Abstract
Several immunomodulatory factors are involved in malaria pathogenesis. Among them, heme has been shown to play a role in the pathophysiology of severe malaria in rodents, but its role in human severe malaria remains unclear. Circulating levels of total heme and its main scavenger, hemopexin, along with cytokine/chemokine levels and biological parameters, including hemoglobin and creatinine levels, as well as transaminase activities, were measured in the plasma of 237 Plasmodium falciparum-infected patients living in the state of Odisha, India, where malaria is endemic. All patients were categorized into well-defined groups of mild malaria, cerebral malaria (CM), or severe noncerebral malaria, which included acute renal failure (ARF) and hepatopathy. Our results show a significant increase in total plasma heme levels with malaria severity, especially for CM and malarial ARF. Spearman rank correlation and canonical correlation analyses have shown a correlation between total heme, hemopexin, interleukin-10, tumor necrosis factor alpha, gamma interferon-induced protein 10 (IP-10), and monocyte chemotactic protein 1 (MCP-1) levels. In addition, canonical correlations revealed that heme, along with IP-10, was associated with the CM pathophysiology, whereas both IP-10 and MCP-1 together with heme discriminated ARF. Altogether, our data indicate that heme, in association with cytokines and chemokines, is involved in the pathophysiology of both CM and ARF but through different mechanisms.
INTRODUCTION
In 2008, India alone accounted for 27% of malaria cases outside Africa (http://www.who.int/malaria/world_malaria_report_2010/worldmalariareport2010.pdf?ua=1). The central and eastern regions of India are the most vulnerable to malaria, with the state of Odisha alone accounting for 40% of all malaria cases and 88% of Plasmodium falciparum infections recorded in India in 2009 (http://nvbdcp.gov.in/malaria-new.html). Although cerebral complications are equally frequent among adults and children, adults are more susceptible to developing multiple malaria-related complications (1). Indeed, a shift in clinical profile has been observed in the last 10 years, with more cases of multiple complications, including malarial acute renal failure (ARF) (1, 2). Mechanisms underlying this shift are not clearly understood and need to be further investigated.
As a consequence of the massive destruction of red blood cells (RBCs) during infection, immunogenic molecules are released into the bloodstream, and they play a major role in determining the clinical outcome (3). Although the malarial parasite uses 60 to 80% of hemoglobin for growth and converts most of the heme into hemozoin, there is also significant release of hemoglobin into the blood from nonparasitized RBCs (4). Cell-free hemoglobin is readily oxidized, releasing its heme prosthetic groups (5). Under aerobic conditions, heme is essential to life when bound to hemoproteins. However, free heme is involved in immune-mediated diseases by inducing the production of reactive oxygen species (ROS) and proinflammatory cytokines (6, 7). Normally, extracellular free heme is neutralized rapidly by hemopexin, forming a heme-hemopexin complex. This complex then is scavenged by macrophages and hepatocytes and further catabolized by heme oxygenase-1 (HO-1) (5). Although the liver is the main site for heme degradation, high levels of cell-free heme can overwhelm its detoxifying capacity during hemolytic pathologies (8, 9).
In mice, injection of heme at later stages of malarial infection triggered cerebral malaria (CM) and also was involved in hepatopathy and dyserythropoiesis (5, 10, 11). Interestingly, during P. vivax infections in Brazil, plasma heme levels were found to increase with disease severity (12). In addition, plasma levels of cell-free hemoglobin increased with severity during P. falciparum infection and could reflect increased systemic levels of heme (13), and more than 50% of P. falciparum-infected children developing CM in Zambia showed the presence of urinary heme, compared to 3.5% for uncomplicated cases (14). However, little is known about heme in the pathophysiology of P. falciparum malaria. In this study, we investigated its role in malarial outcomes of P. falciparum-infected patients from the state of Odisha, India, where malaria is endemic. We also examined the correlation of plasma levels of heme and hemopexin with circulating cytokine profiles and malaria pathology. Our analysis revealed that heme levels increased during CM and malarial ARF, while correlative studies with cytokines suggest that mechanisms involved in the two pathologies are different.
MATERIALS AND METHODS
Ethics.
This study was conducted according to the guidelines set out in the Declaration of Helsinki. The study was approved by the Institutional Human Ethics Committee of SCB Medical College, Cuttack, India, and the Institutional Review Board of all three collaborating institutes: (i) the Institute of Life Sciences, Bhubaneswar, India, (ii) the Tata Institute of Fundamental Research, Mumbai, India, and (iii) Institut Pasteur de Lille, Lille, France. The National Health Office Ethics committee in India approved the study design. All blood samples were collected after obtaining written consent from participants or, in the case of comatose patients, accompanying relatives.
Study site and participants.
The study was conducted in India at the SCB Medical College, Cuttack, Odisha, between 2008 and 2011. Patients (n = 237; age, ≥15 years) with a history of fever and with evidence of altered sensorium, jaundice, oliguria, respiratory distress, shock, and/or bleeding diathesis were screened for P. falciparum infection by Giemsa-stained blood smears and an immune chromatography test (SD Bio Standard Diagnostics India). Patients who were positive for ICT but negative by blood smears were subjected to nested PCR, as described earlier (15). Those with evidence of sepsis were excluded. After diagnosis, all patients received appropriate treatment.
