Abstract
Autism spectrum disorders (ASDs) and related neurological disorders are associated with mutations in many genes affecting the ratio between neuronal excitation and inhibition. However, understanding the impact of these mutations on network activity is complicated by the plasticity of these networks, making it difficult in many cases to separate initial deficits from homeostatic compensation. Here we explore the contrasting evidence for primary defects in inhibition or excitation in ASDs and attempt to integrate the findings in terms of the brain’s ability to maintain functional homeostasis.
A theory of excitatory/inhibitory imbalance in Autism
Over ten years ago John Rubenstein and Michael Merzenich published an influential review (Rubenstein and Merzenich, 2003) suggesting that autism and related disorders might reflect an increase in the ratio between excitation and inhibition leading to hyper-excitability of cortical circuits. The theory was attractive because it provided a potential explanation for the frequent observation of reduced GABAergic signaling in the brains of autistics (Cellot and Cherubini, 2014), as well as their propensity to develop epilepsy. In addition, since inhibition was known or believed to contribute to sharpening the selectivity of excitatory responses in many brain areas, the loss of inhibition could lead to enhanced “noise” and imprecision in learning and cognition. Since this initial formulation, however, other studies have suggested a nearly opposite hypothesis, namely that at least some Autism Spectrum Disorders (ASDs) are characterized by a reduction in the ratio between excitation and inhibition. In this review we first summarize some of the major lines of evidence supporting primary increases and decreases in the ratio of excitatory to inhibitory synaptic transmission in Autism and related disorders. We then argue that a homeostatic view of how activity propagates through cortical circuits predicts such contradictory findings and offers a framework for integrating them.
It is important to acknowledge at the outset that the concept of a single “E/I balance” determining whether brain circuits are normal or “autistic” is obviously overly simplistic. This is true both because different microcircuits in different brain regions may be characterized by different mixtures of excitation and inhibition and because even within a single microcircuit different sources of excitation and inhibition affect different aspects of neuronal function and target distinct cellular compartments. For example, in sensory regions of neocortex, pyramidal neurons receive excitatory synaptic input from different sources on different portions of their dendritic trees (Petreanu et al., 2009). Here, as in the rest of the forebrain, specific populations of interneurons are specialized to regulate distinct subcellular compartments (Kepecs and Fishell, 2014). Therefore, the ratio between excitation and inhibition may vary from one cellular component to another. There is a paucity of studies that have addressed the effects of autism-related mutations on these different components of excitatory and inhibitory transmission. Nor is it straightforward to define a single physiological measurement that accurately captures the balance between excitation and inhibition. One recent study measured both the excitatory and inhibitory evoked synaptic input to visual cortical pyramidal neurons and found that although total input varied between cells the ratio of excitatory and inhibitory input was constant (Xue et al., 2014). Furthermore, perturbing the activity of pyramidal neurons perturbed this balance specifically by changing the strength of perisomatic inhibition mediated by parvalbumin-postive inteurneurons. Despite the complexities of defining and measuring the excitatory/inhibitory ratio, we think there are good reasons that the concept continues to be an influential one in thinking about the misregulation of brain circuits in developmental disorders. Since many of the signaling molecules and activity-dependent processes that affect excitatory synapses (and some classes of inhibitory synapses) are conserved across multiple brain regions, it is not unreasonable to suppose the existence of genetic conditions that could initially affect a distributed set of glutamatergic or GABAergic synapses. In addition, although we are trying to identify pathophysiological threads linking diverse ASDs as well as other developmental disorders, it is clear that these disorders are highly heterogeneous and may have unique mechanisms and consequences. We focus primarily on monogenic syndromes, even though these represent only about 10% of ASDs, because the ability to model these syndromes in animals allows testing of specific hypotheses about circuit dysfunction.
Evidence for primary inhibitory dysfunction in ASD
Autistic patients develop epilepsy at a rate up to 25 times that of the general population (Bolton et al., 2011). Epilepsy is the medical disorder most commonly associated with Autism, occurring in up to one third of affected individuals (Muhle et al., 2004). The prevalence of epileptiform EEG without overt seizures is even higher. Although the association may vary as diagnostic criteria for ASDs are altered, even the exclusion of particular populations such as patients with Rett Syndrome, who have especially high rates of epilepsy (e.g. 70%), is unlikely to dramatically reduce the overall association (Gilby and O’Brien, 2013).
Given the enormous genetic and phenotypic heterogeneity of ASDs, it is perhaps not surprising that individual syndromes vary in the typical age range of onset of epilepsy relative to other aspects of the disorder (Table 1). Some genetic causes of ASDs are virtually invariably associated with early onset epilepsy, and we hypothesize that these are more likely to reflect primary deficits in inhibition because of the lack of an asymptomatic period during which homeostatic compensation could develop. Rescue experiments showing the reversal of symptoms is the gold standard for establishing a causal impact. However, for developmental disorders that affect differentiation and cell migration, reversal could be difficult to achieve in an adult animal. In these cases, the etiology can be analyzed via modeling the disorder in a selective cellular subpopulation. This way, in a number of cases, the etiology can be traced rather directly to failures in the normal neurogenesis, migration, differentiation and/or function of cortical interneurons. One of the defining examples of such a syndrome involves mutation in the transcription factor Aristaless (ARX) in which major subsets of forebrain interneurons fail to migrate into the cortex from the medial ganglionic eminence leading to profound and early onset seizures and major disruptions of cognitive development (for review see Shoubridge et al., 2010). Knocking ARX out selectively in forebrain interneurons recapitulates many symptoms seen in human mutations (Marsh et al., 2009) leading to the idea of an “interneuronopathy” responsible for the epilepsy. Malformation phenotypes (e.g. agenesis of the corpus callosum) are not present in the interneuron-specific KO, presumably reflecting additional roles for this gene in other neuronal subtypes such as excitatory neuron progenitors that cause the malformation phenotype..
Table 1.
