Abstract
Background
Atrioesophageal fistula (AEF) is a rare but serious adverse event of atrial fibrillation (AF) ablation.
Objective
To identify the clinical characteristics of AEF following ablation procedures for AF and determine the associated mortality.
Methods
A systematic review of observational cases of AEF following ablation procedures for AF was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement protocol.
Results
53 cases were identified. Mean age was 54±13 years; 73% (39/53) of cases occurred in males. Mean interval between procedure and presentation was 20±12 days, ranging from 2 to 60 days. AEF was observed in 12 patients who underwent surgical radiofrequency ablation (RFA) and in 41 patients with percutaneous RFA. Fever was the most common presenting symptom (n=44) followed by neurological deficits (n=27) and haematemesis (n=19). CT of the chest (n=27) was the preferred diagnostic test. Patients who did not receive a primary esophageal repair were more likely to have a deadly outcome (34% vs 83%; p<0.05). No difference in mortality rate was found between patients who underwent surgical RFA when compared with percutaneous RFA (58% vs 56%; p=0.579). No association was found between onset of symptoms and mortality (19±10 vs 23±14 days; p=0.355).
Conclusions
AEF following ablation procedures for AF is a serious complication with high mortality rates. Presenting symptoms most often include a triad of fever, neurological deficit and/or haematemesis within 60 days of procedure. The preferred diagnostic test is CT of the chest. The treatments of choice is surgical repair.
Keywords: CARDIAC SURGERY
Key questions.
What is already known about this subject?
Ablation for atrial fibrillation is becoming a mainstream treatment in cases resistant to conventional therapy or with severe symptomatology. As the number of procedures continues to rise, reports of one of the most devastating complications, atrioesophageal fistula, have also increased.
What does this study add?
This is a review of case reports describing the signs and symptoms, diagnosis, management, and the associated mortality of this important complication of ablation procedures for atrial fibrillation. This is the largest review of case reports to date, including 53 cases in 37 peer-reviewed publications.
How might this impact on clinical practice?
We considered it critical to review the clinical findings, diagnostic methods and therapeutic option available for this condition. The early recognition and prompt management might change its serious outcome and high mortality.
Introduction
Catheter ablation for atrial fibrillation (AF) is becoming a mainstream treatment particularly in patients with paroxysmal AF with severe symptomatology.1 As the number of procedures continues to rise, reports of one of the most devastating complications, atrioesophageal fistula (AEF), have also increased. In 2001, Mohr et al,2 reporting outcomes after intraoperative radiofrequency ablation (RFA) of AF in 234 patients at the 81st Annual Meeting of The American Association for Thoracic Surgery, described three patients who developed AEF, one of whom had a fatal outcome.
Since this initial observation, at least 53 cases of AEF following AF ablation procedures have been reported. Although differences in pathogenesis of each case have been reported, the similarities in the clinical presentations allow one to characterise this rare entity. Given its serious outcome and high mortality, we considered it critical to review the clinical findings, diagnostic methods, and therapeutic options available for this condition.
Methods
Search strategy
The objective of this review was to identify the case reports on AEF following ablation procedures for AF. A systematic search of the database PubMed from inception to December 2014 was performed. The search terms included atrioesophageal fistula OR atrio-esophageal fistula OR atrio-oesophageal fistula OR esophagoatrial fistula OR oesophago-atrial fistula. This terms were searched as free text in the title or the abstract.
We limited our search to case reports of humans without timeframe limit. No language restriction was applied. The reference lists of bibliographies of the identified articles were also reviewed.
Selection criteria
To be included in the analysis, a case report had to fulfil the following criteria: (1) report AF as the primary diagnosis for ablation procedure; (2) report clinical presentation; (3) report diagnostic modality used; (4) report management applied and (5) report outcome. Exclusion criteria involved the following: unknown aetiology of AEF and pericardioesophageal fistulas.
Data extraction
The case reports were identified and data extracted using standardised protocol. Disagreements were resolved by arbitration (PC and FHM), and consensus was reached after discussion. We extracted data such as baseline patient demographics, clinical presentation, diagnostic modalities and therapeutic management and outcome.
Statistical analysis
For this systematic review of case reports, we used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement protocol.3 Continuous data are presented as means with SD. Absolute numbers and percentages are presented for categorical data. Comparison between categorical variables was evaluated by using the Fisher exact test (IBM SPSS Statistics V.13 for Windows). Statistical significance was set at 0.05.
Results
Demographics and clinical presentation
Fifty-three cases were identified (table 1). Mean age was 54±13 years; 73% (39/53) of cases occurred in males. Mean interval between procedure and presentation was 20±12 days, ranging from 2 to 60 days.
Table 1.
