Abstract
Mannheimia haemolytica causes pneumonia in domestic and wild ruminants. Leukotoxin (Lkt) is the most important virulence factor of the bacterium. It is encoded within the four-gene lktCABD operon: lktA encodes the structural protoxin, and lktC encodes a trans-acylase that adds fatty acid chains to internal lysine residues in the protoxin, which is then secreted from the cell by a type 1 secretion system apparatus encoded by lktB and lktD. It has been reported that LktC-mediated acylation is necessary for the biological effects of the toxin. However, an LktC mutant that we developed previously was only partially attenuated in its virulence for cattle. The objective of this study was to elucidate the role of LktC-mediated acylation in Lkt-induced cytotoxicity. We performed this study in bighorn sheep (Ovis canadensis) (BHS), since they are highly susceptible to M. haemolytica infection. The LktC mutant caused fatal pneumonia in 40% of inoculated BHS. On necropsy, a large number of necrotic polymorphonuclear leukocytes (PMNs) were observed in the lungs. Lkt from the mutant was cytotoxic to BHS PMNs in an in vitro cytotoxicity assay. Flow cytometric analysis of mutant Lkt-treated PMNs revealed the induction of necrosis. Scanning electron microscopic analysis revealed the presence of pores and blebs on mutant-Lkt-treated PMNs. Mass spectrometric analysis confirmed that the mutant secreted an unacylated Lkt. Taken together, these results suggest that acylation is not necessary for the cytotoxic activity of M. haemolytica Lkt but that it enhances the potency of the toxin.
INTRODUCTION
Mannheimia haemolytica is a respiratory pathogen of domestic and wild ruminants (1–3). It is the most important bacterial pathogen of bovine respiratory disease complex, which costs the U.S. cattle industry alone more than $1 billion (4). M. haemolytica is also an important pathogen of pneumonia in bighorn sheep (Ovis canadensis) (BHS), which is the primary disease responsible for the drastic decline of BHS populations in North America from an estimated 2 million animals in the 1800s to less than 70,000 at the present time (5). Under the experimental conditions, M. haemolytica consistently caused 100% mortality in BHS within 2 to 3 days (6–8). The bacterium possesses several virulence factors, including the capsule, outer membrane proteins, lipopolysaccharide, and leukotoxin (Lkt). Based on the fact that Lkt deletion mutants do not cause mortality (6) or cause reduced mortality and milder lung lesions (9, 10), Lkt has been accepted as the primary virulence factor of M. haemolytica. The 104-kDa Lkt is absolutely specific for ruminant leukocytes (11). Previously, we have shown that the molecular basis for the ruminant specificity of Lkt rests in its binding to the signal peptide of CD18; the signal peptide remains intact in mature CD18 molecules on ruminant leukocytes, unlike that of nonruminants, which is cleaved (12). Although all ruminant leukocyte subsets are susceptible to Lkt-induced cytolysis, polymorphonuclear leukocytes (PMNs) are the most susceptible, due to their higher level of CD18 expression (13, 14). Lkt-induced PMN lysis and degranulation are the primary causes of the acute inflammation and lung injury characteristic of pneumonia caused by M. haemolytica. However, at very low concentrations, Lkt activates target cells to undergo respiratory burst and degranulation. As the concentration of Lkt increases, target cells are induced to undergo apoptosis. At even higher concentrations, necrosis of target cells occurs as a result of membrane damage due to pore formation (15, 16).
Lkt is a member of the repeats-in-toxin (RTX) family of toxins produced by a group of Gram-negative bacteria, including Escherichia coli, Aggregatibacter actinomycetemcomitans, and Actinobacillus pleuropneumoniae. Lkt is encoded by the lktCABD operon, where lktA encodes the inactive protoxin, lktC encodes a trans-acylase that adds fatty acid chains to internal lysine residues in the protoxin, and lktB and lktD encode components of a type 1 secretion system apparatus that, along with the outer membrane protein TolC, secrete the toxin from the cell (17–19). LktC-mediated acylation of LktA protoxin is not required for its expression or secretion (20). It has been reported that LktC-mediated acylation is essential for the biological effects of the toxin, including the induction of apoptosis (20, 21). However, an LktC mutant strain that we (S. K. Highlander) developed previously was only partially attenuated in its virulence in a calf challenge model (22). We reasoned that further elucidation of the role of LktC-induced acylation in the cytotoxic activity of Lkt would be facilitated by characterizing the effects of LktC mutant toxin using target cells that are more susceptible to Lkt than are bovine or ovine cells, which are usually studied in the context of Lkt virulence and activity. PMNs of BHS are 4- to 8-fold more susceptible to M. haemolytica Lkt than are those of domestic sheep (23). Therefore, the objective of this study was to characterize the virulence of the LktC mutant toxin against BHS PMNs in vivo and in vitro.
MATERIALS AND METHODS
Bacteria, cells, and growth conditions.
In this study, M. haemolytica strain SH2099 containing a frameshift mutation in the lktC gene, developed as detailed by Highlander et al. (22), was used. The parental strain SH1217 (22) was used as a wild-type control in all assays. Both of these bacterial strains are of serotype 1. Bacteria were grown in brain heart infusion (BHI) (Remel, Lenexa, KS) medium at 37°C unless otherwise indicated. To prepare inocula for the animal studies, M. haemolytica strain SH2099 was grown on BHI agar plates supplemented with 5% sheep blood (Remel, Lenexa, KS) overnight at 37°C. Bacteria were harvested by scraping with a spreader and were resuspended in BHI broth to obtain an optical density at 600 nm (OD600) of 0.3. The suspension was subcultured for 2 to 3 h at 37°C until the OD600 reached 0.8. The bacterial cells were pelleted by centrifugation at 2,400 × g for 18 min and resuspended in colorless RPMI 1640 medium (Life Technologies, Grand Island, NY) to an approximate concentration of 1 × 104 CFU/5 ml. The bacterial CFU count was extrapolated from a calibrated standard growth curve. Bovine lymphoma 3 (BL3) cells (ATCC, Manassas, VA) were grown in RPMI 1640 medium containing 10% fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA).