Summary of monogenic forms of autism
| Syndrome | Seizure onset | Molecular target | Clinical review | Molecular mechanism review |
|---|---|---|---|---|
| ARX mutations | Neonatal-4 months | ARX | Lux and Osborne, 2006 |
Olivetti and Noebels, 2012 Shoubridge et al., 2010 |
| Dravet Syndrome | First year of life | Scn1a/NAV1.1 | Brunklaus et al., 2012 | Oakley et al., 2011 |
| Tuberous Sclerosis | First year of life | TCS1/2 | Curatolo et al., 2008 | Crino, 2013; Lasarge and Danzer, 2014 |
| Fragile X | Between ages 4 and 10 years | FMRP | Heard et al., 2014 |
Bhakar et al., 2012 Darnell and Klann, 2013 Santoro et al., 2012 |
| Angelman Syndrome | Mean 1 year 1 month “85% of patients within the first three years of life, although less than 25% develop seizures during the first year” | Ube3a |
Valente et al., 2006 Laan and Vein, 2005 Thibert et al., 2013 |
Mabb et al., 2011 |
| Rett Syndrome | Between ages 2 and 5 years | MeCP2 |
Dolce et al., 2013 Neul et al., 2010 |
Guy et al., 2011 Katz et al., 2012 Samaco and Neul, 2011 |
| NRXN1 mutations | Varies with mutation | NRXN1 | Béna et al., 2013 | Südhof, 2008 |
| GPHN mutations | Varies with mutation | GPHN | Lionel et al., 2013 | Tyagarajan and Fritschy, 2014 |
| SHANK mutation, Phelan McDermid Syndrome | Increase with age for PM syndrome, not well characterized for mutations restricted to Shank3 | Shank1,2,3 |
Guilmatre et al., 2014 Sarasua et al., 2014 |
Jiang and Ehlers, 2013 |
| CNTNAP2 mutations | Between ages 2 and 7 years | CNTNAP2 |
Jackman et al., 2009 Lesca et al., 2012 |
Peñagarikano and Geschwind, 2012 |
Another syndrome in which severe childhood epilepsy is linked to autistic symptoms is Dravet’s Syndrome, usually caused by heterozygous loss of function of the sodium channel subunit Scn1a. Recent work indicates that knocking out one copy of the channel selectively in forebrain GABAergic neurons recapitulates all the major symptoms including seizures, hyperactivity, social dysfunction, anxiety, ataxia and sleep disorders (Cheah et al., 2012; Han et al., 2012; Ito et al., 2012; Tai et al., 2014). This appears to be a case in which a channelopathy produces an interneuronopathy since NaV1.1 (the protein product of Scn1a) is localized to the axon initial segments of parvalbumin positive (Pv+) fast-spiking and Somatostatin positive (SST) interneurons in the neocortex and hippocampus, as well as to purkinje neurons of the cerebellum, which also exhibit fast-spiking behavior. Loss of function of one allele of Scn1a prevents sustained fast spiking (FS) in Pv+ neurons (Ogiwara et al., 2007) and decreases seizure threshold even when this manipulation is largely restricted to these neurons (Dutton et al., 2012). Scn1a is only one of several sodium channel subunits which are upregulated in Pv+ FS interneurons during the period of development when they begin to exhibit fast-spiking behavior (Okaty et al., 2009) and several of these including scn1b, scn8a and scn9a have also been associated with Dravet Syndrome itself (Scn1b), or with modifying Dravet Syndrome susceptibility (scn9a) or with other epilepsy syndromes (Meisler et al., 2010). It is important to point out that even the conditional mutants used to analyze Dravet’s Syndrome and Arx, demonstrate only the sufficiency of the loss of function in specific inhibitory neurons to produce particular symptoms. It is not unlikely that for some symptoms of autistic patients with these mutations, alterations in other cells and circuits also contribute and indeed genetic background can influence cellular phenotypes of the haploinsufficiency in mice (Rubinstein et al., 2015) and contributions of the haploinsufficiency in subsets of excitatory neurons modify the seizure phenotype without producing effects on their own (Ogiwara et al., 2013). Demonstrating that the mutation in specific forebrain inhibitory neurons is not only sufficient, but also necessary would require selectively rescuing the behavioral phenotype by rescuing the effects of the mutation in those cells. Although genetic rescue experiments have not yet been performed, behavioral symptoms in a mouse model of Dravet’s syndrome are suppressed by pharmacological increase in GABAergic neurotransmission, pinning a deficit of inhibitory transmission as the cause of these symptoms (Han et al., 2012).
Another genetic disorder associated with seizures and autistic behaviors is Tuberous Sclerosis (TS), named for the presence of cortical malformations called tubers. The disorder is caused by mutations in Tsc1 (hamartin) and Tsc2 (tuberin), which together exist in a complex that inhibits mTOR (mammalian target of rapamycin) signaling, thereby regulating translational machinery and growth in many tissues. Epilepsy is present in the vast majority of patients and ~20–60% of TS patients meet diagnostic criteria for autism (Numis et al., 2011). The pathophysiology of this disorder is still far from clear. For example, although tubers have long been suspected to be the source of epileptic activity and are still removed surgically in TS patients with intractable epilepsy, a number of mouse models of the disorder present with spontaneous seizures, but lack tubers (Goorden et al., 2007; Lozovaya et al., 2014). In addition, recordings in patients suggest tubers are electrically silent, focusing the search for epileptic foci on surrounding tissue (Schwartzkroin and Wenzel, 2012). Deletion of Tsc1 in glia and/or neural progenitors produces seizures (for review see Wong and Crino, 2012), as does deletion in forebrain excitatory neurons, suggesting multiple potential pathways for generating seizures from loss of function of the TS complex. One recent study performed detailed physiological analyses following sparse cre-mediated deletion of a conditional Tsc1 allele in hippocampal neurons. Bateup and colleagues (Bateup et al., 2013) concluded that the primary, cell-autonomous deficit was a reduction in inhibitory input to pyramidal neurons. In addition to cell-autonomous postsynaptic effects (presumed to be on GABA receptors), reduced presynaptic release was also seen with more widespread deletion of Tsc1. Effects on neuronal excitability and on excitatory synapses were also present, but were in the wrong direction to produce circuit hyperexcitability and were instead presumed to reflect homeostatic responses of the circuit to abnormal activity. Although this study demonstrated critical effects of deleting Tsc1 in pyramidal neurons and largely ruled out a contribution to the observed results from loss of mTOR signaling in interneurons, another study recently demonstrated increased mortality and decreased seizure threshold in mice in which Tsc1 was selectively deleted from interneuron progenitors using a Dlx5/6 cre driver strain (Fu et al., 2011). Studies reporting positive effects of deleting Tsc1 in inhibitory neurons and glia highlight the difficulty of teasing apart primary from secondary effects and raise the possibility that disruption of core biological pathways, like the mTOR pathway, can lead to multiple primary effects in different cell types. The approach of cell type specific deletion can help clarify this situation. For example, the Bateup et al. study above reversed earlier conclusions from the same group that the network hyperexcitability was due to a primary deficit in LTD and a corresponding enhancement of excitatory synaptic transmission (Bateup et al., 2011). A similar “embarrassment of riches” in terms of primary and secondary effects is present for other ASDs that result from disruptions of pathways serving important roles in many cell types.
