Abstract
Inflammation caused by infection with Gram-positive bacteria is typically initiated by interactions with Toll-like receptor 2 (TLR2). Endophthalmitis, an infection and inflammation of the posterior segment of the eye, can lead to vision loss when initiated by a virulent microbial pathogen. Endophthalmitis caused by Bacillus cereus develops as acute inflammation with infiltrating neutrophils, and vision loss is potentially catastrophic. Residual inflammation observed during B. cereus endophthalmitis in TLR2−/− mice led us to investigate additional innate pathways that may trigger intraocular inflammation. We first hypothesized that intraocular inflammation during B. cereus endophthalmitis would be controlled by MyD88- and TRIF-mediated signaling, since MyD88 and TRIF are the major adaptor molecules for all bacterial TLRs. In MyD88−/− and TRIF−/− mice, we observed significantly less intraocular inflammation than in eyes from infected C57BL/6J mice, suggesting an important role for these TLR adaptors in B. cereus endophthalmitis. These results led to a second hypothesis, that TLR4, the only TLR that signals through both MyD88 and TRIF signaling pathways, contributed to inflammation during B. cereus endophthalmitis. Surprisingly, B. cereus-infected TLR4−/− eyes also had significantly less intraocular inflammation than infected C57BL/6J eyes, indicating an important role for TLR4 in B. cereus endophthalmitis. Taken together, our results suggest that TLR4, TRIF, and MyD88 are important components of the intraocular inflammatory response observed in experimental B. cereus endophthalmitis, identifying a novel innate immune interaction for B. cereus and for this disease.
INTRODUCTION
Bacillus cereus is a Gram-positive, spore-forming, and beta-hemolytic soil bacterium (1). Commonly identified as a causative agent of foodborne illnesses, B. cereus is also associated with a multitude of clinical conditions, such as central nervous system infections (2), pneumonia (3), endocarditis (4), and gas-gangrene-like cutaneous infections (5). B. cereus also causes a virulent form of endophthalmitis, an intraocular inflammatory condition resulting from the introduction of microorganisms into the posterior segment of the eye following surgery or injury or from a distant site of infection. This infection causes irreversible damage to the retina, often leading to vision loss within 1 or 2 days (6). Typically, B. cereus endophthalmitis occurs following a penetrating eye injury (posttraumatic) with retained intraocular foreign bodies (7, 8) but has also been reported in postoperative patients (9–11). Fewer than 30% of posttraumatic B. cereus endophthalmitis patients retained useful vision, and out of these, only 9% retained 20/70 vision or better (7, 12). Moreover, 48% of B. cereus and other Bacillus species infections required evisceration or enucleation of the eye despite therapeutic intervention (7, 12). Intraocular inflammation that occurs during B. cereus endophthalmitis interferes with the clarity of the visual axis, contributing to disruptions in vision. Characterizing the inflammatory pathways that intensify the infection will help in identifying targets for clinical use. This is especially important as current treatment regimens for B. cereus and other types of endophthalmitis involving the use of corticosteroids are often insufficient in arresting inflammation (12–16).
Initial recognition of bacteria during the early stages of infection is critical in mounting an effective immune response. Toll-like receptors (TLRs) initially recognize bacterial components and induce an inflammatory response in an attempt to clear the pathogen. TLRs are important in mounting an ocular inflammatory response to bacteria during keratitis (17–20), uveitis (21–24), and endophthalmitis (25–27). During Bacillus endophthalmitis, TLR-mediated bacterial recognition results in proinflammatory mediator synthesis and an influx of polymorphonuclear neutrophils (PMN), which have the potential to cause irreversible bystander damage to interior tissues of the eye. We previously reported the significant contribution of TLR2 (25) and a limited role for TLR5 (28) in the pathogenesis of B. cereus endophthalmitis. However, the presence of residual inflammation in the eyes of TLR2−/− mice infected with B. cereus suggested the involvement of additional innate immune recognition and signaling mechanisms in intraocular inflammation during this disease.
To analyze the functional significance of other TLRs and signaling mechanisms in this infection, we first hypothesized that the intraocular inflammatory response to B. cereus would be diminished in the absence of myeloid differentiation primary response gene 88 (MyD88)-mediated signaling, since MyD88 is a major adaptor molecule for all bacterial TLRs (29). We also analyzed the contribution of the adaptor TRIF (TIR domain-containing adapter inducing interferon beta), which also mediates signaling of TLR3 and TLR4 following ligand recognition (30). Using MyD88−/− and TRIF−/− mice, we confirmed a significant role for these TLR adaptors in intraocular inflammation during B. cereus endophthalmitis, as there was a significant reduction in intraocular inflammation in both strains of knockout mice. These findings led to our second hypothesis, that TLR4, which signals through MyD88 and TRIF, was also important in this inflammatory response. Certainly, B. cereus is a Gram-positive bacterium that does not synthesize lipopolysaccharide (LPS), a canonical TLR4 ligand. Surprisingly, intraocular inflammation was significantly reduced in the eyes of B. cereus-infected TLR4−/− mice, suggesting that TLR4 was also important in mediating inflammation during Bacillus endophthalmitis. To our knowledge, this is the first study to implicate the TLR4/TRIF pathway in intraocular infection with Gram-positive bacteria.
MATERIALS AND METHODS
Ethics statement.
The experiments described here involved the use of mice. All procedures were conducted according to guidelines and recommendations of the Guide for the Care and Use of Laboratory Animals (31), the University of Oklahoma Health Sciences Center (OUHSC) IACUC, and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. The OUHSC IACUC approved these studies under protocols 11-068, 11-090, 13-086, and 14-012.
Experimental B. cereus endophthalmitis.
C57BL/6J mice were purchased from commercially available stock colonies (stock no. 000664; Jackson Laboratory, Bar Harbor, ME). The original breeding pair of MyD88−/− mice on the C57BL/6J background was a kind gift from Ira Blader (University at Buffalo, State University of New York). The original breeding pair of TRIF−/− mice on the C57BL/6J background was purchased from the Jackson Laboratory (C57BL/6J-Ticam1Lps2/J, stock no. 005037). The original breeding pair of TLR4−/− mice on the C57BL/6 background was a generous gift from Eric Pearlman (University of California, Irvine). The TLR4−/− and MyD88−/− mice were used with the permission of Shizou Akira (Hyogo College of Medicine, Hyogo, Japan) (32, 33). Following rederivation at the Jackson Laboratory, MyD88−/− and TLR4−/− mice were bred on the C57BL/6J background and were maintained under barrier facility conditions on a 12-h-on/12-h-off light cycle. Vendor-supplied or weaned animals were accustomed to conventional housing under biosafety level 2 conditions for at least 2 weeks before use and were used in experiments at 8 to 10 weeks of age.
Experimental endophthalmitis was initiated in mouse eyes by injecting ∼100 CFU B. cereus strain ATCC 14579 into the midvitreous of one eye by using a sterile glass capillary needle, as previously described (34, 35). During the course of infection, eyes were analyzed by electroretinography (ERG) before harvesting for assays comparing intraocular bacterial growth and inflammation, as described below (34, 35). The time points chosen for analysis are based on those reported in previous studies using this model, which mimics the rapid evolution and severity of human clinical endophthalmitis caused by B. cereus (6–14, 25, 28, 34, 35).
Electroretinography.
Retinal function was analyzed by ERG as previously described (25, 28, 34). ERG is a technique used to analyze retinal function. The retina is stimulated by a transient flash of light, and the response results from electrical responses in the form of graded potentials evoked in each layer of the retina. Following infection and immediately prior to ERG analysis, animals were dark adapted for a minimum of 6 h. Scotopic ERGs were performed at 8 and 12 h postinfection (Espion E2; Diagnosys LLC, Lowell, MA). After dark adaptation, mice were anesthetized, and pupils were dilated with topical phenylephrine (10%; Akorn, Inc., IL). Retinal function was recorded with gold wire electrodes placed on each cornea and a reference electrode. The maximal white light stimulus of 1,200 cd · s/m2 (flash luminance) used to evoke the retinal response was delivered by a white sphere that resembles a Ganzfeld. The interval between two flashes was 45 s to prevent light adaptation to the flashes. The animals were exposed to five flashes of light, and the average amplitude was used. As an indicator of retinal function, the A-wave and B-wave amplitudes were measured from the initiation of the light flash to the trough of the A-wave and from the trough of the A-wave to the peak of the B-wave. The A-wave provides a direct measure of photoreceptor activity, while the B-wave represents the action of Muller cells, bipolar cells, and second-order neurons (amacrine and ganglion cells). A- and B-wave amplitudes were recorded for infected and contralateral uninfected eyes in the same animal. The percentage of retinal function retained in the infected eye compared to that of the uninfected-left-eye controls was computed as 100 − {[1 − (experimental A- or B-wave amplitude/control A- or B-wave amplitude)] × 100}. Values represent the means ± standard errors of the means (SEM) for ≥6 eyes per time point. Experiments were performed at least 3 independent times. Representative wave forms of uninfected and infected eyes are depicted in Fig. S1 in the supplemental material.
Intraocular bacterial growth.
Intraocular growth of B. cereus in infected eyes harvested at 4, 8, and 12 h postinfection was quantified as previously described (25, 28, 34). Eyes were homogenized with 1-mm sterile glass beads (Biospec Products, Inc., Bartlesville, OK) in phosphate-buffered saline (PBS). Suspensions were then track diluted onto brain heart infusion agar and quantified. Values represent the means ± SEM for ≥4 eyes per time point. Experiments were performed at least 3 independent times.
PMN influx.
PMN influx into the eye was estimated by quantifying myeloperoxidase (MPO) levels in whole-eye homogenates at 0, 4, 8, and 12 h postinfection by a sandwich enzyme-linked immunosorbent assay (ELISA) (mouse MPO ELISA kit; Hycult Biotech, Plymouth Meeting, PA), as previously described (25, 28). Homogenates of uninfected eyes served as negative controls. The lower limit of detection for this assay was 2 ng/ml. Values represent the means ± SEM for ≥5 eyes per group per time point. Experiments were performed at least 3 independent times.