Case reports included
| Author | Number of cases | Gender | Age (years) | Procedure | Post procedure day | Clinical presentation | Imaging | Findings | Diagnostic procedure | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sonmez et al4 | 1 | Female | 58 | Surgical: LRFA—melo technique | 22 | Fever, shivers, numbness right arm | TTE | LA thrombus | EGD | Thrombectomy, pericardial sutures | Death |
| Doll et al5 | 1 | Male | 42 | Surgical: IRAAF | 10 | Fever, postprandial TIA | TTE | Normal | Exploratory thoracotomy | Surgical | Survived |
| Doll et al5 | 1 | Female | 62 | Surgical: IRAAF | 6 | Haematemesis | EGD | NA | Pathology | None | Death |
| Doll et al5 | 1 | Male | 59 | Surgical: IRAAF | 12 | Fever, neurological symptoms | CT of the chest | Contrast and free air in the mediastinum | Exploratory Thoracotomy | Surgical | Survived |
| Doll et al5 | 1 | Male | 36 | Surgical: IRAAF | 11 | Chest pain | CT of the chest | Oesophageal perforation | Exploratory thoracotomy | Surgical | Survived |
| Pappone et al6 | 1 | Male | 36 | Percutaneous: CPVA | 3 | Fever, pleuritic chest pain, seizures | CT of the head | Bilateral ischaemia | CT of the chest | Surgical | Survived |
| Pappone et al6 | 1 | Male | 21 | Percutaneous: CPVA | 1 | Fever, grand mal seizure | CT of the head | Unremarkable | TEE | Non-surgical | Death |
| Scanavacca et al7 | 1 | Male | 72 | Percutaneous: RFA | 22 | Seizures, haematemesis | NA | NA | EGD | None | Death |
| Zirlik and Nordt8 | 1 | Male | 66 | Surgical: MVR and Maze procedure | 14 | Collapse | CT of the head | Multiple intracerebral air emboli and infarctions | EGD | Non-surgical | Death |
| Bunch et al9 | 1 | Male | 48 | Percutaneous: RFA | 14 | Fever, chest pain, dysphagia | CT of the chest | 3 mm oesophageal perforation at level of atrium | EGD | Non-surgical | Survived |
| Schley et al10 | 1 | Male | 37 | Percutaneous: RFA | 25 | Fever, grand mal seizure, status epilepticus | CT of the head | Ischaemic lesions | CT of the chest | Surgical | Survived |
| Cummings et al11 | 9 | Male=4 Female=5 | NA | Percutaneous: PRFA | 12.3 (10–16) | Sepsis (n=9), neurological symptoms (n=8); angina (n=2); GI bleed (n=3) | CT of the head | Intravascular air (n=2) | CT of the chest 3/4; autopsy 6/9 | Surgical=3; Non-surgical=6 | Death (n=9) |
| Dagres et al12 | 5 | Male=4 Female | 51 (35–76) | Surgical RFA (n=4); Percutaneous RFA (n=1) | 8–28 | Fever (n=3), chest pain (n=2), hemiparesis (n=3), grand mal seizure (n=1), aphasia (n=1) | NA | NA | CT of the chest | Surgery (n=3); attempted surgery (n=2) | Death (n=2) |
| Preis et al13 | 1 | Male | 56 | Percutaneous: PVI with RFA | 38 | Malaise, chills, bilateral arm weakness | TEE | No vegetations | CT of the chest | Surgical | Survived |
| Malamis et al14 | 1 | Male | 59 | Percutaneous: RFA | 35 | Fever, altered mental status, petechiae | CT of the head | Negative | CT of the chest | Surgical | Death |
| D’Avila et al15 | 1 | Male | 56 | Percutaneous: RFA | 28 | Epigastric pain, dysphagia, tactile fever; focal weakness, anomia, acalculia, agraphia | MRI of the brain | Multiple subacute embolic events | CT of the chest | Surgical | Survived |
| Borchert et al16 | 1 | Male | 59 | Percutaneous: PVI with HIFU ablation catheter | 10 | Chest discomfort and atypical atrial flutter; VF arrest | MRI of the brain | Cerebral and cerebellar ischaemic lesions | CT of the chest | Surgical | Death |
| Ouchikhe et al17 | 1 | Male | 58 | Percutaneous: RFA | 21 | Fever, confusion, meningitis | CT of the head | Bilateral hyperdense lesions (frontal, occipital, parietal and temporal) | TTE | Non-surgical | Death |
| Hazell et al18 | 1 | Male | 72 | Percutaneous: PVI roofline mitral isthmus line CFAE ablation | 16 | Weakness, LOC, chest pain | CT of the head | Right parietal subcortical matter ischaemic changes | CT of the chest | Non-surgical | Death |
| Vijayaraman et al19 | 1 | Male | 45 | Percutaneous: RFA with 3D reconstruction | 10 | Chest pain, low-grade fever, hypotension | CT of the chest | Fluid and air in pericardium and air in right superior mediastinum | Thoracotomy | Surgical | Survived |
| Baker et al20 | 1 | Female | 67 | Surgical: RFA | 20 | Substernal chest pain, nausea, vomiting, confusion, fever, seizures, haematemesis | MRI of the brain | Multiple acute emboli | EGD | Non-surgical | Death |
| Cazavet et al21 | 1 | Male | 35 | Percutaneous: RFA | 38 | Fever, chest pain, vomiting, left hemiplegia and seizures | CT of the head | Initially negative | CT of the chest | Surgical | Survived |
| Gilcrease et al22 | 1 | Male | 61 | Percutaneous: RFA | 10 | Dysphagia, substernal chest pain, fever | CT of the chest | Ulcer at anterior portion oesophagus adjacent to PV | CT of the chest (after 2 months) | Surgical | Death |
| Khandhar et al23 | 1 | Male | 46 | Percutaneous: RFA | 27 | Fever, pericarditis, followed by hemiparesis | CT of the chest | Normal | CT of the chest | Surgical | Survived |