Like TS, Fragile X syndrome (FXS) and Angelman Syndrome (AS) target aspects of protein metabolism critical for synaptic function, and also like TS, both FXS and AS have been associated with abnormalities of both excitatory and inhibitory synaptic transmission. FMRP (Fragile X Mental Retardation Protein) is an RNA binding protein linked to trafficking and translation of synaptic proteins (Darnell et al., 2011) which when knocked out in mouse recapitulates many features of the disorder (Brennan et al., 2006; Musumeci et al., 2000; Bakker et al., 1994). One prominent theory of FXS (see also below) posits that FMRP loss of function leads to exaggerated long-term depression (LTD) of excitatory synapses (Bear et al., 2004). Abnormal long-term plasticity at excitatory synapses is the pathophysiological mechanism pursued in most studies, but large changes in GABAergic transmission have also been reported. For example, in the amygdala GABAergic transmission is reduced, as is the expression of GABA synthesis enzymes and some receptors, although the effects have a complex time course and vary across brain regions (Lozano et al., 2014). Disinhibition may also contribute to the abnormal plasticity seen at excitatory synapses, and the metabotropic glutamate receptors contributing to the pathophysiology of the disease may be located both on glutamatergic and GABAergic neurons (Paluszkiewicz et al., 2011).
Angelman Syndrome is a disorder of protein degradation, rather than synthesis. It is due in large part to loss of function of the E3 ubiquitin ligase UBE3A. In mice, as in humans, Ube3a is imprinted in the brain and the disease arises from loss of expression of the maternal allele. Although there is evidence for reduced excitatory synaptic transmission (see below), a recent study found diminished inhibition occurring late (P80) but not earlier (P25) (Wallace et al., 2012). The reduced inhibition was due to changes in the probability with which FS neurons contacted pyramidal neurons and to presynaptic reductions in the strength of these connections and those made by some other interneuron classes. Excitatory and inhibitory input to FS cells were unaffected. The numbers of interneurons of various classes and the inhibitory quantal amplitude were also not altered. Although genetic lesions restricted to Ube3a can produce AS, more commonly the disorder is caused by a larger maternal deletion affecting other nearby genes. In particular, haploinsufficency of Gabrb3 (a GABA-A beta subunit) is believed to contribute to the much more severe epilepsy seen in these patients than in those with lesions restricted to Ube3a (Tanaka et al., 2012).
A primary deficit in the regulation of inhibitory circuits has also recently been hypothesized to underlie Rett Syndrome (RTT). Initially, symptoms of RTT were believed to reflect primarily defects in glutamatergic (see below) as well as aminergic neurons (Samaco et al., 2009), but more recent work has suggested features of the disorder may arise from actions of MeCP2 in a wide variety of cell types including not only multiple subtypes of neurons, but also astrocytes (Lioy et al., 2011) and microglia (Derecki et al., 2012). Evidence that inhibitory circuits are involved comes most directly from KO of a conditional MeCP2 allele in all GABAergic neurons driven by a Viaat-Cre BAC transgenic (Chao et al., 2010). In keeping with physiological studies of respiratory function in global KO mice (Viemari et al., 2005) these mice have abnormalities of brainstem inhibitory circuits and develop respiratory insufficiency leading to premature death. Respiratory symptoms do not develop in mice in which forebrain-specific deletion of Mecp2 in GABAergic neurons is driven by a Dlx5/6-Cre strain, although this manipulation is reported to produce many other symptoms present in the Viaat cre KO including stereotypies, learning deficits and social abnormalities. Some of these symptoms likely reflect loss in striatal neurons, but changes in inhibitory synaptic transmission were also seen in neocortex, where GABA, its synthesis enzymes, and inhibitory quantal amplitude were reportedly reduced. Changes in inhibitory quantal amplitude were not observed in studies of Mecp2 global KOs (Dani et al., 2005; Nelson et al., 2006), perhaps reflecting differences in the preparations studied (L2/3 vs. L5 or hippocampal cultures). Intriguingly, forebrain selective KO in GABA neurons did not produce seizures or epileptiform activity suggesting that seizures in this disorder arise through a non-cell-autonomous effect on inhibition, or through enhanced excitation in some circuits. In another recent study, cell-type specific deletion of MeCP2 from excitatory neurons either through widely- or sparsely-expressing Cre in neocortex and hippocampus also resulted in hyperexcitation but through a decrease of inhibitory spontaneous and evoked neurotransmission onto MeCP2-deficient excitatory cells (Zhang et al., 2014). Both of these studies use cell-type specific knock-out approaches to conclude that in the absence of MeCP2, the primary defect is decrease of inhibition but whether this defect arises from the loss of MeCP2 function in excitatory, inhibitory or glial cells remains debated. One possibility is that, perhaps as for Tsc1, gene function is needed simultaneously in multiple cell types to maintain normal transmission.
