Abstract
Background
Cardiac catheterization is the standard of care procedure for diagnosis, choice of therapy, and longitudinal follow-up of children and adults with pulmonary hypertension (PH). However, the procedure is invasive and has risks associated with both the procedure and recovery period.
Objectives
Identify risk factors for catastrophic adverse outcome in children with PH undergoing cardiac catheterization.
Methods
We studied children and young adults 0-21 years of age with PH undergoing ≥1 cardiac catheterizations at centers participating in the Pediatric Health Information Systems (PHIS) database between 2007 and 2012. Using mixed effects multivariable regression, we assessed the association between pre-specified subject- and procedure-level covariates and the risk of the composite outcome of death and/or initiation of mechanical circulatory support within 1 day of cardiac catheterization after adjustment for patient- and procedure-level factors.
Results
6,339 procedures performed on 4,401 patients with a diagnosis of PH from 38/43 centers contributing data to the PHIS database were included. The observed risk of composite outcome was 3.5%. In multivariate modeling, the adjusted risk of the composite outcome was 3.3%. Younger age at catheterization, cardiac operation in the same admission as the catheterization, pre-procedural systemic vasodilator infusion, and hemodialysis were independently associated with an increased risk of adverse outcomes. Pre-procedure use of pulmonary vasodilators was associated with reduced risk of composite outcome.
Conclusions
The risk of cardiac catheterization in children and young adults with PH is high relative to previously reported risk in other pediatric populations. The risk is influenced by patient-level factors. Further research is necessary to determine whether knowledge of these factors can be translated into practices that improve outcomes for children with PH.
Keywords: Pediatric Health Information Systems Database, outcomes research, pediatric cardiology, intervention, mortality, extra-corporeal membrane oxygenation
Introduction
Pulmonary hypertension (PH) affects 2.1 to 3.7 children per million (1-3), but remains an extremely morbid condition with a 5-year survival of 65-75% (1,2,4-6). Right heart catheterization is an important tool in the diagnosis, classification, and longitudinal care of these patients (7,8). However, cardiac catheterization in children with PH carries a risk of cardiac arrest of 4.5-5.7 per hundred (9,10), which is >10 times the risk of catheterization in children with other diagnoses and in adults with PH (11-20).
The determinants of peri-procedural morbidity and mortality are not well defined, particularly in children. Relatively small procedural volumes at single centers and differences in practice at different centers are obstacles to the study of outcome in children with PH. Identification of risk factors for adverse outcomes could provide an opportunity to intervene and improve safety of catheterization in children with PH.
We performed a multi-center retrospective cohort study assessing risk factors for catastrophic adverse events using the Pediatric Health Information Systems (PHIS) database, an administrative database. We hypothesized that patient level risk factors, such as age, etiology of PH and indicators of severity of illness, would influence the peri-procedural risk of death and catastrophic outcome.
Methods
Data Source
The PHIS is an administrative database that contains data from inpatient, emergency department, ambulatory surgery, and observation encounters from 43 non-profit, tertiary care pediatric hospitals affiliated with the Children’s Hospital Association (CHA, Overland Park, Kansas) in the United States. Encounters in PHIS include inpatient and observation admissions but exclude outpatient procedures (those without overnight observation). Data quality and reliability are assured through a joint effort between Children’s Hospital Association and participating hospitals. The data warehouse function for the PHIS database is managed by Truven Health Analytics (Ann Arbor, Michigan). For the purposes of external benchmarking, participating hospitals provide discharge/encounter data including demographics, diagnoses, and procedures. Forty-two of these hospitals also submit resource utilization data (e.g., pharmacy products, radiologic studies, and laboratory studies) to the PHIS. Data are de-identified at the time of data submission and are subjected to a number of reliability and validity checks before being included in the database. A data-use agreement was signed between study investigators and CHA. The institutional review board of The Children’s Hospital of Philadelphia reviewed the proposed project and determined that it did not represent human subjects research in accordance with the Common Rule (45 CFR 46.102(f)).
Study Population
Procedures and diagnoses were identified by International Classification of Disease, ninth revision code (ICD-9). We included children and adults, age 0-21 years carrying a diagnosis of PH undergoing cardiac catheterization at any of the 43 PHIS centers between 1/1/2007 and 12/31/2012. We excluded subjects from centers 1) reporting <25 cardiac catheterization procedures per year or 2) not reporting cardiac catheterization procedures in at least 4 of 6 years during the study period to insure that only centers with stable reporting practices were included. Subjects for whom the date of catheterization was missing were also excluded. Subjects undergoing electrophysiology studies and those undergoing cardiac catheterization on mechanical circulatory support were excluded because their risk of adverse event was considered to be qualitatively different.
