Abstract
Pediatric neuro-oncology has undergone an exciting and dramatic transformation during the past 5 years. This article summarizes data from collaborative group and institutional trials that have advanced the science of pediatric brain tumors and survival of patients with these tumors. Advanced genomic analysis of the entire spectrum of pediatric brain tumors has heralded an era in which stakeholders in the pediatric neuro-oncology community are being challenged to reconsider their current research and diagnostic and treatment strategies. The incorporation of this new information into the next-generation treatment protocols will unleash new challenges. This review succinctly summarizes the key advances in our understanding of the common pediatric brain tumors (ie, medulloblastoma, low- and high-grade gliomas, diffuse intrinsic pontine glioma, and ependymoma) and some selected rare tumors (ie, atypical teratoid/rhabdoid tumor and CNS primitive neuroectodermal tumor). The potential impact of this new information on future clinical protocols also is discussed. Cutting-edge genomics technologies and the information gained from such studies are facilitating the identification of molecularly defined subgroups within patients with particular pediatric brain tumors. The number of evaluable patients in each subgroup is small, particularly in the subgroups of rare diseases. Therefore, international collaboration will be crucial to draw meaningful conclusions about novel approaches to treating pediatric brain tumors.
INTRODUCTION
Despite improvement in the cure rates of pediatric brain tumors during the past two decades of the 20th century, which was largely a result of technologic advances in imaging, neurosurgery, and radiation oncology and the introduction of combination chemotherapy, outcomes have remained static for all of these tumors except medulloblastoma.1 This article summarizes key collaborative group protocols and institutional studies that advanced the science of pediatric brain tumors and the survival of patients with these tumors. The lack of advances in treatment of pediatric brain tumors were hindered by our lack of knowledge about the molecular pathogenesis of brain tumors. This deficit is now being overcome by new technologies that facilitate our understanding of the genomic landscape of pediatric brain tumors, international cooperation among leading laboratory and clinical investigators, the availability of well-annotated tumor samples, and generous funding from government and philanthropic sources. The MAGIC (Medulloblastoma Advanced Genomics International Consortium) consortium instituted by the investigators at the Hospital for Sick Children in Toronto revolutionized international cooperation for studying medulloblastoma and set the stage for large-scale genomic studies.2 Armed with this new genomic knowledge, we have renewed enthusiasm to develop novel therapeutic approaches that are tailored to each molecular subtype of disease under the broad umbrellas of medulloblastoma, high-grade glioma, low-grade glioma, ependymoma, and primitive neuroectodermal tumors.
MEDULLOBLASTOMA
Medulloblastoma is a highly malignant embryonal tumor that was first described as a distinct CNS tumor in 1925. Medulloblastoma occurs in infancy, childhood, or adulthood. Clinical heterogeneity has been documented in the clinical presentation, pathology, and cure rate.3 By using combined-modality therapy that includes surgical resection, risk-adjusted irradiation, and adjuvant chemotherapy, approximately 70% of children and adolescents with medulloblastoma can be cured, albeit with debilitating long-term sequelae.4
Molecular Genetics of Medulloblastoma
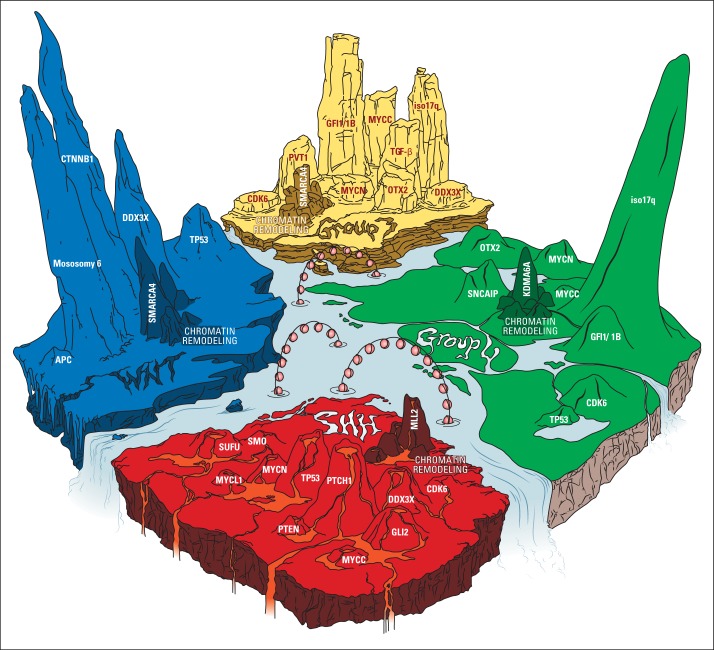
One of the most important discoveries is that medulloblastoma is a heterogeneous disease that consists of four core molecular subgroups identified via transcriptional profiling: wingless (WNT), sonic hedgehog (SHH), group 3, and group 4.5 These subgroups were defined by their unique clinical behavior and outcomes. The WNT-subgroup and SHH-subgroup medulloblastomas are characterized by aberrant activation of the WNT and SHH signaling pathways, respectively. Groups 3 and 4 were so named because of the absence of involvement of any clearly defined signaling pathway. Recently-developed genetic technologies, such as single nucleotide polymorphism gene-mapping arrays to identify somatic copy-number alterations and deep-sequencing studies, have exposed the genetic landscape of medulloblastoma, which thereby expanded our understanding of the molecular subgroups.6 At least 30% to 40% of all medulloblastoma have been demonstrated to harbor somatic alterations (ie, single nucleotide variants, indels, and somatic copy number alterations) targeting a chromatin-modyfing gene, which confirms epigenetic deregulation as a major driver of medulloblastoma (Fig 1).7
Fig 1.
The genetic landscape of medulloblastoma. Recurrent genetic aberrations identified in medulloblastoma (derived from Northcott in 2012,2,7 Robinson et al,11 Pugh et al,12 Jones et al,13 and Northcott et al in 201419) averaged and displayed proportionally by height of terrain peaks. The figure reveals the unique subgroup-specific molecular aberration and highlights chromatin remodeling mutations as the unifying theme among all four medulloblastoma subgroups. Wingless (WNT) medulloblastoma (left; blue icy landscape), the most molecularly homogenous group, consists of CTNNB1 mutations in 85%, monosomy 6 in 80%, DDX3X mutation in 50%, TP53 mutation in 13%, and mutations in chromatin remodeling genes in 49.5% (composed of mutations in SMARCA4 [25%], MLL2 [12.5%], CREBBP [6%], TRAPP [3%], and MED13 [3%]). For the chromatin remodeling peaks (darker colored shading), only the most commonly mutated gene is labeled. Sonic hedgehog (SHH) medulloblastoma (bottom; red volcanic landscape) consists of PTCH1 mutation/deletion in 29%, TP53 mutation in 18%, DDX3X mutation in 11%, GLI2 amplification/mutation in 8%, MYCN amplification in 6%, SUFU mutation in 6%, SMO mutation in 3%, PTEN deletion in 2.5%, MYCL1 amplification in 2%, CDK6 amplification in 1%, MYCC amplification in 0.7%, and mutations in chromatin remodeling genes in 21% (composed of mutations in MLL2 [12%], BCOR [3%], LBD1 [3%], NCOR2 [1.5%], and SMARCA4 [1.5%]). Group 3 medulloblastoma (top; yellow desert rocky terrain) is characterized by GFI1/1B structural variants (eg, inversions, duplications) in 41%, isochromosome (iso) 17q in 26%, transforming growth factor (TGF) -β signaling in 20%, MYCC amplification in 17%, PVT1 alterations in 12%, OTX2 amplification in 8%, MYCN amplification in 4%, DDX3X mutation in 3%, CDK6 amplification in 1%, and mutations in chromatin remodelling genes in 28.5% (composed of mutations in SMARCA4 [10.5%], other KDM family members [5%], MLL2 [4%], KDMA6A [3%], GPS2 [3%], MLL3 [1%], CREBBP [1%], and CHD7 [1%]). Group 4 medulloblastoma (right; green forest mountain terrain) is characterized by iso 17q in 80%, GFI1/1B structural variants in 10%, SNCAIP tandem duplications in 10%, OTX2 amplification in 5.5%, MYCN amplification in 5%, CDK6 amplification in 5%, TP53 mutation in 1%, MYCC amplification in 1%, and mutations in chromatin remodeling genes in 30% (composed of mutations in KDMA6A [13%], other KDM family members [4%], MLL3 [3%], CHD7 [3%], ZMYM3 [3%], MLL2 [2%], GPS2 [1%], and BCOR [1%]).
WNT-subgroup medulloblastoma.
The WNT subgroup accounts for approximately 10% of patients with medulloblastoma, and its cell of origin seems to arise from the lower rhombic lip.8 Consequently, WNT medulloblastomas often develop in a central location, frequently abutting the brainstem. This subgroup generally occurs in older patients (median age, 10 years); almost all WNT tumors display classic histology and are rarely metastatic, and 80% to 85% of occurrences are associated with monosomy 6. Patients with WNT medulloblastoma experience excellent survival with contemporary therapy; their 5-year event-free survival (EFS) is greater than 90%.9,10
The most frequently mutated gene in WNT medulloblastoma is CTNNB1, which occurs in 85% of tumors analyzed. DDX3X mutations were enriched but not exclusive to the WNT subgroup; 11% of SHH tumors and 3% of group 3 tumors also express these mutations.11–13 Approximately 15% of WNT tumors have TP53 mutations, which are not associated with underlying Li-Fraumeni syndrome or a poor prognosis. This finding sharply contrasts with TP53-mutated SHH tumors.14
SHH-subgroup medulloblastoma.
