deposits and concluded that this was representative of the biofilm mode of growth (13, 34). In the current study, we observed that five of five patient samples from group C contained FISH probe targets arranged in aggregates typical of bacterial cells within biofilm microcolonies and that the structure and architecture of these met the Parsek and Singh criteria for biofilm growth, although we obtained no information from patients regarding antibiotic treatment (13, 34). When the microcolonies were examined by FISH probing, we observed that they included both P. acnes and non-P. acnes eubacterial probe targets. From additional samples (group B), we were able to recover 10 different taxonomic groups of bacteria (including P. acnes) and to grow them on artificial medium. Many of the bacteria recovered in culture from atherosclerotic carotid arteries in this study are also known to occupy other locations in the host: Rothia mucilaginosa, Rothia sp., Enterococcus faecalis, and Streptococcus parasanguinis are also found in the oral cavity, Staphylococcus sciuri, Propionibacterium acnes, and Staphylococcus epidermidis are also associated with the skin, and Enterococcus faecalis is also found in the gastrointestinal tract (14, 40–45). These viability data paired with our FISH probe results demonstrated that biofilms within carotid arterial plaque lesions contained viable bacteria that were, at least in some cases, polymicrobial infections. This has important implications for considering treatment strategies for the management of atherosclerosis.
While identifying the presence of viable multispecies biofilms in atherosclerotic carotid arteries is relevant to understanding the components of the disease, what is more important is to understand the impact of these biofilms on the pathogenesis of atherosclerosis. A significant physiological attribute of biofilm bacteria is their ability to undergo a coordinated dispersion event, releasing bacteria from the confines of the biofilm matrix into the surrounding environment. We believe that biofilm dispersion is necessary in order to enable the thinning out of existing and overcrowded microcolonies and to allow the colonization of new locations (46). Our previous work demonstrated that the Gram-negative bacterium Pseudomonas aeruginosa undergoes a biofilm dispersion event when challenged with increased levels of the hormone norepinephrine in the presence of iron-bound transferrin. Recently, norepinephrine was shown to interact in serum with the iron transport protein transferrin, causing transferrin to release its bound ferric iron into the environment as ferrous iron (47). Our findings demonstrated that P. aeruginosa biofilms dispersed in response to increased levels of iron but not norepinephrine or iron-bound transferrin alone (13). The present study demonstrated that the Gram-positive bacterium P. acnes can also be induced to undergo biofilm dispersion in response to an increase in the concentration of free iron in its environment due to the interaction of norepinephrine and iron-bound transferrin. Thus, both Gram-negative and Gram-positive bacteria can be induced to undergo biofilm dispersion via the same inducer, an observation that implies that multispecies biofilms could likewise be dispersion inducible in the presence of iron (an unpublished phenomenon we have observed in our laboratory). The observation that a catecholamine hormone, such as norepinephrine, can induce biofilm dispersion via interaction with transferrin is relevant because both norepinephrine and transferrin have the potential of interacting within the blood system of patients with advanced atherosclerosis. It is potentially significant that these interactions may have an impact on the stability of the biofilms located proximal to the fibrous cap of atherosclerotic lesions. It is intriguing that physical or emotional stress has been linked to both increased levels of norepinephrine (48) and an increased incidence of myocardial infarction, or heart attack (49, 50). The observation that both Gram-negative and Gram-positive bacteria respond to norepinephrine-mediated biofilm dispersion provides one additional piece of information that may link stress to heart attack and stroke. Conclusions based upon this observation must, however, be tempered by the limitation that, to date, these experiments have been performed only in vitro, not in an actual infected carotid artery. We cannot therefore conclude that a similar process occurs in the human host, only that the mechanism that can lead to dispersion has the potential to occur as the result of an increase in the concentration of norepinephrine in the presence of transferrin and P. acnes. At present, it is unclear whether norepinephrine can result in a sufficient increase in free iron within the carotid arterial wall to be able to induce P. acnes to disperse, and it is also unclear whether the complex biology of the diseased carotid artery may have the ability to inhibit this response.