| Siegel et al24 | 1 | Male | 41 | Percutaneous: RFA | 30 | Fever, rigours, near syncope; followed by right-sided hemiparesis | MRI of the brain | Multifocal infarcts | CT of the chest | Surgical | Survived |
| Grubina et al25 | 1 | Male | 72 | Percutaneous: RFA | 9 | Pleuritic chest pain | CT of the chest PAD #15 | Pneumopericardium | EGD | Surgical | Survived |
| St Julien et al26 | 1 | Male | 59 | Percutaneous: transeptal LA ablation with ThermoCool catheter | 42 | Chest pain, diaphoresis, headache, fever, altered mental status | TTE | No vegetations | CT of the chest | Surgical | Survived |
| Zellerhoff et al27 | 1 | Male | 63 | Percutaneous: RFA with 3D mapping | 14 | Muscle weakness, generalised fatigue followed by fever and left-sided hemiparesis | CT of the head | Several large intracerebral lesions suspicious for air embolism | CT of the chest | Non-surgical | Death |
| Purerfellner et al28 | 1 | Male | 49 | Percutaneous: RFA | 29 | Fever, chills, nausea, emesis, altered mental status, athetotic movements; skin changes, haematemesis | EGD | Unable to localise source of bleeding | EGD | Non-surgical | Death |
| Stockigt et al29 | 1 | Male | 78 | Percutaneous: cryoballoon PV isolation | 28 | Fever, shivers, cough for 10 days, followed by neurological symptoms | CT of the chest and abdomen | Negative | Cardiac CT | Non-surgical | Survived |
| Tancevski et al30 | 1 | Male | 45 | Percutaneous: transcatheter ablation | 42 | Fever, weakness, sensory loss of right limbs | CT of the chest and abdomen | CT of the chest: AEF; CT of the abdomen: multiple renal and splenic infarctions | CT surgery | Surgical | Survived |
| Haggerty et al31 | 1 | Male | 27 | Percutaneous: PV RFA | 22 | Fever, chills, hypotension, haematemesis | CT of the chest | Pneumomediastinum adjacent to LA | CT surgery | Surgical | Survived |
| Kanth and Fang32 | 1 | Female | 69 | Percutaneous: RFA | 60 | Sepsis, ischaemic stroke, melena | CT of the chest | AEF | EGD | Non-surgical | Death |
| Ben-David et al33 | 1 | Female | 73 | Percutaneous: RFA | 9 | Pneumomediastinum | UGI series | 4 mm oesophageal perforation at 6 cm from GEJ | EGD | Non-surgical | Death |
| Hartman et al34 | 1 | Male | 62 | Percutaneous: RFA | 30 | Odynophagia, fever, chills, rigours, syncope | Cardiac catheterisation | Negative | CT of the chest | Surgical | Survived |
| Zini et al35 | 1 | Male | 44 | Percutaneous: RFA | – | Altered mental status, stupor | CT of the head | Multifocal air emboli | EGD | Antibiotics, antithrombotics, fistula repair | Death |
| Rivera et al36 | 1 | Female | 50 | Percutaneous: RFA | 28 | Minor haematemesis | CT of the chest | AEF and pleural effusions | EGD | Surgical | Survived |
| Tan and Coffey37 | 1 | Female | 67 | Surgical: MVR and Maze procedure | 20 | Nausea, fever, numbness left foot; unresponsiveness | CT of the head | CT of the head: air embolism RSFA | CT of the chest | Non-surgical | Death |
| Shim et al38 | 1 | Male | 46 | Percutaneous: RFA | 2 | Fever, chills, cough, headache; confusion, generalised tonic-clonic seizures | TTE/TEE | No thrombus | CT of the chest | Surgical | Survived |
| Neven et al39 | 1 | Male | 69 | Percutaneous: HIFU | 31 | Fever, haematemesis, seizures, phrenic nerve palsy | CT of the head | Cerebral embolism | Autopsy | Non-surgical | Death |
| Dixit et al40 | 1 | Female | NA | Percutaneous: PV isolation | 14 | Fever, haematemesis, nausea | EGD | Possible Mallory-Weiss tear | CT of the head | Non-surgical | Death |
AEF, atrioesophageal fistula; CFAE, complex fractionated atrial electrograms; CPVA, circumferential pulmonary vein ablation; EGD, esophagogastroduodenoscopy; GEJ, gastroesophageal junction; HIFU, high-intensity focused ultrasound; IRAAF, intraoperative radiofrequency ablation of atrial fibrillation; LA, left atrium; LOC, loss of consciousness; LRFA, linear radiofrequency ablation; MVR, mitral valve replacement; NA, not available; PAD, postablation day; PV, pulmonary vein; PVI, pulmonary vein ablation; RFA, radiofrequency ablation; RSFA, right superior frontal area; TEE, transesophageal echocardiogram; TIA, transient ischaemic attack; TTE, transthoracic echocardiogram; UGI, upper gastrointestinal; VF, ventricular fibrillation.
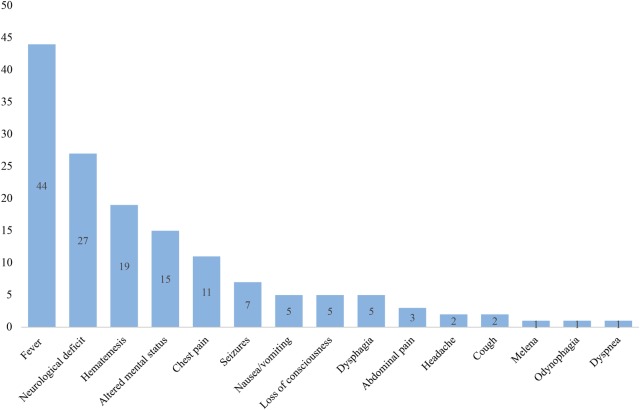
AEF was noticed in 12 patients who underwent surgical RFA and in 41 patients who underwent percutaneous RFA. One case was reported after cryoballoon ablation.29 Fever was the most common presenting symptom (n=44) followed by neurological deficits (n=27; including motor and language impairment), haematemesis (n=19), altered mental status (n=15), chest pain (n=11) and seizures (n=7; figure 1).
Figure 1.
Frequency of symptoms at time of presentation. Neurological deficits include motor and language impairment; altered mental status was also described as confusion.
Diagnostic evaluation, treatment and outcome
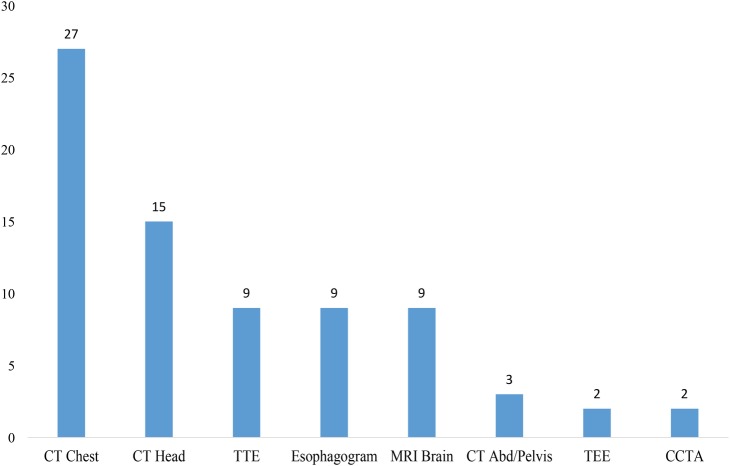
CT of the chest (n=27) and head (n=15) were the preferred diagnostic modalities (figure 2), with multifocal infarcts consistent with air embolism (n=13) and pneumomediastinum (n=12) being the most common findings.
Figure 2.