This subgroup comprises approximately 25% of medulloblastoma occurrences, most of which are of nodular desmoplastic (ND) histology. Indeed, all ND tumors are SHH medulloblastoma, but not all SHH medulloblastomas are ND; they also can have classic or large-cell/anaplastic (LCA) histology.15 Cerebellar granule neuron precursors are the putative cells of origin of SHH medulloblastoma; therefore, these tumors frequently arise in a cerebellar hemisphere.8 Three dominant subcategories prevail: infant SHH (0 to < 4 years), childhood SHH (≥ 4 to 17 years), and adult SHH (> 17 years).16
PTCH1 mutations occur in all three SHH subcategories at approximately equal frequencies (42%, infant SHH; 36%, childhood SHH; and 54%, adult SHH tumors). Infant SHH tumors are the most likely to harbor SUFU mutations (32%). Childhood SHH is typified by TP53 mutations (48%), which are frequently germline (80%) as part of Li-Fraumeni syndrome, and they rarely express SUFU (3%) mutations. This subgroup of SHH tumors with germline TP53-mutated tumors is more frequently associated with amplifications in MYCN (42%) and GLI2 (30%). Adult SHH is characterized by mutations in SMO (30%) and aberrant activation of the phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin (mTOR) –signaling pathway(30%).16
The prognosis of SHH medulloblastoma is quite variable: Patients who have infant SHH have an excellent prognosis when treated with chemotherapy alone17; those who have childhood SHH with TP53 mutations have the worst outcome, especially when MYCN and GLI2 are amplified. Patients with childhood SHH tumors that do not express TP53 mutations and have average-risk features have a 5-year EFS of approximately 60%; those with adult SHH have a 5-year EFS of approximately 40% on the basis of retrospective data.15,18
Groups 3 and 4 medulloblastomas.
Groups 3 and 4 account for 25% and 35% of medulloblastoma occurrences, respectively. They are molecularly distinct but share some overlap. Both have a male preponderance, and isochromosome 17q is confined to these subgroups, though it predominates in group 4 (80% v 26%). Groups 3 and 4 tumors demonstrate a relative paucity of recurrent driver mutations and are characterized by recurrent structural variants, including deletions, duplications, and inversions that place the growth factor–independent family of proto-oncogenes GFI1 and GFI1B next to active enhancer elements, which results in their aberrant activation. This mechanism of enhancer hijacking activates GFI1/GFI1B in approximately 41% and 10% of group 3 and group 4 tumors, respectively.19 The most frequently mutated gene in group 3 is SMARCA4 (11%).
Continuing the theme of deregulated chromatin modifiers, inactivating mutations in the histone lysine (K) -specific demethylase 6A gene (KDM6A) encodes a protein that specifically demethylates the K27 residue of histone H3 (H3K27). Group 3 tumors are associated with LCA histology and metastatic disease (50%). They are also characterized by MYCC overexpression in most instances (approximately 17% have MYCC amplification). In the presence of metastatic disease, isochromosome 17q, or MYCC amplification, group 3 tumors confer a dismal prognosis. In addition to alterations of chromatin-modifying genes, copy number changes that target the transforming growth factor β–signaling pathway occur in 20% of patients with group 3 disease, most commonly through amplification of OTX2 (8%).2,7,20
Group 4 tumors most commonly have classic histology, though some have LCA histology. Group 4 tumors are associated with MYCN amplification, which, in contrast to the SHH subgroup, is not associated with inferior outcome. Patients with group 4 medulloblastoma have an intermediate prognosis. However, those with metastatic disease have a higher risk of relapse, except in the presence of either whole chromosome 11 loss or chromosome 17 gain, which seem to identify a favorable prognostic subgroup. Group 4 has been found in a small number of infants who experience poor survival.21
Current Therapy for Medulloblastoma
During the past three decades, the use of empirically-based craniospinal irradiation (CSI) and chemotherapy after surgical resection has transformed a universally fatal disease into one in which the cure rate is approximately 70%. The application of clinical-risk stratification that is based on the extent of tumor resection and the presence of metastatic disease has refined treatment delivery. Children who are at least 3 years of age, have undergone gross-total resection (< 1.5 cm2 of residual tumor), and have no metastatic disease are classified with average-risk disease; the remainder are classified with high-risk disease. For children with average-risk disease, the introduction of chemotherapy has successfully demonstrated that addition of chemotherapy to CSI improved EFS. This finding has facilitated the reduction of CSI from 36 Gy to 23.4 Gy without adversely affecting outcome.22 Patients with average-risk disease have a 5-year EFS of approximately 80% after 23.4-Gy CSI, with a posterior fossa (PF) boost to 55.8 Gy in conjunction with platinum-based chemotherapy.23 The recently completed Children's Oncology Group (COG) trial for average-risk medulloblastoma investigated the additional reduction of CSI to 18 Gy for children age 3 to 7 years, the age group that is most vulnerable to neurocognitive sequelae. In contrast, patients with high-risk disease have a 5-year EFS between 60% to 70% after they receive various treatment regimens centered on increased radiotherapy (RT) doses and intensified chemotherapy.9,24 A recently published randomized study documented that there is no difference in the outcome for patients with high-risk medulloblastoma who get chemotherapy before RT versus chemotherapy after RT. Infants comprise a distinct risk group, in whom the outcome varies from 20% for those with macrometastatic disease to 90% for those with average-risk features and ND histology who received intensive chemotherapy-only regimens.25
Future Therapies for Medulloblastoma
WNT-subgroup medulloblastoma.
Patients with WNT medulloblastoma are ideal candidates for therapy reduction to minimize the long-term effects of current therapy. However, given the potentially devastating consequences of reducing therapy in a patient incorrectly identified as having the WNT subgroup, accurate tumor assessment is paramount. International cooperative clinical trials are now underway or planned that incorporate molecular profiling to identify patients with WNT-subgroup disease and reduce not only their CSI dose but also their chemotherapy.
SHH-subgroup medulloblastoma.
The potential effectiveness of SMO inhibitors in medulloblastoma was highlighted by the case report of a remarkable initial response of an adult with multiple-relapsed metastatic medulloblastoma treated with the SMO inhibitor GDC-0449.26 Early-phase clinical trials of GDC-0449 for recurrent, SHH-driven medulloblastoma in adults and children revealed that it is well tolerated and has promising efficacy in certain patients.27 Recent evidence suggests that SMO inhibitors demonstrate differential sensitivities depending on SHH subtypes; only patients who harbor upstream SHH-pathway mutations (ie, SMO and PTCH1 mutations) showed sensitivity, whereas those with downstream aberrations (ie, SUFU mutations or GLI2 amplification) demonstrated primary resistance.16 Preclinical studies revealed that arsenic trioxide and itraconazole inhibited SHH signaling in the context of primary and secondary SMO inhibitor–resistant SHH medulloblastoma, an approach that requires additional evaluation for these SHH subtypes.28
Groups 3 and 4 medulloblastoma.
Given the bleak outcome of group 3 patients, therapies that specifically target this subgroup are being sought with murine models that recapitulate the human tumors. By using one of these models and high-throughput screening, investigators identified two cytotoxic drugs, pemetrexed and gemcitabine, as effective and specific for group 3.29 These drugs now are being evaluated in a clinical trial for newly diagnosed patients with medulloblastoma. Bromodomain and extraterminal domain family (BET) bromodomain inhibitors, which suppress MYC-associated transcriptional activity, have emerged as promising compounds targeting group 3 medulloblastoma. BET bromodomain inhibitors modulate GLI transcription downstream of SMO and SUFU; therefore, they may be effective against SHH medulloblastoma that shows primary or secondary resistance to SMO inhibitors.30,31 Group 4–specific therapies remain elusive to date. Epigenetic-based therapies that target chromatin remodeling enzymes, such as demethylating agents (decitabine and azacitidine) and histone deacetylase inhibitors (vorinostat and panobinostat), are currently under preclinical investigation.
HIGH-GRADE GLIOMA AND DIFFUSE INTRINSIC PONTINE GLIOMA
Pediatric high-grade glioma (HGG) and diffuse intrinsic pontine glioma (DIPG) are diffusely infiltrative, malignant glial neoplasms that comprise a spectrum of histologies, and the vast majority are consistent with either anaplastic astrocytoma (WHO grade 3) or glioblastoma (WHO grade 4). Some DIPGs are consistent with diffuse astrocytomas (WHO grade 2) on initial biopsy, which may reflect sampling error. Their molecular genetic signatures and clinical behavior are indistinguishable from histologically higher-grade lesions.