Biofilm dispersion events have been observed to be coordinated with the release of degradative enzymes by bacteria, which will break down the extracellular matrix (ECM), allowing individual cells to escape and a switch to higher growth rates (23–25, 27, 28, 51). The results from this study show that when P. acnes undergoes an iron-induced dispersion event, a statistically significant upregulation of the mRNA transcript for the PPA2105 lipase occurs, as do increased extracellular lipase and protease activities. PPA2105 is a secreted triacylglycerol lipase that cleaves the bonds between the fatty acid side chains and glycerol. Triacylglycerols are blood lipids used for both adipose and glucose transportation (52), and cleavage of the triacylglycerol results in the accumulation of free fatty acids (FFAs), which have been found to stimulate inflammation (53, 54). These FFAs have also been shown by Gribbon et al. (35) to increase the adhesion of P. acnes bacterial cells within the sebaceous follicle to promote colonization. Biofilm dispersion events often lead to downstream biofilm colonization by the newly liberated planktonic bacteria, which begins with the attachment of the released planktonic cells to uninhabited surfaces. The presence of PPA2105 could result in the production of FFAs within the atherosclerotic carotid artery, further propagating colonization by P. acnes biofilms while simultaneously instigating an inflammatory response. It is interesting that findings by Stampfer et al. found a direct correlation to heightened levels of triacylglycerol in the blood of patients who suffered myocardial infarction (52). The higher levels of triacylglycerol in the blood could result in more substrate being available for the lipases, resulting in increased production of FFAs and potentially increased incidences of inflammation and P. acnes colonization.
When the progression of atherosclerosis is assessed, the host immune system should always be taken into account. In a study conducted by van Leeuwen et al., the involvement and location of neutrophils within atherosclerotic low-density lipoprotein receptor-defective (LDLR−/−) mice were assessed, with neutrophils shown to be present in intermediate- and advanced-stage lesions but absent from early-stage lesions (55). In a separate study, Lee et al. used a modified Boyden chemotactic chamber to demonstrate that lipases released by P. acnes acted as a chemoattractant for human neutrophils (56). The neutrophils shown by van Leeuwen et al. to be present within the LDLR−/− mice produced myeloperoxidase, which can contribute to lipid peroxidation and potentially damage tissues surrounding an atherosclerotic lesion (55, 57). Recruitment of neutrophils is likely enhanced by the release of lipases if P. acnes iron-induced dispersion events occur within an atherosclerotic lesion, contributing to tissue damage.
Coenye et al. demonstrated that P. acnes biofilms have increased lipase activity compared to that of planktonic P. acnes (54). We also observed lipolytic activity from P. acnes biofilms, but this was shown to increase along with proteolytic enzyme activity following challenge of these biofilms with free iron. These observations indicate that while dispersion resulted in the release of elevated levels of extracellular enzymes by P. acnes, collateral damage to the surrounding host tissues by nondispersing biofilms resident within atherosclerotic lesions may also have the potential to occur.
The findings of the present study suggest a new perspective on the pathogenesis of atherosclerosis and carotid arterial plaque stability. We believe from the current work and our previous work with carotid arterial tissue samples that polymicrobial biofilm infections play an integral role in atherosclerosis. Our findings further suggest that plaque-associated bacterial biofilms have the capability to respond to the host hormonal state and have the potential to affect local tissues through the release of lytic extracellular enzymes. Proteases and lipases could directly affect arterial tissues and stimulate the recruitment of host immune cells, resulting in further tissue damage. We hypothesize that the potential for tissue damage due to the activity of P. acnes and other bacteria associated with atherosclerotic plaques may play a role in influencing the stability of these lesions and contribute to plaque rupture and atherogenesis.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the participation of UHS Binghamton for their contributions of biological materials for the current study. Particular thanks are due to Terri K. Peters and Cathy Hughes for their assistance with the current project and in organizing the patient study component of this work and to Leonard Anderson for providing patient samples.
Funding for the current work was provided by Binghamton University.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00510-15.