Clinical categorization was based on modified guidelines of the World Health Organization (WHO) (15, 16). Mild malaria (MM) was defined as fever with evidence of P. falciparum infection. Severe malaria (SM) was defined as patients having at least one feature of SM reported by the WHO (16) and were further categorized into four groups based on distinct clinical features: CM, SNCM (severe non-CM), MOD (multiple organ dysfunction), and CM-MOD (CM with MOD). CM was defined as patients with a Glasgow coma score (GCS) of ≤10. SNCM patients had one of the severe manifestations without cerebral involvement, such as severe anemia (hemoglobin, <5 g/dl), ARF (creatinine, >3 mg/dl), jaundice (bilirubin, >3 mg/dl), acute respiratory distress (ARDS; PaO2/FiO2, <200 mm Hg), metabolic acidosis (plasma bicarbonate, <15 mmol/liter), and systolic shock (systolic blood pressure, <80 mm Hg). Hepatopathy was diagnosed either as ALT (alanine transaminase) and AST (aspartate transaminase) at >3 times the normal level or as ALT and AST at >2 times the normal level, with bilirubin of >6 mg/dl and alkaline phosphatase of >100 IU/ml in order to distinguish patients displaying liver dysfunctions from patients having solely jaundice. MOD patients had at least two damaged organs, including ARF, hepatopathy, ARDS, and systolic shock. When more than a single organ failure was associated with CM, patients were considered CM-MOD.
Endemic controls (EC) of identical ethnicity and coming from a similar geographical background were enrolled from among patients' relatives (n = 37). Sepsis was defined as patients showing a systemic inflammatory response syndrome that had a proven or suspected microbial etiology. Sepsis (n = 10) and encephalitis cases (n = 9) were admitted to SCB Medical College. None of the control subjects reported a history of clinical malaria in the 5 years preceding our study.
Blood collection, diagnosis, and biological parameters.
Peripheral venous blood was collected on the day of recruitment prior to initiation of any treatment. Biological parameters such as complete blood count, renal (creatinine) and liver (alkaline phosphatase, bilirubin, ALT, and AST) function tests, blood sugar, electrolytes, and lactate levels were measured for all samples. Patients with bleeding diathesis were assessed for platelet count, prothrombin time, activated partial thromboplastin time, and fibrin degradation products for evidence of disseminated intravascular coagulation. All other patients had their platelets estimated. If there was evidence of thrombocytopenia (<100,000/mm3), a coagulation profile was established to determine subclinical disseminated intravascular coagulation. Viral markers for hepatitis A, E, B, and C were tested in patients with hepatopathy and jaundice, and arterial blood gas analysis was performed during ARDS.
Quantification of HRP-2.
Plasma histidine-rich protein-2 (HRP-2) levels were measured using enzyme-linked immunosorbent assay (ELISA) as described previously (17). An anti-P. falciparum HRP-2 IgM (MPFM-55A; Santa Cruz Biotechnology, France) was used as the capture antibody, an anti-P. falciparum HRP-2 IgG1 was used as a detection antibody (MPFG-55; Santa Cruz Biotechnology, France), and a horseradish peroxidase-coupled monoclonal anti-IgG1 antibody (SB77e; Abcam, France) was used as the secondary antibody for detection. A standard curve was added in duplicate to each plate, and it was obtained through serial dilutions of a serum sample from a previously described cohort with known parasitemia (18). Samples out of range of detection were more or less diluted. The lower limit of detection was 0.5 arbitrary units (AU).
Determination of plasma levels of total heme and hemopexin.
Total heme levels were measured in plasma using the QuantiChrom heme assay kit (BioAssay Systems, USA). Hemopexin levels were measured through ELISA using the human hemopexin ELISA kit (Abcam, France).
Cytokine measurements.
The concentrations of plasma cytokines for each sample were measured in duplicate using the Milliplex MAP multiplex assay kit (Millipore, France). The Bio-Plex 200 system (Bio-Rad, France) was used for detection, and data were analyzed with the Bio-Plex Manager 5.0 software (Bio-Rad, France).
Statistical analysis.
Statistical significance was determined by the nonparametric Mann-Whitney test for pairwise comparisons, or the Kruskal-Wallis test for multiple comparisons, using GraphPad Prism software (version 5; GraphPad Prism Software, USA). Spearman rank correlation analyses and canonical correlations were performed using IgorPro software (WaveMetrics, USA). P < 0.05 was considered significant.
RESULTS
Characteristics of patient groups.
Among P. falciparum-infected patients recruited in the study, 180 (76%) individuals had SM (Table 1). Jaundice, severe anemia, and metabolic acidosis were not considered to be organ dysfunctions but were included in the SNCM group, and they accounted for 21% of SM cases (see Fig. S1A in the supplemental material). Nearly half of the patients with SM (48%) had a single organ dysfunction (see Fig. S1B), with CM being the most common complication (51%), followed by ARF (40%), hepatopathy (7%), and systolic shock (2%) (see Fig. S1B); 23% and 8% had two and three damaged organs, respectively, whereas only one patient had four dysfunctional organs (see Fig. S1A). Among patients having two damaged organs, 83% had ARF, 66% had CM, and 46% had hepatopathy (see Fig. S1C). Only two patients had ARDS that was associated with at least two other damaged organs. It is noteworthy that age was not a predictor of the severity of malaria (Table 1). A higher percentage of males was observed among all malaria groups, which is in agreement with a previous report (19). HRP-2 level has been shown to be a good indicator of total parasite biomass (20). In this study, plasma levels of HRP-2 were higher in SM patients than in MM patients, while only CM and CM-MOD patients had significantly higher HRP-2 levels (Table 1).
TABLE 1.
