Abstract
Objective
The purpose of this study was to investigate regional homogeneity (ReHo) in children with new-onset drug-naïve Benign Epilepsy with Centrotemporal Spikes (BECTS), chronic BECTS and healthy controls (HC) using the Regional Homogeneity (ReHo) method applied to resting state fMRI data.
Methods
Resting state fMRI data was collected from three groups of children aged 6 to13, including new onset drug naïve BECTS, chronic BECTS with medication, and HC; the data analyzed by ReHo method. Mandarin school exams scores were acquired and compared across groups.
Results
There were three main findings. Firstly, compared with HC, abnormally increased ReHo was observed in bilateral sensorimotor regions in new onset BECTS which normalized or even reversed in the chronic BECTS group. Secondly, enhanced ReHo was found in the left frontal language region in the two BECTS groups, with even higher ReHo value in the chronic group. Lastly, decreased ReHo was found in regions of the default mode network (DMN), bilateral occipital lobes and cerebellum in both the new onset and chronic BECTS groups, lower in chronic BECTS. Behavioral analyses of school scores showed the chronic BECTS group presented significantly lower scores compared to HC (p < .05).
Significance
The coherence of low frequency fluctuations is disrupted in sensorimotor, language and DMN-related regions in new-onset BECTS. Some of these effects seem to be selectively normalized in chronic BECTS, thus allowing us to explore possible chronicity and AED-induced effects on BECTS. Abnormal ReHo in left language and DMN regions could be responsible for impairments of cognitive function.
Keywords: Epilepsy, fMRI, Regional Homogeneity, Resting state, BECTS, Pediatric
INTRODUCTION
Benign epilepsy of childhood with centrotemporal spikes (BECTS) is characterized by abnormal nocturnal epileptiform spike activity originating in the rolandic or sensorimotor cortex, without a significant lesion, typically observed in children between 7–10 years of age. Although seizure prognosis is good in children with BECTS, growing evidence suggests that BECTS is associated with abnormalities in cognition, specifically language and language dependent abilities, IQ, visuomotor abilities, and reading disorders/dyslexia. (Nicolai et al., 2006)
Changes in task-related functional activation patterns, alterations in functional connectivity patterns, and structural brain volumes have been reported in both chronic and new-onset pediatric epilepsies. (Bonilha et al., 2014; Datta et al., 2013; Lillywhite et al., 2009; Pardoe et al., 2013) Additionally, functional neuroimaging studies have reported that blood oxygenation level dependent (BOLD) fMRI activity is influenced by epileptiform activity. (Detre, 2006; Mankinen et al., 2011; Salek-Haddadi et al., 2006) There is sparse but growing evidence that BECTS is also characterized by alterations in functional connectivity. (Besseling et al., 2013; Oser et al., 2014) However, all studies to date have examined children with chronic BECTS with or without antiepileptic treatment, thus confounding seizure frequency and severity, chronicity, and cumulative medication exposure which could affect brain activation and network connectivity patterns in neuroimaging studies. (Datta et al., 2013; Lillywhite et al., 2009)
Our objective in this study was to investigate alterations in brain connectivity patterns comparing new-onset drug-naïve children with BECTS to children with chronic BECTS treated with antiepilepsy drugs (AEDs) and healthy controls (HC). As children with BECTS have been reported to have a significantly increased history of need of language-based services antecedent to seizure onset and diagnosis, it is possible that brain connectivity patterns may be abnormal at the time of seizure onset and diagnosis, and may further evolve over time with chronicity of epilepsy and treatment with AEDs. To address these issues we focused on resting state functional connectivity using a novel measure known as regional homogeneity.
Functional connectivity is defined as the temporal correlation or synchronization of low frequency oscillations between anatomically separate brain regions. (Biswal et al., 1995; Constable et al., 2013; Friston, 2011; Negishi et al., 2011) Resting state functional connectivity is increasingly recognized as a useful tool to investigate brain connectivity patterns in patients as no exogenous task demands are made on the subject. This is particularly useful in young children who may or may not comprehend a task well enough to perform reliably in the scanner.
Regional homogeneity (ReHo) analysis evaluates the degree of synchronization between the time-series of a voxel and its neighboring voxels (Liu et al., 2010; Zang et al., 2004) and can be computed from a five minute ‘resting-state’ functional MRI scan when the children are lying in the scanner with eyes closed or fixating on a plus sign. It requires no a priori definition of regions of interest and provides information about the local activity of regions throughout the brain. It is a data-driven method and has been widely used in the literature to characterize changes in the functional integrity of brain regions with aging and disease. (Mankinen et al., 2011; Paakki et al., 2010; Wu et al., 2007; You et al., 2011) We have previously used this technique in a study with adult epilepsy patients and found increased ReHo in a network of regions that may be responsible for seizure genesis and propagation (Zeng et al., 2013).
In the current study we applied this technique to children with new-onset drug-naïve BECTS, children with chronic BECTS on AEDs, and healthy controls. We applied the ReHo method to resting state fMRI data to characterize differences among these groups to provide information regarding the natural history of alterations in brain connectivity patterns.