Many of the ASD models described above involve primary alterations in the transcription, translation, trafficking or degradation of synaptic proteins. Other monogenic models of ASD implicate specific synaptic proteins themselves (Ebert and Greenberg, 2013; De Rubeis et al., 2014). These include ion channels and receptors (like the sodium channels, and glutamate and GABA receptor subunits described above), cell adhesion molecules, that promote the formation and function of synapses between specific classes of pre- and postsynaptic neurons, and synaptic scaffolding molecules that link components of synaptic signaling cascades. In some cases these molecules are differentially expressed at excitatory and inhibitory synapses providing insights into the likely initial pathophysiological deficit. For example, the synaptic cell adhesion molecules Neuroligins1-4 and their binding partners, Neurexins, have been implicated in ASD (Durand et al., 2007; Gauthier et al., 2009; Jamain et al., 2003; Laumonnier et al., 2004; Vaags et al., 2012). Neuroligin 1 is primarily localized to excitatory synapses (Song et al., 1999) while Neuroligin 2 is mostly present at inhibitory synapses (Varoqueaux et al., 2004), Neuroligin 3 is present at both (Budreck and Scheiffele, 2007), and Neuroligin 4 is localized to glycinergic synapses (Hoon et al., 2011). While both Neuroligin 3 and Neuroligin 4 mutations are associated with ASD, the mouse Neuroligin 4 gene exhibits a high degree of sequence divergence from the human orthologs (Bolliger et al., 2008), thus complicating the interpretation of the data from the mouse models. Nevertheless, evidence for primary inhibitory dysfunction comes from mice missing Neuroligins1-3, which have impaired evoked and spontaneous GABAergic/glycinergic transmission in the brainstem (Varoqueaux et al., 2006). Their presynaptic binding partners, Neurexins, also have multiple isoforms (Ullrich et al., 1995) and may differentially regulate synaptic function depending on the neurotransmitter type (Chih et al., 2006). Mutation in contactin associated protein-like 2(CNTNAP2), a protein from the Neurexin superfamily, causes childhood-onset epilepsy along with language regression, intellectual disability, hyperactivity and autism (Strauss et al., 2006). In a mouse model, loss of CNTNAP2 leads to hyperactivity and seizures, defects in neuronal migration, and a reduced number of GABAergic cells (Penagarikano et al., 2011). A related protein, CNTNAP4, is enriched in cortical interneurons and midbrain dopaminergic cells and localizes to synapses and is also associated with ASD (Fernandez et al., 2004; Roohi et al., 2009). Knock-out of this gene results in autistic-like repetitive behaviors and mild epileptiform-like activity in mice. While there is a decrease in the output of the PV-positive interneurons, an increase in dopaminergic neurotransmission is also observed (Karayannis et al., 2014).
Scaffolding proteins are responsible for clustering and localizing postsynaptic receptors and, like adhesion molecules, have synapse-type specificity. Gephyrin is the scaffolding molecule that localizes to the postsynaptic side of inhibitory synapses and controls GABA and glycine receptor localization and clustering (Tyagarajan and Fritschy, 2014). Deletions overlapping with the gephyrin genomic region are associated with ASD and seizure phenotypes (Dejanovic et al., 2014; Lionel et al., 2013). Reduction of Gephyrin levels expression with shRNA knockdown (Jacob et al., 2005) or a mutation (Kneussel et al., 1999) reduced GABA receptor clustering but not overall surface expression leading to decreased GABAergic and glycinergic synaptic currents (Kneussel et al., 1999).
Evidence for primary excitatory dysfunction in ASD
Contrary to Rubenstein and Merzenich’s original hypothesis, work on multiple animal models of ASDs and other developmental disorders suggest a shift in the balance between excitation and inhibition away from excitation. An early suggestion that Autism may be a hypoglutamatergic disorder (Carlsson, 1998) was based (as was a similar hypothesis for schizophrenia) on the preponderance of glutamatergic neurons in implicated brain structures including the amygdala, hippocampus and neocortex, and on the ability of some glutamate antagonists to mimic some symptoms. The complementary idea, namely an excess of inhibition, has been proposed to account for learning deficits in Down syndrome (Belichenko et al., 2009; Fernandez et al., 2007; Kleschevnikov et al., 2004), a disorder sharing many symptomatic components with ASD (Channell et al., 2015). For instance, DS patients also show restricted and repetitive behaviors (Evans et al., 2014), a core symptom in ASD (Leekam et al., 2011). We hypothesize that the similarity in symptoms of this developmental disorder with ASD might indicate the presence of analogous deficits in the brains of autistic patients.
Both enhanced inhibition and reduced excitation have been observed in mouse models of the developmental disorder Rett Syndrome (Dani et al., 2005) although the reduced excitation is a larger effect and has been replicated across a variety of preparations including synaptically connected neocortical pyramidal neurons from knockout mice (Dani and Nelson, 2009), hippocampal neurons cultured from knockout mice (Nelson et al., 2006) and induced pluripotent stem cells from human Rett patients (Marchetto et al., 2010). This effect is cell-autonomous as shown by sparse knock-down in cultured rat neocortical neurons (Blackman et al., 2012) or by recordings from autaptic cultures of hippocampal neurons from knockout mice (Chao et al., 2007). These experiments also link loss of Mecp2 in a specific cell population to the disease symptoms. In order to make a full connection, however, rescue experiments are necessary. In case of Rett syndrome, removing a stop codon or introducing a wild-type version of the gene late in development have been shown to alleviate some of the symptoms in the mouse models (Giacometti et al., 2007; Garg et al., 2013; Guy et al., 2007). However, these experiments are complicated by the need to match the precise level of the MeCP2 gene expression since too much can also lead to deleterious effects (Jiang et al., 2013; Petazzi et al., 2014). Several forms of excitatory synaptic plasticity have been reported to be altered following loss of function of Mecp2. Initial studies observed reductions in LTP at hippocampal and neocortical synapses (Asaka et al., 2006; Moretti et al., 2006), although subsequent work has suggested that this may be secondary to the shift in the ratio between excitation and inhibition, since LTP at neocortical synapses was normal when providing sufficient postsynaptic depolarization (Dani and Nelson, 2009). LTP is also reported to be blocked following overexpression of Mecp2, a manipulation which increases mEPSC frequency without apparent effects on inhibition (Na et al., 2012). Homeostatic synaptic plasticity also appears to be disrupted by loss of Mecp2. Synaptic scaling up in response to reduced activity (Blackman et al., 2012) and scaling down in response to elevated activity (Qiu et al., 2012; Zhong et al., 2012) are both reduced. For scaling up, this has been demonstrated both by cell autonomous knockdown in culture and in slices from knockout mice following sensory manipulations in vivo (Blackman et al., 2012). In the thalamus, strengthening and experience-dependent remodeling of retinogeniculate synapses is altered in Mecp2 KO mice, even though earlier phases of synaptic development in this pathway are normal (Noutel et al., 2011).
Given the fact that Mecp2 has important roles not only in GABAergic neurons and glutamatergic neurons, but also in other neuronal and nonneuronal cell types, how can the net effect on the excitability of brain circuits be predicted? Kron, Katz and colleagues have recently used immediate early gene expression to map overall activity levels throughout the brains of Mecp2 KO mice (Kron et al., 2012). The results are striking and show increased activity within the nucleus tractus solitarus, a structure known to be involved in cardiorespiratory symptoms of the disorder, but overall reduced activity within much of the forebrain including the cerebral cortex and subcortical limbic structures (Figure 1). Hence within the forebrain, reduced excitation appears to predominate over reduced inhibition. A new platform for carrying out this type of analysis brain-wide, but with cellular resolution, may improve our understanding of the net effects on activity of other mutations with complex biology (Kim et al., 2015).
