Abstract
Multicomponent, synergistic and multifunctional nanostructures have taken over the spotlight in the realm of biomedical nanotechnologies. The most prospective materials for bone regeneration today are almost exclusively composites comprising two or more components that compensate for the shortcomings of each one of them alone. This is quite natural in view of the fact that all hard tissues in the human body, except perhaps the tooth enamel, are composite nanostructures. This review article highlights some of the most prospective breakthroughs made in this research direction, with the hard tissues in main focus being those comprising bone, tooth cementum, dentin and enamel. The major obstacles to creating collagen/apatite composites modeled after the structure of bone are mentioned, including the immunogenicity of xenogeneic collagen and continuously failing attempts to replicate the biomineralization process in vitro. Composites comprising a polymeric component and calcium phosphate are discussed in light of their ability to emulate the soft/hard composite structure of bone. Hard tissue engineering composites created using hard material components other than calcium phosphates, including silica, metals and several types of nanotubes, are also discoursed on, alongside additional components deliverable using these materials, such as cells, growth factors, peptides, antibiotics, antiresorptive and anabolic agents, pharmacokinetic conjugates and various cell-specific targeting moieties. It is concluded that a variety of hard tissue structures in the body necessitates a similar variety of biomaterials for their regeneration. The ongoing development of nanocomposites for bone restoration will result in smart, theranostic materials, capable of acting therapeutically in direct feedback with the outcome of in situ disease monitoring at the cellular and subcellular scales. Progress in this research direction is expected to take us to the next generation of biomaterials, designed with the purpose of fulfilling Daedalus’ dream - not restoring the tissues, but rather augmenting them.
Keywords: Apatite, Bone, Calcium phosphate, Nanoparticle, Scaffold, Tissue engineering
Graphical abstract
1. Introduction
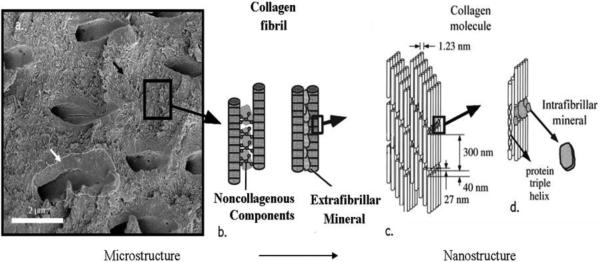
Because of the continuously aging human population on Earth and the corresponding rise in the incidence of hard tissue diseases1, increasing incentives exist to develop methods for minimally invasive regeneration of dysfunctional mineralized tissues. Complications faced by bioengineers in attempts to recreate and regenerate impaired hard tissues are, however, numerous and they appear logical in view of the fact that hard tissues are Nature’s most intricate materials in the classical sense of the word. Their variety within the human body itself is relatively large and the example demonstrating the extraordinary complexity of even arguably the simplest one of them, the tooth enamel, is presented in Fig.1. The microstructure of enamel, the strongest, but also the most brittle hard tissue in the human body2, is dominated by rod-shaped bundles of apatite fibers whose aspect ratio reaches up to 3 · 104 and which are arranged perfectly parallel to each other. Enamel is also 96 - 98 wt% mineral in composition, while water, lipids and various peptides, generally treated as impurities with no functional role at all, account for the rest 2 – 4 wt%. In contrast to enamel, dentin and bone are both collagenous composites and it is usually presumed that there is little or no difference between them at the nanometer scale3. A substantial difference, however, exists at the micro scale. Namely, while bone is composed of parallel arrays of osteons, each one of which is a laminated cylindrical structure, ~ 200 μm in diameter, wherein individual lamellae contain uniaxially oriented collagen fibers mineralized by nanoscopic apatite platelets, dentin is composed of tubules with hypermineralized edges and ~ 1 - 2 μm in diameter interspersed inside of the intertubular matrix whose composition at the submicron scale is identical to that of bone (Fig.2). One finds here type I collagen fibrils reinforced by the nanosized intrafibrillar and extrafibrillar plate-shaped apatite crystals4. Tooth cementum, the fourth and the final apatitic hard tissue in the human body, is another collagenous composite with apatite as the mineral phase5, containing also considerable amounts of proteoglycans and a minor proportion of glycoproteins6.
Fig.1.
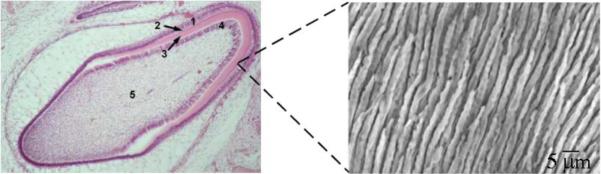
Histological section of the developing human tooth in the maturation stage (left) and a micrograph showing parallel arrangement of enamel rods (right). 1 – ameloblasts; 2 - enamel; 3 – dentin; 4 – odontoblasts; 5 – pulp. Each enamel rod is composed of a myriad of thin apatite fibers, each with approximately 40 – 60 nm in diameter.
Fig.2.
(a) Scanning electron micrograph of a slice of dentin showing tubules, peritubular mineral (white arrow) and the collagenous intertubular matrix (black arrow); (b) Collagen fibrils interconnected by noncollagenous proteins and extrafibrillar mineral; (c) Collagen molecules display a typical spacing of 67 nm. Gap region equals 40 nm and the overlap region is 27 nm in length, which together gives the typical periodicity of 67 nm; (d) Intrafibrillar mineral particles are shown positioned in the gap region between collagen molecules. Reprinted with permission from Ref.7.
All hard tissues in the human body are composites. Even the tooth enamel, whose organic ingredients used to be treated as impurities, is nowadays increasingly classified as a composite structure8. An argument given in support of this is that even the entrapment of a two orders of magnitude lower concentration of macromolecules than that present in enamel would markedly increase its strength9. This particularly applies to its toughness, as a hypothetic enamel made of apatite only is expected to be far more brittle along specific crystallographic directions than natural enamel. A biological material referable to for the sake of supporting this argument is the spine of sea urchin, which contains only 0.02 wt% of glycoproteins (~ 1 protein molecule per each 105 unit cells). Although this amount is significantly lesser than the 3 wt% of organics in nacre10, it appears to be large enough to efficiently absorb the energy from propagating cracks and thus markedly enhance the resistance of the material to fracture11.
All hard tissues in the human body are also nanostructures. For a material to receive the attribute of a “nanostructure” in the domain of chemical engineering, it has to be composed of particulate units whose at least one spatial dimension does not exceed 100 nm. Biomedical engineering has modified this definition over the years by pushing this critical size limit up to 1 μm. Therefore, what regularly classifies as a nanostructure in the medical literature need not be a nanomaterial according to the convention established by materials scientists. As per the standard definition, even though the length of the apatite fibers in enamel reaches between 100 μm and 1 mm, their diameter is in the range of 40 – 60 nm, which classifies enamel as a nanostructure. The same can be said for dentin and bone, both of which are composed of apatite crystals with the average dimensions of 30 × 20 × 2 nm12. Nanocomposites, the subjects of this review, are by definition “multiphase solid materials where one of the phases has one, two or three dimensions of less than 100 nanometers”13. Accordingly, even a material whose bulk is composed of micrograins but whose thin film coating is nanoparticulate in nature classifies as a nanocomposite. More than one of such materials will be elaborated in this review.
Due to the aforementioned versatility of hard tissue structures in the body, including that within bone itself (cancellous, a.k.a. trabecular or spongy bone, comprising 20 % of bone weight, is, for example, far more porous and vascular than cortical bone, having approximately ten times higher specific surface area than the latter), different methods and materials are required to regenerate the impaired hard tissues of different type. To that end, no perfect material exists, as every one of them suffers from specific weaknesses. Rule of thumb, excluding the exceptions, says that metals have superior mechanical properties, but the elastic modulus mismatch can lead to adjacent bone resorption and inferior biomechanical integration, let alone that ultra-corrosive magnesium alloys are the only biodegradable metals that could be used in sustained drug delivery. Ceramics have excellent bioactivity, good tissue integration properties and easily controllable bioresorption profiles, but low tensile strength and unsatisfactory toughness cause problems for load-bearing applications. Polymers are perhaps more versatile than any other type of materials in bone engineering, the reason for which they are more applied as biomaterials than any other material type14, having high flexibility and resistance to failure due to fatigue, but the necessity of sacrificing strength on the account of biodegradability disfavors their sole use as bone substitutes.
Another general bioengineering principle is that a single biomaterial cannot prove to be ideal for two different applications in the body15. Materials successfully applied for the repair of cartilage have been, for example, notoriously inefficient in healing the subchondral bone and vice versa, which has led to the drawing of a firm line between materials for chondral regeneration and the materials for osteochondral regeneration. Drug delivery materials that release drugs at a moderate and sustained rate, with zero-order kinetics, may work well for osteoporotic patients, but may not be applicable in the treatment of osteomyelitis, where a burst release and a higher order kinetics proves to be more desirable16. Relatively small particles (~ 50 nm) tend to have a higher cell uptake efficiency and a greater potential for gene therapy than their larger counterparts, but the cost alongside a lesser retention time17 is their less efficient contravention of the vascular flow and a lower level of control using external fields18, which is why differently sized therapeutic particles may prove to suit the cell-targeting treatment of the less vascular cortical bone and the more vascular trabecular bone. Then, bone is populated by biomolecules and cells specialized for different, often mutually antagonistic roles: bone-depositing osteoblasts and bone-resorbing osteoclasts, nucleation-promoting osteocalcin and nucleation-hindering osteopontin, bone morphogenetic protein-7 and transforming growth factor-β219, proteoglycans and matrix metalloproteases are only some of the examples. For this reason, diametrically opposite stimuli provided by single scaffolds may prove to be necessary to maximize the osteogenic response to an implant, something that could be achieved only using complex composite materials. Nevertheless, with all hard tissues in the body being composite nanostructures, it is expected that an ideal biomaterial applicable in regeneration or substitution of the given tissues should be a composite nanostructure too. The following discourse will highlight some of the most prospective breakthroughs made in this research direction, with the hard tissues in main focus being those comprising bone.
2. Collagen/apatite composites modeled after the structure of bone
The future of all fields of engineering at the nano scale, including bioengineering, can be said to belong to composite, synergetic, multifunctional materials20. As an illustration, Fig.3 correspondingly displays the dramatic annual increase in the number of publications deposited at the US National Library of Medicine for which keywords are “bone engineering” and “composite”, from only 1 in 1980 to 4 in 1990 to 21 in 2000 to 158 in year 2010 to 260 in year 2014, more than in any year before. Single-phase nanomaterials have been explored relatively well in the past, perhaps with the exception of supramolecular constructs and stoichiometrically complex ceramics21. Consequently, as of a few years ago, multi-component nanostructures have taken over the spotlight in the realm of nanotechnologies. Bone is an example of one such material, as the strengths of both of its components compensate for the inevitable weaknesses of each: i.e., apatite crystals, strong to compression, but weak under tension and also brittle, not tough, impart high elastic modulus to the bone, while collagen yields toughness and high tensile strength to it22. The result of this combination is such that the resilience of bone is much greater than the mere sum of the mechanical properties of its basic components; hence, we are free to say that, figuratively, 1 + 1 > 2. As is the case with the tooth enamel, the exceptional stiffness and strength of bone come not only from the synergistic combination of material properties of its mineral and organic components, but from its hierarchical, multi-scale organization as well. In fact, so versatile is the hierarchical organization of bone that it has been proposed that the bone be treated not as a single material, but as a whole family of them23. With the exception of caries-affected hard tissues, which come to contain detectable levels of brushite24, apatite, a nonstoichiometric form of hydroxyapatite (HAp) in which approximately one-fifth of Ca2+ ions are substituted with cationic impurities while carbonate ions contribute to 2 - 8 % of its weight, is the only other mineral component of hard tissues in the human body.
Fig.3.
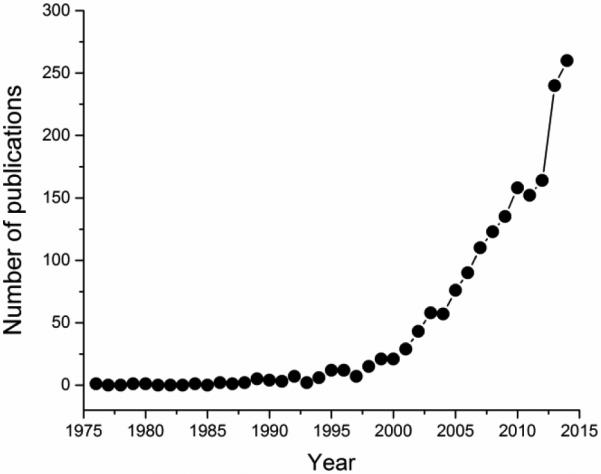
Annual number of publications deposited at the US National Library of Medicine for the period 1976 – 2014 and matching the keywords “bone engineering” and “composite”.
With bone being composed of mainly collagen and apatite, the most logical approach to fabrication of materials for bone replacement may seem to be the structural replication of bone itself25,26. Two main problems are, however, associated with this approach. First, to faithfully replicate a complex composite structure that bone is, entwinement of the intrinsic components at the micro scale is not enough27. What is needed is the replication of their exact interaction at the finest, molecular scale. Only after this is accomplished can more complex bony structures be expected to be built. For, although there is a great variety in the superstructural ordering of mineralized collagen fibers depending on the bone type and species, they are all hypothesized to have been created from the building blocks and interactions that are identical at the molecular scale28. As shown in Fig.2, depending on which one of the two possible sites in the collagen matrix apatite crystals occupy, they could be either intrafibrillar or extrafibrillar, the former of which are particularly important in stabilizing the bone structure, as they fit in the discreet gap between overlapping collagen fibrils and are in no way randomly distributed throughout the protein matrix, as is the case with the extrafibrillar mineral. The filling of intrafibrillar spaces with the mineral particles typically leads to their lesser prominence during high-resolution imaging, and vice versa. There are also indications that, contrary to earlier assumptions, mineralization of collagen fibrils proceeds by filling the overlap region first and the gap region afterwards29. This suggests that the mineralization of the organic matrix of bone is a process far more intricate than it may seem at first and that an unselective deposition of apatite throughout the collagenous matrix cannot be a successful means to replicate the structure of bone at the molecular scale, which is, on the other hand, the basis of the stability of all the higher levels of its superstructural order. In vitro remineralization of dentin has consequently produced markedly weaker materials in cases when the newly deposited mineral was not chemically connected to the already present intrafibrillar apatite particles30.
The c-axis of apatite crystals in bone, [001], is known to be oriented parallel to the long axis of the collagen fibrils31. Examples from catalysis have demonstrated that the exposition of different surface faces can yield a drastically different reactivity of the catalyst, and the precise crystallographic orientation of apatite crystals in bone is probably a crucial factor in determining its unique mechanical properties. The boundary between the 40 nm wide gap and the 27 nm wide overlap zone in collagen fibrils is where the onset of the mineralization is presumed to occur and Ca2+ ions furthermore tend to be positioned in the valleys between the positively charged peaks of collagen molecules. It is possible that if this exact formation mechanism is not perfectly replicated at the molecular scale, the structural failure of the resulting composite will ensue. Due to the complexity of this process, no attempts to reproduce the fine composite structure of bone by artificial means have been successful so far. A corollary of this fact is that no bone substitute for load-bearing applications is available to the orthopedic clinicians, as of today32.
Fig.4 offers a schematic description of stages in the amorphous-cluster-mediated process of incorporation of apatite crystallites into the network of collagen fibrils. Although no agreement could have been reached for more than a decade regarding whether individual ions or amorphous clusters present the growth units during biomineralization events in general, an informal consensus has been reached in the recent years that the latter mechanism more faithfully describes this complex morphogenetic process34. Accordingly, constitutive ions from the extracellular matrix coalesce and form unstable units, so-called Posner’s clusters, with an approximate Ca9(PO4)6 stoichiometry35 and 9 Å in size on average (stage 1). As a result of the inward pull experienced by the atoms attempting to compensate the undersaturated bonds, these anhydrous clusters are more compact than their crystalline counterparts, as measured by shorter Ca-O bond lengths36. The formation of these ionic clusters as precursors for the amorphous intermediates begins in the solution even under undersaturated conditions37. In vitro studies have demonstrated the tendency of these clusters to be attracted to acidic amino acid residues of proteins, in contact with which they start to aggregate, forming amorphous particles with sizes in the order of tens of nanometers38. Thus, having been nucleated in the extracellular matrix, the clusters then approach the organic surface and begin to aggregate near and on it (stage 2). Further aggregation causes densification of the growth units near the surface (stage 3) and is followed by reorganization of the clusters first into amorphous (stage 4) and then into crystalline particles (stage 5), which continue to grow via attachment of clusters or amorphous units. They, however, continuously dissolve and recrystallize, all until a specific crystallographic orientation with respect to the collagen fibrils is reached39. According to an alternative explanation proposed for calcite, amorphous particles form in the solution before they reach the water/organic interface40, with or without the help of extracellular vesicles41. In spite of the nucleation of these metastable clusters in the solution, they are still expected to form predominantly via heterogeneous nucleation under low supersaturation conditions that are present in biological milieus, given that even in the purest solutions nucleation occurs mainly on container walls, dust particles and other impurities, including ions formed by the background cosmic radiation42. Phosphorylated glycoproteins, such as osteocalcin, or proteoglycans with the affinity for Ca2+ ions can act as such feasible heterogeneous nucleation surfaces for the formation of amorphous clusters of apatite, which may subsequently detach, aggregate and become incorporated into the collagen network.
Fig.4.
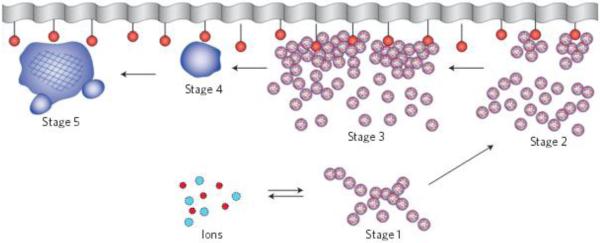
A scheme illustrating the mineralization of collagen fibrils. Reprinted with permission from Ref.33.
