Abstract
Rhesus macaques were studied to directly address the potential for plasmid-deficient Chlamydia trachomatis to serve as a live attenuated vaccine in the genital tract. Five repeated cervical inoculations of rhesus macaques with wild-type serovar D strain D/UW-3/Cx or a plasmid-deficient derivative of this strain, CTD153, resulted in infections with similar kinetics and induced comparable levels of protective immunity. After all animals received five challenges with D/UW-3/Cx, levels of inflammation observed grossly and histologically were similar between the groups. Animals in both groups developed evidence of oviduct dilatation; however, reduced oviduct dilatation was observed for “controllers,” i.e., animals without detectable chlamydial DNA in the fimbriae at weeks 5 and 12. Grouping animals into “ascenders” and “controllers” revealed that elevated early T cell responses were associated with protection, whereas higher antibody responses were associated with ascension. Protected animals shared common major histocompatibility complex (MHC) alleles. Overall, genetic differences of individual animals, rather than the presence or absence of the chlamydial plasmid in the primary infecting strain, appeared to play a role in determining the outcome of infection.
INTRODUCTION
Infections with the obligate intracellular bacterium Chlamydia trachomatis are a major public health concern. In developing nations, repeated conjunctival infections with serovars A to C cause trachoma, the leading cause of preventable blindness worldwide (1). Genitourinary infections with serovars D to K and L1 to L3 are the most prevalent sexually transmitted bacterial infections in the world. An effective vaccine is not available, and increased screening and treatment have been associated with a rise in the incidence of chlamydial genital tract infection (2). Although antibiotic therapy effectively eliminates infection, it does not reverse established pathology. Serious sequelae resulting from genital tract infection with Chlamydia include pelvic inflammatory disease (PID), ectopic pregnancy, chronic pelvic pain, and infertility in women (reviewed in reference 3). Since the majority of infected women are asymptomatic and do not seek treatment, the consequences of infection often do not become apparent until years after infection, when affected women are unable to conceive.
An effective immune response is required for resolution of infection, but overly robust immune activation is responsible for Chlamydia-induced pathology. Studies of mice and guinea pigs have clearly demonstrated that innate immune responses cause tissue damage, while CD4+ T cells and antibody provide protection against challenge infection (reviewed in reference 4). Although specific mediators of protection have been more difficult to delineate for women, correlations between increased CD4+ T cell gamma interferon (IFN-γ) responses and reduced antichlamydial antibody levels have been associated with disease control (reviewed in reference 4). The outcome of a chlamydial infection results from an interplay between the host's ability to control the infection and the bacterium's ability to simultaneously induce inflammation and subvert eradication.
A central regulator of Chlamydia-induced immunopathology is the highly conserved 7.5-kb chlamydial plasmid (5–8). Mice infected with plasmid-deficient Chlamydia muridarum exhibit markedly reduced levels of immunopathology but develop an adaptive immune response that prevents disease upon challenge with virulent C. muridarum (5, 9). In addition, conjunctival inoculation of cynomolgus macaques with plasmid-deficient serovar A has been shown to result in an abbreviated infection that protects against disease in a subset of animals challenged with the virulent parental strain (7). In contrast, curing the plasmid in C. caviae (10) and C. psittaci (11) did not result in significant changes in virulence upon infection of guinea pigs and mice, respectively. The protective capacity of plasmid-deficient strains has not been examined in a rhesus monkey model of Chlamydia genital tract infection.
The rhesus monkey model of trachoma is distinct from the genital tract model both in the mucosal site and in the adaptations of Chlamydia to those sites. In the trachoma model, Chlamydia is inoculated directly onto the conjunctiva, where disease develops. In the genital tract model, Chlamydia is inoculated at the cervix and ascends over time to the oviducts, the site of irreversible immunopathology. This delay in infection of the oviducts allows for priming of Chlamydia-specific T cells and homing of those cells to the genital tract, potentially affecting the dynamics of protection and immunopathology. Genetic differences between ocular and genital biovars may also differentially influence the pathogenesis of infection in these models. C. trachomatis strains exhibit >99% sequence homology, but genes differentially involved in tissue tropism and immune evasion have been identified (reviewed in reference 12). For example, genital but not ocular biovars possess a functional tryptophan operon that permits utilization of indole to synthesize tryptophan (13, 14). This allows evasion of IFN-γ-induced tryptophan degradation by indoleamine 2,3-dioxygenase (IDO) (13, 15). IFN-γ does not induce IDO in the genital tract epithelium of mice (15, 16), which provides a further impetus to explore the potential of plasmid-deficient Chlamydia to serve as a live attenuated vaccine in higher-order mammals.
In the present study, we utilized a plasmid-deficient derivative of C. trachomatis D/UW-3/Cx to infect rhesus macaques at the cervix. This strain, CTD153, has an attachment/uptake defect and induces lower levels of cytokine production in vitro and in the murine genital tract (17). The aims of this study were to use the rhesus monkey model of chlamydial genital tract infection to determine (i) if a plasmid-deficient human C. trachomatis strain induces pathology at lower rates than those of a fully virulent strain, (ii) if “vaccination” with a plasmid-deficient strain is protective against subsequent challenge with a fully virulent strain, and (iii) if immune correlates of protection can be identified. The findings reported in this study are central to the future use of plasmid-deficient Chlamydia strains as live attenuated vaccines.
MATERIALS AND METHODS
Strains, cell lines, and culture conditions.
The C. trachomatis strain D/UW-3/Cx (18) was obtained from the American Type Culture Collection (Manassas, VA) and plaque purified before use (19). The D/UW-3/Cx strain was cured of the plasmid by novobiocin treatment to yield CTD153 (17). The absence of the plasmid from CTD153 was previously verified by PCR and glycogen staining (17). C. trachomatis L2/434/Bu was also used where indicated. Bacteria were propagated in L929 cells (17) and titrated by plaque assay (19) or as inclusion-forming units (IFU) (20), using a fluorescently tagged anti-chlamydial lipopolysaccharide monoclonal antibody (Bio-Rad, Hercules, CA). Chlamydia elementary bodies (EBs) were purified by centrifugation over a discontinuous gradient (21) and inactivated by X-ray irradiation prior to use (17).
Animals.
Ten young female rhesus monkeys (Macaca mulatta) (∼3.5 years of age; ∼4 to 5 kg) were included in the study. They were maintained on a standard colony diet with fruit supplementation per the colony protocol. All procedures conformed to the requirements of the Animal Welfare Act, and protocols were approved prior to implementation by the Institutional Animal Care and Use Committee at the University of California, Davis.
Infection and monitoring.
Animals were divided into two groups (n = 5 animals/group) and were inoculated at the cervical os with the human genital tract serovar D strain D/UW-3/Cx (group 1) or a plasmid-cured derivative of this strain, CTD153 (group 2). After sedation with ketamine hydrochloride (10 mg/kg body weight), they received 5 consecutive inoculations at the cervical os at 1-week intervals (week 0 to week 4), with 1 × 106 IFU of either C. trachomatis D/UW-3/Cx (group 1) or CTD153 (group 2) (Fig. 1). Vaginal swabs and sponges were obtained each week, before inoculation, and were frozen at ≤−80°C until use. At week 5, the reproductive tracts were examined by abdominal ultrasound followed by laparotomy. Gross pathology noted at laparotomy was recorded by an observer who was blinded to the experimental groups. The fimbriae were gently swabbed for titration of Chlamydia. All monkeys were subsequently challenged with 5 consecutive intravaginal inoculations at 1-week intervals (week 7 to week 11), with 1 × 106 IFU of C. trachomatis D/UW-3/Cx. They were euthanized at week 12, and gross pathology was evaluated by an observer who was blinded to the experimental groups. The oviductal diameter was measured at the distal ampulla. The fimbriae were swabbed for titration of Chlamydia. Sections of the fimbriae, oviducts, uterus, cervix, and lymph nodes were collected for further analysis as described below.
FIG 1.