Animal inoculation.
Five adult male BHS, 3 to 5 years of age, from our captive herd at Washington State University were swabbed (nasal and pharyngeal) and bled to identify the presence of Lkt-producing members of the Pasteurellaceae and Lkt-neutralizing antibodies, respectively, as described previously (24, 25). Two animals in a group of four were inoculated intranasally with 5 ml of RPMI 1640 medium containing 1 × 104 CFU of M. haemolytica strain SH2099 on day 0. The uninoculated animals remained as sentinels. When one of the inoculated animals died on day 4, a new animal was added to the group, and all four animals were inoculated with M. haemolytica strain SH2099 as described above on day 103. Nasal swabs, pharyngeal swabs, and blood samples were collected on days 7, 21, 36, 60, 67, and 74 following the first inoculation to evaluate bacterial shedding and transmission and to enumerate Lkt-neutralizing antibody titers, respectively. The animals were housed in Washington State University IACUC-approved facilities and were monitored daily for clinical signs during the course of this experiment.
Production of Lkt.
The protocol for Lkt preparation from M. haemolytica cultures has been described previously (24). Briefly, bacteria were grown to logarithmic phase in BHI broth, pelleted, and resuspended in twice the BHI broth volume of RPMI 1640 medium supplemented with 0.5% bovine serum albumin (Sigma-Aldrich, St. Louis, MO). After 1 to 1.5 h of growth, the bacteria were pelleted by centrifugation and the supernatant obtained was filter sterilized using a 0.22-μm filter. This supernatant preparation containing Lkt was frozen at −20°C until needed. All Lkt functional assays were performed with the same batch of toxin. In the text below, the terms SH1217 Lkt and SH2099 Lkt refer to the Lkt-containing culture supernatant prepared, as described above, from SH1217 and SH2099, respectively.
Isolation of PMNs from peripheral blood of BHS.
PMNs were isolated from peripheral blood of BHS by Ficoll-Paque (Amersham Pharmacia Biotech, Piscataway, NJ) density gradient centrifugation, as previously described (26). PMNs were obtained by hypotonic lysis of the red blood cell pellet.
MTT dye reduction cytotoxicity assay for detection of Lkt-induced cytotoxicity.
Lkt activity was quantitated using the MTT [3-(4,5-dimethylthiazoyl-2-yl)-2,5-diphenyl tetrazolium bromide] dye reduction cytotoxicity assay as described previously (24). Briefly, serial 2-fold dilutions of toxins (50 μl/well) were tested in duplicate on aliquots of either PMNs or BL3 cells (5 × 106 cells/ml in 50 μl/well). Toxin cell treatment was carried out at 37°C for 1 h, following which MTT dye was added to the toxin-treated cells. The plate was then incubated at 37°C for 4 h and 1 h, respectively, for PMNs and BL3 cells. The formazan precipitate that formed was dissolved by the addition of acid isopropanol, and the OD540 of each well was measured using an enzyme-linked immunosorbent assay (ELISA) plate reader. The percent cytotoxicity was determined using the following formula: [1 − (OD given by toxin-treated cells/OD given by untreated control cells)] × 100. The 50% toxicity endpoint was defined as the reciprocal of the highest toxin dilution resulting in at least 50% cytotoxicity. The results reported in this study are the mean percent cytotoxicities of three independent measurements.
Detection of Lkt-neutralizing antibody titers.
The Lkt-neutralizing antibody titer assay was similar to the MTT dye reduction cytotoxicity assay for detection of Lkt-induced cytotoxicity, with the modification that Lkt was preincubated with serially diluted serum (obtained from the inoculated BHS) for 1 h at 4°C before the addition of cells. In this assay, the toxin dilution that produced 50% toxicity was used. The remainder of the assay was identical to that described above. The percent inhibition of cytotoxicity was calculated as follows: [(cytotoxicityLkt+medium − cytotoxicityLkt+ serum)/(cytotoxicityLkt+ medium)] × 100. The Lkt-neutralizing serum endpoint titer of each sample was defined as the reciprocal of the highest test sample dilution resulting in at least 50% neutralization of the toxin.
Flow cytometric analysis.
BHS PMNs treated with SH2099 Lkt or SH1217 Lkt were labeled with annexin V and propidium iodide (PI) (Southern Biotech, Birmingham, AL), according to the manufacturer's instructions. Annexin V specifically binds to phosphatidylserine when it is exposed during apoptosis and necrosis, while PI intercalates with the DNA once a cell has been permeabilized due to pore formation. Dual labeling of cells with annexin V and PI is used to distinguish apoptotic cells (only annexin V positive) from necrotic cells (PI positive). Fifty microliters of SH2099 Lkt or SH1217 Lkt was added to 1 × 106 PMNs and incubated for 1 min at 37°C. The cells were pelleted, rinsed once with colorless RPMI 1640 medium, resuspended in cold annexin V binding buffer containing 10 μl of annexin V (Southern Biotech, Birmingham, AL), and incubated for 15 min on ice in the dark. Subsequently, an additional 380 μl of the annexin V binding buffer was added, along with 10 μl of PI. The samples were then immediately analyzed by flow cytometry. Additionally, serial dilutions of SH2099 Lkt were also assayed to evaluate induction of apoptosis. The flow cytometric measurements were performed using a BD FACSCalibur flow cytometer (BD Biosciences, Mississauga, Ontario, Canada), and the data were analyzed using the FCS Express 4 software (De Novo Software, Glendale, CA).