This model is in concert with the new model of crystal growth applicable presumably to all biomineralization processes and to a large body of synthetic crystal growths too, involving aggregation of amorphous, nano- or subnano-particulate units and their subsequent consolidation and faceting (Fig.5). Broad acceptance of this model, less intuitive than its predecessor, the diffusional one proponed most notably by La Mer et al.45,46, it is important to note, took a painful paradigm shift over two to three decades, perfectly exemplifiable by Kuhn’s classical model of paradigm shifts in science47. Namely, for a long time pioneers who proposed that monodisperse particles most frequently grow by aggregation of separately nucleated subunits48,49 were not given the credit they deserved, especially in the absence of in situ imaging methods. In the last decade, however, the paradigm shift has gradually occurred and now the new paradigm states that most particles form by (a) the growth of primary, usually amorphous units by diffusion, and (b) aggregation and rearrangement of these primary units into more crystalline and bigger particles. Depending on the experimental conditions, the classical, La Mer’s mechanism and the aggregative one are entwined to different extents and dominant at different stages of the process50. Note also that the model involving the aggregation of amorphous precursors is entropically the most favorable pathway since the transient amorphous phase is more similar in structure to the liquid phase from which it is precipitated than to more crystalline particles that present the final product of the reaction.
Fig.5.
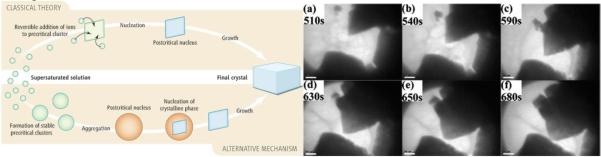
Two basic types of crystal growth: diffusional (“classical”) and aggregative (“alternative”) (left) and in situ TEM imaging of the growth of a CaCO3 crystal via aggregation of amorphous nanoparticulate precursors (right). Reprinted with permissions from Refs. 43 and 44.
That phase transitions should occur by the gradual transformation of the entropically closest phases from one to another is expected from the principle postulated by D’Arcy Thompson51 and reiterated by the Ostwald-Lussac rule52. While the ionic clusters in equilibrium with the solution form on the scale of seconds under the right conditions, the subsequent transformation from nanospherical amorphous calcium phosphate (CAP) units to somewhat uniaxially grown Ca2+-deficient octacalcium phosphate (OCP) to stoichiometric OCP to HAp takes place over the next 3 h or so53. The kinetics of this process, however, greatly depends on (a) thermodynamic factors, including supersaturation of the medium and temperature, and (b) kinetic factors, such as the presence of a foreign surface and its properties, including chemistry, charge and texture. During abrupt precipitation at ultrahigh supersaturations, the transient phases are presumed to be so short-lived that they are virtually undetectable. Collapse of the inherently unstable ionic clusters into amorphous CAP nanospheres is detectable as a drop in pH, while Ca/P ratio grows continuously throughout the process, from ~ 0.3 for the initial clusters to 1 – 1.5 for the amorphous particles to 1.67 for HAp. For this reason, the ideal biomimetic growth of apatite under low supersaturations, when the process is mainly surface-controlled, should start from a solution that possesses high initial concentrations of phosphates and low initial concentrations of Ca2+ ions54. To verify this, an AFM study arrived at conclusion that the surface step propagation velocity as a measure of the crystal growth rate of HAp is inversely proportional to Ca/P ratio in the solution55.
The second problem associated with the application of collagen as a component of bone fillers comes from its intrinsic immunogenicity56, a direct consequence of the fact that it is difficult to obtain directly from the patient and that most of it is xenogeneic in nature, while recombinant technologies as well as the methods to extract the immunogenic, telopeptide portion of collagen molecules are not only of limited availability, but also lead to reduced bioactivity of the protein57. The products of its degradation in vivo, the rate of which is often very variable, depending on the concentration of immunologically activated collagenases in the extracellular matrix surrounding the implant, have frequently been observed to lead to fibrous capsule formation58. Although collagen has been successfully applied topically, e.g., as a wound dressing carrier of antibiotics59,60,61, its mere subcutaneous epithelialization may lead to undesired immunogenic or antigenic responses62. It is also difficult to shape and process for bone graft applications, alongside being mechanically and thermally unstable. Thus, in spite of its superior cell attachment properties and the ability to mimic the extracellular matrix by directing migration, growth, differentiation and organization of cells, it does not present an ideal choice for bone replacement materials.
Neither have bioderived alternatives to collagen proven more adequate for use in bone engineering implants. For example, elastin, another insoluble extracellular matrix protein, adopting a covalently cross-linked random coil conformation, being a key to its rubbery meshwork elasticity, is difficult to obtain free of globular protein contamination, while it has also caused severe calcifications in vivo owing to its excessive propensity to promote mineralization63. Silk fibers produced by Bombyx mori, having five times higher tensile strength (650 MPa) and Young’s modulus (15 GPa) than collagen, are another natural compound considered for bone filling applications64. Silk coatings on biphasic CAP scaffolds, for example, significantly improved their elasticity and toughness, though still far from the range of trabecular bone65. Silk fibers are predominantly composed of two proteins: 75 wt% β-sheet-rich and thus water-insoluble fibroin on the inside and 25 wt% water-soluble sericin on the outside. However, the same problem of immunogenicity evident for xenogeneic collagen has been reported to entail the topical application of sericin on wound sites66. The reason is that silk comes with a large concentration of impurities - 1.5 % being carbohydrates, 0.8 % waxes, 0.7 % inorganic salts and 0.2 % pigments. Spider silk, which is even stronger to tension than that spun by Bombyx mori, having nominal fracture strength of up to 1.1 GPa, suffers from another weakness; namely, unlike Bombyx mori, spiders cannot be directed by domestication to produce large amounts of silk because of their aggressive territoriality67 and attempts to use recombinant techniques in transgenic silkworm, bacteria, yeast and other organisms have been only partially successful68. Chitosan, the water-soluble derivative of chitin, yet another polymer of natural origins and the second most abundant organic material next to cellulose, easily extractable from fungal cell walls and crustacean shells, the byproducts of food industry69, suffers from similar demerits, as expensive purification methods that eliminate impurities that may cause adverse biological reactions both in vitro and in vivo need to be implemented to ensure its safe clinical application. Unlike polyesters, poly(ethylene glycol), gold or silica, chitosan, like poly(L-lysine), is also positively charged, which contributes to its higher reactivity in contact with the oppositely charged cell membrane70,71,72 and the ability to disrupt the epithelial tight junction73,74. Illustrative of the great mechanical potentials of chitosan composites is the fact that the one biological material with the tensile strength higher than that of spider silk, in the range of 3 – 6.5 GPa, are limpet teeth wherein chitin matrix is reinforced by goethite nanofibers75.
This is all to say that biomimetics stands for a very practical idea, although sometimes it is wise to think ahead of Nature and conceive of structures that would be inspired by it and at the same time transcend it. This is in line with the evidence coming from cognitive sciences, showing that no evolution could be made possible if imitation out of empathy and respect were not combined with strivings for originality and uniqueness76. All of this might allure us to think that artificial materials must be more prospective hard tissue engineering options than the natural ones. Still, it would be a mistake to fall prey to the “single compound phobia”77 proponed by the NIH and disvalue the immense potential of natural materials, which in the short run might have even outweighed that of their synthetic counterparts had it not been for the general dislike of the natural medicines approach amongst the synthetically biased biomaterials community. In fact, natural composites comprise a special subset of biomaterials that has been used as bone grafts with success. For example, formation of a silica network in waste obtained from the production of beer, a.k.a. bagasse, yielded a beer-born bone graft usable for a variety of orthopedic treatments78, whereas processed skeleton of marine algae harvested off the coast of South Africa has been used in the clinic as an implant for maxillary sinus floor augmentation and other bone grafting procedures79,80. Moreover, the therapeutic potency of natural materials, many of which could serve as excellent models to replicate in the lab using an array of different biomimetic strategies, has not been tapped into fully yet and countless phycogenic biomaterials could still be made and proven superior over their synthetic analogs, let alone auto-, allo- and xeno-grafts, which still present gold standards for bone replacement materials. Biomaterials obtained from natural sources are typified by an extremely long list of ingredients, most of which are present as trace elements (< 0.01 wt%), acting in synergistic ways that cannot be replicated yet in their synthetic counterparts. However, this versatility of components, mimicking that present in biological systems with which they are meant to interface, presents both their strongest advantage and disadvantage. For, while microelements could foster bone growth by mechanisms not fully elucidated yet, they could also provoke an immune response in the host and lead to severe inflammation and implant failure. Still, despite the problems entailed by the low level of control of their chemical content and biological response, theirs is still a path worth following.
3. Polymeric/CAP composites
As is the case with pure collagen matrix, biodegradable polymers are broken down by hydrolysis into resorbable or excretable segments, but lack the mechanical properties required for load-bearing applications. Their combination with sturdier components is thus necessary to make their application in the clinical repair of most hard tissues feasible. Ideally, the polymeric matrix of higher plasticity and toughness is to inhibit the propagation of dislocations and cracks originating in the core ceramic grains, fibers or layers of higher hardness and brittleness. Following this R&D route, we may come closer to the ideal of a biomaterial that is both biodegradable and mechanically strong to replace bone. Polymer/CAP composites present one class of artificial composite materials that has attracted a particular attention of bone engineers owing to their wide range of unique properties, particularly in terms of their ability to emulate the soft/hard composite structure of bone. The combination of viscoelastic properties of the polymers and osteoconductivity of CAPs has yielded materials that surpassed the resistance to fracture, structural integrity and stiffness of the individual components81, making up for the low compressive strength of the former and brittleness and the lack of malleability of the latter82. With ceramics being generally frail when loaded in tension or shear modes, the preferred form of application of ceramics is particulate, as opposed to fibrous or laminar. Inorganic nanoparticles incorporated to a polymeric network have thus been shown to improve an array of properties of the pure polymer, including stiffness, resistance to wear and crack propagation, compressive load-bearing capacity, overall stability and even tensile strength83. Studies have shown that osteoblasts proliferate better on surfaces stiffer than most polymers84 as well as that mesenchymal stem cells (MSCs) differentiate into neurons on soft surfaces and osteoblasts on the stiff ones85, which explains how come the dispersion of CAP particles throughout a polymeric matrix leads to an increased Young’s modulus and increased bioactivity of the composite material at the same time86. Moreover, it is a rule of thumb that biomaterials in general should be rough so as to promote cell attachment87,88, except in a few cases, including joint and some soft tissue implants for which smooth surfaces are more desirable; impregnation of polymers with inorganic nanoparticles contributes to this surface roughness and makes additional processing steps such as etching or sandblasting unnecessary. Precipitation of HAp throughout an acellular dermal matrix at low supersaturation, the process which is also known as biomimetic mineralization of scaffolds, has correspondingly yielded a markedly more viable surface for the proliferation of periodontal ligament stem cells89. Moreover, less spindle-like and more osteoblastic, polygonal morphological appearance of the cells was in favor of their greater affinity to the surface when the latter contained the ceramic particles dispersed in the matrix, while significant upregulation of two osteogenic markers - the transcription factor Runx2 and osteopontin - was detected on HAp-containing scaffolds compared to HAp-free ones after seven days of incubation. Complementing a ceramic powder with a viscous polymeric phase also allows for the direct injection of the former to the bony defect, bypassing the need for surgical implantation in certain clinical circumstances. In some cases, as when self-setting CAP cements are used in addition to a polymer in the glassy state (e.g., PLLA or PLGA at the physiological temperature) as an additional component, the roles may be reversed, with the ceramic phase being viscous and contributing to the injectable character of the composite. Moreover, the hydrolysis of polyesters exposes carboxylic acid moieties to the local biological environment90 and their potentially unfavorable effect on it could be compensated for by delivering them with the simultaneously degrading alkaline HAp91.
Unlike many other polymeric composites wherein the polymeric phase is the only one that allows for the tuning of structure, properties and performance in the synthesis stage, in the case of polymer/CAP composites it is both phases that could have an array of properties tuned depending on the preparation conditions. Thus, the mechanical and degradation properties of a polymer could be modified by controlling parameters such as cross-linking degree, porosity, the ratio and the arrangement of different monomeric units in copolymers, chain defects, the amount of adsorbed water in hydrogels and the distributions of molecular weight, hydrophobicity and crystallinity, while solubility of CAPs could be similarly tuned by controlling parameters such as phase composition, crystallinity, particle size, stoichiometry, concentration of impurities, lattice strain, etc. Polymers are by default partly crystalline and partly amorphous; the more crystalline the polymer, the higher its brittleness and the lower its degradability tend to be. Highly crystalline polymers do not only have more bonds to hydrolyze compared to their amorphous counterparts, but they also limit the ingress of water to a greater extent, an effect that directly hinders their degradation and lowers the drug release rate in cases when the polymeric particles encapsulate or entrap a drug. Poly(α-hydroxy esters) are a family of biodegradable polymers studied for bone and tooth engineering applications perhaps more than any other synthetic polymer and approved for human use by the US Food and Drug Administration (FDA). The most widely used among them are poly(L-lactic acid) (PLLA), poly(glycolid acid) (PGA), and their combination, poly(lactic-co-glycolic acid) (PLGA). While PLLA has a relatively lengthy degradation time scale, ranging between 10 months and 4 years92 depending on the degree of crystallinity, an increase in the PGA content shortens it down to a couple of months only for PLGA 50:50, before the degradation time begins to soar again at weight ratios approaching that of pure PGA93,94, for which it equals anywhere between 6 and 12 months. Polyurethanes, likewise, offer an easy control over the elasticity and the degradation rate through the carbonate-to-ester bond ratio95 and through the ratio between the aromatic and non-biodegradable, so-called “hard” segments and the aliphatic and biodegradable, so-called “soft” ones. In the case of CAPs, one could control their aqueous solubility within a wide range, spanning from ultrahigh to sparse, using the following phase composition sequence: monocalcium phosphates (MCP) > dicalcium phosphates > calcium pyrophosphate > tricalcium phosphates (TCP) > amorphous CAP > OCP > HAp96. The solubility of different CAP phases is in most cases directly proportional to their degradation rate following implantation. For example, the implantation of a biphasic ceramic composed of HAp and β-TCP into rabbit tibias resulted in the resorption of only about 5 % of HAp and 85 % of β-TCP after three months97. Multiple authors, therefore, consider phase mixtures of various CAPs as ideal bone grafts, including the most commonly applied material of such type, biphasic CAP composed of sparsely soluble HAp and moderately soluble TCP in different proportions. In some cases, however, more soluble CAP phases can be expected to undergo surface reprecipitation of a less soluble phase, which would protect the soluble bulk from contact with water and prolong the period of stability of the material in the body. Alongside its propensity to hinder the implant replacement with the newly ingrown bone, this effect is also particularly critical for drug delivery applications where the biodegradation is a kinetic factor that antecedes the release of the drug into the local environment.
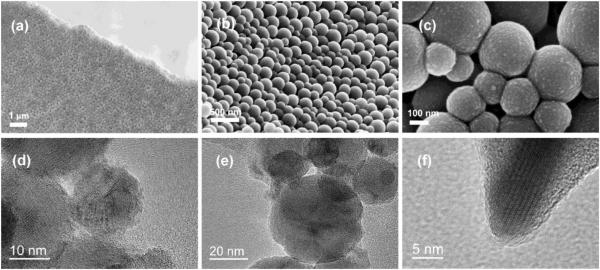
Just as in the case of collagen/CAP composites where the intimacy of the contact between the two phases determines the stability and other properties of the resulting material, the same applies to polymer/ceramic composites in general. Unselectively interspersing CAP particles throughout the polymeric matrix thus presents a less convenient solution than utilizing a more time-consuming approach whereby hydrolysis of the polymer, e.g. PLLA, is allowed to provide nucleation sites for CAP crystallites. Interestingly, irregularly shaped particles are favored because of allowing for the tighter interlocking of polymeric coils around them98, which explains why apatite particles in bone are plate-shaped, not spherical, and typified by ruffled topographies, too. Preventing agglomeration, though, a pervasive problem for most nanoparticulate formulations, driven by unfavorably high surface energies, is an ever present challenge and various surface treatments of CAP were proposed as a solution, notwithstanding the extent to which they would interfere with the impregnation process. These dispersion problems are particularly challenging when the aim is to uniformly intersperse hydrophilic nanoparticles within hydrophobic polymers, a task that routinely requires the usage of surface active agents or chemical functionalization of the polymer with reactive moieties. The combination of solvents and non-solvents of appropriate polarity can transcend these obstacles and provide conditions for simple precipitation of composite structures with a fine level of phase dispersion and homogeneity. Fig.6 correspondingly displays compact and nonporous composite nanoparticles of PLGA and HAp obtained by a sequential precipitation of the two components in a method that is both ecologically and geopolitically sound - the former because of its reliance on eco-friendly chemicals and the latter owing to its inexpensiveness, technological simplicity and the corresponding facileness with which it could be transferred from the developed to the developing world stage settings. Another advantage of this composite comes from its comparatively high drug loading capacity, with both components being able to capture significant amounts thereof – HAp via surface adsorption and PLGA via bulk entrapment. Although the fact that HAp has been used in purification systems and in chromatography for the separation of proteins99, nucleic acids100 and microorganisms101 is already a good indicator of its excellent drug adsorption capacities, perhaps the best illustration of its binding potential comes from Ca2+ ions sandwiched as atomic bridges between two phosphate groups, one from a DNA molecule and another one from zwitterionic dipalmitoyl-phosphatidylcholine, in DNA-immobilized thin films self-assembled using the simple Langmuir-Blodgett technique102. Multiple polymers other than the polymers of lactic and glycolic acids are commonly used in combination with CAP or with other hard components in composites for hard-tissue engineering applications. These include non-biodegradable polymers such as polysiloxanes103, poly(methyl methacrylate)104 and other acrylics105, polyethylene106 and polypropylene107 among olefins and poly(vinyl alcohol)108 among haloalkanes. Biodegradable polymers used in combination with CAP include hyaluronic acid109,110 and chitosan111 as the commonest biological choices, poly(ε-caprolactone)112,113 as another popular polyester, various polyanhydrides and some of the more rarely used polymers, counting poly-p-dioxanone114, poly(trimethylene carbonate)115, poly(ethylene oxide terephthalate)-poly(butylene terephthalate) block copolymer116, and many others.
Fig.6.
FE-SEM (a-c) and HR-TEM (d-f) micrographs of particles comprising HAp nanoparticles interspersed within bigger PLGA spheres. Reprinted with permission from Ref.117.
4. Other composite materials
Hard tissue engineering composites have been created using hard components other than CAPs. As furtherance of the oldest and the most common generation of composites used in reparative dentistry, composites reinforced by silica particles118 or bioactive glass119 present a particularly popular class of such materials. Various forms of bioactive glass have been shown as promising candidates for bone replacement composites; for example, foaming of gelatin and an amorphous mixture of SiO2 and CaO resulted in three-dimensional structures with the pore size in the 100 – 200 μm range, convenient for the internal culturing of stem cells120. Bioactive glass with the SiO2:CaO molar ratio of 5 also slightly improved the compressive strength of PLGA scaffolds and the osteogenic response of MSCs seeded on them121. Compared to CAP nanoparticles typified by swift reorganization of the surface layers and the virtual impossibility of their chemical functionalization, silanol groups on the surface of silica nanoparticles offer a far greater stability and more facile functionalization with organic molecules122. Silicon is also present in the newly formed bone in the amount of 0.5 wt%123, and the biological response to implantation of Si-doped HAp was more positive than for pure HAp124,125.
