Abstract
Survivors of childhood cancer carry a substantial burden of morbidity and are at increased risk for premature death. Furthermore, clear associations exist between specific therapeutic exposures and the risk for a variety of long-term complications. The entire landscape of health issues encountered for decades after successful completion of treatment is currently being explored in various collaborative research settings. These settings include large population-based or multi-institutional cohorts and single-institution studies. The ascertainment of outcomes has depended on self-reporting, linkage to registries, or clinical assessments. Survivorship research in the cooperative group setting, such as the Children's Oncology Group, has leveraged the clinical trials infrastructure to explore the molecular underpinnings of treatment-related adverse events, and to understand specific complications in the setting of randomized risk-reduction strategies. This review highlights the salient findings from these large collaborative initiatives, emphasizing the need for life-long follow-up of survivors of childhood cancer, and describing the development of several guidelines and efforts toward harmonization. Finally, the review reinforces the need to identify populations at highest risk, facilitating the development of risk prediction models that would allow for targeted interventions across the entire trajectory of survivorship.
INTRODUCTION
Childhood cancer survivorship has attracted attention globally, in part, because of the decades of life saved for every child with cancer and an ever-increasing number of survivors, which necessitates an understanding of the quality of survival.1 Cancer therapy at an early age can produce complications that may not become apparent until years later, hence the term late effect for a long-term outcome that persists or that develops several years after cancer is diagnosed. A comprehensive evaluation of these late effects requires carefully constructed cohorts that are sufficiently large and observed for extended periods. Prohibitive costs associated with detailed clinical evaluation of these large cohorts often necessitate reliance on self-reporting or linkage with registries, both of which have attendant limitations. Detailed clinical evaluations have been attempted by using the cooperative group infrastructure, with the accompanying advantage of a multi-institutional setting and an established clinical infrastructure but with the distinct disadvantage of attrition after 5 to 7 years from diagnosis. Detailed clinical evaluations have also been performed in single-institution settings where extensive infrastructure has been developed to observe cohorts for extended time. The single-institution cohorts are generally smaller than the large multi-institutional or population-based cohorts that use self-reporting or registry linkage to ascertain outcomes. Furthermore, single-institution cohorts may suffer from homogeneity of therapeutic exposure, and they are resource intensive. Nonetheless, complementary and often additive information can be gleaned from the various settings, allowing for a comprehensive picture of the landscape in cancer survivorship to emerge. Select findings from such initiatives are described here.
SUVIVORSHIP COHORTS
Collaborative efforts have resulted in the establishment of several survivorship cohorts, such as those of the Childhood Cancer Survivor Study (CCSS); the British Childhood Cancer Survivor Study (BCCSS); the Swiss Childhood Cancer Survivor Study (SCCSS); Adult Life After Childhood Cancer in Scandinavia (ALiCCS); the Dutch Childhood Oncology Group Late Effect Registry (DCOG LATER); the St Jude Lifetime Cohort (SJLIFE); the Childhood, Adolescent, and Young Adult Cancer Survivor Research Program; the Australian cohort of childhood cancer survivors, New South Wales population; and the French and British cohort of childhood cancer survivors. Salient features of select cohorts are summarized in Table 1. Characteristics unique to the cohorts are summarized in the Appendix (online only).
Table 1.
Characteristics of Select Survivorship Cohorts
| Characteristic | CCSS | BCCSS | SCCSS | ALiCCS | DCOG LATER | SJLIFE |
|---|---|---|---|---|---|---|
| Cohort size | 35,937 | 34,569 | 7,600 | 33,160 | 6,168 | 7,825 |
| Years of diagnosis | 1970-1999 | 1940-2006 | 1976-2010 | 1943-2008 | 1963-2002 | 1962-2009 |
| Entry criteria, years from diagnosis | ≥ 5 | ≥ 5 | ≥ 5 | ≥ 1 | ≥ 5 | ≥ 5 |
| Age at diagnosis, years | < 21 | < 15 | < 20 | < 20 | < 18 | < 25 |
| Cancers | Leukemia, CNS tumors, HL, NHL, Wilms tumor, neuroblastoma, soft tissue sarcoma, bone tumors | All | All and Langerhans cell histiocytosis | All | All | All |
| Methods of contact | Longitudinal follow-up, periodic surveys, investigator-initiated ancillary studies | One-time survey, linkage with death, cancer registries, and National Hospital Episode Statistics database | Clinical follow-up, linkage with death and/or cancer registries, periodic surveys | Linkage with death, cancer, and/or hospital registries | Clinic visits, self-reported outcomes, validation of outcomes using medical records, linkage with Dutch registries | Clinic visits, self-reported outcomes, longitudinal follow-up |
| Study design | Hospital based | Population based | Population based | Population based | Nationwide hospital-based cohort | Hospital based |
| Comparison population | Siblings, general population | General population | Siblings, general population | Matched population | General population and matched controls | Frequency-matched community controls |
| Therapeutic exposure data | Yes, > 90% | Limited for cohort but extensive for nested case-control studies | Limited for > 90%, detailed data for focused studies | Limited | 100% | 100% |
| Ascertainment method | ||||||
| Vital status | Linkage with death registries | Linkage with national death registries | Linkage with population registries and death registries | Linkage with death registries | Linkage with death registries | Linkage with death registries |
| Nonmalignant adverse outcomes | Self- or parent report | Self-report and national Hospital Episode Statistics database | Self- or parent report, medical records, hospital statistics planned | National hospital registries | Medical assessment, self-report, medical record validation | Medical assessment, patient report |
| Malignant adverse outcomes | Self- or parent report, validation with pathology report | Linkage with national cancer registries | Self- or parent report, linkage with cancer registries | Linkage with cancer registries | Linkage with cancer registries | Self-report pathology verified and medical assessments |
| Collection of germline DNA | > 60% of participants | No | Currently for focused studies (N ≃ 400), planned for full cohort | No | Yes, planned | > 95% of participants |
Abbreviations: ALiCCS, Adult Life After Childhood Cancer in Scandinavia; BCCSS, British Childhood Cancer Survivor Study; CCSS, Childhood Cancer Survivor Study; DCOG LATER, Dutch Childhood Oncology Group Late Effect Registry; HL, Hodgkin lymphoma; NHL, non-HL; SCCSS, Swiss Childhood Cancer Survivor Study; SJLIFE, St Jude Lifetime Cohort.
COOPERATIVE GROUP STUDIES
Cooperative groups offer unique opportunities to investigate adverse events encountered during treatment and in the first few years after treatment is completed. Cooperative group trials also provide opportunities to investigate treatment-related complications in the context of therapeutic randomization. Children's Oncology Group (COG), a National Cancer Institute–supported clinical trials network, includes more than 200 children's hospitals, universities, and cancer centers committed to conducting translational research to improve outcomes in children with cancer.2 Examples of risk-reduction strategies in COG include random assignment of patients to receive dexamethasone versus prednisone to treat acute lymphoblastic leukemia (ALL), anthracyclines with cardioprotection versus anthracyclines without cardioprotection, and chest irradiation versus no chest irradiation for Hodgkin lymphoma (HL) in girls.3 These trials offer opportunities to understand the role of specific therapeutic exposures in the development of adverse outcomes. Investigators in the COG ALTE11C2 study are using such an opportunity by examining cardiac outcomes in long-term survivors of HL and ALL treated in COG legacy trials Pediatric Oncology Group (POG) 9425, 9426, and 9404 in which patients were randomly selected to receive anthracycline with or without dexrazoxane cardioprotection in the setting of comparable event-free and overall survival in the two arms.4,5 Female adolescents exposed to high-dose alkylating agents and/or ovarian irradiation are at risk for accelerated depletion of ovarian reserve, resulting in premature menopause or acute ovarian failure.6 COG ALTE11C1 researchers are using a prospective longitudinal study design to characterize change in ovarian reserve during and shortly after completion of gonadotoxic therapies. The data will inform the natural history of ovarian function before the onset of clinically apparent premature menopause or acute ovarian failure. These findings can inform counseling and opportunities for the cryopreservation of oocyte and ovarian tissue in individuals at highest risk for ovarian failure.6 Utilizing the well-established clinical trials infrastructure in COG, researchers are currently developing interventional trials COG ALTE1331 and COG ALTE1431 to test strategies to implement before the onset of clinically apparent cardiovascular disease.
