Abstract
Objectives
Cardiovascular disease is an important comorbidity in patients with chronic obstructive pulmonary disease (COPD). We aimed to systematically review the evidence for: (1) risk of myocardial infarction (MI) in people with COPD; (2) risk of MI associated with acute exacerbation of COPD (AECOPD); (3) risk of death after MI in people with COPD.
Design
Systematic review and meta-analysis.
Methods
MEDLINE, EMBASE and SCI were searched up to January 2015. Two reviewers screened abstracts and full text records, extracted data and assessed studies for risk of bias. We used the generic inverse variance method to pool effect estimates, where possible. Evidence was synthesised in a narrative review where meta-analysis was not possible.
Results
Searches yielded 8362 records, and 24 observational studies were included. Meta-analysis showed increased risk of MI associated with COPD (HR 1.72, 95% CI 1.22 to 2.42) for cohort analyses, but not in case–control studies: OR 1.18 (0.80 to 1.76). Both included studies that investigated the risk of MI associated with AECOPD found an increased risk of MI after AECOPD (incidence rate ratios, IRR 2.27, 1.10 to 4.70, and IRR 13.04, 1.71 to 99.7). Meta-analysis showed weak evidence for increased risk of death for patients with COPD in hospital after MI (OR 1.13, 0.97 to 1.31). However, meta-analysis showed an increased risk of death after MI for patients with COPD during follow-up (HR 1.26, 1.13 to 1.40).
Conclusions
There is good evidence that COPD is associated with increased risk of MI; however, it is unclear to what extent this association is due to smoking status. There is some evidence that the risk of MI is higher during AECOPD than stable periods. There is poor evidence that COPD is associated with increased in hospital mortality after an MI, and good evidence that longer term mortality is higher for patients with COPD after an MI.
Strengths and limitations of this study.
This systematic review investigated three important areas relating to the relationship between chronic obstructive pulmonary disease (COPD) and cardiovascular disease: (1) the risk of myocardial infarction (MI) associated with COPD; (2) the risk of MI associated with acute exacerbations of COPD; and (3) the risk of death following MI in patients with COPD compared to patient without COPD.
Strengths of this review were the wide search strategy, broad inclusion criteria and rigorous risk of bias assessment of included studies.
We found strong evidence for an increased risk of MI in people with COPD and an increased risk of longer term death after MI for patients with COPD; however, it is unclear how much of this increased risk may be due to smoking status.
We found poorer evidence for an increased risk of MI during periods of acute exacerbation of COPD compared to stable periods, and for an increased risk of death in hospital after MI for patients with COPD. We make recommendations on how future studies can improve our understanding of these relationships.
Due to statistical and clinical heterogeneity, meta-analysis could only be conducted for some of the research questions.
Introduction
Cardiovascular disease is a common comorbidity and cause of death in people with chronic obstructive pulmonary disease (COPD), with up to one-third dying of cardiovascular disease.1 Reducing the cardiovascular disease in this population is an important strategy for reducing the burden of COPD.
Several studies have shown that people with COPD have a higher risk of myocardial infarction (MI) than people without COPD.2–4 One of the reasons for the increased risk of MI in patients with COPD is the shared major risk factor of smoking. In addition, several other cardiovascular risk factors, including hypertension, diabetes, inactivity, poor diet, and older age, are also prevalent in patients with COPD.5–7 In addition, several studies have found an association between reduced FEV1 (forced expiratory volume1 s) and cardiovascular mortality in the general population.8 However, COPD itself is also thought to be an independent risk factor for MI with increased risk of MI possibly being mediated through increased systemic inflammation or reduced FEV1 in people with COPD.
Acute exacerbations of COPD are events in the natural history of COPD which are characterised by an increase in COPD symptoms such as breathlessness, cough, sputum volume, and sputum purulence. It has recently been suggested that acute exacerbations of COPD (AECOPD) represent a period of increased risk of MI for people with COPD.9 A subtype of patients with COPD appears to have more frequent exacerbations than others. Frequent exacerbators have been defined as individuals who have two or more treated exacerbations per year. Frequent exacerbators may be at higher risk of MI compared to infrequent exacerbators, even during stable periods.
Several investigators have found that patients with COPD have worse mortality in hospital and following discharge after an MI compared to patient without COPD.10–12 However, the finding that patients with COPD have greater in hospital and short-term mortality has not been found by all investigators.13–15
We aimed to systematically review the literature reporting on: (1) the risk of MI in people with COPD; (2) the risk of MI associated AECOPD, either during AECOPD or that associated with the frequent exacerbator phenotype; and (3) the risk of death after MI in people with COPD. These questions represent the most salient aspects of current research into the relationship between COPD and cardiovascular disease, and no systematic reviews have been published on these topics to date.
Methods
Literature search
MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE, BIOSIS & Science Citation Index were searched up to January 2015. A search strategy was devised which would pick up articles relevant to all three research questions. All strategies were based on the MEDLINE search strategy, which is presented in the online supplementary material. In brief, the literature was searched for terms which relate to COPD and terms with relate to MI, and these searches were combined using the AND Boolean logic operator. MeSH terms were combined with natural language searching using truncation where appropriate.
Inclusion and exclusion criteria
Inclusion and exclusion criteria were applied for each of the three research questions. Studies were included if they met the population, exposure, comparator and outcome criteria. These are presented below for each research question. Studies were included from database start date and were not restricted by language.
Risk of MI in people with COPD
The population of interest was the general population. The exposure of interest was diagnosis of COPD. The un-exposed group was people without a diagnosis of COPD. The outcome of interest was acute MI.
Risk of MI associated with AECOPD
The population of interest was people with a diagnosis of COPD. The exposures of interest were either: (1) discrete episodes of AECOPD or periods within 8 weeks of an AECOPD; or (2) frequent exacerbator phenotype. The comparators of interest were either: (1) periods of stable COPD; or (2) infrequent exacerbator phenotype. Studies were included if these reported a relative risk of MI, or if this could be calculated.
Risk of death after MI in people with COPD
The population of interest was those presenting to a hospital with an MI. Studies were included if these compared patients with a diagnosis of COPD to those without a diagnosis of COPD. Outcomes of interest were death in hospital and at any reported time points post discharge. Studies investigating risk of death for patients with COPD after an interventional procedure following an MI (such as percutaneous coronary intervention or coronary artery bypass graft) were specifically excluded under the population criterion.
Selection of included studies
Titles and abstracts, where available, were initially screened for potential inclusion by one reviewer. Full text versions of potentially included studies were then obtained and were screened by two reviewers. Authors were contacted if the information provided in articles was not sufficient to assess whether inclusion criteria were satisfied.
Risk of bias assessment
All included studies, except for those only reported as conference abstracts, were assessed for risk of bias. The risk of bias tool was informed by the Newcastle-Ottawa scale;16 however, we did not make use of a summary score as this is not advisable.17 18 Risk of bias was assessed across the key domains related to selection of participants, comparability of groups, and measurement of outcomes. Several items were included under each domain, and were adapted for different study types. Where reports of studies included more than one analysis (eg, a case control as well as a cohort analysis) the risk of bias for these analyses were conducted separately. Risk of bias assessment was completed by one reviewer and checked by another.