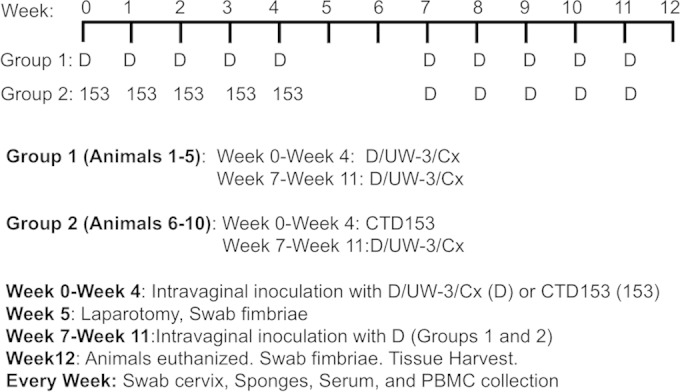
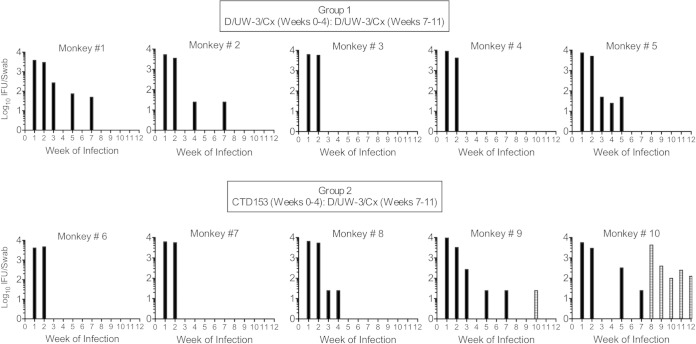
Rhesus monkey inoculation protocol. Monkeys were assigned to two experimental groups (n = 5/group). Group 1 (animals 1 to 5) received 5 weekly intravaginal inoculations with D/UW-3/Cx (D). Group 2 (animals 6 to 10) received 5 weekly inoculations with the plasmid-deficient strain CTD153 (153). No inoculations were conducted at week 5 or 6, and then all animals received 5 weekly inoculations with D/UW-3/Cx. Specimens were collected as indicated.
Quantification of cytokines in vaginal secretions.
An absorbent sponge was inserted into the vaginal vault each week prior to infection and then stored at ≤−80°C prior to analysis. At analysis, sponge contents were eluted as previously described (22). Levels of granulocyte colony-stimulating factor (G-CSF), interleukin-17 (IL-17), IL-1β, IL-6, IL-8, monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1β (MIP-1β), granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-γ, IL-2, MIP-1α, IL-2, tumor necrosis factor alpha (TNF-α), and IL-10 were assayed by using a multiplex cytometric bead array (Millipore, Billerica, MA).
Flow cytometry and cytokine detection.
Mononuclear cells were isolated from the peripheral blood (PBMCs), iliac lymph nodes, and inguinal lymph nodes by density gradient centrifugation, cryopreserved using a controlled-rate protocol, and stored in liquid nitrogen until analysis. Cells were cultured at a concentration of 5 × 105 cells per well, with or without 5 μg/ml of D/UW-3/Cx EBs. After 5 days, the cells were stained with the following antibodies from BD Biosciences (San Jose, CA) (unless otherwise indicated): anti-CD3–fluorescein isothiocyanate (FITC) (clone SP34), anti-CD4–BV 605 (clone L200), anti-CD8–phycoerythrin (PE)–Texas Red (clone 3B5; Life Technologies, Grand Island, NY), anti-CD28–PE (clone CD28.2), anti-CD95–PE–Cy7 (clone dx2), anti-NKG2A–allophycocyanin (APC) (clone z199; Beckman Coulter, Indianapolis, IN), anti-CD69–APC–Cy7 (clone FN50), anti-CCR7–peridinin chlorophyll protein (PerCP)–Cy5.5 (clone 150503), and anti-CXCR3–Alexa Fluor700 (clone 1C6/CXCR3). Cells were also stained with a LIVE/DEAD Aqua Dead cell stain kit (Life Technologies). Cells were permeabilized with a Cytofix/Cytoperm Plus kit according to the manufacturer's instructions (eBiosciences, San Diego, CA) prior to staining with anti-Ki67-V450 (clone B56). Raw cytometric data were acquired with a BD LSRII flow cytometer and analyzed offline using FlowJo software (Tree Star, Ashland, OR). The staining background was minimized by using isotype controls of the antibodies used. Multifluorochrome beads (Bang Laboratories, Fishers, Indiana) were used to calibrate cytometers and to generate offline matrices for signal compensation to eliminate electronic noise. The culture supernatants were harvested for measurements of secreted IFN-γ, TNF-α, and IL-2 by enzyme-linked immunosorbent assay (ELISA) (BD Biosciences).
PBMCs obtained from naive rhesus macaques were plated at 2 × 105 cells per well and stimulated with gradient-purified C. trachomatis D/UW-3/Cx or CTD153 at a final concentration of 5 μg/ml for 24 h. The supernatants were collected and analyzed for IL-6, GM-CSF, and IL-1β production via a multiparametric assay (Bio-Plex; Bio-Rad, Hercules, CA).
Lymph node ELISpot assay.
IFN-γ-specific enzyme-linked immunosorbent spot (ELISpot) assays were performed on mononuclear cells isolated from the iliac and inguinal lymph nodes by using a commercial ELISpot kit according to the manufacturer's instructions (monkey IFN-γ ELISpot kit-ALP; Mabtech, Cincinnati, OH). Cells were cultured in duplicate wells (2 × 105 cells/well) for 24 h at 37°C and 5% CO2. Cells were stimulated with anti-CD28 antibody (1 μg/ml) (clone CD28.2; BD Biosciences) in the presence or absence of inactivated D/UW-3/Cx EBs (2.5 μg/ml). The spots were counted and quality controlled by using a CTL-ImmunoSpot analyzer (C.T.L., Shaker Heights, OH).
Detection of antibodies in sera and vaginal secretions.
Levels of Chlamydia-specific IgA and IgG in sera and vaginal sponge eluates were determined via ELISA at week 0 (negative control), week 6, and week 12, as previously described (22). Plates were coated with inactivated serovar L2/434/Bu EBs (2 μg/ml), and antibody binding was detected with horseradish peroxidase (HRP)-conjugated anti-human IgG or IgA (Biolegend, San Diego, CA).
PCR for amplification of chlamydial DNA.
Fimbrial swabs obtained at weeks 5 and 12 were thawed and vigorously vortexed. Cells were pelleted, and DNA was isolated according to the manufacturer's protocol (DNA isolation kit; Epicentre Biotechnologies, Madison, WI). Nested PCR was used to detect ompA, using the primers and procedure described by Bom et al. (23). Samples were considered positive for ompA if a band of the appropriate size (614 bp) was visible after gel electrophoresis. Chlamydial DNA was quantified via real-time PCR analysis of the 16S rRNA gene as previously described (24).
Detection of Chlamydia in the oviduct.
Portions of the oviducts harvested at week 12 were stored in 500 μl of sucrose phosphate glutamate (SPG) buffer at ≤−80°C until titration. Samples were thawed, minced with scissors, and sonicated in a water bath for 5 min. Serial dilutions were titrated via plaque assay; the limit of detection was 5 PFU per oviduct. An aliquot was also removed for detection of the 16S rRNA gene by PCR as described above (see “PCR for amplification of chlamydial DNA”).
Pathology.
Gross findings were recorded at laparotomy at week 5 and at tissue harvest (week 12). Visible erythema was scored as follows: mild, 1 or 2; moderate, 3 or 4; and severe, 5. Hematoxylin and eosin (H&E)-stained sections of the ectocervix, endocervix, uterus, and oviducts were assessed for the presence of acute inflammation (neutrophils), chronic inflammation (lymphocytes/monocytes), and dilatation. The scoring system used was as follows: 0, normal; 1, rare/minimal; 2, mild; 3, moderate; and 4+, severe. Histopathology was scored by a pathologist who was blinded to the experimental design.
MHC genotyping.
Major histocompatibility complex (MHC) class I and class II DRB alleles were genotyped using 454 pyrosequencing technology (Roche/454 Life Sciences, Branford, CT) as previously described (25).
Statistics.
Flow cytometry, cytokine, and bacterial burden data were compared by using two-way repeated-measure analysis of variance (RM ANOVA) with Bonferroni posttests. Genital tract pathology was compared using the Mann-Whitney U test or one-way ANOVA with Dunn's multiple-comparison test. Antibody levels were compared by using one-way ANOVA with Dunn's multiple-comparison test. Proliferation and cytokine production in the lymph nodes were compared using Student's t test. Where relevant, statistical analysis was conducted on the difference between values obtained for cells cultured in the presence of EBs and those simultaneously cultured in the absence of chlamydial EBs. Prism software (GraphPad, La Jolla, CA) was utilized for all statistical analyses. P values of <0.05 were considered significant.