Inflammatory mediator expression.
Ocular proinflammatory cytokine and chemokine expression was quantified by an ELISA as previously described (25, 28). Eyes were harvested at 4, 8, and 12 h postinfection and homogenized in PBS containing a protease inhibitor cocktail (Roche Diagnostics, IN). Concentrations of interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), IL-1β, or keratinocyte chemoattractant (KC or CXCL1) were quantified by using commercial ELISA kits (Quantikine; R&D Systems, Minneapolis, MN). The lower limit of detection for all ELISAs was 2 pg/ml. Values represent means ± SEM for ≥5 eyes/time point. Experiments were performed at least 2 independent times.
Histology.
Uninfected eyes from each mouse strain and eyes from each infection group were harvested at 4, 8, and 12 h postinfection. Whole globes were incubated in buffered zinc formalin or Davison's fixative for 24 h at room temperature (25, 28). Globes were then transferred to 70% ethanol, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Images are representative of at least 4 eyes per time point from at least 3 independently conducted experiments.
Phagocytosis and killing.
Neutrophils from C57BL/6J and TLR4−/− mice were isolated and purified as described previously (36). Casein was intraperitoneally injected to induce a neutrophil infiltrate in the peritoneal cavity. Neutrophils were then isolated by using Lymphoprep (Axis-Shield PoC, Norway) as the density separating medium. The viability of the purified neutrophils was confirmed by trypan blue viability and Giemsa staining. The viability of these preparations was >95%.
Analysis of phagocytosis and killing activity was performed with freshly isolated neutrophils from C57BL/6J and TLR4−/− mice (37, 38). B. cereus strain ATCC 14579 and neutrophils were incubated separately in PBS containing 10% heat-inactivated C57BL/6J mouse serum at 37°C (Valley Biomedical Products, Winchester, VA). Bacteria and neutrophils were mixed at ratios of 1:1 and 10:1 (bacteria to neutrophils). Bacteria in 10% serum were used as a control. Samples were analyzed at 30 min and 1 h. All samples were centrifuged, and pellets were washed three times with ice-cold PBS. All wash supernatants were pooled. Neutrophil pellets were lysed with ice-cold PBS plus 0.5% saponin (Sigma-Aldrich, St. Louis, MO). Bacteria in the supernatant (extracellular bacteria) and pellet (intracellular bacteria) were quantified by track dilution. These numbers were compared to the value for the control input at each time point to estimate the extent of bacterial killing and phagocytosis. Results are reported as means ± SEM for ≥4 assays per group per time point for at least 3 different experiments.
Statistics.
Data are represented as the arithmetic means ± SEM for all samples in the same experimental group in replicate experiments. The Mann-Whitney rank sum test was used compare experimental groups to determine the statistical significance for all assays. A P value of <0.05 was considered statistically significant. All statistical analyses were performed by using SigmaPlot 13.0 (Systat Software, Inc., San Jose, CA).
RESULTS
Contribution of TLR adaptors MyD88 and TRIF to experimental B. cereus endophthalmitis. (i) Effect of MyD88 or TRIF deficiency on intraocular bacterial growth.
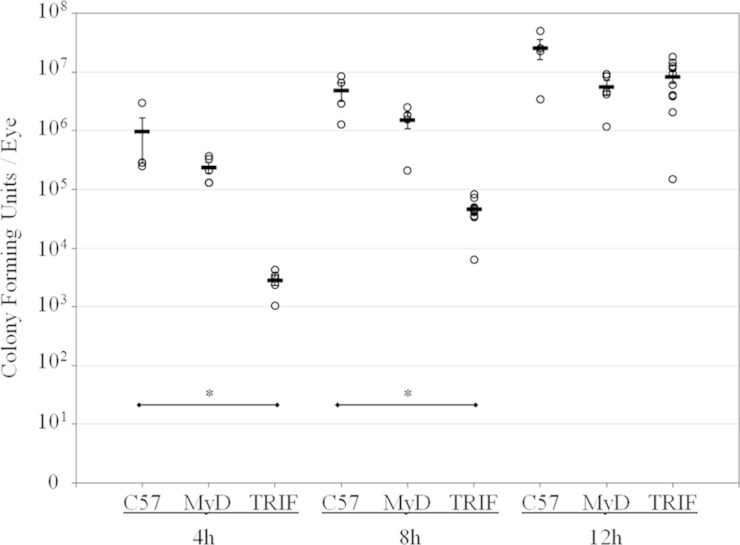
The intraocular bacterial growth during B. cereus infection in C57BL/6J, TRIF−/−, and MyD88−/− eyes is shown in Fig. 1. The starting inoculum for all groups was 113 ± 10 CFU. B. cereus growth in MyD88−/− eyes was similar to that in C57BL/6J controls at 4, 8, and 12 h (P ≥ 0.19). B. cereus growth in infected TRIF−/− eyes was significantly less than that in infected C57BL/6J control and MyD88−/− eyes at 4 and 8 h postinfection (P ≤ 0.016). By 12 h postinfection, B. cereus growth in TRIF−/− eyes was similar to that in infected C57BL/6J and MyD88−/− eyes (P = 0.104). The absence of TRIF, but not MyD88, negatively affected B. cereus growth during the early stages of infection. However, the absence of these adaptors did not significantly affect the overall growth of B. cereus during the later stages of infection.
FIG 1.
Intraocular bacterial growth in MyD88−/− and TRIF−/− eyes infected with B. cereus. C57BL/6J, MyD88−/−, and TRIF−/− mouse eyes were intravitreally injected with 100 CFU B. cereus. Eyes were harvested at 4, 8, and 12 h postinfection, and bacteria were quantified. B. cereus grew to similar concentrations in infected C57BL/6J and MyD88−/− eyes at all time points (P ≥ 0.19). Bacterial counts in infected TRIF−/− eyes were significantly lower than those in infected C57BL/6J eyes at 4 and 8 h postinfection (P ≤ 0.016). At 12 h postinfection, B. cereus concentrations in infected C57BL/6J and TRIF−/− eyes were similar (P = 0.104). Values represent the means ± SEM for ≥4 eyes per time point with at least three independent experiments. *, P ≤ 0.016.
(ii) MyD88- or TRIF-dependent intraocular inflammation.
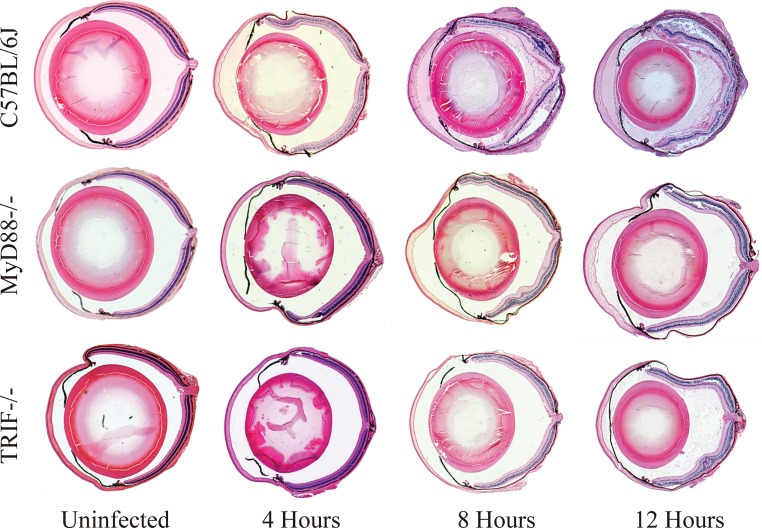
Figure 2 illustrates a whole-eye histological comparison of inflammation in infected eyes of C57BL/6J, MyD88−/−, and TRIF−/− mice during B. cereus endophthalmitis. Uninfected eyes of all mice were normal prior to infection. At 4 h, a fibrin infiltrate was observed in the anterior segment of C57BL/6J eyes, but there was little to no fibrin infiltrate in infected TRIF−/− or MyD88−/− eyes at the same time point. Retinas appeared normal in all infected eyes at 4 h postinfection. At 8 and 12 h, a significant PMN infiltrate, anterior and posterior segment fibrin accumulation, and disrupted retinal layers were observed in infected C57BL/6J eyes. In contrast, there was comparatively less fibrin infiltrate and PMN in infected TRIF−/− and MyD88−/− eyes at the same time points. Infected MyD88−/− and TRIF−/− eyes presented with comparatively less intraocular PMN and fibrin infiltrate, and retinas were intact.
FIG 2.
Whole-eye histology of MyD88−/− and TRIF−/− eyes infected with B. cereus. C57BL/6J, MyD88−/−, and TRIF−/− mouse eyes were intravitreally injected with 100 CFU B. cereus. Uninfected and infected globes were harvested at 4, 8, and 12 h postinfection and processed for staining with hematoxylin and eosin. Uninfected C57BL/6J, MyD88−/−, and TRIF−/− eyes had no inflammation and normal retinal tissue structure. At 4 h, fibrin infiltration was observed in the anterior segment of C57BL/6J eyes, but retinal layers were normal. At 4 h, there was little to no fibrin infiltrate and retinal layers were normal in infected TRIF−/− and MyD88−/− eyes. At 8 and 12 h, infected C57BL/6J eyes had significant posterior segment inflammation and PMN infiltration, significant anterior and posterior segment fibrin accumulation, and disrupted retinal layers. In contrast, there was comparatively less fibrin and PMN infiltrates and retinal damage in infected TRIF−/− and MyD88−/− eyes at the same time points. Sections are representative of 4 eyes per time point with at least three independent experiments. Magnification, ×10.