Electron microscopic analysis.
Twenty microliters of SH2099 Lkt or SH1217 Lkt was added to 1 × 106 PMNs and incubated for 5 min at 37°C. The cells were pelleted, rinsed once with colorless RPMI 1640 medium, and fixed overnight at 4°C in glutaraldehyde-formaldehyde fixative (2% paraformaldehyde, 2% glutaraldehyde, 0.1 M phosphate buffer). The following day, the cells were rinsed twice in 0.1 M phosphate buffer and fixed overnight at 4°C in 1% osmium tetroxide. The cells were rinsed first with 0.1 M phosphate buffer and then with 0.1 M cacodylate buffer and stored in 0.1 M cacodylate buffer containing 3.5% sucrose until they were imaged. Prior to imaging, the cells were rinsed twice in double-distilled water. Images were taken under a low vacuum setting of 130 Pa using an FEI (Hillsboro, OR) SEM Quanta 200F microscope.
MS analysis.
M. haemolytica culture supernatant containing Lkt was concentrated using Amicon Ultra-15 Centrifugal Filter Units (EMD Millipore, Billerica, MA) with a membrane nominal molecular mass limit of 50 kDa. The concentrated SH2099 Lkt or SH1217 Lkt was subjected to SDS-PAGE (12% gels) under nonreducing conditions, followed by Western blotting with monoclonal antibody MM605, which is specific for Lkt (24). Bands corresponding to the Lkt protein were excised and trypsinized in the gel before or after acetic anhydride treatment (acetylation). Acetylation has been used in the past to block trypsin cleavage at lysine residues (27). For acetylation, gel slices were first dehydrated with acetonitrile and subsequently treated with bicarbonate buffer containing 5% acetic anhydride for 5 min. This process of dehydration and acetic anhydride treatment was repeated three more times to acetylate all the lysine residues on Lkt. Subsequently, the gel slices were washed, dehydrated, and dried in a speed-vac prior to addition of trypsin at a concentration of 1 μg/ml for overnight trypsinization at 37°C. The trypsin fragments were analyzed by liquid chromatography-tandem mass spectrometry (LC–MS-MS) as previously described, using an Esquire HCT electrospray ion trap (Bruker Daltonics, Billerica, MA) and an LC Packings Ultimate Nano high-performance liquid chromatography system with minor changes in the LC procedure (28). In MS-MS, a search for peptides containing C12, C13, C13 OH, C14, C14 OH, C15, C16, and C17 fatty acid modifications at lysine residues was performed, using the MASCOT program (Matrix Science Inc., Boston, MA). For the unacetylated peptides, one missed trypsin cut was considered, while for the acetylated peptides, four missed trypsin cuts were considered.
Statistical analysis.
A paired Student t test was used to compare the cytotoxicities of the Lkt produced by the SH2099 M. haemolytica mutant strain on BL3 cells and BHS PMNs. A P value of ≤0.01 was considered significant. A repeated-measures analysis of variance (ANOVA) was used to analyze the percent cytotoxicity data. A P value of ≤0.01 was considered significant. The statistical analyses were performed using the Minitab 17 software (Minitab Inc., State College, PA).
RESULTS
The mutant SH2099 causes fatal pneumonia in two out of five BHS.
One of the two SH2099-inoculated BHS from a group of four BHS died on day 4 postinoculation. The second inoculated animal remained healthy. The two sentinel BHS did not acquire SH2099 from the inoculated animals. When one of the inoculated BHS died, a new BHS was introduced into the group, and all four animals were inoculated with SH2099. Three days later, one more BHS died. In summary, two out of five animals inoculated with SH2099 died. On necropsy, gross pathological examination of their lungs revealed consolidation, hemorrhage, and fibrin deposition. Histopathological examination revealed a large number of necrotic neutrophils (oat cells) in the alveoli, which is pathognomonic of M. haemolytica-caused pneumonia. Swabs collected from lesional lung tissue had large numbers of SH2099 bacteria, as revealed by the CFU assay (data not shown). SH2099 was not isolated from the nasal and pharyngeal swabs collected from the surviving animals throughout the experiment. Only the animal that survived the first inoculation exhibited a Lkt-neutralizing antibody titer of 200 on day 36 postinoculation (data not shown). The other animals did not have significant Lkt-neutralizing antibody titers. None of the surviving animals were euthanized in this study, and hence, no pathological findings are available for them.
Lkt from the LktC mutant SH2099 is cytotoxic for BHS PMNs.
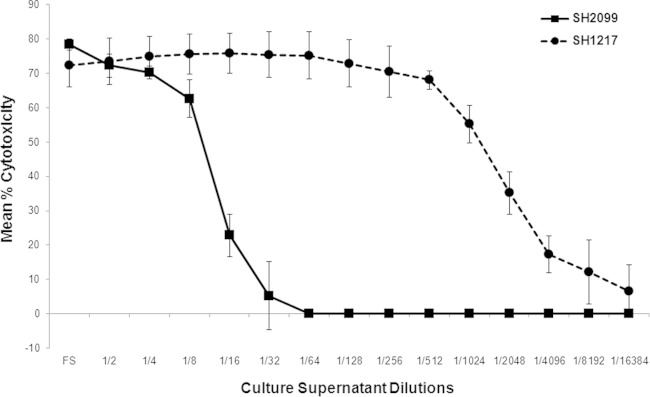
The percent cytotoxicities exhibited by Lkt from the parental strain, SH1217, and the mutant SH2099 are shown in Fig. 1. SH2099 Lkt was cytotoxic for BHS PMNs, albeit to a much lower degree than Lkt from the parental strain. To rule out possible contamination with the parental strain, SH1217, the mutant SH2099 was serially diluted and plated. Thirty single colonies of SH2099 were subcultured, and their identities were verified by PCR assay using specific primers (22) targeting the site of mutation. Lkt from each one of the 30 single colonies was cytotoxic for BHS PMNs and not for BL3 cells (data not shown), confirming the fact that the LktC mutant produced a Lkt that was cytotoxic for BHS PMNs, corroborating the in vivo finding of 40% mortality caused by the mutant in BHS. In our studies, the toxin in the Lkt preparations (from the SH1217 and SH2099 strains) was not quantified due to its extremely labile nature. In the past, functional Lkt units have been used to quantify the toxin in Lkt preparations. In the current scenario, since there were distinct differences in the functional attributes of the two toxins, quantification of the toxins in this manner would not be meaningful. To normalize the preparations, however, similar numbers of CFU of bacteria, volumes of growth medium, and times of incubation were used in our Lkt preparations.