REFERENCES
- 1.Peyser PA. 1997. Genetic epidemiology of coronary artery disease. Epidemiol Rev 19:80–90. doi: 10.1093/oxfordjournals.epirev.a017949. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. 1993. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z, Peto R, Collins R, MacMahon S, Li W. 1991. Serum cholesterol concentration and coronary heart disease in population with low cholesterol concentrations. BMJ 303:276–282. doi: 10.1136/bmj.303.6797.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meir KS, Leitersdorf E. 2004. Atherosclerosis in the apolipoprotein E-deficient mouse: a decade of progress. Arterioscler Thromb Vasc Biol 24:1006–1014. doi: 10.1161/01.ATV.0000128849.12617.f4. [DOI] [PubMed] [Google Scholar]
- 5.Lehtiniemi J, Karhunen PJ, Goebeler S, Nikkari S, Nikkari ST. 2005. Identification of different bacterial DNAs in human coronary arteries. Eur J Clin Invest 35:13–16. [DOI] [PubMed] [Google Scholar]
- 6.Ott SJ, El Mokhtari NE, Musfeldt M, Hellmig S, Freitag S, Rehman A, Kuhbacher T, Nikolaus S, Namsolleck P, Blaut M, Hampe J, Sahly H, Reinecke A, Haake N, Gunther R, Kruger D, Lins M, Herrmann G, Folsch UR, Simon R, Schreiber S. 2006. Detection of diverse bacterial signatures in atherosclerotic lesions of patients with coronary heart disease. Circulation 113:929–937. doi: 10.1161/CIRCULATIONAHA.105.579979. [DOI] [PubMed] [Google Scholar]
- 7.Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. 2007. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect 13:3–10. doi: 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 8.Stoodley P, Sauer K, Davies DG, Costerton JW. 2002. Biofilms as complex differentiated communities. Annu Rev Microbiol 56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida Y, Suzuki N, Nakano Y, Shibuya K, Ogawa Y, Koga T. 2003. Distribution of Actinobacillus actinomycetemcomitans serotypes and Porphyromonas gingivalis in Japanese adults. Oral Microbiol Immunol 18:135–139. doi: 10.1034/j.1399-302X.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- 10.Jander S, Sitzer M, Schumann R, Schroeter M, Siebler M, Steinmetz H, Stoll G. 1998. Inflammation in high-grade carotid stenosis: a possible role for macrophages and T cells in plaque destabilization. Stroke 29:1625–1630. doi: 10.1161/01.STR.29.8.1625. [DOI] [PubMed] [Google Scholar]
- 11.Kurihara N, Inoue Y, Iwai T, Umeda M, Huang Y, Ishikawa I. 2004. Detection and localization of periodontopathic bacteria in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 28:553–558. doi: 10.1016/j.ejvs.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Gibson FC, Hong C, Chou H-H, Yumoto H, Chen J, Lien E, Wong J, Attardo Genco C. 2004. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation 109:2801–2806. doi: 10.1161/01.CIR.0000129769.17895.F0. [DOI] [PubMed] [Google Scholar]
- 13.Lanter BB, Sauer K, Davies DG. 2014. Bacteria present in carotid arterial plaques are found as biofilm deposits which may contribute to enhanced risk of plaque rupture. mBio 5:e01206–14. doi: 10.1128/mBio.01206-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan S-C, Wang J-T, Hsueh P-R, Chang S-C. 2005. Endocarditis caused by Propionibacterium acnes: an easily ignored pathogen. J Infect 51:e229–e231. doi: 10.1016/j.jinf.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Lewis J, Abramson J. 1980. Endocarditis due to Propionibacterium acnes. Am J Clin Pathol 74:690. [DOI] [PubMed] [Google Scholar]
- 16.Günthard H, Hany A, Turina M, Wüst J. 1994. Propionibacterium acnes as a cause of aggressive aortic valve endocarditis and importance of tissue grinding: case report and review. J Clin Microbiol 32:3043–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horner S, Sturridge M, Swanton R. 1992. Propionibacterium acnes causing an aortic root abscess. Br Heart J 68:218–220. doi: 10.1136/hrt.68.8.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renko J, Koskela KA, Lepp PW, Oksala N, Levula M, Lehtimäki T, Solakivi T, Kunnas T, Nikkari S, Nikkari ST. 2013. Bacterial DNA signatures in carotid atherosclerosis represent both commensals and pathogens of skin origin. Eur J Dermatol 23:53–58. doi: 10.1684/ejd.2012.1908. [DOI] [PubMed] [Google Scholar]