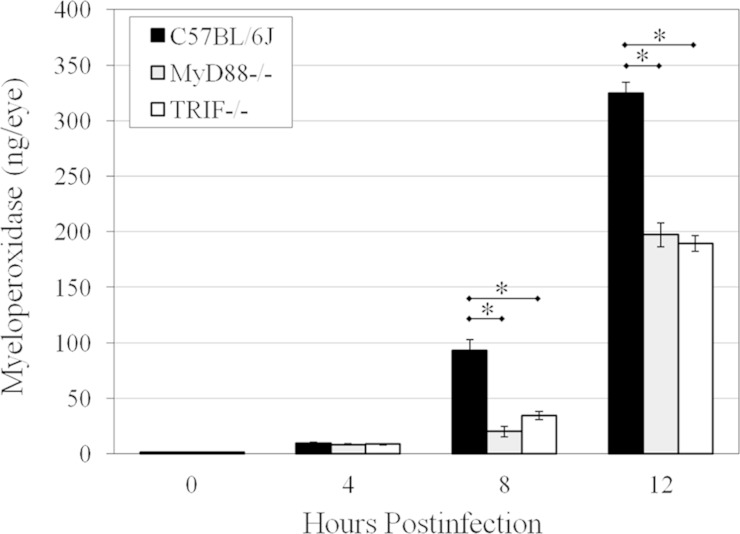
Figure 3 depicts the MPO concentrations of PMN infiltrating into infected C57BL/6J, MyD88−/−, and TRIF−/− eyes. Significantly higher levels of MPO were observed in C57BL/6J eyes than in MyD88−/− eyes at 8 h and 12 h postinfection (P ≤ 0.004). There were also higher concentrations of MPO in infected C57BL/6J eyes than in TRIF−/− eyes at 8 h and 12 h postinfection (P ≤ 0.004). These results corroborated the differing degrees of intraocular inflammation between these groups observed in the histological analysis. MPO levels in infected MyD88−/− and TRIF−/− eyes at 8 and 12 h postinfection were similar (P ≥ 0.065). Taken together, these results confirmed the diminished intraocular inflammation in B. cereus-infected MyD88−/− and TRIF−/− eyes compared to that in infected C57BL/6J eyes.
FIG 3.
PMN infiltration in MyD88−/− and TRIF−/− eyes infected with B. cereus. C57BL/6J, MyD88−/−, and TRIF−/− mouse eyes were injected with 100 CFU B. cereus. PMN infiltration was quantified by measuring MPO levels in mouse eyes by an ELISA. There was not a significant difference in MPO levels in these infected eyes at 4 h postinfection (P ≥ 0.7). However, at 8 and 12 h postinfection, significantly higher levels of MPO were observed in C57BL/6J eyes than in infected MyD88−/− (P ≤ 0.004) and TRIF−/− (P ≤ 0.004) eyes. MPO levels were similar in infected MyD88−/− and TRIF−/− eyes at 8 and 12 h postinfection (P ≥ 0.065). Values represent the means ± SEM for ≥5 eyes per group per time point with at least three independent experiments. *, P ≤ 0.004.
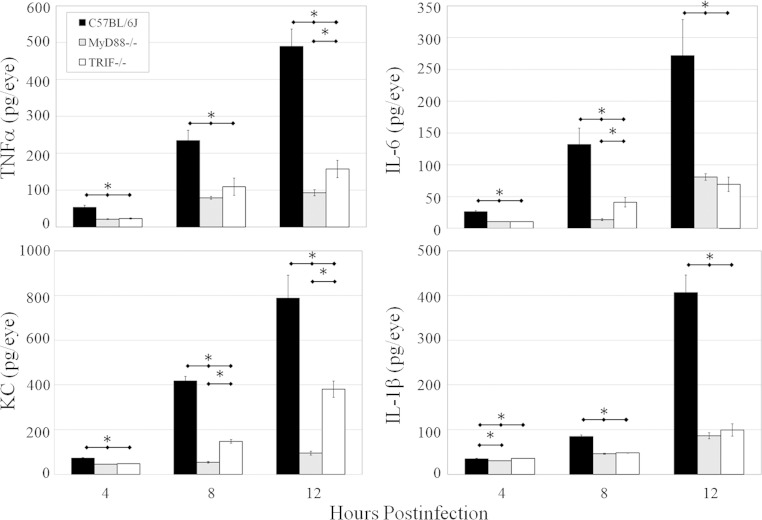
Levels of proinflammatory mediators that recruit neutrophils to an infection site, i.e., IL-6, TNF-α, IL-1β, and KC, were also quantified (Fig. 4). At 4, 8, and 12 h postinfection, there was significantly less TNF-α (P ≤ 0.016) and IL-6 (P ≤ 0.032) in infected MyD88−/− and TRIF−/− eyes than in C57BL/6J eyes. At 8 and 12 h postinfection, IL-1β concentrations in infected MyD88−/− and TRIF−/− eyes were significantly reduced compared to those in C57BL/6J eyes (P ≤ 0.008). The concentrations of TNF-α at 4 and 8 h (P ≥ 0.151), of IL-6 at 4 and 12 h (P ≥ 0.343), and of IL-1β at 8 and 12 h (P ≥ 0.421) were similar between infected MyD88−/− and TRIF−/− eyes. The concentrations of the major recruiting chemokine KC were significantly lower at 4, 8, and 12 h postinfection in infected MyD88−/− eyes than in infected C57BL/6J eyes (P ≤ 0.008). There was also a significant decrease in KC concentrations in infected TRIF−/− eyes at 4, 8, and 12 h postinfection compared to those in infected C57BL/6J eyes (P ≤ 0.032). KC levels at 4, 8, and 12 h postinfection were also significantly lower in infected MyD88−/− eyes than in infected TRIF−/− eyes (P ≤ 0.008). Overall, these results indicate reduced levels of important proinflammatory mediators in MyD88−/− and TRIF−/− eyes infected with B. cereus, which correlates with the histological and neutrophil influx data suggesting a diminished inflammatory environment in eyes deficient in TLR adaptors.
FIG 4.
Proinflammatory mediator expression in MyD88−/− and TRIF−/− eyes infected with B. cereus. C57BL/6J, MyD88−/−, and TRIF−/− mouse eyes were injected with 100 CFU B. cereus. Concentrations of TNF-α, IL-6, IL-1β, and KC were quantified by an ELISA. Overall, higher levels of TNF-α, KC, IL-6, and IL-1β were synthesized in infected eyes of wild-type mice than in infected eyes of MyD88−/− and TRIF−/− mice. Values represent means ± SEM for ≥5 eyes per time point with at least two independent experiments. *, P ≤ 0.02.
(iii) Effect of MyD88 or TRIF deficiency on retinal function.
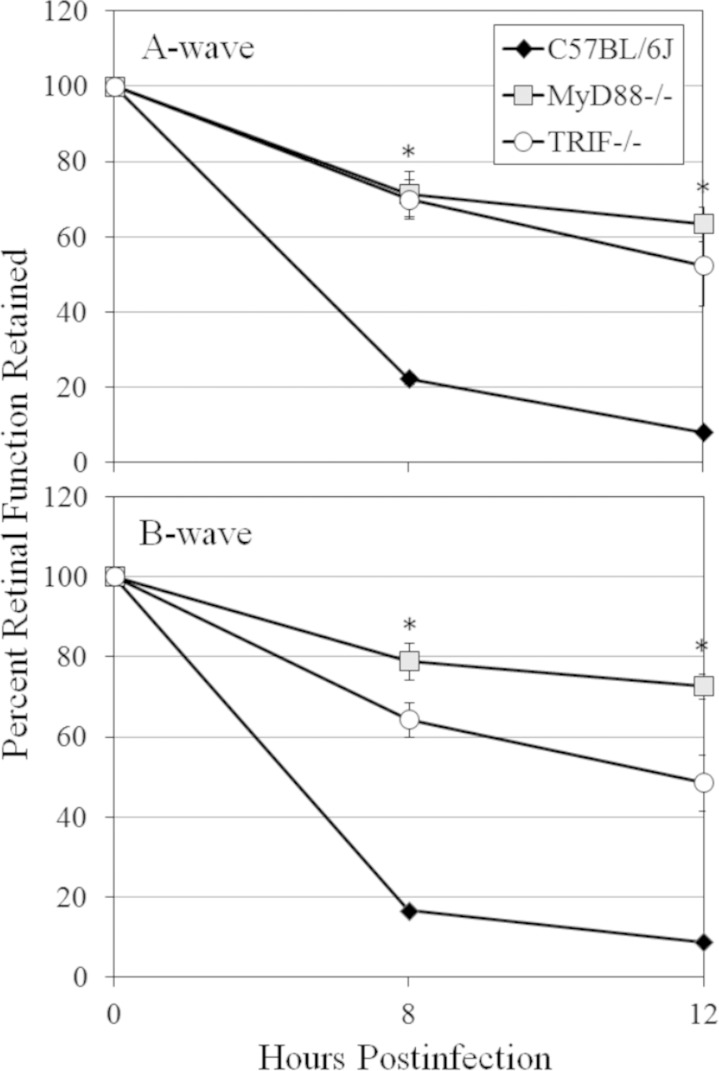
Data from analysis of retinal function of dark-adapted B. cereus-infected C57BL/6J, MyD88−/−, and TRIF−/− mice are summarized in Fig. 5. A-wave and B-wave amplitudes were significantly higher in infected MyD88−/− eyes than in infected C57BL/6J eyes at 8 and 12 h (P ≤ 0.004) postinfection. Similarly, in infected TRIF−/− eyes, high A-wave and B-wave function was retained at 8 and 12 h postinfection (P ≤ 0.008) compared to that in infected C57BL/6J controls. These results demonstrate that retinal function was retained to a greater degree in infected MyD88−/− and TRIF−/− eyes than in C57BL/6J eyes. Retention of A-wave amplitudes in infected MyD88−/− eyes was similar to that in TRIF−/− eyes at 8 and 12 h (P ≥ 0.429). B-wave amplitudes were similar at 8 h (P = 0.082) but reduced at 12 h (P = 0.004) in infected TRIF−/− eyes compared to those in infected MyD88−/− eyes. Taken together, these results demonstrate the importance of MyD88 and TRIF in inflammation and retention of retinal function during experimental B. cereus endophthalmitis.
