Abstract
We describe a case of a 35-year-old woman with a pedunculated uterine leiomyoma with diffuse hydropic degeneration presenting as a giant abdominal mass. The patient was admitted in the emergency department because of diffuse abdominal bloating and discomfort. Ultrasonography (US) showed a heterogeneous abdominopelvic mass. Magnetic resonance imaging (MRI) was performed to further characterise and revealed a myometrial pedunculated tumour. Despite its marked T2-signal heterogeneity and volume, there were no other suspicious findings to suggest a malignant nature; therefore, fertility-sparing myomectomy was performed. Leiomyomas frequently undergo degenerative changes altering their imaging appearances. Leiomyomas with uncommon degenerative changes may be difficult to differentiate from malignant myometrial tumours, based solely on imaging. To the best of our knowledge, a diffuse hydropic degeneration imaging appearance has only been described twice in the literature. We describe the imaging appearance of this rare form of leiomyoma drawing attention to its differential diagnosis.
Background
We describe a case of a woman of childbearing age with a giant abdominal mass diagnosed as a pedunculated uterine leiomyoma with diffuse hydropic change.
Giant pedunculated leiomyomas with degenerative changes may be misinterpreted as ovarian malignant tumours on ultrasound (US) imaging, since they may present as masses with heterogeneous echogenicity. Moreover, they can make it difficult to visualise the adnexa and vascular pedicle between the uterus and juxta-uterine mass. Therefore, MRI is crucial in the preoperative diagnosis of such lesions in order to determine the origin and nature of the tumour.
Typical degenerative forms of leiomyomas have characteristic MRI appearances. However, a leiomyoma with uncommon degenerative changes, such as the tumour presented, may be a diagnostic challenge and sometimes difficult to differentiate from its malignant counterparts, based solely on imaging. This may lead to a more aggressive surgical approach (non-fertility sparing surgery) in a woman of childbearing age.
Leiomyomas with diffuse hydropic changes are rare, with only two cases reported in the English literature. We describe the imaging and histopathological appearance of this rare form of degeneration, drawing attention to its differential diagnosis characteristics.
Case presentation
A 35-year-old nulliparous woman with diffuse abdominal bloating and discomfort was admitted to our hospital emergency department. Her personal medical history was unremarkable.
Investigations
On physical examination, a 20 cm mobile and painless pelvic mass was detected. Other physical assessments were negative. Routine laboratory data were in the normal range, apart from a slightly raised cancer antigen 125 (CA-125) serum level of 123 U/mL (reference range 0–35 U/mL).
Pelvic transabdominal and transvaginal US were performed, revealing a large, relatively well-defined, multilobulated abdominopelvic mass, measuring approximately 20×30×8 cm (figure 1). The mass had heterogeneous echogenicity, with prominent vascularised solid as well as cystic components (figure 1). No apparent relation to the uterus was seen and the ovaries could not be depicted; a small amount of pelvic ascites was also present.
Figure 1.
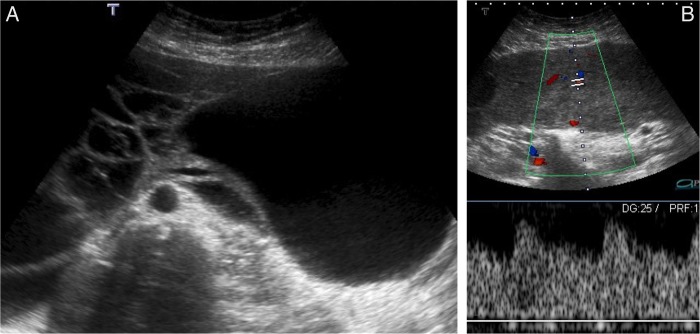
Uterine subserosal leiomyoma with diffuse hydropic degeneration in a 35-year-old woman. Transabdominal ultrasound image in B mode (A); colour and spectral Doppler ultrasound image (B). A giant, well-defined, multilobulated abdominopelvic mass, with prominent vascularised solid as well as cystic components, is seen (A). Note the prominent vessels within the mass, clearly depicted by colour and spectral Doppler ultrasound imaging (B).
Since the ovaries could not be seen and the serum levels of CA-125 were slightly elevated, the possibility of an ovarian neoplasm was raised; therefore, a MRI was performed to identify the origin of the tumour and to further characterise the lesion.