METHODS
Participants
Participants were 84 children, aged 8–12, including 24 children with new-onset drug naïve BECTS, 30 with chronic BECTS, and 30 healthy controls (HC). Children with BECTS were recruited from the Shenzhen Children’s Hospital, Guangdong, China and met the following inclusion criteria: (1) EEG showing classic centro-temporal spikes arising from a normal background, (2) clinical history of at least one seizure that was consistent with the diagnosis of BECTS, and (3) no other clinically diagnosed neurologic disorder. Exclusion criteria were: (1) epilepsy other than BECTS, (2) any parenchymal pathology, for example, pathologic abnormality revealed by magnetic resonance imaging (MRI); and (3) other accompanying neurological disorders such as cerebral palsy, brain tumor, or neurometabolic diseases. Table 1 provides demographics for the three groups of children. All children were diagnosed by the attending pediatric neurologist and all were native Mandarin speakers.
Table 1.
Clinical data
| Group | Enrolled Participants | Number of subjects for whom final results are reported (after excluding for scanner motion) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| N | Gender (M/F) | Age (m±std/y) | Age at onset | School Grade | N | Gende r(M/F) | Age (m±std/y) | Age at onset | School Grade | |
|
| ||||||||||
| BECTS New onset | 24 | 15/9 | 8.9 ±2.1 | 8.7±2.06 | 2.8±1.7 | 16 | 9/7 | 9.2±2.2 | 8.9±2.27 | 2.9±1.8 |
| BECTS Chronic | 30 | 22/8 | 10.9 ±1.9 | 7.8±2.32 | 3.9±1.7 | 17 | 13/4 | 11.1±2.1 | 7.7±2.78 | 4.2±1.7 |
| HC | 30 | 17/13 | 10.2 ±1.8 | 18 | 11/7 | 10.5±1.7 | ||||
The HC were recruited via local primary schools and hospital staff. All controls had acquired Mandarin as their first language and were screened for medical and developmental disorders and had normal structural MRI at the time of recruitment. Middle and final semester Mandarin exam scores (an academic achievement test considered related to language ability, in which lower scores represent lower language ability) for each participant was recorded (Total raw score=100) and the means per group were calculated. This study was approved by Institutional Review Board of both Shenzhen Children’s Hospital and University of Wisconsin-Madison. Written informed assent (from the child) and consent (from the parents) was obtained for each participant.
One way ANOVAs showed statistically significant differences in age represented in Table 1 between new onset BECTS and chronic BECTS group (p=0.009) as well as a trend towards significant difference between new onset BECTS and HC (p=0.056). No statistically significant difference in age was noted between chronic BECTS and HC (p=0.41). There is no significant difference in age of onset between the new onset and chronic BECTS groups (P=0.17, t=1.407).
Medication usage
10 of 17 chronic BECTS took single anti-epileptic drugs (Lamotrigine, n=3; Oxcarbazepine, n=4; levetiracetam, n=2; Depakene, n=1), 2 of the 17 were switched from one drug to another (one from Tegretol to Topiramate, the other from Lamotrigine to Oxcarbazepine). The remaining 5 patients took multiple (2–3) anti-epileptic drugs. The average medication period was 2.73±1.76 years.
MR Data acquisition
Brain imaging data were acquired on a Signa Excite 1.5 T MR imaging system (General Electric, Fairfield, USA) and a standard head coil. Foam pads were used to reduce head motion and scanner noise. All participants were asked to hold still, with eyes open and instructions to stay awake. The eyes open resting-state fMRIs were acquired by using an echo planar imaging sequence with the following parameters: 26 axial slices, thickness/skip = 4/0 mm, in-plane resolution =64 × 64, repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle = 90, field of view = 240 mm × 240 mm, 190 volumes. Axial T1-weighted images (T1WI), were also obtained with a T1 FLAIR sequence with TR = 2307 ms, TE = 10.6 ms, and TI = 620 ms.
Data processing
Data were preprocessed and analyzed using DPARSF based on SPM8 and REST(Chao-Gan and Yu-Feng, 2010; Song et al., 2011) (http://www.restfmri.net, http://www.fil.ion.ucl.ac.uk/spm/). The first 10 time points of each subject’s resting state fMRI data were discarded because of the instability of the initial MRI signal. The remaining 180 images were pre-processed. Preprocessing of the fMRI datasets included standard slice timing, realignment, normalization (voxel size [3,3, 3]), any subjects with head motion greater than 1.5 mm or 1 degree in any of the six parameters (x, y, z, pitch, roll, yaw) were excluded. 8 subjects in new onset drug naive group, 13 in chronic group and 12 in healthy control group were excluded. Linear detrending and temporal band pass filtering (0.01–0.08 Hz) were carried out using DPARSF software. Thus, bandpass (0.01–0.08 Hz) filter was set up to reduce the effect of very low frequency and high frequency physiological noise(Chao-Gan and Yu-Feng, 2010).