FIG 5.
Retinal function in MyD88−/− and TRIF−/− mouse eyes infected with B. cereus. C57BL/6J, MyD88−/−, and TRIF−/− mouse eyes were injected with 100 CFU B. cereus. Retinal function in infected C57BL/6J, MyD88−/−, and TRIF−/− eyes was assessed by ERG at 8 and 12 h postinfection. A-wave and B-wave amplitudes were significantly higher in infected MyD88−/− eyes and TRIF−/− eyes than in C57BL/6J eyes at 8 and 12 h postinfection (P ≤ 0.008). Retention of A-wave amplitudes was similar between infected MyD88−/− and TRIF−/− eyes at 8 and 12 h (P ≥ 0.429). B-wave amplitudes were similar at 8 h (P = 0.082) but reduced at 12 h (P = 0.004) in infected TRIF−/− eyes compared to those in MyD88−/− eyes. Values represent means ± SEM for ≥6 eyes per time point with at least three independent experiments. *, P < 0.008 versus C57BL/6J eyes.
Contribution of TLR4 to experimental B. cereus endophthalmitis. (i) Effect of TLR4 deficiency on bacterial growth.
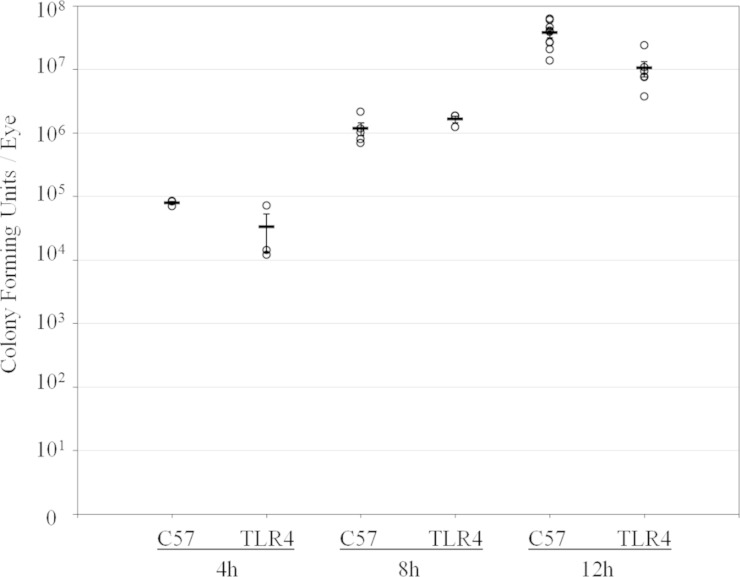
Intraocular growth of B. cereus in infected C57BL/6J and TLR4−/− eyes is depicted in Fig. 6. The starting inoculum for all groups was 116 ± 10 CFU. Intraocular bacterial growth in infected C57BL/6J eyes was similar to that in TLR4−/− eyes at all time points postinfection (P ≥ 0.053). This result indicated that the absence of TLR4 did not affect bacterial growth in the eye during endophthalmitis.
FIG 6.
Intraocular bacterial growth in TLR4−/− eyes infected with B. cereus. C57BL/6J and TLR4−/− mouse eyes were infected with 100 CFU B. cereus. Intraocular bacterial growth in infected C57BL/6J eyes was similar to that in infected TLR4−/− eyes at all time points postinfection (P ≥ 0.053). Values represent means ± SEM for ≥4 eyes per time point with at least three independently conducted experiments.
(ii) TLR4-dependent intraocular inflammation.
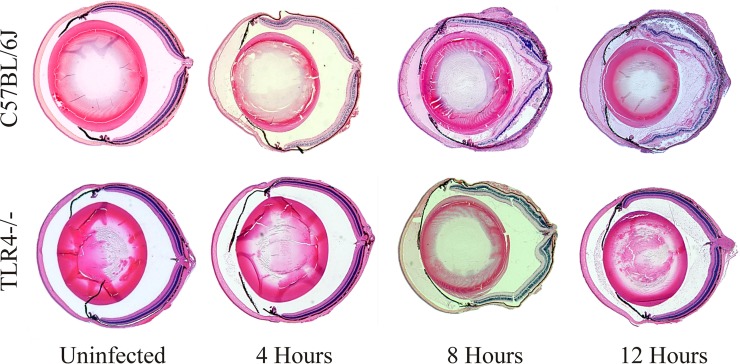
Figure 7 depicts whole-eye histological comparisons of inflammation in uninfected and infected C57BL/6J and TLR4−/− eyes. Uninfected eyes of all mice were normal prior to infection. At 4 h postinfection, there was fibrin infiltration in the anterior segment of C57BL/6J eyes. This was not observed in TLR4−/− eyes. Compared to infected C57BL/6J eyes, infected TLR4−/− eyes exhibited less fibrin infiltration in the anterior and posterior segments, less PMN influx, and preserved retinal architecture at both 8 and 12 h postinfection. In infected TLR4−/− eyes, the lack of inflammation and intact retinal layers were similar to those observed in infected MyD88−/− and TRIF−/− eyes at 8 and 12 h postinfection.
FIG 7.
Whole-eye histology of TLR4−/− eyes infected with B. cereus. C57BL/6J and TLR4−/− mouse eyes were infected with 100 CFU B. cereus. Histology of uninfected C57BL/6J and TLR4−/− eyes showed no difference in globe or retinal architecture and no fibrin deposition or neutrophil infiltration. Compared to infected C57BL/6J eyes, infected TLR4−/− eyes exhibited less fibrin infiltration in both the anterior and posterior segments, less PMN influx, and preserved retinal architecture at 4, 8, and 12 h postinfection. Sections are representative of 4 eyes per time point from at least three independent experiments. Magnification, ×10.
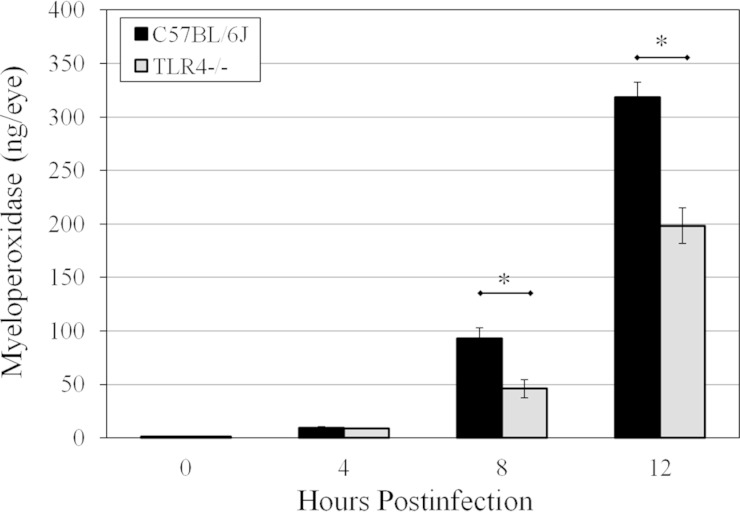
Figure 8 illustrates the MPO concentrations in infected C57BL/6J and TLR4−/− eyes. MPO concentrations at 8 and 12 h postinfection were significantly higher in infected C57BL/6J eyes than in infected TLR4−/− eyes (P ≤ 0.016), suggesting comparatively less PMN influx into infected TLR4−/− eyes. These data correlated with the histology data demonstrating reduced posterior segment inflammation that resulted in the absence of functional TLR4. In infected TLR4−/− eyes, the lack of MPO was similar to that observed in infected MyD88−/− and TRIF−/− eyes at 8 and 12 h postinfection.
FIG 8.
PMN infiltration in TLR4−/− eyes infected with B. cereus. C57BL/6J and TLR4−/− mouse eyes were infected with 100 CFU B. cereus. PMN infiltration was quantified by quantifying the MPO concentrations in infected C57BL/6J and TLR4−/− eyes by an ELISA. MPO concentrations at 8 and 12 h postinfection were significantly higher in infected C57BL/6J eyes than in infected TLR4−/− eyes (P ≤ 0.016). Values represent means ± SEM for ≥5 eyes per time point with at least three independent experiments. *, P ≤ 0.016.
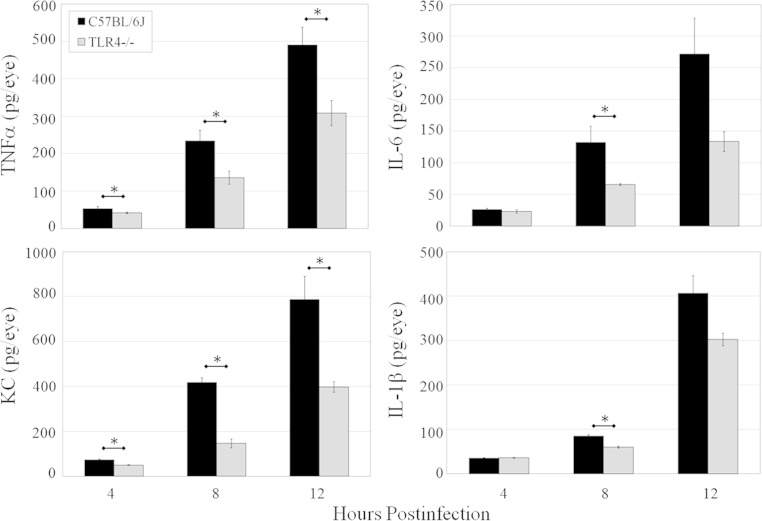
Figure 9 depicts concentrations of the proinflammatory mediators IL-6, TNF-α, IL-1β, and KC in infected C57BL/6J and TLR4−/− eyes. Infected TLR4−/− eyes had significantly less TNF-α at 4, 8, and 12 h postinfection than did infected C57BL/6J eyes (P ≤ 0.036). IL-6 and IL-1β concentrations were significantly lower in TLR4−/− than in C57BL/6J eyes at 8 h postinfection (P ≤ 0.008). Mean concentrations of IL-6 and IL-1β were obviously lower in TLR4−/− eyes than in C57BL/6J eyes at 12 h postinfection, but this difference was not statistically significant (P = 0.095). Chemokine KC concentrations in infected TLR4−/− eyes were significantly lower than those in infected in C57BL/6J eyes at 4, 8, and 12 h postinfection (P ≤ 0.008). The reduction in the levels of proinflammatory mediators in TLR4−/− eyes was similar to that observed in infected MyD88−/− and TRIF−/− eyes. These results, along with those of histological comparisons and quantification of MPO levels, demonstrated reduced intraocular inflammation in infected TLR4−/− eyes during experimental B. cereus endophthalmitis.