- 19.Armingohar Z, Jørgensen JJ, Kristoffersen AK, Abesha-Belay E, Olsen I. 2014. Bacteria and bacterial DNA in atherosclerotic plaque and aneurysmal wall biopsies from patients with and without periodontitis. J Oral Microbiol 6:23408. doi: 10.3402/jom.v6.23408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tunney MM, Patrick S, Curran MD, Ramage G, Hanna D, Nixon JR, Gorman SP, Davis RI, Anderson N. 1999. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J Clin Microbiol 37:3281–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passerini L, Phang P, Jackson F, Lam K, Costerton J, King E. 1987. Biofilms on right heart flow-directed catheters. Chest 92:440–446. doi: 10.1378/chest.92.3.440. [DOI] [PubMed] [Google Scholar]
- 22.Guío L, Sarriá C, de las Cuevas C, Gamallo C, Duarte J. 2009. Chronic prosthetic valve endocarditis due to Propionibacterium acnes: an unexpected cause of prosthetic valve dysfunction. Rev Esp Cardiol 62:167–177. doi: 10.1016/S0300-8932(09)70159-9. [DOI] [PubMed] [Google Scholar]
- 23.Boyd A, Chakrabarty AM. 1994. Role of alginate lyase in cell detachment of Pseudomonas aeruginosa. Appl Environ Microbiol 60:2355–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gacesa P. 1987. Alginate-modifying enzymes: a proposed unified mechanism of action for the lyases and epimerases. FEBS Lett 212:199–202. doi: 10.1016/0014-5793(87)81344-3. [DOI] [Google Scholar]
- 25.Xun L, Mah RA, Boone DR. 1990. Isolation and characterization of disaggregatase from Methanosarcina mazei LYC. Appl Environ Microbiol 56:3693–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vats N, Lee SF. 2000. Active detachment of Streptococcus mutans cells adhered to epon-hydroxylapatite surfaces coated with salivary proteins in vitro. Arch Oral Biol 45:305–314. doi: 10.1016/S0003-9969(99)00139-9. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan JB, Ragunath C, Ramasubbu N, Fine DH. 2003. Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous β-hexosaminidase activity. J Bacteriol 185:4693–4698. doi: 10.1128/JB.185.16.4693-4698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itoh Y, Wang X, Hinnebusch BJ, Preston JF, Romeo T III. 2005. Depolymerization of β-1,6-N-acetyl-d-glucosamine disrupts the integrity of diverse bacterial biofilms. J Bacteriol 187:382–387. doi: 10.1128/JB.187.1.382-387.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greisen K, Loeffelholz M, Purohit A, Leong D. 1994. PCR primers and probes for the 16S rRNA gene of most species of pathogenic bacteria, including bacteria found in cerebrospinal fluid. J Clin Microbiol 32:335–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McBain AJ, Bartolo RG, Catrenich CE, Charbonneau D, Ledder RG, Rickard AH, Symmons SA, Gilbert P. 2003. Microbial characterization of biofilms in domestic drains and the establishment of stable biofilm microcosms. Appl Environ Microbiol 69:177–185. doi: 10.1128/AEM.69.1.177-185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexeyev OA, Marklund I, Shannon B, Golovleva I, Olsson J, Andersson C, Eriksson I, Cohen R, Elgh F. 2007. Direct visualization of Propionibacterium acnes in prostate tissue by multicolor fluorescent in situ hybridization assay. J Clin Microbiol 45:3721–3728. doi: 10.1128/JCM.01543-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J Bacteriol 184:1140–1154. doi: 10.1128/jb.184.4.1140-1154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar V, Abbas AK, Aster JC, Perkins JA (ed). 2014. Robbins and Cotran pathologic basis of disease. Elsevier Health Sciences Division, Philadelphia, PA. [Google Scholar]
- 34.Parsek MR, Singh PK. 2003. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol 57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 35.Gribbon E, Cunliffe W, Holland K. 1993. Interaction of Propionibacterium acnes with skin lipids in vitro. J Gen Microbiol 139:1745–1751. doi: 10.1099/00221287-139-8-1745. [DOI] [PubMed] [Google Scholar]
- 36.Steiner B, Romero-Steiner S, Cruce D, George R. 1997. Cloning and sequencing of the hyaluronate lyase gene from Propionibacterium acnes. Can J Microbiol 43:315–321. doi: 10.1139/m97-044. [DOI] [PubMed] [Google Scholar]
- 37.Meijer A, Roholl P, Gielis-Proper S, Ossewaarde J. 2000. Chlamydia pneumoniae antigens, rather than viable bacteria, persist in atherosclerotic lesions. J Clin Pathol 53:911–916. doi: 10.1136/jcp.53.12.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kozarov EV, Dorn BR, Shelburne CE, Dunn WA, Progulske-Fox A. 2005. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler Thromb Vasc Biol 25:e17–e18. doi: 10.1161/01.ATV.0000155018.67835.1a. [DOI] [PubMed] [Google Scholar]