Figure 1. Summary of brain activity mapping in Mecp2 mutant mice based on Fos expression.
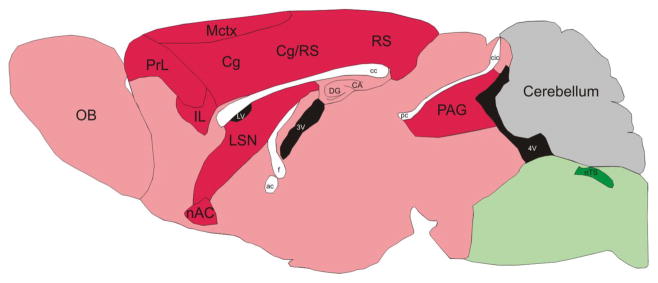
Low Fos expression in the motor cortex (Mctx) and adjacent regions indicates decreased activity while there is higher Fos expression in the nucleus of the solitary tract (nTS) and nearby areas. Differences in Fos expression in the Mecp2 null brain compared to wild-type are color coded as follows: Red, Null < Wt; Green, Null > Wt. Reproduced with permission from (Kron et al., 2012).
Abnormalities of excitatory synaptic function in Angelman Syndrome (AS) include changes in the threshold for LTP and LTD induction, more transient LTP, reduced density and size of dendritic spines, reduced mEPSC frequency, and reductions in the AMPA/NMDA ratio (for review see Mabb et al., 2011). Each of these impairments of excitatory synaptic transmission may be limited to particular sets of synapses, ages or conditions, but may contribute to impaired learning and sensory plasticity. Specific substrates of ubiquitination underlying these abnormalities include Arc, an immediate early gene important for multiple forms of Hebbian and homeostatic plasticity at excitatory synapses (Greer et al., 2010), and Ephexin-5 (Margolis et al., 2010) which regulates excitatory synapse number during development (Kaphzan et al., 2012).
Rodent models of TS were initially found to exhibit reduced LTP (Ehninger et al., 2008) but more recent studies have demonstrated a profound loss of protein synthesis-dependent, mGluR-mediated LTD (Auerbach et al., 2011; Bateup et al., 2011) leading to upregulation of excitatory synaptic transmission. However, as noted above, cell type specific knockout suggest these changes in excitation may be secondary to reduced inhibition. MGluR-mediated LTD is also the form of synaptic plasticity most closely implicated in the pathogenesis of FXS (Waung and Huber, 2009) and the two mutations were found to produce opposing effects on synaptic plasticity and learning behavior in mice (Auerbach et al., 2011). A recent sequencing study identified mTOR and Tsc2 as targets of FMRP and hypothesized that increased FMRP regulation is induced by upregulation of the mTOR pathway following loss of TSC signaling (Ascano et al., 2012). This would provide a mechanistic explanation for the ability of removal of fmr1 to normalize the phenotype of TS.
The idea that FXS is due to overactive LTD implies that excitatory synaptic transmission is weakened in this syndrome. There is ample evidence that this is the case, but the defects observed vary developmentally and with the specific pathway studied. In the somatosensory cortex, the first major intracortical relay linking L4 neurons to L2/3 is initially weaker and more diffuse but this regularizes with development (Bureau et al., 2008). At the same time, there is a potent and lasting reduction in the excitatory drive from L4 neurons onto FS interneurons, thereby disinhibiting L4 excitatory neurons (Gibson et al., 2008; Patel et al., 2013). The change in excitatory input to FS neurons is dependent on presynaptic FMRP expression as was also found in a separate study of the reduction in excitatory connectivity between CA3 neurons in slice culture (Hanson and Madison, 2007). Although similar in their reliance on presynaptic Fmr1, the two forms of excitatory synaptic weakening differ in that the former is due primarily to a reduction in the probability of release, while the latter is due to a reduced probability of connectivity. The two also differ in their presumed functional consequences as one will lead to disinhibition and circuit hyperexcitability, while the other will lead to reduced circuit excitability. Both are also likely distinct from the postsynaptic effects of Fmr1 on mGluR-dependent LTD. These studies highlight the circuit level complexities that arise when a single gene product can produce multiple distinct cellular and synaptic phenotypes when acting in different cell types or even in different compartments within the same cell.
Reduced excitation has also been posited as a key pathophysiological mechanism in the autistic syndromes associated with Shank3, PSD95 and a variety of associated PSD proteins. (For reviews of the PSD and synaptic protein involved in developmental disorders see (Ebert and Greenberg, 2013; Ting et al., 2012). Specifically, deletion of neurexin-1β in adult mice results in impaired glutamatergic transmission onto cortical pyramidal neurons (Rabaneda et al., 2014) and neurexin-1α deficient mice also have a decrease in excitatory synapse function (Etherton et al., 2009). Knock-in of a Neuroligin-3 mutation associated with autism, R704C, leads to a decrease in AMPA-mediated transmission with no effect on NMNDA or GABAergic neurotransmission in the hippocampus (Etherton et al., 2011).
The Neuroligin-Neurexin complex binds to the synaptic scaffolding proteins SHANK1-3 at the postsynaptic densities of excitatory synapses (Jiang and Ehlers, 2013). Deletions that encompass SHANK3 and other genes produce Phelan-McDermid syndrome associated with Autism, intellectual disability, hypotonia and seizures. These features are also present in deletions and other mutations restricted to Shank3. Autism-associated Shank3 mutant mice have normal basal synaptic transmission but have reduced post-tetanic potentiation and LTP in the CA1 area of the hippocampus which correlates with reductions in GluA1 subunits and other PSD proteins (Wang et al., 2011). Two independent models of Shank3 insufficiency found a decrease in excitatory synaptic transmission and plasticity in the hippocampus (Bozdagi et al., 2010) and the striatum (Peça et al., 2011). Intringuingly, duplications of Shank3 produce hyperactivity, mania and seizures in human patients and mice engineered with duplications display similar symptoms associated with enhanced excitatory and reduced inhibitory synaptic transmission (Han et al., 2013).