Evidence synthesis
Characteristics and findings of studies were tabulated and compared. Data on severity of COPD was extracted as GOLD stage or FEV1% predicted, where available. Information was also extracted on smoking status and previous cardiovascular disease. Estimates of effect were extracted or calculated, and are presented as ORs, risk ratios (RR), incidence rate ratios (IRR) or HRs.
Where included studies were reasonably statistically and clinically similar, we pooled results using random effects meta-analysis. We used the generic inverse variance method to pool maximally adjusted effect estimates. Analysis was conducted in Review Manager 5.3. Where studies were too statistically (I2 over 75%) or clinically heterogeneous, meta-analysis was not conducted, but study summary results were graphed on forest plots without pooling the results. Studies which were not adjusted at all were not included in forest plots. For the question on risk of MI associated with COPD, studies were stratified by adjustment for smoking status (yes or no) and study design (cohort or case–control). For the question on risk of death following MI in COPD compared to patient without COPD, studies were stratified by outcome time point (in-hospital mortality or follow-up mortality). For follow-up mortality, studies were further stratified by analysis.
Results
Identified studies
Literature searches yielded 8362 records. After title and abstract screening, 49 records were selected for full-text assessment, which resulted in the inclusion of 24 studies. The inclusion and exclusion process is summarised in figure 1. Of the 24 included studies, 9 investigated the risk of MI in patients with COPD compared to patient without COPD; 2 investigated the risk of MI associated with AECOPD; no studies were found which investigated the risk of MI associated with the frequent exacerbator phenotype; and 12 investigated outcomes after MI for patients with COPD compared to patient without COPD. Summary characteristics of included studies are presented in tables 1–3.
Figure 1.
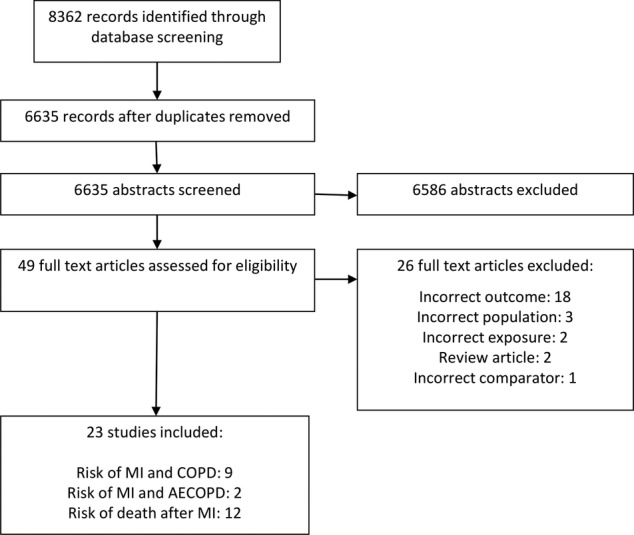
Study selection. AECOPD, acute exacerbation of COPD; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction.
Table 1.
Characteristics of included studies – risk of MI associated with COPD
| Study | Design and setting | Population | Characteristics of COPD patients | MI definition | Maximally adjusted estimate (95% CI) | Factors adjusted for |
|---|---|---|---|---|---|---|
| Curkendall et al 2 30 | Cohort in the Saskatchewan Health databases 1998–2001. | 11 493 COPD patients ≥40 years, identified by physician claim or hospital discharge COPD code and at least two prescriptions for COPD medicines within 6 months of the index COPD code. 22 986 age and sex matched non-COPD patients |
Age NR Sex NR COPD severity NR Current smokers 16% History of CVD Previous MI—2.3% Previous angina—6.6% |
Any MI during follow up: any inpatient or outpatient diagnosis of MI Hospitalisation due to MI: primary hospital discharge diagnosis of MI Fatal MI: underlying cause of death which initiated the sequence of events that lead to death recorded as MI |
Any MI during follow up (period prevalence): OR 1.61 (1.43–1.81) Hospitalisation due to MI: IRR 1.49 (0.71–3.13) Fatal MI: IRR 1.51 (1.14–2.01) |
Period prevalence of MI: Age, sex, history of cardiovascular disease, diabetes, hypertension, hypercholesterolaemia Hospitalisation for MI: adjusted for history of cardiovascular events, diabetes, hypertension, and hypercholesterolemia using Poisson regression, age and sex by matching. Fatal MI: age and sex by matching only. |
| Feary et al 3 | Cohort in The Health Improvement Network, 2005–2007 | 29 870 COPD patients >35 years identified by COPD diagnostic code. 1 174 240 non-COPD patients |
Age 35–44–1.8% 45–54–7.0% 55–64–20.5% 65–74–31.7% ≥75–39.0% Sex 48.1% male COPD severity FEV1 % predicted 50–80%–37.5% 30–49%–19.1% <30%–5.3% Current smokers 65.3% History of CVD Prior CVD–28.0% |
Diagnostic code for MI in primary care record |
35–44 years: HR 10.34 (3.28–32.6) 45–54 years: HR 1.22 (0.55–2.74) 55–64 years: HR 1.55 (1.07–2.26) 65–74 years: HR 1.78 (1.37–2.31) ≥75 years: 1.34 (1.03–1.73) |
Age, sex and smoking status |
| Huiart et al 28 | Cohort in the Saskatchewan Health databases 1990–1999 | 5 648 COPD patients ≥50 years, identified by prescription of three or more bronchodilators within the period of one year. Rates of MI compared to those of general Saskatchewan population |
Age NR Sex NR COPD severity NR Current smokers NR History of CVD NR Characteristics were not split by COPD status. |
Primary hospital discharge diagnosis of MI | Standardised IRR: 1.30 (1.15–1.44) | Age and sex by standardisation |
| Mapel et al 28 | Cohort in the Veterans Administration Medical System, 1991–1999 | COPD patients identified by discharge codes (1991–1999) and/or outpatient codes (1997–1999) Age and sex matched controls without COPD |
Age Median 60 (IQR, 49–62) Sex 95.7% male COPD severity NR Current smokers NR History of CVD Cardiovascular disease—71.2% |
Specific ICD-9-CM code for MI during 1999 that was not present in 1998 |
COPD patients identified using discharge codes IRR: 1.28 (1.18–1.38) COPD patients identified using outpatient codes IRR: 5.31 (4.54–6.21) |
Age and sex by matching |
| Rodriguez et al 19 | Cohort and case-control study in the General Practice Research Database, 1996–2001 | 1532 patients with a first COPD diagnosis in 1996, and no history of cardiovascular disease 13 500 age and sex matched non-COPD patients, with no history of cardiovascular disease |