Baseline characteristics
| Characteristic | No. of patients | % male | Agee (yr) | Hemoglobine (g/dl) | HRP-2e (AU) | Creatininee (mg/dl) | ALTe (IU/liter) | GCSe |
|---|---|---|---|---|---|---|---|---|
| MM | 57 | 78.9 | 29 (22–39) | 10.8 (9.6–12.6) | 9.8 (1.1–20.6) | 1.3 (1.0–1.5) | 39.0 (28.7–51.0) | 15 |
| Severe malaria | 180 | 82.2 | 29 (22–45) | 10.0 (8.0–11.4) | 22.8 (8.0–40.8)*** | 2.2 (1.2–5.4) | 58.0 (39.0–85.0) | 11 |
| CMa | 48 | 81.3 | 28 (20–40) | 10.2 (8.7–11.9) | 22.5 (4.3–40.7)# | 1.3 (1.1–1.7) | 46.0 (33.3–60.8) | 9 |
| ARFb | 23 | 91.3 | 30 (25–45) | 9.6 (8.0–10.2) | 28.1 (10.9–42.6) | 7.0 (5.8–9.1) | 41.0 (33.3–55.5) | 15 |
| Hepatopathyc | 12 | 75.0 | 29 (26–40) | 10.3 (9.2–11.0) | 13.3 (1.6–25.0) | 1.0 (0.8–1.2) | 131.5 (99.8–189.0) | 15 |
| Systolic shockd | 4 | 50.0 | 40 (40–46) | 12.7 (7.4–13.6) | 23.7 (20.2–37.4) | 2.3 (1.3–2.7) | 60.5 (44.3–70.8) | 15 |
| Other SNCMf | 37 | 73.0 | 28 (22–40) | 9.5 (6.4–12.0) | 14.5 (5.1–29.3) | 1.2 (0.9–1.6) | 53.0 (33.0–72.0) | 15 |
| MOD | 14 | 85.7 | 27 (22–53) | 10.2 (8.7–11.6) | 20.7 (3.0–34.6) | 4.3 (3.0–8.6) | 136.0 (93.3–160.0) | 15 |
| CM-MOD | 42 | 90.5 | 35 (23–47) | 9.6 (8.0–11.4) | 34.1 (20.9–54.2)### | 5.4 (3.8–8.1) | 68.0 (50.5–104.5) | 9 |
| Total | 237 | 76.6 | 29 (22–41) | 10.0 (8.3–12.0) | 20.5 (5.6–34.5) | 1.6 (1.1–4.2) | 52.0 (36.0–79.5) | 15 |
CM was diagnosed as a GCS of ≤10.
ARF was diagnosed as plasma creatinine levels of >3 mg/dl.
Hepatopathy was diagnosed as ALT and AST at >3 times the normal level or as AST at >2 times the normal level and bilirubin levels of >6 mg/dl.
Systolic shock was diagnosed as systolic blood pressure of <80 mmHg.
Values are medians (interquartile ranges). ***, P ≤ 0.001 for comparison of mild malaria (MM) and severe malaria cases using the Mann-Whitney test. P ≤ 0.05 (#) and P ≤ 0.001 (###) for comparisons of mild malaria cases, patients affected with cerebral malaria (CM), acute renal failure (ARF), hepatopathy, systolic shock, other sever noncerebral malaria cases (SNCM), multiple organ dysfunction (MOD), and CM with MOD (CM-MOD) using the Kruskal-Wallis test with a Dunn's posttest.
Jaundice, severe anemia, acidosis, and disseminated intravascular coagulation.
Heme and hemopexin levels in severe malaria.
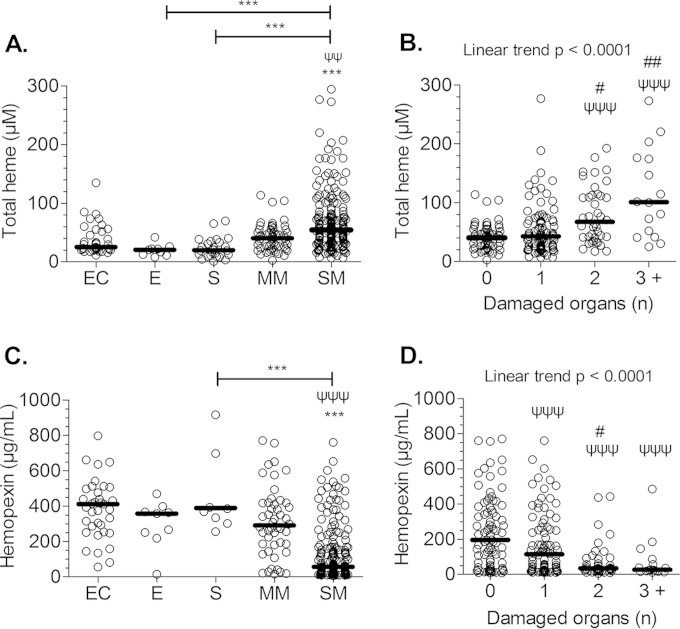
To understand the impact of heme in the pathophysiology of severe P. falciparum malaria, we measured plasma levels of heme, which include both free and protein-bound forms, in P. falciparum-infected patients and correlated them with disease severity. Patients with systemic (sepsis; n = 10) and brain (encephalitis; n = 9) inflammatory disease also were included to assess whether any observed association was malaria specific. We found no change in heme levels in cases of encephalitis and sepsis. However, heme levels increased with malarial disease severity as well as with the number of damaged organs (Fig. 1A and B). Mortality was observed only during CM (n = 6) and CM-MOD (n = 9), and CM/CM-MOD patients who did not survive had a 2-fold increase in heme levels compared to those who survived (P < 0.01; Mann-Whitney test). Also, we noted an inverse correlation between heme and hemopexin levels (R = −0.416; P < 0.0001). There was a 6-fold decrease in the plasma levels of hemopexin in SM patients compared to that of MM patients or ECs, and hemopexin levels decreased significantly with the number of damaged organs (Fig. 1C and D).
FIG 1.
Total heme levels and malaria severity. Total heme and hemopexin levels were measured in the plasma of P. falciparum-infected patients and were compared according to the different clinical outcomes, i.e., endemic controls (EC), encephalitis (E), severe sepsis (S), mild malaria (MM), severe malarial (SM) patients (A and C), and the number of damaged organs during malaria (B and D). The dark lines indicate the medians. Data were analyzed using the Kruskal-Wallis test with a Dunn's posttest or, for panel B, with a linear trend analysis. Symbols: ψ, differences with the MM or with the group of patients having 0 damaged organ; #, differences with the group of patients having 1 damaged organ; *, differences between selected groups. **, P ≤ 0.01; ***, P ≤ 0.001.
Heme/hemopexin levels and organ injuries.