BURDEN OF MORBIDITY
Investigators from several studies have described the burden of morbidity that survivors of childhood cancer carry by using methods that range from self-reporting to comprehensive clinical assessment. The magnitude of this burden has varied across the studies and has depended on the method of ascertainment of long-term health conditions and the length of follow-up. Common Terminology Criteria for Adverse Events from the National Cancer Institute were applied to the CCSS cohort to characterize the overall chronic disease burden borne by survivors. Overall, 62.3% of survivors had at least one chronic condition, placing the cohort at a 3.3-fold increased risk of any long-term health condition compared with age- and sex-matched siblings. Of more importance, 27.5% of the CCSS cohort had a severe and/or life-threatening condition, placing them at an 8.2-fold increased risk compared with that of the sibling comparison group.7 The risk of severe and/or life-threatening conditions in the CCSS cohort was highest among survivors of bone tumor (38.9-fold increased risk) and lowest among survivors of leukemia or Wilms tumor (4.1-fold increased risk) compared with siblings. Risk of severe and/or life-threatening conditions increased 12.6-fold for survivors of CNS tumors, 10.2-fold for survivors of HL, 8.9-fold for survivors of sarcoma, 6.8-fold for survivors of NHL, and 4.7-fold for survivors of neuroblastoma. The SCCSS revealed severe outcomes in one third of survivors.8,9 By the age of 50 years, the cumulative incidence of severe, disabling, life-threatening, and/or fatal health conditions in the CCSS cohort was 53.6%.10 Furthermore, among survivors who reached age 35 years without a previous severe and/or life-threatening condition, 25.9% experienced a subsequent condition within 10 years.
The overall burden of chronic disease has also been evaluated at single institutions, with detailed medical assessment of survivors. In a cohort of 1,362 survivors of childhood cancer treated at the Emma Children's Hospital/Academic Medical Center,11 75% had one or more adverse events, and 40% had one or more severe, life-threatening, and/or disabling adverse events. On the basis of data from medical assessments of the SJLIFE cohort, the estimated cumulative prevalence for a serious and/or disabling or life-threatening chronic condition was 80.5% (95% CI, 73.0% to 86.6%) by the of age 45 years (Fig 1).
Fig 1.
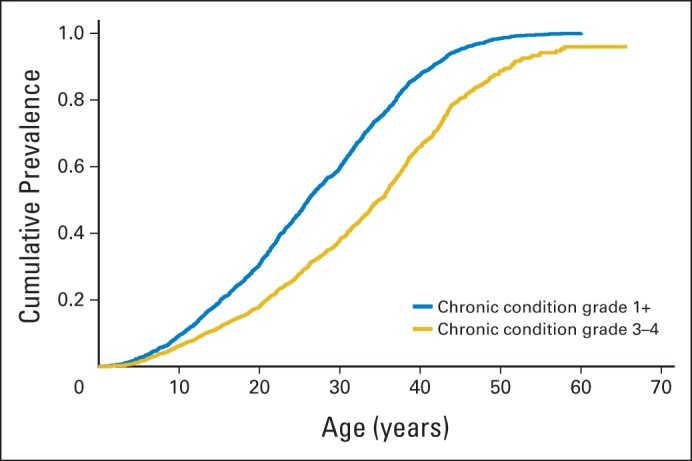
Cumulative prevalence of health conditions in 1,713 adults from the St Jude Lifetime Cohort.
SUBSEQUENT MALIGNANT NEOPLASM
Subsequent malignant neoplasms (SMNs) are histologically distinct malignancies that develop among patients treated for a primary malignancy. Although a few SMNs are attributable to heritable conditions such as retinoblastoma or neurofibromatosis, the vast majority of SMNs observed in survivors of childhood cancer are largely attributed to the genotoxic insult resulting from therapeutic exposures. The unique role played by specific therapeutic exposures in the development of SMNs has resulted in their classification into two distinct categories. The first is chemotherapy-related myelodysplasia and acute myeloid leukemia (t-MDS/AML), and the second is radiation-related solid SMNs. Characteristics of t-MDS/AML include a short latency of < 3 years after the diagnosis of primary cancer diagnosis and an association with alkylating agents and/or topoisomerase II inhibitors.12 Solid SMNs are strongly associated with irradiation and are characterized by a latency that exceeds 10 years.12 Nonmelanoma skin cancers (NMSC), breast cancer, CNS tumors, thyroid cancer, genitourinary cancers, digestive tract tumors, bone tumors and SMNs of respiratory sites are the most common solid SMNs observed among survivors of childhood cancer.13–16
Table 2 summarizes the magnitude of the risk of subsequent neoplasms reported by survivorship cohorts. The ALiCCS cohort had a 3.3-fold increased risk of SMNs,13 the BCCSS cohort had a four-fold increased risk,14 the CCSS cohort had a six-fold increased risk,15 and the DCOG LATER cohort had an 11.2-fold increased risk.16 The study-specific variability in the observed excess risk of SMNs compared with that of the general population is attributable to the difference in the length of follow-up and the age of the survivors cohort. The 30-year cumulative incidence in the CCSS cohort was 9.1% for NMSC, 7.9% for SMN excluding NMSC, and 3.1% for meningioma.15 Population-based registry data from the ALiCCS yielded an absolute excess risk (AER) of three to six per 1,000 person-years of follow-up.13 The BCCSS cohort demonstrated that 52% of the excess cancers observed among those age 40 years or older was attributable to digestive, genitourinary, breast, or respiratory sites.14 Finally, the CCSS cohort showed that patients with SMNs remain at considerable risk for additional subsequent neoplasms.17 Survivors of medulloblastoma had the highest 30-year cumulative incidence of meningioma (16.4%).15
Table 2.
Subsequent Neoplasms Among Survivors of Childhood Cancer
| Cohort | Cohort Size | No. of Patients with Subsequent Neoplasms | Follow-Up | Cumulative Incidence | Standardized Incidence Ratio |
Absolute Excess Risk, per 1,000 person-years |
||
|---|---|---|---|---|---|---|---|---|
| Value | 95% CI | Value | 95% CI | |||||
| CCSS15 | 14,359 | 1,402 | Mean, 22.7 years; SD, 6.8 | 20.5% at 30 years after diagnosis of childhood cancer, 7.9% for SMNs excluding NMSC, 9.1% for NMSC, 3.1% for meningioma | 6.0 | 5.5 to 6.4 | 2.6 | 2.4 to 2.9 |
| ALiCCS13 | 47,697 | 1,088 | 476,289 person-years | ≃ 13.5% by age 50 years | 3.3 | 3.1 to 3.5 | 1.73 | 1.68 to 1.77 |
| BCCSS14 | 17,981 | 1,354* | Median, 24.3 years | 13.8% by age 60 years | 3.9 | 3.6 to 4.2 | 1.68 | 15.3 to 18.3 |
| DCOG LATER16 | 1,368 | 62 | Median, 16.8 years | 11.1% at 30 years after primary cancer diagnosis | 11.2 | 8.5 to 14.4 | 3.2 | Data not available |
Abbreviations: ALiCCS, Adult Life After Childhood Cancer in Scandinavia; BCCSS, British Childhood Cancer Survivor Study; CCSS, Childhood Cancer Survivor Study; DCOG LATER, Dutch Childhood Oncology Group Late Effect Registry; NMSC, nonmelanoma skin cancer; SMN, subsequent malignant neoplasm.