Individual ReHo maps were generated by calculating Kendall’s coefficient concordance (KCC, also called ReHo value) of the time series of a given voxel with those of its nearest neighbors (26 voxels), on a voxel-wise basis. (Zang et al., 2004) Then, the data were smoothed with a Gaussian filter of 4 mm full width at half-maximum (FWHM) to reduce noise and residual differences in gyral anatomy. The ReHo maps were generated for each subject in each group.
2.5. Second-level analysis
One-sample t-tests were performed within each group to show where in the brain the standardized KCC value was larger than one. The significant threshold was set at p<0.05 and results were corrected for multiple comparison using the false discovery rate (FDR) criterion. (Genovese et al., 2002) Then, the second-level random effects two-sample t-tests were performed to compare the ReHo results between the patient and HC within a conjunction mask. A conjunction mask was generated by combining the voxels in 2 of the 3 compared groups (new-onset drug-naïve, chronic patients or HC), which were obtained from one-sample t-test results. The t-map was set at a threshold of p<0.05 (combined height threshold p<0.01 and a minimum cluster size of 10 voxels), using the AlphaSim program in the REST software.
RESULTS
New-onset drug-naïve BECTS vs. HC
New-onset drug-naïve BECTS patients, relative to HC, showed increased ReHo in sensorimotor regions including precentral and postcentral gyri; language regions including left middle and inferior frontal regions; right superior and middle temporal regions, and the cuneus. In contrast, decreased ReHo was observed in cerebellar and occipital regions, superior parietal, and cuneus/precuneus -which composed regions of the default model network (DMN)-compared to HC. Figure 1 shows regions exhibiting significantly different ReHo values between the groups.
Figure 1. New-onset drug-naïve BECTS vs. HC.
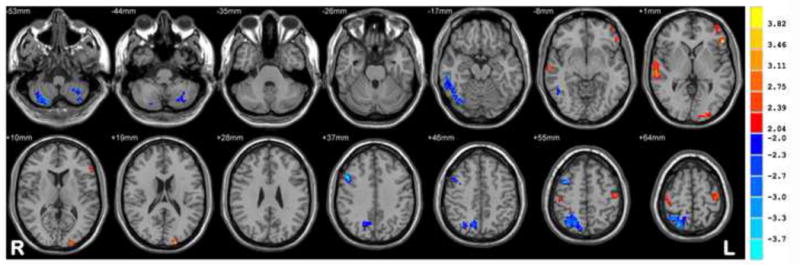
Statistic t-map showing the difference between the new onset BECTS group and HC (two sample t-test, p < 0.005, voxel >10). Warm colors indicate new-onset group > HC, whereas cool colors indicate new-onset group < HC.
Chronic BECTS on AEDs vs HC
Chronic BECTS patients relative to HC showed increased ReHo in language regions including middle and inferior frontal regions, superior and middle temporal regions, and the temporal pole. Decreased ReHo was observed in cerebellar and occipital regions as well as in regions of the DMN including the posterior cingulate, precuneus, and parietal regions. Figure 2 depicts the regions exhibiting significantly different ReHo values for the chronic BECTS and HC groups.
Figure 2. Chronic BECTS on AEDs vs HC.
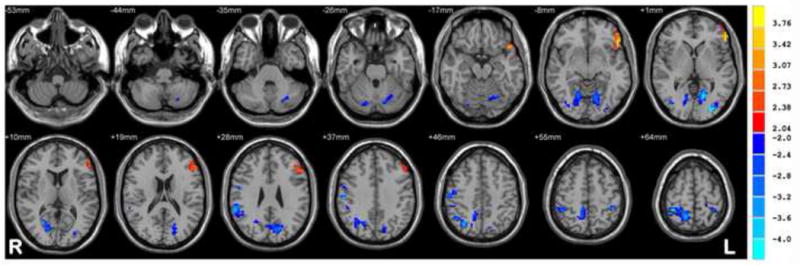
Statistic t-map showing the difference between the chronic BECTS group and HC (two sample t-test, p < 0.005, voxel >10). Warm colors indicate chronic > HC, whereas cool colors indicate chronic < HC.
Chronic vs. new-onset BECT groups
Increased ReHo was observed in language related regions including the left inferior and middle frontal gyrus and inferior temporal regions in the chronic BECTS group relative to new-onset drug naïve BECTS children. Additionally, increased regional homogeneity was seen in the right inferior frontal lobe in patients with chronic versus new onset BECTS. Decreased ReHo was observed in cerebellar and occipital regions as well as regions of the DMN (posterior cingulate, precuneus and cuneus) and parietal regions in chronic BECTS. Table 2 and Figure 3 depict the significantly different ReHo values for the new onset and chronic BECTS groups.
Table 2.