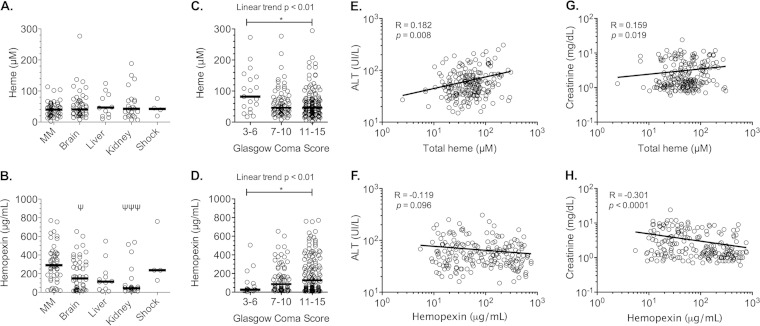
Systolic shock cases were excluded from analysis because of the small number of patients (n = 4). Similar total heme levels were obtained in patients with a single organ dysfunction and those with MM (Fig. 1B and 2A). However, hemopexin levels decreased significantly in patients having one damaged organ (Fig. 1C), especially when the brain and kidney were involved but not the liver (Fig. 2B). We also compared total heme and hemopexin levels according to the GCS. Considering a GCS of ≤6 to be indicative of a poor prognosis (21), we grouped patients into three GCS categories: ≤6, 7 to 10, and >10. Levels of total heme significantly increased when the GCS was ≤6 (Fig. 2C) and were inversely correlated to plasma hemopexin levels (Fig. 2D). Additionally, hemopexin levels were lower in patients with malarial ARF but not with hepatopathy (Fig. 2B). According to a Spearman's rank correlation analysis, creatinine levels correlated negatively with hemopexin and positively with total heme, whereas ALT correlated with total heme levels only (Fig. 2E to H).
FIG 2.
Correlation between clinical features and biological parameters with total heme and hemopexin levels. (A and B) Total heme and hemopexin levels in the plasma of P. falciparum patients were compared between mild malaria (MM) cases and individuals having only a single complication: cerebral malaria (brain), hepatopathy (liver), and malarial acute renal failure (kidney). (C and D) Total heme levels and hemopexin levels were compared according to the Glasgow coma score (GCS) and also were correlated using a Spearman's rank correlation test to alanine transaminase levels (ALT) (E and F) and creatinine levels (G and H) of P. falciparum-infected patients. The dark lines indicate the medians. Data shown in panels A to D were analyzed using the Kruskal-Wallis test with a Dunn's posttest. In panel B, ψ indicates differences with MM, and in panels A to D, an asterisk indicates differences between selected groups. *, P ≤ 0.05; ***, P ≤ 0.001.
Correlation of heme and hemopexin levels with cytokines in severe malaria.
In order to assess whether plasma levels of total heme and hemopexin were related to cytokine profiles in malaria, we measured the plasma concentrations of the following cytokines: granulocyte macrophage colony-stimulating factor, granulocyte colony-stimulating factor, alpha 2 interferon (IFN-α2), IFN-γ, interleukin-1α (IL-1α), IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17, IFN-γ-induced protein 10 (IP-10), macrophage inflammatory protein 1α (MIP-1α), MIP-β, monocyte chemotactic protein 1 (MCP-1), tumor necrosis factor alpha (TNF-α), and TNF-β. IL-10, IP-10, TNF-α, and MCP-1 correlated with both heme and hemopexin levels (see Table S1 in the supplemental material).
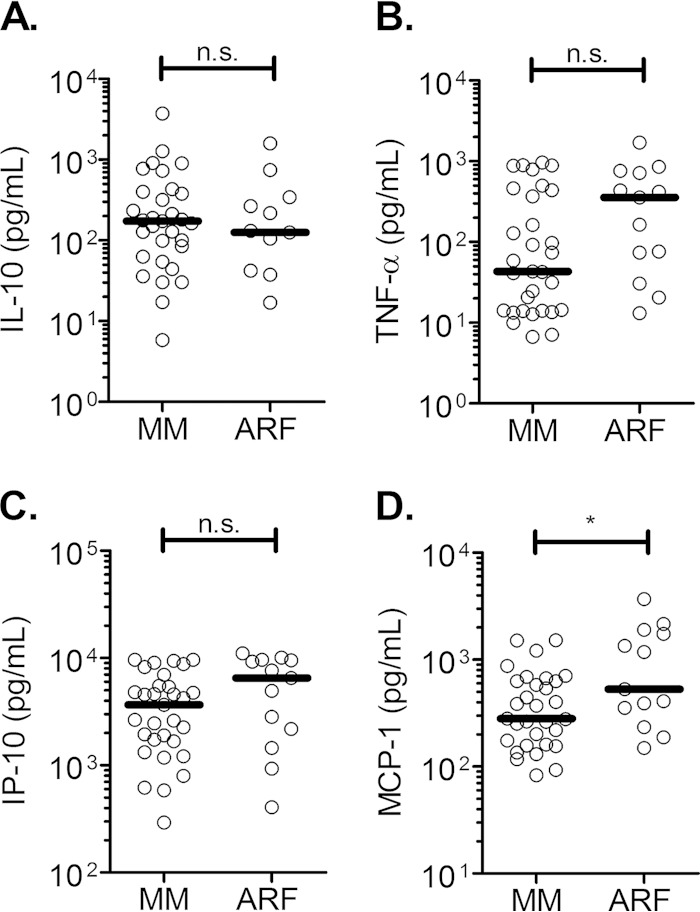
To understand the networks involved among these different factors, we performed a canonical correlation analysis between cytokine levels and biological parameters by including all study patients. Canonical analysis is a multivariate analysis that distinguishes components of relationships between two sets of variables. It can further describe, by coefficients, how variables combined in one set explain a corresponding variable combination in a second set and in each component, also called the canonical variate. The negative or positive coefficient assigned for each variable predicts the way of its contribution in the component. The two sets of variables used in our analysis were the following: set X included IL-10, IP-10, MCP-1, and TNF-α, and set Y included creatinine, heme, and hemopexin (Table 2). Analysis revealed two separate significant canonical variates (first, P < 0.0001; second, P = 0.0021). Total heme and creatinine contributed negatively to the first canonical variate, while all cytokines were positively related to it. Within each set of variables, IP-10 and hemopexin had the highest coefficients, suggesting that they were major contributors to this component of the relationship (Table 2). IL-10 and IP-10 were positively associated with the second significant canonical variate. The same also was true for heme, hemopexin, and creatinine, suggesting a positive correlation between these variables. Interestingly, within this second canonical variate, MCP-1 and creatinine had the highest coefficients (Table 2). The canonical analysis indicates the existence of complex networks, while overall only the plasma levels of MCP-1 were significantly higher during malarial ARF than MM (Fig. 3A to D).