FIG 1.
Lkt produced by the LktC mutant strain SH2099 is cytotoxic for BHS PMNs in vitro. PMNs from the parent strain, SH1217, and the mutant SH2099 were tested for Lkt-induced cytotoxicity by the MTT dye reduction cytotoxicity assay. The results shown are the means of three independent experiments. The error bars indicate standard deviations of the means.
Lkt from the LktC mutant SH2099 induces necrosis in BHS PMNs.
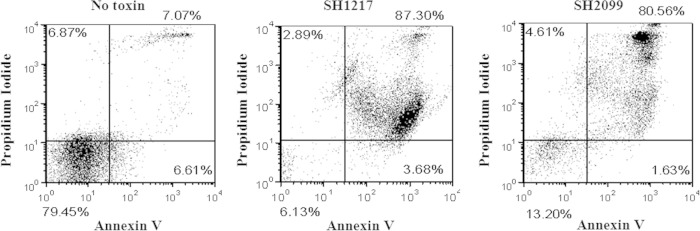
Induction of 40% mortality in inoculated BHS by the mutant and the in vitro cytotoxicity of its Lkt to BHS PMNs prompted us to confirm its ability to induce apoptosis and necrosis in BHS PMNs. Dual annexin V and PI labeling was used to determine the occurrence of apoptosis and/or necrosis, using flow cytometry. Following 1 min incubation of BHS PMNs with either the wild-type or the mutant toxin, a massive shift in the cell numbers toward a population of annexin V- and PI-positive necrotic cells was observed (Fig. 2). This pattern did not change even when serially diluted Lkt was incubated with BHS PMNs (data not shown).
FIG 2.
Lkt produced by the LktC mutant SH2099 induces necrosis in BHS PMNs. BHS PMNs were labeled with annexin V and PI following treatment with SH2099 or SH1217 leukotoxin-containing culture fluid. The flow cytometry scatter plots show the percentages of cells that are positive for PI alone (upper left quadrant), for annexin alone (lower right quadrant), and for PI and annexin (upper right quadrant) and negative for PI and annexin (lower left quadrant). The results shown are representative of the results of three independent experiments.
Lkt from the LktC mutant SH2099 causes budding and pore formation in plasma membranes of BHS PMNs.
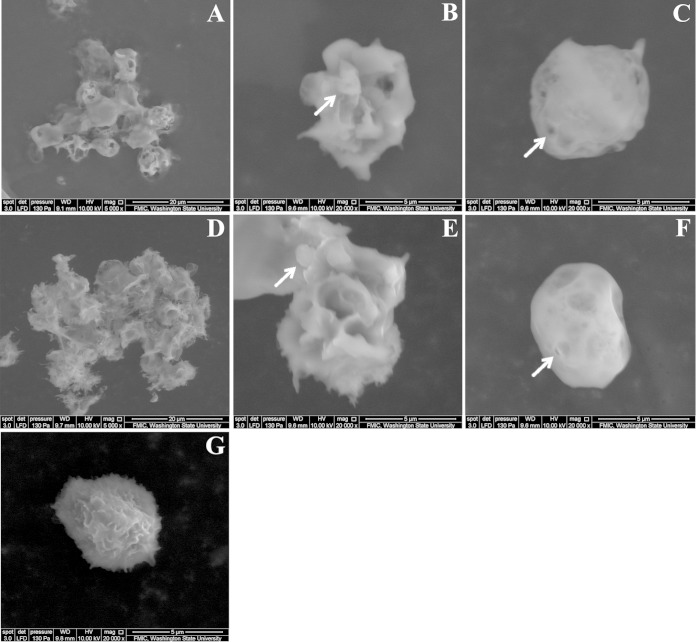
Scanning electron micrographs of BHS PMNs treated with SH2099 Lkt or SH1217 Lkt are shown in Fig. 3. Membrane budding indicative of apoptosis and pore formation indicative of necrosis were observed following treatment of cells with either toxin (Fig. 3). At a lower magnification (×5,000), extensive cell-cell adhesion, indicative of leaky cells, was also observed (Fig. 3).
FIG 3.
Mutant Lkt-treated cells exhibit pores and blebs on their membranes. Scanning electron microscopy was used to analyze BHS PMNs treated with Lkt from the parent, SH1217 (A, B, and C), or the mutant SH2099 (D, E, and F) strain. Extensive cell-cell adhesion in SH1217 Lkt-treated cells (A) and SH2099 Lkt-treated cells (D) was observed at ×5,000 magnification. Blebs (B and E) and pores (C and F) are indicated by arrows on the membranes of SH1217 and SH2099 Lkt-treated cells (×20,000 magnification). (G) An untreated BHS PMN.
The parental strain, SH1217, Lkt, but not the mutant strain SH2099 Lkt, is acylated at lysine554.