The no. was 837 after patients with NMSC and meningioma were excluded.
Exquisitely detailed therapeutic exposures enabled detailed assessment of the dose-response relationship between radiation exposure and the risk for breast cancer (Fig 2A),18 CNS SMNs (Fig 2B),19 thyroid tumors,20 and NMSC21 in the CCSS cohort. Furthermore, both the CCSS22 and the BCCSS14 have shown that the risk of site-specific SMNs is comparable with that observed among individuals at increased risk for cancer because of genetic susceptibility. Therefore, among female survivors of HL exposed to chest irradiation in the CCSS cohort, the cumulative incidence of breast cancer was 35% by age 50 years, a rate comparable with that of carriers of a BRCA mutation in the general population.22 The cumulative incidence of colorectal cancer by age 50 years after exposure to abdominopelvic irradiation was 1.4% in the BCCSS cohort. This was comparable with the 1.2% risk observed in individuals with two or more age- and sex-matched first-degree relatives with colorectal cancer.14
Fig 2.
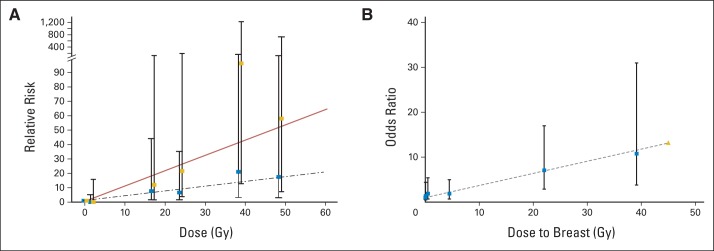
Risk of subsequent malignant neoplasms by radiation dose for (A) brain tumors and (B) breast cancer.
HL
Survivors of HL in the CCSS cohort had the highest 30-year cumulative incidence of SMNs excluding NMSCs (18.4%).15 The incidence of invasive solid SMN surpasses the risk of relapse by 20 years after diagnosis. The median time to the first solid SMN was 18.7 years after diagnosis.23 The cumulative incidence was higher for female survivors (26.1%) than for male survivors (10.9% at 30 years). The higher incidence among female survivors primarily derived from their high incidence of breast cancer. Compared with the general US population, the standardized incidence ratios (SIRs) were highest for the solid epithelial cancers, bone cancer (22.3), thyroid cancer (17.6), and breast cancer (17.0). The cumulative incidence of invasive breast cancer was 18.3% at 30 years. The cumulative incidence of NMSC was 16.7% at 30 years. The BCCSS showed the survivors of childhood HL had a 5.6-fold increased risk of subsequent neoplasms compared with the general population.14 The risk was 8.9× that of the general population for breast cancer, and 27.7× that of the general population for bone tumors.
ALL
The CCSS demonstrated that survivors of childhood ALL had a 4.4-fold increased risk of invasive SMNs. The excess absolute risk was 1.4 per 1,000 person-years of observation, and the 30-year cumulative incidence was 5.2%.15 The most common subsequent neoplasm was NMSC (40%), followed by meningioma (20%), glial tumors (8%), breast cancer (3%), soft tissue sarcomas (3%), and other tumors (26%).
A cohort of 8,831 children with ALL given legacy COG therapeutic protocols between 1983 and 1995 had a risk of SMN that was 7.9× higher than that observed in the general population.24 The risk was significantly increased for t-MDS/AML (SIR, 52.3), NHL (SIR, 20.8), parotid gland tumors (SIR, 33.4), thyroid cancer (SIR, 13.3), brain tumors (SIR, 10.1), and soft tissue sarcoma (SIR, 9.1). The cumulative incidence of t-MDS/AML approached 0.2% at 10 years, with 13 of 14 events reported in the first 5 years.
CARDIOVASCULAR OUTCOMES
The CCSS cohort has demonstrated that aging adult survivors are at increasing risk for treatment-associated cardiotoxicity25 and stroke.26 Among recipients of cardiotoxic agents in the SJLIFE cohort, the cumulative prevalence of select cardiac outcomes by age 50 years was 24% for cardiomyopathy, 25% for coronary artery disease, 86% for valve disorders, and 42% for conduction disorders.27 In a cohort of 1,362 survivors of childhood cancer treated at Emma Children's Hospital/Academic Medical Center, the 30-year cumulative incidence of symptomatic cardiac events was 12.6% after exposure to anthracyclines and chest irradiation; it was 7.3% after anthracycline exposure alone and 4.0% after chest irradiation alone.28 An exponential relationship was demonstrated between the risk of cardiac event and cardiotoxic therapeutic exposure (cumulative dose of anthracyclines and/or radiation dose).28 Again, the observed variability in the prevalence of cardiac disorders among various studies likely depends on the method by which outcomes were ascertained, that is, self-reporting versus clinical screening.
Survivors of childhood cancer are at risk for a constellation of cardiovascular risk factors, such as hypertension, diabetes, and dyslipidemia.29,30 Survivors of ALL from the SJLIFE cohort had a higher risk of the metabolic syndrome (relative risk [RR], 1.4), hypertension (RR, 2.4), low HDL (RR, 1.4), obesity (RR, 1.5) and insulin resistance (RR, 1.6) than that of age-, sex-, and race-matched control subjects from the National Health and Nutrition Examination.31 Metabolic syndrome was associated with older age and previous cranial radiotherapy. In another single-institution study, the prevalence of metabolic syndrome was 13%, and survivors of ALL treated with cranial irradiation were at increased risk for metabolic syndrome.32 Survivors in the CCSS who received total-body irradiation (odds ratio [OR], 7.2), abdominal irradiation (OR, 2.7), or alkylating agents (OR, 1.7) at a young age were at increased risk for diabetes mellitus.33
Data from the CCSS demonstrated that cardiovascular outcomes experienced by survivors of childhood cancer are potentiated by modifiable cardiovascular risk factors that are components of metabolic syndrome.34 Observations from nononcology populations support the benefits of interventions to reduce these modifiable risk factors, which include obesity, smoking, hypertension, diabetes, and dyslipidemia.35,36 In line with this observation, adherence to a heart-healthy lifestyle was associated with lower risk of metabolic syndrome in the oncology population.37 Findings from studies in nononcology populations suggest that routine screening for these risk factors may be beneficial, setting the stage for possible interventions to mitigate adverse cardiovascular outcomes in survivors of cancer.
NEUROMUSCULAR OUTCOMES
Data from the SJLIFE cohort support an association between exposure to vinca alkaloids and motor impairment, and between cisplatin exposure and sensory impairment. Patients exposed to vinca alkaloids had a 1.7-fold increased risk of motor neuropathy, and those exposed to cisplatin had a 1.6-fold increased risk of sensory neuropathy compared with unexposed individuals.38 Findings from the SJLIFE cohort also demonstrated that the prevalence of frailty among survivors of childhood cancer in their 30s was comparable to that of adults older than 65 years.39 An important finding was that frail survivors have a 2.6-fold higher risk of death and a 2.2-fold higher risk of developing a new chronic condition than that of nonfrail survivors.