Age NR Sex NR COPD severity NR Current smokers NR History of CVD NR |
Diagnostic code for MI in primary care record |
Cohort analysis: IRR 1.18 (0.81–1.71) Case-control analysis: OR 0.93 (0.62–1.39) |
Cohort analysis: Age and sex Case control analysis: Age, sex, smoking and number of primary care physician visits |
| Schneider et al 4 | Cohort and nested case-control study in the General Practice Research Database, 1995–2005 | 35 772 patients with a first COPD diagnosis between 1995–2005 35 772 non-COPD patients matched on age, sex and calendar time and general practice |
Age 40–49–6.8% 50–59–19.9% 60–69–33.8% >70–39.6% Sex 51.3% male COPD severity NR Current smokers 43.3% History of CVD Prior MI/CHD—18.3% Prior CHF—8.4% |
Diagnostic code for MI along with death or hospitalisation within 30 days of the diagnosis; and/or start of new treatment with ACE antagonist, β-blocker, statin, vitamin K antagonist, platelet aggregation inhibitor or aspirin within 90 days of the diagnosis in primary care record |
Cohort analysis: IRR 1.56 (1.43–1.75) Case control analysis: Any COPD : OR 1.40 (1.13–1.73) Mild COPD: OR 1.79 (1.12–2.86) Moderate COPD: OR 1.30 (1.04–1.62) Severe COPD: OR 3.00 (1.53–5.86) |
Cohort analysis: matched on age, sex, calendar time and general practice Case-control analysis: Smoking status, BMI, hypertension, hyperlipidaemia, diabetes and NSAID use |
| Sidney et al 29 | Cohort in health insurance database. North Carolina, 1996–1999 | COPD defined as: hospitalisation or outpatient diagnosis of COPD, two or more prescriptions for COPD medicines, aged over 40 years. Non-COPD patients matched on age, sex and length of care plan membership. |
Age 40–59–35% 60–79–55% >80–10% Sex 55.4% male COPD severity NR Current smokers NR History of CVD Prior MI—1.8% Prior angina—1.0% Prior CHF—7.2% |
ICD code for acute MI |
Overall: IRR 1.89 (1.71–2.09) Men: IRR 1.77 (1.56–2.01) Women: IRR 2.09 (1.78–2.46) 40–64 years: IRR 2.43 (1.98–2.98) ≥65 years: IRR 1.73 (1.54–1.94) |
Age, sex and baseline cardiovascular risk profile. |
| Sode et al 31 | Cohort study within the National Danish patient registry, 1980–2006 | Entire Danish population. COPD identified through hospital admission codes or COPD as cause of death |
Age <30–7% 30–59–54% 60–79 –35 % >80–3% Sex 55% male COPD severity NR Current smokers NR History of CVD NR |
Discharge diagnosis of MI or cause of death from Danish Causes of Death Registry listed as MI | HR 1.26 (1.25–1.27) | Age, sex, Danish ancestry, geographical residency (rurality), and level of education |
| Yin et al 32 | Cohort of all residents of Sweden aged over 18, July 2005- December 2008. | 51 348 COPD patients identified by diagnostic codes from patient records. 6 743 342 non-COPD patients. |
Those with no previous MI or stroke Age Mean 71.1 Sex 44.3% male COPD severity NR Current smokers NR History of CVD No previous MI Those with previous MI Age Mean 69.2 Sex 58.4% male COPD severity NR Current smokers NR History of CVD All had previous MI |
Diagnostic code for MI, or primary cause of death listed as MI |
No previous MI or stroke: HR 1.47 (1.41–1.55)* Previous MI: HR 1.33 (1.23–1.43)* |
Age, sex, socioeconomic status, use of cardiovascular and respiratory medicines. |
*Data from personal communication (Magnus Back. Email communication. 18/08/2014).
COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; FEV1, forced expiratory volume in 1 s; ICD, International Classification of Diseases; IRR, incidence rate ratios; MI, myocardial infarction.
Table 2.
Characteristics of included studies—risk of MI associated with AECOPD
| Study | Design and setting | Population | Characteristics | AECOPD definition | MI definition | Risk periods | Risk estimate (95% CI) |
|---|---|---|---|---|---|---|---|
| Donaldson et al9 | Self-controlled case series in The Health Improvement Network, 2003–2005 | 426 patients with COPD and MI during study period. COPD defined using Quality and Outcomes Framework codes |
Age: Median 74 years (IQR, 67 to 80) Sex: 61% male Current smokers: NR COPD severity: Median FEV1% predicted: 55.9% (IQR, 43% to 73%) History of CVD: NR |
Three definitions used:
|
Diagnostic code for MI in primary care record | 1–5 days, 6–10 days, 11–15 days, 16–49 days, and 1–49 days | Antibiotics and steroids definition: 1–5 days: IRR 2.27 (1.10 to 4.70) 6–10 days: IRR 1.74 (0.80 to 4.0) 11–15 days: IRR 0.90 (0.30 to 2.90) 16–49 days: IRR 0.83 (0.50 to 1.40) 1–49 days: IRR 1.11(0.70 to 1.70) |
| Halpin et al21 | Secondary analysis of patients in UPLIFT RCT | 3 512 Patients with COPD who survived at least their first AECOPD. COPD defined as age ≥40 years, smoking history ≥10 pack-years, FEV1 ≤70% predicted, and FEV1/FVC ≤70% |
Age: Mean 64 (SD, 8) Sex: 74% male COPD severity GOLD stage II 43% GOLD stage III 46% GOLD stage IV 9%Mean FEV1% predicted 38% (SD, 12) Current smokers: 29%History of CVD: NR |
Increase in or new onset of more than one of: cough, sputum, sputum purulence, wheezing or dyspnoea; lasting three or more days and requiring treatment with an antibiotic or oral steroid. Data on timing of AECOPD collected at study visits | MI ascertained during RCT follow-up and recorded as a serious adverse event | 30 days after AECOPD, compared to 30 days before AECOPD | IRR 13.04 (1.71 to 99.7) |
AECOPD, acute exacerbation of COPD; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IRR, incidence rate ratios; MI, myocardial infarction; RCT, randomised control trial.
Table 3.