Study Measures
Data were extracted from the PHIS database by direct query using ICD-9 codes and Clinical Transaction Codes for pharmaceutical products as previously described (Online Table 1). The primary outcome was a composite of death or initiation of mechanical circulatory support (extracorporeal membrane oxygenation, percutaneous ventricular assist device, or balloon pump) within 1 day of cardiac catheterization. Patient-level data included subject age, sex, race, insurance payer (private, public, other), presence of genetic syndrome (21), presence of non-cardiac congenital anomalies, history of prematurity (defined as gestational age <34 weeks in patients <1 year of age), location of patient prior to the procedure (outpatient, NICU, ICU, CICU, and step down unit/cardiac unit), and receipt of mechanical ventilation prior to catheterization, receipt of inotropic agents, systemic vasodilators, and pulmonary vasodilators. Sub-classification of PH was limited in this database to 1) idiopathic pulmonary arterial hypertension (IPAH), 2) pulmonary arterial hypertension associated with congenital heart disease (APAH-CHD), 3) PH with cardiomyopathy, 4) PH in the context of a heart transplant, and 5) chronic thromboembolic pulmonary hypertension (CTEPH). Diagnoses are based on ICD-9 codes extracted by coders based on physician documentation and sent to CHCA. Further detail regarding diagnoses and clinically relevant data such as oximetry, pressure data, laboratory, and imaging data are not available. Procedural data included whether a transcatheter intervention was performed during the case.
For some covariates identified prior to analysis (such as race and cardiac diagnosis), data were missing in <10% of cases. For these subjects, data were coded as “missing,” and included in analysis. There were no other missing data.
Statistical Analysis
Descriptive statistics were expressed as mean ± standard deviation, median (range and inter-quartile range [IQR]), and percentages and counts as appropriate. Multiple catheterizations were performed on individual subjects over the study period. All eligible procedures were included, and all statistics are reported per procedure except where noted.
The association between patient level characteristics and composite outcome was assessed using mixed-effects multivariate generalized linear models (with logistic link) (22). Fixed effects for the pre-specified covariates (listed above) were included. To account for covariance within centers and between multiple procedures performed in a single individual, random intercepts were added to the model for each center and patient (23). An additional analysis restricted to the first catheterization for each subject during the study period was performed as an alternative means of accounting for bias introduced by including multiple catheterizations per subject. Adjusted risks of outcomes were estimated using conditional standardization (i.e., the risk estimated by the model if variables are set at either the mean values for the cohort for continuous variables and at the referent group for categorical variables) to provide a more clinically applicable estimation of risk. Based on previous research (24), an interaction term for age category and history of prematurity was included.
Several pre-specified sensitivity and subset analyses were performed. A model including PH medications by drug class (calcium channel blockers [CCB], phosphodiesterase-5 [PDE-5] inhibitors, endothelin receptor antagonists [ERA], and prostacyclin analogues) was performed. We also adjusted for the number of classes of PH medications to assess whether this provided pre-procedural risk stratification.
Post-hoc analyses included: 1) characteristics and outcomes of subjects in whom a catheter-based intervention was performed vs. those without an intervention, 2) outcomes stratified by quintiles of center annual catheterization volume (quintiles determined as previous described [24]), and 3) outcomes excluding subjects who underwent a cardiac operation within 1 day of catheterization. The latter analysis was performed to assess the effect of pre-operative catheterizations at centers utilizing routine post-operative ECMO in high-risk patients on observed results. This may also eliminate subjects who experienced clinical decompensation and subsequent ECMO.
All analyses were performed using Stata MP v13 (Statacorp, College Station, Texas). The threshold for statistical significance was set at p <0.05.
Results
Study population
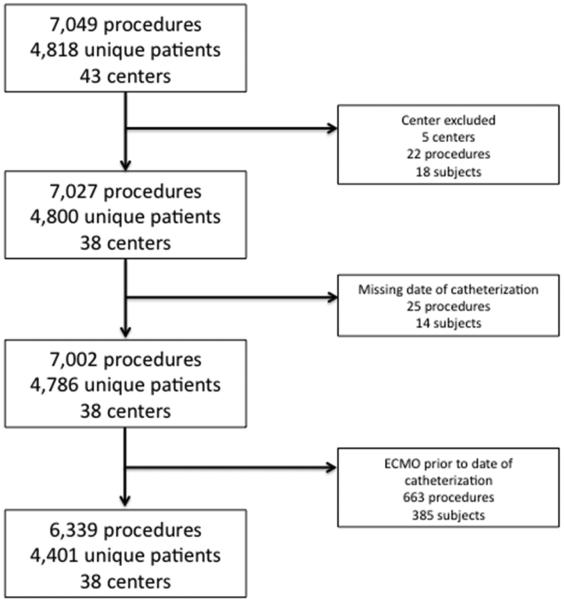
The initial query yielded data from 7,049 procedures performed in 4,818 subjects during 6,180 hospitalizations at 43 centers (Figure 1). Twenty-two (0.3 %) procedures on 18 subjects from 5 centers were excluded because the centers reported less than 25 catheterizations per year or did not report any catheterization procedures in at least 4 of 6 years of the study period. Twenty-five (0.4%) procedures in 14 subjects were excluded because of missing date of catheterization, and 663 (9%) procedures on 385 subjects were excluded due to mechanical circulatory support prior to and on the day of catheterization. Thus, the study sample included 6,339 procedures in 4,401 unique subjects from 5,651 hospitalizations across 38 centers.
Figure 1. Study population.