Nanotubes present another class of materials considered for application in composites for hard tissue engineering. For example, carbon nanotube dispersion in a fumarate-based polymer yielded a material with excellent mechanical performance126; however, the fate of such nanotubes in the body is uncertain, as it is difficult to predict based on the implant location whether they would degrade, migrate elsewhere or be inert. Tungsten disulfide127 and titania128 nanotubes have also been investigated for a potential application in polymeric composites for bone replacement. Titania nanotubes have attracted a particular attention because of the comparative ease with which they could be created as a coating on the titanium implant surface via electrochemical anodization129. This process is both (a) an alternative to regular etching procedures that are supposed to increase the intimacy of the tissue/implant interface and that precede a typical surgical implantation and (b) a mechanism to enable the implant to be loaded with sustainably releasing drugs. In support of this approach, a significantly lower thrombogenicity was detected on titania nanotube arrays, as compared to biomedical-grade titanium, after contact with blood plasma130. Titania nanotubes have also been shown to be capable of releasing proteins or antibiotics for hours when immersed in the physiological solution131,132. The main problem associated with drug delivery using titania nanotubes comes from their practical limitation to the first-order release kinetics, typified by the burst release of the drug in the first couple of hours. Despite the claims that nanotube diameters comparable to the size of the drug molecules would result in zero-order release kinetics133, no such release profiles have been reported to date. Coating the titania nanotube arrays with natural or synthetic polymers that would somewhat mitigate the burst release was proposed as a solution to this problem134. As for composites comprising titania nanotubes, another interesting approach is that based on arrays of titania nanotubes loaded with both regular and reverse polymeric micelles135. They were proven as capable of simultaneously delivering hydrophobic drugs, such as indomethacin and itraconazole, and hydrophilic ones, such as gentamicin, over a period of 10 days, with the drug release profiles for each drug being highly dependent on the polymeric micelle ratio.
A special family of composite materials is that comprising titanium as the main component. Although evidenced for their biocompatibility, superior elastic properties over ceramics and relatively high toughness compared to other commonly used metals, their main downside comes from their non-biodegradable nature and an extremely low level of bioactivity. Also, similar to most metals and alloys, titanium is corrosion-resistant, but not wear-resistant too, and surface scratches and erosion following implantation serve as an evidence for this effect. Although clinicians maintain that contamination with metals is mainly due to instrumentation rather than the implanted metal, long-term exposure to wear debris has caused patients to develop inflammatory reactions136,137, frequently followed by periprosthetic osteolysis and implant loosening, eventually requiring arthroplasty revision138. In spite of its proven biocompatibility, the wear of titanium implants, especially in highly load-bearing applications, such as artificial hips, has led to severe inflammation and subsequent bone loss as the result of the migration of the eroding particles to regions contiguous with bone139. Moreover, an ideal biomaterial from the contemporary tissue engineering standpoint is meant to degrade at the implantation site at the rate that matches the bone ingrowth rate. However, with the exception of magnesium, currently the only biodegradable metal researched for its use in drug delivery and other biomedical applications140,141, the traditional metallic implants, including those made of iron, cobalt or zirconium, do not satisfy this criterion. On the other side of the spectrum lie calcium sulfates, carbonates and very soluble orthophosphate phases, which typically degrade faster than the rate of new bone formation, leaving gaps in the bony structure and causing severe drainage at the wound site142. Weak or nonexistent union with the surrounding bone, corrosion, fatigue and absorption of mechanical stimuli to such a degree that the adjacent biomechanical structure partially atrophies or becomes completely resorbed, requiring the surgical removal of the implant, are all documented in the literature. These disadvantages are attempted to be overcome by coating titanium with CAP143,144 or with biopolymers145,146, hoping that the latter would promote bone-implant bonding and a more intimate tissue/material interface. Metallic and ceramic phases in contact, however, have a relatively high tendency to separate, which explains many instances of delamination and spallation of plasma-sprayed CAP coating layers on titanium surfaces observed even under comparatively mild testing conditions147, let alone in much harsher in vivo environment148.
Multiple other composites have been investigated for their ability to act as bone grafts and bone-regeneration implants. An aligned fibrous mesh obtained by electrospinning of poly(ε-caprolactone) and reinforced by 10 wt% of magnesium silicate nanopowder has, for example, improved the tensile strength and elastic modulus of the pure polymer and provided a surface for an enhanced cell response when compared to the unreinforced meshes149. Interestingly, poly(ε-caprolactone) fibers obtained by the same technique and reinforced by hardystonite, a mineral with the chemical formula Ca2ZnSi2O7, did not only exhibit a better mechanical performance compared to the same scaffolds reinforced by HAp, but they also enhanced cell proliferation and infiltration and promoted a more intense mineralization of the matrix by adipose-tissue-derived stem cells150. Siloxane-doped vaterite is another ceramic phase that has been used as an alternative to CAP in cotton-like PLLA-based scaffolds151. Composites enriched with silver nanoparticles have also attracted a particular attention owing to the antibacterial properties of silver and its consideration as a viable alternative to traditional antibiotics, whose efficacy in eradicating microbial sources of infection has reached an all-time low. Supplementation of gelatin/HAp scaffolds with silver nanoparticles has thus resulted in composite materials with 80% of porosity, able to promote the proliferation of osteoblasts while exerting a strong bactericidal effect against both gram positive and gram negative bacterial strains152. Unlike silver ions, which owe their antibacterial efficacy to the ability to interact with thiol groups in bacterial enzymes, denature them and induce the disintegration of the cell membrane and the lethal leakage of its cytoplasmic content153, other, less popular antimicrobial ions, rely on different mechanisms to eradicate bacteria. Selenium, for instance, inhibits the S. aureus biofilm formation and also initiates an oxidative cascade by being reduced to elemental form once metabolized154, while gallium serves as an irreducible iron analog under physiological conditions155,156. Other antibacterial ions include copper, zinc, cerium, strontium, europium, titanium and cobalt and all of them are easily incorporable into the lattice of HAp as calcium ion substitutes157.
One of the central advantages offered by ceramics as components of composites for hard tissue engineering comes from the possibility to control an array of their properties by controlling their stoichiometry, foreign ion inclusion and other parameters that define their total phase composition, be it isotropic, modular or functionally gradient. Lanthanum-strontium manganites (La1−xSrxMnO3+δ) exemplify this well, with variations in La3+/Sr2+ molar ratio being an excellent means of modifying the intensity of superexchange interaction158 and concentration of holes in the electronic structure, which affect an array of magnetic and charge transport properties159, respectively, endowing the material with a broad application potential. Another example comes from lanthanum-strontium cuprates (La2−xSrxCuO4), where minor amounts of dopants could be used to control the ratio between antiferromagnetic, superconductive and metallic phases of the compound160. Similarly, CAPs are found in one or multiple phases whose solubility, alongside mechanical and other properties, ranges from very high, as for MCPs, to sparsely soluble, as for OCP or HAp. This intrinsic versatility of CAPs and most ceramics in general allows for tailoring of their properties to match those of hard or soft tissues that they are meant to replace or regenerate.
On the other hand, one of the basic premises of materials science is that the structure of a material is an equally essential determinant of its properties and the performance as its chemical composition is. For example, a chemically identical metallic material can act as a conductor, a semiconductor or an isolator depending on the concentration of grain boundaries and similarly broad effects of the microstructure have been observed with respect to the biological response to materials. Whether a compound is predisposed to exert a toxic, a neutral or a healing effect on the body is, thus, oftentimes determined by its structural parameters, such as the grain size161, crystallinity162, the dominant polymorph163, porosity164, and the morphology of pores165 and particles166. This principle becomes even more accentuated for composite materials wherein the number of possible interactions between the components is multifold. Just like randomly combining HAp and collagen is no guarantee that a composite structure as stable and sturdy as bone will be produced, so does it frequently occur that two composites formed from identical components, but by slightly different means, yield drastically different end results. Such was the case with chitosan-HAp scaffolds whereby the air-dried ones demonstrated a threefold increase in compressive strength over the freeze-dried ones167, suggesting that even at the final processing stages changes can occur that dramatically affect the interaction of the components at the nano and molecular scales. After all, if sheer solvent desorption and resorption was enough to cause reversible structural transformations in 3-nm-sized zinc sulfide particles168, the structure of softer nanoparticles must be even more amenable to such post-processing effects. Naturally, also, with an increase in the number of the components, the assortment of the possible symmetries of their ordering increases too. An additional advantage offered by composite materials is the possibility of creating different symmetries at different scales, from the molecular to the micro scale, an effect that is achieved much more facilely than by single-phase materials.
5. Additional common ingredients of composite biomaterials
Unlike cartilage, which is home to a single cell type, multiple types of cells populate bone, including, most importantly, osteoblasts, osteoclasts and osteocytes. Osteoblasts are cells that do not only secrete proteins that constitute the bone matrix and induce calcium deposition within the fibrillar collagen network, thus promoting mineralization of bone, but also have immune functions, as they express cytokines to attract leukocytes and phagocytes to the wound or infection site. Yet, although they complement fibroblasts in making hard tissues (rather than the soft ones that fibroblasts make), both genotypically and morphologically they are hardly distinguishable from them. Namely, all the genes expressed in fibroblasts are expressed in osteoblasts too, while only two osteoblast-specific RNA transcripts have been identified so far: one encoding for Runx2, a.k.a. Cbfa1, a transcription factor and a key regulator of osteoblast differentiation, and the other one encoding for osteocalcin, the most abundant non-collagenous protein of the bone matrix, which serves as a nucleation center for the mineral particles and which, conversely, inhibits osteoblast function and attracts the antagonistic cells known as osteoclasts169. A considerable difference in stiffness between the two cell types exists though: while the elastic modulus of fibroblasts is ~ 3 kPa, that of osteoblasts is ~ 20 kPa170. Osteoclasts secrete lactic acid and proteolytic enzymes that dissolve the mineral and digest the organic matrix of bone. These multinuclear cells derived from hematopoietic stem cells (HSCs) and related to macrophages also phagocytize mineral particles in addition to dissolving them via acid secretion. In coordination with each other, these two mutually antagonistic types of cells, osteoblasts and osteoclasts, contribute to constant remodeling of bone in response to various biochemical and mechanical stimuli, an effect that is perhaps best demonstrated by the significant bone loss and osteopenia experienced by bedridden patients and astronauts who spend prolonged periods of time in microgravity conditions171. Surrounded by osteoid, the organic matrix of bone, osteoblasts stop creating the bone matrix and become quiescent osteocytes, cells involved in signal transduction of mechanical stimuli, the task that they achieve by secreting hormones, e.g. sclerostin, which inhibits bone formation in response to mechanical stress172. Osteocytes are essentially progenitory cells, which, in contrast to multipotent and indefinitely replicable stem cells, are oligopotent at best and can replicate only a limited number of times. Bone marrow does contain MSCs, although to a very minor extent: only 0.001% of the cellular content of bone marrow, with their proportion decreasing with age. Still, isolated MSCs could undergo over 30 passages, or more than a billion-fold expansion, without losing their osteogenic potential173. In addition to these pluripotent cells, bone marrow is also a reservoir for HSCs that differentiate into erythrocytes and leukocytes, cells involved in the transport of nutrients and immune resistance, respectively174. Correspondingly, viable interaction with cells evaluable in vitro presents the first step in estimating the clinical suitability of a biomaterial and Fig.7 shows an example in favor of composite nanoparticles. Namely, when vitamin D3 is delivered directly off the surface of HAp nanoparticles, on which it was physisorbed, the effect on the osteoblastic cell is nowhere as viable as that when the delivery is mediated by a surface layer of polymer, in this case PLGA175.
Fig.7.
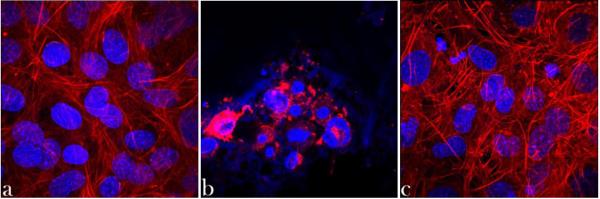
Z-stacked confocal optical micrographs of osteoblastic MC3T3-E1 cells incubated with no nanoparticles (a), with HAp nanoparticles loaded with vitamin D3 (b), and with HAp nanoparticles loaded with vitamin D3 and coated with PLGA (c), fluorescently stained for the cell nucleus (blue) and cytoskeletal f-actin (red) following 4 days of incubation. The size of each image is 300 × 300 μm.
Therefore, the first and the foremost additional component of materials for bone tissue engineering applications includes cells, on whose type, density, cell cycle stage and other genotypic and phenotypic characteristics the biological performance of the composite will come to depend. Most often, cells sucked into the scaffold are autologously derived so as to avoid the immunological response. When cells are delivered together with an implant, sufficient porosity and pore connectivity must be embedded in the material, lest the cells in its interior undergo necrosis due to the hindered transport of nutrients and waste products. No cell in the body is farther than a couple of hundreds of microns from a blood vessel and the same principle is to be applied in the design of biomaterial scaffolds. Note that microporous materials are defined as those with the pore diameters of less than 2 nm; macroporous materials have the pore diameters larger than 50 nm, while the mesoporous category lies in the middle176. However, the size of the pores in scaffolds has to be much larger, beyond the “macroporous”, in the order of tens or hundreds of microns, for the cells to be accommodated therein. Moreover, creating a surface-to-bulk gradient of the pore size may prove to be favorable, as suggested by the study that demonstrated the most successful implants in regenerating osteochondral defects in rabbits to be PLGA scaffolds with 100-200 μm pores in the chondral layer and 300-450 μm pores in the osseous layer177. Scaffolds, ideally, should not only possess a multimodal distribution of pores, but the right connections between pores could be considered to be of an even greater importance. The intercellular fluid and cells need to flow and communicate through signaling, respectively, and there are ongoing research efforts aimed at finding the most optimal networks of pores for the proliferation of cells within. Until now, electrospinning has been by far the most common method used to make porous polymeric scaffolds, with particulate leaching and thermally-induced phase separation being the other two most frequent synthesis routes178.
The second type of commonly used ingredients belongs to growth factors and particularly bone morphogenetic proteins (BMPs), which promote osteogenic differentiation of the cells and the building of new bony tissues179,180,181. The central problems associated with their application spring from (a) their sensitivity and proneness to environmental degradation, (b) necessity of accomplishing precisely tuned and optimized delivery profiles, and (c) expensiveness of production through recombinant techniques, all of which limits the scope of their practical applicability. The burst release of growth factors is, additionally, considered unfavorable from the point of view of optimal bone regeneration, as their controlled and prolonged release is of vital importance to make the material osteoinductive182,183. Also, tumorigenesis, ectopic expression and other forms of ill-directed growth resulting from the uncontrolled dosage or location of delivered BMPs and other growth factors, including platelet-derived growth factor-BB, and present serious potential problems pertaining to their usage184,185,186,187. Strictly speaking, with the healing process following injury that the implantation of a hard tissue substitute is consisting of multiple steps, including clotting, angiogenesis, inflammation, scar formation, etc., each one of which occurs at a narrowly defined time scale, it makes sense that regulatory molecules targeting these specific events should be released within similarly narrowly defined time windows. For example, blood clotting occurs in the first few hours following surgery and any sustained delivery of a molecule affecting this biological process over a period of days or weeks would not make much sense. Similarly, the immediate, burst release of a drug targeting inflammation, which takes over the biomaterial/tissue interface gradually, usually intensifying itself during the first couple of days after the implantation, would be similarly illogical from this point of view that necessitates precise tuning of the drug release profiles to the corresponding physiological events.
Antibiotics and other pharmaceutics present an important class of compounds frequently delivered using bone implants188. As for the former, their purpose is either prophylactic, that is, to prevent a postsurgical infection from developing, or deliberately antimicrobial in the cases when an infection has already been diagnosed. Composite nanostructures accommodating HAp in combination with polyesters, such as PLGA, have thus been intensely researched for their potential use in the delivery of antibiotics to infected bone and the treatment of osteomyelitis189. Capable of being loaded with both hydrophilic and hydrophobic drugs, ranging from those effective in the treatment of infections caused by opportunistic S. aureus and S. epidermis to anti-tuberculosis drugs, they are also formable as scaffolds with accurately designed porosities using 3D printing techniques190. An important advantage of this family of composite materials is their potentially allowing for tunable multiple-stage release profiles even more complex than the three-phase kinetic profiles typical for polymeric microparticles. In those cases, the initial burst release (~ 20 % in the first 4 – 6 h) results from the drug either adsorbed on the surface or entrapped within the first few surface layers of the degrading particles. The second phase involves sustained release dependent on the mechanism of degradation of the polymeric matrix. Polyesters, such as PLLA or PLGA, degrade through bulk erosion whereby the water uptake is faster than the degradation of the polymer and the drug release occurs without a significant loss of mass or volume of the polymer191,192. In contrast, polyanhydrides degrade via surface erosion whereby the hydrolysis proceeds faster than the water ingress into the interior of the polymeric particle193. The third stage is characterized by a slight increase in the release rate and it takes place as the degradation nears its completion194. In the case of acidic polyesters or polyphosphazenes, the release of acidic byproducts is responsible for the autocatalytic acceleration of the degradation process at this stage. It has been argued that these multiple-stage release profiles can have great benefits compared to solely linear profiles. For example, it was shown that the rapid initial release of paclitaxel stopped the proliferation of a tumor, while a more sustained, second phase of release allowed for its complete gradual eradication195.