The lysine residue at amino acid position 554 in M. haemolytica Lkt has been predicted to be acylated based on homology to other RTX toxins and an observed decrease in toxin activity following amino acid changes at the residue (29). In this study, we also investigated plausible fatty acid modifications on lysine669, which, in alignment with E. coli hemolysin, is the closest glycine-lysine pair to the second acylated lysine residue on hemolysin. Hence, we used mass spectrometry to analyze the tryptic fragments of Lkt produced by SH1217 and SH2099 to determine whether lysine554 and lysine669 are acylated. The mass spectrometric analysis of acylated proteins requires consideration of the fact that the fatty acid chains on the acylated lysine residues (and arginine residues) prevent cleavage carboxy-terminal to modified lysine (and arginine) residues. Furthermore, if tryptic digestion is performed following in vitro acetylation, tryptic cleavage occurs following arginine residues, but not after lysine residues, because acetylation of lysine prevents cleavage carboxy terminal to lysine residues. Therefore, tryptic digestion of an acetylated protein would confirm the presence of unacylated lysines in the protein. Hence, acylation of lysine554 in Lkt would result in a tryptic peptide spanning amino acids (aa) 550 to 560 containing the acylated lysine554. On the other hand, lack of acylation of lysine554 would result in tryptic cleavage C terminal to lysine554, which would not result in a tryptic peptide spanning aa 550 to 560. As shown in Table 1, tryptic cleavage of Lkt from SH1217, but not SH2099, resulted in peptide 550–560, indicating that lysine554 was acylated in Lkt from the parental strain, SH1217, but not in Lkt from the mutant SH2099. The lysine554 on this peptide was modified with the addition of either a hydroxy myristic acid or myristic acid side chain. Moreover, tryptic digestion of in vitro acetylated Lkt resulted in the 550-to-565 fragment containing acetylated lysine554 from SH2099 Lkt, but not from SH1217 Lkt, further confirming the presence of free lysine554 in SH2099 Lkt and acylated lysine554 in SH1217 Lkt.
TABLE 1.
Summary of LC–MS-MS studies on SH2099 Lkt
| Lysine position | Leukotoxin | Tryptic fragment observed | Sequence | Lysine modification |
|---|---|---|---|---|
| 554 | SH2099 | None | ||
| SH1217 | 550–560 | VQTGKYEYITK | Lys 554 C14 | |
| Lys 554 C14 OH | ||||
| SH2099 acetylated | 550–565 | VQTGKYEYITKLNIVR | Lys 554 acetyl | |
| Lys 560 acetyl | ||||
| SH1217 acetylated | 550–565 | VQTGKYEYITKLNIVR | Lys 554 C14 | |
| Lys 554 C14 OH | ||||
| Lys 560 acetyl | ||||
| 669 | SH2099 | 670–685a | ALHEVTSTHTALVGNR | Not observed |
| SH1217 | 670–685a | ALHEVTSTHTALVGNR | Not observed | |
| SH2099 acetylated | 664–685 | FVETGKALHEVTSTHTALVGNR | Lys 669 acetyl | |
| SH1217 acetylated | 664–685 | FVETGKALHEVTSTHTALVGNR | Lys 669 acetyl |
Peptide fragment produced due to lysine669.
In contrast, the lysine669 was not acylated in Lkt from mutant SH2099 or the parent SH1217, as revealed by the absence of the tryptic 670-to-685 fragment. Lack of acylation on both toxins was confirmed by the presence of the tryptic 664-to-685 fragment containing acetylated lysine669.
DISCUSSION
Functional in vitro studies with an LktA toxin produced from an LktC deletion mutant strain evaluating the production of reactive oxygen metabolites in bovine PMNs and interleukin 8 (IL-8) in bovine alveolar macrophages (BAMs) and release of Ca2+ ions in PMNs and BAMs suggested that LktC-mediated acylation was necessary to activate the LktA protoxin (20). Another study with an isogenic LktC mutant showed that the mutant toxin had minimal to no cell cytotoxicity and was incapable of inducing apoptosis on bovine peripheral blood lymphocytes, again suggesting that LktC-mediated acylation is necessary for the activation of the LktA protoxin (21). In summary, while the ability of neutrophils and macrophages to form extracellular traps is not affected by the acylation status of Lkt (30, 31), acylation of LktA is required for IL-8 cytokine production, Ca2+ ion release, apoptosis induction, and cytotoxicity in bovine leukocytes. However, the LktC mutant SH2099 previously developed by us (S. K. Highlander) by creating a frameshift mutation in lktC, was only partially attenuated in its virulence against cattle (22). Therefore, it was of interest to us to elucidate the role of acylation in Lkt-induced cytotoxicity. We reasoned that a more susceptible target cell would be a better candidate for elucidating the molecular basis underlying the activation of LktA protoxin by LktC-mediated acylation. The rationale for using BHS in this study is that PMNs of the species are more susceptible to Lkt than those of other ruminants, including domestic sheep and cattle (23).
Our observation that 40% of the mutant-inoculated BHS developed fatal pneumonia was the first indication that acylation may not be absolutely necessary for the toxic activity of Lkt. The presence of a large number of necrotic PMNs in the lungs of dead BHS supported this notion. It is noteworthy that these animals were inoculated with 1 × 104 CFU, a dose 10-fold lower than the lethal dose of 1 × 105 CFU previously established in our laboratory (25). Due to limited availability of BHS, we could not include control groups of animals that were inoculated with the SH1217 parental wild-type strain or RPMI medium, which could be a possible caveat in interpreting our data. Nevertheless, in our earlier studies involving serotype A1 of M. haemolytica, we demonstrated that Lkt of M. haemolytica is necessary for the induction of fatal pneumonia in BHS (6). In our animal experiments, it was also of interest to us to evaluate whether the LktC mutant SH2099 would be attenuated enough to serve as a possible vaccine candidate in BHS, although with the progression of our animal experiments, it became clearly evident that the mutant strain was unsuitable. The surviving animal was reinoculated merely with the intention of administering a booster inoculation.