Flow-chart detailing progress from initial data query to the analytic cohort based on exclusion criteria for centers, missing date of catheterization, and receipt of ECMO prior to catheterization.
The median age at catheterization was 2.2 years (range: 2 days to 21 years) (Table 1). 51% of catheterizations were performed in subjects who were male, 62% white, and 38% with private insurance. 21% of procedures were in subjects with IPAH, 69% with APAH-CHD, 6% with PH and cardiomyopathy, 4% with PH following an orthotopic heart transplant, and 1% with CTEPH. Prior to catheterization, 5% were receiving CCB, 10% were receiving PDE-5 inhibitors, 6% were receiving ERA, 1.1% were receiving continuous intravenous prostacyclin analogues, and 0.1% were receiving inhaled nitric oxide. Overall, 13% were receiving inotropes and 9% systemic vasodilators. During the same hospitalization and prior to catheterization, 5% of subjects had undergone a cardiac operation. During the catheterization, 23% of subjects underwent a transcatheter intervention.
Table 1.
Study Population
| Catheterization Procedures (n) | 6,339 |
| Individual Patients (n) | 4,401 |
| Age (years) | 2 .2 (IQR: 210 days–9.5 years Range: 2 days-21 years) |
| Age categories: | |
| Neonate with prematurity | 0.3% (21) |
| Neonate without prematurity | 3.3% (207) |
| Infant (30 days to 1 year) with prematurity | 3.7% (236) |
| Infant (30 days to 1 year) without prematurity | 28% (1,827) |
| 1-8 years | 36% (2,279) |
| 8-18 years | 24% (1,503) |
| >18 years | 4.2% (266) |
| Male sex % (n) | 51% (3,236) |
| Race % (n) | |
| White | 62% (3,920) |
| Black | 14% (917) |
| Asian | 3% (216) |
| Other | 16% (998) |
| Missing | 5% (288) |
| Payor % (n) | |
| Private | 38% (2,430) |
| Medicaid | 43% (2,716) |
| Other | 19% (1,193) |
| Diagnosis % (n) | |
| IPAH | 21% (1,304) |
| APAH-CHD | 69% (4,357) |
| Cardiomyopathy with PH | 6% (359) |
| Chronic thromboembolic pulmonary hypertension | 1% (49) |
| PH with orthotopic heart transplant | 4% (270) |
| Genetic syndrome % (n) | 18% (1,126) |
| Non-cardiac congenital anomaly | 12% (752) |
| Mechanical ventilation prior to catheterization | 16% (1,038) |
| Medications prior to catheterization | |
| PDE-5 inhibitors | 10% (624) |
| Calcium channel blockers | 5% (335) |
| Prostacyclin analogues | 1.1% (77) |
| ETA antagonists | 6% (349) |
| Inhaled nitric oxide | 0.1% (5) |
| Inotropic agents | 13% (817) |
| Systemic vasodilators | 10% (641) |
| Hemodialysis | 0.2% (15) |
| Cardiothoracic operation prior to catheterization during the same hospitalization |
5% (324) |
| Transcatheter intervention during catheterization | 23% (1,441) |
| Outcomes % (n) | |
| Composite outcome within 1 day | 3.5% (222) |
| Death within 1 day | 0.3% (17) |
| ECMO within 1 day | 3.3% (206) |
| Composite outcome on day of catheterization | 1.0% (61) |
| Death on day of catheterization | 0.1% (9) |
| ECMO on day of catheterization | 0.8% (52) |
Catheterizations including transcatheter intervention were performed in subjects who were younger (p <0.001), more likely male, and of “other race. They also had a higher proportion of APAH-CHD (89% vs. 62.8%), lower proportion of IPAH (6.9% vs. 24.6%, p <0.001), lower proportion receiving pulmonary vasodilator (14.9% vs. 19.0%, p <0.001), higher proportion receiving systemic vasodilators (12.7% vs. 9.4%, p <0.001), and higher proportion who underwent cardiac operation in the same hospitalization (8.7% vs. 4.1%, p <0.001) compared to catheterizations without an intervention (Online Table 2). The risk of composite outcome, death, and initiation of ECMO were not significantly different on day of catheterization or within 1 day of catheterization.
The risk of the composite outcome within 1 day of catheterization was 3.5% (n = 222) (Table 1). 206 (3.3%) subjects underwent initiation of mechanical circulatory support and 17 (0.3%) died). The risk of the composite outcome was 1.0% (n = 61) on the day of cardiac catheterization; 52 (0.8%) underwent initiation of mechanical circulatory support and 9 (0.1%) died. The risk of death prior to discharge was 6.6% (n = 416), with an increased risk of death if placed on mechanical support within 1 day of catheterization 15.1% (p <0.001). In the subgroup of procedures in subjects with IPAH, 1.8% (25/1416) underwent ECMO and 36% of these (9/16) died prior to discharge.