MRI showed a giant pedunculated abdominopelvic mass arising from the posterior uterine fundus, which was clearly demonstrated by the presence of multiple vessels between the uterus and the juxta-uterine mass (‘bridging vessels’ sign) (figure 2A; arrow); these findings were consistent with the presence of a tumour of myometrial origin, such as a leiomyoma on the benign side or a leiomyosarcoma on the malignant side.
Figure 2.
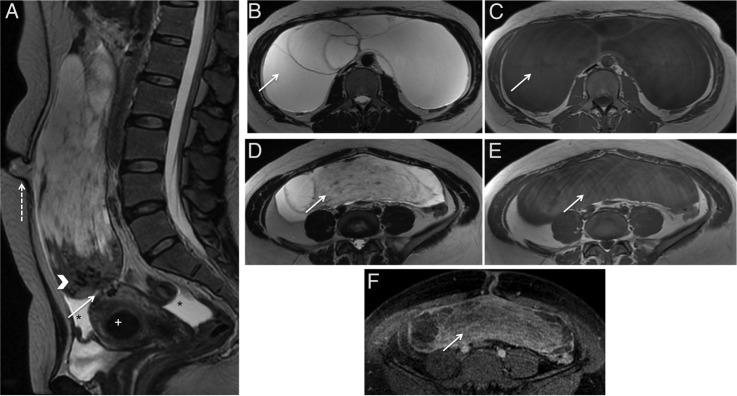
Uterine subserosal leiomyoma with diffuse hydropic degeneration in a 35-year-old woman. Sagittal T2-weighted MRI (A); axial T2-weighted MRI (B and D); axial T1-weighted MRI (C and E); axial gadolinium-enhanced fat-suppressed T1-weighted MRI (F). A giant pedunculated abdominopelvic mass is seen arising from the posterior uterine fundus (A). The tumour is lobulated, very well-defined and encapsulated with a small protruding anterior segment herniating through an umbilical defect (A; dashed arrow). Note the presence of multiple vessels between the uterus and the juxta-uterine mass (‘bridging vessels’ sign) (A; arrow). It showed an inferior solid component with multiple signal voids within (A; arrowhead). In its middle aspect, an enhancing cord-like solid component was seen showing moderate T2 hypointense signal and iso-signal to the muscle in T1-weighted images (D–F; arrows). Its superior part was composed of multiple large cystic areas showing low signal on T1-weighted images and high T2 signal (B and C; arrows). A small amount of ascites was detected only in the pelvis (A; asterisks).
The mass was lobulated, very well-defined and encapsulated, with a small protruding anterior segment herniating through an umbilical defect (figure 2A; dashed arrow). There were no signs of invasion of the adjacent structures.
Its appearance was markedly heterogeneous (figure 2). It showed an inferior solid component with multiple signal voids within, displaying iso-signal to the muscle in both T1 and T2 sequences and enhancing markedly after contrast administration in the equilibrium phase (figure 2A; arrowhead). In its middle aspect, an enhancing cord-like solid component was seen showing moderate T2 hypo-intense signal and iso-signal to the muscle in T1-weighted images (figures 2D–F; arrows). Its superior part was composed of multiple large cystic areas showing low signal on T1-weighted images and high T2 signal (figures 2B, C; arrows). No necrosis or areas of increased T1 signal that could suggest haemorrhage were seen.
A small amount of ascites was detected only in the pelvis (figure 2A; asterisks).
No inguinal, pelvic or para-aortic lymphadenopathies, and no peritoneal implants or hepatic metastases were detected.
Both ovaries were within their normal appearance and a submucosal myometrial leiomyoma, displaying low T2 signal was seen in the anterior uterine corpus, measuring 2.4×1.5 cm (figures 2A(cross), B).
Differential diagnosis
The differential diagnosis of a giant heterogeneous myometrial pedunculated subserosal tumour should include leiomyomas with degenerative changes on the benign side and leiomyosarcomas on the malignant side.
The definite diagnosis of a leiomyosarcoma is histopathological (based on the assessment of nuclear atypia, mitotic activity and tumour cell necrosis). However, apart from the presence of a large intermediate to high signal T2 mass, there were no imaging findings to suggest this diagnosis (irregular or infiltrative margins; inhomogeneity with necrosis and/or haemorrhage; lymphadenopathies and/or distant metastasis).