Diagnostic modalities on presentation to the emergency room (CT abd/pelvis, CT of the abdomen and pelvis with contrast; CT chest, CT of the chest with intravenous contrast; CT head, CT of the head without contrast; CCTA, computed cardiac tomographic angiograph; .MRI brain, MRI of the brain; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography).
Among the CT of the chest findings pneumomediastinum was a strong indicator of esophageal injury, as well as hemopericardium and pneumopericardium.5 10 14 31 At least two cases reported pneumopericardium and four cases reported intracardiac air. Transthoracic echocardiography was performed in 11 cases and transesophageal echocardiography (TEE) in three cases.
No difference in mortality rate was found between patients who underwent surgical RFA when compared with percutaneous RFA (10/29, 58% vs 20/24, 56%; p=0.579). Patients who did not receive a primary esophageal repair were more likely to have a deadly outcome (34% with surgical treatment vs 83% with conservative treatment; p<0.05). In those cases that underwent corrective surgical intervention, the left atrium (LA) was identified, exposing the fistula between the atrium and esophagus.5 No association was found between onset of symptoms and mortality (19±10 vs 23±14 days; p=0.355).
Discussion
AEF can be defined as an abnormal communication between the atrium and the esophagus as a result of a trauma, although idiopathic fistulas have been described.41 Literature reports a 15% rise in the rates of AF ablations resulting in an increase from 0.06% to 0.79% over 15 years (1990–2005 period), which is parallel to a rise in the prevalence of AF itself—from 270 000 to over 2.2 million people affected—a number that continues to grow.42 The incidence of AEF varies from 0.03% to 1.5%; however, its true incidence may be under-reported.5 43–45
Prior reports have evaluated this topic. Finsterer et al46 and Stöllberger et al47 focused on the neurological manifestations of AEF after RFA. Nair et al48 performed a review of the epidemiology, clinical features, aetiopathogenesis and management of AEF after RFA. Singh et al49 reported a review of the principles of AEF repair and clinical outcomes in 29 patients. We describe the largest case report review to date, evaluating 53 cases of AEF after RFA in 37 peer-reviewed publications.
Demographics and clinical presentation
AEF has been reported to be more prevalent in males than females.50 We found similar results, with 73% of the cases occurring in males. This could partially be explained by the fact that more men undergo RFA and that women are less likely get invasive treatment.46 AEF typically presented 20±12 days post-RFA, ranging from 2 to 60 days. Occasionally the patient might present repeatedly before a definitive diagnosis is made.11 The presenting symptoms can involve different organs and systems, including fever, neurological deficit, haematemesis, altered mental status, chest pain or a combination of these (figure 1). No association was found between onset of symptoms and mortality (19±10 vs 23±14 days; p=0.355). Finsterer et al46 suggested that the latency between initial insult and the development of symptoms may depend on the fistula size, the treatment initially provided, and the number of additional complications. A high index of suspicion for this catastrophic complication is required for patients with a recent history of RFA in order to achieve a correct diagnosis and prompt management.
Diagnostic procedures
CT of the chest with intravenous contrast has shown to be the most useful diagnostic tool.22 23 Other diagnostic techniques, such as CT of the head, can be useful. In this review, the most common findings were multifocal air embolism of the brain and pneumomediastinum. Several radiological features have been reported, including pericardial effusion and the obvious communication between the atrium and the pericardium or the esophagus.12 51 TEE and/or esophagogastroduodenoscopy (EGD) are precluded at any suspicion of AEF. Air insufflation during TEE or EGD may lead to massive embolisation, resulting in severe neurological injury and death.4 5 7 8 11–13 15 18 21 24 26 28 30 32 35 36 38 44 If systemic bacterial endocarditis is suspected, avoiding TEE could prove lifesaving.13
Anatomical contributing factors
Unfortunately, despite efforts to determine how this complication occurs, there is little understanding in the pathogenesis of AEF.
Gillinov et al52 considered that body size, when extremely small, may contribute to perforation, assuming thinner patients are more likely to have a thin left posterior atrial wall.14 Sonmez et al4 suggested that a thin atrial wall could also result from atrial enlargement (>60 mm in diameter). Paradoxically, others like Lemola et al53 proposed that a small LA might be at higher risk for fistula formation because the esophagus may occupy a larger relative area of the posterior LA, where much of the ablation is performed.
The absence of a fat layer between the esophagus and atrium may identify patients at higher risk of esophageal injury53; the distance is often <5 mm from the esophagus to the endocardial layer of the atrium.14 This hypothesis is supported by a cadaver study that showed marked individual variation of thickness of the posterior left atrial wall and the fibrofatty layer between the atrium and the esophagus.9 54
Procedural contributing factors
The incidence of AEF following percutaneous ablation has ranged from 0.01% to 0.2%, and is as high as 1–1.5% for patients undergoing surgical ablations.2 5 6 12 45 55–57 This report includes 12 surgical and 41 percutaneous cases of AEF after RFA for AF. The higher number of percutaneous cases could be explained by the increased use of this therapeutic modality. However, given the overall small numbers of cases reported in the literature, it is not known whether the incidence of AEF differs when done surgically or percutaneously.12
The accountability of an individual operator technique is inevitably implicit. In early cases, TEE was used as a standard imaging aid during ablation procedures. When the probe is left during the procedure, it mechanically displaces the esophagus towards the ablation catheter, increasing the heat transfer to the esophageal mucosa.58 Attempts to reduce temperature have been achieved with the use of cryoballoon technology for pulmonary vein isolation; however, despite this, AEF may still occur.29
Given that direct thermal injury may account for the development of AEF, it seems critical to determine the role of intraoesophageal temperatures during ablation procedures. There are, however, case reports of AEF without significant change in esophageal temperature.5 9 15 16 18 37 Changes in the esophageal mucosa consistent with thermal injury are commonly seen in about 47% cases, while ulceration may occur in 14–18% cases.59 60 One case series reporting the development of fistula in four patients showed no statistically significant difference when comparing AEF to AEF-free cases, although mean maximum temperature and total energy appeared slightly higher in the esophageal injury group.5 The risk for developing AEF is augmented by magnitude and duration of local heating, which is related to catheter tip size, contact pressure, catheter orientation, the number of linear lesions sets in the posterior wall, as well as the power output and duration associated with each lesion. Furthermore, general anaesthesia during catheter ablation may increase the risk of esophageal wall injury given the alteration in the physiological motility of the esophagus.61
Treatment
Surgical intervention has been considered the standard of care, though isolated cases of successful conservative management have been reported.21 Only two of five cases treated with esophageal stenting survived after the procedure.8 9 18 21 27 Nevertheless, pericardioesophageal fistulas have been reported to be successfully managed with esophageal stenting when detected early (at days 26, 9 and 18 after the ablation procedure in the cases reported).51 Broad-spectrum antibiotics should be started concomitantly. Complications, such as stent dislocation, embolic and/or septic events, and stenosis may follow; therefore, patients should be closely followed.