Characteristics of included studies – risk of death after MI
| Study | Design and setting | Population | COPD patient characteristics | Maximally adjusted estimate for mortality (95% CI) | Factors adjusted for |
|---|---|---|---|---|---|
| Andell et al 10 | Cohort study within the Swedish SWEDEHEART registry between 2005–2010. | Consecutive patients admitted to Swedish coronary care units. COPD diagnosis ascertained through linkage to the Swedish National Patient Registry. |
Age Mean 75 years (SD, 9) Sex 54% male COPD severity NR Current smokers 32.9% History of CVD Prior MI 13.7% Prior HF 20.2% |
Mortality at one year: HR 1.14 (1.07–1.21) |
Age, sex, smoking, comorbidity (previous MI, previous stroke, heart failure, renal failure, hypertension, diabetes, peripheral artery disease, cancer and previous bleeding), in hospital treatment and discharge medications (heparin, fondaparinux, dalteparin, enoxaparin, glycoprotein IIb/IIa inhibitors, angioplasty, coronary stenting, β-blockers, aspirin, clopidogrel, prasugrel, calcium channel blockers, digoxin, diuretics, statins, nitrates and warfarin). |
| Behar et al 22 | Cohort study in Israel between 1981–1983 | 2276 consecutive patients surviving an MI after admission to 13 coronary care units. Patients with a history of chronic bronchitis or chronic airways obstruction and clinical and/or radiographic findings compatible with COPD during hospitalisation for MI were included. |
Age Mean 66.8 years (SD, 9.7) Sex 79.3% male COPD severity NR Current smokers 43.3% History of CVD Prior MI—28.8% Prior angina—55.4% |
Unadjusted:* In –hospital RR 1.39 (1.16–1.67) 1 year RR 1.34 (1.16–1.55) 5 years RR 1.28 (1.18–1.40) |
|
| Bursi et al 23 | Cohort study of the population in the Rochester Epidemiology project involving residents in Olmsted County, Minnesota from 1979 to 2007 | Local residents in Olmsted County. MI ascertained from medical records compatible with ICD criteria. Information on COPD was also obtained from ICD codes. |
Age Mean 73 years (SD, 11) Sex 59% male COPD severity NR Current smokers 35% History of CVD Those with prior CVD excluded |
HR 1.30 (1.10 to 1.54), mean follow up 4.7 years. | Age, sex, smoking, hypertension, MI type (STEMI/non-STEMI), creatine kinase level, killip class, reperfusion treatment in hospital, use of drugs on discharge (β-blockers, ACEi, diuretics) |
| Dziewierz et al 24 | Cohort study within Krakow Registry of ACS in February 2005-March 2005 and December 2005-January 2006 | 1414 patients with MI admitted to hospital in Krakow, Poland. Those with a previous history of COPD and current treatment with a steroid or bronchodilator were classified as COPD patients. |
Age Mean 71.8 years (SD, 11) Sex 62% male COPD severity NR Current smokers 40.7% History of CVD MI 34.6% Angina 80.2% HF 30.9% |
HR 2.15 (1.30–3.55) | Age, sex, BMI, diabetes, hypertension, hyperlipidaemia, prior angina, prior MI, prior heart failure, left ventricular ejection fraction, prior PCI, prior CABG, prior stroke or transient ischaemic attack, smoking status, peripheral arterial disease, chronic renal insufficiency, parameters on admission (chest pain, cardiogenic shock, heart rate, systolic blood pressure, diastolic blood pressure), time from chest pain onset to admission and type of MI (STEMI or NSTEMI) |
| Enriquez et al 11 | Cross sectional study of National Cardiovascular Data Registry in the USA between January 2008 and December 2010 | 158 890 patients admitted to one of 445 sites with an MI. COPD patients had a history of COPD or were using long term inhaled or oral β-agonists, inhaled anti-inflammatory agents, leukotriene receptor antagonists or inhaled steroids. |
Age STEMI—median 66 years nSTEMI—median 70 years Sex STEMI—60.4% male nSTEMI—57.5% male COPD severity NR Current smokers STEMI—57.0% nSTEMI—41.9% History of CVD STEMI Prior MI—29.7% Prior CHF—15.3% nSTEMI Prior MI—39.% Prior CHF—33.3% |
In-hospital mortality STEMI OR 1.05 (0.95–1.17) Non-STEMI OR 1.21 (1.11–1.33) |
Age, serum creatinine, systolic blood pressure, troponin elevation, heart failure or cardiogenic shock at presentation, ST-segment changes, heart rate and prior peripheral arterial disease. |
| Hadi et al 15 | Cross sectional study of patients hospitalised with ACS in May 2006 and January 2007 to June 2007 in six Middle Eastern countries | 8169 consecutive patients in the Gulf RACE registry presenting with ACS at 65 centres across six countries. COPD patients were identified from 1) medical records or 2) use of COPD medicines. |
Age Median 64 (IQR, 56–71) Sex NR COPD severity NR Current smokers 38.7% History of CVD Prior MI—34.8% Prior angina – 54.4% |
In hospital mortality: OR 0.40 (0.20–1.24) |
Age, sex, cardiogenic shock, use of thrombolysis, use of aspirin, use of β-blocker, use of ACEi |
| Hawkins et al 25 | Cohort study of patients with acute MI enrolled in VALIANT trial | Patients with MI complicated by LVSD and HF. COPD was identified by a questionnaire completed by trial site investigators. |
Age Mean 68.1 (SD, 9.9) Sex 71.1% male COPD severity NR Current smokers 42.0% History of CVD Prior MI—39.9% Prior angina—46.1% Prior HF—27.3% |
HR 1.14 (1.02–1.28) | Age, heart rate, systolic and diastolic blood pressure, weight, baseline creatinine, smoking status, diabetes, dyslipidaemia, hypertension, killip classification, anterior MI, new lower bundle branch block, thrombolytic therapy, primary PCI, coronary artery bypass graft, history of heart failure, atrial fibrillation, previous MI, angina, previous stroke, peripheral arterial disease, renal insufficiency, alcohol abuse, country of enrolment, beta blocker use, randomised treatment |
| Kjoller et al 13 | Cohort study of consecutive patients recruited 1–6 days after an MI | Danish hospitals between May 1990 and July 1992 as part of TRACE study. COPD was identified using either 1) medical records or 2) patient report in addition to use of COPD medicines |
Age Median 70.5 (5–95 percentiles, 50.7–83.5) Sex 68.2% men COPD severity NR Current smokers 60.0% History of CVD Previous MI—25.1% Previous angina—43.9 Previous CHF—28.2% |
Cohort entry to 30 days: HR 0.89 (0.68–1.11) Cohort entry to 7 years: HR 1.15 (1.04–1.28) |
Age, sex, BMI, hypertension, diabetes, smoking status, previous angina, wall motion index, angina, history of CHF, new CHF, atrial fibrillation, bundle branch block, wall motion index, use of thrombolytic therapy |
| Quint et al 26 (abstract) |
Cohort study of patients admitted after a first MI using data from the UK CALIBER database | 8 065 patients admitted to UK hospitals with a first MI between Jan 2003-Dec 2008. COPD was identified using primary care records. |
Age NR Sex NR COPD severity NR Current smokers NR History of CVD NR |
Mortality up to 7 years: HR 1.37 (1.23–1.52) |
Age and sex |
| Raposeiras et al 12 (abstract) |
Cross sectional and cohort study of patients with ACS | 4 497 consecutive patients admitted to Spanish hospitals for ACS. The ascertainment method for COPD was unclear. |
Age NR Sex NR COPD severity NR Current smokers NR History of CVD NR |
In-hospital death OR 1.04 (1.03–1.04) Follow up mortality HR 1.69 (1.41–2.03), median follow up 3.1 years |
GRACE score β-blocker therapy |
| Rha et al 33 (abstract) |
Case control study in Korea AMI registry from 2005 to 2007 | AMI patients in KAMIR |
Age Mean 71.7 (SD 10.0) Sex NR COPD severity NR Current smokers NR History of CVD NR |
Mortality at 8 months OR 2.69, 95% CI could not be calculated from reported information. |
Unadjusted |
| Salisbury et al 27 | 19 centre prospective study of patients presenting with MI in a cohort study | MI patients in PREMIER study restricted to patients discharged alive after MI. Patients were considered to have COPD if they had a documented history of obstructive pulmonary disease (COPD or asthma) or had therapy specific for obstructive pulmonary disease. |
Age Mean 64.5 (SD, 12.4) Sex 61.8% male COPD severity NR Current smokers 37.6% History of CVD Previous MI—29.7% Previous HF—24.3% |
Mortality up to 1 year HR 2.00 (1.44–2.79) |
Age, gender, race, avoidance of health care due to cost, smoking, diabetes, hypertension, CHF, ejection fraction, previous CVD, MI diagnosis type, new onset HF after MI, diseased vessels on angiogram, enrolling site, percentage of MI quality of care indicators of the centre, treatment type |
| Stefan et al 14 | Cross sectional study with follow up of patients hospitalised with AMI at greater Worcester, Massachusetts between 1997–2007 | Patients hospitalised with AMI in greater Worcester, Massachusetts medical centres. COPD patients were identified by previous mention of clinical or radiographic evidence for COPD in their medical record. |
Age Mean 74 years Sex 52.4% male COPD severity NR Current smokers 27.3% History of CVD Prior angina—22.3% Prior HF—38.6% |
In hospital: OR 1.25 (0.97–1.34) 30 day mortality: OR 1.31 (1.10–1.58) |
Age, sex, year of hospitalisation, history of CVD, history of renal failure, type of MI (STEMI/non-STEMI), length of stay, smoking status used in secondary analysis |
*Calculated from reported data.