Molecular Genetics of Pediatric HGG and DIPG
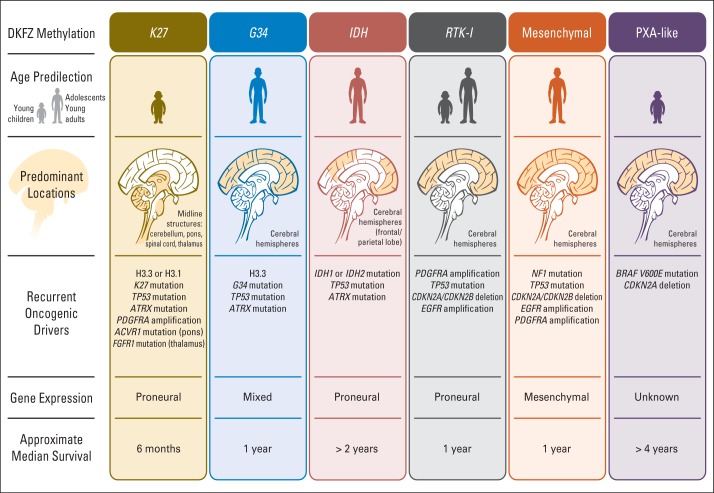
Subgroups of pediatric HGG and DIPG have been distinguished on the basis of recurrent combinations of genomic and/or epigenomic features with distinct biologic and clinical characteristics.32–37 Key findings include the discovery of novel oncogenic driver mutations in histones H3.1 (position K27) and H3.3 (positions K27 and G34) as well as in the activin A receptor, type I (ACVR1). Genome-wide methylation-profiling data from the German Cancer Research Center and the Cancer Genome Atlas study groups support six epigenetically distinct subgroups of glioblastoma, five of which include pediatric patients (younger than 21 years, in descending order of prevalence): K27, G34, receptor tyrosine kinase (M6), mesenchymal (M1/M2), and isocitrate dehydrogenase (IDH; CpG island methylator phenotype).38 An additional subgroup with glioblastoma histology but a distinct methylation signature that resembles pleomorphic xanthoastrocytoma (PXA) with frequent BRAF V600E mutations and a more favorable outcome was recently described and termed PXA-like.39
The K27 subgroup is characterized by its midline location (midbrain, brainstem, spinal cord), whereas the G34 subgroup most commonly arises in the cerebral white matter. The IDH subgroup, which is common in secondary glioblastomas that arise in adults and is characterized by mutations in IDH (IDH1 or IDH2) as well as longer survival, is rarely seen in children. Published retrospective data indicate that the IDH, PXA-like, and G34 subgroups are associated with longer overall survival (OS), whereas the K27, receptor tyrosine kinase inhibitor, and mesenchymal subgroups are associated with the shortest OS.33,39 In addition, the presence of amplified oncogenes, such as EGFR, PDGFR, and MYCN, was associated with a particularly poor outcome across subgroups. These data confirm the considerable molecular, biologic, and clinical heterogeneity of pediatric HGG and provide invaluable information for designing future clinical trials that involve a rational selection and stratification of patients on the basis of molecular subgroups, which are summarized in Figure 2.39
Fig 2.
Subgroups of pediatric high-grade glioma that are based on German Cancer Research Center (DKFZ) methylation, age at onset, tumor location, oncogenic drives, gene expression, and median survival. IDH, isocitrate dehydrogenase; PXA, pleomorphic xanthoastrocytoma; RTK-I, receptor tyrosine kinase (subgroup 1).
Current Therapies for Pediatric HGG and DIPG
The outcome for children with HGG or DIPG remains dismal and has been virtually unchanged for decades. For pediatric HGG, the strongest clinical prognostic factors are extent of resection and histologic grade. Single-agent temozolomide, when administered during and after RT, significantly prolongs EFS and OS in adults with glioblastoma40; however, similar treatment strategies used in a COG phase II trial ACNS0126 (RT and temozolomide) did not improve the outcome in pediatric HGG compared with previous studies that used different adjuvant chemotherapy regimens.41 Given the biologic heterogeneity of pediatric HGG, any clinical benefit of adjuvant chemotherapy may be confined to a particular subset. In pediatric patients with DIPG or HGG treated in arm C of the German Hirntumor cooperative group study for GBM (HIT-GBM-C) with intensive chemotherapy during and after RT, survival was better than that seen in prior HIT-GBM studies in the subgroup of patients with HGG who had undergone gross-total resection.42 For DIPG, no improvement in outcome, compared with that achieved with RT alone, has been seen in any prospective clinical trial using RT plus adjuvant chemotherapy, including temozolomide and radiation sensitizers (ie, motexafin-gadolinium) and intensified chemotherapy regimens (Table 1). Thus, the standard of care for patients with DIPG remains RT alone.
Table 1.
Outcomes for Cooperative Group Studies
| Protocol by Pediatric Brain Tumor Type | Study Hypothesis | Radiation Therapy | Chemotherapy | Planned Duration of Therapy | Accrual (No. of patients) | Age | EFS |
|---|---|---|---|---|---|---|---|
| Medulloblastoma | |||||||
| Standard risk | |||||||
| A 996123 | To determine efficacy of cyclophosphamide-based regimen versus standard regimen | 23.4 Gy CSI; 55.8 Gy PF | Weekly VCR during RT; randomly assigned chemotherapy: CDDP/CCNU/VCR v CDDP/Cyclo/VCR | 56 weeks | 379 | 3-18 years | 81% ± 2.1% (5 year); no difference between chemotherapy arms |
| SIOP III43 | Randomized study to determine the efficacy of RT alone versus chemotherapy + RT | 35 Gy CSI; 55 Gy PF | Weekly VCR during RT; carboplatin and VP16 alternating with cyclophosphamide and VP16 | 6 weeks for RT alone; 20 weeks for RT + chemotherapy | 179 | 3-16 years | 67% (5 year); 74.2% (chemotherapy + RT); 59.8%(RT alone) |
| High risk | |||||||
| POG 903124 | Efficacy of pre-RT chemotherapy on the EFS of high-risk medulloblastoma | 35.2-44.0 Gy CSI; 53.2-54.4 Gy PF | Three cycles of pre-RT chemotherapy with CDDP/VP16 followed by seven cycles of Cyclo/VCR v same chemotherapy given post-RT | 47 weeks | 224 | 3-18 years | 68.1% ± 3% (5 year); no difference between the two arms |
| High-grade glioma | |||||||
| ACNS012640 | Temozolomide administered during and after RT will improve EFS compared with historical controls | 54.0 Gy | Temozolomide during RT and followed by RT for 10 cycles | 50 weeks | 107 | 3 to ≤ 22 years | 11% ± 3% (3 year); no improvement |
| HIT-GBM-C41 | Intensive chemotherapy during and after RT, followed by valproate maintenance therapy, will improve OS compared with historical controls | 54 Gy | Two cycles of PEV and PEI, respectively, during RT, followed by six cycles of PEI alternating with monthly VCR, followed by continuous valproate maintenance therapy | 30 weeks, followed by continuous valproate maintenance therapy | 60 | 3-17 years | OS: 67% ± 10% (1 year) and 63% ± 12% (5 year) for patients with complete resection only; improvement compared with historical controls; no improvement for incomplete resection |
| Diffuse pontine glioma | |||||||
| ACNS012644 | Temozolomide administered during RT and post-RT will improve EFS compared with historical controls | 59.4 Gy | Temozolomide during RT and followed by RT for 10 cycles | 46 weeks | 63 | 3-21 years | 14% ± 5.5% (1 year); no improvement |
| ACNS022245 | Motexafin-gadolinum administered during RT will improve EFS | 54 Gy | Motexafin-gadolinium administered with daily RT | 6 weeks | 60 | < 22 years | 18% ± 5% (1 year); no improvement |
| HIT-GBM-C41 | Intensive chemotherapy during and after RT, followed by valproate maintenance therapy will improve OS compared with historical controls | 59.4 Gy | Two cycles of PEV and PEI, respectively, during RT, followed by six cycles of PEI alternating with monthly VCR, followed by continuous valproate maintenance therapy | 30 weeks, followed by continuous valproate maintenance therapy | 37 | 3-17 years | 0.40 ± 0.07 years (median ± SD EFS); no improvement compared with control (0.55 ± 0.098 median ± SD EFS) |
| Low-grade glioma | |||||||
| A 995246 | Compare the efficacy of two active chemotherapy regimens for LGG | — | Carbo/VCR v CCNU/procarbazine/TG/VCR | 52 weeks | 274 | < 10 years | 45% ± 3.2% (5 year); no difference in the two regimens |
| Ependymoma | |||||||
| ACNS0121* | Efficacy of conformal RT in ependymoma | 59.4 Gy (> 18 months) | Only for patients with subtotal resection | 6 weeks | 355 | > 12 months to < 21 years | 62.6 ± 2.7% (5 year); similar to highly selected single-institution series |
Abbreviations: Carbo, carboplatin; CCNU, lomustine; CDDP, cisplatin; CSI, craniospinal irradiation; Cyclo, cyclophosphamide; EFS, event-free survival; HIT-GBM-C, arm C of Hirntumor study for GBM; LGG, low-grade glioma; OS, overall survival; PEI, cisplatin, etoposide, and ifosfamide; PEV, cisplatin, etoposide, and vincristine; PF, posterior fossa; RT, radiotherapy; SD, standard deviation; TG, thioguanine; VCR, vincristine; VP16, etoposide.
T. Merchant, personal communication, July 2015.
The most recent COG HGG trial, ACNS0822, compared two combined-modality experimental arms that included either vorinostat or bevacizumab with standard therapy (ie, combined-modality that includes temozolomide). This study was recently closed after interim analysis showed that the predefined end point (ie, improved 1-year EFS compared with standard therapy) could not be met. In the context of the disappointing results of first-line therapy with bevacizumab in adults47,48 and the lack of efficacy of bevacizumab-containing regimens in children with recurrent disease,49,50 bevacizumab will probably not play a substantial role in future pediatric HGG and DIPG trials.