The four main degenerative forms of leiomyomas are: hyaline; myxoid; haemorrhagic and cystic. Rare forms include: lipomatous variants and diffuse hydropic leiomyomas.
The mass showed multiple large cystic areas with low signal on T1-weighted images and high T2 signal, therefore a leiomyoma with partial cystic degeneration could be considered. However, there were also enhancing cord-like solid components with moderate T2 hypo-intense signal and iso-signal to the muscle in T1-weighted images, which are not characteristic of any of the commonest degenerative forms of leiomyomas. Histopathologically, these were revealed to be strand-like areas of tumour cells within a vast oedematous stroma that was sometimes combined with hyalinisation, which is characteristic of hydropic degeneration. Therefore, the tumour was diagnosed as a leiomyoma with diffuse hydropic degeneration. The US and MRI characteristics of this type of leiomyoma have only been previously described twice in the literature.
Treatment
Since the tumour had no MRI features to suggest a malignant myometrial tumour and seemed resectable, fertility-sparing myomectomy was performed. The pelvic and abdominal cavities were explored during surgery, and neither peritoneal implants nor lymphadenopathies were found.
Outcome and follow-up
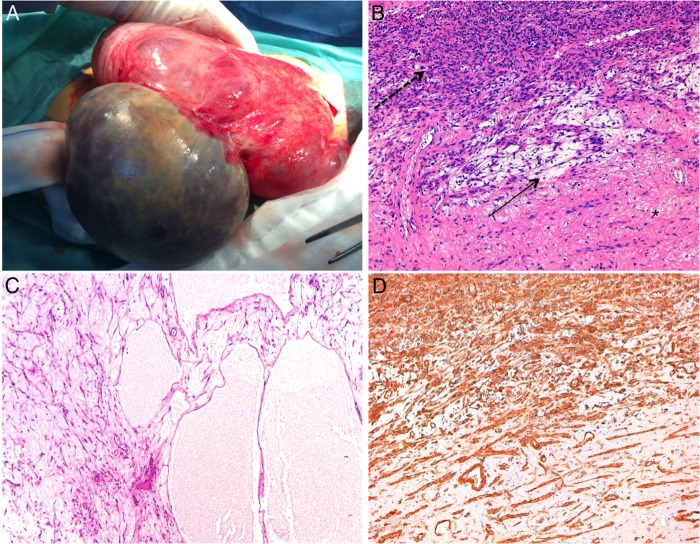
The tumour, weighing 2801 g and measuring 24×30×8 cm, exhibited a smooth, shiny surface. On cut surface, it was a solid, multicystic whitish-grey mass with smooth and thin walls. Watery fluid was seen extruding from it.
The histopathological examination revealed a tumour composed of hypercellular areas of intersecting fascicles of spindle cells with eosinophilic fibrillary cytoplasm and cigar-shaped nuclei (figure 3). These cells were within a vast oedematous stroma where some vessels and collagen deposition could be seen (figure 3). Moreover, hypocellular and pseudomulticystic areas, without epithelial covering, were seen (figure 3). These areas were filled with pale eosinophilic fluid.
Figure 3.
Uterine subserosal leiomyoma with diffuse hydropic degeneration in a 35-year-old woman. Surgical specimen (A). Microscopic examination with H&E stained section (10×10) (B)- Hypercellular area of the tumour (dashed arrow) and a small oedematous focus (arrow) within a collagenous stroma with some scattered vessels (asterisk). Microscopic examination with H&E stained section (10×4) (C)- Hypocellular pseudocystic area filled with pale pink oedematous fluid. Microscopic examination with smooth muscle actin stains section (10×10) (D)- Highlight the tumoural smooth muscle differentiation.
No cytological atypia, mitosis or necrosis was identified.
The diagnosis of a leiomyoma with extensive hydropic degeneration was made.
To date, the patient has undergone 7 months of follow-up, without major complications.
Discussion
Uterine leiomyomas are the most common uterine neoplasms. They are more likely to occur as asymptomatic masses in women in their fourth and fifth decades of life.1 The presence of symptoms is related to the tumoural size, with up to one-third of women presenting with uterine bleeding, pelvic pain and pressure.1 Infertility is also associated with the presence of leiomyomas, especially those of a submucosal type.