AMI, acute myocardial infarction; BMI, body mass index; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; HF, heart failure; LVSD, left ventricular systolic dysfunction; MeSH, Medical Subject Headings; MI, myocardial infarction; NR, not reported; PCI, percutaneous coronary intervention; NSTEMI, non–ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction.
All of the included studies which investigated risk of MI in people with COPD used data from either routine clinical or administrative databases. COPD was defined using diagnostic codes; these varied between COPD diagnosis in primary care, outpatient departments, hospital admission or discharge codes, and cause of death codes. Three studies also required that patients with COPD had been prescribed COPD medicines. One of the studies, Rodriguez et al,19 included only patients with a recent diagnosis of COPD and followed up to 5 years after this diagnosis to identify MI. Only one study3 reported a summary of COPD severity, and only two reported prevalence of current smokers. Four studies reported a cohort analysis only. Two studies4 19 reported a cohort analysis as well as a case–control analysis. One study reported the results of a cohort analysis and an analysis of period prevalence. One study20 compared rates of MI in patients with COPD to standardised populations rates of MI.
Two studies9 21 were identified which investigated the risk of MI associated with AECOPD. Both studies defined risk periods after the onset of AECOPD and used within person designs to compare the risk to a baseline period.
Nine studies reported mortality for patients with COPD after an MI compared to patient without COPD. Five studies11 12 14 15 22 reported a comparison of in-hospital mortality after an MI between patients with COPD and patient without COPD. Eight studies10 12 13 23–27 used a time-to-event analysis to investigate death after discharge from a hospital admission for MI.
Risk of bias assessment
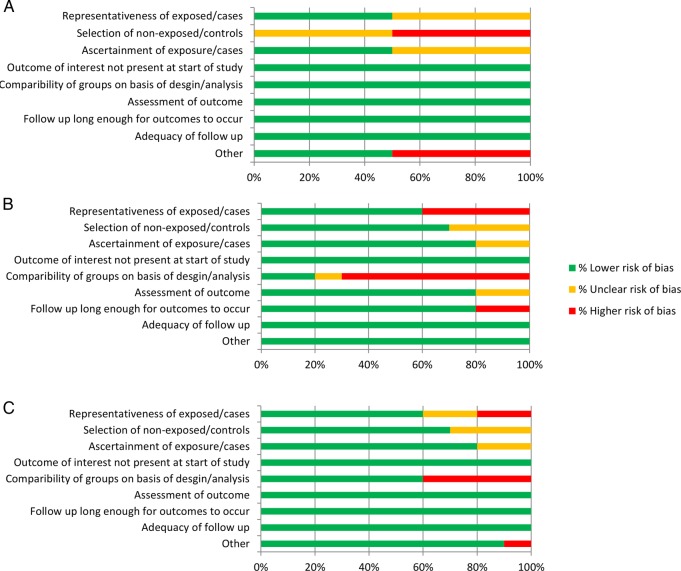
The proportion of studies (or analyses, where appropriate) which were assessed as having either lower, unclear or higher risk of bias for each of the research questions is presented in figure 2. Detailed results from the risk of bias assessment for individual studies are presented in the appendix.
Figure 2.
Summary of risk of bias for risk assessments for: A studies investigating risk of MI associated with COPD; B studies investigating risk of MI associated with AECOPD; and C studies investigating risk of death following MI in people with COPD. AECOPD, acute exacerbation of COPD; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction.
Risk of MI in people with COPD
Of nine included studies, eight found a higher risk of MI in patients with COPD compared to patient without COPD. Six studies estimated the ratio of incidence rates of MI in patients with COPD compared to patient without COPD. Five studies4 19 20 28 29 estimated this for all MIs, this ranged from IRR 1.18 (95% CI 0.81 to 1.71) to 5.31 (4.54 to 6.21). One study2 30 estimated the IRR for hospitalisation due to MI (IRR 1.49, 95% CI 0.71 to 3.13) and fatal MIs (1.51, 1.14 to 2.01). Two studies31 32 estimated the ratio of hazard of MI in patients with COPD compared to patient without COPD one study estimated this to be HR 1.26 95%, CI 1.25 to 1.27, while the other study estimated this to be HR 1.47 (1.41 to 1.55) for those with no previous MI, and HR 1.33 (1.23 to 1.43) for those with a previous MI. One study2 30 estimated the ratio of odds of period prevalence over 5 years of acute MI in patients with COPD compared to patient without COPD (OR 1.61, 95% CI 1.43 to 1.81). Only one3 of the included cohort studies compared risk of MI in people with COPD and people without COPD adjusted for smoking status. This study reported results stratified by age groups. Meta-analysis of these results showed an increased risk of MI for people with COPD (HR 1.72, 95% CI 1.22 to 2.42) (figure 3). Two of the included case–control studies adjusted for smoking status. Meta-analysis of these results did not show an increased risk of MI for people with COPD (OR 1.18, 95% CI 0.80 to 1.76) (figure 4). Meta-analysis was not conducted for the studies which did not adjust for smoking as heterogeneity was too high (I2=93%). These results are graphically summarised in figure 5.
Figure 3.