ENDOCRINE AND REPRODUCTIVE OUTCOMES
ALiCCS reports confirm that endocrine disorders are a major health problem in survivors of childhood cancer, with an AER of four excess endocrine disorders per 1,000 person-years of follow-up.40,41 A variety of endocrine and reproductive outcomes have been described in the SJLIFE cohort.27,42–44 Survivors exposed to radiation developed hypothalamic-pituitary (56.4%), thyroid (13.8%), testicular (66.4%), or ovarian (11.8%) impairment.45 The SJLIFE study demonstrated that serum inhibin B and follicle-stimulating hormone lack specificity as a surrogate for sperm concentration.43 A dose-related decline in sperm concentration is associated with increasing cyclophosphamide equivalent dose and an absence of a threshold dose predictive of impaired spermatogenesis or azoospermia.42
LATE MORTALITY
The CCSS cohort had an 8.4-fold increased risk of premature death compared with the general population.46 Figure 3 displays the all-cause mortality of 5-year survivors of childhood cancer compared with age-adjusted expected survival rates in the US population. The BCCSS cohort had a 10.7-fold increased risk of death,47 and the ALiCCS cohort had an 8.3-fold increased risk of death.48 A single-center cohort from DCOG LATER demonstrated a 17-fold increased risk of late mortality.49 Both the CCSS and BCCSS cohorts demonstrated changing patterns of cancer-specific mortality with increasing time from diagnosis (Fig 3B).47,50 In the BCCSS cohort, the AER for deaths from recurrence declined with time, whereas the AER for deaths from SMNs and cardiovascular causes increased. Subsequent to 45 years after diagnosis, recurrence accounted for only 7% of the excess number of deaths, whereas SMNs and cardiovascular causes accounted for 51% and 26% of the excess number of deaths, respectively.47 The CCSS cohort had a 15.2-fold increased risk of SMN-related deaths, an 8.8-fold increased risk of pulmonary deaths, and a seven-fold increased risk of death from cardiac causes compared with the general population.46
Fig 3.
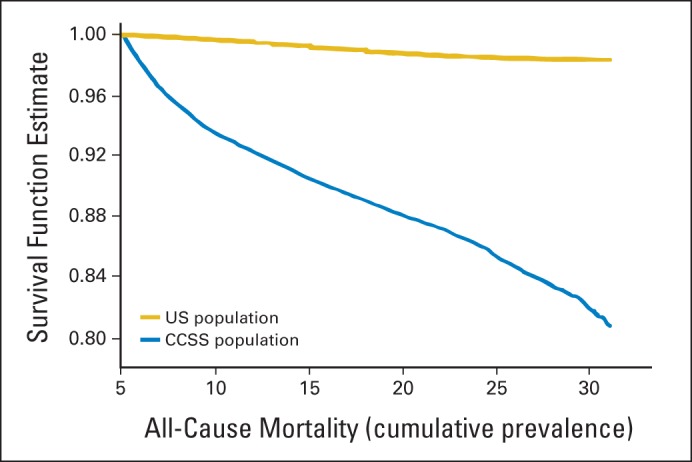
All-cause mortality among 5-year survivors from the Childhood Cancer Survivor Study.
GENETIC SUSCEPTIBILITY TO TREATMENT-RELATED ADVERSE OUTCOMES
The observed interindividual variability in the risk for treatment-related complication suggests a role for individual genetic susceptibility.51 A large multi-institutional case-control study within COG, COG ALTE03N1, is being conducted to address this question in patients with SMNs, stroke, cardiomyopathy, or osteonecrosis. DNA and RNA are procured from case patients with the aforementioned adverse events and matched control subjects who are survivors of cancer with no adverse events to examine the role of genetic susceptibility and gene-environment interactions in the development of treatment-related adverse events. CCSS researchers, in collaboration with the Division of Cancer Epidemiology and Genetics at the National Cancer Institute, have studied the genotypes of almost 6,000 survivors of childhood cancer using the Illumina HumanOmni5Exome microarray to investigate genetic contributions to the development of subsequent neoplasms and specific SMNs. This resource will be made available to investigators interested in genetic susceptibility to nonmalignant late effects.
Regarding genetic susceptibility to anthracycline-related cardiomyopathy, carbonyl reductases catalyze the reduction of anthracyclines to cardiotoxic alcohol metabolites; polymorphisms in CBR3 influence synthesis of these metabolites. Recent findings from study COG ALTE03N1 suggest that homozygosis for the G allele of CBR3 contributes to an increased risk of cardiomyopathy associated with low-to-moderate dose anthracyclines (Fig 4A).52 COG ALTE03N1 investigators also identified the common single nucleotide polymorphism rs2232228 in the hyaluronan synthase gene HAS3 that exerts a modifying effect on anthracycline dose-dependent risk of cardiomyopathy (Fig 4B).53 In individuals exposed to high-dose anthracyclines > 250 mg/m2, the rs2232228 AA genotype conferred an 8.9-fold increased risk of cardiomyopathy compared with the GG genotype. The high cardiomyopathy risk associated with the AA genotype could be a result of inadequate remodeling and/or inadequate protection of the heart from reactive oxygen species–mediated injury induced by high anthracycline exposure. Finally, in a study representing a collaboration between Canada and the Netherlands, investigators identified multiple genetic variants in SLC28A3 and other genes associated with anthracycline-related cardiotoxicity.54 Combined with clinical risk factors, genetic risk profiles were predictive of cardiotoxicity in 75% of the individuals at high risk.54
Fig 4.
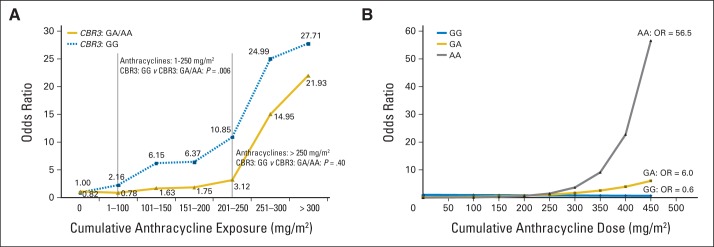
Genetic susceptibility to anthracycline-related cardiotoxicity: (A) CBR3 genotype and risk of cardiomyopathy after exposure to low-to-moderate dose anthracyclines and (B) HAS3 genotype and risk of cardiomyopathy after exposure to high-dose anthracyclines.
LONG-TERM FOLLOW-UP GUIDELINES
The Institute of Medicine strongly recommends that all survivors of childhood cancer receive regular medical care that is adapted to the specific risks that arise from their previous cancer therapy, genetic predispositions, lifestyle, and any comorbid health conditions.55 CCSS data suggest that patients examined by a primary care clinician are less likely than those evaluated at a cancer center to receive indicated echocardiography (22% v 53%) or mammography (35% v 62%).56 Similar findings from SCCSS demonstrate that only a minority of adult survivors of childhood cancer undergo regular follow-up, and few receive written documentation on their diagnosis, therapy, and advice for future medical check-ups.57 These findings suggest that survivors of childhood cancer and their healthcare providers could benefit from standardized, risk-based recommendations for early detection of long-term complications. Both oncology and nononcology providers have endorsed the need for such guidelines because of their lack of familiarity with the unique health risks associated with treatment for childhood cancer.58–60
Several groups, such as the US COG, Scottish Intercollegiate Guidelines Network, DCOG LATER, and the United Kingdom Children's Cancer Study Group Late Effects Group, have developed recommendations for follow-up of survivors of childhood cancer.61–64 Evidence from literature is used to identify high-risk groups, whereas screening recommendations are derived by means of expert consensus to match the magnitude of risk with the intensity of screening recommendations.65 In addition, guidelines also provide resources for patient counseling and education. Standardizing the follow-up care of survivors of childhood cancer provides opportunities to refine guidelines by examining the yield from observing them and by determining their cost effectiveness, as described next.