Clusters with significantly different ReHo values in Chronic BECTS on medication vs. New onset drug naïve BECTS.
| Brian regions | H* | Brodman n’s Area | Peak MNI coordinate | Cluster voxel | Peak t Value | |||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| 1 | Cerebellum Posterior Lobe | R | 33 | −66 | −30 | 84 | −3.7228 | |
| 2 | Lingual Gyrus, Cerebellum Posterior Lobe, Calcarine, Culmen, Cerebellum Anterior Lobe | L | 18,19 | −15 | −78 | −15 | 294 | −4.0962 |
| 3 | Frontal_Inf_Orb_R, Middle Frontal Gyrus, Inferior Frontal Gyrus | R | 47 | 36 | 27 | −18 | 59 | 4.8762 |
| 4 | Rectal Gyrus, Medial Frontal Gyrus, | L | 11 | −3 | 36 | −21 | 73 | 3.5491 |
| 5 | Posterior Cingulate, Lingual Gyrus | R | 30 | 24 | −66 | 6 | 89 | −3.3827 |
| 6 | Inferior Temporal Gyrus, Fusiform Gyrus | R | 20,37 | 57 | −57 | −15 | 60 | 3.2162 |
| 7 | Frontal_Inf_Orb, Inferior Frontal Gyrus | L | 47 | −48 | 24 | −12 | 64 | 3.1368 |
| 8 | Middle Occipital Gyrus, Inferior Occipital Gyrus | L | 18 | −33 | −75 | 6 | 164 | −4.1231 |
| 9 | Inferior Temporal Gyrus, Middle Occipital Gyrus | R | 42 | −72 | −3 | 58 | −3.0802 | |
| 10 | Inferior Frontal Gyrus, Rolandic_Oper_R, Precentral Gyrus | R | 44,45 | 57 | 9 | 24 | 89 | 3.3935 |
| 11 | Superior Frontal Gyrus, | L | 10 | −12 | 69 | 6 | 56 | −3.3635 |
| 12 | Middle Frontal Gyrus, | L | 10 | −36 | 54 | 3 | 58 | −3.9499 |
| 13 | Middle Temporal Gyrus | L | −36 | −63 | 21 | 62 | −3.8091 | |
| 14 | Middle Temporal Gyrus | R | 45 | −69 | 27 | 69 | −3.1477 | |
| 15 | Precuneus, Cingulate | R | 31 | 3 | −69 | 24 | 134 | −4.3865 |
| 16 | Middle Frontal Gyrus | R | 9 | 30 | 33 | 42 | 66 | 3.6563 |
| 17 | Precentral Gyrus, Postcentral Gyrus | R | 3,4 | 24 | −30 | 72 | 172 | −3.6089 |
Positive T value indicates greater ReHo value in chronic group.
H mean hemisphere
Figure 3. Chronic vs. new-onset BECT groups.
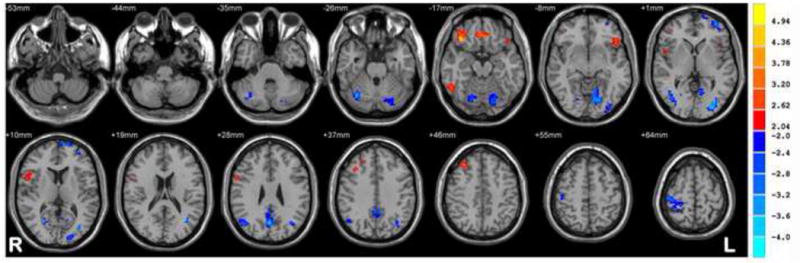
Statistic t-map showing the difference between the chronic group and new onset BECTS (two sample t-test, p < 0.005, voxel >10). Warm colors indicate chronic > new onset, whereas cool colors indicate chronic < new onset.
Behavioral performance/academic achievement
The mean score on the annual Mandarin school exam was 87.2±9.2 for HC, 80.5±11.6 for new onset BECTS, and 76.0±12.9 in chronic BECTS. Statistical analysis showed that there was a significant difference only between the chronic BECTS and HC groups (t=2.946, p=0.006), with no significant differences between the new onset BECTS groups versus chronic BECTS and controls (p> 0.10).
Discussion
This investigation of children with new-onset BECTS, chronic BECTS and HC yielded three major findings: 1) In new-onset BECTS, abnormally increased ReHo was observed in bilateral sensorimotor regions, which was normalized or even reversed in the chronic BECTS group. 2) ReHo was abnormally higher in a left frontal language region in both new-onset and chronic BECTS, which was even higher in the latter. 3) Compared with HC, decreased ReHo was found in regions of DMN, bilateral occipital lobes (prominently in right side) and cerebellum in both BECTS groups, while ReHo value was lower in chronic compared to new onset BECTS. Behavioral analyses of school scores showed that the chronic BECTS group got lower scores t compared to HC (p<0.05), with no other pairwise differences. For the first time we show significant differences of the coherence of low frequency fluctuations in the early course of the disorder.