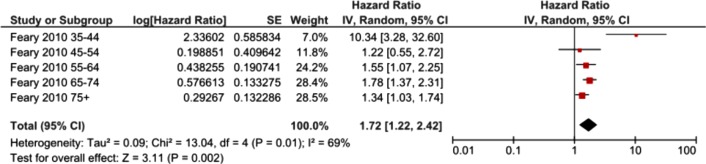
Forest plot showing risk of MI associated with COPD in cohort studies which adjusted for smoking status. CIs may vary slightly from those quoted in tables due to transformation during meta-analysis. COPD, chronic obstructive pulmonary disease; MI, myocardial infarction.
Figure 4.
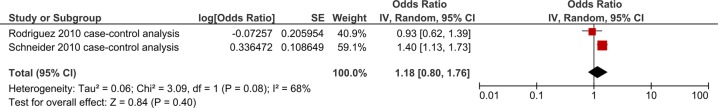
Forest plot showing risk of MI associated with COPD in case-control studies which are adjusted for smoking status. CIs may vary slightly from those quoted in tables due to transformation during meta-analysis. COPD, chronic obstructive pulmonary disease; MI, myocardial infarction.
Figure 5.
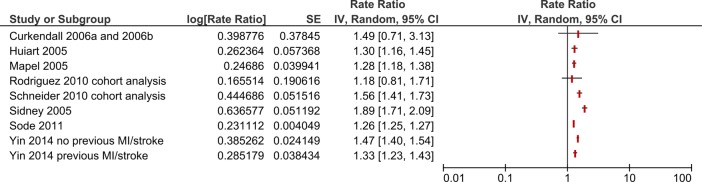
Forest plot showing risk of MI associated with COPD in cohort studies which did not adjust for smoking status. CIs may vary slightly from those quoted in tables due to transformation during meta-analysis. COPD, chronic obstructive pulmonary disease; MI, myocardial infarction.
Some studies investigated whether the effect of COPD on the risk of MI was different in terms of age and severity of airflow obstruction. Feary et al3 found that the effect of COPD on risk of MI was higher in the 35–44 year age group (HR 10.34, 95% CI 3.28 to 32.6) compared to older age groups (45–54 years: HR 1.22 (95% CI 0.55–2.74), 55–64 years: HR 1.55 (95% CI 1.07 to 2.26), 65–74 years: HR 1.78 (95% CI 1.37 to 2.31), ≥75 years: HR 1.34 (95% CI 1.03 to 1.73)). Sidney 200529 reported similar findings; the effect of COPD on risk of MI was higher in those who were aged 40–64 years (HR 2.43, 95% CI 1.98 to 2.98) compared to those who were aged over 64 years (HR 1.73, 95% CI 1.54 to 1.94). Schneider et al4 investigated the risk of MI by sub-group of COPD severity. They found that the effect of COPD on the risk of MI was greater in those with severe COPD (OR 3.00, 95% CI 1.53 to 5.86) compared to those with moderate COPD (OR 1.30, 95% CI 1.04 to 1.62) or mild COPD (OR 1.79, 95% CI 1.12 to 2.86).
Risk of MI associated with AECOPD
Donaldson 20109 conducted a self-controlled case series using data from The Health Improvement Network (THIN). They used prescription of antibiotics and steroids in patient with COPD to identify AECOPD, and report an increased risk of MI in the 1–5 days following the onset of AECOPD (IRR 2.27, 95% CI 1.10 to 4.70). No difference in the risk of MI was found for the period 6–49 days, or at any time point when the alternative definitions of AECOPD of prescription of steroids alone or antibiotics alone were used. Halpin et al21 reported a secondary analysis of the UPLIFT trial, which was an RCT comparing inhaled tiotropium and placebo in patients with COPD with a primary outcome of reduction in FEV1 decline. Time to first AECOPD was a secondary outcome. AECOPD were identified using a symptom-based definition and were reported to trial staff at regular study visits. Data on MI were collected as serious adverse events. This study found that compared to the 30 days prior to AECOPD, risk of MI in the 30 days following AECOPD was increased (IRR 13.04; 95% CI 1.71 to 99.7). These results are graphically summarised in figure 6. Owing to different exposure time periods, the results for within person studies investigating the risk of MI associated with AECOPD were not pooled in meta-analysis.
Figure 6.
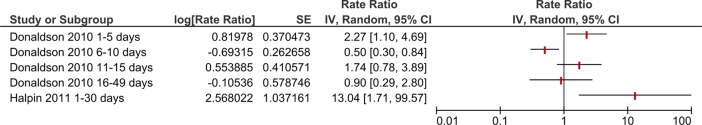
Forest plot showing risk of MI associated with acute exacerbations of COPD. CIs may vary slightly from those quoted in tables due to transformation during meta-analysis. COPD, chronic obstructive pulmonary disease; MI, myocardial infarction.
Risk of death after MI in people with COPD
Of the studies investigating differences in in-hospital mortality after an MI, two12 22 found an increased risk of mortality for patients with COPD (RR 1.39, 95% CI 1.16 to 1.67 (unadjusted); and OR 1.04, 95% CI 1.03 to 1.04). Two studies14 15 did not find evidence for increased in-hospital mortality for patients with COPD (OR 0.40, 95% CI 0.20 to 1.24; OR 1.25, 95% CI 0.97 to 1.34). One study11 reported results split by type of MI: it did not find an increased in-hospital mortality for patients with COPD after a STEMI (OR 1.05, 95% CI 0.95 to 1.17), but did find increased in-hospital death for patients with COPD after a non-STEMI (OR 1.21, 95% CI 1.11 to 1.33). Meta-analysis of adjusted results showed weak evidence for an increased risk of in-hospital death for patients with COPD (OR 1.13, 95% CI 0.97 to 1.31) (figure 7).
Figure 7.
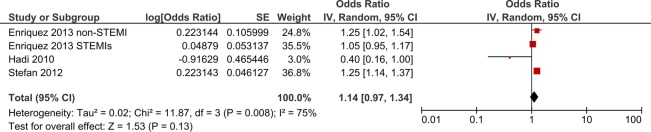
Forest plot showing risk of in-hospital death following MI for patients with COPD compared to patients without COPD. CIs may vary slightly from those quoted in tables due to transformation during meta-analysis. COPD, chronic obstructive pulmonary disease; MI, myocardial infarction.
One study14 reported mortality at 30 days for patients with COPD compared to patient without COPD. This study found increased mortality for patients with COPD (OR 1.31, 1.10 to 1.58). Another study33 reported mortality at 8 months, and in an unadjusted analysis, found increased mortality for patients with COPD compared to patients without COPD (OR 2.69, 95% CI was not reported and could not be calculated). One study22 also found, on unadjusted analysis, that mortality was greater for patients with COPD at 1 year (RR 1.34, 95% CI 1.16 to 1.55) and 5 years (RR 1.28, 95% CI 1.18 to 1.40) after MI.