YIELD OF RISK-BASED SCREENING FOR LONG-TERM COMPLICATIONS
Using the COG Long-Term Follow-Up Guidelines,64 investigators at a single institution conducted 4,992 tests in survivors of childhood cancer during 1,188 clinic visits to determine the yield of exposure-driven, risk-based screening for the early detection of long-term complications.66 High-yield tests included thyroid function assessment, which revealed hypothyroidism in 10.1% of survivors; audiometry, which showed hearing loss in 22.6%; dual-energy x-ray absorptiometry, which yielded low bone mineral density in 23.2%; serum ferritin testing, which demonstrated iron overload in 24.0%; and pulmonary function testing, which indicated pulmonary dysfunction in 84.1%. Screening tests with a negligible or negative yield of < 1% included complete blood counts to detect therapy-related leukemia; dipstick urinalysis to detect proteinuria; serum blood urea nitrogen and/or creatinine assessment to detect glomerular defects, microscopic urinalysis to detect hematuria indicative of hemorrhagic cystitis or bladder cancer; and ECG for anthracycline-related conduction disorder. A similar evaluation among SJLIFE cohort members demonstrated that the highest prevalence of adverse outcomes affected pulmonary, neurosensory, endocrine, reproductive, cardiovascular, and neurocognitive function.27 These findings led to refinement of COG Long-Term Follow-Up Guidelines with the elimination of screening tests with low yield.
COST-EFFECTIVENESS OF LONG-TERM FOLLOW-UP GUIDELINES
The COG Long-Term Follow-Up Guidelines recommend lifetime echocardiographic screening for asymptomatic left ventricular dysfunction. The effectiveness and cost-effectiveness of the COG guidelines was evaluated by simulating life histories by using Markov health states.67 COG guidelines reduced the risk of heart failure in survivors of childhood cancer at a cost of less than $100,000 per quality-adjusted life year. Furthermore, the study revealed less-frequent screening schedules that achieved most of the benefits and that were more cost-effective than those stated in the COG guidelines.
INTERNATIONAL LATE EFFECTS OF CHILDHOOD CANCER GUIDELINE HARMONIZATION GROUP
In 2010, the International Late Effects of Childhood Cancer Guideline Harmonization Group (IGHG) was organized to establish an integrated strategy for the surveillance of late effects in survivors of childhood cancer worldwide.68 The IGHG uses a consistent, evidence-based method that adheres to the Appraisal of Guidelines for Research and Evaluation Collaboration69 and the standards for Developing Trustworthy Clinical Practice Guidelines of the Institute of Medicine.70 In the IGHG, concordance and discordance and evidence that support recommendations in existing national guidelines are evaluated for each topic. The literature is systematically searched for relevant questions, and extensive evidence summaries for the risk groups, accuracy of screening tests, and potential treatment options are organized.68 Screening and/or surveillance recommendations are developed and graded by using elements of established recommendations.71,72 Harmonization has been completed and its results published for the surveillance of breast cancer73 and cardiomyopathy.74 Harmonization efforts are ongoing for the surveillance of gonadal toxicity, thyroid cancer, and CNS neoplasm.
CONCLUSIONS AND FUTURE DIRECTIONS
Improved understanding of treatment-related toxicity has guided not only the adoption of less-toxic therapies but also the development of treatment summaries, survivorship plans, and efforts to harmonize survivorship guidelines worldwide.68 Research into cancer survivorship will continue to evolve as better treatment options, new agents, and combinations of agents are developed. Although targeted therapies will likely prolong survival, many of the agents used in the past 40 years will continue to be included in the treatment of most pediatric patients with cancer.75 Refinements in radiation therapy and minimally invasive surgeries are intended to minimize late effects. More recent cohorts of children with cancer must still be observed to determine how modifications to therapy affect the prevalence and spectrum of late effects. The growing population of survivors provides numerous opportunities for research related to the etiology of cancer and other late effects. Detailed knowledge of therapeutic exposures coupled with close follow-up after exposure enable researchers to study testable hypotheses and to determine the effects of host and therapy-related factors in the development of adverse outcomes ranging from carcinogenesis to organ dysfunction.
Opportunities also exist to explore gene-environment interactions that may modify susceptibility to adverse outcomes and thus provide insights into the identification of high-risk populations. It is also important to develop and test interventions that can reduce the effect of cancer and its treatment on morbidity and mortality in survivors of childhood cancer. For example, survivors of HL treated with chest irradiation are at risk for developing lung cancer, and tobacco use increases this risk 20-fold. Successful smoking prevention and/or cessation strategies among survivors can decrease their risk while also decreasing the development and progression of atherosclerosis and other SMNs. Female survivors of cancer treated with chest irradiation are at increased risk for breast cancer, providing an opportunity to develop therapeutic strategies to reduce this risk. These strategies can be surgical intervention, such as prophylactic mastectomy or bilateral oophorectomy, or hormonal chemoprevention.
Survivors of childhood cancer, particularly those who received irradiation of the hypothalamic-pituitary axis, are also at risk for obesity. Obesity, in turn, can exacerbate the already-increased risks of cardiovascular disease associated with anthracycline therapy and radiation therapy to the chest. Modifiable cardiovascular risk factors, such as hypertension, can potentiate the risk of major cardiac events, and both behavioral and medical interventions can potentially reduce these risks. Clinical trials of these strategies could be performed to test their effectiveness and provide evidence to disseminate these findings as standards of care, helping to reduce morbidity and mortality, and, ultimately, improve overall quality of life for survivors of childhood cancer.
Acknowledgment
We thank all of the survivors who completed a 40-page questionnaire and all general practitioners who returned consent forms. The British Childhood Cancer Survivor Study would not have been possible without the support of its funders: University of Birmingham, Cancer Research UK, the Kay Kendall Leukaemia Fund, and the European Commission to whom we offer our profound thanks. Finally, we thank all BCCSS staff who have given many years of dedicated work to bring the BCCSS to fruition.
Appendix
Childhood Cancer Survivor Study
The Childhood Cancer Survivor Study (CCSS) is a multi-institutional, multidisciplinary collaborative research resource funded by the National Cancer Institute. It initially comprised 20,691 survivors of childhood cancers and a comparison sibling cohort (Leisenring WM et al: J Clin Oncol 27:2319-2327, 2009; Robison LL et al: J Clin Oncol 27:2308-2318, 2009). Eligibility for participation in the CCSS included a diagnosis of the commonly occurring childhood cancers before age 21 years; treatment between January 1, 1970, and December 31, 1986; and survival for at least 5 years after diagnosis. The primary method of eliciting health-related information was through completion by patients or their parents of a detailed baseline questionnaire with four subsequent follow-up questionnaires. In addition, the CCSS has established a biorepository of biologic specimens. The CCSS is currently expanding the cohort to include 15,247 eligible 5-year survivors whose disease was diagnosed between 1987 and 1999. At completion, the cohort will include 35,937 survivors and siblings. The adult survivors of childhood cancer in this cohort represent approximately 11% of such survivors in the United States. The 30-year span covered with the expansion will allow CCSS investigator to understand the effect of both reduction in therapy for patients at low risk for disease recurrence and intensification of therapy for patients at high risk of relapse. The CCSS distinguishes itself by its large sample, the exquisitely detailed therapeutic exposure data for all patients, and a large biorepository of germline DNA.
British Childhood Cancer Survivor Study
The original British Childhood Cancer Survivor Study (BCCSS) population-based cohort comprised 17,981 individuals with a diagnosis of cancer (all diagnoses) younger than 15 years of age in England, Wales, or Scotland between 1940 and 1991 and surviving at least 5 years from diagnosis (Hawkins MM et al: Pediatr Blood Cancer 50:1018-1025, 2008). The BCCSS cohort was recently expanded to include survivors who received a diagnosed between 1992 and 2006, increasing the total cohort to 34,490. Patient records are linked with both the national death and cancer registries to ascertain underlying causes of all deaths and all incident subsequent malignant neoplasms. A survey was mailed to all those alive, age 16 years or older, by means of their primary care physician from the original cohort. For the expanded cohort, adverse health outcomes will be ascertained by linking with electronic records in the National Hospital Episode Statistics database. The BCCSS distinguishes itself by being the largest population-based cohort that spans the longest period from 1940 to 2006.