Future Therapies for Pediatric HGG and DIPG
The overarching theme that has emerged in pediatric HGG and DIPG biology is the disruption of multiple epigenetic regulatory processes by affecting histone modification, DNA methylation, and chromatin remodeling.38 These data provide a foundation for the development of novel treatments that target the genetic and epigenetic drivers of pediatric HGG initiation and progression. Several agents that target specific chromatin modifiers are in preclinical and/or early clinical development, and such strategies will hopefully mature efficiently to enter clinical studies.51,52
The molecular heterogeneity of pediatric HGG and DIPG provides the impetus to stratify patients into biologically and clinically relevant molecular subgroups at the time of diagnosis.39 It also motivates the identification of potentially actionable driver mutations, especially in the context of future clinical trials with novel, molecularly targeted drugs, including epigenetic modifiers. Preliminary data have demonstrated the efficacy of V600E inhibitors in recurrent HGG that harbors the mutation, which thus provides early proof of principal for treating HGG on the basis of molecular subgroups.53
LOW-GRADE GLIOMA
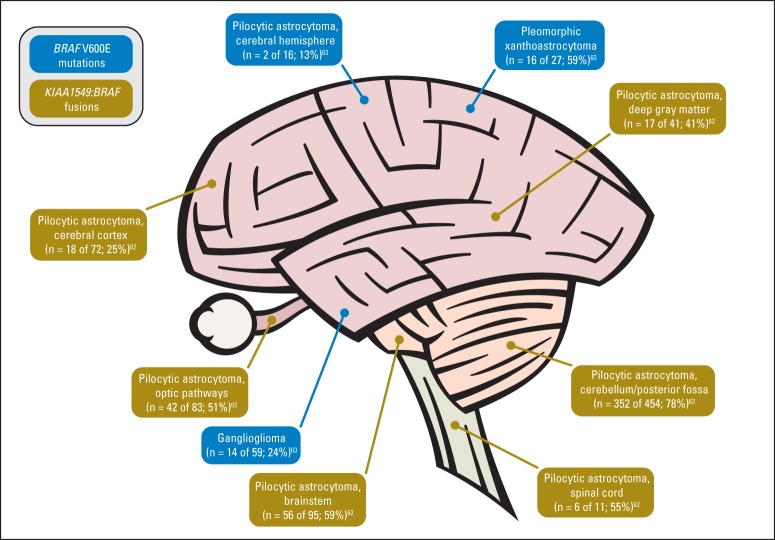
Low-grade gliomas (LGGs) represent multiple tumor subtypes and are the most common brain tumor of childhood. Two autosomal-dominant cancer-predisposition syndromes, tuberous sclerosis complex and neurofibromatosis type-1 (NF-1), are associated with an increased frequency of LGGs (subependymal giant cell astrocytomas and pilocytic astrocytomas of the optic pathways/hypothalamus, respectively). Despite many similarities, pediatric LGG subtypes have distinct predilections for specific locations within the CNS, which often correlate with specific genomic alterations and the ability to achieve gross-total resection (Fig 3).
Fig 3.
BRAF mutations and fusions by tumor histology and tumor location in pediatric low-grade gliomas.
BRAF oncogene mutations that activate the mitogen-activated protein kinase (MAPK) pathway represent the most frequent genomic alteration in pediatric LGGs.54–56 Furthermore, KIAA1549–BRAF gene fusions are common among pilocytic astrocytomas in the cerebellum but not in the cerebral cortex,56–62 whereas BRAF V600E mutations are more frequent among pleomorphic xanthoastrocytomas, gangliogliomas, and a subset of extracerebellar pilocytic astrocytomas.63 Other alterations that activate the MAPK pathway, such as SRGAP3–RAF1 and FAM131B–BRAF fusions and a 3-bp insertion at position 599 (BRAFinsT), have been identified in pediatric LGGs.64,65
Unlike LGGs among older adolescents and adults, childhood LGGs almost never express IDH1 or IDH2 mutations and rarely undergo malignant transformation into higher-grade neoplasms.66,67
Current Therapy for LGGs
Most children with LGGs undergo surgical resection followed by observation; carboplatin-containing chemotherapy and/or conformal RT are reserved for recurrent or progressive tumors.68–71 Treatment decisions largely are based on the tumor's location and the patient's age at diagnosis rather than on the glioma histologic subtype or tumor biology. With such strategies, the 10- to 20-year OS for children with LGGs is 83% to 94%.72–75
Randomized, phase III clinical trials for children with LGGs have recently been conducted by COG and the International Society of Pediatric Oncology (SIOP). COG A9952 was a prospective, randomized trial for children younger than 10 years with LGGs. Enrolled patients without NF-1 were randomly assigned to receive carboplatin and vincristine (CV; n = 137) or thioguanine, procarbazine, CCNU (lomustine), and vincristine (n = 137). The 5-year EFS (± standard deviation) was 39% ± 4% for patients randomly assigned to CV and was 52% ± 5% for those who received thioguanine, procarbazine, lomustine, and vincristine (P = .10).46
The SIOP clinical trial LGG-2004 is a comprehensive treatment strategy for children with LGGs and includes a randomized chemotherapy trial for children without NF-1. The study compares CV versus CV plus etoposide. Results of the SIOP-LGG-2004 clinical trial are anticipated in the near future.
Future Therapies for LGGs
BRAF duplication/MAPK pathway–targeting agents.
Considerable interest exists in the targeted inhibition of the MAPK pathway as therapy for pediatric LGGs. A recent phase II study of sorafenib was associated with an unexpected and unprecedented acceleration of LGG growth, irrespective of the tumor's BRAF status and most likely caused by paradoxical ERK activation, which led to early closure of this study.76,77 In addition, an ongoing phase II study from the Pediatric Brain Tumor Consortium (NCT01089101) is examining the activity of selumetinib (AZD4266), an MEK1/2 inhibitor, against pediatric LGGs on the basis of efficacy seen in a phase I study. As the results of the phase II study become available, BRAF/MAPK/ERK pathway inhibitors are expected to be examined in future trials designed for patients with newly diagnosed LGG.
BRAF V600E–targeting agents.
Vemurafenib and dabrafenib are competitive small molecules that bind and inhibit the ATP-binding domain of mutant BRAF V600E but not other mutant forms of BRAF.78 Vemurafenib and dabrafenib are approved by the US Food and Drug Administration for unresectable or metastatic melanoma with BRAF V600E mutations. Furthermore, trametinib, a MEK inhibitor, is also approved by the US Food and Drug Administration for BRAF V600E–mutated metastatic melanoma. Dabrafenib is currently being examined as therapy for BRAF V600E–mutant pediatric tumors, including LGGs (NCT01677741).
AKT/mTOR pathway–targeting agents.
Subependymal giant cell astrocytoma, an LGG subtype found nearly exclusively among children with tuberous sclerosis complex, has an activated AKT/mTOR pathway. Clinical trials have demonstrated that mTOR inhibitors (eg, sirolimus and everolimus) have activity against this LGG subtype.79,80 Everolimus has received approval as a treatment for subependymal giant cell astrocytomas that cannot be surgically resected.
Two clinical trials of mTOR inhibitors have demonstrated activity against progressive pediatric LGGs, regardless of NF-1 status. Yalon et al81 reported the activity of sirolimus and everolimus against recurrent pediatric LGG. Among 19 evaluable patients, one experienced partial response (PR; > 50% decrease in tumor size), five experienced stable disease (SD), and 10 experienced progressive disease (PD); three patients discontinued the study therapy before being evaluated. Six patients (n =1, PR and n = 5, SD) experienced tumor stabilization for 12 months or greater, and two patients with PD experienced tumor control for at least 12 months after completion of therapy. Kieran et al82,83 reported the activity of everolimus against pediatric LGGs. Of the 23 patients enrolled, four experienced PR, 13 had SD, and six experienced PD.82,83 These responses justify additional exploration of mTOR inhibitors against pediatric LGGs.
EPENDYMOMA
Ependymoma is the second-most-common malignant brain tumor in childhood. Ependymoma can arise intracranially, in the supratentorial compartment (ST), and within the PF and spinal cord.84 Despite our recent identification of driver oncogenes and molecular subtypes of ependymoma, the treatment of children and adolescents with the disease remains challenging. The outcome of ependymoma in children, especially in infants, is poor; almost half die as a result of their disease.85
In most pediatric ependymoma protocols, patients are stratified on the basis of the disease WHO grade and/or patient age at diagnosis. Histopathologic diagnosis, including WHO grading,86 is still challenging and has been controversial with regard to classification and reproducibility.87
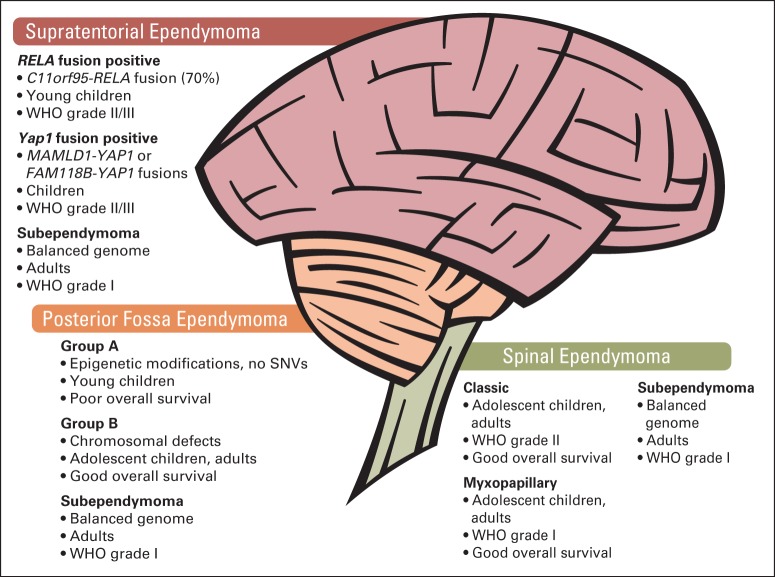
Several recent studies have provided reproducible evidence of distinct molecular subtypes of ependymomas.88–93 Two molecular subtypes, although histologically similar within the PF, were discovered.89 These distinct diseases differ on the basis of age at diagnosis, copy-number aberrations, gene expression, and outcome. Group-A PF ependymomas show flat genomes, occur predominantly in infants, and show activation of cancer-related signaling pathways (eg, vascular endothelial growth factor, platelet-derived growth factor receptor, integrin, MAPK). Group-B PF tumors occur in adolescents and young adults and frequently display large genomic aberrations. These ependymomas can be cured by RT alone.