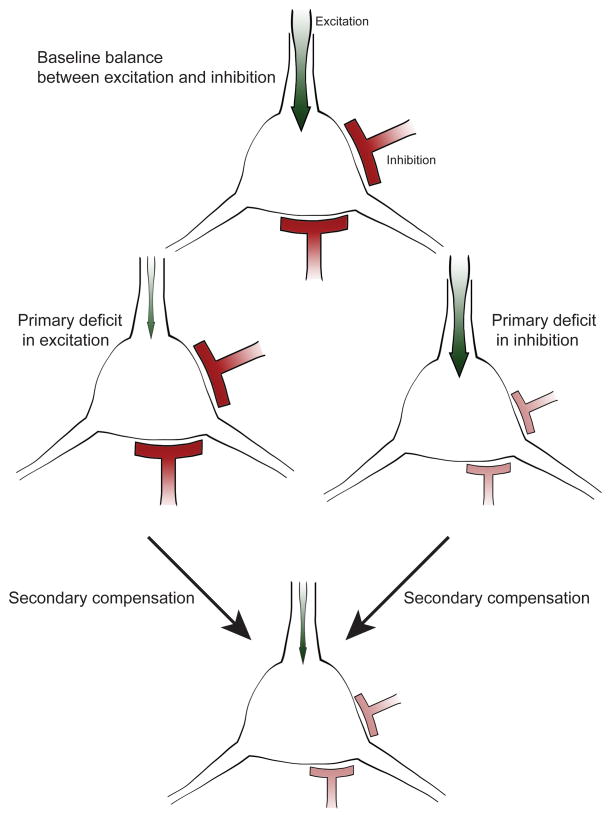
A homeostatic resolution
A common theme in the physiological studies of developmental disorders reviewed above is that both excitatory and inhibitory synapses are functionally altered. One difficulty in resolving this issue arises in part because single genes that play major roles in fundamental cellular processes like translation, RNA trafficking, protein degradation and the epigenetic regulation of transcription are likely to have complex effects in multiple cell types that together contribute to the network phenotype (Ramocki and Zoghbi, 2008), but this pleiotropy is only part of the problem. Another explanation for these disparities is that primary changes in excitation or inhibition alter network activity, and this change in activity can itself induce secondary changes. One particularly powerful set of secondary changes is that engaged by homeostatic plasticity mechanisms (Figure 2). Studies in a variety of invertebrate (Davis, 2006; Marder and Goaillard, 2006) and vertebrate (Pozo and Goda, 2010; Turrigiano, 2011; Turrigiano and Nelson, 2004) model systems have identified a family of mechanisms that adjust neuronal and synaptic function in order to homeostatically regulate circuit activity. These act to attempt to return network activity to a predetermined set point following perturbations of activity. They occur via changes in intrinsic neuronal excitability and in the strength and number of excitatory and inhibitory synapses. Given the existence of powerful homeostatic plasticity mechanisms, it can be difficult to separate primary effects of disease causing mutations from compensatory changes in circuit function. In some cases, like mutations of ARX, or Dravet’s Syndrome, it is fairly clear there are primary disruptions of critical functions of specific subtypes of GABAergic interneurons. In other cases, like those mutations in the SHANK proteins, or some of their PSD binding partners, the primary deficit is fairly clearly at excitatory synapses on glutamatergic neurons. In many other cases, however, it is still hard to discern which changes are the “chicken” and which are the “egg.”
Figure 2. Homeostatic compensation regulates excitation/inhibition ratio in cortical networks.
Proper neural network function relies on the balance between excitatory (green) and inhibitory (red) input. Primary defects in excitation or inhibition can be corrected via secondary compensatory mechanisms to restore balance and maintain network function. When a cell receives reduced excitation, secondary mechanisms down regulate the amount of inhibitory input onto this cell. Similarly, the excitatory input is decreased in response to a deficit in inhibition. Hence changes in both classes of synapses can appear similar following disease mechanisms that initially affect only one or the other.
Initial deficits and homeostatic responses could be hard to separate from the network and cellular homeostatic mechanisms. Sparse, cell-type specific knock-out strategies can be helpful for distinguishing cell autonomous from network effects (Bateup et al., 2013; Blackman et al., 2012; Chao et al., 2007; Zhang et al., 2014) while temporal control of deletion (Cheval et al., 2012) or rescue experiments (Garg et al., 2013; Giacometti et al., 2007; Guy et al., 2007) can overcome developmental compensation and uncover cell-autonomous homeostatic effects. However, even these refined genetic strategies must be bolstered by careful functional analysis to distinguish, for example, effects on output vs. input synapses, or even postsynaptic vs. retrograde presynaptic effects. A part of the issue is that the sparse cell-type specific studies are necessary to understand the primary deficit, but they will not recapitulate the behavioral phenotype. On the other hand, full knock-out approaches that do capture the behavioral aspects of a disorder lack the brain region selectivity and are confounded by network and homeostatic effects.
Understanding which homeostatic changes occur in ASD models is challenging even if the initial insult is known. Although there is an enormous diversity of synaptic connections, they may be broadly categorized in most brain areas into five main types of connections: glutamatergic synapses that excite other glutamatergic neurons (recurrent excitatory synapses), or that excite inhibitory neurons, GABAergic synapses that inhibit excitatory neurons, or that inhibit other GABAergic neurons (disinhibitory synapses); and finally modulatory synapses (such as monoaminergic synapses). Each major connection type is likely to have a separate set of homeostatic mechanisms that can be altered or spared by a single mutation in ASD. Molecular mechanisms specifying each connection type are likely to be modified independently of the other types. Below we review examples of the molecular mechanisms underlying these processes for each of the glutamatergic and GABAergic connection types. Homeostatic plasticity of modulatory neurons and synapses have been well studied in invertebrate model systems (see Marder and Goaillard 2006) but have been much less studied in mammals.
Recurrent excitation
Homeostatic mechanisms regulating recurrent excitatory connections were first studied in the neocortex, spinal cord, hippocampus and other structures (see Turrigiano and Nelson, 2004 for review). Molecular mechanisms that regulate the formation, function and modification of the excitatory synapses onto other excitatory cells have been extensively described. For example, cell adhesion proteins not only regulate the formation of excitatory synapses but also contribute to homeostatic changes that these synapses undergo (Thalhammer and Cingolani, 2014). Other studies have identified the kinases and phosphatases involved in homeostatic plasticity and have identified many of the core signaling pathways involved in multiple forms of plasticity. These include calcium signaling pathways such as calcineurin, which has been shown to regulate synaptic scaling (Kim and Ziff, 2014) and network adaptation to activity changes (Casanova et al., 2013), Ca(2+)/calmodulin-dependent protein kinase II and IV (Hell, 2014), which can target Ubiquitin-proteasome system to regulate synaptic strength (Djakovic et al., 2012). There are numerous other pathways and molecules that are important homeostatic regulators of the recurrent excitatory connections and are a subject of many good reviews. What is clear, however, is that many of these, like cell adhesion molecules, the ubiquitin-proteasome system, and calcium have been also implicated in ASD (Krey and Dolmetsch, 2007; Mabb et al., 2011; Yang et al., 2014).