Compounds that stimulate the production of BMPs and thus indirectly enhance bone formation have been a part of the repertoire of additional ingredients of composites for bone regeneration and have predominantly included statins196. Icaritin, an exogenous phytoestrogenic compound, was combined into a composite PLGA/TCP scaffold and its sustained release enhanced the bone defect repair in the rabbit model of steroid-associated osteonecrosis197. Plasmids are rarely delivered by means of hard tissue replacement materials, though it has been shown that both CAPs198,199 and polycationic polymers, e.g. polyethylenimine/hyaluronic acid200, can complex DNA and be used as non-viral gene delivery carriers, though still in a less efficient way than it can be achieved by means of the viral ones. Cytokines and antibodies present other deliverable biomolecules of interest capable of fostering a favorable cellular response that promotes new bone formation. Attaching cell-binding peptides to the surface of the particles or entrapping them in their interiors so as to promote a more favorable cell/material interaction also presents a potentially valuable approach. Various host-cell specific moieties and cell-penetrating peptides could be employed as functional surface groups with the aim to promote a more intimate tissue/material interface. As for the latter, enrichment of composites with analogues of lipofectamine, a positively charged compound used in gene transfection protocols to facilitate the diffusion of exogenous oligonucleotides across the cell membrane201, can be considered as an option. One of the peptides commonly used to improve the biocompatibility of the implant is Arg-Gly-Asp (RGD)202,203. A part of many proteins of the extracellular matrix, this peptide has been shown to promote cell adhesion204, proliferation205 and differentiation206 in multiple cell types. Next, despite the fact that wound healing following bone injury and placement of an implant involves blood clot formation, which has a significant effect on the outcome of the healing process, “most research in bone tissue engineering virtually ignores the important role of a blood clot in supporting healing”207. To that end, it is only a question of time when the control over this physiological process will be exerted using additional ingredients of regenerative constructs in hard tissue engineering. Tissue plasminogen activator (t-PA), a protein involved in dissolving blood clots, was, for example, conjugated to the cationic polyurethane surfaces and gold nanoparticles capped with poly(vinyl pyrrolidone) and lysine, which had a multifold effect on the extension of its half-life208,209, while another approach pertains to the fabrication of clot-lysing surfaces as those having selective affinity for plasminogen and t-PA, which, when combined, yield plasmin able to dissolve the fibrin clot. Using such agents in combination with their antagonists, the blood coagulation process in the vicinity of the implant could be optimized, having a positive effect on bone regeneration.
Functionalizing the composite systems with antagonistic pairs of peptides allows for the use of such composites as smart therapeutic devices, capable of releasing drugs in direct feedback with the demands of the biological microenvironment. As suggested by a study by Wu et al.210, reservoirs holding an antiresorptive drug, aimed to diminish the activity of osteoclasts, could be linked to (Asp)8 sequence, whereas those storing an anabolic drug, aimed to stimulate osteoblasts, could be linked to (Asp-Ser-Ser)6 targeting peptide. It is worth noting that antiresorptive drugs per se, including primarily estrogen receptor agonists and various bisphosphonates, such as alendronate211, clondronate212 and others, have also been successfully co-delivered together with other therapeutics. Bisphosphonates are also interesting because of their ability to act as bone-targeting agents, ensuring the localization of the nanoparticles at the bone interface even when injected into the bloodstream213. Still, the effects of coupling such agents to antifouling peptides214, capable of exhibiting either a prophylactic or a straightforwardly antibiotic effect by preventing the formation of the biofilm or breaking it down, respectively, have yet to be explored. Some proteins could be reduced to only a dozen of amino acid residues long peptides and still maintain the gross of their functionality215 - such methods could prove to be practical in the design of peptides capable of inducing the right cell response. The benefits of the delivery of peptides come from their structural sturdiness compared to large proteins, which have low cellular permeability and low stability, being sensitive to mildest deviations from the physiological conditions and/or the action of proteases. Even when they reach the interior of a cell, they may be detected as foreign entities and degraded as such in the lysosome. Yet, it is nowadays known that proteins can also cause the cells to change the identity through the epigenetic route216, the role that was prior to this discovery thought to have been reserved for nucleic acids only. This also explains why a large body of research is being dedicated to vesicular capsules and other protein carriers217. Note that the time scale for the adsorption or chemisorption of peptides onto inorganic particles is different depending on the complexity and the level of symmetry of the interacting protein assemblies. Side chains typically bind on the scale of nanoseconds, individual protein molecules on the scale of seconds, clusters of peptides require minutes, and layers of peptides may need hours. The choice between the bulk entrapment, nonselective physisorption and covalent binding depends on the type of molecule in question. In the case of nonspecific adsorption, electrostatic attraction, van der Waals forces and entropic driving forces, as in the hydrophobic effect, are relied on to achieve stable binding. Typical chemical conjugation mechanisms involve reactions between amines and activated carboxylic acid groups as esters with a good leaving group (e.g., using alginic acid to form carboxyl surface groups and then n-hydroxysuccinimide or trifluoroacetic anhydride to deprotonate them into reactive carboxylate groups) yielding amide bonds, between pyridyldithiols and thiols yielding disulfide bonds, between acetyls and azides yielding triazole bonds, between maleimide derivatives and thiols yielding thioether bonds, between amines and thiocyanates yielding isothiourea bonds, between surface p-nitrophenylcarbonyl terminal groups and amine ligands yielding carbamate bonds, and so forth218. Noted in the literature are also a variety of azide-alkyne cycloadditions, a.k.a. “click” chemical reactions used to bind drugs or targeting moieties to a plethora of polymeric micelles and nanoparticles, liposomes, metallic and silica nanoparticles and carbon nanotubes219,220. Note also that different materials require different methods to achieve stable surface functionalization: co-precipitation or ion exchange may be sufficient for CAPs, chemisorption is most optimal for polymers, while functionalization of carbon-based materials, including nanotubes and graphene, requires derivatization via carboxylic moieties introduced by a combined acid and permanganate ion oxidation treatment221 and is greatly facilitated if structural defects are created in the first place222.
Functionalization with poly(ethylene glycol) (PEG) presents another routine approach in the drug delivery field. The rationale is that passivation with hydrophilic PEG produces random coiling at the surface, which entraps water and prevents opsinization and rapid clearance by the reticuloendothelial system. As a result, conjugation with PEG is able to: (a) sterically stabilize particles and prevent the binding of plasma protein, thereby prolonging the pharmacokinetic half-life in circulation and increasing the bioavailability of the drug; (b) reduce immunogenicity; and (c) enhance permeability and retention effect223. Labeling the particles with technetium-99, indium-111 or iodine-125224 for radiological bone scintigraphy, doping them with luminescent ions for imaging purposes or with magnetic ions, ranging from iron to gadolinium to cobalt225, for use in MRI applications, in hyperthermia treatments and for magnetic-field-assisted bone growth enhancement are other interesting, though largely unexplored research avenues, even though they could present steps forward in the direction of smart, theranostic biomaterials for bone regeneration, capable of acting therapeutically in direct feedback with the outcome of in situ disease monitoring at the cellular and subcellular scales. Which brings us over to the central advantage offered by the future generation of composite therapeutic systems: smart, synergistic and multifunctional response to demands of the local microenvironment.
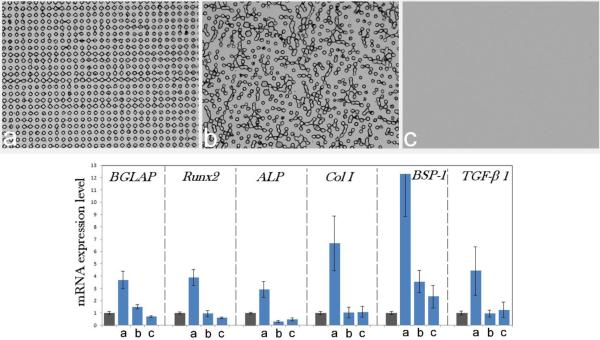
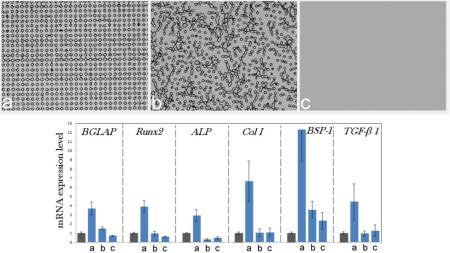
Interestingly, the recently made composite particles comprising silver nanoparticles coated with PGA and embedded together with ascorbic acid in submicron PLGA spheres, possessing a triple functionality – antibacterial, osteoinductive and antioxidative – upregulated the expression of osteocalcin and type I procollagen without the release of any growth factors226. Transcending the need for the delivery of chemical agents as sources of bone growth marker upregulation by the right structural synergy between the composite components is bound to present an ever more exciting topic of research in this field. Advancements along this line of research have the potential to finally erase the traditional division of biomaterials for bone engineering applications to three categories: osteogenic, osteoinductive, and osteoconductive. As a reminder, osteoconduction is a process whereby an implant facilitates the ingrowth of blood vessels and the migration of bone cells capable of osteogenesis onto it. Therefore, a material that supports bone growth on itself by definition demonstrates osteoconductivity. Osteoinduction, in turn, implies the recruitment of pluripotent MSCs from the surrounding tissue and induction of their differentiation into osteoblasts, a process that is mediated by the growth factors, specifically bone morphogenic proteins. Accordingly, osteoinduction is thought to be achievable only with the delivery of osteoinductive factors, which include bioactive chemicals capable of inducing recruitment, differentiation and proliferation of bone cells. CAPs per se are, according to this definition, only osteoconductive and only an addition of growth factors is supposed to be able to render them osteoinductive. Yet, their ability to upregulate the expression of osteogenic markers and boost the osteoblastic differentiation, making them effectively osteoinductive too, has been reported on numerous occasions227,228,229. The addition of growth factors, such as bone morphogenetic proteins, has indeed made CAPs osteoinductive230,231, although the same osteoinductive effect achieved by BMP-2 on human MSCs was accomplished by nanosized HAp particles, for example232. Surface features and precisely set morphologies are also capable of compensating for the effects of sole chemistry and acting as critical osteoinductive agents. Not only do the size and the geometry of the surface features and the distances between them matter in this sense, but the order/disorder at the level of their translational symmetry matters too, as shown in Fig.8. Namely, the gene expression of osteocalcin, osteopontin, procollagen type I, alkaline phosphatase, and the transcription factors Runx2 and TGFβ-1 was consistently upregulated only in osteoblastic cells seeded on translationally ordered composite films made by dispersing HAp nanoparticles in a 80 kDa poly(ε-caprolactone) matrix, as opposed to translationally disordered and flat surfaces of the same composition233. Many prior studies have shown that topography can be a more important determinant of the viability of the biomaterial/tissue interface than the surface chemistry or stiffness234,235,236. This finding has, however, implied that the order at the level of the distribution of topographic features can be a more decisive factor than their surface density, size and geometry. This is all to say that the research possibilities in the design of advanced nanocomposites for bone tissue engineering really seem limitless, concealing unforeseen clinical potentials, the unleashing of which the devotees to this exciting field of materials science, as of today, unreservedly anticipate.
Fig.8.
Upregulated mRNA expression of osteocalcin (BGLAP), osteopontin (BSP-1), procollagen type I (Col I), alkaline phosphatase (ALP), and the transcription factors Runx2 and TGFβ-1 in osteoblastic MC3T3-E1 cells grown on (a) topographically ordered nanocomposite poly(ε-caprolactone)/HAp films compared to the same cells grown on the control cell culture polystyrene (gray bars) and on (b) topographically disordered and (c) flat poly(ε-caprolactone)/HAp films. The photolithographically fabricated cylindrical surface features have 10 μm in diameter and 10 μm in height and are spaced by 10 μm (edge-to-edge) on average in both films (b) and (c). mRNA expression was measured by quantitative RT-polymerase chain reaction relative to the housekeeping gene β-actin (ACTB). Data first normalized to the expression of ACTB and then to the gene expression of the control group are shown as averages with error bars representing standard deviation. Genes significantly (p < 0.05) upregulated with respect to the control group are marked with *. Genes significantly (p < 0.05) downregulated with respect to the control group are marked with †.
6. Outlook
The demand for a new generation of bone replacement materials has never been higher, given more than 2 million bone graft operations performed annually worldwide237 (600,000+ in the US alone)238 and the constant increase in this number owing to the aging population of the Earth. The medical costs of bone graft placement have reached $1.3 billion in 2010 and continue to rise at the 7.4 % annual rate239. Bone graft business has simultaneously thrived in the recent years, generating revenues in the excess of $2.5 billion per annum240. As of today, however, only about twenty tissue engineering applications, mainly for skin and muscoskeletal regeneration, have been approved by the FDA and made available on the market. The current generation of bone substitutes suffers from numerous demerits, including insufficient strength for load-bearing applications, widely varied resorption times, insensitivity with respect to the application zone in the bone and in the body, insufficient drug release tunability, and the lack of control over the fate of internalized and adhered cells and biomolecules. Despite the fact that there is not too many sites in the body from which bone could be harvested without causing a biomechanical dysfunction or unaesthetic disfigurement, allografts and autografts as parts of hard tissues taken from donors or the patient itself, respectively, still constitute the greatest majority of materials used as bone grafts in the orthopedic surgery, with only 5 % or less being synthetic materials. While the downside of autografts is the necessity for two separate surgical procedures to be performed, increasing the risk of infection and failure, allografts pose a risk of implant rejection and disease transmission, including the need for an extensive sterilization procedure during which the properties of the filler are often degraded. If we add the relative inflexibility of autografts in filling irregular defects and their propensity to be resorbed too rapidly, we could come to conclusion that there is a serious discrepancy between the bone substitutes routinely applied in the clinic today and the quality and potentials of those lying scattered across the ‘valley of dreams’ of the research stage and the so-called ‘valley of death’ of the translational, preclinical stages.
Materials for hard tissue engineering applications in the research phase, on the other hand, greatly outnumber the limited number of those that are currently available to the clinicians. And with no material able to replicate bone anywhere in sight, we could be certain that the research race will continue towards the destination that is a perfect biomaterial for the regeneration of hard tissues. Considering a variety of hard tissues within the organism, we could be equally certain that more than one such perfect biomaterial awaits us at this ultimate aim of our research ventures. Understanding this is equally enlightening as the realization that not a single worldview could be enough to reflect the phenomenal complexity of life. Rather, only a combination of mutually differing points of view can account for a view of life that is truly complete. Of course, endorsing composite replacements of naturally composite boney tissues comes at the cost of a pervasive concern that an undesirable synergy among the multiple particle components might be produced, the reason for which natural medicines have been traditionally disfavored over monomolecular synthetics. Such synergies resemble double-edged swords in a sense that they could be positive, as in the case when the delivery of clindamycin with CAP nanoparticles canceled out the negative effect that the administration of the pure antibiotic had on the osteogenesis of the bone tissue241 or in the case when the co-delivery of rapamycin and paclitaxel using nanoparticulate poly(ethylene glycol)-poly(ε-caprolactone) block copolymers had a greater antitumor efficacy than the delivery of the two drugs alone242, but they could also be detrimental, as in the cases when increases in toxicity entailed combination drug therapies243,244 or when binding of fluorophores caused the unintended deformation of the labeled biomolecules245, including even the crystal lattice of CAPs246. Whatever the case, synergies are inescapable in composite systems and their protean effects, rising steeply in proportion with the number of interactive components, must be carefully assessed prior to the medical application of their therapeutic carriers.
Great disparity exists between the current generation of composite materials clinically applied in the restoration of hard tissues and the far more sophisticated types of composites elaborated in this discourse. This gap becomes clearly obvious when we put side by side the rather rudimentary composites used today by dentists, maxillofacial surgeons and orthopedists and the fantastic, ultra-finely structured and multifunctional composites towards which the future of the materials science is streaming. For example, titanium implants coated with the bioactive layer of HAp have a favorable effect on bone integration, but they do not overcome the problem of elastic modulus mismatch between the implant and the bone and the corresponding stress-shielding effect, which tends to lead to bone resorption, the loss of tissue/implant union and an increased likelihood of fracture. Also, the rather weakly bound, thin and porous bioactive HAp layer is only temporarily existent in vivo and after only a few weeks or months at best all but the ideal titanium/tissue interface is restored and with it all the imperfections tied to it. Its long-term benefits are questionable because, although it does speed up the host response, the tissue interface eventually looks the same, with or without it. Another type of clinically applied nanocomposites, based on the combination of PLGA and nanostructured HAp, suffers from the insufficient strength as the result of the low mechanical synergy between the hard and the soft components as well as from the risks of bone resorption and inflammation associated with the burst release of acidic products of the autocatalytic degradation of the polymer.
Demerits of the current generation of nanocomposites used in bone replacement procedures are actually too many to fit the content of this review. For example, biodegradable load-bearing bone replacement materials are neither available on the market nor existent on the horizon. Then, practically all of these options are isotropic, which contrasts the fact that the mechanical properties of compact human bones are anisotropic and depend on the direction of the applied force, being stronger in both tension and compression modes when the load is applied parallel to the direction of its long axis than when it is applied normal to it. Also, even though they are drastically different in porosity, strength, vascularity, turnover rate and mechanical properties247, the cortical and the trabecular bone are usually treated with identical fillers. This is all to say that every single composite used in bone restoration today suffers from obvious deficiencies. In contrast, as we could have seen from the present discourse, the composites currently in the research stage outweigh in their complexity and the potential those in use today. Once we see the former fully transitioned to the bedside, it will certainly be a great triumph for this loose confederation of materials scientists and medical professionals that we have today.
The most revolutionary breakthroughs in the development of nanocomposites for bone regeneration will certainly belong to smart, multicomponent systems capable of delivering therapeutic payloads at the ideal location by means of the right targeting agents and at the ideal time points through release triggered by an in situ detection of disease markers. This perfectly accurate spatial and temporal delivery of therapeutics is in need of theranostic systems guidable by inbuilt molecular recognition codes to the right location in the body as well as programmed to release the therapeutic cargos in an environmentally sensitive way, in direct feedback with the outcomes of in situ disease monitoring via an embedded diagnostic module. Nacre-mimicking, multilayered particles with preset layer degradation rates, functionally gradient particles with structure and properties scaled to provide an ideal biological response, and Janus particles with either/or logic of release present some of the exciting nanocomposite forms that will overcrowd the literature in the coming decades. Beaver’s enamel owing its exceptional hardness and resistance to acid attack to the atomically thin, graded layers of ferrihydrite and amorphous iron-rich CAP enveloping the apatite nanorods248 or Pt3Ni alloys displaying enhanced catalytic properties compared to pure Pt owing to the Ni-free first atomic surface layer249 could be instructive examples in terms of fostering the atomically precise surface design of composite structures. Because nanoparticles may display a wide spectrum of properties in a narrow window of sizes250 and abruptly transition between states under marginal changes in size251, tuning nanoparticulate ingredients of biocomposites to precisely optimized sizes (alongside other physical features - shape, topology, microporosity, surface potential, etc.) so as to harness their unique properties is an approach that will lead to exciting biomaterials in the times ahead of us. We know now that cell-cell communication is essential to prevent the development of malignancies, yet we are nowhere near the design of intelligent nanoparticles that share information with one another, which is yet another breakthrough that we are bound to witness in the foreseeable future. Seeing particles as predominantly relational entities may also entail the substitution of the frequently militaristic metaphors describing their therapeutic role in the body with organic ones, more conducive to the healing process252. All hard tissues are also hierarchical structures, possessing distinct symmetries at different spatial scales, and their artificial replicas may need to be similar in superstructure to serve as their ideal substitutes - i.e., isotropic and with unique orderings of nanoparticulate units and their assemblies at the molecular, nano, micro and macro levels. It is important to note that synergy between the individual components in these forthcoming composite materials will stem from their entwinement at ultrafine scales. Of course, the clinical prospect of such sophisticated structures - which may eventually really earn the right to be called devices - will critically depend on the ability to design and create them by elegantly simple and cost-effective means.