TABLE 2.
Canonical correlation analysis of cytokine, total heme, hemopexin, and creatinine levelsa
| Variable and set | Canonical variate |
|
|---|---|---|
| 1 | 2 | |
| X | ||
| IL-10 | 0.0308 | 0.0814 |
| TNF-α | 0.0071 | −0.0103 |
| IP-10 | 0.0751 | 0.0576 |
| MCP-1 | 0.0605 | −0.1582 |
| Y | ||
| Heme | −0.0524 | 0.0419 |
| Hemopexin | 0.1207 | 0.1016 |
| Creatinine | −0.0376 | 0.2166 |
Canonical correlation analysis was done using the data for all of the patients and was calculated between the two sets of variables. Analysis revealed two separate significant canonical variates (first, P < 0.0001; second, P = 0.0021). Each column describes one of the two total canonical variates, with the respective coefficients for the variables in each set. IL-10, IP-10, heme, hemopexin, and creatinine were positively correlated with the second canonical variate, whereas MCP-1 and TNF-α were negatively correlated with it.
FIG 3.
Cytokine levels during MM and ARF. IL-10 (A), TNF-α (B), IP-10 (C), and MCP-1 (D) levels were measured in the plasma of P. falciparum-infected patients having mild malaria (MM) or only malarial acute renal failure (ARF). The dark lines indicate the medians. Data were analyzed using the Mann-Whitney test. *, P ≤ 0.05; n.s., nonsignificant difference.
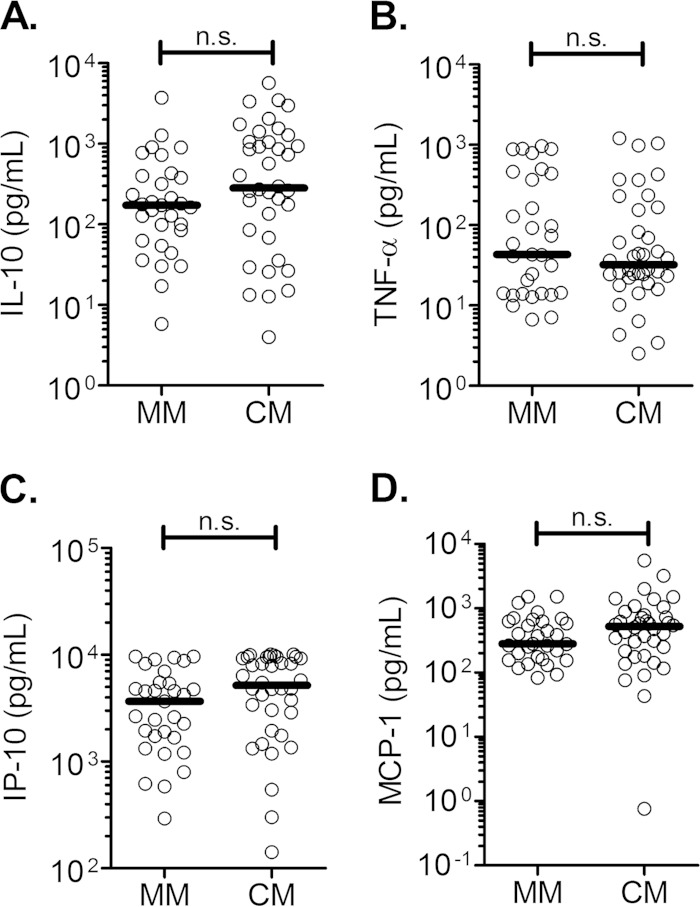
It is noteworthy that the plasma cytokine profile in CM patients was different from that observed in malarial ARF. Plasma IL-10, IP-10, MCP-1, and TNF-α levels were comparable between CM and MM patients (Fig. 4A to D). Therefore, canonical correlations between IL-10, IP-10, MCP-1, and TNF-α (set X) and heme, hemopexin, and the CM-specific GCS (set Y) also were calculated (Table 3). Only one significant canonical variate was found (P < 0.0001), within which heme levels were negatively associated with the component, while GCS and hemopexin were positively associated with it. In addition, all of the cytokines from the first set of variables were positively associated with the component. This suggests that low plasma levels of heme are associated with an increase in GCS and increased cytokine levels. In each set of variables, IP-10 and hemopexin had the highest coefficients, suggesting their major effect in the CM-related network.
FIG 4.
Cytokine levels during MM and CM. IL-10 (A), TNF-α (B), IP-10 (C), and MCP-1 (D) levels were measured in the plasma of mild malaria (MM) and cerebral malaria (CM) patients. The dark lines indicate the medians. Data were analyzed using the Mann-Whitney test. **, P ≤ 0.01; ***, P ≤ 0.001; n.s., nonsignificant difference.
TABLE 3.
Canonical correlation analysis of cytokine, total heme, and hemopexin levels and GCSa
| Variable and set | Canonical variate 1 |
|---|---|
| X | |
| IL-10 | 0.0420 |
| TNF-α | 0.0009 |
| IP-10 | 0.0753 |
| MCP-1 | 0.0457 |
| Y | |
| Heme | −0.0616 |
| Hemopexin | 0.1225 |
| GCS | 0.0578 |
Canonical correlation analysis was done using the data for all patients and was calculated between the two sets of variables. Analysis revealed one significant canonical variate (P < 0.0001). Heme was negatively associated with the component, whereas all of the other variables from both sets were positively associated with it.