Transvaginal US in conjunction with transabdominal US is the first imaging modality to assess a pelvic mass. The US diagnosis of typical leiomyomas is usually straightforward, as they usually present as well-defined, solid hypoechoic masses in a submucosal, intramural or subserosal localisation. However, pedunculated leiomyomas may pose diagnostic problems on US, as occurred in our case.
Pedunculated leiomyomas are tumours that are connected to the uterus via a vascular pedicle. They can be submucosal or subserosal. We show the latter type, which was confused with an ovarian mass on transabdominal and transvaginal B mode, as well as on Doppler US imaging.
This misinterpretation especially occurs when large tumours make it difficult to visualise the adnexa, and to depict the vascular pedicle between the uterus and the juxta-uterine mass on Doppler imaging. Moreover, some of these tumours may detach from their stalk, forming independent masses (the so-called parasitic leiomyomas).
When the adnexal or uterine origin of the tumour cannot be determined by US imaging, MRI should be performed. In these cases, oblique sequences (ie, the axial plan of the ovary, which corresponds to the parallel plan of the endometrial cavity) are a problem-solving tool.
Furthermore, MRI in this particular case was also useful to further characterise the lesion, since it showed marked US heterogeneous echogenicity, displaying both cystic and vascular solid areas.
It is well known that 10% of leiomyomas are variant forms represented by different types of histological degeneration or cellular histological subtypes.1 Unlike typical leiomyomas, which characteristically display low signal on T2-weighted images, the different degenerating forms of leiomyoma have a variable appearance on MRI. These forms tend to occur in large tumours that enlarge and outgrow their blood supply.2 3
The most common type of degeneration is hyaline, which can be present in up to 60% of cases.3 Hyaline degeneration usually shows low signal on T2-weighted images, and sometimes a ‘cobblestone appearance’ and variable enhancement on postcontrast imaging.4
Leiomyomas with myxoid degeneration show the presence of soft mucoid areas with hyaluronic acid-rich mucopolysaccharide gelatinous foci, sometimes intermixed with cystic areas. These typically show very high T2 signal and mild enhancement after contrast administration.2
Red degeneration usually occurs in pregnancy and is represented by multiple haemorrhagic infarctions. On MRI, leiomyomas with red degeneration tend to present peripheral or diffuse high signal on T1-weighted images and variable signal intensity on T2-weighted images.
Cystic degenerated leiomyomas show non-enhancement and high T2 signal.
Diffuse hydropic change is a very rare form of degeneration characterised by watery oedema that may be combined with hyalinisation, resulting in a cord-like pattern growth of the tumoural cells.1 The presence of a large amount of fluid may result in large tumours.3 The accumulation of watery oedema can be sometimes be misinterpreted as a myxoid matrix and, therefore, these types of leiomyomas may be misdiagnosed as myxoid leiomyomas or as leiomyosarcomas. Another confounding factor is, when the extensive hydropic degeneration presents beyond the limits of a leiomyoma, it mimics an infiltrative border of a myxoid leiomyosarcoma.5
To the best of our knowledge, this is the third case where imaging findings of diffuse hydropic leiomyomas are shown. Both previous reports described a diffuse hydropic leiomyoma in pregnancy.3 6
In one of the cases, the tumour appeared as a unilocular cystic mass with irregular walls on both US and MRI.3 The other case reported a large and lobulated complex appearing mass on US that on MRI displayed heterogeneous mainly high T2 signal with strands of low signal within.6 Thick blood vessels were present but no fatty components were seen. This radiological appearance was similar to the case we present here.
Atypical degenerating and cellular forms of leiomyomas with high signal on T2-weighted images can be a diagnostic challenge for radiologists, as they can mimic malignant tumours.7 “Pseudo-Meigs’ syndrome” has been associated with hydropic leiomyomas, and the case we report was also associated with ascites and elevated serum levels of CA-125, which complicated the differential diagnosis.3
Uterine sarcomas account for approximately 1–3% of uterine tumours, of which leiomyosarcoma is the most frequent.1 7 The reported incidence of sarcomatous degeneration of leiomyomas is 0.1–0.8%.8
As atypical forms of leiomyomas, leiomyosarcomas present as intermediate to high signal T2 masses. They tend to be irregular, infiltrative, inhomogeneous enhancing tumours associated with necrosis and haemorrhage.7 8
These tumours usually invade the myometrium and adjacent pelvic structures, and demonstrate lymphatic and haematogenous spread mainly to the lungs.8
None of these features were present, and since the patient was young and expressed a desire to preserve fertility, a myomectomy was performed.