BCCSS has received grants from Cancer Research UK, the Kay Kendall Leukaemia Fund, and the European Commission under FP7 for projects PanCareSurFup, PROCARDIO, and CEREBRAD.
BCCSS is a national collaborative undertaking guided by a Steering Group that comprises Douglas Easton, PhD (chair), Michael Hawkins, PhD, Helen Jenkinson, MD, Meriel Jenney, MD, Raoul Reulen, PhD, Kathryn Pritchard-Jones, PhD, Michael Stevens, MD, Elaine Sugden, MD, Andrew Toogood, MD, and Hamish Wallace, MD. BCCSS benefits from the contributions of the Officers, Centres, and individual members of the Children's Cancer and Leukaemia Group and the Regional Paediatric Cancer Registries. The BCCSS acknowledges the collaboration of the Office for National Statistics, the General Register Office for Scotland, the Welsh Cancer Intelligence and Surveillance Unit, the National Health Service Information Centre, the regional cancer registries, health authorities, and area health boards for providing general practitioner names and addresses and the general practitioners nationwide who facilitated direct contact with survivors.
Swiss Childhood Cancer Survivor Study
The Swiss Childhood Cancer Survivor Study (SCCSS) is an ongoing national population-based cohort of children and adolescents treated for cancer before the age of 21 years (Kuehni CE et al: Int J Epidemiol 41:1553-1564, 2012) and registered in the Swiss Childhood Cancer Registry (SCCR; Michel G et al: Swiss Med Wkly 137:502-509, 2007). The SCCR was founded in 1976 and registers childhood cancer (all diagnoses) and Langerhans cell histiocytosis. At present, the SCCR includes 7,600 individuals treated in pediatric cancer centers, approximately 90%, or elsewhere, approximately 10%. The CCSS relies on self-reporting, and the BCCSS relies on self-reporting and record linkage. By contrast, main notification sources for adverse outcomes in the SCCSS include clinical follow-up at centers of the Swiss Pediatric Oncology Group (www.spog.ch); self-reported surveys of survivors and parents to assess morbidity, psychosocial outcomes, and health behaviors; review of population registries for vital status; evaluation of mortality statistics for causes of death; and search of regional cancer registries for subsequent neoplasms. Linkage with national hospital statistics is planned. Results are compared with findings from siblings and the general population.
SCCSS is funded by research Grants No. KLS 01605-10-2004, KLS 2215-02-2008, KLS 02631-08-2010, KFS-2783-02-2011, and KLS-3412-02-2014 from the Swiss Cancer League; the cancer leagues of the cantons Bern, Zurich and Aargau; the European Union FP7 projects, PanCareSurfUp, and PanCareLife; and the Swiss National Science Foundation Grants No. PZ00P3_121682/1 and SNSF-323 630-133897. The work of the Swiss Childhood Cancer Registry is supported by the Swiss Paediatric Oncology Group, Schweizerische Konferenz der kantonalen Gesundheitsdirektorinnen und-direktoren, Swiss Cancer Research, Kinderkrebshilfe Schweiz, Ernst-Göhner Stiftung, Stiftung Domarena, CSL Behring, and the National Institute of Cancer Epidemiology and Registration.
Adult Life After Childhood Cancer in Scandinavia
The inter-Nordic collaborative Adult Life After Childhood Cancer in Scandinavia (ALiCCS) program is an ongoing, population-based follow-up study of cancer and noncancer morbidity and late mortality in individuals treated for cancer during childhood. Each study includes the latest updates from the cancer registries of the five Nordic countries. The cohort consists of individuals with a diagnosis of cancer before 20 years of age since the start of the cancer registries in Denmark in 1943, in Finland and Norway in 1953, in Iceland in 1955, and in Sweden in 1958. The ALiCCS database for studies of noncancer morbidity was established in 2012.40 As of 2008, a total of 33,160 1-year survivors of childhood cancer and 212,892 population comparisons were included in the cohort. Vital status information was obtained from population registries, and a full hospital history for each person was derived from the national hospital registries. Case-cohort studies are under way in which therapeutic exposures are being abstracted from medical records.
ALiCCS is funded by research Grant No. 09-066899/DSF from the Danish Council for Strategic Research.
Dutch Childhood Oncology Group Late Effect Registry
The Dutch Childhood Oncology Group Late Effect Registry (DCOG LATER) is a collaborative group of clinicians, researchers, and representatives from the Dutch organization of survivors of childhood cancer. The DCOG LATER program includes 6,168 5-year survivors treated at seven pediatric oncology centers between 1963 and 2002 before the age of 18 years. Baseline demographics and therapeutic exposures have been collected in a Web database. A general health and risk-factor questionnaire was sent to survivors of the DCOG LATER cohort in 2013. Questionnaire results will be validated by general physicians and medical records. Linkage with the Dutch Cancer Registry and the pathology report registry called PALGA will be used to assess subsequent neoplasms and mortality. DCOG LATER aims to invite all 5,545 survivors of childhood cancer for a clinic visit to validate questionnaire results, to collect data about surrogate outcomes, and to gain insights regarding the additional value of screening tests.
DCOG LATER is funded by the Dutch Cancer Society, Quality of Life Foundation, KiKa, and projects of the European Union FP7 projects, PanCareSurfUp, and PanCareLife. It is a collaboration of Pediatric Oncology Centers in the Netherlands: Princess Maxima Center for childhood cancer (Dr L.C. Kremer, Dr M. van den Heuvel-Eibrink), Emma Children's Hospital/AMC Amsterdam (Dr L. Kremer, Dr H. van der Pal, Professor M. Grootenhuis, Professor M. Jaspers, Professor H. Caron, Dr C. Ronckers), Sophia Children's Hospital/Erasmus Medical Center, Rotterdam (Dr van den Heuvel-Eibrink, Dr S. Neggers), University Medical Center Groningen (Dr W. Tissing, Dr W. Dolsma, Dr A. Postma), University Medical Center St Radboud, Nijmegen (Dr. J. Loonen), UMCU/Wilhelmina Utrecht (Dr B. Versluys), Willem Alexander Kinder-en Jeugdcentrum, LUMC Leiden (Dr D. Bresters), VU University Medical Center Amsterdam (Dr E. van Dulmen-den Broeder, Dr A. van der Steeg), DCOG (Dr H. de Ridder), and the Netherlands Cancer Institute (Professor F. van Leeuwen).
St Jude Lifetime Cohort
In 2007, St Jude Children's Research Hospital initiated a medically evaluated cohort to undertake lifelong evaluation of health-related outcomes in aging survivors of childhood cancer (Hudson MM et al: Pediatr Blood Cancer 56:825-836, 2011). Eligibility for participation in the St Jude Lifetime Cohort (SJLIFE) included survival longer than 10 years after diagnosis and age greater than 18 years. Eligibility was recently modified to 5-year survival. The SJLIFE study includes all diagnoses as well as frequency-matched community controls with protocol-based medical assessments, patient-reported outcomes, periodic longitudinal evaluations, and biologic specimen procurement. The SJLIFE study aims to define excess lifetime morbidity associated with cancer and its therapy.
Footnotes
Supported by Grants No. U10CA098543 (S.B. and S.H.A.) and CA55727 from the National Cancer Institute; by Cancer Center Support CORE Grant No. CA21765 to St Jude Children's Research Hospital; and by the American Lebanese Syrian Associated Charities (G.T.A., L.L.R., and M.M.H.).