DISCUSSION
Hemolytic conditions are responsible for the release of heme into circulation. Despite its critical role in many biological processes, free heme can be involved in the induction of pathogenic responses (7). Therefore, to assess the role of heme in human malaria, we measured the plasma levels of total heme and hemopexin in P. falciparum-infected patients. We found that the highest total heme levels were correlated inversely with hemopexin levels in both CM and ARF patients. Thus, our results provide evidence that the severity of P. falciparum malaria is associated with an increase in total heme levels in plasma. Accordingly, heme levels were particularly high in fatal cases with an increasing number of affected organs but not in cases of hepatopathy. To our knowledge, this study is the first to emphasize the deleterious effects of heme in P. falciparum infection.
Hemopexin is the most important element of the heme-detoxifying system because of its high binding affinity to heme that allows it to ferry heme from the bloodstream to scavenger cells (9). The degradation of the heme-hemopexin complex is likely to result in a decrease in hemopexin levels during hemolysis, thereby contributing to the systemic accumulation of heme (22). We have shown previously that intraperitoneal injections of heme in mice resulted in a decrease in hemopexin levels (10). Therefore, we propose that hemopexin levels reflect free heme levels. Interestingly, there is a negative correlation between total heme and hemopexin levels in malaria, suggesting that during infection, mainly unbound heme is found in plasma. Hemopexin is a positive acute-phase protein, and previous studies have reported an increase in the circulating levels of hemopexin and its precursor during sepsis or in MM (23, 24). However, decreased levels of hemopexin during severe sepsis also were associated with a low survival prognosis, highlighting the importance of hemopexin in the scavenging of free heme (23, 25). Our observation that heme and hemopexin levels remained unchanged in the severe sepsis and encephalitis groups strongly supports the association of systemic accumulation of heme in malarial infection with the lysis of infected RBCs but not with inflammatory conditions. This assumption is reinforced by the significant correlation of heme with HRP-2 (R = 0.475; P < 0.001), as HRP-2 levels are a good indicator of the total body parasite biomass (20). Even though we did not observed any correlation between total heme and hemoglobin levels (R = 0.015; P = 0.839), we cannot exclude the contribution of healthy RBCs to the systemic release of heme.
Our findings reveal that heme plays a role in the pathophysiology of human CM, which also has been confirmed previously by studies in mice (5). In India, CM is often associated with MOD, including malarial ARF (26). In this study, 31% of SM patients had more than two damaged organs, and ARF was the second most common cause of SM (see Fig. S1 in the supplemental material). Despite the increasing incidence of malarial ARF in India, little is known about its pathophysiology (2). ARF is a frequent complication in disorders associated with high levels of heme (8, 25, 27). To our knowledge, we are the first to report increased heme levels during malarial ARF, pointing to a possible toxic effect of heme on the kidneys during P. falciparum infections. During CM and malarial ARF, it is likely that the heme-mediated pathophysiology involves mechanical as well as immunological processes (8, 28, 29). Moreover, as a catalyzer of ROS generation, heme decreases the intracellular levels of reduced glutathione, which is associated with the modulation of the immune response through the polarization of T lymphocytes and macrophages toward T-helper 2 and M2 profiles (10, 30).
Cumulative evidence in our study strongly indicates an immunological network involving heme and cytokines, such as IL-10, IP-10, MCP-1, and TNF-α, in the pathophysiology of CM and malarial ARF. As suggested previously, IP-10 levels seemed to be regulated by hemopexin (31), but cytokine levels were not associated with the outcome of CM (32, 33). In fact, we have shown that IP-10 alone was not a predictor of CM in the Odishi population (F. Herbert, N. Tchitchek, D. Bansal, J. Jacques, S. Pathak, C. Bécavin, C. Fesel, E. Dalko, P.-A. Cazenave, C. Preda, B. Ravindran, S. Sharma, B. Das, and S. Pied, submitted for publication). In this context and as suggested by canonical correlation analyses, IP-10 might be a major component of the complex immune network involving heme and hemopexin in the pathophysiology of CM. Despite a positive correlation between heme and creatinine levels, malarial ARF was associated with increased levels of MCP-1 only, indicating different immune-mediated mechanisms in the pathophysiology of these two malaria-driven complications. Heme- and hemoglobin-induced renal damage is further associated with the production of high levels of ROS and with an overexpression of HO-1, which inhibits MCP-1 expression through a negative loop (27, 34). In our canonical analysis, this may correspond to the second canonical variate, where low levels for MCP-1 and TNF-α were related to high levels of heme. In addition, increased levels of IL-10 were associated with increased levels of creatinine, which is in agreement with the protective effect of IL-10 during ARF in murine models (35). In contrast with this observation, the first canonical variate was related to overall high cytokine/chemokine levels rather than to high hemopexin, low heme, and low creatinine levels, highlighting the complexity of the networks involved in the pathophysiology of ARF.
Although the pathophysiology of CM and malarial ARF could, in part, be driven by genetic and environmental factors, our results suggest that IL-10, IP-10, MCP-1, and TNF-α, along with heme and hemopexin, contribute to the development of these conditions via different regulatory mechanisms. We also suggest that plasma heme and hemopexin levels are good indicators for differentiating severe outcomes of P. falciparum malaria. To gain better insight into the complex mechanisms underlying these regulations, we will investigate, in further studies, the polymorphism in the promoter of the HO-1 gene, HMOX1, that influences its expression and circulating levels of heme (36, 37).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Indo-French Centre for the Promotion of Advanced Research, the Associated International Laboratory Systems (LIA; CNRS), Immunology and Genetics of Infectious Diseases (SIGID), the Department of Biotechnology from the Ministry of Science and Technology of India (DBT), and intramural funds from Tata Institute of Fundamental Research (TIFR). E.D. was supported by a doctoral contract with Université Lille 1, the Raman-Charpak award from IFCPAR, the AAP n10 award from the College Doctoral Lille Nord de France, and awards from the Fondation des Treilles and the Conseil Régional du Nord-Pas de Calais.