Multivariate model for risk factors associated with death or mechanical circulatory support within 1 day of cardiac catheterization
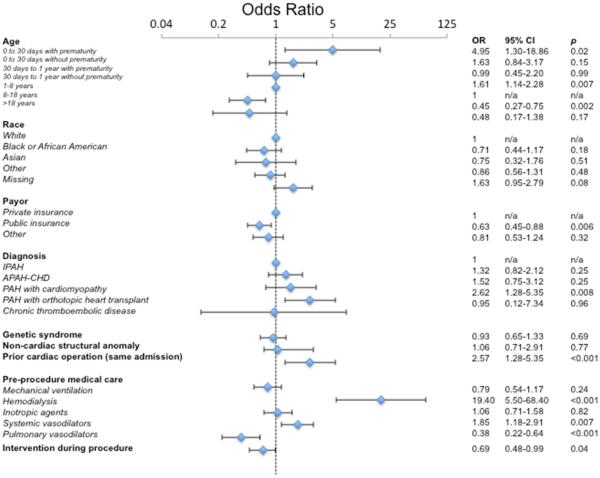
Table 2 and Figure 2 show the results of a mixed effects multivariate regression model of the risk factors for death or mechanical circulatory support within one day after catheterization. Using conditional standardization, the adjusted risk of composite primary outcome within one day of catheterization was 3.3% (95% CI: 1.9-5.5%). Several factors were independently associated with an increased risk. Neonates with history of prematurity (OR: 4.95, p = 0.02) and infants without prematurity (OR: 1.61, p = 0.007) were associated with an increased risk of the composite outcome relative to subjects between >1 and ≤8 years of age. Similarly, older children (age >8 and ≤18) had a reduced risk of the composite outcome (OR: 0.45, p = 0.02). Age >18 years was not associated with a significant change in the risk of adverse events. In terms of diagnosis, there were no significant differences in risk of composite outcome for subjects with APAH-CHD, cardiomyopathy, or chronic thromboembolic pulmonary hypertension compared to IPAH. However, PH after heart transplant, however, was associated with significantly increased risk (OR: 2.78, p = 0.005).
Table 2.
Multivariate model of risk factors for combined outcome (random intercept, with interaction between age category and prematurity)
| Odds Ratio |
95% CI | p | |
|---|---|---|---|
| Age | |||
| 0 to 30 days with prematurity | 4.95 | 1.30-18.86 | 0.02 |
| 0-30 days without prematurity | 1.63 | 0.84-3.17 | 0.15 |
| 30 days to 1 year with prematurity | 0.99 | 0.45-2.20 | 0.99 |
| 30 days to 1 year without prematurity | 1.61 | 1.14-2.28 | 0.007 |
| 1-8 years | 1 | n/a | n/a |
| 8-18 years | 0.45 | 0.27-0.75 | 0.002 |
| >18 years | 0.48 | 0.17-1.38 | 0.17 |
| Race | |||
| White | 1 | n/a | n/a |
| Black or African-American | 0.71 | 0.44-1.17 | 0.18 |
| Asian | 0.75 | 0.32-1.76 | 0.51 |
| Other | 0.86 | 0.56-1.31 | 0.48 |
| Missing | 1.63 | 0.95-2.79 | 0.08 |
| Payor | |||
| Private insurance | 1 | n/a | n/a |
| Public insurance | 0.63 | 0.45-0.88 | 0.006 |
| Other | 0.81 | 0.53-1.24 | 0.32 |
| Diagnosis | |||
| IPAH | 1 | n/a | n/a |
| APAH-CHD | 1.32 | 0.82-2.12 | 0.25 |
| PAH with cardiomyopathy | 1.52 | 0.75-3.12 | 0.25 |
| PAH with orthotopic heart transplant | 2.62 | 1.28-5.35 | 0.008 |
| Chronic thromboembolic pulmonary hypertension | 0.95 | 0.12-7.34 | 0.96 |
| Genetic syndrome | 0.93 | 0.65-1.33 | 0.69 |
| Non-cardiac structural anomaly | 1.06 | 0.71-2.91 | 0.77 |
| Cardiac operation prior to catheterization | 2.57 | 1.28-5.35 | <0.001 |
| Pre-procedural medical care | |||
| Mechanical ventilation | 0.79 | 0.54-1.17 | 0.24 |
| Hemodialysis | 19.40 | 5.50-68.40 | <0.001 |
| Inotropic agents | 1.06 | 0.71-1.58 | 0.82 |
| Systemic vasodilators | 1.85 | 1.18-2.91 | 0.007 |
| Pulmonary vasodilators | 0.38 | 0.22-0.64 | <0.001 |
| Intervention during procedure | 0.69 | 0.48-0.99 | 0.04 |
Adjusted risk of composite outcome is 3.3% (95% CI:1.9-5.5%).
Akaike information criteria: 1802
Figure 2. Forest plot of multivariable analysis of risk factors for catastrophic adverse outcome <1 day from catheterization.
Odds ratios from mixed-effects multivariate generalized linear models are depicted (blue diamonds) along with 95% confidence intervals (brackets).