Surgical repairs require cardiopulmonary bypass in order to first excise and replace the necrotic tissue in an intracardiac fashion.5 This method allows abolition of gaseous and bacteremic contamination within a locally aseptic, blood-rich, and tissue-friendly environment.34
Esophageal resections constitute the second step of treatment. Stenting is not considered as first-line therapy as yet, but has been reported as a temporary measure in bridging to definitive surgical intervention and lately as an alternative management therapy when patients are unable to undergo surgery.51 Only one case reported successful stenting as an end point.9 33 Novel alternatives have been proposed such as cervical esophageal ligation and decompression.26
Conservative management of esophageal perforation remains controversial with mortality rates ranging from 20% to 45%.9 According to the included cases (table 1), patients who did not receive a primary esophageal repair were more likely to have a deadly outcome (34% with surgical treatment vs 83% with conservative treatment; p<0.05).This may be owing to the critical status at presentation of this group of patients. All the patients with suspected AEF should be transferred to a hospital equipped with cardiothoracic surgery facilities.
Outcome
AEF has reportedly been associated with a mortality rate of 40–80%.12 45 In this review, we found no difference in mortality rate between patients who underwent surgical RFA when compared with percutaneous RFA (58% vs 56%; p=0.579). Prior reports evidence lower mortality rates in the surgical RFA group, suggesting that there is a higher awareness of a complication when the procedure is done surgically.47 The complications following AEF, if survival is achieved, include multiple septicemias and even Guillain-Barré syndrome.12 No instances of spontaneous resolution have been reported.
Procedural suggestions and prevention
Technology advances now allow detailed mapping of the cardiac–esophageal interface by preprocedural and/or intraprocedural imaging; and energy delivery may be guided by intracardiac echocardiography.25 Nonetheless, cases of esophageal perforation, seen when using robotic mapping methods, have been reported.61
Patwardhan et al62 hinted that the bipolar mode of RF would be safer than the unipolar mode, since it—in theory—prevents energy dispersion and thus the formation of AEF.37 Lower power setting and shorter lesion durations in the posterior aspect of the LA have been suggested as possible ways to avoid this complication. However, power has been shown to be a weak predictor of intraesophageal temperature during ablation and even power settings <10W may increase luminal temperatures in the esophagus and cause AEF.63 Intraoperative esophageal temperature monitoring has emerged as a method that allows the operator to stop the delivery of energy when increasing esophageal temperatures are detected.19 64 Since the esophageal temperature can continue to increase for few seconds after discontinuation of energy delivery, immediate discontinuation of radiofrequency application has been suggested if esophageal temperatures increase rapidly or reach more than an absolute temperature of 39°C.9
RFA uses a point-by-point system that entails absorptive heating and induction of thermal necrosis as mechanisms of action. Using an open-irrigated catheter lowers the energy output when compared to a standard 8 mm tip catheter and was found to decrease the rate of esophageal ulceration; however, all the ablation catheters still carry the risk of AEF.65
Newer imaging techniques, such as combining the use of barium sulfate paste during CT or gadolinium diglutamate during MRI, are currently used to visualise the anatomical relationship between esophagus, pulmonary veins and LA position wall. The integration of these imaging modalities and current 3-D mapping systems (CARTO-3 or EnSite Velocity) provides a visualisation tool to understand the complex anatomy, and can play an important role in prevention of esophageal injury. Piorkowski et al66 reported a high accuracy in visualising the true anatomic relationship of the esophagus and LA by preprocedural CT scan, and its intraprocedural position by using electro-anatomic mapping systems.
Conclusion
AEF following ablation procedures for AF is a serious complication with high mortality rates. It is critical to be aware of this complication in the outcome sequence of catheter ablation. Presenting symptoms most often include a triad of fever, neurological deficit and/or haematemesis. Prompt diagnostic work-up should include a CT-chest. TEE is contraindicated even when endocarditis is suspected. Survival depends on rapid diagnosis and intervention. When untreated, the outcome is more often fatal. Thorough patient education regarding signs and symptoms of esophageal injury upon discharge is warranted.
Footnotes
Contributors: PC, FHM, ACD, EFA, TS, DG, CDB and SD were responsible for conception and design of study; analysis and interpretation of data and drafting of the manuscript; final approval of the manuscript submitted and agreement to be accountable for all aspects of the work.