Lysis of BHS PMNs by the mutant toxin in in vitro cytotoxicity assays (Fig. 1) further confirmed the notion that acylation is not necessary for the toxic activity of Lkt. Lkts from all 30 of the single colonies obtained by subculturing the mutant were cytotoxic to BHS PMNs and not to BL3 cells, ruling out the possibility that the mutant was contaminated with the parental strain. These observations prompted us to further characterize the mutant Lkt. Our finding that the mutant Lkt induced necrosis in BHS PMNs comparable to that induced by its wild-type parental strain (Fig. 2) suggested that acylation is not necessary for the toxic activity of LktA protoxin. This view was further supported by scanning electron microscopy on mutant Lkt-treated cells, which revealed pores and blebs on the membranes of dead/dying cells. However, direct comparisons between the two toxins cannot be made, since the toxin preparations were not quantified. Nevertheless, we did use similar numbers of CFU of bacteria, growth medium volumes, and incubation times in our Lkt preparations from either the SH1217 or SH2099 strain to normalize them. Moreover, the same batch of Lkt was used in all the in vitro assays. The toxin produced by the SH1217 strain was used only as a positive control for the assay.
Taken together, these observations strongly suggested that acylation is not absolutely necessary for Lkt-induced apoptosis and necrosis of target cells. However, we wanted to confirm that the frameshift mutation that was used to create the mutant in fact abrogated acylation of the Lkt. Mass spectrometric analysis indicated that the toxin produced by the wild-type SH1217 strain of M. haemolytica has only one lysine residue (lysine554) modified by C14 and C14 OH fatty acid. This is in contrast to other members of the RTX toxin family, including A. actinomycetemcomitans leukotoxin, E. coli hemolysin (HlyA), and Bordetella pertussis adenylate cyclase toxin-hemolysin (CyaA), where two lysine residues are acylated (32–34). The fact that peptide 550–560 resulted from tryptic cleavage of the parental strain, SH1217, but not the mutant SH2099 confirmed that lysine554 was acylated by C14 and C14 OH fatty acid in Lkt from the parent, but not from the mutant strain SH2099. The presence of peptide 550–565 containing acetylated lysine554 in the tryptic digest of acetylated Lkt from the mutant further confirmed the presence of unacylated lysine554 in the mutant toxin.
The SH2099 mutant, when cultured on blood agar plates, produces nonhemolytic colonies, in contrast to the parent strain, SH1217 (22). We and others have previously shown that Lkt-induced cytotoxicity of leukocytes is mediated by the interaction of Lkt with CD18, the β subunit of β2-integrins (13, 35). However, similar ligand-receptor interaction has not been identified for the hemolytic activity. Moreover, loss of activity on erythrocytes following abrogation of acylation has been reported for other RTX toxins, including B. pertussis toxin CyaA and E. coli toxin HlyA (31, 36). Therefore, it is tempting to speculate that the posttranslational modification of LktA by LktC-induced acylation confers hemolytic ability on LktA.
The previous studies that found LktC-mediated acylation necessary for Lkt-induced cytotoxicity were performed with serotype 1 which is the predominant serotype of M. haemolytica in bovine pneumonia (37, 38). Serotype 2 is predominant in pneumonia in domestic sheep (37, 38). However, BHS are susceptible to both serotypes 1 and 2 of M. haemolytica (6, 25, 39–41). This study was conducted with serotype 1. It is unlikely that different serotypes possess different activation pathways for the Lkt. There are no reports in the literature that suggest otherwise. This is true for other RTX toxins, as well.
In summary, we propose that LktC-mediated acylation of the LktA protoxin is unlikely to confer any qualitative characteristic necessary for the toxic activity of the protoxin. Rather, the added fatty acids, by interacting with the lipids in the plasma membrane, may facilitate the binding of Lkt to its receptor, CD18, thereby enhancing its toxic activity. Thus, acylation is not required for the cytotoxic activity of M. haemolytica leukotoxin but enhances its potency.
ACKNOWLEDGMENTS
This research was supported by funds from the Wild Sheep Foundation and its state chapters, Rocky Mountain Bighorn Society, Nevada Bighorns Unlimited, and the U.S. Forest Service. Sai Arun Batra was supported by a Graduate Training Fellowship from Morris Animal Foundation.
Help with flow cytometric analysis provided by Reginaldo Bastos and the Flow Cytometry Laboratory at the Washington State University Department of Veterinary Microbiology and Pathology is gratefully acknowledged. Assistance with electron microscopic analysis provided by Valerie Jean Lynch-Holm and Christine Mary Davitt at the Franceschi Microscopy and Imaging Center is also appreciated.