Inhibition
Early studies revealed that inhibitory synapses also undergo homeostatic changes, although these in general occur in the opposite direction from changes at excitatory synapses (Kilman et al., 2002). A long literature beginning with studies by Jones and colleagues (Jones, 1993) has established that ongoing cortical activity is required for the normal development and maintenance of interneuron input and output synapses, intrinsic firing properties, morphology and expression of markers including GAD, parvalbumin and others. In a number of cases, these activity-dependent effects have been traced to release of BDNF from excitatory neurons (Hong et al., 2008; Peng et al., 2010; Woo and Lu, 2006), and deletion of TrkB, the high affinity receptor for BDNF, selectively in forebrain interneurons reduces their expression of key components of GABA synthesis and release such as GAD65. However, the signaling mechanisms by which activity in pyramidal neurons drives homeostatic changes in inhibition are more complex. First, BDNF itself has a plurality of actions including apparently cell-autonomous effects on spine density in excitatory neurons (English et al., 2012) as well as acute suppressive effects on inhibitory transmission (Frerking et al., 1998), both of which may contribute to its net effects in some preparations being pro-epileptic, rather than homeostatic (McNamara and Scharfman, 2012). Second, BDNF is not the only activity-dependent signaling molecule regulating forebrain inhibitory circuits, (see below). Some of the complexity of BDNF signaling reflects the complexity of its transcriptional regulation. BDNF is transcribed from 8 different promoters. One of these, promoter IV, is the major activity-dependent promoter active in the neocortex. Selective disruption of promoter IV (Jiao et al., 2011; Sakata et al., 2009) or of the ability of the activity dependent transcription factor CREB to bind to promoter IV (Hong et al., 2008) selectively reduces inhibitory, but not excitatory synaptic transmission. Synapses from parvalbumin-positive interneurons are selectively disrupted (Jiao et al., 2011). Presumably, it is this signaling pathway that permits the precise matching of PV+ inhibitory to excitatory synaptic strength onto pyramidal cells, and which permits this balance to be adjusted following perturbations that alter sensory drive or pyramidal neuron activity (House et al., 2011; Xue et al., 2014).
Altered BDNF signaling has been implicated in mouse models of Fragile X, Rett, and Angelman Syndrome (Cao et al., 2013; Chang et al., 2006; Lauterborn et al., 2007). These are among the disorders that are characterized by a period of normal development followed by regression and later seizure onset (Table 1). The case of Rett Syndrome illustrates the complexity of understanding how disease causing mutations interact with activity-dependent signaling pathways. BDNF was first identified as a direct target of repression by the DNA-binding protein MeCP2, the gene mutated in the overwhelming majority of Rett Syndrome cases. Studies in cultured neurons confirmed that activity (depolarization) caused phosphorylation of MeCP2, which, in turn, derepressed BDNF expression (Zhou et al., 2006). Subsequent in vivo studies found, however, that rather than contributing to the symptoms of Mecp2 KO mice, BDNF overexpression alleviated them, while reduced BDNF signaling exacerbated them (Chang et al., 2006). Presumably, in vivo, diminished activity-dependent release of BDNF (due to reduced activity) outweighs the direct derepression mediated by loss of MeCP2. This pathway could account for the cell autonomous ability of deleting Mecp2 (Zhang et al., 2014) or possibly TSC1 (Bateup et al., 2013) to reduce inhibitory input to pyramidal neurons.
Another molecule that links excitatory network activity with structural and functional changes in inhibitory function is the transcription factor NPAS4 (Lin et al., 2008; Ramamoorthi et al., 2011). Upregulation of NPAS4 following activity in CA1 pyramidal neurons results in enhancement of inhibitory connections onto these excitatory neurons through transcription of late-response genes, including Bdnf, (Lin et al., 2008). Thus, activity in the excitatory cells is essential for proper inhibitory synapse formation onto these cells. Consistent with this, NPAS4 knock-out mice appear to be hyperactive, are prone to seizures and have deficits in social behaviors and cognitive functions (Coutellier et al., 2012; Lin et al., 2008). On the other hand, NPAS4 is also upregulated and induces a distinct transcriptional program following activity in inhibitory neurons, which then increases excitatory input onto these inhibitory cells (Spiegel et al., 2014). Thus, the same transcription factor regulates synaptic function in excitatory and inhibitory cells to achieve a homeostatic resolution in response to increased activity. Presumably, there are additional factors yet to be identified, contributing to activity-dependent regulation of cortical inhibition, particularly with respect to other subtypes of interneurons besides FS cells.
Excitatory synapses onto inhibitory cells
The strength of inhibition depends not only on regulation of pre- and postsynaptic properties of inhibitory synapses, but also on the excitatory synapses driving the firing of inhibitory neurons. The best characterized inter-cellular signaling mechanism regulating these synapses is that initiated by binding of the ligand neuregulin 1 (Nrg1) to its receptor ErbB4 on parvalbumin positive, fast-spiking (FS) interneurons. During early cortical development, ErbB4 is critical for the proper migration of FS interneurons. Subsequently ErbB4 regulates both the inhibitory outputs and excitatory inputs of FS neurons (for reviews see Buonanno, 2010; Rico and Marín, 2011). Specifically, loss of ErbB4 in interneurons decreases the number of axo-axonic synapses made by chandelier cells, and decreases the number of excitatory synapses made onto FS neurons. Conversely, overexpression of Nrg1 by pyramidal neurons or treatment with exogenous Nrg1 can increase axo-axonic inhibitory synapses and increase excitatory synapses onto FS neurons. A subsequent study in prefrontal cortex confirmed a critical role for ErbB4 in regulating excitatory synapses onto FS interneurons, but failed to find an effect of embryonic interneuron-specific deletion of the receptor on interneuron migration or on interneuron output synapses (Yang et al., 2013). Nrg1-ErB4 signaling may also acutely regulate GABA release (Woo et al., 2007) and interneuron excitability (Li et al., 2012; Tan et al., 2012).