We thank Devendra Bansal for his help in assaying cytokine levels and Gauri Pathak for kindly reviewing the manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00531-15.
REFERENCES
- 1.Kumar A, Valecha N, Jain T, Dash AP. 2007. Burden of malaria in India: retrospective and prospective view. Am J Trop Med Hyg 77:69–78. [PubMed] [Google Scholar]
- 2.Das BS. 2008. Renal failure in malaria. J Vector Borne Dis 45:83–97. [PubMed] [Google Scholar]
- 3.Schofield L, Grau GE. 2005. Immunological processes in malaria pathogenesis. Nat Rev Immunol 5:722–735. doi: 10.1038/nri1686. [DOI] [PubMed] [Google Scholar]
- 4.Pamplona A, Hanscheid T, Epiphanio S, Mota MM, Vigario AM. 2009. Cerebral malaria and the hemolysis/methemoglobin/heme hypothesis: shedding new light on an old disease. Int J Biochem Cell Biol 41:711–716. doi: 10.1016/j.biocel.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira A, Balla J, Jeney V, Balla G, Soares MP. 2008. A central role for free heme in the pathogenesis of severe malaria: the missing link? J Mol Med 86:1097–1111. doi: 10.1007/s00109-008-0368-5. [DOI] [PubMed] [Google Scholar]
- 6.Graca-Souza AV, Arruda MA, de Freitas MS, Barja-Fidalgo C, Oliveira PL. 2002. Neutrophil activation by heme: implications for inflammatory processes. Blood 99:4160–4165. doi: 10.1182/blood.V99.11.4160. [DOI] [PubMed] [Google Scholar]
- 7.Larsen R, Gouveia Z, Soares MP, Gozzelino R. 2012. Heme cytotoxicity and the pathogenesis of immune-mediated inflammatory diseases. Front Pharmacol 3:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinchi F, Gastaldi S, Silengo L, Altruda F, Tolosano E. 2008. Hemopexin prevents endothelial damage and liver congestion in a mouse model of heme overload. Am J Pathol 173:289–299. doi: 10.2353/ajpath.2008.071130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiabrando D, Vinchi F, Fiorito V, Tolosano E. 2011. Haptoglobin and hemopexin in heme detoxification and iron recycling. [In] Veas F. (ed), Acute phase proteins–regulation and functions of acute phase proteins. InTech, Rijeka, Croatia. [Google Scholar]
- 10.Dalko E, Gaudreault V, Sanchez Dardon J, Moreau R, Scorza T. 2013. Preconditioning with hemin decreases Plasmodium chabaudi adami parasitemia and inhibits erythropoiesis in BALB/c mice. PLoS One 8:e54744. doi: 10.1371/journal.pone.0054744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dey S, Bindu S, Goyal M, Pal C, Alam A, Iqbal MS, Kumar R, Sarkar S, Bandyopadhyay U. 2012. Impact of intravascular hemolysis in malaria on liver dysfunction: involvement of hepatic free heme overload, NF-kappaB activation, and neutrophil infiltration. J Biol Chem 287:26630–26646. doi: 10.1074/jbc.M112.341255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrade BB, Araujo-Santos T, Luz NF, Khouri R, Bozza MT, Camargo LM, Barral A, Borges VM, Barral-Netto M. 2010. Heme impairs prostaglandin E2 and TGF-beta production by human mononuclear cells via Cu/Zn superoxide dismutase: insight into the pathogenesis of severe malaria. J Immunol 185:1196–1204. doi: 10.4049/jimmunol.0904179. [DOI] [PubMed] [Google Scholar]
- 13.Yeo TW, Lampah DA, Tjitra E, Gitawati R, Kenangalem E, Piera K, Granger DL, Lopansri BK, Weinberg JB, Price RN, Duffull SB, Celermajer DS, Anstey NM. 2009. Relationship of cell-free hemoglobin to impaired endothelial nitric oxide bioavailability and perfusion in severe falciparum malaria. J Infect Dis 200:1522–1529. doi: 10.1086/644641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thuma PE, van Dijk J, Bucala R, Debebe Z, Nekhai S, Kuddo T, Nouraie M, Weiss G, Gordeuk VR. 2011. Distinct clinical and immunologic profiles in severe malarial anemia and cerebral malaria in Zambia. J Infect Dis 203:211–219. doi: 10.1093/infdis/jiq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panda AK, Panda SK, Sahu AN, Tripathy R, Ravindran B, Das BK. 2011. Association of ABO blood group with severe falciparum malaria in adults: case control study and meta-analysis. Malar J 10:309. doi: 10.1186/1475-2875-10-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. 2010. Guidelines for the treatment of malaria. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf?ua=1. [Google Scholar]
- 17.Noedl H, Bronnert J, Yingyuen K, Attlmayr B, Kollaritsch H, Fukuda M. 2005. Simple histidine-rich protein 2 double-site sandwich enzyme-linked immunosorbent assay for use in malaria drug sensitivity testing. Antimicrob Agents Chemother 49:3575–3577. doi: 10.1128/AAC.49.8.3575-3577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bansal D, Herbert F, Lim P, Deshpande P, Becavin C, Guiyedi V, de Maria I, Rousselle JC, Namane A, Jain R, Cazenave PA, Mishra GC, Ferlini C, Fesel C, Benecke A, Pied S. 2009. IgG autoantibody to brain beta tubulin III associated with cytokine cluster-II discriminate cerebral malaria in central India. PLoS One 4:e8245. doi: 10.1371/journal.pone.0008245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pathak S, Rege M, Gogtay NJ, Aigal U, Sharma SK, Valecha N, Bhanot G, Kshirsagar NA, Sharma S. 2012. Age-dependent sex bias in clinical malarial disease in hypoendemic regions. PLoS One 7:e35592. doi: 10.1371/journal.pone.0035592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dondorp AM, Desakorn V, Pongtavornpinyo W, Sahassananda D, Silamut K, Chotivanich K, Newton PN, Pitisuttithum P, Smithyman AM, White NJ, Day NP. 2005. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med 2:e204. doi: 10.1371/journal.pmed.0020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patankar TF, Karnad DR, Shetty PG, Desai AP, Prasad SR. 2002. Adult cerebral malaria: prognostic importance of imaging findings and correlation with postmortem findings. Radiology 224:811–816. doi: 10.1148/radiol.2243010588. [DOI] [PubMed] [Google Scholar]