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Smita Bhatia
Administrative support: Smita Bhatia
Provision of study materials or patients: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Collaborative Research in Childhood Cancer Survivorship: The Current Landscape
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Smita Bhatia
No relationship to disclose
Saro H. Armenian
No relationship to disclose
Gregory T. Armstrong
No relationship to disclose
Eline van Dulmen-den Broeder
No relationship to disclose
Michael M. Hawkins
No relationship to disclose
Leontien C.M. Kremer
No relationship to disclose
Claudia E. Kuehni
No relationship to disclose
Jørgen H. Olsen
No relationship to disclose
Leslie L. Robison
No relationship to disclose
Melissa M. Hudson
No relationship to disclose
REFERENCES
- 1.American Cancer Society. Global Cancer Facts and Figures (ed 2) Atlanta, GA: American Cancer Society; 2011. [Google Scholar]
- 2.Adamson PC. The Children's Oncology Group's 2013 five year blueprint for research. Pediatr Blood Cancer. 2013;60:955–956. doi: 10.1002/pbc.24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armenian SH, Landier W, Hudson MM, et al. Children's Oncology Group's 2013 blueprint for research: Survivorship and outcomes. Pediatr Blood Cancer. 2013;60:1063–1068. doi: 10.1002/pbc.24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tebbi CK, London WB, Friedman D, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol. 2007;25:493–500. doi: 10.1200/JCO.2005.02.3879. [DOI] [PubMed] [Google Scholar]
- 5.Vrooman LM, Neuberg DS, Stevenson KE, et al. The low incidence of secondary acute myelogenous leukaemia in children and adolescents treated with dexrazoxane for acute lymphoblastic leukaemia: A report from the Dana-Farber Cancer Institute ALL Consortium. Eur J Cancer. 2011;47:1373–1379. doi: 10.1016/j.ejca.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine J, Canada A, Stern CJ. Fertility preservation in adolescents and young adults with cancer. J Clin Oncol. 2010;28:4831–4841. doi: 10.1200/JCO.2009.22.8312. [DOI] [PubMed] [Google Scholar]
- 7.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 8.von der Weid N, Beck D. Late sequelae in a group of children and adolescents treated for cancer between 1981 and 1986 in Switzerland. Schweizerische medizinische Wochenschrift. 1993;123:1293–1299. [PubMed] [Google Scholar]
- 9.von der Weid N, Mosimann I, Hirt A, et al. Intellectual outcome in children and adolescents with acute lymphoblastic leukaemia treated with chemotherapy alone: Age- and sex-related differences. Eur J Cancer. 2003;39:359–365. doi: 10.1016/s0959-8049(02)00260-5. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32:1218–1227. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297:2705–2715. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 12.Bhatia S, Sklar C. Second cancers in survivors of childhood cancer. Nat Rev Cancer. 2002;2:124–132. doi: 10.1038/nrc722. [DOI] [PubMed] [Google Scholar]
- 13.Olsen JH, Moller T, Anderson H, et al. Lifelong cancer incidence in 47,697 patients treated for childhood cancer in the Nordic countries. J Natl Cancer Inst. 2009;101:806–813. doi: 10.1093/jnci/djp104. [DOI] [PubMed] [Google Scholar]
- 14.Reulen RC, Frobisher C, Winter DL, et al. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA. 2011;305:2311–2319. doi: 10.1001/jama.2011.747. [DOI] [PubMed] [Google Scholar]
- 15.Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102:1083–1095. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardous-Ubbink MC, Heinen RC, Bakker PJ, et al. Risk of second malignancies in long-term survivors of childhood cancer. Eur J Cancer. 2007;43:351–362. doi: 10.1016/j.ejca.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong GT, Liu W, Leisenring W, et al. Occurrence of multiple subsequent neoplasms in long-term survivors of childhood cancer: A report from the childhood cancer survivor study. J Clin Oncol. 2011;29:3056–3064. doi: 10.1200/JCO.2011.34.6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inskip PD, Robison LL, Stovall M, et al. Radiation dose and breast cancer risk in the childhood cancer survivor study. J Clin Oncol. 2009;27:3901–3907. doi: 10.1200/JCO.2008.20.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neglia JP, Robison LL, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98:1528–1537. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 20.Sigurdson AJ, Ronckers CM, Mertens AC, et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): A nested case-control study. Lancet. 2005;365:2014–2023. doi: 10.1016/S0140-6736(05)66695-0. [DOI] [PubMed] [Google Scholar]
- 21.Watt TC, Inskip PD, Stratton K, et al. Radiation-related risk of basal cell carcinoma: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2012;104:1240–1250. doi: 10.1093/jnci/djs298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217–2223. doi: 10.1200/JCO.2013.54.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: A report from the Childhood Cancer Survivor Study. Blood. 2011;117:1806–1816. doi: 10.1182/blood-2010-04-278796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatia S, Sather HN, Pabustan O, et al. Low incidence of second neoplasms among children diagnosed with acute lymphoblastic leukemia after 1983. Blood. 2002;99:4257–4264. doi: 10.1182/blood.v99.12.4257. [DOI] [PubMed] [Google Scholar]
- 25.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowers DC, Liu Y, Leisenring W, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:5277–5282. doi: 10.1200/JCO.2006.07.2884. [DOI] [PubMed] [Google Scholar]
- 27.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Pal HJ, van Dalen EC, van Delden E, et al. High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol. 2012;30:1429–1437. doi: 10.1200/JCO.2010.33.4730. [DOI] [PubMed] [Google Scholar]
- 29.Meacham LR, Chow EJ, Ness KK, et al. Cardiovascular risk factors in adult survivors of pediatric cancer: A report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2010;19:170–181. doi: 10.1158/1055-9965.EPI-09-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oeffinger KC, Adams-Huet B, Victor RG, et al. Insulin resistance and risk factors for cardiovascular disease in young adult survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;27:3698–3704. doi: 10.1200/JCO.2008.19.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nottage KA, Ness KK, Li C, et al. Metabolic syndrome and cardiovascular risk among long-term survivors of acute lymphoblastic leukaemia: From the St Jude Lifetime Cohort. Br J Haematol. 2014;165:364–374. doi: 10.1111/bjh.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Waas M, Neggers SJ, Pieters R, et al. Components of the metabolic syndrome in 500 adult long-term survivors of childhood cancer. Ann Oncol. 2010;21:1121–1126. doi: 10.1093/annonc/mdp414. [DOI] [PubMed] [Google Scholar]
- 33.Meacham LR, Sklar CA, Li S, et al. Diabetes mellitus in long-term survivors of childhood cancer: Increased risk associated with radiation therapy—A report for the Childhood Cancer Survivor Study. Arch Intern Med. 2009;169:1381–1388. doi: 10.1001/archinternmed.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith SC, Jr, Collins A, Ferrari R, et al. Our time: A call to save preventable death from cardiovascular disease (heart disease and stroke) J Am Coll Cardiol. 2012;60:2343–2348. doi: 10.1016/j.jacc.2012.08.962. [DOI] [PubMed] [Google Scholar]
- 36.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:e584–e636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 37.Smith WA, Li C, Nottage KA, et al. Lifestyle and metabolic syndrome in adult survivors of childhood cancer: A report from the St Jude Lifetime Cohort Study. Cancer. 2014;120:2742–2750. doi: 10.1002/cncr.28670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ness KK, Jones KE, Smith WA, et al. Chemotherapy-related neuropathic symptoms and functional impairment in adult survivors of extracranial solid tumors of childhood: Results from the St Jude Lifetime Cohort Study. Arch Phys Med Rehabil. 2013;94:1451–1457. doi: 10.1016/j.apmr.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: A report from the St Jude Lifetime cohort study. J Clin Oncol. 2013;31:4496–4503. doi: 10.1200/JCO.2013.52.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Fine Licht F, Winther JF, Gudmundsdottir T, et al. Hospital contacts for endocrine disorders in Adult Life after Childhood Cancer in Scandinavia (ALiCCS): A population-based cohort study. Lancet. 2014;383:1981–1989. doi: 10.1016/S0140-6736(13)62564-7. [DOI] [PubMed] [Google Scholar]