RESULTS
Rhesus macaques infected with wild-type or plasmid-deficient serovar D develop immunity to repeated infection.
In this study, we utilized a repeated infection approach that was previously shown to induce oviduct disease in pig-tailed macaques (26). Animals were divided into two groups (n = 5 animals/group) that were inoculated at the cervical os five times on a weekly basis with the human genital tract serovar D strain D/UW-3/Cx (group 1) or a plasmid-deficient derivative of this strain, CTD153 (group 2) (Fig. 1). After the first five inoculations, no inoculations were performed for a period of 2 weeks. Animals in both groups were then inoculated on a weekly basis for 5 weeks with D/UW-3/Cx.
No differences in bacterial burden in the lower genital tract were found for animals in group 1 or group 2 on individual days or over the course of infection (Fig. 2). All monkeys became infected, and animals in both groups had titers of approximately 104 Chlamydia organisms at weeks 1 and 2. Reduced bacterial burdens were noted beginning at week 3, with only 2 of 5 animals in both groups being positive for infection at that time point (Fig. 2 and 3). Four animals appeared to develop complete resistance to reinfection by week 3 (animals 3, 4, 6, and 7) (Fig. 3). Only two animals, animals 9 and 10, had detectable chlamydiae over the course of the last 5 weeks of infection with D/UW-3/Cx, with animal 10 remaining positive through the end of the study.
FIG 2.
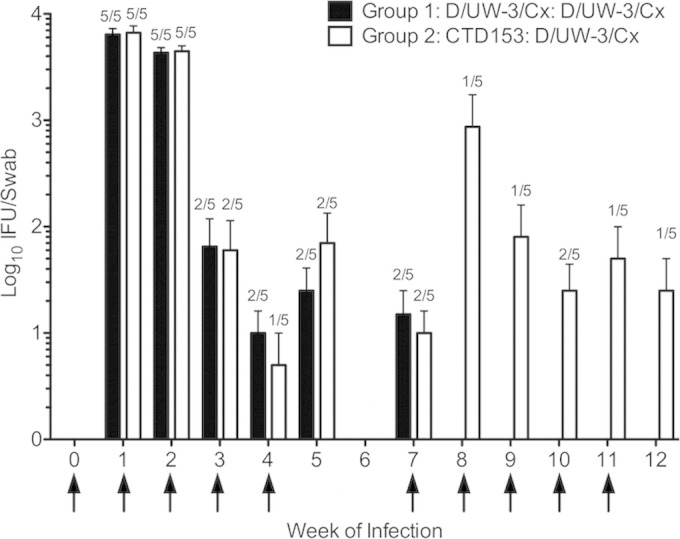
Bacterial burdens in the lower genital tract did not differ between groups. For the first 5 weeks, group 1 (black bars) was inoculated with D/UW-3/Cx, whereas group 2 (white bars) was inoculated with CTD153. All monkeys were inoculated with D/UW-3/Cx for the last 5 weeks. Bars represent the means ± standard errors of the means (SEM) for log10 IFU/swab. Fractions represent frequencies of monkeys that were positive for infection. Arrows indicate weeks when monkeys were inoculated. P values were >0.05 on individual days and over the entire interval as measured via two-way RM ANOVA with Bonferroni posttests.
FIG 3.
The degree of resistance to repeated infections varied between individual monkeys. Four animals had no detectable bacteria after the first 2 weeks: two in group 1 (animals 3 and 4) and two in group 2 (animals 6 and 7). Five animals appeared to develop an intermediate level of protective immunity in the lower genital tract (animals 1, 2, 5, 8, and 9), and one animal did not develop resistance to infection (animal 10). Bars represent the log10 IFU/swab of the cervix each week. Black bars represent titers resulting from the first five inoculations, which were performed with D/UW-3/Cx for group 1 and CTD153 for group 2. Striped bars represent bacteria detectable after animals in group 2 were inoculated with D/UW-3/Cx.
Detection of Chlamydia in the upper genital tract is associated with increased inflammation.
Infection of the upper genital tract was monitored by swabs of the fimbriae at laparotomy (week 5) and at tissue harvest (week 12). DNA isolated from swab eluates was used as the template for a nested PCR assay targeting ompA (23). At week 5, ompA-specific PCR products were detected via agarose gel electrophoresis in samples obtained at laparotomy for two animals in group 1 (animals 1 and 5) and two animals in group 2 (animals 9 and 10) (Table 1). At week 12, DNA was again detected in at least one oviduct from monkeys 1 and 9 and was also detected for the first time in oviducts from monkeys 3, 7, and 8. The chlamydial 16S rRNA gene was quantified via real-time PCR, and although this test was less sensitive than ompA nested PCR, the 16S rRNA gene was detected in 8 of 12 oviducts that had tested positive for ompA (Table 1). There was no evidence of chlamydial DNA in the fimbriae at week 5 or 12 for animal 2, 4, or 6. Live bacteria were not detected in the oviductal sections collected at tissue harvest for any monkey.
TABLE 1.
Summary of upper genital tract gross pathology and bacterial loadsa
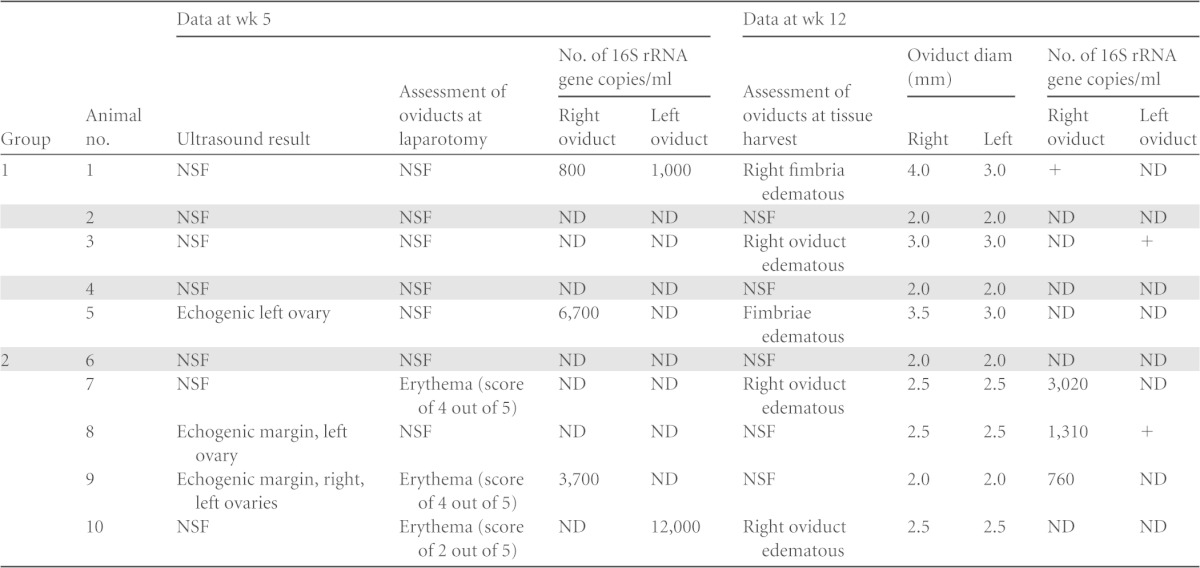
Genital tracts were analyzed via ultrasound and laparotomy at 5 weeks and at harvest (week 12). Fimbriae were swabbed at both time points. Chlamydia was found in swabs of the fimbriae via nested PCR for all swabs for which the 16S rRNA gene was enumerated. Three swabs had chlamydial DNA detected only via nested PCR (+). Shaded rows indicate animals (animals 2, 4, and 6) with no detectable chlamydial DNA or evidence of gross pathology. Group 1, infected with D/UW-3/Cx and D/UW-3/Cx; group 2, infected with CTD153 and D/UW-3/Cx. NSF, no significant findings; ND, not detected; +, positive by nested PCR but not positive for the 16S rRNA gene.