- 39.Rafferty B, Jönsson D, Kalachikov S, Demmer RT, Nowygrod R, Elkind MSV, Bush H, Kozarov E. 2011. Impact of monocytic cells on recovery of uncultivable bacteria from atherosclerotic lesions. J Intern Med 270:273–280. doi: 10.1111/j.1365-2796.2011.02373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamane K, Nambu T, Yamanaka T, Mashimo C, Sugimori C, Leung K-P, Fukushima H. 2010. Complete genome sequence of Rothia mucilaginosa DY-18: a clinical isolate with dense meshwork-like structures from a persistent apical periodontitis lesion. Sequencing 2010:457236. [Google Scholar]
- 41.Collins M, Hutson R, Båverud V, Falsen E. 2000. Characterization of a Rothia-like organism from a mouse: description of Rothia nasimurium sp. nov. and reclassification of Stomatococcus mucilaginosus as Rothia mucilaginosa comb. nov. Int J Syst Evol Microbiol 50:1247–1251. doi: 10.1099/00207713-50-3-1247. [DOI] [PubMed] [Google Scholar]
- 42.Rams T, Feik D, Young V, Hammond B, Slots J. 1992. Enterococci in human periodontitis. Oral Microbiol Immunol 7:249–252. doi: 10.1111/j.1399-302X.1992.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 43.Socransky S, Manganiello A, Propas D, Oram V, van Houte J. 1977. Bacteriological studies of developing supragingival dental plaque. J Periodontal Res 12:90–106. doi: 10.1111/j.1600-0765.1977.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 44.Kloos WE, Schleifer KH, Smith RF. 1976. Characterization of Staphylococcus sciuri sp. and its subspecies. Int J Syst Bacteriol 26:22–37. doi: 10.1099/00207713-26-1-22. [DOI] [Google Scholar]
- 45.Fey PD, Olson ME. 2010. Current concepts in biofilm formation of Staphylococcus epidermidis. Future Microbiol 5:917–933. doi: 10.2217/fmb.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davies DG, Marques CNH. 2009. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J Bacteriol 191:1393–1403. doi: 10.1128/JB.01214-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandrini SM, Shergill R, Woodward J, Muralikuttan R, Haigh RD, Lyte M, Freestone PP. 2010. Elucidation of the mechanism by which catecholamine stress hormones liberate iron from the innate immune defense proteins transferrin and lactoferrin. J Bacteriol 192:587–594. doi: 10.1128/JB.01028-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pacak K, Palkovits M, Kopin IJ, Goldstein DS. 1995. Stress-induced norepinephrine release in the hypothalamic paraventricular nucleus and pituitary-adrenocortical and sympathoadrenal activity: in vivo microdialysis studies. Front Neuroendocrinol 16:89–150. doi: 10.1006/frne.1995.1004. [DOI] [PubMed] [Google Scholar]
- 49.Tofler GH, Stone PH, Maclure M, Edelman E, Davis VG, Robertson T, Antman EM, Muller JE. 1990. Analysis of possible triggers of acute myocardial infarction (the MILIS study). Am J Cardiol 66:22–27. doi: 10.1016/0002-9149(90)90729-K. [DOI] [PubMed] [Google Scholar]
- 50.Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. 1993. Triggering of acute myocardial infarction by heavy physical exertion—protection against triggering by regular exertion. N Engl J Med 329:1677–1683. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- 51.Sauer K, Cullen M, Rickard A, Zeef L, Davies D, Gilbert P. 2004. Characterization of nutrient-induced dispersion in Pseudomonas aeruginosa PAO1 biofilm. J Bacteriol 186:7312–7326. doi: 10.1128/JB.186.21.7312-7326.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH. 1996. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA 276:882–888. [PubMed] [Google Scholar]
- 53.Jappe U. 2003. Pathological mechanisms of acne with special emphasis on Propionibacterium acnes and related therapy. Acta Derm Venereol 83:241–248. doi: 10.1080/00015550310016463. [DOI] [PubMed] [Google Scholar]
- 54.Coenye T, Peeters E, Nelis HJ. 2007. Biofilm formation by Propionibacterium acnes is associated with increased resistance to antimicrobial agents and increased production of putative virulence factors. Res Microbiol 158:386–392. doi: 10.1016/j.resmic.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 55.van Leeuwen M, Gijbels MJ, Duijvestijn A, Smook M, van de Gaar MJ, Heeringa P, de Winther MP, Tervaert JWC. 2008. Accumulation of myeloperoxidase-positive neutrophils in atherosclerotic lesions in LDLR−/− mice. Arterioscler Thromb Vasc Biol 28:84–89. [DOI] [PubMed] [Google Scholar]
- 56.Lee W, Shalita A, Suntharalingam K, Fikrig S. 1982. Neutrophil chemotaxis by Propionibacterium acnes lipase and its inhibition. Infect Immun 35:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Podrez EA, Schmitt D, Hoff HF, Hazen SL. 1999. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest 103:1547–1560. doi: 10.1172/JCI5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.