Abbreviations: IPAH: idiopathic pulmonary arterial hypertension, APAH-CHD: Pulmonary arterial hypertension associated with congenital heart disease, PAH: pulmonary arterial hypertension
Several aspects of pre-procedural medical care were independently associated with the risk of catastrophic adverse outcome. Hemodialysis was associated with increased risk (OR: 19.40, p <0.001), as was receipt of systemic vasodilators (OR: 1.85, p = 0.007). Receipt of any PH medications was associated with reduced risk of adverse outcome (OR: 0.38, p <0.001). Mechanical ventilation and receipt of inotropes were not associated with the risk of composite outcome.
We also assessed the associated risk of catastrophic adverse outcome with treatment with PH medications by class (Online Table 3). The point estimates for CCB, PDE-5 inhibitors, and ERA were similar to that of PH medications overall (but with decreased precision). The association between PDE-5 inhibitors and reduced risk remained significant (OR: 0.44, p = 0.02), while the observed reduction in risk with CCB (OR: 0.47, p = 0.08) and ERA (OR: 0.63, p = 0.34) were not statistically significant. The number of PH medications received did not appear to affect the results (Online Table 4).
Several post-hoc analyses were performed. Excluding patients who underwent transcatheter interventions did not change the standardized risk of composite outcome or the previously observed associations with other covariates (data not shown). An analysis restricted to the first catheterization for each subject during the study period demonstrated no significant changes in the adjusted risk or the odds ratios for selected covariates, suggesting that the initial method for accounting for this bias was effective (Online Table 5). A sensitivity analysis was performed restricting analysis to procedures in subjects who did not undergo an operation within 1 day of catheterization (n = 6,207/6,339 cases) to preoperative catheterization with subsequent post-operative ECMO support. The standardized risk of composite outcome was 2.6% (95% CI: 1.5-4.7%) with similar predictors as seen in the main analysis (Online Table 6).
The association between catheterization lab volume and adverse outcomes was measured. The risk of composite outcome was highest in the lowest volume quintile (6.1%) and lowest in the highest volume quintile (2.4%) with a statistically significant association across quintiles (p <0.001) (Online Table 7). After adjusting for other covariates, adjusted risk of death was significantly higher at low volume centers (Quintiles 1 and 2) than at higher volume centers. There was no significant difference between outcomes at centers with higher volume (Quintiles 3, 4, and 5), though a non-significant trend towards reduced risk with higher volume was observed (Online Table 7 and 8).
Discussion
Despite advances in non-invasive imaging technology, cardiac catheterization remains the gold standard for initial diagnosis, choice of initial pharmacotherapy, and longitudinal assessment of patients with PH (7,8). The risk of mortality with catheterization in children (across all diagnoses) has been measured in several single center series with estimates between 0.3 and 0.8% (12-15,19,24,25). Catheterization in adults with PH has been associated with a risk of mortality of 0.05% (26). The risk of cardiac arrest during cardiac catheterization in children with PH in single center series is >10-fold higher than in other children undergoing cardiac catheterization (9,10,27). The single multicenter registry study measuring the risk of adverse event in cardiac catheterizations in children with PH reported no deaths >177 cases, but the relatively small study population introduces uncertainty, with a 1-sided 97.5% confidence interval for the mortality risk of 2.1% (28).
These studies emphasize the challenge of measuring the risk of mortality in PH. As PH is a rare condition, even large volume primary children’s hospitals perform relatively few cardiac catheterization procedures in children and young adults with PH. In addition, the wide spectrum of disease severity makes appropriate adjustment for pre-procedural risk necessary, which is challenging in small study samples. Several registries have collected data on patients with PH (4,6). A single study from the Tracking Outcomes in Pediatric Pulmonary Hypertension (TOPP) registry demonstrated a risk of death following catheterization of 0.6% (95% CI: 0.2-1.3%) (29), but was not sufficiently powered to define risk factors for catastrophic adverse outcome. Also, TOPP is a voluntary registry requiring informed consent for inclusion, which potentially excluded the most severely affected patients. In the current study, we used administrative data to perform a retrospective cohort study, which, with some limitations, allowed us to measure risk of cardiac catheterization without this bias from a broad range of pediatric hospitals and to adjust for patient characteristics. Our population may differ in ways from those in other series, especially those focused on IPAH, but may be more representative of the burden of disease that is seen in practice by the tertiary care centers who submit data to PHIS. The adjusted risk of death or initiation of mechanical circulatory support within 1 day of cardiac catheterization was 2.6-3.3%, significantly higher than previously reported risk of similar outcomes with cardiac catheterization in children (24) and that reported in adults with PH (11-20). The discrepancy between the high risk of catastrophic adverse outcome and the relative technical simplicity of these procedures (unlike right heart catheterization and endomyocardial biopsy in children following orthotopic heart transplantation, which has a very low risk of adverse outcome [18,24,30]) highlighted that patient-associated factors are of greater import than procedural characteristics in determining overall risk in this case.
This study identified patient-specific factors that may be useful in risk-stratifying patients. As noted in previous studies (17,31-33), infants and especially premature infants with PH had a higher risk than other children with generally lower risks with increasing age. Exceptions to this pattern were neonates without prematurity, infants with history of prematurity, and subjects >18 years of age, in whom risk was not significantly different from school-age children. These subpopulations were relatively small and (<5% of the total study population), and the failure to demonstrate differences may have been due to insufficient statistical power.