FIG 9.
Proinflammatory mediator expression in TLR4−/− eyes infected with B. cereus. C57BL/6J and TLR4−/− mouse eyes were infected with 100 CFU B. cereus. Concentrations of TNF-α, IL-6, IL-1β, and KC were quantified by an ELISA. Overall, higher levels of TNF-α, KC, IL-6, and IL-1β were synthesized in infected eyes of C57BL/6J mice than in infected eyes of TLR4−/− mice. Values represent means ± SEM for ≥5 eyes per time point with at least two independent experiments. *, P ≤ 0.036.
(iii) Effect of TLR4 deficiency on retinal function.
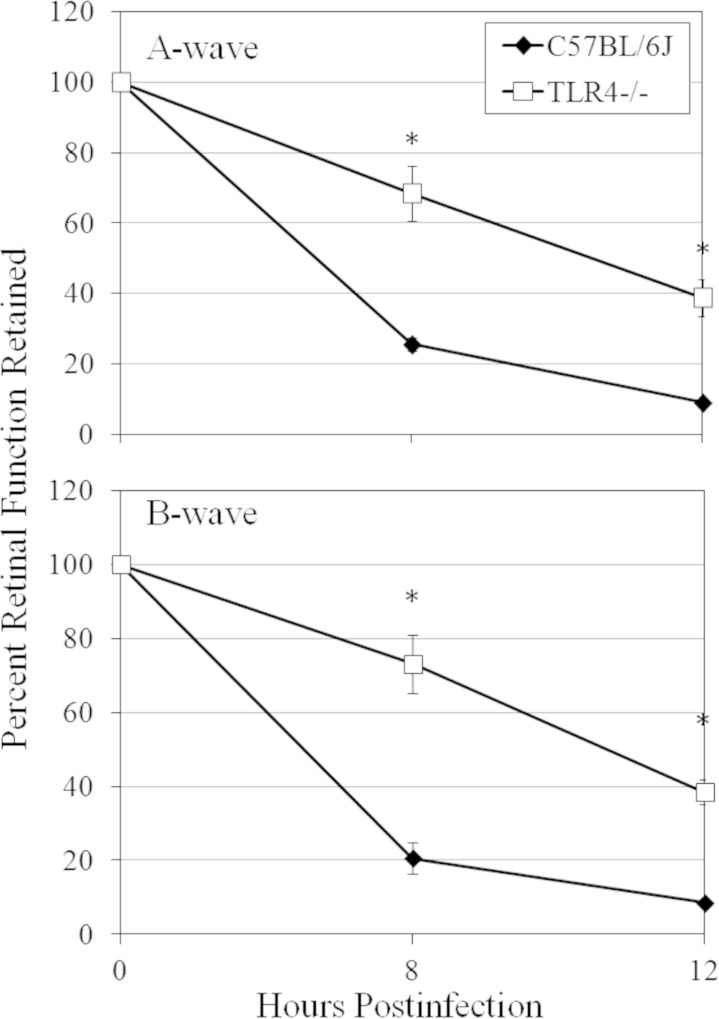
Analysis of retinal function in infected C57BL/6J and TLR4−/− eyes is summarized in Fig. 10. At 8 and 12 h, there was significantly greater A-wave and B-wave function retained in infected TLR4−/− eyes than in infected C57BL/6J eyes (P ≤ 0.029). By 12 h postinfection, A- and B-wave function in infected C57BL/6J eyes was <10%, but in infected TLR4−/− eyes, retinal responses were >50%. This reduced decrease in retinal function was similar to that observed in infected MyD88−/− and TRIF−/− eyes at 12 h postinfection. Taken together, these results demonstrate the importance of TLR4 in inflammation and retention of retinal function during experimental B. cereus endophthalmitis.
FIG 10.
Retinal function in TLR4−/− eyes infected with B. cereus. C57BL/6J, MyD88−/−, and TRIF−/− mouse eyes were injected with 100 CFU B. cereus. Retinal function in infected C57BL/6J and TLR4−/− eyes at 8 and 12 h postinfection was assessed by ERG. At 8 and 12 h, there was significantly higher retained A-wave and B-wave function in infected TLR4−/− eyes than in infected C57BL/6J eyes (P ≤ 0.001). Values represent means ± SEM for ≥7 eyes per time point with at least three independent experiments. *, P ≤ 0.001.
(iv) Effect of TLR4 deficiency on neutrophil function.
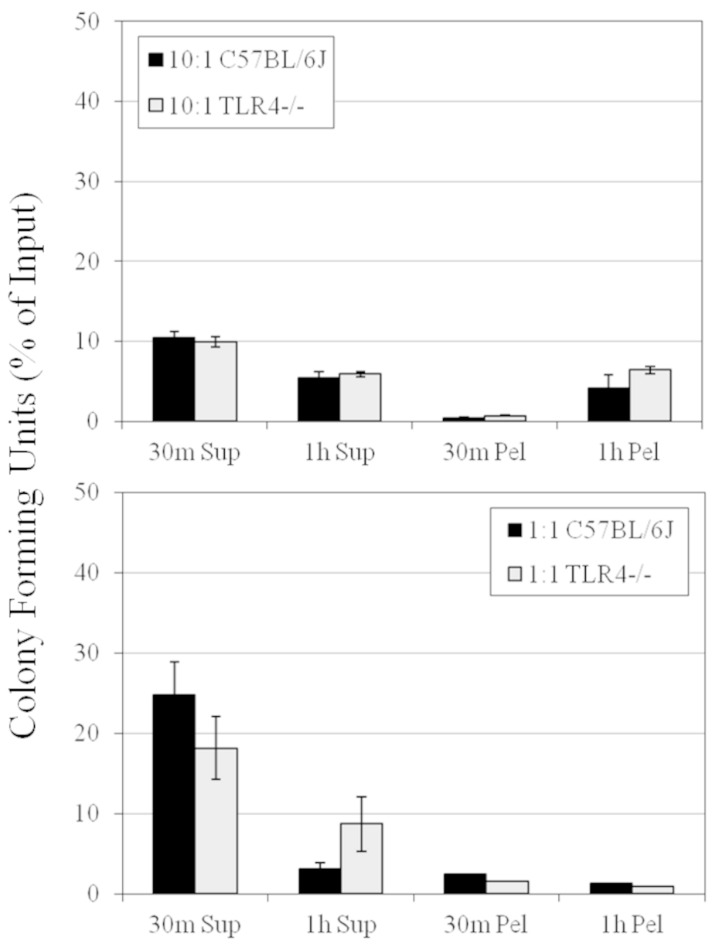
Neutrophils are the predominant infiltrating immune cells in the eye during B. cereus endophthalmitis (34). To ensure that the absence of TLR4 did not affect neutrophil function, we determined the efficiency of phagocytosis and intracellular killing of B. cereus by C57BL/6J and TLR4−/− neutrophils. The results are summarized in Fig. 11. We observed similar extracellular bacterial counts 30 min and 1 h following incubation of B. cereus with TLR4−/− and C57BL/6J neutrophils at a 10:1 ratio (P ≥ 0.514). Similar intracellular bacterial counts from neutrophils at a 10:1 ratio were also observed (P ≥ 0.342). We also determined the efficacy of phagocytosis with equivalent numbers of PMN (at a 1:1 ratio). There were similar numbers of extracellular bacteria 30 min and 1 h following incubation of B. cereus with TLR4−/− and C57BL/6J neutrophils at a 1:1 ratio (P ≥ 0.200). Similar numbers of intracellular bacteria following incubation with neutrophils at a 1:1 ratio were observed at 30 min and at 1 h (P ≥ 0.114). Overall, these results suggest that TLR4 did not influence the phagocytosis and killing ability of neutrophils.
FIG 11.
Phagocytosis and intracellular killing of B. cereus by C57BL/6J and TLR4−/− neutrophils. The efficiency of phagocytosis and intracellular killing of B. cereus by C57BL/6J and TLR4−/− neutrophils was estimated. Thirty minutes and 1 h following incubation of B. cereus with TLR4−/− and C57BL/6J neutrophils at a 10:1 ratio, extracellular (P ≥ 0.514) and intracellular (P ≥ 0.342) bacterial counts were similar. Thirty minutes and 1 h following incubation of B. cereus with TLR4−/− and C57BL/6J neutrophils at a 1:1 ratio, extracellular (P ≥ 0.200) and intracellular (P ≥ 0.114) bacterial counts were similar. Values represent the means ± SEM for ≥4 assays per group per time point from at least 3 different experiments. The P value was >0.05 for all groups. Sup, supernatant; Pel, pellet.