Competing interests: FHM Ad hoc consultant for the following organisations: Daiichi Sankyo, Pfizer, Takeda, Abbott, Servier, Medtronic, Ipca Laboratories Ltd.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Fisher JD, Spinelli MA, Mookherjee D et al. . Atrial fibrillation ablation: reaching the mainstream. Pacing Clin Electrophysiol 2006;29:523–37. 10.1111/j.1540-8159.2006.00388.x [DOI] [PubMed] [Google Scholar]
- 2.Mohr FW, Fabricius AM, Falk V et al. . Curative treatment of atrial fibrillation with intraoperative radiofrequency ablation: short-term and midterm results. J Thorac Cardiovasc Surg 2002;123:919–27. 10.1067/mtc.2002.120730 [DOI] [PubMed] [Google Scholar]
- 3.Moher D, Liberati A, Tetzlaff J et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 4.Sonmez B, Demirsoy E, Yagan N et al. . A fatal complication due to radiofrequency ablation for atrial fibrillation: atrio-esophageal fistula. Ann Thorac Surg 2003;76:281–3. 10.1016/S0003-4975(03)00006-7 [DOI] [PubMed] [Google Scholar]
- 5.Doll N, Borger MA, Fabricius A et al. . Esophageal perforation during left atrial radiofrequency ablation: is the risk too high? J Thorac Cardiovasc Surg 2003;125:836–42. 10.1067/mtc.2003.165 [DOI] [PubMed] [Google Scholar]
- 6.Pappone C, Oral H, Santinelli V et al. . Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation 2004;109:2724–6. 10.1161/01.CIR.0000131866.44650.46 [DOI] [PubMed] [Google Scholar]
- 7.Scanavacca MI, D'Avila A, Parga J et al. . Left atrial-esophageal fistula following radiofrequency catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2004;15:960–2. 10.1046/j.1540-8167.2004.04083.x [DOI] [PubMed] [Google Scholar]
- 8.Zirlik A, Nordt TK. Massive air embolism after Maze. Heart 2005;91:736 10.1136/hrt.2004.043901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunch TJ, Nelson J, Foley T et al. . Temporary esophageal stenting allows healing of esophageal perforations following atrial fibrillation ablation procedures. J Cardiovasc Electrophysiol 2006;17:435–9. 10.1111/j.1540-8167.2006.00464.x [DOI] [PubMed] [Google Scholar]
- 10.Schley P, Gulker H, Horlitz M. Atrio-oesophageal fistula following circumferential pulmonary vein ablation: verification of diagnosis with multislice computed tomography. Europace 2006;8:189–90. 10.1093/europace/euj050 [DOI] [PubMed] [Google Scholar]
- 11.Cummings JE, Schweikert RA, Saliba WI et al. . Brief communication: atrial-esophageal fistulas after radiofrequency ablation. Ann Intern Med 2006;144:572–4. 10.7326/0003-4819-144-8-200604180-00007 [DOI] [PubMed] [Google Scholar]
- 12.Dagres N, Kottkamp H, Piorkowski C et al. . Rapid detection and successful treatment of esophageal perforation after radiofrequency ablation of atrial fibrillation: lessons from five cases. J Cardiovasc Electrophysiol 2006;17:1213–15. 10.1111/j.1540-8167.2006.00611.x [DOI] [PubMed] [Google Scholar]
- 13.Preis O, Digumarthy SR, Wright CD et al. . Atrioesophageal fistula after catheter pulmonary venous ablation for atrial fibrillation: imaging features. J Thorac Imaging 2007;22:283–5. 10.1097/RTI.0b013e318054e26f [DOI] [PubMed] [Google Scholar]
- 14.Malamis AP, Kirshenbaum KJ, Nadimpalli S. CT radiographic findings: atrio-esophageal fistula after transcatheter percutaneous ablation of atrial fibrillation. J Thorac Imaging 2007;22:188–91. 10.1097/01.rti.0000213569.63538.30 [DOI] [PubMed] [Google Scholar]
- 15.D'Avila A, Ptaszek LM, Yu PB et al. . Images in cardiovascular medicine. Left atrial-esophageal fistula after pulmonary vein isolation: a cautionary tale. Circulation 2007;115:e432–3. 10.1161/CIRCULATIONAHA.106.680181 [DOI] [PubMed] [Google Scholar]
- 16.Borchert B, Lawrenz T, Hansky B et al. . Lethal atrioesophageal fistula after pulmonary vein isolation using high-intensity focused ultrasound (HIFU). Heart Rhythm 2008;5:145–8. 10.1016/j.hrthm.2007.08.023 [DOI] [PubMed] [Google Scholar]
- 17.Ouchikhe A, Maindivide J, Le Bivic JL et al. . Atrio-oesophageal fistula after radiofrequency ablation: predominant neurological symptoms. Ann Fr Anesth Reanim 2008;27:499–501. 10.1016/j.annfar.2008.03.011 [DOI] [PubMed] [Google Scholar]
- 18.Hazell W, Heaven D, Kazemi A et al. . Atrio-oesophageal fistula: an emergent complication of radiofrequency ablation. Emerg Med Australas 2009;21:329–32. 10.1111/j.1742-6723.2009.01205.x [DOI] [PubMed] [Google Scholar]
- 19.Vijayaraman P, Netrebko P, Geyfman V et al. . Esophageal fistula formation despite esophageal monitoring and low-power radiofrequency catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2009;2:e31–3. 10.1161/CIRCEP.109.883694 [DOI] [PubMed] [Google Scholar]
- 20.Baker MJ, Panchal PC, Allenby PA. Life-threatening GI hemorrhage caused by atrioesophageal fistula: a rare complication after catheter ablation for atrial fibrillation. Gastrointest Endosc 2010;72:887–9. 10.1016/j.gie.2010.01.001 [DOI] [PubMed] [Google Scholar]
- 21.Cazavet A, Muscari F, Marachet MA et al. . Successful surgery for atrioesophageal fistula caused by transcatheter ablation of atrial fibrillation. J Thorac Cardiovasc Surg 2010;140:e43–5. 10.1016/j.jtcvs.2010.02.032 [DOI] [PubMed] [Google Scholar]
- 22.Gilcrease GW, Stein JB. A delayed case of fatal atrioesophageal fistula following radiofrequency ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2010;21:708–11. 10.1111/j.1540-8167.2009.01688.x [DOI] [PubMed] [Google Scholar]