Contemporary studies highlighted the genetic and epigenetic alterations as therapeutic targets of different subtypes of ependymoma.92,93 By using whole-exome and whole-genome sequencing technologies, the researchers found that no single recurrent somatic mutations could be identified in PF ependymoma. The mutation rates in groups A and B were low (average, n = 5 somatic mutations per occurrence). Accentuating the DNA-methylation pattern, group-A ependymomas displayed a slightly higher proportion of methylated CpG islands within the promoter region than did group B ependymomas. Group-A tumors showed a greater extent of epigenetic silencing of targets of the polycomb repressive complex 2, including downregulation of differentiation genes through H3K27 trimethylation. In vitro preclinical studies that used epigenetic demethylating drugs have shown responses in isolated ependymoma cells. These results are promising treatment strategies that target DNA CpG methylation, polycomb repressive complex 2/enhancer of zeste homolog 2 (EZH2), and/or histone deacetylases (Fig 4).
Fig 4.
Several subtypes of ependymomas, including WHO grades 1 to 3 disease within all three compartments of the CNS—supratentorial (ST), posterior fossa (PF), and spinal (SP)—are illustrated. RELA-positive ependymomas, including YAP1 fusion–positive ependymomas and subependymomas, arise within the ST region of the brain. Both fusion-positive subtypes display histopathologic features of WHO grades 2 and 3 ependymomas. In the PF, the majority of ependymomas belong to subtype group A, and group B tumors are more infrequent. Both subtypes display the histologic pattern of anaplastic and WHO grade 2 ependymomas; in contrast, subependymomas can be classified as WHO grade 1. SP tumors are diagnosed as classic ependymomas that are WHO grade 2 or 3; myxopapillary ependymoma and spinal subependymomas are WHO grade 1. In children, group A and RELA-positive tumors are diagnosed most often and are associated with poor overall survival. SNV, single nucleotide variant.
Parker et al93 recently discovered that greater than 70% of ST ependymomas express the C11orf95–RELA gene fusion within chromosome 11q, which was possibly caused by chromothripsis. RELA is a downstream target of nuclear factor-κB signaling that acts as a transcription factor and regulates cell maintenance.
A study that used data from 500 ependymal brain tumors presented a molecular-based classification scheme of nine molecular subgroups by using DNA methylation arrays. This molecular classification outperforms the current histopathologic grading in the risk stratification of patients94 (Fig 4). Within each anatomic region, three subgroups were identified, including subependymomas (WHO grade 1) that can be diagnosed predominantly in adults, which affect the spinal cord, PF, and ST areas. Molecular subtypes of the spinal cord correlated with histologic diagnoses myxopapillary ependymoma and grade 2 ependymomas. Within the PF, previously described molecular subtypes could be confirmed, namely, group A (PF-EPN-A) and group B (PF-EPN-B). Tumors of the two remaining ST subtypes originate by tumorigenic gene fusions that affect the gene RELA (ST-EPN-RELA) and Yes-associated protein 1 (YAP1 [ST-EPN-YA]). The integration of molecular subtypes and clinical follow-up data revealed a strong association with poor OS of patients with ST-EPN-RELA and PF-EPN-A tumors, who are usually children.94
Current Therapy for Ependymoma
Treatment of all ependymomas consists of attempting complete neurosurgical resection and adjuvant RT. Study protocols differ in terms of eligibility criteria for RT; some centers start RT at 1 year of age or younger, and others start at 18 months. A recently concluded COG study that used adjuvant RT after surgical resection documented no improvement in outcome versus historical data, though the subset of patients with gross-total resection had an improved EFS (Table 1). The benefit of addition of adjuvant chemotherapy to surgical resection and RT is being investigated in the current COG trial for newly diagnosed ependymoma (NCT01096368).
The current European Ependymoma Study (SIOP Ependymoma II; EudraCT No. 2013-002766-39) will open for patient enrollment in late 2014. The study has three strata: Stratum 1 includes patients older than 1 year, who have had gross-total resection of a WHO grade 2 to 3 tumor. These children will receive conformal RT followed by random assignment with or without maintenance chemotherapy (vincristine, cyclophosphamide, etoposide, and cisplatin). Stratum II includes patients older than 1 year who have residual disease. These patients will receive chemotherapy (vincristine, cyclophosphamide, and etoposide), including random assignment with or without high-dose methotrexate, before a second resection and conformal RT. Stratum III includes patients younger than 1 year or those not eligible to receive RT. Patients in this group will be treated with standard chemotherapy (vincristine, cyclophosphamide, and cisplatin) and will be randomly assigned with or without treatment with the histone deacetylase inhibitor valproate.
Future Therapies for Ependymoma
Genetically engineered mouse models of ependymoma offer excellent opportunities for preclinical drug testing in vivo. The Ephb2 mouse model was used in a high-throughput screen that identified fluorouracil as an effective therapy for ST ependymoma.95 Efficacy data in humans are currently being evaluated.
RARE EMBRYONAL TUMORS
Molecular subtyping of pediatric brain tumors revealed more than a decade ago that atypical teratoid/rhabdoid tumors (ATRTs) and other CNS primitive neuroectodermal tumors (CNS PNETs) are biologically distinct from medulloblastoma.96 Because the next generation of clinical trials aims to stratify and treat tumors on the basis of molecular subtypes, it is of paramount importance to additionally characterize these rare embryonal tumors as distinct diseases.
ATRT
ATRT was initially described as a universally fatal tumor of young children (Table 2). It is associated with a deletion on chromosome 22 and, in addition, is characterized by the loss of SMARCB1 expression.97 ATRT arises in infratentorial or ST locations with an approximately equal proportion and rarely arises in the spine.98–100 SMARCB1 expression should be evaluated in all young patients with embryonal tumors to confirm the diagnosis of ATRT rather than medulloblastoma or other CNS PNETs.101 The diagnosis of ATRT has implications for constitutional testing102 and should lead to appropriately aggressive therapy that results in increased survival.103
Table 2.
Patient Demographics and Molecular and Clinical Features of ATRT and CNS PNET
| Feature | ATRT | ETMR* | CNS PNET |
|
|---|---|---|---|---|
| Group 2 | Group 3 | |||
| Median patient age | 18 months | 3 years | 8 years | 6 years |
| Sex ratio | Male > female | Female > male | Male > female | Male > female |
| IHC marker | SMARCB1 negative | LIN28 positive | Olig2 positive | LIN28 and Olig2 negative |
| Metastatic disease at presentation, % of patients | 30 | 25 | 15 | 50 |
| Molecular marker | SMARCB1-inactivating genetic alteration (mutation, deletion, or insertion) | C19MC miRNA amplicon | CDKN2A/B loss; Chr 8p, 13, 20 gain | CDKN2A/B loss; Chr 14 loss |
Abbreviations: ATRT, atypical teratoid rhabdoid tumor; Chr, chromosome; CNS PNET, CNS primitive neuroectodermal tumor; ETMR, embryonal tumor with multilayered rosettes; IHC, immunohistochemistry.
Also known as embryonal tumor with abundant neuropil and true rosettes, medulloepithelioma or ependymoblastoma.
Current therapy for ATRT.
A significant proportion of ATRTs arise in children younger than 3 years. Treatment with conventional postoperative chemotherapy alone results in less than 20% survival.104–106 Small cohorts of patients treated with regimens designed specifically for ATRT have achieved survival rates greater than 50%.104,107 Treatment factors that predict survival have included the use of multimodality regimens containing RT, intrathecal chemotherapy, and/or high-dose therapy with stem cell rescue.99,100,104,107,108 Ongoing, prospective studies will more precisely define the outcome of children with ATRT in the current era. Current curative therapy for ATRT is perhaps excessively toxic, including the acute toxicity of high-dose chemotherapy108 and long-term toxicity of RT in young children. Novel therapy that improves outcomes while it decreases toxicity is greatly needed.
Future clinical trials for ATRT.
The availability of ATRT cell lines and accurate preclinical mouse models have enhanced the discovery of novel therapeutic targets for ATRT. Current targets under consideration are aurora A kinase, cyclin D1, EZH2, and insulin-like growth factor-1. The availability of aurora A kinase inhibitors has facilitated the development of a phase II trial for patients with recurrent ATRT and malignant rhabdoid tumor (NCT02114229). If this trial demonstrates the efficacy of this agent, it will most likely be incorporated into therapy for patients with newly diagnosed ATRT.
CNS PNETs
International collaboration and advanced genomics have supported the recent discovery of diagnostic and prognostic factors for CNS PNET. A comprehensive analysis of PNET tumors of cortical origin defined three distinct molecular subtypes, initially termed groups 1, 2, and 3.109 Group 1 tumors, which have been the most well defined to date, are characterized by an amplification on chromosome 19q13.42 that contains a microRNA cluster that has a functional association with oncogenesis110 and diffuse expression of the LIN28A protein, both of which may facilitate diagnosis by using standard pathology methods (ie, immunohistochemistry and fluorescent in situ hybridization).111 The term embryonal tumor with multilayered rosettes (ETMR) has been proposed as a unifying diagnostic term for group 1 tumors, including the previously described embryonal tumor with abundant neuropil and true rosettes, ependymoblastoma, and medulloepithelioma, because genome-wide DNA methylation and copy-number analysis has demonstrated biologic similarity between these three histologic entities.112
ETMR occurs in the youngest age group and has been associated with the poorest prognosis of all CNS PNET in retrospective studies. However, our understanding of the biology of ETMR has rapidly facilitated preclinical modeling and evaluation of potential novel therapeutics; inhibitors of the insulin-like growth factor/phosphatidylinositol 3-kinase/mTOR pathway have shown early promise in a preclinical study.113 Group 2 tumors may be defined by the expression of the Olig2 protein, and both groups 2 and 3 are associated with older age at diagnosis and frequent chromosome 9p loss centered on the CDKN2A/B locus.109 Additional molecular study of these subtypes is warranted, as is additional study of CNS PNET that occurs in noncortical areas of the brain (Table 1).