Finally, note that most of the composite materials elaborated in this review piece were biodegradable in nature. Such a choice is in line with the contemporary belief that an ideal biomaterial is to become fully replaced by the healthy tissue(s) and, ideally, convert the regenerated area of the body to its original, fully functional state. Regenerative approach to tissue engineering has been, in fact, the paradigm of our times. It, itself, is an inverted version of the paradigm that surrounded the birth of the field of biomaterials in the form that we know it today, that is, around the time the term “biomaterials” was coined at Clemson University symposia in the 1960s. Namely, although materials science aficionados amongst surgeons are credited for devising the first biomaterials in the real sense of the word, the first research in “biomaterials” was done by traditional materials science expatriates, who brought along with them the central materials science paradigm and instated it on this new territory: “Materials should be made as stable, sturdy and durable as possible”. As a result, for better or worse, metals became the first biomaterials for the replacement of hard tissues and improving their resistance to corrosion and wear (i.e., structural stability in biological environments) the object of the first research in this field. Right after them on the list of interest were initially non-biodegradable polymers, such as poly(methyl methacrylate), polyethylene and poly(vinyl chloride). Degradation of biomaterials went on to be viewed as an unfavorable process by causing the properties and the performance of the materials to deteriorate with time. It took a whole new look at things, stemming, interestingly, from a wish not to correct a problem, but to use it as a solution for a problem existing in a different domain, before biodegradable materials gained value in the biomedical community. While not disputing the definite merits, alongside an intrinsic beauty, of this new paradigm, this author, however, believes that an interest for a new generation of materials will be discovered in the near future. These materials would be fundamentally different from their biodegradable predecessors and intended to be superior in performance in comparison with the substituted tissues, be they fully functional or defective.
This viewpoint echoes the dreams of Daedalus, who created wings for his son so as to make him fly, or, in other words, go beyond nature. Fulfillment of these Daedalus’ dreams would give a whole new meaning to the equation placed in the title of this work: 1 + 1 > 2. Namely, this next generation of biomaterials envisaged hereby would be designed with the purpose of not restoring the tissues, but rather augmenting them. As an intended result, the combination of a biomaterial and the body would yield something more and beyond the body in question, let alone a simple sum of the two. Biology has inspired the design of numerous advanced materials in practical use, but ever since steel and titanium alloys were introduced to bone engineering from aerospace industry, no advanced materials made it through the translational path from the materials science lab into the orthopedic clinic despite their great promises, with quantum dots, carbon nanotubes and fullerenes being some of the notable examples. Even worse, from the ideological standpoint, biomaterials science has distanced itself from Daedalus’ dream and embraced a more down-to-earth and organic perspective, attempting to restore the biology at birth instead of boldly go beyond it in structure and functionality. Needless to add, this is in direct disparity with the point of view held by molecular biology, where genotypic and phenotypic manipulations for the purpose of eliciting responses vaguely dormant in cells are regularly targeted. In view of this, one thing is certain: ‘tis the idea whose time has yet to come and, like many wonderful ideas, this one, too, takes us to the moment of the conception of the field discoursed on, drawing a full circle between the beginnings and ends. The exigency of its elaboration cannot be overstated, yet in spite of the author’s belief in its critical importance for the future of the field, it will present the topic of some future treatises.
Highlights.
1) Most prospective breakthroughs made in composites for bone engineering.
2) Smart materials acting therapeutically in feedback with in situ disease monitoring.
3) Bone as an example of a composite where strengths compensate for weaknesses.
4) Ceramic/polymer composites modeled after the structure of bone.
5) Future generation of biomaterials that restore, but also augment repaired tissues.
Acknowledgments
Writing of this review article was supported by the NIH grant R00-DE021416.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Del Pozo JL, Patel R. Infection Associated with Prosthetic Joints. The New England Journal of Medicine. 2009;361:787–794. doi: 10.1056/NEJMcp0905029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uskoković V. Prospects and Pits on the Path of Biomimetics: The Case of Tooth Enamel. Journal of Biomimetics, Biomaterials and Tissue Engineering. 2010;8:45–78. doi: 10.4028/www.scientific.net/JBBTE.8.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uskoković V. Biomineralization and Biomimicry of Tooth Enamel. In: Vallittu Pekka., editor. Non-Metallic Biomaterials for Tooth Repair and Replacement. Woodhead Publishing; Cambridge, UK: 2013. pp. 20–89. [Google Scholar]
- 4.Bertassoni LE, Habelitz S, Kinney JH, Marshall SJ, Marshall GW., Jr. Biomechanical Perspective on the Remineralization of Dentin. Caries Research. 2009;43(1):70–77. doi: 10.1159/000201593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goncalves PF, Sallum EA, Sallum AW, Casati MZ, de Toledo S, Nociti FH., Junior Dental Cementum reviewed: development, structure, composition, regeneration and potential functions. Braz J Oral Sci. 2005;4:651–658. [Google Scholar]
- 6.Ho SP, Yu B, Yun W, Marshall GW, Ryder MI, Marshall SJ. Structure, chemical composition and mechanical properties of human and rat cementum and its interface with root dentin. Acta Biomaterialia. 2009;5(2):707–18. doi: 10.1016/j.actbio.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uskoković V, Bertassoni LE. Nanotechnology in Dental Sciences: Moving towards a Finer Way of Doing Dentistry. Materials. 2010;3(3):1674–1691. doi: 10.3390/ma3031674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White SN, Luo W, Paine ML, Fong H, Sarikaya M, Snead ML. Biological Organization of Hydroxyapatite Crystallites into a Fibrous Continuum Toughens and Controls Anisotropy in Human Enamel. Journal of Dental Research. 2001;80:321–326. doi: 10.1177/00220345010800010501. [DOI] [PubMed] [Google Scholar]
- 9.He LH, Swain MV. Understanding the mechanical behaviour of human enamel from its structural and compositional characteristics. Journal of the Mechanical Behavior of Biomedical Materials. 2008;1:18–29. doi: 10.1016/j.jmbbm.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Bonderer LJ, Studart AR, Gauckler LJ. Bioinspired Design and Assembly of Platelet Reinforced Polymer Films. Science. 2008;319:1069–1073. doi: 10.1126/science.1148726. [DOI] [PubMed] [Google Scholar]
- 11.Stupp SI, Braun PV. Molecular Manipulation of Microstructures: Biomaterials, Ceramics, and Semiconductors. Science. 1997;277:1242–1248. doi: 10.1126/science.277.5330.1242. [DOI] [PubMed] [Google Scholar]
- 12.Burger C, Zhou H-W, Wang H, Sics I, Hsiao BS, Chu B, Graham L, Glimcher MJ. Lateral Packing of Mineral Crystals in Bone Collagen Fibrils. Biophys J. 2008 Aug 15;95(4):1985–1992. doi: 10.1529/biophysj.107.128355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davim JP, Charitidis CA. Walter de Gruyter; Boston, MA: 2013. Nanocomposites: Materials, Manufacturing and Engineering. [Google Scholar]
- 14.Sáenz A, Rivera-Muñoz E, Brostow W, Castañ VM. Ceramic Biomaterials: An Introductory Overview. Journal of Materials Education. 1999;21:297–306. [Google Scholar]
- 15.Uskoković V, Košak A, Drofenik M. Preparation of Silica-Coated Lanthanum-Strontium Manganite Particles with Designable Curie Point, for Application in Hyperthermia Treatments. International Journal of Applied Ceramic Technology. 2006;3(2):134–143. [Google Scholar]
- 16.Uskoković V, Desai TA. Calcium Phosphate Nanoparticles: A Future Therapeutic Platform for the Treatment of Osteomyelitis? Therapeutic Delivery. 2013;4(6):643–645. doi: 10.4155/tde.13.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang L, Yang X, Yin Q, Cai K, Wang H, Chaudhury I, Yao C, Zhou Q, Kwon M, Hartman JA, Dobrucki IT, Dobrucki LW, Borst LB, Lezmi S, Helferich WG, Ferguson AL, Fan TM, Cheng J. Investigating the optimal size of anticancer nanomedicine. Proc Natl Acad Sci U S A. 2014;111(43):15344–9. doi: 10.1073/pnas.1411499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis EE, Child HW, Hursthouse A, Stirling D, McCully M, Paterson D, Mullin M, Berry CC. The influence of particle size and static magnetic fields on the uptake of magnetic nanoparticles into three dimensional cell-seeded collagen gel cultures. J Biomed Mater Res B Appl Biomater. 2014 Oct 31; doi: 10.1002/jbm.b.33302. doi: 10.1002/jbm.b.33302. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Fuchshofer R, Yu AH, Welge-Lüssen U, Tamm ER. Bone morphogenetic protein-7 is an antagonist of transforming growth factor-beta2 in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007;48(2):715–26. doi: 10.1167/iovs.06-0226. [DOI] [PubMed] [Google Scholar]
- 20.Uskoković V. Entering the Era of Nanoscience: Time to Be So Small. Journal of Biomedical Nanotechnology. 2013;9:1441–1470. doi: 10.1166/jbn.2013.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uskoković V. Theoretical and Practical Aspects of Colloid Science and Self-Assembly Phenomena Revisited. Reviews in Chemical Engineering. 2007;23(5):301–372. [Google Scholar]
- 22.Ritchie RO. How does human bone resist fracture? Annals of the New York Academy of Sciences. 2010;1192:72–80. doi: 10.1111/j.1749-6632.2009.05232.x. [DOI] [PubMed] [Google Scholar]
- 23.Weiner S, Wagner HD. The Material Bone: Structure-Mechanical Function Relations. Annual Review of Materials Science. 1998;28:271–298. [Google Scholar]
- 24.Giocondi JL, El-Dasher BS, Nancollas GH, Orme CA. Molecular mechanisms of crystallization impacting calcium phosphate cements. Philosophical Transactions of the Royal Society A: Mathematical, Physical & Engineering Sciences. 2010;368(1917):1937–61. doi: 10.1098/rsta.2010.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uskoković V, Ignjatović N, Petranović N. Synthesis and Characterization of Hydroxyapatite-Collagen Biocomposite Materials. Materials Science Forum. 2003;413:269–274. [Google Scholar]
- 26.O'Hara RM, Orr JF, Buchanan FJ, Wilcox RK, Barton DC, Dunne NJ. Development of a bovine collagen-apatitic calcium phosphate cement for potential fracture treatment through vertebroplasty. Acta Biomaterialia. 2012;8(11):4043–52. doi: 10.1016/j.actbio.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Uskoković V, Ignjatović N, Petranović N. Synthesis and Characterization of Hydroxyapatite-Collagen Biocomposite Materials. Materials Science Forum. 2003;413:269–274. [Google Scholar]
- 28.Nair AK, Gautieri A, Chang S-W, Buehler MJ. Molecular mechanics of mineralized collagen fibrils in bone. Nature Communications. 2013;4:1724. doi: 10.1038/ncomms2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nudelman F, Sommerdijk NA. Biomineralization as an inspiration for materials chemistry. Angewandte Chemie International Edition English. 2012;51(27):6582–96. doi: 10.1002/anie.201106715. [DOI] [PubMed] [Google Scholar]
- 30.Bertassoni LE, Habelitz S, Pugach M, Soares PC, Marshall SJ, Marshall GW., Jr. Evaluation of surface structural and mechanical changes following remineralization of dentin. Scanning. 2010;32(5):312–9. doi: 10.1002/sca.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nudelman F, Lausch AJ, Sommerdijk NA, Sone ED. In vitro models of collagen biomineralization. Journal of Structural Biology. 2013 doi: 10.1016/j.jsb.2013.04.003. pii: S1047-8477(13)00089-0. [DOI] [PubMed] [Google Scholar]
- 32.Bohner M. Bioresorbable Ceramics. In: Buchanan Fraser., editor. Degradation Rate of Bioresorbable Materials. Woodhead; Cambridge, UK: 2008. pp. 95–114. [Google Scholar]
- 33.Cölfen H. Biomineralization: A crystal-clear view. Nature. 2010;9:960–961. doi: 10.1038/nmat2911. [DOI] [PubMed] [Google Scholar]
- 34.Gower LB. Biomimetic Model Systems for Investigating the Amorphous Precursor Pathway and its Role in Biomineralization. Chemical Reviews. 2008;108(11):4551–4627. doi: 10.1021/cr800443h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eanes ED, Gillessen H, Posner AS. Intermediate states in the precipitation of hydroxyapatite. Nature. 1965;208:365–367. doi: 10.1038/208365a0. [DOI] [PubMed] [Google Scholar]
- 36.Yin X, Stott MJ. Biological calcium phosphates and Posner’s cluster. Journal of Chemical Physics. 2003;118:3717–3723. [Google Scholar]
- 37.Gebauer D, Volkel A, Colfen H. Stable Prenucleation Calcium Carbonate Clusters. Science. 2008;322(5909):1819–1822. doi: 10.1126/science.1164271. [DOI] [PubMed] [Google Scholar]
- 38.Dey A, Bomans PHH, Muller FA, Will J, Frederik PM, de With G, Sommerdijk NAJM. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nature Materials. 2010;9:1010–1014. doi: 10.1038/nmat2900. [DOI] [PubMed] [Google Scholar]
- 39.Pouget EM, Bomans PHH, Goos JACM, Frederik PM, de With G, Sommerdijk NAJM. The initial stages of template-controlled CaCO3 formation revealed bNanoclustery cryo-TEM. Science. 2009;323(5920):1455–8. doi: 10.1126/science.1169434. [DOI] [PubMed] [Google Scholar]
- 40.Politi Y, Arad T, Klein E, Weiner S, Addadi L. Sea Urchin Spine Calcite Forms via a Transient Amorphous Calcium Carbonate Phase. Science. 2003;306(5699):1161–4. doi: 10.1126/science.1102289. [DOI] [PubMed] [Google Scholar]
- 41.Mahamid J, Addadi L, Weiner S. Crystallization pathways in bone. Cells Tissues Organs. 2011;194:92–97. doi: 10.1159/000324229. [DOI] [PubMed] [Google Scholar]
- 42.Pound GM. Liquid and Crystal Nucleations. Ind. Eng. Chem. 1952;44(6):1278–1283. [Google Scholar]
- 43.Meldrum FC, Sear RP. Now You See Them. Science. 2008;322:1802–1803. doi: 10.1126/science.1167221. [DOI] [PubMed] [Google Scholar]
- 44.Nielsen MH, Lee JRI, Hu Q, Han TY-J, De Yoreo JJ. Structural evolution, formation pathways and energetic controls during template-directed nucleation of CaCO3. Faraday Discuss. 2012;159:105–121. [Google Scholar]
- 45.La Mer VK, Dinegar RH. Theory, Production and Mechanism of Formation of Monodispersed Hydrosols. J. Am. Chem. Soc. 1950;72:4847–4854. [Google Scholar]
- 46.La Mer VK. Nucleation in Phase Transitions. Ind. Eng Chem. 1952;44(6):1270–1277. [Google Scholar]
- 47.Kuhn T. The Structure of Scientific Revolutions. Nolit; Belgrade: 1969. [Google Scholar]
- 48.Bogush GH, Zukoski CF. Uniform Silica Particle Precipitation: An Aggregative Growth Model. J Colloid Interface Sci. 1991;142:19–34. [Google Scholar]
- 49.Matijevic E. Uniform Inorganic Colloid Dispersions. Achievements and Challenges. Langmuir. 1994;10:8–16. [Google Scholar]
- 50.Giesche H. Medical and Technological Applications of Monodispersed Colloidal Silica Particles. In: Matijevic Egon., editor. Medical Applications of Colloids. Springer; New York, NY: 2008. p. 43. [Google Scholar]
- 51.Thompson DW. On Growth and Form. Dover; London, UK: 1917. [Google Scholar]
- 52.Mann S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry. Oxford University Press; Oxford, UK: 2001. [Google Scholar]
- 53.Nudelman F, Pieterse K, George A, Bomans PH, Friedrich H, Brylka LJ, Hilbers PA, de With G, Sommerdijk NA. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nature Materials. 2010;9(12):1004–9. doi: 10.1038/nmat2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uskoković V, Li W, Habelitz S. Amelogenin as a Promoter of Nucleation and Crystal Growth of Apatite. Journal of Crystal Growth. 2011;316:106–117. doi: 10.1016/j.jcrysgro.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orme CA, Giocondi JL. The Use of Scanning Probe Microscopy to Investigate Crystal-Fluid Interfaces. In: Skowronski M, DeYoreo JJ, Wang CA, editors. Perspectives on Inorganic, Organic, and Biological Crystal Growth: From Fundamentals to Applications. Springer; Berlin: 2007. [Google Scholar]
- 56.Kawalec-Carroll JS, Hetherington VJ, Dockery DS, Shive C, Targoni OS, Lehmann PV, Nadler D, Prins D. Immunogenicity of unprocessed and photooxidized bovine and human osteochondral grafts in collagen-sensitive mice. BMC Musculoskeletal Disorders. 2006;7:32. doi: 10.1186/1471-2474-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Browne S, Zeugolis DI, Pandit A. Collagen: Finding a Solution for the Source. Tissue Engineering Part A. 2013 doi: 10.1089/ten.tea.2012.0721. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wheeler TS, Sbravati ND, Janorkar AV. Mechanical & Cell Culture Properties of Elastin-Like Polypeptide, Collagen, Bioglass, and Carbon Nanosphere Composites. Annals of Biomedical Engineering. 2013;41(10):2042–2055. doi: 10.1007/s10439-013-0825-3. [DOI] [PubMed] [Google Scholar]