- 23.Khandhar S, Nitzschke S, Ad N. Left atrioesophageal fistula following catheter ablation for atrial fibrillation: off-bypass, primary repair using an extrapericardial approach. J Thorac Cardiovasc Surg 2010;139:507–9. 10.1016/j.jtcvs.2008.12.036 [DOI] [PubMed] [Google Scholar]
- 24.Siegel MO, Parenti DM, Simon GL. Atrial-esophageal fistula after atrial radiofrequency catheter ablation. Clin Infect Dis 2010;51:73–6. 10.1086/653425 [DOI] [PubMed] [Google Scholar]
- 25.Grubina R, Cha YM, Bell MR et al. . Pneumopericardium following radiofrequency ablation for atrial fibrillation: insights into the natural history of atrial esophageal fistula formation. J Cardiovasc Electrophysiol 2010;21:1046–9. 10.1111/j.1540-8167.2010.01740.x [DOI] [PubMed] [Google Scholar]
- 26.St Julien J, Putnam JB Jr, Nesbitt JC et al. . Successful treatment of atrioesophageal fistula by cervical esophageal ligation and decompression. Ann Thorac Surg 2011;91:e85–6. 10.1016/j.athoracsur.2011.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zellerhoff S, Lenze F, Schulz R et al. . Fatal course of esophageal stenting of an atrioesophageal fistula after atrial fibrillation ablation. Heart Rhythm 2011;8:624–6. 10.1016/j.hrthm.2010.10.041 [DOI] [PubMed] [Google Scholar]
- 28.Purerfellner H, Stöllberger C, Finsterer J. Meningo-encephalitis as initial manifestation of a fatal atrio-oesophageal fistula after atrial fibrillation ablation. Acta Cardiol 2011;66:555–7. [DOI] [PubMed] [Google Scholar]
- 29.Stockigt F, Schrickel JW, Andrie R et al. . Atrioesophageal fistula after cryoballoon pulmonary vein isolation. J Cardiovasc Electrophysiol 2012;23:1254–7. 10.1111/j.1540-8167.2012.02324.x [DOI] [PubMed] [Google Scholar]
- 30.Tancevski I, Hintringer F, Stuehlinger M et al. . Atrioesophageal fistula after percutaneous transcatheter ablation of atrial fibrillation. Circulation 2012;125:966 10.1161/CIRCULATIONAHA.111.044438 [DOI] [PubMed] [Google Scholar]
- 31.Haggerty KA, George TJ, Arnaoutakis GJ et al. . Successful repair of an atrioesophageal fistula after catheter ablation for atrial fibrillation. Ann Thorac Surg 2012;93:313–15. 10.1016/j.athoracsur.2011.05.050 [DOI] [PubMed] [Google Scholar]
- 32.Kanth P, Fang J. Cerebral air embolism: a complication of a bleeding atrioesophageal fistula. Clin Gastroenterol Hepatol 2012;10:A22 10.1016/j.cgh.2011.10.013 [DOI] [PubMed] [Google Scholar]
- 33.Ben-David K, Rosenthal M, Chauhan SS. A novel strategy for the management of acute hemorrhage from an atrio-esophageal fistula after atrial ablation. Am Surg 2012;78:E286–7. [PubMed] [Google Scholar]
- 34.Hartman AR, Glassman L, Katz S et al. . Surgical repair of a left atrial-esophageal fistula after radiofrequency catheter ablation for atrial fibrillation. Ann Thorac Surg 2012;94:e91–3. 10.1016/j.athoracsur.2012.04.052 [DOI] [PubMed] [Google Scholar]
- 35.Zini A, Carpeggiani P, Pinelli G et al. . Brain air embolism secondary to atrial-esophageal fistula. Arch Neurol 2012;69:785 10.1001/archneurol.2011.1896 [DOI] [PubMed] [Google Scholar]
- 36.Rivera GA, David IB, Anand RG. Successful atrioesophageal fistula repair after atrial fibrillation ablation. J Am Coll Cardiol 2013;61:1204 10.1016/j.jacc.2012.09.068 [DOI] [PubMed] [Google Scholar]
- 37.Tan C, Coffey A. Atrioesophageal fistula after surgical unipolar radiofrequency atrial ablation for atrial fibrillation. Ann Thorac Surg 2013;95:e61–2. 10.1016/j.athoracsur.2012.08.066 [DOI] [PubMed] [Google Scholar]
- 38.Shim HB, Kim C, Kim HK et al. . Successful management of atrio-esophageal fistula after cardiac radiofrequency catheter ablation. Korean J Thorac Cardiovasc Surg 2013;46:142–5. 10.5090/kjtcs.2013.46.2.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neven K, Schmidt B, Metzner A et al. . Fatal end of a safety algorithm for pulmonary vein isolation with use of high-intensity focused ultrasound. Circ Arrhythm Electrophysiol 2010;3:260–5. 10.1161/CIRCEP.109.922930 [DOI] [PubMed] [Google Scholar]
- 40.Dixit S, Gerstenfeld EP, Ratcliffe SJ et al. . Single procedure efficacy of isolating all versus arrhythmogenic pulmonary veins on long-term control of atrial fibrillation: a prospective randomized study. Heart Rhythm 2008;5:174–81. 10.1016/j.hrthm.2007.09.024 [DOI] [PubMed] [Google Scholar]
- 41.Aghasadeghi K, Aslani A. Aquarium sign in the left atrium. Cardiology 2007;107:411 10.1159/000099061 [DOI] [PubMed] [Google Scholar]
- 42.Kneeland PP, Fang MC. Trends in catheter ablation for atrial fibrillation in the United States. J Hosp Med 2009;4:E1–5. 10.1002/jhm.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pappone C, Santinelli V. Atrial fibrillation ablation: state of the art. Am J Cardiol 2005;96:59L–64L. 10.1016/j.amjcard.2005.09.063 [DOI] [PubMed] [Google Scholar]
- 44.Sonmez B, Demirsoy E, Yilmaz O. Atrioesophageal fistula: is it an unavoidable complication of radiofrequency ablation? J Thorac Cardiovasc Surg 2003;126:1662–3; author reply 63 10.1016/S0022-5223(03)01049-3 [DOI] [PubMed] [Google Scholar]
- 45.Ghia KK, Chugh A, Good E et al. . A nationwide survey on the prevalence of atrioesophageal fistula after left atrial radiofrequency catheter ablation. J Interv Card Electrophysiol 2009;24:33–6. 10.1007/s10840-008-9307-1 [DOI] [PubMed] [Google Scholar]