- 41.Holmqvist AS, Olsen JH, Andersen KK, et al. Adult life after childhood cancer in Scandinavia: Diabetes mellitus following treatment for cancer in childhood. Eur J Cancer. 2014;50:1169–1175. doi: 10.1016/j.ejca.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Green DM, Liu W, Kutteh WH, et al. Cumulative alkylating agent exposure and sperm parameters among adult survivors of childhood cancer: A report from the St Jude Lifetime Cohort Study. Lancet Oncol. 2014;15:1215–1223. doi: 10.1016/S1470-2045(14)70408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green DM, Zhu L, Zhang N, et al. Lack of specificity of plasma concentrations of inhibin B and follicle-stimulating hormone for identification of azoospermic survivors of childhood cancer: A report from the St Jude lifetime cohort study. J Clin Oncol. 2013;31:1324–1328. doi: 10.1200/JCO.2012.43.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gurney JG, Kaste SC, Liu W, et al. Bone mineral density among long-term survivors of childhood acute lymphoblastic leukemia: Results from the St Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2014;61:1270–1276. doi: 10.1002/pbc.25010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chemaitilly W, Li Z, Huang S, et al. Anterior hypopituitarism in adult survivors of childhood cancers treated with cranial radiotherapy: A report from the St Jude Lifetime Cohort Study. J Clin Oncol. 2015;33:492–500. doi: 10.1200/JCO.2014.56.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reulen RC, Winter DL, Frobisher C, et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA. 2010;304:172–179. doi: 10.1001/jama.2010.923. [DOI] [PubMed] [Google Scholar]
- 48.Garwicz S, Anderson H, Olsen JH, et al. Late and very late mortality in 5-year survivors of childhood cancer: Changing pattern over four decades-experiences from the Nordic countries. Int J Cancer. 2012;131:1659–1666. doi: 10.1002/ijc.27393. [DOI] [PubMed] [Google Scholar]
- 49.Cardous-Ubbink MC, Heinen RC, Langeveld NE, et al. Long-term cause-specific mortality among five-year survivors of childhood cancer. Pediatr Blood Cancer. 2004;42:563–573. doi: 10.1002/pbc.20028. [DOI] [PubMed] [Google Scholar]
- 50.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: A summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Armenian SH, Bhatia S. Chronic health conditions in childhood cancer survivors: Is it all treatment-related—Or do genetics play a role? J Gen Intern Med. 2009;24(suppl 2):S395–S400. doi: 10.1007/s11606-009-0995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blanco JG, Sun CL, Landier W, et al. Anthracycline-related cardiomyopathy after childhood cancer: Role of polymorphisms in carbonyl reductase genes—A report from the Children's Oncology Group. J Clin Oncol. 2012;30:1415–1421. doi: 10.1200/JCO.2011.34.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, Liu W, Sun CL, et al. Hyaluronan synthase 3 variant and anthracycline-related cardiomyopathy: A report from the Children's Oncology Group. J Clin Oncol. 2014;32:647–653. doi: 10.1200/JCO.2013.50.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Visscher H, Ross CJ, Rassekh SR, et al. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol. 2012;30:1422–1428. doi: 10.1200/JCO.2010.34.3467. [DOI] [PubMed] [Google Scholar]
- 55.Hewitt M, WS, Simone JV, editors. Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 56.Nathan PC, Ness KK, Mahoney MC, et al. Screening and surveillance for second malignant neoplasms in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Ann Intern Med. 2010;153:442–451. doi: 10.1059/0003-4819-153-7-201010050-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rebholz CE, von der Weid NX, Michel G, et al. Follow-up care amongst long-term childhood cancer survivors: A report from the Swiss Childhood Cancer Survivor Study. Eur J Cancer. 2011;47:221–229. doi: 10.1016/j.ejca.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 58.Henderson TO, Hlubocky FJ, Wroblewski KE, et al. Physician preferences and knowledge gaps regarding the care of childhood cancer survivors: A mailed survey of pediatric oncologists. J Clin Oncol. 2010;28:878–883. doi: 10.1200/JCO.2009.25.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nathan PC, Daugherty CK, Wroblewski KE, et al. Family physician preferences and knowledge gaps regarding the care of adolescent and young adult survivors of childhood cancer. J Cancer Surviv. 2013;7:275–282. doi: 10.1007/s11764-013-0271-0. [DOI] [PubMed] [Google Scholar]
- 60.Suh E, Daugherty CK, Wroblewski K, et al. General internists' preferences and knowledge about the care of adult survivors of childhood cancer: A cross-sectional survey. Ann Intern Med. 2014;160:11–17. doi: 10.7326/M13-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scottish Intercollegiate Guidelines Network. Long-Term Follow-Up of Survivors of Childhood Cancer: A National Clinical Guideline. Edinburgh, Scotland: Scottish Intercollegiate Guidelines Network; 2004. [Google Scholar]
- 62.Dutch Childhood Oncology Group. Richtlijn follow-up na kinderkanker meer dan 5 jaar na diagnose. https://www.skion.nl/workspace/uploads/richtlijn_follow-up_na_kinderkanker_deel_1.pdf.
- 63.United Kingdom Children's Cancer Study Group Late Effects Group. Therapy-based long-term follow-up. http://www.cclg.org.uk/dynamic_files/LTFU-full.pdf.
- 64.Children's Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers: version 4.0, 2014. http://www.survivorshipguidelines.org. [PubMed]
- 65.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children's Oncology Group long-term follow-up guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 66.Landier W, Armenian SH, Lee J, et al. Yield and utility of screening for long-term complications using the Children's Oncology Group long-term follow-up guidelines. J Clin Oncol. 2012;30:4401–4408. doi: 10.1200/JCO.2012.43.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong FL, Bhatia S, Landier W, et al. Cost-effectiveness of the Children's Oncology Group long-term follow-up screening guidelines for childhood cancer survivors at risk for treatment-related heart failure. Ann Intern Med. 2014;160:672–683. doi: 10.7326/M13-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kremer LC, Mulder RL, Oeffinger KC, et al. A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer. 2013;60:543–549. doi: 10.1002/pbc.24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II. 1. Performance, usefulness and areas for improvement. CMAJ. 2010;182:1045–1052. doi: 10.1503/cmaj.091714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Graham R, Mancher M, Miller Wolman DM, et al., editors. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 71.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gibbons RJ, Smith SCJ, Antman E, et al. American College of Cardiology/American Heart Association clinical practice guidelines. II. Evolutionary changes in a continuous quality improvement project. Circulation. 2003;107:3101–3107. doi: 10.1161/01.CIR.0000079017.53579.9C. [DOI] [PubMed] [Google Scholar]
- 73.Mulder RL, Kremer LC, Hudson MM, et al. Recommendations for breast cancer surveillance for female survivors of childhood, adolescent, and young adult cancer given chest radiation: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2013;14:e621–e629. doi: 10.1016/S1470-2045(13)70303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Armenian SH, Hudson MM, Mulder RL, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16:e123–e136. doi: 10.1016/S1470-2045(14)70409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hudson MM, Neglia JP, Woods WG, et al. Lessons from the past: Opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer. 2012;58:334–343. doi: 10.1002/pbc.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]