- 59.Patino MG, Neiders ME, Andreana S, Noble B, Cohen RE. Collagen as an implantable material in medicine and dentistry. Journal of Oral Implantology. 2002;28(5):220–5. doi: 10.1563/1548-1336(2002)028<0220:CAAIMI>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 60.Park SN, Kim JK, Suh H. Evaluation of antibiotic-loaded collagen-hyaluronic acid matrix as a skin substitute. Biomaterials. 2004;25(17):3689–98. doi: 10.1016/j.biomaterials.2003.10.072. [DOI] [PubMed] [Google Scholar]
- 61.Cui F, Li G, Huang J, Zhang J, Lu M, Lu W, Huan J, Huang Q. Development of chitosan-collagen hydrogel incorporated with lysostaphin (CCHL) burn dressing with anti-methicillin-resistant Staphylococcus aureus and promotion wound healing properties. Drug Delivery. 2011;18(3):173–80. doi: 10.3109/10717544.2010.509363. [DOI] [PubMed] [Google Scholar]
- 62.Lynn AK, Yannas IV, Bonfield W. Antigenicity and immunogenicity of collagen. Journal of Biomedical Materials Research B: Applied Biomaterials. 2004;71:343–354. doi: 10.1002/jbm.b.30096. [DOI] [PubMed] [Google Scholar]
- 63.Nimni ME, Myers D, Ertl D, Han B. Factors which affect the calcification of tissue-derived bioprostheses. J Biomed Mater Res. 1997;15:531–7. doi: 10.1002/(sici)1097-4636(19970615)35:4<531::aid-jbm13>3.0.co;2-e. 35(4) [DOI] [PubMed] [Google Scholar]
- 64.Kurland NE, Kundu J, Pal S, Kundu SC, Yadavalli VK. Self-assembly mechanisms of silk protein nanostructures on two-dimensional surfaces. Soft Matter. 2012;8:4952–4959. [Google Scholar]
- 65.Li JJ, Gil ES, Hayden RS, Li C, Roohani-Eshafani SI, Kaplan DL, Zreiqat H. Multiple silk coatings on biphasic calcium phosphate scaffolds: effect on physical and mechanical properties and in vitro osteogenic response on human mesenchymal stem cells. Biomacromolecules. 2013;14:2179–2188. doi: 10.1021/bm400303w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akturk O, Tezcaner A, Bilgili H, Deveci MS, Gecit MR, Keskin D. Evaluation of sericin/collagen membranes as prospective wound dressing biomaterial. Journal of Bioscience and Bioengineering. 2011;112(3):279–88. doi: 10.1016/j.jbiosc.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 67.Agrawal CM, Ong JL, Appleford MR, Mani G. Introduction to Biomaterials: Basic Theory with Engineering Applications. Cambridge University Press; Cambridge, UK: 2014. [Google Scholar]
- 68.Heidebrecht A, Schiebel T. Recombinant production of spider silk proteins. Adv Appl Microbiol. 2013;82:115–153. doi: 10.1016/B978-0-12-407679-2.00004-1. [DOI] [PubMed] [Google Scholar]
- 69.Liu SQ. Bioregenerative Engineering: Principles and Applications. John Wiley & Sons; Hoboken, NJ: 2007. [Google Scholar]
- 70.Berknop-Schnurch A, Dunnhaupt S. Chitosan-Based Drug Delivery Systems. Eur J Pharm Biopharm. 2012;81:463–469. doi: 10.1016/j.ejpb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 71.Singha K, Namgung R, Kim WJ. Polymers in Small-Interfering RNA Delivery. Nucleic Acid Ther. 2011;21:133–147. doi: 10.1089/nat.2011.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duceppe N, Tabrizian M. Advances in using chitosan-based nanoparticles for in vitro and in vivo drug and gene delivery. Expert Opin Drug Deliv. 2010;7:1191–1207. doi: 10.1517/17425247.2010.514604. [DOI] [PubMed] [Google Scholar]
- 73.Yeh TH, Hsu LW, Tseng MT, Lee PL, Sonjae K, Ho YC, Sung HW. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials. 2011;32(26):6164–73. doi: 10.1016/j.biomaterials.2011.03.056. [DOI] [PubMed] [Google Scholar]
- 74.Kowapradit J, Opanasopit P, Ngawhirunpat T, Rojanarata T, Ruktanonchai U, Sajomsang W. Methylated N-(4-N,N-dimethylaminocinnamyl) chitosan enhances paracellular permeability across Caco-2 cells. Drug Deliv. 2010;17(5):301–12. doi: 10.3109/10717541003706273. [DOI] [PubMed] [Google Scholar]
- 75.Barber AH, Lu D, Pugno NM. Extreme Strength Observed in Limpet Teeth. Journal of the Royal Society Interface. 2015;12:20141326. doi: 10.1098/rsif.2014.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Uskoković V. Co-Creation of Experiential Qualities. Pragmatics & Cognition. 2011;19(3):562–589. [Google Scholar]
- 77.Atta-ur-Rahman Some Adventures in Natural Product Chemistry and Higher Education. Lecture given at the 7th World Congress on Preventive & Regenerative Medicine; Taipei. 2014. [Google Scholar]
- 78.Rojo ES, Ramos M, Yates M, Martin-Luengo MA, Serrano AMM, Civantos A, López-Lacomba JL, Reilly G, Vervaet C, Tarterra JL, Luis BF, Argomaniz LV. Preparation, characterization and in vitro osteoblast growth of waste-derived biomaterials. ASC Advances. 2014;4:12630–12639. [Google Scholar]
- 79.Ewers R. Maxilla sinus grafting with marine algae derived bone forming material: a clinical report of long-term results. J Oral Maxillofac Surg. 2005;63:1712–23. doi: 10.1016/j.joms.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 80.Wanschitz F, Figl M, Wagner A, Rolf E. Measurement of volume changes after sinus floor augmentation with a phycogenic hydroxyapatite. Int J Oral Maxillofac Implants. 2006 May-Jun;21(3):433–8. [PubMed] [Google Scholar]
- 81.Rezwan K, Chen Q, Blaker J, Boccaccini A. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(18):3413–31. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 82.Bucholz RW. Nonallograft osteoconductive bone graft substitutes. Clinical Orthopaedics and Related Research. 2002;395:44–52. doi: 10.1097/00003086-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 83.Ba Linh NT, Lee KH, Lee BT. Functional nanofiber mat of polyvinyl alcohol/gelatin containing nanoparticles of biphasic calcium phosphate for bone regeneration in rat calvaria defects. Journal of Biomedical Materials Research A. 2013;101(8):2412–23. doi: 10.1002/jbm.a.34533. [DOI] [PubMed] [Google Scholar]
- 84.Pandit V, Zuidema JM, Venuto KN, Macione J, Dai G, Gilbert RJ, Kotha SP. Evaluation of multifunctional polysaccharide hydrogels with varying stiffness for bone tissue engineering. Tissue Engineering Part A. 2013;19(21-22):2452–63. doi: 10.1089/ten.tea.2012.0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 86.Liu Y, Lim J, Teoh S-H. Review: Development of Clinically Relevant Scaffolds for Vascularised Bone Tissue Engineering. Biotechnology Advances. 2013;31:688–705. doi: 10.1016/j.biotechadv.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 87.Zamani F, Amani-Tehran M, Latifi M, Shokrgozar MA. The influence of surface nanoroughness of electrospun PLGA nanofibrous scaffold on nerve cell adhesion and proliferation. Journal of Materials Science: Materials in Medicine. 2013;24(6):1551–60. doi: 10.1007/s10856-013-4905-6. [DOI] [PubMed] [Google Scholar]
- 88.Gongadze E, Kabaso D, Bauer S, Slivnik T, Schmuki P, van Rienen U, Iglič A. Adhesion of osteoblasts to a nanorough titanium implant surface. Int J Nanomedicine. 2011;6:1801–16. doi: 10.2147/IJN.S21755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ge S, Zhao N, Wang L, Liu H, Yang P. Effects of hydroxyapatite nanostructure on channel surface of porcine acellular dermal matrix scaffold on cell viability and osteogenic differentiation of human periodontal ligament stem cells. International Journal of Nanomedicine. 2013;8:1887–1895. doi: 10.2147/IJN.S44695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Siparsky GL, Voorhees KJ, Miao F. Hydrolysis of Polylactic Acid (PLA) and Polycaprolactone (PCL) in Aqueous Acetonitrile Solutions: Autocatalysis. Journal of Environmental Polymer Degradation. 1998;6:31–41. [Google Scholar]
- 91.Vukomanović M, Škapin S, Jančar B, Maksin T, Ignjatović N, Uskoković V, Uskoković D. Poly(D,L-Lactide-Co-Glycolide)/Hydroxyapatite Core-Shell Nanospheres. Part 1: A Multifunctional System for Controlled Drug Delivery. Colloids and Surfaces B: Biointerfaces. 2011;82(2):404–413. doi: 10.1016/j.colsurfb.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 92.Lunt J. Polylactic acid polymers for fibers and nonwovens. International Fiber Journal. 2000;15:48–52. [Google Scholar]
- 93.Sabir MI, Xu X. A review of biodegradable polymeric materials for bone tissue engineering applications. J Mater Sci. 2009;44:5713–24. [Google Scholar]
- 94.Cameron RE, Kamvari-Moghaddam A. Synthetic bioresorbable polymers. In: Buchanan F, editor. Degradation rate of bioresorbable materials. Woodhead; Cambridge: 2008. pp. 43–66. [Google Scholar]
- 95.Hong Y, Guan J, Fujimoto KL, Hashizume R, Pelinescu AL, Wagner WR. Tailoring the degradation kinetics of poly(ester-carbonate urethane)urea thermoplastic elastomers for tissue engineering scaffolds. Biomaterials. 2010;31(15):4249–4258. doi: 10.1016/j.biomaterials.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Uskoković V, Desai TA. Phase Composition Control of Calcium Phosphate Nanoparticles for Tunable Drug Delivery Kinetics and Treatment of Osteomyelitis. Part 1: Preparation and Drug Release. Journal of Biomedical Materials Research Part A. 2013;101(5):1416–1426. doi: 10.1002/jbm.a.34426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eggli PS, Muller W, Schenk RK. Porous hydroxyapatite and tricalcium phosphate cylinders with two different pore size ranges implanted in the cancellous bone of rabbits. A comparative histomorphometric and histologic study of bony ingrowth and implant substitution. Clinical Orthopaedics and Related Research. 1988;232:127–138. [PubMed] [Google Scholar]
- 98.Supová M. Problem of hydroxyapatite dispersion in polymer matrices: a review. J Mater Sci Mater Med. 2009;20(6):1201–13. doi: 10.1007/s10856-009-3696-2. [DOI] [PubMed] [Google Scholar]
- 99.Jungbauer A, Hahn R, Deinhofer K, Luo P. Performance and characterization of a nanophased porous hydroxyapatite for proten chromatography. Biotechnol Bioeng. 2004;87:364–75. doi: 10.1002/bit.20121. [DOI] [PubMed] [Google Scholar]
- 100.Yu S, Geng J, Zhou P, Wang J, Chen X, Hu J. New hydroxyapatite monolithic column for DNA extraction and its application in the purification of Bacillus subtilis crude lysate. J Chromatography A. 2008;1183:29–37. doi: 10.1016/j.chroma.2007.11.120. [DOI] [PubMed] [Google Scholar]
- 101.Lobaina T, Zhurbenko R, Alfonso I, Rodríguez C, Gala-García A, Gontijo SL, Cortés ME, Gomes A, Sinisterra RD. Efficacy of coral-hydroxyapatite and biphasic calcium phosphate for early bacterial detection. Biointerphases. 2014;9(2):029018. doi: 10.1116/1.4880616. [DOI] [PubMed] [Google Scholar]
- 102.Cristofolini L, Berzina T, Erokhina S, Konovalov D, Erokhin V. Structural study of the DNA dipalmitoylphosphatidylcholine complex at the air-water interface. Biomacromolecules. 2007;8(7):2270–5. doi: 10.1021/bm070322w. [DOI] [PubMed] [Google Scholar]
- 103.Bareiro O, Santos LA. Tetraethylorthosilicate (TEOS) applied in the surface modification of hydroxyapatite to develop polydimethylsiloxane/hydroxyapatite composites. Colloids Surf B Biointerfaces. 2014;115:400–5. doi: 10.1016/j.colsurfb.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 104.Arabmotlagh M, Bachmaier S, Geiger F, Rauschmann M. PMMA-hydroxyapatite composite material retards fatigue failure of augmented bone compared to augmentation with plain PMMA: in vivo study using a sheep model. J Biomed Mater Res B Appl Biomater. 2014;102(8):1613–9. doi: 10.1002/jbm.b.33140. [DOI] [PubMed] [Google Scholar]
- 105.Lukaszczyk J, Janicki B, López A, Skołucka K, Wojdyła H, Persson C, Piaskowski S, Smiga-Matuszowicz M. Novel injectable biomaterials for bone augmentation based on isosorbide dimethacrylic monomers. Mater Sci Eng C Mater Biol Appl. 2014;40:76–84. doi: 10.1016/j.msec.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 106.Hild N, Fuhrer R, Mohn D, Bubenhofer SB, Grass RN, Luechinger NA, Feldman K, Dora C, Stark WJ. Nanocomposites of high-density polyethylene with amorphous calcium phosphate: in vitro biomineralization and cytocompatibility of human mesenchymal stem cells. Biomedical Materials. 2012;7(5):054103. doi: 10.1088/1748-6041/7/5/054103. [DOI] [PubMed] [Google Scholar]
- 107.Liao CZ, Wong HM, Yeung KW, Tjong SC. The development, fabrication, and material characterization of polypropylene composites reinforced with carbon nanofiber and hydroxyapatite nanorod hybrid fillers. Int J Nanomedicine. 2014;9:1299–310. doi: 10.2147/IJN.S58332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nie L, Chen D, Suo J, Zou P, Feng S, Yang Q, Yang S, Ye S. Physicochemical characterization and biocompatibility in vitro of biphasic calcium phosphate/polyvinyl alcohol scaffolds prepared by freeze-drying method for bone tissue engineering applications. Colloids and Surfaces B: Biointerfaces. 2012;100:169–76. doi: 10.1016/j.colsurfb.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 109.Ohba S, Wang W, Itoh S, Takagi Y, Nagai A, Yamashita K. Efficacy of platelet-rich plasma gel and hyaluronan hydrogel as carriers of electrically polarized hydroxyapatite microgranules for accelerating bone formation. Journal of Biomedical Materials Research A. 2012;100(11):3167–76. doi: 10.1002/jbm.a.34250. [DOI] [PubMed] [Google Scholar]
- 110.Chen JP, Tsai MJ, Liao HT. Incorporation of biphasic calcium phosphate microparticles in injectable thermoresponsive hydrogel modulates bone cell proliferation and differentiation. Colloids and Surfaces B Biointerfaces. 2013;110C:120–129. doi: 10.1016/j.colsurfb.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 111.Uskoković V, Desai TA. In vitro Analysis of Nanoparticulate Hydroxyapatite/Chitosan Composites as Potential Drug Delivery Platforms for the Sustained Release of Antibiotics in the Treatment of Osteomyelitis. Journal of Pharmaceutical Sciences. 2014;103(2):567–579. doi: 10.1002/jps.23824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim MJ, Koh YH. Synthesis of aligned porous poly(ε-caprolactone) (PCL)/hydroxyapatite (HA) composite microspheres. Materials Science and Engineering C: Materials for Biological Applications. 2013;33(4):2266–72. doi: 10.1016/j.msec.2013.01.051. [DOI] [PubMed] [Google Scholar]
- 113.Fu S, Yang L, Fan J, Wen Q, Lin S, Wang B, Chen L, Meng X, Chen Y, Wu J. In vitro mineralization of hydroxyapatite on electrospun poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) fibrous scaffolds for tissue engineering application. Colloids and Surfaces B: Biointerfaces. 2013;107:167–73. doi: 10.1016/j.colsurfb.2013.01.068. [DOI] [PubMed] [Google Scholar]
- 114.Rodriguez IA, Madurantakam PA, McCool JM, Sell SA, Yang H, Moon PC, Bowlin GL. Mineralization Potential of Electrospun PDO-Hydroxyapatite-Fibrinogen Blended Scaffolds. Int J Biomater. 20122012:159484. doi: 10.1155/2012/159484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van Leeuwen AC, Bos RR, Grijpma DW. Composite materials based on poly(trimethylene carbonate) and β-tricalcium phosphate for orbital floor and wall reconstruction. Journal of Biomedical Materials Research B: Applied Biomaterials. 2012;100(6):1610–20. doi: 10.1002/jbm.b.32729. [DOI] [PubMed] [Google Scholar]
- 116.Nandakumar A, Barradas A, de Boer J, Moroni L, van Blitterswijk C, Habibovic P. Combining technologies to create bioactive hybrid scaffolds for bone tissue engineering. Biomatter. 2013;3(2):e23705. doi: 10.4161/biom.23705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Uskoković V, Hoover C, Vukomanović M, Uskoković DP, Desai TA. Osteogenic and Antimicrobial Nanoparticulate Calcium Phosphate and/or Poly-Lactide-Co-Glycolide Powders for the Treatment of Osteomyelitis. Materials Science and Engineering C: Materials for Biological Applications. 2013;33(6):3362–3373. doi: 10.1016/j.msec.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ganesh N, Jayakumar R, Koyakutty M, Mony U, Nair SV. Embedded silica nanoparticles in poly(caprolactone) nanofibrous scaffolds enhanced osteogenic potential for bone tissue engineering. Tissue Engineering Part A. 2012;18(17-18):1867–81. doi: 10.1089/ten.TEA.2012.0167. [DOI] [PubMed] [Google Scholar]
- 119.Wang C, Lin K, Chang J, Sun J. Osteogenesis and angiogenesis induced by porous β-CaSiO(3)/PDLGA composite scaffold via activation of AMPK/ERK1/2 and PI3K/Akt pathways. Biomaterials. 2013;34(1):64–77. doi: 10.1016/j.biomaterials.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 120.Nadeem D, Kiamehr M, Yang X, Su B. Fabrication and in vitro evaluation of a sponge-like bioactive-glass/gelatin composite scaffold for bone tissue engineering. Materials Science and Engineering C: Materials for Biological Applications. 2013;33(5):2669–78. doi: 10.1016/j.msec.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 121.Filipowska J, Pawlik J, Cholewa-Kowalska K, Tylko G, Pamula E, Niedzwiedzki L, Szuta M, Laczka M, Osyczka AM. Incorporation of sol–gel bioactive glass into PLGA improves mechanical properties and bioactivity of composite scaffolds and results in their osteoinductive properties. Biomed. Mater. 2014;9:065001. doi: 10.1088/1748-6041/9/6/065001. [DOI] [PubMed] [Google Scholar]