REFERENCES
- 1.Ackermann MR, Brogden KA. 2000. Response of the ruminant respiratory tract to Mannheimia (Pasteurella) haemolytica. Microbes Infect 2:1079–1088. doi: 10.1016/S1286-4579(00)01262-4. [DOI] [PubMed] [Google Scholar]
- 2.Miller MW. 2008. Pasteurellosis, p 330–339. In Williams ES, Barker IK (ed), Infectious diseases of wild mammals. Iowa State University Press, Ames, IA. [Google Scholar]
- 3.Mosier DA. 1997. Bacterial pneumonia. Vet Clin North Am Food Anim Pract 13:483–493. [DOI] [PubMed] [Google Scholar]
- 4.Griffin D. 1997. Economic impact associated with respiratory disease in beef cattle. Vet Clin North Am Food Anim Pract 13:367–377. [DOI] [PubMed] [Google Scholar]
- 5.Valdez R, Krausman PR. 1999. Mountain sheep of North America. University of Arizona Press, Tucson, AZ. [Google Scholar]
- 6.Dassanayake RP, Shanthalingam S, Herndon CN, Lawrence PK, Frances Cassirer E, Potter KA, Foreyt WJ, Clinkenbeard KD, Srikumaran S. 2009. Mannheimia haemolytica serotype A1 exhibits differential pathogenicity in two related species, Ovis canadensis and Ovis aries. Vet Microbiol 133:366–371. doi: 10.1016/j.vetmic.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Onderka DK, Wishart WD. 1988. Experimental contact transmission of Pasteurella haemolytica from clinically normal domestic sheep causing pneumonia in Rocky Mountain bighorn sheep. J Wildl Dis 24:663–667. doi: 10.7589/0090-3558-24.4.663. [DOI] [PubMed] [Google Scholar]
- 8.Foreyt WJ. 1994. Effects of controlled contact exposure between healthy bighorn sheep and llamas, domestic goats, mountain goats, cattle, domestic sheep, or mouflon sheep. Biennial Symp North Wild Sheep Goat Council 9:7–14. [Google Scholar]
- 9.Petras SF, Chidambaram M, Illyes EF, Froshauer S, Weinstock GM, Reese CP. 1995. Antigenic and virulence properties of Pasteurella haemolytica leukotoxin mutants. Infect Immun 63:1033–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tatum FM, Briggs RE, Sreevatsan SS, Zehr ES, Ling Hsuan S, Whiteley LO, Ames TR, Maheswaran SK. 1998. Construction of an isogenic leukotoxin deletion mutant of Pasteurella haemolytica serotype 1: characterization and virulence. Microb Pathog 24:37–46. doi: 10.1006/mpat.1997.0181. [DOI] [PubMed] [Google Scholar]
- 11.Kaehler KL, Markham RJ, Muscoplat CC, Johnson DW. 1980. Evidence of species specificity in the cytocidal effects of Pasteurella haemolytica. Infect Immun 30:615–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanthalingam S, Srikumaran S. 2009. Intact signal peptide of CD18, the β-subunit of β2-integrins, renders ruminants susceptible to Mannheimia haemolytica leukotoxin. Proc Natl Acad Sci U S A 106:15448–15453. doi: 10.1073/pnas.0906775106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deshpande MS, Ambagala TC, Ambagala AP, Kehrli ME Jr, Srikumaran S. 2002. Bovine CD18 is necessary and sufficient to mediate Mannheimia (Pasteurella) haemolytica leukotoxin-induced cytolysis. Infect Immun 70:5058–5064. doi: 10.1128/IAI.70.9.5058-5068.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shewen PE, Wilkie BN. 1982. Cytotoxin of Pasteurella haemolytica acting on bovine leukocytes. Infect Immun 35:91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maheswaran SK, Weiss DJ, Kannan MS, Townsend EL, Reddy KR, Whiteley LO, Srikumaran S. 1992. Effects of Pasteurella haemolytica A1 leukotoxin on bovine neutrophils: degranulation and generation of oxygen-derived free radicals. Vet Immunol Immunopathol 33:51–68. doi: 10.1016/0165-2427(92)90034-N. [DOI] [PubMed] [Google Scholar]
- 16.Yoo HS, Rajagopal BS, Maheswaran SK, Ames TR. 1995. Purified Pasteurella haemolytica leukotoxin induces expression of inflammatory cytokines from bovine alveolar macrophages. Microb Pathog 18:237–252. doi: 10.1016/S0882-4010(05)80001-4. [DOI] [PubMed] [Google Scholar]
- 17.Strathdee CA, Lo RY. 1989. Cloning, nucleotide sequence, and characterization of genes encoding the secretion function of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol 171:916–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Highlander SK, Chidambaram M, Engler MJ, Weinstock GM. 1989. DNA sequence of the Pasteurella haemolytica leukotoxin gene cluster. DNA 8:15–28. doi: 10.1089/dna.1.1989.8.15. [DOI] [PubMed] [Google Scholar]
- 19.Highlander SK, Engler MJ, Weinstock GM. 1990. Secretion and expression of the Pasteurella haemolytica leukotoxin. J Bacteriol 172:2343–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thumbikat P, Briggs RE, Kannan MS, Maheswaran SK. 2003. Biological effects of two genetically defined leukotoxin mutants of Mannheimia haemolytica. Microb Pathog 34:217–226. doi: 10.1016/S0882-4010(03)00033-0. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, Clinkenbeard KD, Clarke C, Cudd L, Highlander SK, Dabo SM. 1999. Pasteurella haemolytica leukotoxin induced apoptosis of bovine lymphocytes involves DNA fragmentation. Vet Microbiol 65:153–166. doi: 10.1016/S0378-1135(98)00286-7. [DOI] [PubMed] [Google Scholar]