DISCUSSION
The eye is an immune-privileged site. Under ideal conditions, the ocular immune defense mechanisms responding to invading pathogens clear infecting microbes without overwhelming inflammation that can perturb the clarity of the visual axis. However, these defense mechanisms can be compromised by infections with pathogens that cannot be cleared from the eye. The severity of inflammation within the eye typically depends on the virulence of the infecting pathogen. In the case of B. cereus intraocular infection, the host inflammatory response is greatly accelerated compared to that for endophthalmitis caused by other pathogens, such as Staphylococcus epidermidis, Propionibacterium acnes, and Candida (39, 40). In B. cereus endophthalmitis, PMN migrate into the vitreous in as early as 4 h and into the retinal layers by 8 h, filling the vitreous and blocking the clarity of the visual axis, contributing to decreases in retinal function (34). Although Bacillus intraocular infections are rare, the potential for these and other intraocular infections to cause blindness is high, and better therapeutic strategies are needed to improve visual outcomes.
The innate immune response is the first line of defense against invading pathogens, and TLRs are an important contributor in many inflammatory diseases. Kumar et al. proposed that TLR2 ligand-induced activation prior to infection can reduce bacterial loads and inflammation, suggesting the importance of TLR2 during Staphylococcus aureus endophthalmitis (26). We previously reported the importance of TLR2 in driving inflammation during B. cereus endophthalmitis. In that study, although inflammation was significantly reduced in infected TLR2−/− eyes, there was residual inflammation and delayed neutrophil influx into the eye (25), indicating that there are additional inflammatory pathways that are active during this infection. We reported that although B. cereus possesses flagella, TLR5 did not contribute to inflammation during B. cereus endophthalmitis (28). However, because inflammation is typically a combinatorial effect of several innate recognition mechanisms, we further examined the involvement of other TLRs and adaptor molecules in the pathogenesis of B. cereus endophthalmitis.
Our initial results suggest the importance of both MyD88- and TRIF-mediated signaling pathways in B. cereus endophthalmitis. Our MyD88 data agree with those of Talreja et al., who recently reported on the importance of MyD88 in experimental S. aureus endophthalmitis (41). There was a significant reduction of inflammatory cell influx in MyD88−/− and TRIF−/− eyes, likely due to significantly decreased concentrations of proinflammatory mediators in these eyes compared to those in C57BL/6J controls. The chemokine KC, which recruits neutrophils to an inflamed site, is synthesized in part via the MyD88 pathway (71). This explains the significantly reduced concentrations of KC in infected MyD88−/− eyes compared to those in infected TRIF−/− eyes. There was also greater retinal function in these adaptor knockouts than in C57BL/6J controls, which may be due to decreased retinal bystander damage by lower numbers of infiltrating PMN. The intraocular growth of B. cereus was not altered by the absence of MyD88, indicating that differences in inflammation were not due to arrested intraocular bacterial growth.
Intraocular bacterial growth was reduced in the absence of TRIF at 4 and 8 h postinfection, but by 12 h, bacterial counts were similar to those in C57BL/6J controls. Overall, the rate of increase in bacterial growth in TRIF−/− eyes between 8 and 12 h postinfection (2.09 × 106 average CFU/h) was higher than the rate of increase in MyD88−/− eyes (1.02 × 106 average CFU/h) over the same time period. This may explain why the B-wave amplitude in TRIF−/− mice decreased rapidly throughout 12 h compared to the decrease in the B-wave amplitude in MyD88−/− mice. These results also suggest that the observed differences in retinal function loss were due to differences in inflammatory responses in infected MyD88−/− and TRIF−/− eyes. It is not clear why bacterial replication was limited in TRIF−/− eyes compared to that in MyD88−/− and wild-type eyes at 4 and 8 h postinfection. One would expect a higher bacterial burden in tissue lacking a sufficient inflammatory response, but we did not observe this in our studies. We speculate that there may be differences in the intraocular environment in TRIF−/− mice. Talreja et al. (41) recently reported that a deficiency in cathelicidin-related antimicrobial peptide (CRAMP) led to an increased S. aureus burden in mouse eyes with endophthalmitis, so it would be reasonable to speculate that increased levels of AMP would lead to a lower bacterial burden. However, Stockinger et al. (42) reported that TRIF−/− mice had low AMP expression levels but in the intestine. If inflammation in this model is dictated solely by bacterial burden, one would expect that an infection that was slow to evolve (perhaps in TRIF−/− eyes) might result in less inflammation at later time points. However, if the CFU counts alone dictated inflammation, one would expect the 12-h TRIF−/− and 12-h MyD88−/− eyes to exhibit pathology as severe as that in 8-h wild-type eyes. This was not the case. While the course of inflammation and retinal function loss in MyD88−/− and TRIF−/− eyes were similar, the rates of bacterial growth in these groups were not. This leads us to posit that a unique environment existed in these TRIF−/− eyes during infection, which may have limited bacterial growth.
The roles of the TLR adaptor molecules MyD88 and TRIF have been studied in other ocular inflammation and infection models to identify potential targets for the development of more efficient therapeutics. In an endotoxin-induced uveitis (EIU) mouse model, the TLR4/MyD88-dependent pathway contributed to inflammation (43–45). However, in the EIU model, some of TRIF-dependent cytokines were found to be proinflammatory (46), while other TRIF-dependent cytokines were anti-inflammatory (43, 47). In Pseudomonas aeruginosa keratitis models, MyD88- and TRIF-mediated signaling following LPS recognition by TLR4 has been reported (48–50). MyD88 has also been reported to be an important contributor in other retinal diseases, such as diabetic retinopathy (51) and age-related macular degeneration (AMD) (52). In diabetic retinopathy, MyD88 was important for the induction of proinflammatory proteins (51). In AMD, MyD88 promoted death signals from mobile element transcripts that led to retinal degeneration (52). Our results emphasize the importance of MyD88 and TRIF pathways in mediating the intraocular inflammatory response to Bacillus during endophthalmitis.
The importance of TLR4 in inflammation during bacterial corneal infections and in Gram-negative bacterial endophthalmitis has also been analyzed. We recently reported that the absence of TLR4 resulted in a delayed inflammatory response in experimental Klebsiella pneumoniae endophthalmitis (38). Tullos et al. also proposed that TLR2 and TLR4 modulated the immune response in a Streptococcus pneumoniae keratitis model, with TLR2 aiding in bacterial clearance and the absence of TLR4 contributing to reduced disease pathogenesis (53). Moreover, in that model, there were decreases in cytokine and innate immune signaling gene expression levels in TLR4−/− corneas, suggesting that TLR4 contributed to the corneal inflammatory response against S. pneumoniae (53).
Our interest in TLR4-mediated inflammation in B. cereus endophthalmitis arose after utilizing TLR4−/− mice as a negative control for our previous TLR2 studies (25). To our knowledge, this is the first study reporting the involvement of TLR4 in the context of Gram-positive intraocular inflammation. In the present study, differences in intraocular bacterial burden and neutrophil function between C57BL/6J and TLR4−/− mice were not observed, suggesting that differences in inflammation were not due to these factors. Overall, the results indicated a diminished inflammatory environment with higher retinal function in TLR4−/− mice that can be attributed to decreased PMN influx and reduced levels of proinflammatory mediators in the absence of TLR4.
It is interesting to note that the intraocular bacterial burden was not greater in MyD88−/−, TRIF−/−, or TLR4−/− mice despite the absence of PMN recruited to the site of infection. A similar finding was reported for experimental B. cereus endophthalmitis in TLR2−/− mice (25). B. cereus replicates rapidly in the eye even when PMN are present in large numbers. Sun et al. (54) reported that PMN are inefficient in clearing Pseudomonas from corneas due to this organism's toxin activities. Our data suggest that the host response is not very effective in controlling B. cereus after a threshold is reached in the eye, perhaps due to B. cereus toxin-mediated evasion of the host inflammatory response.
The cholesterol-dependent cytolysins (CDCs) are the major virulence factors associated with the disease pathogenesis of Listeria monocytogenes (listeriolysin) (55), Streptococcus pyogenes (streptolysin) (72), Streptococcus pneumoniae (pneumolysin) (56–58), and Bacillus anthracis (anthrolysin) (59). It has been proposed that bacterial CDCs can cause an increased production of proinflammatory cytokines through a TLR4-mediated response (60, 61). In B. anthracis, anthrolysin and lethal toxin acted to induce macrophage apoptosis through TLR4 (61). Similarly, pneumolysin induced TLR4-dependent apoptosis as part of the host immune response in a model of pneumococcal pneumonia (62). These studies challenge the paradigm that TLR4 can recognize only Gram-negative ligands such as LPS (60–62). B. cereus synthesizes cereolysin, a CDC. Not much is known about the role of cereolysin in disease pathogenesis. Our study suggests that there are agonists for TLR4 in the B. cereus intraocular infection environment, and cereolysin may be one of these.
With the increased numbers of cataract surgeries, pars plana vitrectomies, and anti-vascular endothelial growth factor (VEGF) injections for other ocular diseases, the risk of endophthalmitis associated with these conditions is increasing (63–68). In the context of blinding eye infections, TLRs have been investigated for their anti-inflammatory therapeutic properties. TLR-based therapeutic approaches have also been investigated in P. aeruginosa keratitis models (69, 70). A prophylactic approach to prevent S. aureus endophthalmitis using the TLR2 ligand Pam3Cys has been reported (26). The absence of TLR2 delayed inflammation in B. cereus endophthalmitis; however, TLR2 and TLR4 pathways each contributed to intraocular inflammation. Furthermore, a complete absence of inflammation was not observed for MyD88 or TRIF adaptor knockout mice, suggesting that there still may be other unidentified innate receptors that can compensate for the loss of TLR signaling.