- 122.Uskoković V, Lee PP, Walsh L, Fischer KE, Desai TA. Silicon Nanowire Coated Microparticles as Epithelial Drug Delivery Devices. The Effect of PEGylation on Particle-Epithelium Interactions. Biomaterials. 2012;33(5):1663–1672. doi: 10.1016/j.biomaterials.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carlisle EM. Proceedings: Silicon as an essential element. Federation Proceedings. 1974;33(6):1758–66. [PubMed] [Google Scholar]
- 124.Porter AE, Botelho CM, Lopes MA, Santos JD, Best SM, Bonfield W. Ultrastructural comparison of dissolution and apatite precipitation on hydroxyapatite and silicon-substituted hydroxyapatite in vitro and in vivo. Journal of Biomedical Materials Research A. 2004;69(4):670–9. doi: 10.1002/jbm.a.30035. [DOI] [PubMed] [Google Scholar]
- 125.Li Y, Li Q, Zhu S, Luo E, Li J, Feng G, Liao Y, Hu J. The effect of strontium-substituted hydroxyapatite coating on implant fixation in ovariectomized rats. Biomaterials. 2010;31(34):9006–14. doi: 10.1016/j.biomaterials.2010.07.112. [DOI] [PubMed] [Google Scholar]
- 126.Lalwani G, Henslee AM, Farshid B, Lin L, Kasper FK, Qin YX, Mikos AG, Sitharaman B. Two-dimensional nanostructure-reinforced biodegradable polymeric nanocomposites for bone tissue engineering. Biomacromolecules. 2013;14(3):900–9. doi: 10.1021/bm301995s. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Surnamelalwani G, Surnamehenslee GM, Surnamefarshid G, Surnameparmar G, Surnamelin G, Surnameqin GX, Kasper GS, Surnamemikos GG, Surnamesitharaman G. Tungsten Disulfide Nanotubes Reinforced Biodegradable Polymers for Bone Tissue Engineering. Acta Biomaterialia. 2013 doi: 10.1016/j.actbio.2013.05.018. doi:pii: S1742-7061(13)00267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jia H, Kerr LL. Sustained ibuprofen release using composite poly(lactic-co-glycolic acid)/titanium dioxide nanotubes from Ti implant surface. Journal of Pharmaceutical Sciences. 2013;102(7):2341–8. doi: 10.1002/jps.23580. [DOI] [PubMed] [Google Scholar]
- 129.Smith YR, Sarma B, Mohanty SK, Misra M. Light-assisted anodized TiO2 nanotube arrays. ACS Applied Materials and Interfaces. 2012;4(11):5883–90. doi: 10.1021/am301527g. [DOI] [PubMed] [Google Scholar]
- 130.Smith BS, Popat KC. Titania nanotube arrays as interfaces for blood-contacting implantable devices: a study evaluating the nanotopography-associated activation and expression of blood plasma components. Journal of Biomedical Nanotechnology. 2012;8(4):642–658. doi: 10.1166/jbn.2012.1421. [DOI] [PubMed] [Google Scholar]
- 131.Song YY, Schmidt-Stein F, Bauer S, Schmuki P. Amphiphilic TiO2 nanotube arrays: an actively controllable drug delivery system. Journal of the American Chemical Society. 2009;131:4230–4342. doi: 10.1021/ja810130h. [DOI] [PubMed] [Google Scholar]
- 132.Popat K, Eltgroth M, LaTempa T, Grimes C, Desai T. Decreased Staphylococcus epidermis adhesion and increased osteoblast functionality on antibiotic-loaded titania nanotubes. Biomaterials. 2007;28:4880–4888. doi: 10.1016/j.biomaterials.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 133.Bernards DA, Desai TA. Nanotemplating of Biodegradable Polymer Membranes for Constant-Rate Drug Delivery. Advanced Materials. 2010;22:2358–2362. doi: 10.1002/adma.200903439. [DOI] [PubMed] [Google Scholar]
- 134.Hu Y, Cai K, Luo Z, Xu D, Xie D, Huang Y, Yang W, Liu P. TiO2 nanotubes as drug nanoreservoirs for the regulation of mobility and differentiation of mesenchymal stem cells. Acta Biomaterialia. 2012;8:439–448. doi: 10.1016/j.actbio.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 135.Aw MS, Addai-Mensah J, Losic D. A multi-drug delivery system with sequential release using titania nanotube arrays. Chemical Communications. 2012;48(27):3348–3350. doi: 10.1039/c2cc17690d. [DOI] [PubMed] [Google Scholar]
- 136.Bainbridge JA, Revell PA, Al-Saffar N. Costimulatory molecule expression following exposure to orthopaedic implants wear debris. Journal of Biomedical Materials Research. 2001;54(3):328–34. doi: 10.1002/1097-4636(20010305)54:3<328::aid-jbm30>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 137.Kretzer JP, Reinders J, Sonntag R, Hagmann S, Streit M, Jeager S, Moradi B. Wear in total knee arthroplasty--just a question of polyethylene?: Metal ion release in total knee arthroplasty. Int Orthop. 2014;38(2):335–40. doi: 10.1007/s00264-013-2162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Desai MA, Bancroft LW. Periprosthetic Osteolysis. Orthopedics. 2008;31(6) doi: 10.3928/01477447-20080601-07. [DOI] [PubMed] [Google Scholar]
- 139.Grosse S, Haugland HK, Lilleng P, Ellison P, Hallan G, Høl PJ. Wear particles and ions from cemented and uncemented titanium-based hip prostheses-A histological and chemical analysis of retrieval material. J Biomed Mater Res B Appl Biomater. 2014 Jul 23; doi: 10.1002/jbm.b.33243. doi: 10.1002/jbm.b.33243. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Persaud-Sharma D, McGoron A. Biodegradable Magnesium Alloys: A Review of Material Development and Applications. J Biomim Biomat Tissue Eng. 2012;12:25–39. doi: 10.4028/www.scientific.net/JBBTE.12.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Campos CM, Muramatsu T, Iqbal J, Zhang Y-J, Onuma Y, Garcia-Garcia Hector M., Haude M, Lemos PA, Warnack B, Serruys PW. Bioresorbable Drug-Eluting Magnesium-Alloy Scaffold for Treatment of Coronary Artery Disease. Int. J. Mol. Sci. 2013;14(12):24492–24500. doi: 10.3390/ijms141224492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ziran BH, Smith WR, Morgan SJ. Use of calcium-based demineralized bone matrix/allograft for nonunions and posttraumatic reconstruction of the appendicular skeleton: preliminary results and complications. Journal of Trauma. 2007;63(6):1324–8. doi: 10.1097/01.ta.0000240452.64138.b0. [DOI] [PubMed] [Google Scholar]
- 143.Lee JS, Suarez-Gonzalez D, Murphy WL. Mineral Coatings for Temporally Controlled Delivery of Multiple Proteins. Advanced Materials. 2011;23:4279–4284. doi: 10.1002/adma.201100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang GL, He FM, Song E, Hu JA, Wang XX, Zhao SF. In vivo comparison of bone formation on titanium implant surfaces coated with biomimetically deposited calcium phosphate or electrochemically deposited hydroxyapatite. International Journal of Oral and Maxillofacial Implants. 2010;25(4):669–80. [PubMed] [Google Scholar]
- 145.Vanderleyden E, Mullens S, Luyten J, Dubruel P. Implantable (bio)polymer coated titanium scaffolds: a review. Current Pharmaceutical Design. 2012;18(18):2576–90. doi: 10.2174/138161212800492903. [DOI] [PubMed] [Google Scholar]
- 146.Nayak S, Dey T, Naskar D, Kundu SC. The promotion of osseointegration of titanium surfaces by coating with silk protein sericin. Biomaterials. 2013;34(12):2855–64. doi: 10.1016/j.biomaterials.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 147.Laonapakul T, Nimkerdphol AR, Otsuka Y, Mutoh Y. Failure behavior of plasma-sprayed HAp coating on commercially pure titanium substrate in simulated body fluid (SBF) under bending load. Journal of Mechanical Behavior of Biomedical Materials. 2012;15:153–66. doi: 10.1016/j.jmbbm.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 148.Dalat F, Barnoud R, Fessy MH, Besse JL. Histologic study of periprosthetic osteolytic lesions after AES total ankle replacement. A 22 case series. Orthop Traumatol Surg Res. 2013;99(6 Suppl):S285–95. doi: 10.1016/j.otsr.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 149.Kharaziha M, Fathi MH, Edris H. Development of novel aligned nanofibrous composite membranes for guided bone regeneration. Journal of Mechanical Behavior of Biomedical Materials. 2013;24:9–20. doi: 10.1016/j.jmbbm.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 150.Jaiswal AK, Chhabra H, Kadam SS, Londhe K, Soni VP, Bellare JR. Hardystonite improves biocompatibility and strength of electrospun polycaprolactone nanofibers over hydroxyapatite: a comparative study. Materials Science and Engineering C: Materials for Biological Applications. 2013;33(5):2926–36. doi: 10.1016/j.msec.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 151.Obata A, Ozasa H, Kasuga T, Jones JR. Cotton wool-like poly(lactic acid)/vaterite composite scaffolds releasing soluble silica for bone tissue engineering. Journal of Materials Science: Materials in Medicine. 2013;24(7):1649–58. doi: 10.1007/s10856-013-4930-5. [DOI] [PubMed] [Google Scholar]
- 152.Marsich E, Bellomo F, Turco G, Travan A, Donati I, Paoletti S. Nano-composite scaffolds for bone tissue engineering containing silver nanoparticles: preparation, characterization and biological properties. Journal of Materials Science: Materials in Medicine. 2013;24(7):1799–807. doi: 10.1007/s10856-013-4923-4. [DOI] [PubMed] [Google Scholar]
- 153.Jung WK, Koo HC, Kim KW, Shin S, Kim SH, Park YH. Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli. Appl Environ Microbiol. 2008;74:2171–2178. doi: 10.1128/AEM.02001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rodrıguez-Valencia C, Lopez-Alvarez M, Cochon-Cores B, Pereiro I, Serra J, Gonzalez P. Novel selenium doped hydroxyapatite coatings for biomedical applications. Journal of Biomedical Materials Research A. 2013;101:853–861. doi: 10.1002/jbm.a.34387. [DOI] [PubMed] [Google Scholar]
- 155.Kelson AB, Carnevali M, Truong-Le V. Gallium-Based Anti-Infectives: Targeting Microbial Iron-Uptake Mechanisms. Curr Opin Pharmacol. 2013;13:707–716. doi: 10.1016/j.coph.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 156.Minandri F, Bonchi C, Frangipani E, Imperi F, Visca P. Promises and Failures of Gallium as an Antibacterial Agent. Future Microbiol. 2014;9:379–397. doi: 10.2217/fmb.14.3. [DOI] [PubMed] [Google Scholar]
- 157.Kolmas J, Groszyk E, Kwiatkowska-Rózycka D. Substituted Hydroxyapatites with Antibacterial Properties. BioMed Research International ID. 2014:178123. doi: 10.1155/2014/178123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Uskoković V, Košak A, Drofenik M. Silica-Coated Lanthanum-Strontium Manganites for Hyperthermia Treatments. Materials Letters. 2006;60(21-22):2620–2622. [Google Scholar]
- 159.Pavone M, Munoz-Garcia AN, Ritzmann AM, Carter EA. First-Principles Study of Lanthanum Strontium Manganite: Insights into Electronic Structure and Oxygen Vacancy Formation. Journal of Physical Chemistry. 2014;C118:13346–13356. [Google Scholar]
- 160.Gozar A, Logvenov G, Fitting Kourkoutis L, Bollinger AT, Giannuzzi LA, Muller DA, Bozovic I. High-temperature interface superconductivity between metallic and insulating copper oxides. Nature. 2008;455:782–785. doi: 10.1038/nature07293. [DOI] [PubMed] [Google Scholar]
- 161.Estrin Y, Ivanova EP, Michalska A, Truong VK, Lapovok R, Boyd R. Accelerated stem cell attachment to ultrafine grained titanium. Acta Biomater. 2011;7(2):900–6. doi: 10.1016/j.actbio.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 162.Smith AM, Paxton JZ, Hung YP, Hadley MJ, Bowen J, Williams RL, Grover LM. Nanoscale crystallinity modulates cell proliferation on plasma sprayed surfaces. Materials Science and Engineering C: Materials for Biological Applications. 2015;48:5–10. doi: 10.1016/j.msec.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 163.Wang J, Fan J. Lung Injury Induced by TiO2 Nanoparticles Depends on Their Structural Features: Size, Shape, Crystal Phases, and Surface Coating. Int. J. Mol. Sci. 2014;15:22258–22278. doi: 10.3390/ijms151222258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–91. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 165.Xu M, Zhai D, Chang J, Wu C. In vitro assessment of three-dimensionally plotted nagelschmidtite bioceramic scaffolds with varied macropore morphologies. Acta Biomater. 2014;10(1):463–76. doi: 10.1016/j.actbio.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 166.Silva RM, Teesy C, Franzi L, Weir A, Westerhoff P, Evans JE, Pinkerton KE. Biological response to nano-scale titanium dioxide (TiO2): role of particle dose, shape, and retention. J Toxicol Environ Health A. 2013;76(16):953–72. doi: 10.1080/15287394.2013.826567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nguyen DT, McCanless JD, Mecwan MM, Noblett AP, Haggard WO, Smith RA, Bumgardner JD. Balancing mechanical strength with bioactivity in chitosan-calcium phosphate 3D microsphere scaffolds for bone tissue engineering: air- vs. freeze-drying processes. Journal of Biomaterial Science Polymer Edition. 2013;24(9):1071–83. doi: 10.1080/09205063.2012.735099. [DOI] [PubMed] [Google Scholar]
- 168.Zhang H, Gilbert B, Huang F, Banfield JF. Water-Driven Structural Transformation in Nanoparticles at Room Temperature. Nature. 2003;424:1025–1029. doi: 10.1038/nature01845. [DOI] [PubMed] [Google Scholar]
- 169.Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science. 2000;289(5484):1501–1504. doi: 10.1126/science.289.5484.1501. [DOI] [PubMed] [Google Scholar]
- 170.Butcher DT, Alliston T, Weaver VM. A Tense Situation: Forcing Tumour Progression. Nature Reviews Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Uddin SM, Qin YX. Dynamic acoustic radiation force retains bone structural and mechanical integrity in a functional disuse osteopenia model. Bone. 2015 doi: 10.1016/j.bone.2015.01.020. pii: S8756-3282(15)00034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nguyen J, Tang SY, Nguyen D, Alliston T. Load regulates bone formation and Sclerostin expression through a TGFb-dependent mechanism. PLoS One. 8(1):e53813. doi: 10.1371/journal.pone.0053813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bruder SP, Jaiswal N, Ricalton NS, Mosca JD, Kraus KH, Kadiyala S. Mesenchymal stem cells in osteobiology and applied bone regeneration. Clinical Orthopaedics and Related Research. 1998;(355 Suppl):S247–56. doi: 10.1097/00003086-199810001-00025. [DOI] [PubMed] [Google Scholar]
- 174.Mistry AS, Mikos AG. Tissue Engineering Strategies for Bone Regeneration. Advanced Biochemical Engineering/Biotechnology. 2005;94:1–22. doi: 10.1007/b99997. [DOI] [PubMed] [Google Scholar]
- 175.Ignjatović N, Uskoković V, Ajduković Z, Uskoković D. Multifunctional Hydroxyapatite and Poly(D,L-Lactide-co-Glycolide) Nanoparticles for the Local Delivery of Cholecalciferol. Materials Science and Engineering C: Materials for Biological Applications. 2013;33(2):943–950. doi: 10.1016/j.msec.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rouquerol J, Avnir D, Fairbridge CW, Everett DH, Haynes JH, PErnicone N, Ramsay JDF, Sing KSW, Unger KK. Recommendations for the characterization of porous solids. Pure & Applied Chemistry. 1994;66(8):1739–1758. [Google Scholar]
- 177.Duan P, Pan Z, Cao L, He Y, Wang H, Qu Z, Dong J, Ding J. The effects of pore size in bilayered poly(lactide-co-glycolide) scaffolds on restoring osteochondral defects in rabbits. Journal of Biomedical Materials Research A. 2013 doi: 10.1002/jbm.a.34683. in press; doi: 10.1002/jbm.a.34683. [DOI] [PubMed] [Google Scholar]
- 178.Holzwarth JM, Ma PX. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials. 2011;32:9622–9629. doi: 10.1016/j.biomaterials.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Miki T, Imai Y. Osteoinductive potential of freeze-dried, biodegradable, poly (glycolic acid-co-lactic acid) disks incorporated with bone morphogenetic protein in skull defects of rats. International Journal of Oral and Maxillofacial Surgery. 1996;25(5):402–6. doi: 10.1016/s0901-5027(06)80042-1. [DOI] [PubMed] [Google Scholar]
- 180.Jensen T, Baas J, Dolathshahi-Pirouz A, Jacobsen T, Singh G, Nygaard JV, Foss M, Bechtold J, Bünger C, Besenbacher F, Søballe K. Osteopontin functionalization of hydroxyapatite nanoparticles in a PDLLA matrix promotes bone formation. Journal of Biomedical Materials Research A. 2011;99(1):94–101. doi: 10.1002/jbm.a.33166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ji Y, Xu GP, Zhang ZP, Xia JJ, Yan JL, Pan SH. BMP-2/PLGA delayed-release microspheres composite graft, selection of bone particulate diameters, and prevention of aseptic inflammation for bone tissue engineering. Annals in Biomedical Engineering. 2010;38(3):632–9. doi: 10.1007/s10439-009-9888-6. [DOI] [PubMed] [Google Scholar]
- 182.Lasserre A, Bajpai PK. Ceramic Drug-Delivery Devices. Critical Reviews in Therapeutic Drug Carrier Systems. 1998;15(1):1–56. [PubMed] [Google Scholar]
- 183.Liu Y, Hunziker EB, Randall NX, de Groot K, Layrolle P. Proteins incorporated into biomimetically prepared calcium phosphate coatings modulate their mechanical strength and dissolution rate. Biomaterials. 2003;24:65–70. doi: 10.1016/s0142-9612(02)00252-1. [DOI] [PubMed] [Google Scholar]
- 184.Tannoury CA, An HS. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014;14(3):552–9. doi: 10.1016/j.spinee.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 185.Yang X, Wang YP, Liu FX, Zeng K, Qian MQ, Chen G, Shi L, Zhu GX. Increased invasiveness of osteosarcoma mesenchymal stem cells induced by bone-morphogenetic protein-2. In Vitro Cell Dev Biol Anim. 2013;49(4):270–8. doi: 10.1007/s11626-013-9598-0. [DOI] [PubMed] [Google Scholar]