- 22.Highlander SK, Fedorova ND, Dusek DM, Panciera R, Alvarez LE, Rinehart C. 2000. Inactivation of Pasteurella (Mannheimia) haemolytica leukotoxin causes partial attenuation of virulence in a calf challenge model. Infect Immun 68:3916–3922. doi: 10.1128/IAI.68.7.3916-3922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silflow RM, Foreyt WJ, Leid RW. 1993. Pasteurella haemolytica cytotoxin-dependent killing of neutrophils from bighorn and domestic sheep. J Wildl Dis 29:30–35. doi: 10.7589/0090-3558-29.1.30. [DOI] [PubMed] [Google Scholar]
- 24.Gentry MJ, Srikumaran S. 1991. Neutralizing monoclonal antibodies to Pasteurella haemolytica leukotoxin affinity-purify the toxin from crude culture supernatants. Microb Pathog 10:411–417. doi: 10.1016/0882-4010(91)90086-P. [DOI] [PubMed] [Google Scholar]
- 25.Subramaniam R, Shanthalingam S, Bavananthasivam J, Kugadas A, Potter KA, Foreyt WJ, Hodgins DC, Shewen PE, Barrington GM, Knowles DP, Srikumaran S. 2011. A multivalent Mannheimia-Bibersteinia vaccine protects bighorn sheep against Mannheimia haemolytica challenge. Clin Vaccine Immunol 18:1689–1694. doi: 10.1128/CVI.05276-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ambagala TC, Ambagala APN, Srikumaran S. 1999. The leukotoxin of Pasteurella haemolytica binds to β2 integrins on bovine leukocytes. FEMS Microbiol Lett 179:161–167. [DOI] [PubMed] [Google Scholar]
- 27.Plazas-Mayorca MD, Zee BM, Young NL, Fingerman IM, LeRoy G, Briggs SD, Garcia BA. 2009. One-pot shotgun quantitative mass spectrometry characterization of histones. J Proteome Res 8:5367–5374. doi: 10.1021/pr900777e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macmillan H, Brayton KA, Palmer GH, McGuire TC, Munske G, Siems WF, Brown WC. 2006. Analysis of the Anaplasma marginale major surface protein 1 complex protein composition by tandem mass spectrometry. J Bacteriol 188:4983–4991. doi: 10.1128/JB.00170-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellett S, Welch RA. 1996. Escherichia coli hemolysin mutants with altered target cell specificity. Infect Immun 64:3081–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aulik NA, Hellenbrand KM, Klos H, Czuprynski CJ. 2010. Mannheimia haemolytica and its leukotoxin cause neutrophil extracellular trap formation by bovine neutrophils. Infect Immun 78:4454–4466. doi: 10.1128/IAI.00840-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aulik NA, Hellenbrand KM, Czuprynski CJ. 2012. Mannheimia haemolytica and its leukotoxin cause macrophage extracellular trap formation by bovine macrophages. Infect Immun 80:1923–1933. doi: 10.1128/IAI.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanley P, Packman LC, Koronakis V, Hughes C. 1994. Fatty acylation of two internal lysine residues required for the toxic activity of Escherichia coli hemolysin. Science 266:1992–1996. doi: 10.1126/science.7801126. [DOI] [PubMed] [Google Scholar]
- 33.Masin J, Basler M, Knapp O, El-Azami-El-Idrissi M, Maier E, Konopasek I, Benz R, Leclerc C, Sebo P. 2005. Acylation of lysine 860 allows tight binding and cytotoxicity of Bordetella adenylate cyclase on CD11b-expressing cells. Biochemistry 44:12759–12766. doi: 10.1021/bi050459b. [DOI] [PubMed] [Google Scholar]
- 34.Fong KP, Tang HY, Brown AC, Kieba IR, Speicher DW, Boesze-Battaglia K, Lally ET. 2011. Aggregatibacter actinomycetemcomitans leukotoxin is post-translationally modified by addition of either saturated or hydroxylated fatty acyl chains. Mol Oral Microbiol 26:262–276. doi: 10.1111/j.2041-1014.2011.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Clinkenbeard KD, Ritchey JW. 1999. Bovine CD18 identified as a species specific receptor for Pasteurella haemolytica leukotoxin. Vet Microbiol 67:91–97. doi: 10.1016/S0378-1135(99)00040-1. [DOI] [PubMed] [Google Scholar]
- 36.Valeva A, Walev I, Kemmer H, Weis S, Siegel I, Boukhallouk F, Wassenaar TM, Chavakis T, Bhakdi S. 2005. Binding of Escherichia coli hemolysin and activation of the target cells is not receptor-dependent. J Biol Chem 280:36657–36663. doi: 10.1074/jbc.M507690200. [DOI] [PubMed] [Google Scholar]
- 37.Highlander SK. 2001. Molecular genetics analysis of virulence in Mannheimia (Pasteurella) haemolytica. Front Biosci 6:D1128–D1150. doi: 10.2741/Highland. [DOI] [PubMed] [Google Scholar]
- 38.Zecchinon L, Fett T, Desmecht D. 2005. How Mannheimia haemolytica defeats host defence through a kiss of death mechanism. Vet Res 36:133–156. doi: 10.1051/vetres:2004065. [DOI] [PubMed] [Google Scholar]
- 39.Dassanayake RP, Shanthalingam S, Herndon CN, Subramaniam R, Lawrence PK, Bavananthasivam J, Cassirer EF, Haldorson GJ, Foreyt WJ, Rurangirwa FR, Knowles DP, Besser TE, Srikumaran S. 2010. Mycoplasma ovipneumoniae can predispose bighorn sheep to fatal Mannheimia haemolytica pneumonia. Vet Microbiol 145:354–359. doi: 10.1016/j.vetmic.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Foreyt WJ, Snipes KP, Kasten RW. 1994. Fatal pneumonia following inoculation of healthy bighorn sheep with Pasteurella haemolytica from healthy domestic sheep. J Wildl Dis 30:137–145. doi: 10.7589/0090-3558-30.2.137. [DOI] [PubMed] [Google Scholar]
- 41.Foreyt WJ, Silflow RM. 1996. Attempted protection of bighorn sheep (Ovis canadensis) from pneumonia using a nonlethal cytotoxic strain of Pasteurella haemolytica, biotype A, serotype. J Wildl Dis 32:315–321. doi: 10.7589/0090-3558-32.2.315. [DOI] [PubMed] [Google Scholar]