Although a retrospective study conducted by Thomassin-Naggara et al7 suggested that the combination of T2 signal intensity with the analysis of diffusion-weighted imaging would be the best way to differentiate leiomyosarcomas from atypical forms of leiomyomas, both conventional and functional MRI have limited value in the diagnosis of these types of tumours since there is a significant overlap with their benign counterparts.9 10 Therefore, the definite diagnosis is exclusively histopathological and based on the assessment of three major histological features: mitotic activity, nuclear atypia and tumour cell necrosis. None of these characteristics were observed in the case we here present.
Learning points.
Pedunculated subserosal leiomyomas may be difficult to distinguish from adnexal masses by US imaging alone, therefore MRI with oblique sequences should be performed to determine the origin of the tumour.
The diagnosis of a typical leiomyoma is usually straightforward on US imaging. However, tumours with degenerating changes and those showing rapid growth in postmenopausal women should be further characterised by MRI.
Leiomyomas with atypical degenerating changes such as diffuse hydropic change, usually present as a diagnostic challenge for the radiologist, since they can mimic malignancy.
Although there are imaging criteria that can suggest the diagnostic of leiomyosarcomas, there is significant overlap with atypical forms of their benign counterpart in both conventional and functional MRI. Therefore, their diagnosis is exclusively histopathological.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kurman RJ, Carcangiu ML, Herrington CS et al. . Classification of tumours of the ovary. In: WHO classification of tumours. Vol 6 4th edn Lyon: IARC, 2014:135– 41. [Google Scholar]
- 2.Mazziotti S, Ascenti G, Racchiusa S et al. . Retroperitoneal growth of degenerated myxoid uterine leiomyoma mimicking sarcoma. Clin Radiol 2012;67:616–17. 10.1016/j.crad.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 3.Awad EE, El-agwani AS, Elhabashy AM et al. . A giant uterine myometrium cyst mimicking an ovarian cyst in pregnancy: an uncommon presentation of hydropic degeneration of uterine fibroid. Egypt J Radiol Nucl Med 2015;46:529–34. 10.1016/j.ejrnm.2015.03.003 [DOI] [Google Scholar]
- 4.Murase E, Siegelman ES, Outwater EK et al. . Uterine leiomyomas: histopathologic features, MR imaging findings, differential diagnosis, and treatment. Radiographics 1999;19:1179–97. 10.1148/radiographics.19.5.g99se131179 [DOI] [PubMed] [Google Scholar]
- 5.Toledo G, Oliva E. Smooth muscle tumors of the uterus: a practical approach. Arch Pathol Lab Med 2008;132:595–605. doi:10.1043/1543-2165(2008)132[595:SMTOTU]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 6.Heffernan E, Kobel M, Spielmann A. Hydropic leiomyoma of the uterus presenting in pregnancy: imaging features. Br J Radiol 2009;82:e164–7. 10.1259/bjr/50866065 [DOI] [PubMed] [Google Scholar]
- 7.Thomassin-Naggara I, Dechoux S, Bonneau C et al. . How to differentiate benign from malignant myometrial tumours using MR imaging. Eur Radiol 2013;23:2306–14. 10.1007/s00330-013-2819-9 [DOI] [PubMed] [Google Scholar]
- 8.Rha SE, Byun JY, Jung SE et al. . CT and MRI of uterine sarcomas and their mimickers. AJR Am J Roentgenol 2003;181:1369–74. 10.2214/ajr.181.5.1811369 [DOI] [PubMed] [Google Scholar]
- 9.Cornfeld D, Israel G, Martel M et al. . MRI appearance of mesenchymal tumors of the uterus. Eur J Radiol 2010;74:241–9. 10.1016/j.ejrad.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 10.Tamai K, Koyama T, Saga T et al. . The utility of diffusion-weighted MR imaging for differentiating uterine sarcomas from benign leiomyomas. Eur Radiol 2008;18:723–30. 10.1007/s00330-007-0787-7 [DOI] [PubMed] [Google Scholar]