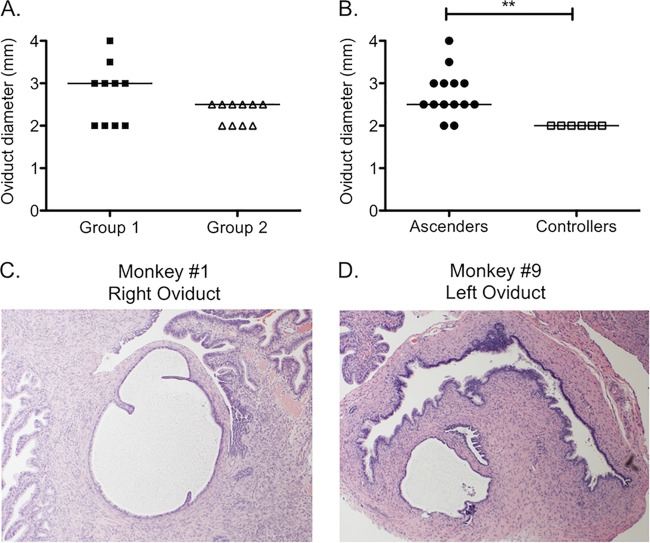
Upper genital tract inflammation and pathology were evaluated at week 5 by abdominal ultrasound followed by laparotomy and again at week 12. All animals with detectable chlamydial DNA in the upper genital tract displayed abnormal findings at one of these examinations (Table 1). Findings at week 5 included echogenic ovaries (animals 5, 8, and 9) and/or oviduct erythema (animals 7, 9, and 10). At tissue harvest, fimbrial (animals 1 and 5) or oviductal (animals 3, 7, and 10) edema was observed for 5 of the monkeys. We found no difference in the pattern of these findings between group 1 and group 2. In addition, measurement of the oviduct diameter at tissue harvest did not reveal differences between the groups (Fig. 4A), with a median diameter of 3 mm for animals in group 1 and 2.5 mm for animals in group 2. However, when animals were separated into those that had detectable DNA via PCR and abnormalities by gross examination, termed “ascenders” (animals 1, 3, 5, 7, 8, 9, and 10), and those that had no detectable chlamydial DNA or pathology, termed “controllers” (animals 2, 4, and 6), significant differences in oviduct diameter were observed (Fig. 4B) (P < 0.01). Dilated sections of oviducts were detected in two of the ascenders (animals 1 and 9) on histopathology (Fig. 4C and D). All of the “controllers” had an oviduct diameter of 2 mm, with no evidence of dilatation (Fig. 4B).
FIG 4.
Oviduct dilatation at week 12 was increased in monkeys with evidence of ascension of Chlamydia to the oviducts. (A) No significant difference in oviduct diameter was detected after 5 weeks of challenge with D/UW-3/Cx between animals infected with D/UW-3/Cx for the first 5 weeks (group 1; black squares) and animals infected with CTD153 for the first 5 weeks (group 2; white triangles). (B) The oviduct diameter was increased for monkeys who had detectable Chlamydia in their oviducts (ascenders; black circles) at either week 5 or week 12 compared to monkeys with nondetectable levels of Chlamydia (controllers; white squares), regardless of whether they were initially infected with D/UW-3/Cx or CTD153. Symbols represent individual oviducts. Horizontal lines indicate the medians. **, P < 0.01 by the Mann-Whitney U test for ascenders versus controllers. Hydrosalpinx was visible on histopathology (magnification, ×4) on the right oviduct for animal 1 (C) and on the left oviduct for animal 9 (D).
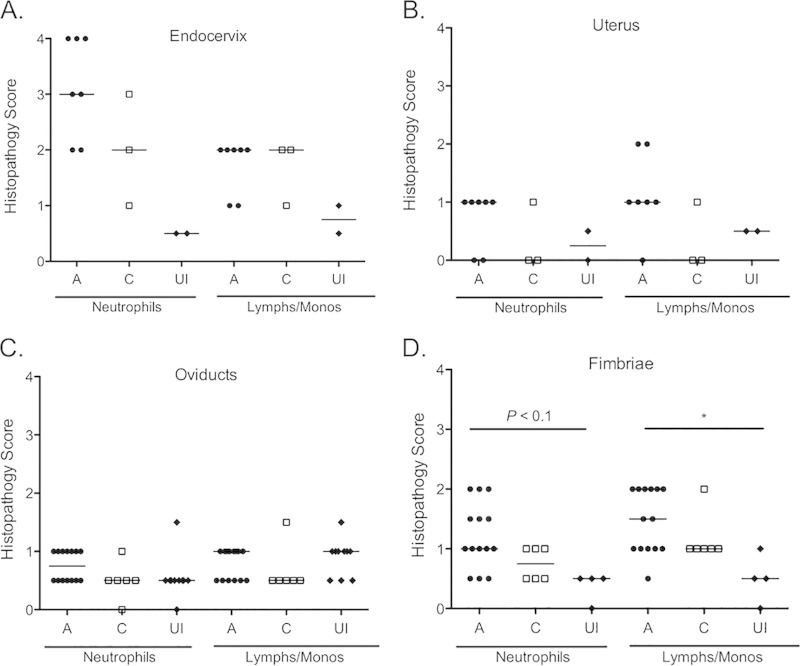
Sections of the ectocervix, endocervix, uterus, oviducts, and fimbriae were also examined for the presence of neutrophils and mononuclear cells (lymphocytes/monocytes). Tissues from control rhesus macaques were obtained for comparison. Levels of acute endocervical inflammation were increased for animals in group 1 (median, 4) compared to those in group 2 (median, 2) and uninfected animals (median, 0.5), although the differences were significant only for the experimental groups compared to uninfected animals (P < 0.05) (see Fig. S1A in the supplemental material). Levels of acute and chronic inflammation were otherwise very similar between these groups (see Fig. S1). When animals were assessed as ascenders and controllers, inflammation levels were also similar, although the median scores were consistently 0.5 to 1 point lower for the controllers (Fig. 5). In addition, animals classified as ascenders displayed higher inflammation scores. In particular, all 6 fimbriae that scored higher than 1 for neutrophils were from the ascender group (Fig. 5D). A similar trend was observed for lymphocytes/monocytes, with 8 of 9 fimbriae from the ascender group having scores higher than 1 (Fig. 5D). A significantly higher score for mononuclear cells was noted for ascenders (median, 1.5) than for uninfected animals (median, 0.5; P < 0.05) (Fig. 5D). Dilated sections of the oviducts were also detected on histopathology for two ascenders, i.e., animal 1 (right oviduct) and animal 9 (left oviduct) (Fig. 4C and D). Dilation of the oviductal lumen was otherwise not observed via histopathology. Despite the detection of inflammation, none of the cytokines assayed in the genital tract secretions were present at levels above those detected at week zero (data not shown).
FIG 5.
Histopathology of the genital tracts of ascenders and controllers at week 12. Genital tract tissues obtained from ascenders (A; black circles), controllers (C; white squares), and uninfected controls (UI; black diamonds) at tissue harvest were analyzed histologically. (A) The highest levels of inflammation were detected in the endocervix. Overall, inflammation was low in the uterus (B), oviducts (C), and fimbriae (D), although ascenders consistently exhibited the highest inflammation scores. Horizontal lines indicate the medians. Symbols represent individual animals (A and B) or oviducts (C and D). *, P < 0.05 by one-way ANOVA with Dunn's multiple-comparison test.
Chlamydia-specific antibodies serve as markers of increased infection.
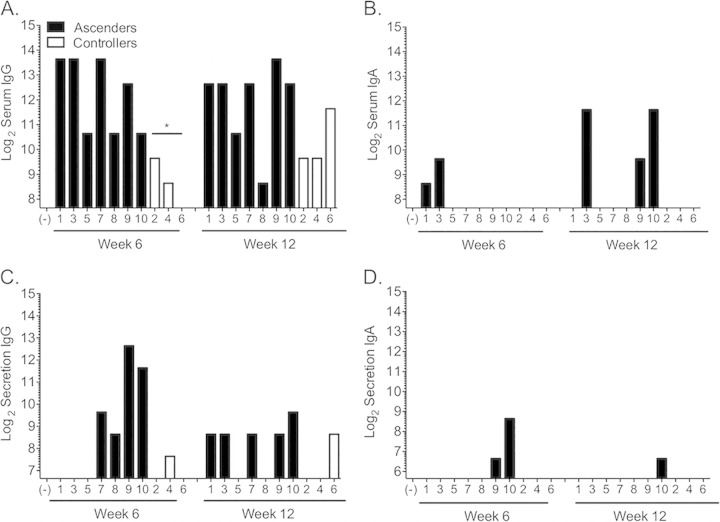
Levels of IgG and IgA in sera and vaginal secretions were measured at weeks 6 and 12. There was no difference in antibody levels between group 1 and group 2 (see Fig. S2 in the supplemental material). However, we detected a significantly lower level of serum IgG at week 6 for controllers than for ascenders (Fig. 6A) (P < 0.05). Levels of IgA in the serum were detectable for only 4 animals, all of which were in the ascender group (Fig. 6B). In the secretions, the two animals with the lowest level of resistance to reinfection (animals 9 and 10) had the highest levels of IgG (Fig. 6C) and were the only animals with detectable IgA (Fig. 6D).