In addition, several markers of increased severity of illness prior to catheterization were independently associated with adverse outcome, including hemodialysis, receipt of systemic vasodilators, and previous cardiac operation during the same admission. These presumably are markers of hemodynamic instability and general frailty.
Transcatheter intervention was associated with a lower risk of adverse outcome. This is counter-intuitive since conventional wisdom suggests that risk should be tied to intervention. However, we found a similar decrease in risk in a broader cohort of children undergoing catheterization (24). Interventions may have been more likely in patients who are relatively well (with less chance of attempting an intervention in patients with more severe illness). Performance of an intervention may have indicated a reversible anatomic issue, with the performance of an intervention improving patient outcome.
The association of chronic medications with decreased risk of adverse events should be interpreted with care. Diagnostic and treatment algorithms recommend that patients undergo catheterization prior to initiation of these therapies, so patients receiving these treatments prior to catheterization have necessarily survived at least one catheterization and have likely demonstrated some responsiveness to pulmonary vasodilators, leading to selection bias. Patients receiving PH medications and undergoing subsequent catheterization may also have a different risk profile. Further measures to account for confounding by indication are necessary to clarify the relationship between pulmonary vasodilators and risk of catastrophic adverse outcome.
A secondary analysis identified an association between center volume and the risk of early catastrophic adverse outcome. The protective effect of center catheterization volume for catheterization has been demonstrated previously across the range of procedures and diagnoses (24). Additional research is necessary to identify whether this protective effect is due to dispersible best practices or economy of scale.
Limitations
There are several limitations to this study. Identification of subjects relied on ICD-9 codes from administrative data. As evidenced by a large number of subjects not receiving medications and a large number of APAH-CHD patients, the population described in this study may differ from those cited in other studies (especially those focused on IPAH). The detail available especially in regards to the specific anatomy of patients with APAH-CHD was limited. Post hoc analyses were performed to determine the degree to which APAH-CHD patients influenced the risk of catastrophic outcome. The PHIS database is limited to catheterizations that are part of an observation or inpatient admission. It does notably include procedures with subsequent hospital admission or death in the catheterization laboratory. This may have resulted in an over-estimation of risk. Although most procedures for PH are performed with post-procedural inpatient observation, there are centers that do not admit patients for observation post-catheterization, especially those with mild disease. Hemodynamic and oximetric data from the procedure as well as additional procedural (e.g., method of anesthesia) and additional clinical data (e.g., imaging studies), all of which might affect risk, were not available. The database has a temporal resolution of a single day of service. Choosing death or initiation ECMO on the day of catheterization and the following date of service as the primary outcome allowed us to capture all events that occurred from the date of catheterization to >24 hours (while including events that might be as much as >48 hours). Finally, it was not possible to determine the degree to which observed adverse events are the direct result of the catheterization (as opposed to the result of progressive disease or other events). In a previous study death before discharge was attributable to catheterization in 10% of cases (33). Thus, the risks calculated are necessarily inflated, but we hoped to mitigate this by limiting the time horizon as described previously (24).
Conclusions
The risk of catastrophic adverse outcome following cardiac catheterization in children with PH was significantly higher than that in children with other diagnoses. Younger age, pre-procedural systemic vasodilators, and cardiac operation within the same admission all increased the risk of an adverse event, while treatment with PH medications was associated with reduced risk. Further study is necessary to determine how these factors interrelate and whether a predictive would improve outcomes of children and young adults undergoing cardiac catheterization for PH. Although administrative databases have limitations, this study provides information that may inform design and interpretation of future studies, whether they use multicenter registry data or datasets that combine clinical and administrative data.
Supplementary Material
PERSPECTIVES.
Competency in Medical Knowledge
Cardiac catheterization in children and young adults with pulmonary hypertension is associated with higher risks of death or circulatory collapse than when performed for other diagnoses. Adverse outcomes are more frequent in younger patients and less common in those receiving pulmonary vasodilator medications.
Competency Interpersonal and Communication Skills
When discussing cardiac catheterization procedures for children with pulmonary hypertension, physicians should explain the risk of catastrophic outcomes to patients (when appropriate) and their families in the context of balancing risks against potential benefits.
Translational Outlook
This multicenter cohort study may lead to a better understanding of the factors that are associated with risk of adverse events with catheterization in children with pulmonary hypertension.
Central Illustration: Catheterization in Children with Pulmonary Hypertension: Forest Plot of Multivariable Analysis of Risk Factors for Catastrophic Adverse Outcome.
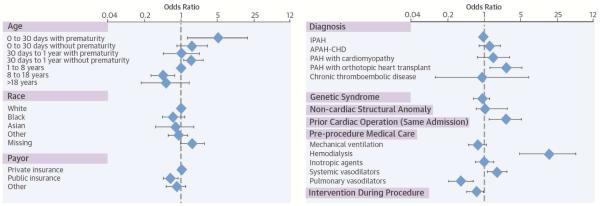
These results assess process within 1 day from catheterization, and depict odds ratios from mixed-effects multivariate generalized linear models.