- 46.Finsterer J, Stöllberger C, Pulgram T. Neurological manifestations of atrio-esophageal fistulas from left atrial ablation. Eur J Neurol 2011;18:1212–19. 10.1111/j.1468-1331.2011.03375.x [DOI] [PubMed] [Google Scholar]
- 47.Stöllberger C, Pulgram T, Finsterer J. Neurological consequences of atrioesophageal fistula after radiofrequency ablation in atrial fibrillation. Arch Neurol 2009;66:884–7. 10.1001/archneurol.2009.105 [DOI] [PubMed] [Google Scholar]
- 48.Nair GM, Nery PB, Redpath CJ et al. . Atrioesophageal fistula in the era of atrial fibrillation ablation: a review. Can J Cardiol 2014;30:388–95. 10.1016/j.cjca.2013.12.012 [DOI] [PubMed] [Google Scholar]
- 49.Singh SM, d'Avila A, Singh SK et al. . Clinical outcomes after repair of left atrial esophageal fistulas occurring after atrial fibrillation ablation procedures. Heart Rhythm 2013;10:1591–7. 10.1016/j.hrthm.2013.08.012 [DOI] [PubMed] [Google Scholar]
- 50.Gerstenfeld EP, Callans D, Dixit S et al. . Characteristics of patients undergoing atrial fibrillation ablation: trends over a seven-year period 1999–2005. J Cardiovasc Electrophysiol 2007;18:23–8. 10.1111/j.1540-8167.2006.00662.x [DOI] [PubMed] [Google Scholar]
- 51.Eitel C, Rolf S, Zachaus M et al. . Successful nonsurgical treatment of esophagopericardial fistulas after atrial fibrillation catheter ablation: a case series. Circ Arrhythm Electrophysiol 2013;6:675–81. 10.1161/CIRCEP.113.000384 [DOI] [PubMed] [Google Scholar]
- 52.Gillinov AM, Pettersson G, Rice TW. Esophageal injury during radiofrequency ablation for atrial fibrillation. J Thorac Cardiovasc Surg 2001;122:1239–40. 10.1067/mtc.2001.118041 [DOI] [PubMed] [Google Scholar]
- 53.Lemola K, Sneider M, Desjardins B et al. . Computed tomographic analysis of the anatomy of the left atrium and the esophagus: implications for left atrial catheter ablation. Circulation 2004;110:3655–60. 10.1161/01.CIR.0000149714.31471.FD [DOI] [PubMed] [Google Scholar]
- 54.Sanchez-Quintana D, Cabrera JA, Climent V et al. . Anatomic relations between the esophagus and left atrium and relevance for ablation of atrial fibrillation. Circulation 2005;112:1400–5. 10.1161/CIRCULATIONAHA.105.551291 [DOI] [PubMed] [Google Scholar]
- 55.Dagres N, Hindricks G, Kottkamp H et al. . Complications of atrial fibrillation ablation in a high-volume center in 1,000 procedures: still cause for concern? J Cardiovasc Electrophysiol 2009;20:1014–19. 10.1111/j.1540-8167.2009.01493.x [DOI] [PubMed] [Google Scholar]
- 56.Cappato R, Calkins H, Chen SA et al. . Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol 2009;53:1798–803. 10.1016/j.jacc.2009.02.022 [DOI] [PubMed] [Google Scholar]
- 57.Ren JF, Lin D, Marchlinski FE et al. . Esophageal imaging and strategies for avoiding injury during left atrial ablation for atrial fibrillation. Heart Rhythm 2006;3:1156–61. 10.1016/j.hrthm.2006.06.006 [DOI] [PubMed] [Google Scholar]
- 58.Bunch TJ, Day JD. Examining the risks and benefits of transesophageal echocardiogram imaging during catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2012;5:621–3. 10.1161/CIRCEP.112.973297 [DOI] [PubMed] [Google Scholar]
- 59.Halm U, Gaspar T, Zachaus M et al. . Thermal esophageal lesions after radiofrequency catheter ablation of left atrial arrhythmias. Am J Gastroenterol 2010;105:551–6. 10.1038/ajg.2009.625 [DOI] [PubMed] [Google Scholar]
- 60.Schmidt M, Nolker G, Marschang H et al. . Incidence of oesophageal wall injury post-pulmonary vein antrum isolation for treatment of patients with atrial fibrillation. Europace 2008;10:205–9. 10.1093/europace/eun001 [DOI] [PubMed] [Google Scholar]
- 61.Shalaby A, Refaat M, Sebastien G et al. . Conservative management of pericardial-esophageal fistula complicating robotic atrial fibrillation ablation. Heart Rhythm 2011;8:905–8. 10.1016/j.hrthm.2011.01.035 [DOI] [PubMed] [Google Scholar]
- 62.Patwardhan AM, Dave HH, Tamhane AA et al. . Intraoperative radiofrequency microbipolar coagulation to replace incisions of maze III procedure for correcting atrial fibrillation in patients with rheumatic valvular disease. Eur J Cardiothorac Surg 1997;12:627–33. 10.1016/S1010-7940(97)00222-4 [DOI] [PubMed] [Google Scholar]
- 63.Cummings JE, Schweikert RA, Saliba WI et al. . Assessment of temperature, proximity, and course of the esophagus during radiofrequency ablation within the left atrium. Circulation 2005;112:459–64. 10.1161/CIRCULATIONAHA.104.509612 [DOI] [PubMed] [Google Scholar]
- 64.Redfearn DP, Trim GM, Skanes AC et al. . Esophageal temperature monitoring during radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2005;16:589–93. 10.1111/j.1540-8167.2005.40825.x [DOI] [PubMed] [Google Scholar]
- 65.Martinek M, Bencsik G, Aichinger J et al. . Esophageal damage during radiofrequency ablation of atrial fibrillation: impact of energy settings, lesion sets, and esophageal visualization. J Cardiovasc Electrophysiol 2009;20:726–33. 10.1111/j.1540-8167.2008.01426.x [DOI] [PubMed] [Google Scholar]
- 66.Piorkowski C, Hindricks G, Schreiber D et al. . Electroanatomic reconstruction of the left atrium, pulmonary veins, and esophagus compared with the “true anatomy” on multislice computed tomography in patients undergoing catheter ablation of atrial fibrillation. Heart Rhythm 2006;3:317–27. 10.1016/j.hrthm.2005.11.027 [DOI] [PubMed] [Google Scholar]