FIG 6.
Levels of Chlamydia-specific IgG in sera and secretions were inversely associated with the degree of infection control. (A) Levels of IgG in sera were significantly higher at 6 weeks for ascenders (black bars) than for controllers (white bars). There were no significant differences in levels of IgA in sera (B), IgG in secretions (C), or IgA in secretions (D). *, P < 0.05 by one-way ANOVA with Dunn's multiple-comparison test. Bars represent the log2 values of the highest antibody dilutions where antibody to chlamydial elementary bodies was detected at a level above that of the negative control (−). The origin of the y axis was set at the lowest dilution of the negative-control sample. Numbers on the x axis indicate animal numbers.
Early Chlamydia-specific T cell responses are associated with enhanced control of infection, and late responses are associated with increased infection.
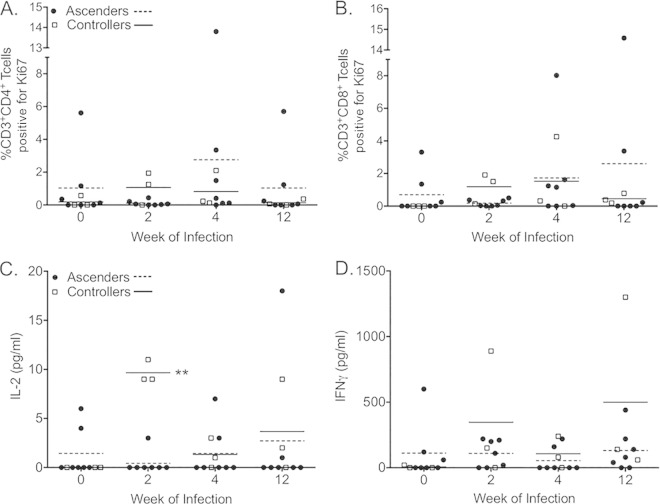
PBMCs isolated at weeks 0, 2, 4, and 12 were stimulated for 5 days in the presence and absence of chlamydial EBs. Proliferation was determined by upregulation of Ki67 (see Fig. S3 in the supplemental material). At week 2, the highest levels of Chlamydia-specific proliferation by CD4+ (Fig. 7A) and CD8+ (Fig. 7B) T cells were observed for the animals with the greatest degree of infection control, i.e., animals 4 and 6 (Fig. 3). Increased proliferation at weeks 4 and 12 was noted for animals with poor control and continued infection (Fig. 7A and B), with the highest levels observed for animal 10, the only monkey remaining infected over the entire study (Fig. 3). Consistent with the increased proliferation for controllers at week 2, significantly increased IL-2 levels were measured from the supernatants harvested at day 5 of stimulation (Fig. 7C). There were no significant differences in PBMC IFN-γ production at any time point (Fig. 7D). When animals were grouped according to the initial strain of infection, no significant differences were detected for CD4+ T cell proliferation (see Fig. S4A), CD8+ T cell proliferation (see Fig. S4B), or IL-2 production (see Fig. S4C). The only significant difference observed was increased IFN-γ production by PBMCs isolated from animals in group 1 at week 12 (see Fig. S4D). Stimulation of naive PBMCs from three different animals with CTD153 (5 μg/ml) resulted in slightly lower levels of cytokines than those seen on stimulation with D/UW-3/Cx, but the differences were not significant (data not shown). The average percent decrease for PBMCs stimulated with CTD153 compared to those stimulated with D/UW-3/Cx was 34% for IL-1β, 15% for IL-6, and 2% for GM-CSF after 24 h.
FIG 7.
Chlamydia-specific responses were increased in the peripheral blood of controllers at week 2. Proliferation of CD3+ CD4+ T cells (A) and CD3+ CD8+ T cells (B) was determined by analysis of Ki67 expression by flow cytometry after 5 days of incubation with EBs. Levels of IL-2 (C) and IFN-γ (D) were measured in the supernatants, with significantly higher levels of IL-2 detected in the supernatants of controllers (white squares; solid lines) than in those of ascenders (black circles; dashed lines) at week 2. Data points indicate values obtained for PBMCs incubated with EBs for 5 days after subtraction of values for PBMCs incubated without EBs. Horizontal lines indicate the means. **, P < 0.01 by two-way RM ANOVA with Bonferroni posttests.
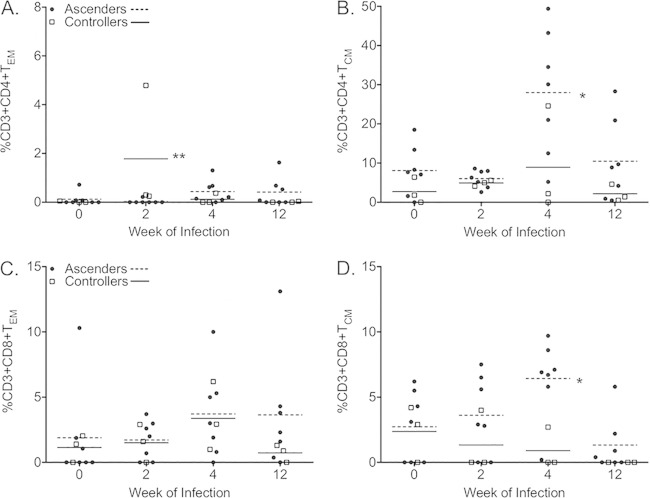
TEM are increased in the peripheral blood of controllers, while TCM are increased in that of ascenders.
CD3+ T cells can be subdivided into types of memory cells based on surface marker expression. Central memory T cells (TCM) are CD28+ CD95+, and effector memory T cells (TEM) are CD28− CD95+ (see Fig. S5 in the supplemental material) (27, 28). The frequency of Chlamydia-specific CD4+ TEM was significantly increased in the peripheral blood of controllers at week 2 (Fig. 8A), but no difference in the frequency of CD8+ TEM (Fig. 8C) was observed between ascenders and controllers at any point. In contrast, CD4+ TCM (Fig. 8B) and CD8+ TCM (Fig. 8D) frequencies were increased for ascenders at week 4. We did not detect differences in the frequencies of TEM or TCM between group 1 and group 2 (see Fig. S6). Other parameters analyzed that did not differ between the groups included CXCR3, CD69, and CCR7, as well as the frequency of NK cells (CD3− CD8+ NKG2a+) (data not shown).
FIG 8.
Comparison of effector and central memory T cells in the peripheral blood of ascenders and controllers. Frequencies of Chlamydia-specific CD4+ effector memory cells (A), but not CD8+ effector memory cells (C), were increased in the peripheral blood of controllers (white squares; solid lines) at week 2, while frequencies of both CD4+ central memory cells (B) and CD8+ central memory cells (D) were increased in the peripheral blood of ascenders (black circles; dashed lines) at week 4. TCM, CD28+ CD95+ cells; TEM, CD28− CD95+ cells. Data points indicate frequencies obtained for PBMCs incubated with EBs for 5 days after values for PBMCs incubated without EBs were subtracted. Horizontal lines indicate the means. *, P < 0.05; **, P < 0.01 (two-way RM ANOVA with Bonferroni posttests).
Analysis of Chlamydia-specific responses in the iliac lymph nodes.