- 22.Muller-Eberhard U, Javid J, Liem HH, Hanstein A, Hanna M. 1968. Plasma concentrations of hemopexin, haptoglobin and heme in patients with various hemolytic diseases. Blood 32:811–815. [PubMed] [Google Scholar]
- 23.Janz DR, Bastarache JA, Sills G, Wickersham N, May AK, Bernard GR, Ware LB. 2013. Association between haptoglobin, hemopexin and mortality in adults with sepsis. Crit Care 17:R272. doi: 10.1186/cc13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray S, Renu D, Srivastava R, Gollapalli K, Taur S, Jhaveri T, Dhali S, Chennareddy S, Potla A, Dikshit JB, Srikanth R, Gogtay N, Thatte U, Patankar S, Srivastava S. 2012. Proteomic investigation of falciparum and vivax malaria for identification of surrogate protein markers. PLoS One 7:e41751. doi: 10.1371/journal.pone.0041751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen R, Gozzelino R, Jeney V, Tokaji L, Bozza FA, Japiassu AM, Bonaparte D, Cavalcante MM, Chora A, Ferreira A, Marguti I, Cardoso S, Sepulveda N, Smith A, Soares MP. 2010. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med 2:51ra71. [DOI] [PubMed] [Google Scholar]
- 26.Jain V, Nagpal AC, Joel PK, Shukla M, Singh MP, Gupta RB, Dash AP, Mishra SK, Udhayakumar V, Stiles JK, Singh N. 2008. Burden of cerebral malaria in central India (2004-2007). Am J Trop Med Hyg 79:636–642. [PMC free article] [PubMed] [Google Scholar]
- 27.Tracz MJ, Alam J, Nath KA. 2007. Physiology and pathophysiology of heme: implications for kidney disease. J Am Soc Nephrol 18:414–420. doi: 10.1681/ASN.2006080894. [DOI] [PubMed] [Google Scholar]
- 28.Nuchsongsin F, Chotivanich K, Charunwatthana P, Omodeo-Sale F, Taramelli D, Day NP, White NJ, Dondorp AM. 2007. Effects of malaria heme products on red blood cell deformability. Am J Trop Med Hyg 77:617–622. [PubMed] [Google Scholar]
- 29.Chiu DT, van den Berg J, Kuypers FA, Hung IJ, Wei JS, Liu TZ. 1996. Correlation of membrane lipid peroxidation with oxidation of hemoglobin variants: possibly related to the rates of hemin release. Free Radic Biol Med 21:89–95. doi: 10.1016/0891-5849(96)00035-4. [DOI] [PubMed] [Google Scholar]
- 30.Cambos M, Bazinet S, Abed E, Sanchez-Dardon J, Bernard C, Moreau R, Olivier M, Scorza T. 2010. The IL-12p70/IL-10 interplay is differentially regulated by free heme and hemozoin in murine bone-marrow-derived macrophages. Int J Parasitol 40:1003–1012. doi: 10.1016/j.ijpara.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Wilson NO, Solomon W, Anderson L, Patrickson J, Pitts S, Bond V, Liu M, Stiles JK. 2013. Pharmacologic inhibition of CXCL10 in combination with anti-malarial therapy eliminates mortality associated with murine model of cerebral malaria. PLoS One 8:e60898. doi: 10.1371/journal.pone.0060898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain V, Armah HB, Tongren JE, Ned RM, Wilson NO, Crawford S, Joel PK, Singh MP, Nagpal AC, Dash AP, Udhayakumar V, Singh N, Stiles JK. 2008. Plasma IP-10, apoptotic and angiogenic factors associated with fatal cerebral malaria in India. Malar J 7:83. doi: 10.1186/1475-2875-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prakash D, Fesel C, Jain R, Cazenave PA, Mishra GC, Pied S. 2006. Clusters of cytokines determine malaria severity in Plasmodium falciparum-infected patients from endemic areas of central India. J Infect Dis 194:198–207. doi: 10.1086/504720. [DOI] [PubMed] [Google Scholar]
- 34.Pittock ST, Norby SM, Grande JP, Croatt AJ, Bren GD, Badley AD, Caplice NM, Griffin MD, Nath KA. 2005. MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice: pathophysiologic correlates. Kidney Int 68:611–622. doi: 10.1111/j.1523-1755.2005.00439.x. [DOI] [PubMed] [Google Scholar]
- 35.Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA. 2001. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int 60:2118–2128. doi: 10.1046/j.1523-1755.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 36.Takeda M, Kikuchi M, Ubalee R, Na-Bangchang K, Ruangweerayut R, Shibahara S, Imai S, Hirayama K. 2005. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to cerebral malaria in Myanmar. Jpn J Infect Dis 58:268–271. [PubMed] [Google Scholar]
- 37.Walther M, De Caul A, Aka P, Njie M, Amambua-Ngwa A, Walther B, Predazzi IM, Cunnington A, Deininger S, Takem EN, Ebonyi A, Weis S, Walton R, Rowland-Jones S, Sirugo G, Williams SM, Conway DJ. 2012. HMOX1 gene promoter alleles and high HO-1 levels are associated with severe malaria in Gambian children. PLoS Pathog 8:e1002579. doi: 10.1371/journal.ppat.1002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.