ReHo measures the coherence of the low frequency fluctuations in the BOLD signal in neighboring voxels and likely represents spontaneous neuronal activity. Increased ReHo is indicative of abnormal enhancement of local neuronal activity while reduced ReHo is indicative of decreased neuronal activity that could lead to local brain dysfunction. (Sheng et al., 2014; Zang et al., 2004)
BECTS has historically been viewed as a “benign” disorder, mainly because seizures remit in adolescence (Loiseau and Duché, 1989) and patients go on to lead a relatively normal life. (Camfield and Camfield, 2014) However, there is growing evidence that points toward a less benign nature of this epilepsy syndrome in which affected children exhibit a variety of cognitive impairments (Danielsson and Petermann, 2009) linked largely but not exclusively to language function. (Datta et al., 2013; Overvliet et al., 2011b; Overvliet et al., 2013) Here we found that, compared to HC, new-onset drug-naïve BECTS patients exhibited increased ReHo while chronic BECTS on medication exhibited decreased ReHo in bilateral sensorimotor regions including the rolandic cortex, the putative source for abnormal epileptiform activity in BECTS. This result suggests that antiepileptic drugs (AEDs) may reverse this abnormal activity observed in the new-onset drug-naïve BECTS and even reach a lower level when compared with HC. These cross-sectional findings, which require confirmation in prospective studies, suggest evidence for exploration of AEDs effects on the unmedicated neural-network level. Another possible explanation is that the natural course of the disorder contributed to a reverse in the increased ReHo rather than an effect of the AEDs. Further studies comparing chronic BECTS with and without AEDs would provide valuable clues to explore the more specific reasons for the decreased ReHo.
In the present study both groups of children with BECTS showed increased ReHo in left inferior frontal gyrus (IFG; Broca’s area) and middle frontal gyrus (MFG) when compared to controls, areas that are established regions in the language network in both healthy children and adults as well as in patients with epilepsy. (Friederici and Gierhan, 2013; Scharff et al., 2013; Swanson et al., 2007) The increased ReHo value in these language regions might indicate abnormal enhanced coherence patterns which might serve to interrupt normal language network and function. More interestingly, the ReHo values in these regions of the chronic group were higher than new-onset drug-naïve BECTS patients, which might suggest more severe interruption in language function. These findings raise the possibility that increased ReHo value in these language regions cannot be normalized by AEDs, with ReHo even higher in the chronic BECTS group. Evidence that lowers performance on the annual Mandarin school exam in chronic BECTS group provides some support for this inference. As the Mandarin exam score was considered related to language ability, so lower scores meant lower language ability, indicating lower language and cognitive function. More interestingly, increased ReHo in the right inferior frontal lobe in patients with chronic versus new onset BECTS was found, which could represent a compensatory/adaptive mechanism in language networks which are known to be bilateral, albeit with a left hemispheric predominance.
Lilly white et al. found that differences in functional activation patterns between BECTS and controls were related to poor performance only when high-level cognition was required, and speculated that language difficulties in BECTS are a regional rather than a global problem. (2009) However, Datta et al. found significant differences in language fMRI laterality indices between BECTS and controls and suggested that differences in functional activation patterns may be due to compensatory mechanisms in the patients given that there were no significant behavioral differences between the two groups. (Datta et al., 2013)
Additionally, the rolandic cortex has been reported to play an important role in motor preparation,(Tzagarakis et al., 2010) as well as in the coordination of articulation and production of words,(Bouchard et al., 2013) suggesting that integrity of functional processes in this area is critical for word generation. Some studies have found correlations between motor and language processes in children with BECTS suggesting that disruptions in sensorimotor regions are indeed negatively affecting aspects of language. (Besseling et al., 2013; Overvliet et al., 2011b) Specifically, Besseling et al. found a reduction in functional connectivity between Broca’s and the rolandic network, which they hypothesized, was due to the link between epileptiform activity in sensorimotor cortex and language difficulties. (2013) Our results show that regional coherence in treated chronic BECTS patients approach normal patterns in the sensorimotor regions but continue to exhibit abnormal coherence patterns in the language regions. BECTS patients have seizures originating in and around the rolandic cortex, and it is likely that the AEDs primarily exert maximal effects on these regions leading to normalization of local synchrony in these areas, while other regions such as language may show abnormal increased activity and function due to effects from AEDs.
Both groups of children with BECTS showed decreased ReHo in DMN regions (posterior cingulate, precuneus and cuneus) when compared to HC. However, chronic BECTS presented the lowest ReHo in some DMN areas when compared to both new-onset BECTS and HC, which could be due to the chronicity of the disorder. This abnormal reduction in DMN activity in BECTS during rest is different from what has been reported during task performance. There is evidence that there may be intrinsic behavioral competition between attentional resources directed to a specific task and processes subserving the DMN(Fox and Raichle, 2007). Oser et al. (2014) reported that BECTS patients had problems deactivating DMN regions only when the language task presented a high level of difficulty(2014). An inability to successfully suppress DMN activity in BECTS coupled with increased neural activity in language-related regions could be associated with poor performance on language tasks generally reported in BECTS(Besseling et al., 2013; Overvliet et al., 2011a).