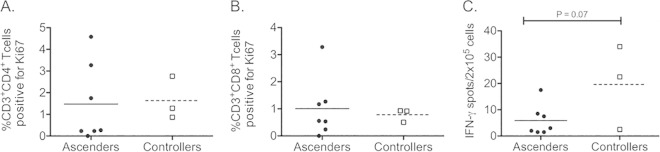
Studies of mice have shown that Chlamydia-specific IFN-γ-producing T cells are detectable in the iliac lymph nodes during infection. Analysis of the frequency of CD4+ (Fig. 9A) or CD8+ (Fig. 9B) T cells proliferating in response to Chlamydia in the iliac lymph nodes of ascenders and controllers at week 12 revealed detectable responses but no difference between the groups. We found a trend toward an increased frequency of cells producing IFN-γ in response to Chlamydia in the iliac lymph nodes of controllers at week 12 (Fig. 9C) (P = 0.07). There were no differences in the inguinal lymph nodes, and no differences were detected when animals in group 1 and group 2 were compared (data not shown).
FIG 9.
T cell proliferation and IFN-γ production by mononuclear cells isolated from the iliac lymph nodes at week 12. Levels of proliferation of CD3+ CD4+ T cells (A) and CD3+ CD8+ T cells (B), as determined by analysis of Ki67 expression by flow cytometry after 5 days of incubation with EBs, were similar for ascenders and controllers. (C) Analysis of the frequency of IFN-γ-producing cells in the iliac lymph nodes by ELISpot assay revealed a trend toward an increased frequency in controllers at week 12 (P = 0.07). Data points indicate values obtained for incubation with EBs for 5 days (A and B) or 24 h (C) after subtraction of values obtained after incubation without EBs. Horizontal lines indicate the means. The data were analyzed via Student's t test.
Controllers share a subset of MHC class I and class II alleles.
Pyrosequencing was used to genotype the MHC class I A/B and class II DRB loci (Table 2). We found that the two animals with the greatest ability to control infection (animals 4 and 6) shared a total of 10 MHC class I alleles and 3 MHC class II alleles. Animal 2, the third monkey in the controller group, also shared four MHC class I and one MHC class II allele with animals 4 and 6. Genes completely unique to animals in the controller group were the MHC class 1B gene B049 and the class II DRB gene W2, which were detected in animals 4 and 6 but not animal 2.
TABLE 2.
Summary of MHC class 1 and class II DRB pyrosequencing results for the rhesus macaques in this studya
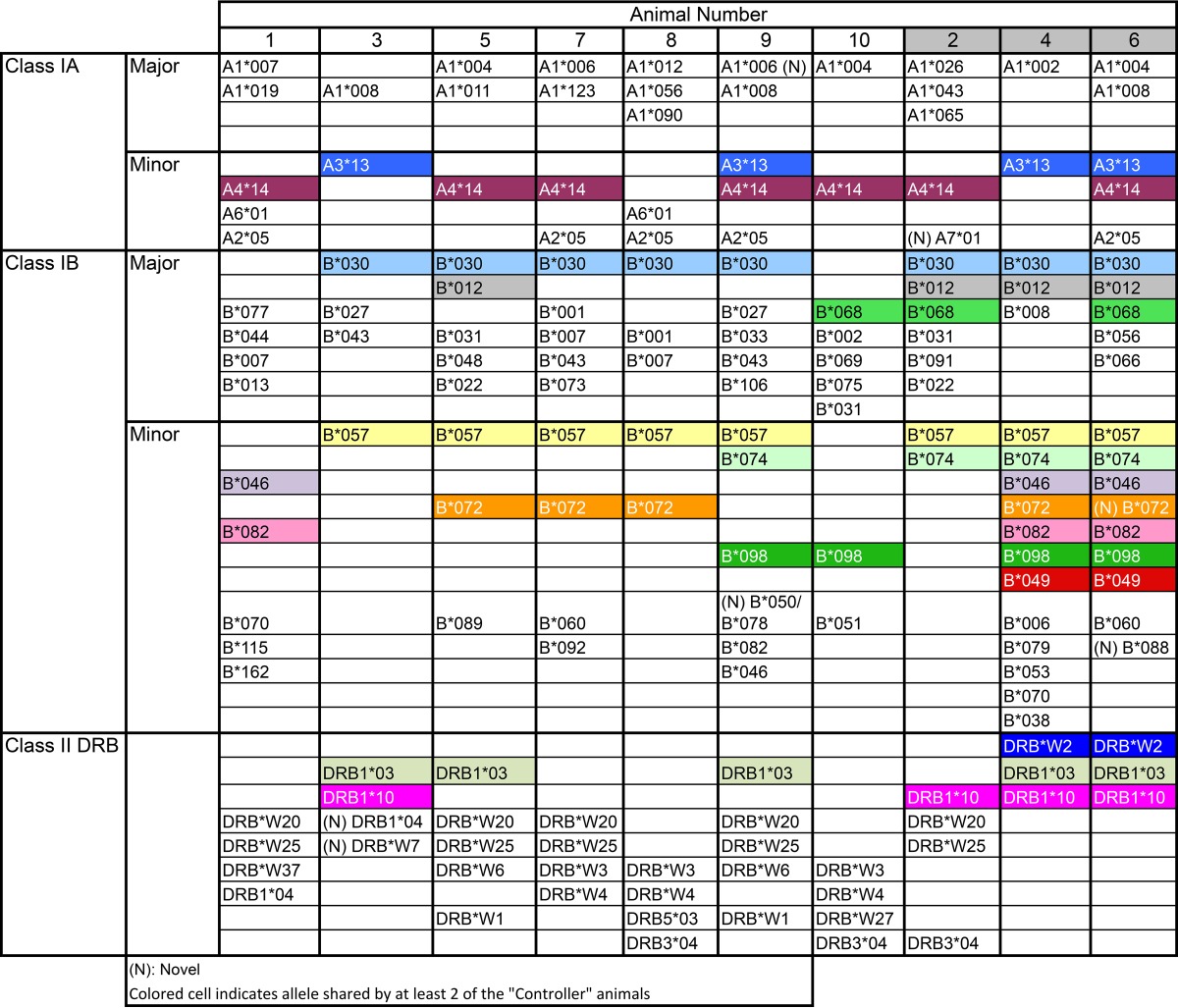
aThe table shows the allelic diversity at the MHC class I and class II loci. Animals 2, 4, and 6 represent the “controllers.” Alleles shared by at least two of the “controllers” are highlighted by unique colors. There were 5 alleles shared by all three animals in this group. The two most protected monkeys, with the shortest duration of infection and no detectable bacteria in the fimbriae, shared 13 alleles, two of which were unique to these monkeys.
DISCUSSION
In these studies, we directly addressed the potential for plasmid-deficient C. trachomatis to serve as a live attenuated vaccine to prevent genital tract infection and disease. Five repeated cervical inoculations with wild-type serovar D strain D/UW-3/Cx and a plasmid-deficient derivative of this strain, CTD153, resulted in infections with similar kinetics and induced comparable levels of protective immunity. After all macaques were challenged five times with D/UW-3/Cx, similar levels of inflammation and pathology were observed in both groups. However, grouping animals into “ascenders” and “controllers” based on the presence or absence of chlamydial DNA in the fimbriae at weeks 5 and 12 revealed reduced oviduct dilatation as well as lower antichlamydial antibody titers and increased early T cell responses for “controllers.” Thus, the characteristics of individual animals were the key determinants of the outcome of infection, rather than the presence or absence of the chlamydial plasmid in the strain used for the first set of infections.
In contrast to the similar bacterial burdens we observed upon inoculation with D/UW-3/Cx and CTD153 in these studies, both human (8, 29) and murine (5) strains of plasmid-deficient Chlamydia are less infectious in the mouse model of genital tract infection. It is possible that we did not detect subtle differences in infection that would have been observed in the first 6 days after each inoculation, since endocervical swabs were obtained only every 7 days. For example, in the mouse model, a reduced bacterial burden was only detectable in the lower genital tract in the first several days after infection with a plasmid-deficient strain of C. muridarum (5). In addition, weekly inoculations would prevent the detection of differences related to the duration of infection. In the trachoma model, the infection course with plasmid-deficient serovar A was significantly abbreviated (7). Lastly, differences related to the infectivity of the plasmid-deficient strain, as observed for plasmid-deficient L2 (29) and serovar F (8) in mice, would likely not have been detectable with repeated inoculations of 1 × 106 organisms.