Eight studies10 12 13 23–27 reported results of survival analysis of mortality during follow-up after an MI. All of the studies reported higher mortality for patients with COPD compared to patients without COPD during follow-up after discharge following an MI. HRs ranged from 1.15 (95% CI 1.04 to 1.28) to 2.15 (95% CI 1.30 to 3.55). However, one of these studies13 found no evidence of a difference in mortality when restricting the time period to the first 30 days following discharge (HR 0.89, 95% CI 0.68 to 1.11). Meta-analysis of studies which reported adjusted results showed an increased risk of death after discharge following MI for patients with COPD compared to patients without COPD (HR 1.26, 1.13 to 1.40) (figure 8). Four of the studies included under this question were excluded from meta-analysis for methodological12 33 or clinical heterogeneity.25 27
Figure 8.
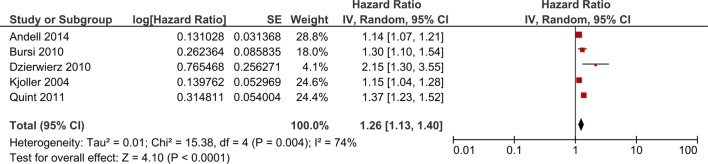
Forest plot showing risk of death after discharge following MI for patients with COPD compared to patients without COPD. Cis may vary slightly from those quoted in tables due to transformation during meta-analysis. COPD, chronic obstructive pulmonary disease; MI, myocardial infarction.
Discussion
Main findings
Most studies which investigated the risk of MI in people with COPD found that those with COPD have higher risk of MI than people who do not have COPD; however, it is unclear how much of this increased risk is due to smoking status. The included cohort study which adjusted for smoking status showed an increased risk of MI in people with COPD, but this was not apparent in pooled analysis of the case–control studies which adjusted for smoking status. Both of the included studies which investigated the risk of MI associated with AECOPD found an increased risk of MI in the weeks following AECOPD. Most studies which investigated mortality after an MI for patients with COPD as compared to patients without COPD found that mortality after discharge was greater for those with COPD, and an increased risk of death was found on pooled analysis. However, findings on in-hospital mortality after an MI were mixed, and there was only weak evidence for increased risk of death in hospital for patients with COPD on pooled analysis.
Limitations of included studies and future work
One common limitation among the included studies, particularly those which investigated the risk of MI associated with COPD, was missing information on smoking status. As smoking is very strongly associated with COPD and risk of MI, it is likely to be a major confounder in all studies investigating this association. All of the studies in this review which investigated this association used either clinical or administrative routine data sources. Routine data are a potentially rich source of information about huge numbers of patients. However, data on smoking are not routinely recorded in all administrative databases. Indeed, all of those studies which did not have data for smoking in this question used administrative databases. Future studies on the association between COPD and cardiovascular disease should use data sources which contain reliable information on smoking status.
Further studies should be carried out to confirm findings that AECOPD are periods of increased risk of MI for people with COPD. These studies should ensure they use validated exposure measures and are adequately powered. Possible reasons for an increased risk of MI during AECOPD include increased inflammation and the potential cardiovascular effects of the drugs used to treat AECOPD. If indeed the finding of increased risk during AECOPD is confirmed, future studies should attempt to disentangle the reasons for increased risk of MI. In addition, studies should investigate factors which might modify this relationship, such as drugs used for treatment of COPD and cardiovascular prevention. Another potential bias in studies which investigate the relationship between AECOPD and MI, which could explain some of the increased risk of MI after AECOPD, is differential misclassification of episodes of angina as AECOPD.
No studies were found which investigated the risk of MI associated with the frequent exacerbator phenotype. The frequent exacerbator phenotype may prove to be a useful characteristic for stratifying cardiovascular risk among patients with COPD. Future cohort studies of cardiovascular disease in people with COPD should, where possible, phenotype participants and investigate the relationship between exacerbator phenotype and risk of MI. Few included studies assessed the influence of severity of COPD on risk of MI; further research should investigate this relationship as well as the influence of severity of COPD on risk of death following MI.
A further limitation of several of the included studies on death following MI was availability of information on cause of death. Collection of information on cause of death in future studies would allow investigators to draw more confident conclusions about the reasons for increased risk of death following MI for people with COPD.
Strengths and limitations of this review
This review benefitted from using a comprehensive search strategy which covered several bibliographic databases. As the relationship between AECOPD and MI has not been extensively studied, the inclusion criteria for this research question were kept purposively broad. This allowed all information pertaining to this relationship to be included in the evidence synthesis. One potential limitation of systematic reviews is publication bias. The potential for publication bias was highest for the review of outcomes after MI. In order to reduce the risk of this bias, we only included studies which specifically investigated the risk of COPD on MI rather than several different potential prognostic factors, as studies which investigated several factors which did not find an association between COPD and MI may not have reported this in the abstract or even in the text. Owing to clinical and statistical heterogeneity, meta-analysis could only be conducted for some of the research questions. Where meta-analysis was conducted, statistical heterogeneity was generally high, and this may limit the generalisability of pooled estimates.
Conclusions
There is good evidence of an increased risk of MI in people with COPD; however, it is unclear to what extent this association is due to smoking status.
There is some evidence that among people with COPD, AECOPD represent periods of increased risk of MI. However, further larger studies using validated exposure methods are needed to support this finding.
There is weak evidence that in-hospital mortality is higher for people with COPD after an MI. There is good evidence that postdischarge mortality after an MI is higher for people with COPD.
Footnotes
Contributors: KJR, LS and JKQ conceived and designed the study. KJR and RY screened abstracts and full text articles for inclusion, extracted data, and assessed the risk of bias of included studies. KJR conducted the meta-analysis. KJR, RY, LS and JKQ interpreted findings. KJR wrote the first draft. KJR, RY, LS and JKQ revised the article critically for important intellectual content. KJR, RY, LS and JKQ approved the final version of the article.
Funding: JKQ is supported by an MRC Population Health Scientist fellowship [G0902135]. LS is supported by a Wellcome Trust Senior Research Fellowship in Clinical Science [098504/Z/12/Z].
Competing interests: JKQ reports grants from Medical Research Council, grants from GSK—MRC and the British Lung Foundation during the conduct of the study; personal fees from GSK, Almirall and AstraZeneca, outside the submitted work. LS reports grants from Wellcome Trust during the conduct of the study; grants from Wellcome Trust, grants from MRC, grants from NIHR, personal fees from GSK, outside the submitted work. KJR has no conflicts of interest to disclose. RY has no conflicts of interest to disclose.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: This is a systematic review of previously published studies.