In our study, both BECTS groups presented decreased ReHo compared to controls in bilateral occipital and cerebellar regions, with the chronic BECTS group presenting the lowest values. Again, this might be due to chronicity effects. There is evidence that children with BECTS tend to engage visual regions in order to perform in a semantic decision task while HC engaged regions involved in attention and response monitoring (i.e. cingulate cortex). (Vannest et al., 2013) This could be indicative that children with BECTS are relying on regions outside the network of interest (e.g. language network) for the successful completion of a given cognitive task, utilizing less efficient strategies. The cerebellum, is involved in motor control as well as higher cognitive processes, including aspects of language. (Buckner, 2013; Buckner et al., 2011; Hubrich-Ungureanu et al., 2002) Therefore, the known problems encountered by subjects with BECTS could be linked to reduced regional synchrony as measured by ReHo in bilateral cerebellar areas.
Previous fMRI studies have failed to find functional differences between non-medicated BECTS and BECTS on AEDs. For example, Datta et al. failed to find correlations between performance and language reorganization in non-medicated children with BECTS and BECTS on AEDs. (2013) Similarly Oser et al. found no differences in activation between BECTS on AEDs and those not on AEDs in an independent component analysis of task fMRI data. (2014) It is possible that low to modest sample sizes and the task demands may have obscured any significant differences in these studies. Our results suggest that both drug-naïve and chronic medicated BECTS groups have differential effects at the level of low frequency neuronal fluctuations as measured by ReHo compared to controls, suggesting that medication and/or chronicity has a definite impact at the level of neuronal oscillations. Furthermore, functional differences between both groups of BECTS may be taking place at a regional rather than global level. In our study, chronic BECTS also performed significantly worse than HC in the Mandarin school test while there was no statistically significant difference between new-onset BECTS and HC.
Limitations
There are several limitations associated with our study. We were not able to correlate abnormal ReHo in patients with behavioral performance which precludes drawing any definite conclusions regarding abnormal ReHo and cognitive impairments in BECTS patients. Our sample size was relatively limited given that we had large number of participants (~40%) with excessive motion who were excluded from the analyses. The new-onset BECTS were significantly younger in age than chronic BECTS and were showing a trend towards significance in terms of being younger in age than healthy controls. One possible explanation for the ReHo changes between new-onset and chronic BECTS is that it could be due to developmental changes, given patient’s age difference between the two groups; but given these ReHo changes were mostly different from what was seen when comparing new-onset BECTS with older healthy controls this is less likely the case. There was also no significant difference in age between chronic BECTS and healthy controls, yet ReHo changes were noted between these two groups making ReHo changes more likely due to seizure and medication effects. Our design was cross-sectional and while we inferred chronicity effects the answers to these issues can only come from a prospective investigation. Nonetheless, questions to address in a longitudinal study are clearly raised here. Resample voxel size was set as 3 mm × 3 mm × 3 mm, which is a very common resampling voxel size for ReHo analysis (Chen et al., 2015; Ni et al., 2012; Ping et al., 2013). In this study, its original voxel size was 3.75 mm × 3.75 mm × 4 mm. Slightly increased ReHo value would be expected. There was one study, which analyzed the parameters that influenced the sensitivity of BOLD signal and found that smaller resampled voxel size did produce a significantly greater sensitivity.
CONCLUSION
At present, no study has examined children with new onset and chronic BECTS. We demonstrate, for the first time, that regional homogeneity or the coherence of low frequency fluctuations is disrupted in sensorimotor, language and default mode network regions in new-onset drug-naïve BECTS which appears to normalize or even reverse in the chronic BECTS group. Enhanced ReHo is observed in the left frontal language region in both new onset and chronic BECTS, with even higher or greater abnormality in the chronic BECTS group. Lastly, decreased ReHo was found in regions of default model network (DMN) and bilateral occipital lobes and cerebellum in both the new onset and chronic BECTS groups. These results provide a new light on this common childhood epilepsy syndrome and possible changes that occur over time.
Abnormal ReHo values were found in new-onset drug-naïve and chronic BECTS groups.
Abnormal ReHo values were seen in Sensorimotor, Language and Default Mode regions.
Impairment of function may be due to abnormal ReHo values in brain regions.
Acknowledgments
This project was supported by RSNA Seed Grant (No. RSD1214), NIH Grants UL1TR000427, K23NS086852, R01NS044351-09, and Medical Science Grant of Guangdong Province (No. B2013359).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Contributor Information
Hongwu Zeng, Email: homerzeng@126.com.
Camille Garcia Ramos, Email: camille.garcia23@gmail.com.
Veena A. Nair, Email: vnair@uwhealth.org.
Yan Hu, Email: 2680224170@qq.com.
Jianxiang Liao, Email: liaojianxiang@vip.sina.com.
Christian La, Email: cla.wisc@gmail.com.
Li Chen, Email: chenli2000@126.com.
Yungen Gan, Email: ganyungen@yahoo.com.
Feiqiu Wen, Email: fwen62@126.com.
Bruce Hermann, Email: hermann@neurology.wisc.edu.
Vivek Prabhakaran, Email: vprabhakaran@uwhealth.org.