In mice, infection with plasmid-deficient C. muridarum does not cause oviduct pathology and prevents the development of immunopathology upon challenge with fully virulent C. muridarum (5). In the present macaque study, there were no differences in immunopathology between animals infected with plasmid-deficient serovar D and those infected with wild-type serovar D for the first 5 weeks. At week 5, although only gross examinations were conducted for pathology, 4 of 5 monkeys infected with CTD153 for the first 5 weeks exhibited evidence of oviduct inflammation. It is possible that differences in inflammatory infiltrates would have been observed histologically or via flow cytometry at week 5, but we did not obtain biopsy specimens at that time point due to the potential for oviduct damage. In addition, none of the animals developed evidence of severe disease over the 12-week course of the experiment, in contrast to the high degree of pathology observed in the mouse model upon infection with Chlamydia muridarum. It is possible that differences between plasmid-deficient and wild-type C. muridarum are magnified in the mouse model due to the highly virulent and pathogenic nature of C. muridarum. In the current study, only two animals exhibited evidence of tubal dilatation on histologic sections, and six animals had grossly visible oviduct/fimbrial edema at the time of euthanasia. The frequency of these findings was consistent with that of results described after 5 weekly cervical inoculations of rhesus monkeys with a serovar E strain (26). In addition, a previous study showed that after a single cervical inoculation with serovar D, neither Chlamydia nor severe pathology was detected in the upper genital tract of pig-tailed macaques (30). Repeated direct injection of genital tract serovars into the oviducts was necessary to induce severe disease, including adhesions, scarring, and systemic symptoms (31).
Although we did not detect differences between macaques originally infected with CTD153 or D/UW-3/Cx, we did detect interanimal variability in regard to the duration of lower genital tract infection, presence of bacteria in the upper genital tract, and degree of inflammation. We identified three animals that appeared to be more resistant to upper genital tract infection/inflammation than the others. This is consistent with the findings for the trachoma model, which showed that three monkeys previously infected with plasmid-deficient serovar A were protected against disease to a greater extent upon challenge with wild-type serovar A than the other three monkeys in the study (7). These differences were attributed to variability in the MHC haplotype. In addition, early studies of pig-tailed macaques revealed that the degree of protective immunity that developed after resolution of a single intravaginal inoculation with serovar D varied between animals (30). Later studies found that MHC class I alleles were associated with resistance and susceptibility to the development of peritubal adhesions following multiple simultaneous inoculations of the fimbriae and cervix with serovar D (32). Our genetic analysis did not detect any alleles that were exclusive to all three controller monkeys, although we did detect an MHC class I allele and an MHC class II allele that were present only in the two controllers with the shortest course of lower genital tract infection.
Analysis of immune responses in the controller animals suggested possible mechanisms of protection from disease. Findings included a trend toward increased Chlamydia-specific CD4+ and CD8+ T cells in the peripheral blood at week 2 and increased numbers of IFN-γ-producing cells in the iliac lymph nodes at week 12. We also found a significantly increased level of Chlamydia-specific IL-2 production and a higher frequency of Chlamydia-specific CD4+ effector memory cells in the blood at week 2. Early induction of these T cells likely allows for homing and establishment of a protective population of Chlamydia-specific cells both at the site of infection and in the draining lymph nodes. Genital tract-resident memory CD4+ T cells are protective in both the murine herpes model and the murine chlamydial model of infection (33, 38). Detection of increased Chlamydia-specific responses in animals with the highest levels of infection at later time points is likely indicative of the development of an ineffective response that did not effectively eliminate bacteria. This is also likely the case for the detection of increased levels of antibody in animals that did not effectively control infection. We have no data to suggest that these T cell and antibody responses were harmful to the host. In addition, future studies will be necessary to explore the protective capacity of T cells and antibodies specific for individual chlamydial antigens. Our current study, involving 10 monkeys, was underpowered to find significant differences in those parameters.
Over 95% of women infected with C. trachomatis are asymptomatic, and although complications can be severe, they are relatively rare (3, 34). Thus, the low degree of pathogenicity observed for C. trachomatis in the rhesus monkey model mirrors human infection more closely than infection of mice with C. muridarum, although important differences remain. The animals in this study were inoculated with a much higher bacterial load (1 × 106 IFU) than what likely occurs during human sexual transmission (35). Despite the high inoculum, four animals became culture negative after 2 weeks, and infection resolved in all but one of the animals by the end of the 12-week monitoring period. This contrasts with observations in women, in whom untreated infection may continue for months to years (36, 37). The shorter duration of infection may explain why none of the animals developed marked upper genital tract inflammation or disease. Nevertheless, nonhuman primates are important for examining the role of genetic diversity in the immune response to C. trachomatis infection (7). It is possible that the lab-passaged clonal C. trachomatis strains used in this study were not optimal for producing disease and that use of a low-passage C. trachomatis isolate obtained from women with symptomatic infection may prove more virulent in macaques.
The data presented here demonstrate that resistance to reinfection develops in the macaque genital tract after multiple challenge infections irrespective of the presence or absence of the plasmid. This study provides evidence against the use of plasmid-deficient Chlamydia as a live attenuated vaccine for genital tract infection. Our data indicate that early induction of Chlamydia-specific T cells helps to control the ascension of bacteria to the upper genital tract and thus prevents disease. Future studies will focus on identifying protective T cell antigens and testing vaccines in nonhuman primates with a diverse array of MHC haplotypes in order to mirror the genetic diversity of humans.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from The Hartwell Foundation (to T.D.) and by a California National Primate Research Center base operating grant (grant P51-OD011107).
We thank the animal care and veterinary staff at the California National Primate Research Center.
This article is dedicated to Alison Logar.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00841-15.
REFERENCES
- 1.Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. 2004. Global data on visual impairment in the year 2002. Bull World Health Organ 82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.Rekart ML, Gilbert M, Meza R, Kim PH, Chang M, Money DM, Brunham RC. 2013. Chlamydia public health programs and the epidemiology of pelvic inflammatory disease and ectopic pregnancy. J Infect Dis 207:30–38. doi: 10.1093/infdis/jis644. [DOI] [PubMed] [Google Scholar]
- 3.Haggerty CL, Gottlieb SL, Taylor BD, Low N, Xu F, Ness RB. 2010. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis 201(Suppl 2):S134–S155. doi: 10.1086/652395. [DOI] [PubMed] [Google Scholar]
- 4.Darville T, Hiltke TJ. 2010. Pathogenesis of genital tract disease due to Chlamydia trachomatis. J Infect Dis 201(Suppl 2):S114–S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Connell CM, Ingalls RR, Andrews CW Jr, Skurlock AM, Darville T. 2007. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J Immunol 179:4027–4034. doi: 10.4049/jimmunol.179.6.4027. [DOI] [PubMed] [Google Scholar]