In conclusion, our study highlights the importance of TLR4 and its signaling adaptor molecules in driving the intraocular inflammatory response and overall disease pathology during experimental B. cereus endophthalmitis. The current study paves the way for identifying potential TLR4 ligands that may serve as targets to reduce bystander damage caused by acute inflammation. As B. cereus endophthalmitis is one of the most rapidly blinding forms of intraocular infection, it is critical to develop therapeutics that address the damaging effects of both the bacterium and the host.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by the National Institutes of Health (grants R01EY024140 and R01EY012985 to M.C.C.). Our research is also supported in part by NIH grants R21EY022466 (to M.C.C.) and P30EY021725 (NEI Core grant to Robert E. Anderson, OUHSC) and an unrestricted grant to the Dean A. McGee Eye Institute from Research To Prevent Blindness.
We thank Ira Blader (University at Buffalo, State University of New York) for the original breeding pair of MyD88−/− mice and Eric Pearlman (University of California, Irvine) for the original breeding pair of TLR4−/− mice. We thank Feng Li, Nanette Wheatley (OUHSC Live Animal Imaging and Analysis Core Facility), and Mark Dittmar (Dean McGee Eye Institute Animal Facility) for their helpful technical assistance. We also thank Jonathan Hunt (University of Oklahoma Health Sciences Center) for invaluable suggestions and Paula Pierce (Excalibur Pathology, Moore, OK) and Linda Boone (OUHSC Cellular Imaging Core Facility, Dean McGee Eye Institute) for histology expertise.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00502-15.
REFERENCES
- 1.Bottone EJ. 2010. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev 23:382–398. doi: 10.1128/CMR.00073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hilliard NJ, Schelonka RL, Waites KB. 2003. Bacillus cereus bacteremia in a preterm neonate. J Clin Microbiol 41:3441–3444. doi: 10.1128/JCM.41.7.3441-3444.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller JM, Hair JG, Hebert M, Hebert L, Roberts FJ, Weyant RS. 1997. Fulminating bacteremia and pneumonia due to Bacillus cereus. J Clin Microbiol 35:504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Block C, Levy M, Fritz V. 1978. Bacillus cereus endocarditis—a case report. S Afr Med J 53:556–557. [PubMed] [Google Scholar]
- 5.Fitzpatrick DJ, Turnbull PCB, Keane CT, English LF. 1979. Two gas-gangrene-like infections due to Bacillus cereus. Br J Surg 66:577–579. doi: 10.1002/bjs.1800660819. [DOI] [PubMed] [Google Scholar]
- 6.Callegan MC, Engelbert M, Parke DW, Jett BD, Gilmore MS. 2002. Bacterial endophthalmitis: epidemiology, therapeutics, and bacterium-host interactions. Clin Microbiol Rev 15:111–124. doi: 10.1128/CMR.15.1.111-124.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.David DB, Kirkby GR, Noble BA. 1994. Bacillus cereus endophthalmitis. Br J Ophthalmol 78:577–580. doi: 10.1136/bjo.78.7.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davey RJ, Tauber WB. 1987. Posttraumatic endophthalmitis: the emerging role of Bacillus cereus infection. Rev Infect Dis 9:110–123. doi: 10.1093/clinids/9.1.110. [DOI] [PubMed] [Google Scholar]
- 9.Chan WM, Liu DT, Chan CK, Chong KK, Lam DS. 2003. Infective endophthalmitis caused by Bacillus cereus after cataract extraction surgery. Clin Infect Dis 37:e31–e34. doi: 10.1086/375898. [DOI] [PubMed] [Google Scholar]
- 10.Roy M, Chen JC, Miller M, Boyaner D, Kasner O, Edelstein E. 1997. Epidemic Bacillus endophthalmitis after cataract surgery. I. Acute presentation and outcome. Ophthalmology 104:1768–1772. doi: 10.1016/S0161-6420(97)30028-1. [DOI] [PubMed] [Google Scholar]
- 11.Chen JC, Roy M. 2000. Epidemic Bacillus endophthalmitis after cataract surgery. II. Chronic and recurrent presentation and outcome. Ophthalmology 107:1038–1041. doi: 10.1016/S0161-6420(00)00099-3. [DOI] [PubMed] [Google Scholar]
- 12.Wiskur BJ, Robinson ML, Farrand AJ, Novosad BD, Callegan MC. 2008. Toward improving therapeutic regimens for Bacillus endophthalmitis. Invest Ophthalmol Vis Sci 49:1480–1487. doi: 10.1167/iovs.07-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu SM, Way T, Rodrigues M, Steidl SM. 2000. Effects of intravitreal corticosteroids in the treatment of Bacillus cereus endophthalmitis. Arch Ophthalmol 118:803–806. doi: 10.1001/archopht.118.6.803. [DOI] [PubMed] [Google Scholar]
- 14.Pollack J, Beecher D, Pulido J, Lee Wong A. 2004. Failure of intravitreal dexamethasone to diminish inflammation or retinal toxicity in an experimental model of Bacillus cereus endophthalmitis. Curr Eye Res 29:253–259. doi: 10.1080/02713680490516701. [DOI] [PubMed] [Google Scholar]
- 15.Graham RO, Peyman GA. 1974. Intravitreal injection of dexamethasone. Treatment of experimentally induced endophthalmitis. Arch Ophthalmol 92:149–154. [DOI] [PubMed] [Google Scholar]
- 16.Ermis SS, Cetinkaya Z, Kiyici H, Inan UU, Ozturk F. 2007. Effects of intravitreal moxifloxacin and dexamethasone in experimental Staphylococcus aureus endophthalmitis. Curr Eye Res 32:337–344. doi: 10.1080/02713680701215595. [DOI] [PubMed] [Google Scholar]
- 17.Mohammed I, Abedin A, Tsintzas K, Abedin SA, Otri AM, Hopkinson A, Dua HS. 2011. Increased expression of hepcidin and Toll-like receptors 8 and 10 in viral keratitis. Cornea 30:899–904. doi: 10.1097/ICO.0b013e31820126e5. [DOI] [PubMed] [Google Scholar]
- 18.Yuan X, Wilhelmus KR. 2010. Toll-like receptors involved in the pathogenesis of experimental Candida albicans keratitis. Invest Ophthalmol Vis Sci 51:2094–2100. doi: 10.1167/iovs.09-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao J, Wu XY, Fu-Shin XY. 2009. Activation of Toll-like receptors 2 and 4 in Aspergillus fumigatus keratitis. Innate Immun 15:155–168. doi: 10.1177/1753425908101521. [DOI] [PubMed] [Google Scholar]
- 20.Huang X, Barrett RP, McClellan SA, Hazlett LD. 2005. Silencing Toll-like receptor-9 in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci 46:4209–4216. doi: 10.1167/iovs.05-0185. [DOI] [PubMed] [Google Scholar]
- 21.Allensworth JJ, Planck SR, Rosenbaum JT, Rosenzweig HL. 2011. Investigation of the differential potentials of TLR agonists to elicit uveitis in mice. J Leukoc Biol 90:1159–1166. doi: 10.1189/jlb.0511249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang JH, Hampartzoumian T, Everett B, Lloyd A, McCluskey PJ, Wakefield D. 2007. Changes in Toll-like receptor (TLR)-2 and TLR4 expression and function but not polymorphisms are associated with acute anterior uveitis. Invest Ophthalmol Vis Sci 48:1711–1717. doi: 10.1167/iovs.06-0807. [DOI] [PubMed] [Google Scholar]
- 23.Chang JH, McCluskey PJ, Wakefield D. 2012. Recent advances in Toll-like receptors and anterior uveitis. Clin Experiment Ophthalmol 40:821–828. doi: 10.1111/j.1442-9071.2012.02797.x. [DOI] [PubMed] [Google Scholar]
- 24.Pratap DS, Lim LL, Wang JJ, Mackey DA, Kearns LS, Stawell RJ, Wellcome Trust Case Control Consortium 2, Brudon KP, Mitchell P, Craig JE, Hall AJ, Hewitt AW. 2011. The role of Toll-like receptor variants in acute anterior uveitis. Mol Vis 17:2970–2977. [PMC free article] [PubMed] [Google Scholar]
- 25.Novosad BD, Astley RA, Callegan MC. 2011. Role of Toll-like receptor (TLR) 2 in experimental Bacillus cereus endophthalmitis. PLoS One 6:e28619. doi: 10.1371/journal.pone.0028619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A, Singh CN, Glybina IV, Mahmoud TH, Fu-Shin XY. 2010. Toll-like receptor 2 ligand-induced protection against bacterial endophthalmitis. J Infect Dis 201:255–263. doi: 10.1086/649589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kochan T, Singla A, Tosi J, Kumar A. 2012. Toll-like receptor 2 ligand pretreatment attenuates retinal microglial inflammatory response but enhances phagocytic activity toward Staphylococcus aureus. Infect Immun 80:2076–2088. doi: 10.1128/IAI.00149-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parkunan SM, Astley R, Callegan MC. 2014. Role of TLR5 and flagella in Bacillus intraocular infection. PLoS One 9:e100543. doi: 10.1371/journal.pone.0100543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda K, Akira S. 2004. TLR signaling pathways. Semin Immunol 16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Akira S. 2003. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science 301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 31.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 32.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide. Evidence for TLR4 as the Lps gene product. J Immunol 162:3749–3752. [PubMed] [Google Scholar]
- 33.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143–150. doi: 10.1016/S1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 34.Ramadan RT, Ramirez R, Novosad BD, Callegan MC. 2006. Acute inflammation and loss of retinal architecture and function during experimental Bacillus endophthalmitis. Curr Eye Res 31:955–965. doi: 10.1080/02713680600976925. [DOI] [PubMed] [Google Scholar]