References
- Besseling RM, Overvliet GM, Jansen JF, van der Kruijs SJ, Vles JS, Ebus SC, Hofman PA, de Louw AJ, Aldenkamp AP, Backes WH. Aberrant functional connectivity between motor and language networks in rolandic epilepsy. Epilepsy Res. 2013;107:253–262. doi: 10.1016/j.eplepsyres.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bonilha L, Tabesh A, Dabbs K, Hsu DA, Stafstrom CE, Hermann BP, Lin JJ. Neurodevelopmental alterations of large-scale structural networks in children with new-onset epilepsy. Hum Brain Mapp. 2014 doi: 10.1002/hbm.22428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard KE, Mesgarani N, Johnson K, Chang EF. Functional organization of human sensorimotor cortex for speech articulation. Nature. 2013;495:327–332. doi: 10.1038/nature11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80:807–815. doi: 10.1016/j.neuron.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of neurophysiology. 2011;106:2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camfield CS, Camfield PR. Rolandic epilepsy has little effect on adult life 30 years later: a population-based study. Neurology. 2014;82:1162–1166. doi: 10.1212/WNL.0000000000000267. [DOI] [PubMed] [Google Scholar]
- Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Frontiers in systems neuroscience. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Zhang J, Li XW, Xia W, Feng X, Qian C, Yang XY, Lu CQ, Wang J, Salvi R, Teng GJ. Altered intra- and interregional synchronization in resting-state cerebral networks associated with chronic tinnitus. Neural plasticity. 2015;2015:475382. doi: 10.1155/2015/475382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable RT, Scheinost D, Finn ES, Shen X, Hampson M, Winstanley FS, Spencer DD, Papademetris X. Potential use and challenges of functional connectivity mapping in intractable epilepsy. Frontiers in neurology. 2013;4:39. doi: 10.3389/fneur.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson J, Petermann F. Cognitive deficits in children with benign rolandic epilepsy of childhood or rolandic discharges: a study of children between 4 and 7 years of age with and without seizures compared with healthy controls. Epilepsy Behav. 2009;16:646–651. doi: 10.1016/j.yebeh.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Datta AN, Oser N, Bauder F, Maier O, Martin F, Ramelli GP, Steinlin M, Weber P, Penner IK. Cognitive impairment and cortical reorganization in children with benign epilepsy with centrotemporal spikes. Epilepsia. 2013;54:487–494. doi: 10.1111/epi.12067. [DOI] [PubMed] [Google Scholar]
- Detre JA. Clinical applicability of functional MRI. J Magn Reson Imaging. 2006;23:808–815. doi: 10.1002/jmri.20585. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Gierhan SM. The language network. Current opinion in neurobiology. 2013;23:250–254. doi: 10.1016/j.conb.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity: a review. Brain connectivity. 2011;1:13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Hubrich-Ungureanu P, Kaemmerer N, Henn FA, Braus DF. Lateralized organization of the cerebellum in a silent verbal fluency task: a functional magnetic resonance imaging study in healthy volunteers. Neurosci Lett. 2002;319:91–94. doi: 10.1016/s0304-3940(01)02566-6. [DOI] [PubMed] [Google Scholar]
- Lillywhite LM, Saling MM, Harvey AS, Abbott DF, Archer JS, Vears DF, Scheffer IE, Jackson GD. Neuropsychological and functional MRI studies provide converging evidence of anterior language dysfunction in BECTS. Epilepsia. 2009;50:2276–2284. doi: 10.1111/j.1528-1167.2009.02065.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Yan C, Ren J, Yao L, Kiviniemi VJ, Zang Y. Using coherence to measure regional homogeneity of resting-state FMRI signal. Frontiers in systems neuroscience. 2010;4:24. doi: 10.3389/fnsys.2010.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankinen K, Long XY, Paakki JJ, Harila M, Rytky S, Tervonen O, Nikkinen J, Starck T, Remes J, Rantala H, Zang YF, Kiviniemi V. Alterations in regional homogeneity of baseline brain activity in pediatric temporal lobe epilepsy. Brain Res. 2011;1373:221–229. doi: 10.1016/j.brainres.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Negishi M, Martuzzi R, Novotny EJ, Spencer DD, Constable RT. Functional MRI connectivity as a predictor of the surgical outcome of epilepsy. Epilepsia. 2011;52:1733–1740. doi: 10.1111/j.1528-1167.2011.03191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L, Qi R, Zhang LJ, Zhong J, Zheng G, Zhang Z, Zhong Y, Xu Q, Liao W, Jiao Q, Wu X, Fan X, Lu GM. Altered regional homogeneity in the development of minimal hepatic encephalopathy: a resting-state functional MRI study. PLoS One. 2012;7:e42016. doi: 10.1371/journal.pone.0042016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolai J, Aldenkamp AP, Arends J, Weber JW, Vles JS. Cognitive and behavioral effects of nocturnal epileptiform discharges in children with benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav. 2006;8:56–70. doi: 10.1016/j.yebeh.