Acknowledgements
The authors acknowledge Jin Long (Center for Pediatric Clinical Effectiveness Healthcare Analytics Unit at The Children’s Hospital of Philadelphia) for his role as a programmer who performed the query from PHIS.
Funding sources: Dr. O’Byrne receives support from the NIH [T32 HL007915] and Entelligence Young Investigator grant. Dr. Kawut is supported by the National Institutes of Health [K24 HL103844].
Abbreviations
- CCB
calcium channel blockers
- CTEPH
chronic thromboembolic pulmonary hypertension
- ERA
endothelin receptor antagonists
- IPAH
idiopathic pulmonary arterial hypertension
- ICD-9
International Classification of Disease, ninth revision code
- IQR
interquartile range
- PHIS
Pediatric Health Information Systems Database
- PDE-5
Phosphodiesterase-5
- PH
pulmonary hypertension
- APAH-CHD
pulmonary arterial hypertension associated with congenital heart disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Relationship with industry: Dr. Dori receives research support from Siemens. Dr. Gillespie is a consultant for Medtronic. Doctors Glatz and Rome are consultants for Bristol Myers Squib Inc. Dr. Hanna receives research support from Ely Lily, United Therapeutics, Gilead, and Actelion. The other authors have nothing to disclose.
Reference
- 1.van Loon RLE, Roofthooft MTR, Hillege HL, et al. Pediatric Pulmonary Hypertension in the Netherlands Clinical Perspective Epidemiology and Characterization During the Period 1991 to 2005. Circulation. 2011;124:1755–1764. doi: 10.1161/CIRCULATIONAHA.110.969584. [DOI] [PubMed] [Google Scholar]
- 2.Moledina S, Hislop AA, Foster H, et al. Childhood idiopathic pulmonary arterial hypertension: a national cohort study. Heart. 2010;96:1401–1406. doi: 10.1136/hrt.2009.182378. [DOI] [PubMed] [Google Scholar]
- 3.Fraisse A, Jais X, Schleich J-M, et al. Characteristics and prospective 2-year follow-up of children with pulmonary arterial hypertension in France. Arch Cardiovasc Dis. 2010;103:66–74. doi: 10.1016/j.acvd.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Barst RJ, McGoon MD, Elliott CG, et al. Survival in Childhood Pulmonary Arterial Hypertension: Insights From the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management. Circulation. 2012;125:113–122. doi: 10.1161/CIRCULATIONAHA.111.026591. [DOI] [PubMed] [Google Scholar]
- 5.Haworth SG, Hislop AA. Treatment and survival in children with pulmonary arterial hypertension: the UK Pulmonary Hypertension Service for Children 2001-2006. Heart. 2008;95:312–317. doi: 10.1136/hrt.2008.150086. [DOI] [PubMed] [Google Scholar]
- 6.Berger RMF, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379:537–546. doi: 10.1016/S0140-6736(11)61621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonneau G, Galiè N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:S5–S12. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 8.Galiè N, Torbicki A, Barst R, et al. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Eur Heart J. 2004;25:2243–2278. doi: 10.1016/j.ehj.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Carmosino MJ, Friesen RH, Doran A, et al. Perioperative Complications in Children with Pulmonary Hypertension Undergoing Noncardiac Surgery or Cardiac Catheterization. Anesth Analg. 2007;104:521–527. doi: 10.1213/01.ane.0000255732.16057.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor CJ, Derrick G, McEwan A, et al. Risk of cardiac catheterization under anaesthesia in children with pulmonary hypertension. Br J Anaesth. 2007;98:657–661. doi: 10.1093/bja/aem059. [DOI] [PubMed] [Google Scholar]
- 11.Bennett D, Marcus R, Stokes M. Incidents and complications during pediatric cardiac catheterization. Paediatr Anaesth. 2005;15:1083–1088. doi: 10.1111/j.1460-9592.2005.01677.x. [DOI] [PubMed] [Google Scholar]
- 12.Cassidy SC, Schmidt KG, Van Hare GF, et al. Complications of Pediatrc Cardiac Catheterization: A 3-Year Study. J Am Col. Cardiol. 1992;19:1285–1293. doi: 10.1016/0735-1097(92)90336-l. [DOI] [PubMed] [Google Scholar]
- 13.Kirkpatrick SE, Takahashi M, Petry EL, et al. Percutaneous heart catheterization in infants and children: II. Prospective study of results and complications in 127 consecutive cases. Circulation. 1970;42:1049–1056. doi: 10.1161/01.cir.42.6.1049. [DOI] [PubMed] [Google Scholar]
- 14.Mehta R, Lee K-J, Chaturvedi R, et al. Complications of pediatric cardiac catheterization: A review in the current era. Cathet Cardiovasc Intervent. 2008;72:278–285. doi: 10.1002/ccd.21580. [DOI] [PubMed] [Google Scholar]
- 15.Vitiello R, McCrindle BW, Nykanen D, et al. Complications associated with pediatric cardiac catheterization. J Am Coll. Cardiol. 1998;32:1433–1440. doi: 10.1016/s0735-1097(98)00396-9. [DOI] [PubMed] [Google Scholar]