- 186.Eguchi Y, Wakitani S, Imai Y, Naka Y, Hashimoto Y, Nakamura H, Takaoka K. Antitumor necrotic factor agent promotes BMP-2-induced ectopic bone formation. J Bone Miner Metab. 2010;28(2):157–64. doi: 10.1007/s00774-009-0127-x. [DOI] [PubMed] [Google Scholar]
- 187.Baldo BA. Side effects of cytokines approved for therapy. Drug Saf. 2014;37(11):921–43. doi: 10.1007/s40264-014-0226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Uskoković V, Batarni SS, Schweicher J, King A, Desai TA. Effect of Calcium Phosphate Particle Shape and Size on their Antibacterial and Osteogenic Activity in the Delivery of Antibiotics in vitro. ACS Applied Materials & Interfaces. 2013;5(7):2422–2431. doi: 10.1021/am4000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Uskoković V. Nanostructured Platforms for the Sustained and Local Delivery of Antibiotics in the Treatment of Osteomyelitis. Critical Reviews in Therapeutic Drug Carrier Systems. 2015;32(1):1–59. doi: 10.1615/critrevtherdrugcarriersyst.2014010920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dong J, Zhang S, Liu H, Li X, Liu Y, Du Y. Novel alternative therapy for spinal tuberculosis during surgery: reconstructing with anti-tuberculosis bioactivity implants. Expert Opin Drug Deliv. 2014;11(3):299–305. doi: 10.1517/17425247.2014.872625. [DOI] [PubMed] [Google Scholar]
- 191.Siepmann J, Gopferich A. Mathematical Modeling of Bioerodible, Polymeric Drug Delivery Systems. Advanced Drug Delivery Reviews. 2001;48:229–247. doi: 10.1016/s0169-409x(01)00116-8. [DOI] [PubMed] [Google Scholar]
- 192.Alexis F. Facors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly(lactic acid)-co-(glycolic acid) Polymer International. 2005;54:36–46. [Google Scholar]
- 193.Jain JP, Chitkara D, Kumar N. Polyanhydrides as localized drug delivery carrier: an update. Expert Opinion in Drug Delivery. 2008;5:889–907. doi: 10.1517/17425247.5.8.889. [DOI] [PubMed] [Google Scholar]
- 194.Lemaire V, Belair J, Hildgen P. Structural modeling of drug release from biodegradable porous matrices based on a combined diffusion/erosion process. International Journal of Pharmaceutics. 2003;258(1-2):95–107. doi: 10.1016/s0378-5173(03)00165-0. [DOI] [PubMed] [Google Scholar]
- 195.Lu Z, Tsai M, Lu D, Wang J, Wientjes MG, Au JL-S. Tumor-Penetrating Microparticles for Intraperitoneal Therapy of Ovarian Cancer. Journal of Pharmacology and Experimental Therapeutics. 2008;327:673–682. doi: 10.1124/jpet.108.140095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G. Stimulation of Bone Formation in Vitro and in Rodents by Statins. Science. 1999;286:1946–9. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 197.Wang XL, Xie XH, Zhang G, Chen SH, Yao D, He K, Wang XH, Yao XS, Leng Y, Fung KP, Leung KS, Qin L. Exogenous phytoestrogenic molecule icaritin incorporated into a porous scaffold for enhancing bone defect repair. J Orthop Res. 2013;31(1):164–72. doi: 10.1002/jor.22188. [DOI] [PubMed] [Google Scholar]
- 198.Graham FL, van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 199.Bisht S, Bhakta G, Mitra S, Maitra A. DNA loaded calcium phosphate nanoparticles: highly efficient non-viral vector for gene delivery. International Journal of Pharmaceutics. 2005;288:157–168. doi: 10.1016/j.ijpharm.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 200.Needham CJ, Williams AK, Chew SA, Kasper FK, Mikos AG. Engineering a polymeric gene delivery vector based on poly(ethylenimine) and hyaluronic acid. Biomacromolecules. 2012;13(5):1429–37. doi: 10.1021/bm300145q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Covello G, Siva K, Adami V, Denti MA. An Electroporation Protocol for Efficient DNA Transfection in PC12 Cells. Cytotechnology. 2014;66(4):543–53. doi: 10.1007/s10616-013-9608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wu TY, Zhou ZB, He ZW, Ren WP, Yu XW, Huang Y. Reinforcement of a new calcium phosphate cement with RGD-chitosan-fiber. Journal of Biomedical Materials Research A. 2014;102(2):315–23. doi: 10.1002/jbm.a.34669. [DOI] [PubMed] [Google Scholar]
- 203.Bongio M, van den Beucken JJ, Nejadnik MR, Leeuwenburgh SC, Kinard LA, Kasper FK, Mikos AG, Jansen JA. Biomimetic modification of synthetic hydrogels by incorporation of adhesive peptides and calcium phosphate nanoparticles: in vitro evaluation of cell behavior. European Cell and Materials. 2011;17(22):359–76. doi: 10.22203/ecm.v022a27. [DOI] [PubMed] [Google Scholar]
- 204.D'Souza SE, Ginsberg MH, Plow EF. Arginyl-glycyl-aspartic acid (RGD): a cell adhesion motif. Trends in Biochemical Sciences. 1991;16(7):246–50. doi: 10.1016/0968-0004(91)90096-e. [DOI] [PubMed] [Google Scholar]
- 205.Jeon O, Alsberg E. Photofunctionalization of alginate hydrogels to promote adhesion and proliferation of human mesenchymal stem cells. Tissue Engineering Part A. 2013;19(11-12):1424–32. doi: 10.1089/ten.tea.2012.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Chung TW, Lai DM, Chen SD, Lin YI. Poly (ε-caprolactone) scaffolds functionalized by grafting NGF and GRGD promote growth and differentiation of PC12 cells. Journal of Biomedical Materials Research A. 2013 doi: 10.1002/jbm.a.34693. doi: 10.1002/jbm.a.34693. [DOI] [PubMed] [Google Scholar]
- 207.Shiu HT, Goss B, Lutton C, Crawford R, Xiao Y. Formation of blood clot on biomaterial implants influences bone healing. Tissue Eng Part B Rev. 2014;20(6):697–712. doi: 10.1089/ten.TEB.2013.0709. [DOI] [PubMed] [Google Scholar]
- 208.Li D, Wang S, Wu Z, Chen H, Brash JL. A new t-PA releasing concept based on protein–protein displacement. Soft Matter. 2013;9:2321–2328. [Google Scholar]
- 209.Wu Z, Chen H, Li D, Brash JL. Tissue plasminogen activator-containing polyurethane surfaces for fibrinolytic activity. Acta Biomater. 2011;7(5):1993–8. doi: 10.1016/j.actbio.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 210.Liu J, Zhang H, Dong Y, Jin Y, Hu X, Cai K, Ma J, Wu G. Bi-directionally selective bone targeting delivery for anabolic and antiresorptive drugs: a novel combined therapy for osteoporosis? Med Hypotheses. 2014;83(6):694–6. doi: 10.1016/j.mehy.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 211.Wu H, Xu Y, Liu G, Ling J, Dash BC, Ruan J, Zhang C. Emulsion cross-linked chitosan/nanohydroxyapatite microspheres for controlled release of alendronate. J Mater Sci Mater Med. 2014;25(12):2649–58. doi: 10.1007/s10856-014-5289-y. [DOI] [PubMed] [Google Scholar]
- 212.Zou Y, Brooks JL, Talwalkar V, Milbrandt TA, Puleo DA. Development of an injectable two-phase drug delivery system for sequential release of antiresorptive and osteogenic drugs. J Biomed Mater Res B Appl Biomater. 2012;100(1):155–62. doi: 10.1002/jbm.b.31933. [DOI] [PubMed] [Google Scholar]
- 213.Hirabayashi H, Takahashi T, Fujisaki J, Masunaga T, Sato S, Hiroi J, Tokunaga Y, Kimura S, Hata T. Bone-specific delivery and sustained release of diclofenac, a non-steroidal anti-inflammatory drug, via bisphosphonic prodrug based on the Osteotropic Drug Delivery System (ODDS) J Control Release. 2001;70(1-2):183–91. doi: 10.1016/s0168-3659(00)00355-2. [DOI] [PubMed] [Google Scholar]
- 214.Campoccia D, Montanaro L, Arciola CR. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials. 2013;34(34):8533–54. doi: 10.1016/j.biomaterials.2013.07.089. [DOI] [PubMed] [Google Scholar]
- 215.de Trad CH, Fang Q, Ćosić I. The Resonant Recognition Model (RRM) Predicts Amino Acid Residues in Highly Conservative Regions of the Hormone Prolactin (PRL) Biophysical Chemistry. 2000;84:149–157. doi: 10.1016/s0301-4622(00)00109-5. [DOI] [PubMed] [Google Scholar]
- 216.Lunyak VL, Rosenfeld MG. Epigenetic regulation of stem cell fate. Human Molecular Genetics. 2008;17(R1):R28–R36. doi: 10.1093/hmg/ddn149. [DOI] [PubMed] [Google Scholar]
- 217.Bysell H, Månsson R, Hansson P, Malmsten M. Microgels and microcapsules in peptide and protein drug delivery. Advanced Drug Delivery Reviews. 2011;63(13):1172–85. doi: 10.1016/j.addr.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 218.Torchilin VP. Drug Delivery – Handbook of Experimental Pharmacology. Vol. 197. Springer-Verlag; Berlin: 2010. Passive and Active Drug Targeting: Drug Delivery to Tumors as an Example. [DOI] [PubMed] [Google Scholar]
- 219.Lallana E, Sousa-Herves A, Fernandez-Trillo F, Riguera R, Fernandez-Megia E. Click chemistry for drug delivery nanosystems. Pharmaceutical Research. 2012;29(1):1–34. doi: 10.1007/s11095-011-0568-5. [DOI] [PubMed] [Google Scholar]
- 220.Pechar M, Pola R, Laga R, Ulbrich K, Bednárová L, Maloň P, Sieglová I, Král V, Fábry M, Vaněk O. Coiled coil peptides as universal linkers for the attachment of recombinant proteins to polymer therapeutics. Biomacromolecules. 2011;12(10):3645–3655. doi: 10.1021/bm200897b. [DOI] [PubMed] [Google Scholar]
- 221.Collins PG. Defects and Disorder in Carbon Nanotubes. In: Narlikar AV, Fu YY, editors. Oxford Handbook of Nanoscience and Technology, Volume 2: Materials: Structures, Properties and Characterization Techniques. Oxford University Press; Oxford, UK: 2010. pp. 23–78. [Google Scholar]
- 222.Georgakilas V, Otyepka M, Bourlinos AB, Chandra V, Kim N, Kemp KC, Hobza P, Zboril R, Kim KS. Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chemical Reviews. 2012;112(11):6156–214. doi: 10.1021/cr3000412. [DOI] [PubMed] [Google Scholar]
- 223.Fishburn CS. The Pharmacology of PEGylation: Balancing PD with PK to generate novel therapeutics. Journal of Pharmaceutical Science. 2008;97:4167–4183. doi: 10.1002/jps.21278. [DOI] [PubMed] [Google Scholar]
- 224.Ignjatović N, Vranješ-Djurić S, Mitić Z, Janković D, Uskoković D. Investigating an organ-targeting platform based on hydroxyapatite nanoparticles using a novel in situ method of radioactive 125Iodine labeling. Mater Sci Eng C Mater Biol Appl. 2014;43:439–46. doi: 10.1016/j.msec.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 225.Ignjatović N, Ajduković Z, Savić V, Najman S, Mihailović D, Vasiljević P, Stojanović Z, Uskoković V, Uskoković D. Nanoparticles of Cobalt-Substituted Hydroxyapatite in Regeneration of Mandibular Osteoporotic Bones. Journal of Materials Science: Materials in Medicine. 2013;24(2):343–354. doi: 10.1007/s10856-012-4793-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Stevanović M, Uskoković V, Filipović M, Škapin SD, Uskoković DP. Composite PLGA/AgNpPGA/AscH Nanospheres with Combined Osteoinductive, Antioxidative and Antimicrobial Activities. ACS Applied Materials and Interfaces. 2013;5(18):9034–9042. doi: 10.1021/am402237g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tan Y, Wang G, Fan H, Wang X, Lu J, Zhang X. Expression of core binding factor 1 and osteoblastic markers in C2C12 cells induced by calcium phosphate ceramics in vitro. Journal of Biomedical Materials Research A. 2007;82(1):152–9. doi: 10.1002/jbm.a.31125. [DOI] [PubMed] [Google Scholar]
- 228.Rupani A, Hidalgo-Bastida LA, Rutten F, Dent A, Turner I, Cartmell S. Osteoblast activity on carbonated hydroxyapatite. Journal of Biomedical Materials Research A. 2012;100(4):1089–96. doi: 10.1002/jbm.a.34037. [DOI] [PubMed] [Google Scholar]
- 229.Song JH, Kim JH, Park S, Kang W, Kim HW, Kim HE, Jang JH. Signaling responses of osteoblast cells to hydroxyapatite: the activation of ERK and SOX9. Journal of Bone and Mineral Metabolism. 2008;26(2):138–42. doi: 10.1007/s00774-007-0804-6. [DOI] [PubMed] [Google Scholar]
- 230.Zhang W, Tsurushima H, Oyane A, Yazaki Y, Sogo Y, Ito A, Matsumura A. BMP-2 gene-fibronectin-apatite composite layer enhances bone formation. Journal of Biomedical Science. 2011;18(62) doi: 10.1186/1423-0127-18-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bae SE, Choi J, Joung YK, Park K, Han DK. Controlled release of bone morphogenetic protein (BMP)-2 from nanocomplex incorporated on hydroxyapatite-formed titanium surface. Journal of Controlled Release. 2012;160(3):676–684. doi: 10.1016/j.jconrel.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 232.Lock J, Liu H. Nanomaterials enhance osteogenic differentiation of human mesenchymal stem cells similar to a short peptide of BMP-7. International Journal of Nanomedicine. 2011;6:2769–2777. doi: 10.2147/IJN.S24493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Uskoković V, Desai TA. Does translational symmetry matter? Fibroblastic and Osteoblastic Interactions with the Topographically Distinct Poly(ε-Caprolactone)/Hydroxyapatite Thin Films. ACS Applied Materials and Interfaces. 2014;6(15):13209–13220. doi: 10.1021/am503043t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Fiedler J, Ozdemir B, Bartholomä J, Plettl A, Brenner RE, Ziemann P. The effect of substrate surface nanotopography on the behavior of multipotnent mesenchymal stromal cells and osteoblasts. Biomaterials. 2013;34(35):8851–9. doi: 10.1016/j.biomaterials.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 235.Zhao L, Liu L, Wu Z, Zhang Y, Chu PK. Effects of micropitted/nanotubular titania topographies on bone mesenchymal stem cell osteogenic differentiation. Biomaterials. 2012;33(9):2629–41. doi: 10.1016/j.biomaterials.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 236.Wu YN, Law JB, He AY, Low HY, Hui JH, Lim CT, Yang Z, Lee EH. Substrate topography determines the fate of chondrogenesis from human mesenchymal stem cells resulting in specific cartilage phenotype formation. Nanomedicine. 2014;10(7):1507–16. doi: 10.1016/j.nano.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 237.Jahangir AA, Nunley RM, Mehta S, Sharan A, Jahangir AA, Nunley RM, Kusuma SK, Genuario JW, Ranawat A, Covey A, Flint J. Bone-graft substitutes in orthopaedic surgery. American Academy of Orthopaedic Surgeons Now. 2008 Jan; retrieved from http://www.aaos.org/news/aaosnow/jan08/reimbursement2.asp.
- 238.Wu S, Liu X, Yeung KWK, Liu C, Yang X. Biomimetic porous scaffolds for bone tissue engineering. Mat Sci Eng R. 2014;80:1–36. [Google Scholar]
- 239.Orthoworld, Inc. Orthopaedic Industry Annual Report. 2011 [Google Scholar]
- 240.Desai BM. Osteobiologics. Am J Orthop. 2007;36:8–11. [PubMed] [Google Scholar]
- 241.Uskoković V, Desai TA. Phase Composition Control of Calcium Phosphate Nanoparticles for Tunable Drug Delivery Kinetics and Treatment of Osteomyelitis. Part 2: Antibacterial and Osteoblastic Response. Journal of Biomedical Materials Research Part A. 2013;101(5):1427–1436. doi: 10.1002/jbm.a.34437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Blanco E, Sangai T, Wu S, Hsiao A, Ruiz-Esparza GU, Gonzalez-Delgado CA, Cara FE, Granados-Principal S, Evans KW, Akcakanat A, Wang Y, Do KA, Meric-Bernstam F, Ferrari M. Colocalized delivery of rapamycin and paclitaxel to tumors enhances synergistic targeting of the PI3K/Akt/mTOR pathway. Mol. Ther. 2014;22:1310–9. doi: 10.1038/mt.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Trosset JY, Carbonell P. Synergistic Synthetic Biology: Units in Concert. Front Bioeng Biotechnol. 2013;1:11. doi: 10.3389/fbioe.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tamma PD, Cosgrove SE, Maragakis LL. Combination Therapy for Treatment of Infections with Gram-Negative Bacteria. Clinical Microbiology Reviews. 2012;25:450–470. doi: 10.1128/CMR.05041-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Japaridze A, Benke A, Renevey S, Benadiba C, Dietler G. Influence of DNA Binding Dyes on Bare DNA Structure Studied with Atomic Force Microscopy. Macromolecules. 2015 in press. doi: 10.1021/ma502537g. [Google Scholar]
- 246.Ibsen CJ, Birkedal H. Modification of bone-like apatite nanoparticle size and growth kinetics by alizarin red S. Nanoscale. 2010;2:2478–2486. doi: 10.1039/c0nr00488j. [DOI] [PubMed] [Google Scholar]
- 247.Wu S, Liu X, Yeung KWK, Liu C, Yang X. Biomimetic Porous Scaffolds for Bone Tissue Engineering. Materials Science and Engineering R. 2014;80:1–36. [Google Scholar]
- 248.Gordon LM, Cohen MJ, MacRenaris KW, Pasteris JD, Seda T, Joester D. Amorphous Intergranular Phases Control the Properties of Rodent Tooth Enamel. Science. 2015;347:746–750. doi: 10.1126/science.1258950. [DOI] [PubMed] [Google Scholar]
- 249.Stamenković VR, Fowler B, Mun BS, Wang G, Ross PN, Lucas CA, Marković NM. Improved Oxygen Reduction Activity on Pt3Ni(111) via Increased Surface Site Availability. Science. 2007;315:493–497. doi: 10.1126/science.1135941. [DOI] [PubMed] [Google Scholar]
- 250.Mistry H, Reske R, Zeng Z, Zhao Z-J, Greeley J, Strasser P, Cuenya BR. Exceptional size-dependent activity enhancement in the electroreduction of CO2 over Au nanoparticles. Journal of the American Chemical Society. 2014;136:16473–16476. doi: 10.1021/ja508879j. [DOI] [PubMed] [Google Scholar]
- 251.Mustalahti S, Myllyperkiö P, Malola S, Lahtinen T, Salorinne K, Koivisto J, Häkkinen H, Pettersson M. Molecule-like Photodynamics of Au102(pMBA)44 Nanocluster. ACS Nano. 2015;9:2328–2335. doi: 10.1021/nn506711a. [DOI] [PubMed] [Google Scholar]
- 252.Vincent BB, Loeve S. Metaphors in Nanomedicine: The Case of Targeted Drug Delivery. NanoEthics. 2014;8:1–17. [Google Scholar]