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Oser N, Hubacher M, Specht K, Datta AN, Weber P, Penner IK. Default mode network alterations during language task performance in children with benign epilepsy with centrotemporal spikes (BECTS) Epilepsy Behav. 2014;33:12–17. doi: 10.1016/j.yebeh.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Overvliet GM, Aldenkamp AP, Klinkenberg S, Nicolai J, Vles JS, Besseling RM, Backes W, Jansen JF, Hofman PA, Hendriksen J. Correlation between language impairment and problems in motor development in children with rolandic epilepsy. Epilepsy Behav. 2011a;22:527–531. doi: 10.1016/j.yebeh.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Overvliet GM, Aldenkamp AP, Klinkenberg S, Vles JS, Hendriksen J. Impaired language performance as a precursor or consequence of Rolandic epilepsy? Journal of the neurological sciences. 2011b;304:71–74. doi: 10.1016/j.jns.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Overvliet GM, Besseling RM, van der Kruijs SJ, Vles JS, Backes WH, Hendriksen JG, Ebus SC, Jansen JF, Hofman PA, Aldenkamp AP. Clinical evaluation of language fundamentals in Rolandic epilepsy, an assessment with CELF-4. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2013;17:390–396. doi: 10.1016/j.ejpn.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Paakki JJ, Rahko J, Long X, Moilanen I, Tervonen O, Nikkinen J, Starck T, Remes J, Hurtig T, Haapsamo H, Jussila K, Kuusikko-Gauffin S, Mattila ML, Zang Y, Kiviniemi V. Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain Res. 2010;1321:169–179. doi: 10.1016/j.brainres.2009.12.081. [DOI] [PubMed] [Google Scholar]
- Pardoe HR, Berg AT, Archer JS, Fulbright RK, Jackson GD. A neurodevelopmental basis for BECTS: evidence from structural MRI. Epilepsy Res. 2013;105:133–139. doi: 10.1016/j.eplepsyres.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping L, Su-Fang L, Hai-Ying H, Zhang-Ye D, Jia L, Zhi-Hua G, Hong-Fang X, Yu-Feng Z, Zhan-Jiang L. Abnormal Spontaneous Neural Activity in Obsessive-Compulsive Disorder: A Resting-State Functional Magnetic Resonance Imaging Study. PLoS One. 2013;8:e67262. doi: 10.1371/journal.pone.0067262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salek-Haddadi A, Diehl B, Hamandi K, Merschhemke M, Liston A, Friston K, Duncan JS, Fish DR, Lemieux L. Hemodynamic correlates of epileptiform discharges: an EEG-fMRI study of 63 patients with focal epilepsy. Brain Res. 2006;1088:148–166. doi: 10.1016/j.brainres.2006.02.098. [DOI] [PubMed] [Google Scholar]
- Scharff C, Friederici AD, Petrides M. Neurobiology of human language and its evolution: primate and non-primate perspectives. Frontiers in evolutionary neuroscience. 2013;5:1. doi: 10.3389/fnevo.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng K, Fang W, Su M, Li R, Zou D, Han Y, Wang X, Cheng O. Altered spontaneous brain activity in patients with Parkinson’s disease accompanied by depressive symptoms, as revealed by regional homogeneity and functional connectivity in the prefrontal-limbic system. PLoS One. 2014;9:e84705. doi: 10.1371/journal.pone.0084705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson SJ, Sabsevitz DS, Hammeke TA, Binder JR. Functional magnetic resonance imaging of language in epilepsy. Neuropsychology review. 2007;17:491–504. doi: 10.1007/s11065-007-9050-x. [DOI] [PubMed] [Google Scholar]
- Tzagarakis C, Ince NF, Leuthold AC, Pellizzer G. Beta-band activity during motor planning reflects response uncertainty. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:11270–11277. doi: 10.1523/JNEUROSCI.6026-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannest J, Szaflarski JP, Eaton KP, Henkel DM, Morita D, Glauser TA, Byars AW, Patel K, Holland SK. Functional magnetic resonance imaging reveals changes in language localization in children with benign childhood epilepsy with centrotemporal spikes. Journal of child neurology. 2013;28:435–445. doi: 10.1177/0883073812447682. [DOI] [PubMed] [Google Scholar]
- Wu T, Zang Y, Wang L, Long X, Li K, Chan P. Normal aging decreases regional homogeneity of the motor areas in the resting state. Neurosci Lett. 2007;423:189–193. doi: 10.1016/j.neulet.2007.06.057. [DOI] [PubMed] [Google Scholar]
- You H, Wang J, Wang H, Zang YF, Zheng FL, Meng CL, Feng F. Altered regional homogeneity in motor cortices in patients with multiple system atrophy. Neurosci Lett. 2011;502:18–23. doi: 10.1016/j.neulet.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Zeng H, Pizarro R, Nair VA, La C, Prabhakaran V. Alterations in regional homogeneity of resting-state brain activity in mesial temporal lobe epilepsy. Epilepsia. 2013;54:658–666. doi: 10.1111/epi.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]