References
- 1.Sin DD, Anthonisen NR, Soriano JB et al. Mortality in COPD: role of comorbidities. Eur Respir J 2006;28:1245–57. 10.1183/09031936.00133805 [DOI] [PubMed] [Google Scholar]
- 2.Curkendall SM, DeLuise C, Jones JK et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol 2006;16:63–70. 10.1016/j.annepidem.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 3.Feary JR, Rodrigues LC, Smith CJ et al. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax 2010;65:956–62. 10.1136/thx.2009.128082 [DOI] [PubMed] [Google Scholar]
- 4.Schneider C, Bothner U, Jick SS et al. Chronic obstructive pulmonary disease and the risk of cardiovascular diseases. Eur J Epidemiol 2010;25:253–60. 10.1007/s10654-010-9435-7 [DOI] [PubMed] [Google Scholar]
- 5.Divo M, Cote C, de Torres JP et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;186:155–61. 10.1164/rccm.201201-0034OC [DOI] [PubMed] [Google Scholar]
- 6.Mannino DM, Thorn D, Swensen A et al. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 2008;32:962–9. 10.1183/09031936.00012408 [DOI] [PubMed] [Google Scholar]
- 7.Pitta F, Troosters T, Spruit MA et al. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;171:972–7. 10.1164/rccm.200407-855OC [DOI] [PubMed] [Google Scholar]
- 8.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality*: a population-based study and a systematic review of the literature. Chest 2005;127:1952–9. 10.1378/chest.127.6.1952 [DOI] [PubMed] [Google Scholar]
- 9.Donaldson GC, Hurst JR, Smith CJ et al. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest 2010;137:1091–7. 10.1378/chest.09-2029 [DOI] [PubMed] [Google Scholar]
- 10.Andell P, Koul S, Martinsson A et al. Impact of chronic obstructive pulmonary disease on morbidity and mortality after myocardial infarction. Open Heart 2014;1:e000002 10.1136/openhrt-2013-000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enriquez JR, de Lemos JA, Parikh SV et al. Association of chronic lung disease with treatments and outcomes patients with acute myocardial infarction. Am Heart J 2013;165:43–9. 10.1016/j.ahj.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 12.Raposeiras Roubin S, Abu Assi E, Alvarez Alvarez B et al. In-hospital and follow-up mortality associated to chronic obstructive pulmonary disease in a contemporary cohort of patients with acute coronary syndrome. Eur Heart J 2012;33:1084 [Google Scholar]
- 13.Kjoller E, Kober L, Iversen K et al. Importance of chronic obstructive pulmonary disease for prognosis and diagnosis of congestive heart failure in patients with acute myocardial infarction. Eur J Heart Fail 2004;6:71–7. 10.1016/j.ejheart.2003.09.008 [DOI] [PubMed] [Google Scholar]
- 14.Stefan MS, Bannuru RR, Lessard D et al. The impact of COPD on management and outcomes of patients hospitalized with acute myocardial infarction: a 10-year retrospective observational study. Chest 2012;141:1441–8. 10.1378/chest.11-2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadi HA, Zubaid M, Al Mahmeed W et al. Prevalence and prognosis of chronic obstructive pulmonary disease among 8167 Middle Eastern patients with acute coronary syndrome. Clin Cardiol 2010;33:228–35. 10.1002/clc.20751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Secondary The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 17.Jüni P, Witschi A, Bloch R et al. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999;282:1054–60. 10.1001/jama.282.11.1054 [DOI] [PubMed] [Google Scholar]
- 18.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. Chichester, UK: John Wiley & Sons Ltd., 2008. [Google Scholar]
- 19.Rodriguez LA, Wallander MA, Martin-Merino E et al. Heart failure, myocardial infarction, lung cancer and death in COPD patients: a UK primary care study. Respir Med 2010;104:1691–9. 10.1016/j.rmed.2010.04.018 [DOI] [PubMed] [Google Scholar]
- 20.Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest 2005;128:2640–6. 10.1378/chest.128.4.2640 [DOI] [PubMed] [Google Scholar]
- 21.Halpin DM, Decramer M, Celli B et al. Risk of nonlower respiratory serious adverse events following COPD exacerbations in the 4-year UPLIFT Trial. Lung 2011;189:261–8. 10.1007/s00408-011-9301-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Behar S, Panosh A, Reicher-Reiss H et al. Prevalence and prognosis of chronic obstructive pulmonary disease among 5,839 consecutive patients with acute myocardial infarction. SPRINT Study Group. Am J Med 1992;93:637–41. 10.1016/0002-9343(92)90196-I [DOI] [PubMed] [Google Scholar]
- 23.Bursi F, Vassallo R, Weston SA et al. Chronic obstructive pulmonary disease after myocardial infarction in the community. Am Heart J 2010;160:95–101. 10.1016/j.ahj.2010.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dziewierz A, Siudak Z, Rakowski T et al. Relationship between chronic obstructive pulmonary disease and in-hospital management and outcomes in patients with acute myocardial infarction. Kardiol Pol 2010;68:294–301. [PubMed] [Google Scholar]
- 25.Hawkins NM, Huang Z, Pieper KS et al. Chronic obstructive pulmonary disease is an independent predictor of death but not atherosclerotic events in patients with myocardial infarction: analysis of the Valsartan in Acute Myocardial Infarction Trial (VALIANT). Eur J Heart Fail 2009;11:292–8. 10.1093/eurjhf/hfp001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quint JK, Herrett E, Timmis A et al. Survival after the first myocardial infarction is shorter in patients with COPD compared to the general population. Thorax 2011;66:A45–6. 10.1136/thoraxjnl-2011-201054b.96 [DOI] [Google Scholar]
- 27.Salisbury AC, Reid KJ, Spertus JA. Impact of chronic obstructive pulmonary disease on post-myocardial infarction outcomes. Am J Cardiol 2007;99:636–41. 10.1016/j.amjcard.2006.09.112 [DOI] [PubMed] [Google Scholar]
- 28.Mapel DW, Dedrick D, Davis K. Trends and cardiovascular co-morbidities of COPD patients in the veterans administration medical system, 1991–1999. COPD 2005;2:35–41. 10.1081/COPD-200050671 [DOI] [PubMed] [Google Scholar]
- 29.Sidney S, Sorel M, Quesenberry CP Jr et al. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest 2005;128:2068–75. 10.1378/chest.128.4.2068 [DOI] [PubMed] [Google Scholar]
- 30.Curkendall SM, Lanes S, de Luise C et al. Chronic obstructive pulmonary disease severity and cardiovascular outcomes. Eur J Epidemiol 2006;21:803–13. 10.1007/s10654-006-9066-1 [DOI] [PubMed] [Google Scholar]
- 31.Sode BF, Dahl M, Nordestgaard BG. Myocardial infarction and other co-morbidities in patients with chronic obstructive pulmonary disease: a Danish nationwide study of 7.4 million individuals. Eur Heart J 2011;32:2365–75. 10.1093/eurheartj/ehr338 [DOI] [PubMed] [Google Scholar]
- 32.Yin L, Lensmar C, Ingelsson E et al. Differential association of chronic obstructive pulmonary disease with myocardial infarction and ischemic stroke in a nation-wide cohort. Int J Cardiol 2014;173:601–3. 10.1016/j.ijcard.2014.03.140 [DOI] [PubMed] [Google Scholar]
- 33.Rha SW, Li YJ, Chen KY et al. Impact of chronic obstructive pulmonary disease on the clinical characteristics and outcomes in propensity-matched patients with acute myocardial infarction. Am J Cardiol 2009;103:36–37B. 10.1016/j.amjcard.2009.01.12519101226 [DOI] [Google Scholar]