Current clinical trials for CNS PNET.
CNS PNETs can occur in any region of the CNS. Radiologically distinct tumors that arise in the pineal region are termed pineoblastomas. Although rare, CNS PNET can also arise in the brainstem.114 The remaining CNS PNETs originate in other midline or, more commonly, cortical regions. Children with CNS PNET have been historically treated on medulloblastoma regimens but have had inferior outcomes. The 5-year survival of approximately 50% after conventional CSI and chemotherapy has not improved in two decades.115,116
Reports on whether survival of pineoblastoma is superior to that of other CNS PNETs are conflicting, which may be partially explained by the high likelihood of pineoblastoma to present or recur in a disseminated pattern.116–118 A recent series has suggested that reduced CSI (23.4 Gy) followed by high-dose chemotherapy with stem-cell rescue offers an improved outcome in patients with localized surgically resected CNS PNET.119
Future clinical trials for CNS PNET.
The molecular characterization of CNS PNET is an inspiring example of international cooperation and collaboration facilitating the study of the rarest diseases. There is an equally great clinical research challenge still before us: to conduct cooperative clinical trials that incorporate patients with newly diagnosed CNS PNET in appropriate studies of molecularly based therapy.
In conclusion, cooperation among investigators from around the globe has facilitated genomic analysis of pediatric brain tumors. This information has revolutionized our understanding of the underlying pathogenesis of pediatric brain tumors. Our laboratory-based research colleagues have done an admirable job in defining a path forward for the next generation of clinical studies. The challenge is now back to the clinicians and regulatory agencies to design and fund the next generation of clinical protocols that will facilitate rapid accrual of patients to protocols that promise to improve the cure rate while minimizing toxicities.120
Acknowledgment
We thank David Jones and Dominik Sturm for critical review of Figure 2. We also thank Angela McArthur for editing the manuscript and Joshua Stokes for providing the illustrations.
Footnotes
Supported by St Jude Children's Research Hospital with Grant No. CA21765 (A.G.) provided by the Cancer Center Support, the Noyes Brain Tumor Foundation, Musicians Against Childhood Cancer, and the American Lebanese-Syrian Associated Charities. N.G.G. is supported by The Raine Medical Research Foundation's Clinical Research Fellowship Program.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pediatric Brain Tumors: Innovative Genomic Information Is Transforming the Diagnostic and Clinical Landscape
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Amar Gajjar
Consulting or Advisory Role: AstraZeneca, Celgene
Research Funding: Genentech (Inst)
Daniel C. Bowers
Research Funding: Novartis
Matthias A. Karajannis
Consulting or Advisory Role: MEDACorp
Research Funding: GlaxoSmithKline (Inst), Novartis (Inst), Bayer AG (Inst), Pfizer (Inst)
Sarah Leary
No relationship to disclose
Hendrik Witt
No relationship to disclose
Nicholas G. Gottardo
Research Funding: Pfizer (Inst)
REFERENCES
- 1.Pui CH, Gajjar AJ, Kane JR, et al. Challenging issues in pediatric oncology. Nat Rev Clin Oncol. 2011;8:540–549. doi: 10.1038/nrclinonc.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Northcott PA, Shih DJ, Peacock J, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488:49–56. doi: 10.1038/nature11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellison DW, Kocak M, Dalton J, et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol. 2011;29:1400–1407. doi: 10.1200/JCO.2010.30.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gajjar AJ, Robinson GW. Medulloblastoma: Translating discoveries from the bench to the bedside. Nat Rev Clin Oncol. 2014;11:714–722. doi: 10.1038/nrclinonc.2014.181. [DOI] [PubMed] [Google Scholar]
- 5.Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol. 2012;123:465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Northcott PA, Tuka JT, Taylor MD. Genomics of medulloblastoma: From Giemsa-banding to next-generation sequencing in 20 years. Neurosurg Focus. 2010;28:E6. doi: 10.3171/2009.10.FOCUS09218. [DOI] [PubMed] [Google Scholar]
- 7.Northcott PA, Jones DT, Kool M, et al. Medulloblastomics: The end of the beginning. Nat Rev Cancer. 2012;12:818–834. doi: 10.1038/nrc3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson P, Tong Y, Robinson G, et al. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010;468:1095–1099. doi: 10.1038/nature09587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 10.Ellison DW, Onilude OE, Lindsey JC, et al. beta-Catenin status predicts a favorable outcome in childhood medulloblastoma: The United Kingdom Children's Cancer Study Group Brain Tumour Committee. J Clin Oncol. 2005;23:7951–7957. doi: 10.1200/JCO.2005.01.5479. [DOI] [PubMed] [Google Scholar]
- 11.Robinson G, Parker M, Kranenburg TA, et al. Novel mutations target distinct subgroups of medulloblastoma. Nature. 2012;488:43–48. doi: 10.1038/nature11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pugh TJ, Weeraratne SD, Archer TC, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488:106–110. doi: 10.1038/nature11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones DT, Jäger N, Kool M, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488:100–105. doi: 10.1038/nature11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhukova N, Ramaswamy V, Remke M, et al. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J Clin Oncol. 2013;31:2927–2935. doi: 10.1200/JCO.2012.48.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kool M, Korshunov A, Remke M, et al. Molecular subgroups of medulloblastoma: An international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, group 3, and group 4 medulloblastomas. Acta Neuropathol. 2012;123:473–484. doi: 10.1007/s00401-012-0958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kool M, Jones DT, Jäger N, et al. Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell. 2014;25:393–405. doi: 10.1016/j.ccr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352:978–986. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 18.Kool M, Korshunov A, Pfister SM. Update on molecular and genetic alterations in adult medulloblastoma. Memo. 2012;5:228–232. doi: 10.1007/s12254-012-0037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Northcott PA, Lee C, Zichner T, et al. Enhancer hijacking activates GFI1 family oncogenes in medulloblastoma. Nature. 2014;511:428–434. doi: 10.1038/nature13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Northcott PA, Nakahara Y, Wu X, et al. Multiple recurrent genetic events converge on control of histone lysine methylation in medulloblastoma. Nat Genet. 2009;41:465–472. doi: 10.1038/ng.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shih DJ, Northcott PA, Remke M, et al. Cytogenetic prognostication within medulloblastoma subgroups. J Clin Oncol. 2014;32:886–896. doi: 10.1200/JCO.2013.50.9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Packer RJ, Goldwein J, Nicholson HS, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children's Cancer Group Study. J Clin Oncol. 1999;17:2127–2136. doi: 10.1200/JCO.1999.17.7.2127. [DOI] [PubMed] [Google Scholar]
- 23.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 24.Tarbell NJ, Friedman H, Polkinghorn WR, et al. High-risk medulloblastoma: A Pediatric Oncology Group randomized trial of chemotherapy before or after radiation therapy (POG 9031) J Clin Oncol. 2013;31:2936–2941. doi: 10.1200/JCO.2012.43.9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutkowski S, von Hoff K, Emser A, et al. Survival and prognostic factors of early childhood medulloblastoma: An international meta-analysis. J Clin Oncol. 2010;28:4961–4968. doi: 10.1200/JCO.2010.30.2299. [DOI] [PubMed] [Google Scholar]
- 26.Rudin CM, Hann CL, Laterra J, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361:1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gajjar A, Stewart CF, Ellison DW, et al. Phase I study of vismodegib in children with recurrent or refractory medulloblastoma: A Pediatric Brain Tumor Consortium study. Clin Cancer Res. 2013;19:6305–6312. doi: 10.1158/1078-0432.CCR-13-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Aftab BT, Tang JY, et al. Itraconazole and arsenic trioxide inhibit Hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23:23–34. doi: 10.1016/j.ccr.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morfouace M, Shelat A, Jacus M, et al. Pemetrexed and gemcitabine as combination therapy for the treatment of group 3 medulloblastoma. Cancer Cell. 2014;25:516–529. doi: 10.1016/j.ccr.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bandopadhayay P, Bergthold G, Nguyen B, et al. BET bromodomain inhibition of MYC-amplified medulloblastoma. Clin Cancer Res. 2014;20:912–925. doi: 10.1158/1078-0432.CCR-13-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang Y, Gholamin S, Schubert S, et al. Epigenetic targeting of Hedgehog pathway transcriptional output through BET bromodomain inhibition. Nat Med. 2014;20:732–740. doi: 10.1038/nm.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 33.Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22:425–437. doi: 10.1016/j.ccr.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 34.Fontebasso AM, Papillon-Cavanagh S, Schwartzentruber J, et al. Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nat Genet. 2014;46:462–466. doi: 10.1038/ng.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu G, Diaz AK, Paugh BS, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014;46:444–450. doi: 10.1038/ng.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buczkowicz P, Hoeman C, Rakopoulos P, et al. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet. 2014;46:451–456. doi: 10.1038/ng.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor KR, Mackay A, Truffaux N, et al. Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat Genet. 2014;46:457–461. doi: 10.1038/ng.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sturm D, Bender S, Jones DT, et al. Paediatric and adult glioblastoma: Multiform (epi)genomic culprits emerge. Nat Rev Cancer. 2014;14:92–107. doi: 10.1038/nrc3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korshunov A, Ryzhova M, Hovestadt V, et al. Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol. 2015;129:669–678. doi: 10.1007/s00401-015-1405-4. [DOI] [PubMed] [Google Scholar]