- 16.Bergersen L, Gauvreau K, Lock JE, Jenkins KJ. A risk adjusted method for comparing adverse outcomes among practitioners in pediatric and congenital cardiac catheterization. Cong Heart Dis. 2008;3:230–240. doi: 10.1111/j.1747-0803.2008.00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergersen L, Gauvreau K, Foerster SR, et al. Catheterization for Congenital Heart Disease Adjustment for Risk Method (CHARM) JACC Cardiovasc Interv. 2011;4:1037–1046. doi: 10.1016/j.jcin.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Bergersen L, Gauvreau K, Marshall A, et al. Procedure-type risk categories for pediatric and congenital cardiac catheterization. Circulation: Cardiovascular Interventions. 2011;4:188–194. doi: 10.1161/CIRCINTERVENTIONS.110.959262. [DOI] [PubMed] [Google Scholar]
- 19.Bergersen L, Marshall A, Gauvreau K, et al. Adverse event rates in congenital cardiac catheterization - a multi-center experience. Catheter Cardiovasc Interv. 2010;75:389–400. doi: 10.1002/ccd.22266. [DOI] [PubMed] [Google Scholar]
- 20.Bergersen L, Gauvreau K, Jenkins KJ, et al. Adverse event rates in congenital cardiac catheterization: a new understanding of risks. Cong Heart Dis. 2008;3:90–105. doi: 10.1111/j.1747-0803.2008.00176.x. [DOI] [PubMed] [Google Scholar]
- 21.Pasquali SK, Hall M, Li JS, et al. Corticosteroids and outcome in children undergoing congenital heart surgery: analysis of the Pediatric Health Information Systems database. Circulation. 2010;122:2123–2130. doi: 10.1161/CIRCULATIONAHA.110.948737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Applied Longitudinal Analysis (Kindle Edition) 2nd Wiley; Hoboken: 2011. Overview of Linear Models for Longitudinal Data; pp. 1961–2888. [Google Scholar]
- 23.Applied Longitudinal Analysis (Kindle Edition) 2nd Wiley; 2011. Generalized Linear Mixed Effects Models; pp. 10334–11453. [Google Scholar]
- 24.O'Byrne ML, Glatz AC, Shinohara R, et al. Effect of Center Catheterization Volume on Risk of Catastrophic Adverse Event Following Cardiac Catheterization in Children. American Heart Journal. doi: 10.1016/j.ahj.2015.02.018. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohn HE, Freed MD, Hellenbrand WF, et al. Complications and mortality associated with cardiac catheterization in infants under one year: a prospective study. Pediatr Cardiol. 1985;6:123–131. doi: 10.1007/BF02336550. [DOI] [PubMed] [Google Scholar]
- 26.Hoeper MM, Lee SH, Voswinckel R, et al. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J. Am. Coll. Cardiol. 2006;48:2546–2552. doi: 10.1016/j.jacc.2006.07.061. [DOI] [PubMed] [Google Scholar]
- 27.Williams GD, Maan H, Ramamoorthy C, et al. Perioperative complications in children with pulmonary hypertension undergoing general anesthesia with ketamine. Paediatr Anaesth. 2010;20(1):28–37. doi: 10.1111/j.1460-9592.2009.03166.x. [DOI] [PubMed] [Google Scholar]
- 28.Hill KD, Lim DS, Everett AD, et al. Assessment of pulmonary hypertension in the pediatric catheterization laboratory: Current insights from the magic registry. Cathet. Cardiovasc. Intervent. 2010;76:865–873. doi: 10.1002/ccd.22693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barst RJ, Beghetti M, Berger R, et al. Complications related to heart catheterization (HC) in children with Pulmonary Hypertension (PH): Factors identified from the TOPP Registry (Tracking Outcomes and Practice in Pediatric PH); Abstract presented at the American Thoracic Society Meeting; Philadelphia PA. 2013. [Google Scholar]
- 30.Daly KP, Marshall AC, Vincent JA, et al. Endomyocardial biopsy and selective coronary angiography are low-risk procedures in pediatric heart transplant recipients: results of a multicenter experience. J Heart Lung Transplant. 2012;31:398–409. doi: 10.1016/j.healun.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutton N, Lock JE, Geggel RL. Cardiac catheterization in infants weighing less than 1,500 grams. Cathet Cardiovasc Intervent. 2006;68:948–956. doi: 10.1002/ccd.20905. [DOI] [PubMed] [Google Scholar]
- 32.Schneider DJ, Moore JW. Interventional cardiac catheterization in very small infants. Prog Pediatr Cardiol. 2001;14:27–33. [Google Scholar]
- 33.Backes CH, Cua C, Kreutzer J, et al. Low weight as an independent risk factor for adverse events during cardiac catheterization of infants. Catheter Cardiovasc Interv. 2013;82:786–794. doi: 10.1002/ccd.24726. [DOI] [PubMed] [Google Scholar]
- 34.Backes CH, Bergersen L, Rome JJ, et al. Quality metrics in cardiac catheterization for congenital heart disease: Utility of 30-day mortality. Cathet Cardiovasc Interv. 2014:1–7. doi: 10.1002/ccd.25683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.