- 6.Russell M, Darville T, Chandra-Kuntal K, Smith B, Andrews CW Jr, O'Connell CM. 2011. Infectivity acts as in vivo selection for maintenance of the chlamydial cryptic plasmid. Infect Immun 79:98–107. doi: 10.1128/IAI.01105-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kari L, Whitmire WM, Olivares-Zavaleta N, Goheen MM, Taylor LD, Carlson JH, Sturdevant GL, Lu C, Bakios LE, Randall LB, Parnell MJ, Zhong G, Caldwell HD. 2011. A live-attenuated chlamydial vaccine protects against trachoma in nonhuman primates. J Exp Med 208:2217–2223. doi: 10.1084/jem.20111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sigar IM, Schripsema JH, Wang Y, Clarke IN, Cutcliffe LT, Seth-Smith HM, Thomson NR, Bjartling C, Unemo M, Persson K, Ramsey KH. 2014. Plasmid deficiency in urogenital isolates of Chlamydia trachomatis reduces infectivity and virulence in a mouse model. Pathog Dis 70:61–69. doi: 10.1111/2049-632X.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riley MM, Zurenski MA, Frazer LC, O'Connell CM, Andrews CW Jr, Mintus M, Darville T. 2012. The recall response induced by genital challenge with Chlamydia muridarum protects the oviduct from pathology but not from reinfection. Infect Immun 80:2194–2203. doi: 10.1128/IAI.00169-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frazer LC, Darville T, Chandra-Kuntal K, Andrews CW Jr, Zurenski M, Mintus M, AbdelRahman YM, Belland RJ, Ingalls RR, O'Connell CM. 2012. Plasmid-cured Chlamydia caviae activates TLR2-dependent signaling and retains virulence in the guinea pig model of genital tract infection. PLoS One 7:e30747. doi: 10.1371/journal.pone.0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyairi I, Laxton JD, Wang X, Obert CA, Arva Tatireddigari VR, van Rooijen N, Hatch TP, Byrne GI. 2011. Chlamydia psittaci genetic variants differ in virulence by modulation of host immunity. J Infect Dis 204:654–663. doi: 10.1093/infdis/jir333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdelsamed H, Peters J, Byrne GI. 2013. Genetic variation in Chlamydia trachomatis and their hosts: impact on disease severity and tissue tropism. Future Microbiol 8:1129–1146. doi: 10.2217/fmb.13.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldwell HD, Wood H, Crane D, Bailey R, Jones RB, Mabey D, Maclean I, Mohammed Z, Peeling R, Roshick C, Schachter J, Solomon AW, Stamm WE, Suchland RJ, Taylor L, West SK, Quinn TC, Belland RJ, McClarty G. 2003. Polymorphisms in Chlamydia trachomatis tryptophan synthase genes differentiate between genital and ocular isolates. J Clin Invest 111:1757–1769. doi: 10.1172/JCI17993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fehlner-Gardiner C, Roshick C, Carlson JH, Hughes S, Belland RJ, Caldwell HD, McClarty G. 2002. Molecular basis defining human Chlamydia trachomatis tissue tropism. A possible role for tryptophan synthase. J Biol Chem 277:26893–26903. [DOI] [PubMed] [Google Scholar]
- 15.Nelson DE, Virok DP, Wood H, Roshick C, Johnson RM, Whitmire WM, Crane DD, Steele-Mortimer O, Kari L, McClarty G, Caldwell HD. 2005. Chlamydial IFN-gamma immune evasion is linked to host infection tropism. Proc Natl Acad Sci U S A 102:10658–10663. doi: 10.1073/pnas.0504198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roshick C, Wood H, Caldwell HD, McClarty G. 2006. Comparison of gamma interferon-mediated antichlamydial defense mechanisms in human and mouse cells. Infect Immun 74:225–238. doi: 10.1128/IAI.74.1.225-238.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Connell CM, AbdelRahman YM, Green E, Darville HK, Saira K, Smith B, Darville T, Scurlock AM, Meyer CR, Belland RJ. 2011. Toll-like receptor 2 activation by Chlamydia trachomatis is plasmid dependent, and plasmid-responsive chromosomal loci are coordinately regulated in response to glucose limitation by C. trachomatis but not by C. muridarum. Infect Immun 79:1044–1056. doi: 10.1128/IAI.01118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephens RS, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, Mitchell W, Olinger L, Tatusov RL, Zhao Q, Koonin EV, Davis RW. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 19.O'Connell CM, Nicks KM. 2006. A plasmid-cured Chlamydia muridarum strain displays altered plaque morphology and reduced infectivity in cell culture. Microbiology 152:1601–1607. doi: 10.1099/mic.0.28658-0. [DOI] [PubMed] [Google Scholar]
- 20.Kelly KA, Robinson EA, Rank RG. 1996. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatis following genital infection. Infect Immun 64:4976–4983. (Erratum, 65:2508, 1997.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He X, Nair A, Mekasha S, Alroy J, O'Connell CM, Ingalls RR. 2011. Enhanced virulence of Chlamydia muridarum respiratory infections in the absence of TLR2 activation. PLoS One 6:e20846. doi: 10.1371/journal.pone.0020846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darville T, Andrews CW Jr, Laffoon KK, Shymasani W, Kishen LR, Rank RG. 1997. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect Immun 65:3065–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bom RJ, Christerson L, Schim van der Loeff MF, Coutinho RA, Herrmann B, Bruisten SM. 2011. Evaluation of high-resolution typing methods for Chlamydia trachomatis in samples from heterosexual couples. J Clin Microbiol 49:2844–2853. doi: 10.1128/JCM.00128-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scurlock AM, Frazer LC, Andrews CW Jr, O'Connell CM, Foote IP, Bailey SL, Chandra-Kuntal K, Kolls JK, Darville T. 2011. Interleukin-17 contributes to generation of Th1 immunity and neutrophil recruitment during Chlamydia muridarum genital tract infection but is not required for macrophage influx or normal resolution of infection. Infect Immun 79:1349–1362. doi: 10.1128/IAI.00984-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiseman RW, Karl JA, Bimber BN, O'Leary CE, Lank SM, Tuscher JJ, Detmer AM, Bouffard P, Levenkova N, Turcotte CL, Szekeres E Jr, Wright C, Harkins T, O'Connor DH. 2009. Major histocompatibility complex genotyping with massively parallel pyrosequencing. Nat Med 15:1322–1326. doi: 10.1038/nm.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patton DL, Sweeney YT, Stamm WE. 2005. Significant reduction in inflammatory response in the macaque model of chlamydial pelvic inflammatory disease with azithromycin treatment. J Infect Dis 192:129–135. doi: 10.1086/431365. [DOI] [PubMed] [Google Scholar]
- 27.Soloff AC, Liu X, Gao W, Day RD, Gambotto A, Barratt-Boyes SM. 2009. Adenovirus 5- and 35-based immunotherapy enhances the strength but not breadth or quality of immunity during chronic SIV infection. Eur J Immunol 39:2437–2449. doi: 10.1002/eji.200839130. [DOI] [PubMed] [Google Scholar]
- 28.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. 2002. Development and homeostasis of T cell memory in rhesus macaque. J Immunol 168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 29.Carlson JH, Whitmire WM, Crane DD, Wicke L, Virtaneva K, Sturdevant DE, Kupko JJ III, Porcella SF, Martinez-Orengo N, Heinzen RA, Kari L, Caldwell HD. 2008. The Chlamydia trachomatis plasmid is a transcriptional regulator of chromosomal genes and a virulence factor. Infect Immun 76:2273–2283. doi: 10.1128/IAI.00102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolner-Hanssen P, Patton DL, Holmes KK. 1991. Protective immunity in pig-tailed macaques after cervical infection with Chlamydia trachomatis. Sex Transm Dis 18:21–25. doi: 10.1097/00007435-199101000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Patton DL, Kuo CC, Wang SP, Halbert SA. 1987. Distal tubal obstruction induced by repeated Chlamydia trachomatis salpingeal infections in pig-tailed macaques. J Infect Dis 155:1292–1299. doi: 10.1093/infdis/155.6.1292. [DOI] [PubMed] [Google Scholar]
- 32.Lichtenwalner AB, Patton DL, Cosgrove Sweeney YT, Gaur LK, Stamm WE. 1997. Evidence of genetic susceptibility to Chlamydia trachomatis-induced pelvic inflammatory disease in the pig-tailed macaque. Infect Immun 65:2250–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iijima N, Iwasaki A. 2014. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 346:93–98. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oakeshott P, Kerry S, Aghaizu A, Atherton H, Hay S, Taylor-Robinson D, Simms I, Hay P. 2010. Randomised controlled trial of screening for Chlamydia trachomatis to prevent pelvic inflammatory disease: the POPI (prevention of pelvic infection) trial. Br Med J 340:c1642. doi: 10.1136/bmj.c1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geisler WM, Suchland RJ, Whittington WL, Stamm WE. 2001. Quantitative culture of Chlamydia trachomatis: relationship of inclusion-forming units produced in culture to clinical manifestations and acute inflammation in urogenital disease. J Infect Dis 184:1350–1354. doi: 10.1086/323998. [DOI] [PubMed] [Google Scholar]
- 36.Molano M, Meijer CJ, Weiderpass E, Arslan A, Posso H, Franceschi S, Ronderos M, Munoz N, van den Brule AJ. 2005. The natural course of Chlamydia trachomatis infection in asymptomatic Colombian women: a 5-year follow-up study. J Infect Dis 191:907–916. doi: 10.1086/428287. [DOI] [PubMed] [Google Scholar]
- 37.Geisler WM. 2010. Duration of untreated, uncomplicated Chlamydia trachomatis genital infection and factors associated with chlamydia resolution: a review of human studies. J Infect Dis 201(Suppl 2):S104–S113. doi: 10.1086/652402. [DOI] [PubMed] [Google Scholar]
- 38.Stary G, Olive A, Radovic-Moreno AF, Gondek D, Alvarez D, Basto PA, Perro M, Vrbanac VD, Tager AM, Shi J, Yethon JA, Farokhzad OC, Langer R, Starnbach MN, von Andrian UH. 2015. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 348:aaa8205. doi: 10.1126/science.aaa8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.