- 40.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 41.Cohen KJ, Pollack IF, Zhou T, et al. Temozolomide in the treatment of high-grade gliomas in children: A report from the Children's Oncology Group. Neuro Oncol. 2011;13:317–323. doi: 10.1093/neuonc/noq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolff JE, Driever PH, Erdlenbruch B, et al. Intensive chemotherapy improves survival in pediatric high-grade glioma after gross total resection: Results of the HIT-GBM-C protocol. Cancer. 2010;116:705–712. doi: 10.1002/cncr.24730. [DOI] [PubMed] [Google Scholar]
- 43.Taylor RE, Bailey CC, Robinson K, et al. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The International Society of Paediatric Oncology/United Kingdom Children's Cancer Study Group PNET-3 study. J Clin Oncol. 2003;21:1581–1591. doi: 10.1200/JCO.2003.05.116. [DOI] [PubMed] [Google Scholar]
- 44.Cohen KJ, Heideman RL, Zhou T, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: A report from the Children's Oncology Group. Neuro Oncol. 2011;13:410–416. doi: 10.1093/neuonc/noq205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bradley KA, Zhou T, McNall-Knapp RY, et al. Motexafin-gadolinium and involved field radiation therapy for intrinsic pontine glioma of childhood: A children's oncology group phase 2 study. Int J Radiat Oncol Biol Phys. 2013;85:e55–e60. doi: 10.1016/j.ijrobp.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ater JL, Zhou T, Holmes E, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: A report from the Children's Oncology Group. J Clin Oncol. 2012;30:2641–2647. doi: 10.1200/JCO.2011.36.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 49.Narayana A, Kunnakkat S, Chacko-Mathew J, et al. Bevacizumab in recurrent high-grade pediatric gliomas. Neuro Oncol. 2010;12:985–990. doi: 10.1093/neuonc/noq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gururangan S, Fangusaro J, Poussaint TY, et al. Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas: A Pediatric Brain Tumor Consortium study. Neuro Oncol. 2014;16:310–317. doi: 10.1093/neuonc/not154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dhanak D, Jackson P. Development and classes of epigenetic drugs for cancer. Biochem Biophys Res Commun. 2014;455:58–69. doi: 10.1016/j.bbrc.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 52.Dawson MA, Kouzarides T. Cancer epigenetics: From mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 53.Robinson GW, Orr BA, Gajjar A. Complete clinical regression of a BRAF V600E-mutant pediatric glioblastoma multiforme after BRAF inhibitor therapy. BMC Cancer. 2014;14:258. doi: 10.1186/1471-2407-14-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pfister S, Janzarik WG, Remke M, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118:1739–1749. doi: 10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bar EE, Lin A, Tihan T, et al. Frequent gains at chromosome 7q34 involving BRAF in pilocytic astrocytoma. J Neuropathol Exp Neurol. 2008;67:878–887. doi: 10.1097/NEN.0b013e3181845622. [DOI] [PubMed] [Google Scholar]
- 56.Sievert AJ, Jackson EM, Gai X, et al. Duplication of 7q34 in pediatric low-grade astrocytomas detected by high-density single-nucleotide polymorphism-based genotype arrays results in a novel BRAF fusion gene. Brain Pathol. 2009;19:449–458. doi: 10.1111/j.1750-3639.2008.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin A, Rodriguez FJ, Karajannis MA, et al. BRAF alterations in primary glial and glioneuronal neoplasms of the central nervous system with identification of 2 novel KIAA1549:BRAF fusion variants. J Neuropathol Exp Neurol. 2012;71:66–72. doi: 10.1097/NEN.0b013e31823f2cb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forshew T, Tatevossian RG, Lawson AR, et al. Activation of the ERK/MAPK pathway: A signature genetic defect in posterior fossa pilocytic astrocytomas. J Pathol. 2009;218:172–181. doi: 10.1002/path.2558. [DOI] [PubMed] [Google Scholar]
- 59.Hawkins C, Walker E, Mohamed N, et al. BRAF-KIAA1549 fusion predicts better clinical outcome in pediatric low-grade astrocytoma. Clin Cancer Res. 2011;17:4790–4798. doi: 10.1158/1078-0432.CCR-11-0034. [DOI] [PubMed] [Google Scholar]
- 60.Jacob K, Albrecht S, Sollier C, et al. Duplication of 7q34 is specific to juvenile pilocytic astrocytomas and a hallmark of cerebellar and optic pathway tumours. Br J Cancer. 2009;101:722–733. doi: 10.1038/sj.bjc.6605179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cin H, Meyer C, Janzarik WG, et al. Oncogenic FAM131B-BRAF fusion resulting from 7q34 deletion comprises an alternative mechanism of MAPK pathway activation in pilocytic astrocytoma. Acta Neuropathol. 2011;121:763–774. doi: 10.1007/s00401-011-0817-z. [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez EF, Scheithauer BW, Giannini C, et al. PI3K/AKT pathway alterations are associated with clinically aggressive and histologically anaplastic subsets of pilocytic astrocytoma. Acta Neuropathol. 2011;121:407–420. doi: 10.1007/s00401-010-0784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121:397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 64.Jones DT, Kocialkowski S, Liu L, et al. Oncogenic RAF1 rearrangement and a novel BRAF mutation as alternatives to KIAA1549:BRAF fusion in activating the MAPK pathway in pilocytic astrocytoma. Oncogene. 2009;28:2119–2123. doi: 10.1038/onc.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eisenhardt AE, Olbrich H, Röring M, et al. Functional characterization of a BRAF insertion mutant associated with pilocytic astrocytoma. Int J Cancer. 2011;129:2297–2303. doi: 10.1002/ijc.25893. [DOI] [PubMed] [Google Scholar]
- 66.Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118:401–405. doi: 10.1007/s00401-009-0550-z. [DOI] [PubMed] [Google Scholar]
- 67.Buccoliero AM, Castiglione F, Degl'Innocenti DR, et al. IDH1 mutation in pediatric gliomas: Has it a diagnostic and prognostic value? Fetal Pediatr Pathol. 2012;31:278–282. doi: 10.3109/15513815.2012.659383. [DOI] [PubMed] [Google Scholar]
- 68.Wisoff JH, Sanford RA, Heier LA, et al. Primary neurosurgery for pediatric low-grade gliomas: A prospective multi-institutional study from the Children's Oncology Group. Neurosurgery. 2011;68:1548–1554. doi: 10.1227/NEU.0b013e318214a66e. discussion 1554-1555. [DOI] [PubMed] [Google Scholar]
- 69.Merchant TE, Kun LE, Wu S, et al. Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol. 2009;27:3598–3604. doi: 10.1200/JCO.2008.20.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Packer RJ, Lange B, Nicholson HS, et al. Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J Clin Oncol. 1993;11:850–856. doi: 10.1200/JCO.1993.11.5.850. [DOI] [PubMed] [Google Scholar]
- 71.Müller K, Gnekow A, Falkenstein F, et al. Radiotherapy in pediatric pilocytic astrocytomas: A subgroup analysis within the prospective multicenter study HIT-LGG 1996 by the German Society of Pediatric Oncology and Hematology (GPOH) Strahlenther Onkol. 2013;189:647–655. doi: 10.1007/s00066-013-0357-7. [DOI] [PubMed] [Google Scholar]
- 72.Bandopadhayay P, Bergthold G, London WB, et al. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: An analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer. 2014;61:1173–1179. doi: 10.1002/pbc.24958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fisher PG, Tihan T, Goldthwaite PT, et al. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer. 2008;51:245–250. doi: 10.1002/pbc.21563. [DOI] [PubMed] [Google Scholar]
- 74.Gnekow AK, Falkenstein F, von Hornstein S, et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012;14:1265–1284. doi: 10.1093/neuonc/nos202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gajjar A, Sanford RA, Heideman R, et al. Low-grade astrocytoma: A decade of experience at St Jude Children's Research Hospital. J Clin Oncol. 1997;15:2792–2799. doi: 10.1200/JCO.1997.15.8.2792. [DOI] [PubMed] [Google Scholar]
- 76.Karajannis MA, Legault G, Fisher MJ, et al. Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro Oncol. 2014;16:1408–1416. doi: 10.1093/neuonc/nou059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sievert AJ, Lang SS, Boucher KL, et al. Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc Natl Acad Sci U S A. 2013;110:5957–5962. doi: 10.1073/pnas.1219232110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Franz DN, Leonard J, Tudor C, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol. 2006;59:490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- 80.Krueger DA, Care MM, Agricola K, et al. Everolimus long-term safety and efficacy in subependymal giant cell astrocytoma. Neurology. 2013;80:574–580. doi: 10.1212/WNL.0b013e3182815428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yalon M, Rood B, MacDonald TJ, et al. A feasibility and efficacy study of rapamycin and erlotinib for recurrent pediatric low-grade glioma (LGG) Pediatr Blood Cancer. 2013;60:71–76. doi: 10.1002/pbc.24142. [DOI] [PubMed] [Google Scholar]
- 82.Kieran MW, Yao X, Macy M, et al. Prospective multi-institutional phase II study of everolimus (RAD001), an mTOR inhibitor, in pediatric patients with recurrent or progressive low-grade glioma. Pediatr Blood Cancer, SIOP 2013 Scientific Programme. 2013;60 O-0068. [Google Scholar]
- 83. Reference deleted.