- 35.Ramadan RT, Moyer AL, Callegan MC. 2008. A role for tumor necrosis factor-α in experimental Bacillus cereus endophthalmitis pathogenesis. Invest Ophthalmol Vis Sci 49:4482–4489. doi: 10.1167/iovs.08-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo Y, Dorf ME. 2001. Isolation of mouse neutrophils. Curr Protoc Immunol Chapter 3:Unit 3.20. doi: 10.1002/0471142735.im0320s22. [DOI] [PubMed] [Google Scholar]
- 37.Hampton MB, Vissers MC, Winterbourn CC. 1994. A single assay for measuring the rates of phagocytosis and bacterial killing by neutrophils. J Leukoc Biol 55:147–152. [DOI] [PubMed] [Google Scholar]
- 38.Hunt JJ, Astley R, Wheatley N, Wang JT, Callegan MC. 2014. TLR4 contributes to the host response to Klebsiella intraocular infection. Curr Eye Res 39:790–802. doi: 10.3109/02713683.2014.883412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durand ML. 2013. Endophthalmitis. Clin Microbiol Infect 19:227–234. doi: 10.1111/1469-0691.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandelbaum S, Forster RK. 1996. Exogenous endophthalmitis, p 1298–1320. In Pepose JS, Holland GN, Wilhelmus KR (ed), Ocular infection and immunity. Mosby, St Louis, MO. [Google Scholar]
- 41.Talreja D, Singh PK, Kumar A. 2015. In vivo role of TLR2 and MyD88 signaling in eliciting innate immune responses in staphylococcal endophthalmitis. Invest Ophthalmol Vis Sci 56:1719–1732. doi: 10.1167/iovs.14-16087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stockinger S, Duerr CU, Fulde M, Dolowschiak T, Pott J, Yang I, Eibach D, Bäckhed F, Akira S, Suerbaum S, Brugman M, Hornef MW. 2014. TRIF signaling drives homeostatic intestinal epithelial antimicrobial peptide expression. J Immunol 193:4223–4234. doi: 10.4049/jimmunol.1302708. [DOI] [PubMed] [Google Scholar]
- 43.Kezic J, Taylor S, Gupta S, Planck SR, Rosenzweig HL, Rosenbaum JT. 2011. Endotoxin-induced uveitis is primarily dependent on radiation-resistant cells and on MyD88 but not TRIF. J Leukoc Biol 90:305–311. doi: 10.1189/jlb.0111036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su SB, Silver PB, Grajewski RS, Agarwal RK, Tang J, Chan CC, Caspi RR. 2005. Essential role of the MyD88 pathway, but nonessential roles of TLRs 2, 4, and 9, in the adjuvant effect promoting Th1-mediated autoimmunity. J Immunol 175:6303–6310. doi: 10.4049/jimmunol.175.10.6303. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Lu H, Hu X, Chen W, Xu Z, Li S, Xu Y. 2011. Nuclear factor translocation and acute anterior uveitis. Mol Vis 17:170–176. [PMC free article] [PubMed] [Google Scholar]
- 46.Tuaillon N, Shen DF, Berger RB, Lu B, Rollins BJ, Chan CC. 2002. MCP-1 expression in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci 43:1493–1498. [PubMed] [Google Scholar]
- 47.Sonoda KH, Sasa Y, Qiao H, Tsutsumi C, Hisatomi T, Komiyama S, Kubota T, Sakamoto T, Kawano Y, Ishibashi T. 2003. Immunoregulatory role of ocular macrophages: the macrophages produce RANTES to suppress experimental autoimmune uveitis. J Immunol 171:2652–2659. doi: 10.4049/jimmunol.171.5.2652. [DOI] [PubMed] [Google Scholar]
- 48.Huang X, Du W, McClellan SA, Barrett RP, Hazlett LD. 2006. TLR4 is required for host resistance in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci 47:4910–4916. doi: 10.1167/iovs.06-0537. [DOI] [PubMed] [Google Scholar]
- 49.Sun Y, Karmakar M, Roy S, Ramadan RT, Williams SR, Howell S, Pearlman E. 2010. TLR4 and TLR5 on corneal macrophages regulate Pseudomonas aeruginosa keratitis by signaling through MyD88-dependent and-independent pathways. J Immunol 185:4272–4283. doi: 10.4049/jimmunol.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaidi TS, Zaidi T, Pier GB. 2010. Role of neutrophils, MyD88-mediated neutrophil recruitment, and complement in antibody-mediated defense against Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci 51:2085–2093. doi: 10.1167/iovs.09-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang J, Lee CA, Du Y, Sun Y, Pearlman E, Sheibani N, Kern TS. 2013. MyD88-dependent pathways in leukocytes affect the retina in diabetes. PLoS One 8:e68871. doi: 10.1371/journal.pone.0068871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tarallo V, Hirano Y, Gelfand BD, Dridi S, Kerur N, Kim Y, Ambati J. 2012. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell 149:847–859. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tullos NA, Thompson HW, Taylor SD, Sanders M, Norcross EW, Tolo I, Moore Q, Marquart ME. 2013. Modulation of immune signaling, bacterial clearance, and corneal integrity by Toll-like receptors during Streptococcus pneumoniae keratitis. Curr Eye Res 38:1036–1048. doi: 10.3109/02713683.2013.804094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun Y, Karmakar M, Taylor PR, Rietsch A, Pearlman E. 2012. ExoS and ExoT ADP ribosyltransferase activities mediate Pseudomonas aeruginosa keratitis by promoting neutrophil apoptosis and bacterial survival. J Immunol 188:1884–1895. doi: 10.4049/jimmunol.1102148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cossart P, Vicente MF, Mengaud J, Baquero F, Perez-Diaz JC, Berche P. 1989. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect Immun 57:3629–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Canvin JR, Marvin AP, Sivakumaran M, Paton JC, Boulnois GJ, Andrew PW, Mitchell TJ. 1995. The role of pneumolysin and autolysin in the pathology of pneumonia and septicemia in mice infected with a type 2 pneumococcus. J Infect Dis 172:119–123. doi: 10.1093/infdis/172.1.119. [DOI] [PubMed] [Google Scholar]
- 57.Hirst RA, Kadioglu A, O'Callaghan C, Andrew PW. 2004. The role of pneumolysin in pneumococcal pneumonia and meningitis. Clin Exp Immunol 138:195–201. doi: 10.1111/j.1365-2249.2004.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubins JB, Charboneau D, Fasching C, Berry AM, Paton JC, Alexander JE, Janoff EN. 1996. Distinct roles for pneumolysin's cytotoxic and complement activities in the pathogenesis of pneumococcal pneumonia. Am J Respir Crit Care Med 153:1339–1346. doi: 10.1164/ajrccm.153.4.8616564. [DOI] [PubMed] [Google Scholar]
- 59.Mosser EM, Rest RF. 2006. The Bacillus anthracis cholesterol-dependent cytolysin, anthrolysin O, kills human neutrophils, monocytes and macrophages. BMC Microbiol 6:56. doi: 10.1186/1471-2180-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, Golenbock DT. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci U S A 100:1966–1971. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park JM, Ng VH, Maeda S, Rest RF, Karin M. 2004. Anthrolysin O and other Gram-positive cytolysins are Toll-like receptor 4 agonists. J Exp Med 200:1647–1655. doi: 10.1084/jem.20041215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Srivastava A, Henneke P, Visintin A, Morse SC, Martin V, Watkins C, Malley R. 2005. The apoptotic response to pneumolysin is Toll-like receptor 4 dependent and protects against pneumococcal disease. Infect Immun 73:6479–6487. doi: 10.1128/IAI.73.10.6479-6487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hatch WV, Cernat G, Wong D, Devenyi R, Bell CM. 2009. Risk factors for acute endophthalmitis after cataract surgery: a population-based study. Ophthalmology 116:425–430. doi: 10.1016/j.ophtha.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 64.Vaziri K, Schwartz SG, Kishor K, Flynn HW Jr. 2015. Endophthalmitis: state of the art. Clin Ophthalmol 9:95–108. doi: 10.2147/OPTH.S76406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramulu PY, Do DV, Corcoran KJ, Corcoran SL, Robin AL. 2010. Use of retinal procedures in Medicare beneficiaries from 1997 to 2007. Arch Ophthalmol 128:1335–1340. doi: 10.1001/archophthalmol.2010.224. [DOI] [PubMed] [Google Scholar]
- 66.Dave VP, Pathengay A, Schwartz SG, Flynn HW Jr. 2014. Endophthalmitis following pars plana vitrectomy: a literature review of incidence, causative organisms, and treatment outcomes. Clin Ophthalmol 8:2183–2188. doi: 10.2147/OPTH.S71293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheung CS, Wong AW, Lui A, Kertes PJ, Devenyi RG, Lam WC. 2012. Incidence of endophthalmitis and use of antibiotic prophylaxis after intravitreal injections. Ophthalmology 119:1609–1614. doi: 10.1016/j.ophtha.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 68.McCannel CA. 2011. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina 31:654–661. doi: 10.1097/IAE.0b013e31820a67e4. [DOI] [PubMed] [Google Scholar]
- 69.Kumar A, Gao N, Standiford TJ, Gallo RL, Yu FS. 2010. Topical flagellin protects the injured corneas from Pseudomonas aeruginosa infection. Microbes Infect 12:978–989. doi: 10.1016/j.micinf.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar A, Hazlett LD, Yu FS. 2008. Flagellin suppresses the inflammatory response and enhances bacterial clearance in a murine model of Pseudomonas aeruginosa keratitis. Infect Immun 76:89–96. doi: 10.1128/IAI.01232-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Filippo K, Henderson RB, Laschinger M, Hogg N. 2008. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J Immunol 180:4308–4315. doi: 10.4049/jimmunol.180.6.4308. [DOI] [PubMed] [Google Scholar]
- 72.Limbago B, Penumalli V, Weinrick B, Scott JR. 2000. Role of streptolysin O in a mouse model of invasive group A streptococcal disease. Infect Immun 68:6384–6390. doi: 10.1128/IAI.68.11.6384-6390.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.