- 84.McGuire CS, Sainani KL, Fisher PG. Incidence patterns for ependymoma: A Surveillance, Epidemiology, and End Results study. J Neurosurg. 2009;110:725–729. doi: 10.3171/2008.9.JNS08117. [DOI] [PubMed] [Google Scholar]
- 85.Gatta G, Botta L, Rossi S, et al. Childhood cancer survival in Europe 1999-2007: results of EUROCARE-5–a population-based study. Lancet Oncol. 2014;15:35–47. doi: 10.1016/S1470-2045(13)70548-5. [DOI] [PubMed] [Google Scholar]
- 86.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ellison DW, Kocak M, Figarella-Branger D, et al. Histopathological grading of pediatric ependymoma: Reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed. 2011;10:7. doi: 10.1186/1477-5751-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnson RA, Wright KD, Poppleton H, et al. Cross-species genomics matches driver mutations and cell compartments to model ependymoma. Nature. 2010;466:632–636. doi: 10.1038/nature09173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Witt H, Mack SC, Ryzhova M, et al. Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell. 2011;20:143–157. doi: 10.1016/j.ccr.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wani K, Armstrong TS, Vera-Bolanos E, et al. A prognostic gene expression signature in infratentorial ependymoma. Acta Neuropathol. 2012;123:727–738. doi: 10.1007/s00401-012-0941-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoffman LM, Donson AM, Nakachi I, et al. Molecular sub-group-specific immunophenotypic changes are associated with outcome in recurrent posterior fossa ependymoma. Acta Neuropathol. 2014;127:731–745. doi: 10.1007/s00401-013-1212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mack SC, Witt H, Piro RM, et al. Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature. 2014;506:445–450. doi: 10.1038/nature13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parker M, Mohankumar KM, Punchihewa C, et al. C11orf95-RELA fusions drive oncogenic NF-kappaB signalling in ependymoma. Nature. 2014;506:451–455. doi: 10.1038/nature13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pajtler KW, Witt H, Sill M, et al. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015;27:728–743. doi: 10.1016/j.ccell.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Atkinson JM, Shelat AA, Carcaboso AM, et al. An integrated in vitro and in vivo high-throughput screen identifies treatment leads for ependymoma. Cancer Cell. 2011;20:384–399. doi: 10.1016/j.ccr.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pomeroy SL, Tamayo P, Gaasenbeek M, et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415:436–442. doi: 10.1038/415436a. [DOI] [PubMed] [Google Scholar]
- 97.Rorke LB, Packer R, Biegel J. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood. J Neurooncol. 1995;24:21–28. doi: 10.1007/BF01052653. [DOI] [PubMed] [Google Scholar]
- 98.von Hoff K, Hinkes B, Dannenmann-Stern E, et al. Frequency, risk-factors and survival of children with atypical teratoid rhabdoid tumors (AT/RT) of the CNS diagnosed between 1988 and 2004, and registered to the German HIT database. Pediatr Blood Cancer. 2011;57:978–985. doi: 10.1002/pbc.23236. [DOI] [PubMed] [Google Scholar]
- 99.Athale UH, Duckworth J, Odame I, et al. Childhood atypical teratoid rhabdoid tumor of the central nervous system: A meta-analysis of observational studies. J Pediatr Hematol Oncol. 2009;31:651–663. doi: 10.1097/MPH.0b013e3181b258a9. [DOI] [PubMed] [Google Scholar]
- 100.Lafay-Cousin L, Hawkins C, Carret AS, et al. Central nervous system atypical teratoid rhabdoid tumours: The Canadian Paediatric Brain Tumour Consortium experience. Eur J Cancer. 2012;48:353–359. doi: 10.1016/j.ejca.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 101.Pfister SM, Korshunov A, Kool M, et al. Molecular diagnostics of CNS embryonal tumors. Acta Neuropathol. 2010;120:553–566. doi: 10.1007/s00401-010-0751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Biegel JA, Zhou JY, Rorke LB, et al. Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res. 1999;59:74–79. [PubMed] [Google Scholar]
- 103.Slavc I, Chocholous M, Leiss U, et al. Atypical teratoid rhabdoid tumor: Improved long-term survival with an intensive multimodal therapy and delayed radiotherapy—The Medical University of Vienna Experience, 1992 to 2012. Cancer Med. 2014;3:91–100. doi: 10.1002/cam4.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tekautz TM, Fuller CE, Blaney S, et al. Atypical teratoid/rhabdoid tumors (ATRT): Improved survival in children 3 years of age and older with radiation therapy and high-dose alkylator-based chemotherapy. J Clin Oncol. 2005;23:1491–1499. doi: 10.1200/JCO.2005.05.187. [DOI] [PubMed] [Google Scholar]
- 105.Geyer JR, Sposto R, Jennings M, et al. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: A report from the Children's Cancer Group. J Clin Oncol. 2005;23:7621–7631. doi: 10.1200/JCO.2005.09.095. [DOI] [PubMed] [Google Scholar]
- 106.Grundy RG, Wilne SH, Robinson KJ, et al. Primary postoperative chemotherapy without radiotherapy for treatment of brain tumours other than ependymoma in children under 3 years: Results of the first UKCCSG/SIOP CNS 9204 trial. Eur J Cancer. 2010;46:120–133. doi: 10.1016/j.ejca.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 107.Chi SN, Zimmerman MA, Yao X, et al. Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol. 2009;27:385–389. doi: 10.1200/JCO.2008.18.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gardner SL, Asgharzadeh S, Green A, et al. Intensive induction chemotherapy followed by high dose chemotherapy with autologous hematopoietic progenitor cell rescue in young children newly diagnosed with central nervous system atypical teratoid rhabdoid tumors. Pediatr Blood Cancer. 2008;51:235–240. doi: 10.1002/pbc.21578. [DOI] [PubMed] [Google Scholar]
- 109.Picard D, Miller S, Hawkins CE, et al. Markers of survival and metastatic potential in childhood CNS primitive neuro-ectodermal brain tumours: An integrative genomic analysis. Lancet Oncol. 2012;13:838–848. doi: 10.1016/S1470-2045(12)70257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li M, Lee KF, Lu Y, et al. Frequent amplification of a chr19q13.41 microRNA polycistron in aggressive primitive neuroectodermal brain tumors. Cancer Cell. 2009;16:533–546. doi: 10.1016/j.ccr.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Korshunov A, Ryzhova M, Jones DT, et al. LIN28A immunoreactivity is a potent diagnostic marker of embryonal tumor with multilayered rosettes (ETMR) Acta Neuropathol. 2012;124:875–881. doi: 10.1007/s00401-012-1068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Korshunov A, Sturm D, Ryzhova M, et al. Embryonal tumor with abundant neuropil and true rosettes (ETANTR), ependymoblastoma, and medulloepithelioma share molecular similarity and comprise a single clinicopathological entity. Acta Neuropathol. 2014;128:279–289. doi: 10.1007/s00401-013-1228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Spence T, Perotti C, Sin-Chan P, et al. A novel C19MC amplified cell line links Lin28/let-7 to mTOR signaling in embryonal tumor with multilayered rosettes. Neuro Oncol. 2014;16:62–71. doi: 10.1093/neuonc/not162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fangusaro JR, Jubran RF, Allen J, et al. Brainstem primitive neuroectodermal tumors (bstPNET): Results of treatment with intensive induction chemotherapy followed by consolidative chemotherapy with autologous hematopoietic cell rescue. Pediatr Blood Cancer. 2008;50:715–717. doi: 10.1002/pbc.21032. [DOI] [PubMed] [Google Scholar]
- 115.Reddy AT, Janss AJ, Phillips PC, et al. Outcome for children with supratentorial primitive neuroectodermal tumors treated with surgery, radiation, and chemotherapy. Cancer. 2000;88:2189–2193. doi: 10.1002/(sici)1097-0142(20000501)88:9<2189::aid-cncr27>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 116.Gerber NU, von Hoff K, Resch A, et al. Treatment of children with central nervous system primitive neuroectodermal tumors/pinealoblastomas in the prospective multicentric trial HIT 2000 using hyperfractionated radiation therapy followed by maintenance chemotherapy. Int J Radiat Oncol Biol Phys. 2014;89:863–871. doi: 10.1016/j.ijrobp.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 117.Cohen BH, Zeltzer PM, Boyett JM, et al. Prognostic factors and treatment results for supratentorial primitive neuroectodermal tumors in children using radiation and chemotherapy: A Childrens Cancer Group randomized trial. J Clin Oncol. 1995;13:1687–1696. doi: 10.1200/JCO.1995.13.7.1687. [DOI] [PubMed] [Google Scholar]
- 118.Perreault S, Lober RM, Carret AS, et al. Relapse patterns in pediatric embryonal central nervous system tumors. J Neurooncol. 2013;115:209–215. doi: 10.1007/s11060-013-1213-4. [DOI] [PubMed] [Google Scholar]
- 119.Chintagumpala M, Hassall T, Palmer S, et al. A pilot study of risk-adapted radiotherapy and chemotherapy in patients with supratentorial PNET. Neuro Oncol. 2009;11:33–40. doi: 10.1215/15228517-2008-079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gottardo NG, Hansford SJ, McGlade JP, et al. Medulloblastoma down under 2013: A report from the third annual meeting of the International Medulloblastoma Working Group. Acta Neuropathol. 2014;127:189–201. doi: 10.1007/s00401-013-1213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]