Mutations in Nrg1 and ErbB4 are associated with schizophrenia and deletion of ErbB4 has also recently been identified in a case of profound developmental delay in speech and cognitive abilities (Kasnauskiene et al., 2013). Studies of mutant mice have also implicated enhanced Nrg1/ErbB4 signaling in AS (Kaphzan et al., 2012). The recent history of attempts to unravel how this pathway contributes to schizophrenia and cognitive function is illustrative of the difficulty of disentangling direct and secondary effects of disruptions in one leg of the excitatory-inhibitory circuit in cortex. Initial studies focused on the role played by ErbBr and Nrg1 on excitatory synaptic transmission between pyramidal neurons. ErbB4 is present at glutamatergic synapses onto FS neurons and interacts biochemically with PSD-95. Loss of function studies suggested loss of ErbB4 caused reduction in the number of dendritic spines (Barros et al., 2009) and alterations of excitatory synapse function (Li et al., 2007). Application of Nrg1 could block the induction or expression of hippocampal LTP and could depotentiate LTP after induction (Kwon et al., 2005). Careful studies excluding expression of ErbB4 in pyramidal neurons of neocortex and hippocampus led several to conclude that perhaps the effects of Nrg1 and ErbB4 on excitatory synapses were not cell autonomous. Recently two groups directly tested for the depotentiation phenotype. Both found that conditional KO of ErbB4 restricted to interneurons blocked the ability of Nrg1 to produce depotentiation of excitatory synapses. Although the same genetic tests have not been carried out for changes in spines, it has been suggested that these effects are also not cell autonomous (Rico and Marín, 2011).
Disinhibition
Although numerous studies have highlighted the importance of GABAergic interneurons for regulating network activity in the forebrain, very few of these have focused on inhibitory connections between interneurons. Early paired recording studies demonstrated that within major interneuron classes cells are linked both by electrical (gap junction) and chemical (inhibitory) synapses (Galarreta and Hestrin, 1999; Gibson et al., 1999). Disinhibitory synapses between interneurons of distinct classes have also recently been studied. Somatostatin-expressing interneurons disinhibit cortical pyramidal neurons by directly inhibiting fast-spiking parvalbumin-positive interneurons in layer 4 (Xu et al., 2013). In auditory and medial prefrontal cortices, vasoactive intestinal peptide-expressing interneurons provide inhibition to somatostatin and parvalbumin-positive interneurons (Pi et al., 2013). In the visual cortex, parvalbumin-expressing interneurons inhibit each other while somatostatin-expressing interneurons strongly inhibit other types of inhibitory neurons (Pfeffer et al., 2013). The behavioral and network effects of deficits in disinhibition are not well studied although there is evidence that disinhibition is important for network synchrony (Hu et al., 2011) and associative fear learning (Letzkus et al., 2011). Unfortunately, there is very little known about homeostatic regulation of these various types of disinhibitory connections.
Circuit Homeostasis in ASD: inadequate, maladaptive and targeted
The mechanisms mediating homeostatic plasticity are diverse and include at a minimum changes in intrinsic excitability resulting from changing numbers, properties or localization of voltage gated ion channels; pre- and postsynaptic changes in the strength of excitatory and inhibitory synapses, and changes in the numbers of these synapses. Given this large number of homeostatic mechanisms, one might wonder why they fail to regularize activity during developmental disorders? Why aren’t primary changes in inhibitory or excitatory circuits fully compensated by changes in other parts of the network? Here we consider three possible explanations.
First, compensation may be initially adequate but may fail as developmental abnormalities accumulate. This could occur if the magnitude of the relevant homeostatic mechanisms are insufficient relative to the magnitude of the initial pathological change (e.g. scaling up of excitatory synapses in response to global reductions in activity is typically 25–50% over the course of a 1–2 day activity manipulation). The inability of compensation to keep up may explain, for example, why symptoms in some ASDs are relatively mild in early development and only become pronounced later (Table 1). In addition, changes in activity may engage Hebbian synaptic plasticity, which acts to enhance or decrease synaptic transmission in the same direction as the change in the input (i.e. strengthening synapses between highly active neurons and weakening those between inactive or less active neurons). Thus, Hebbian plasticity mechanisms will act in the opposite direction of homeostatic compensation and exacerbate the defect. For example, if inhibitory transmission is reduced, this is likely to enhance the ability of excitatory synapses to undergo Hebbian LTP which will in turn create a further imbalance in activity making compensation all the more difficult. This nonlinear interaction could contribute to the frequent switch-like onset and subsequent persistence of seizures, although the question of how or even whether “seizures beget seizures” is controversial (Ben-Ari, 2008; Blume, 2006; Hauser and Lee, 2002). Perhaps more importantly, compensation mechanisms may themselves be targets of the underlying pathology. For example, at least some of the complex single gene disorders (Rett, FRX, TS, ARX) result in blockade of synaptic scaling. As noted above, this has been demonstrated for Rett. For FRX, blockade of scaling up has also been described (Soden and Chen, 2010). Other studies have shown that both pre (Doyle et al., 2010) and postsynaptic (Goold and Nicoll, 2010) forms of scaling down depend on transcription and/or protein synthesis and so might be expected to be altered by disorders that affect translation and RNA and protein stability.
A second reason that homeostatic mechanisms may not alleviate developmental disorders is that they can become maladaptive. For example, since inhibitory neurons receive input from excitatory neurons, in response to a decrease in excitatory activity, the output of the inhibitory neurons is also decreased via direct loss of input or through secondary homeostatic changes to maintain the balance of inhibition and excitation (Hartman et al., 2006; Xue et al., 2014). This response, initially helpful, can potentially move the excitation/inhibition ratio past the balance point and result in the opposite problem: too little inhibition to prevent runaway synchronous activity (Figure 3). Theoretical studies have shown that epilepsy-like bursting activity in isolated neocortex following loss of afferent signals can be accounted for by homeostatic plasticity (Houweling et al., 2005). In addition, a similar phenomenon has been demonstrated in a central pain syndrome model in which a spinothalamic tract lesion results in hyperexcitation in the thalamus (Wang and Thompson, 2008). We hypothesize that late seizures occurring in developmental disorders like Rett Syndrome that are characterized by overall decreases in forebrain activity may reflect this kind of maladaptive compensation.
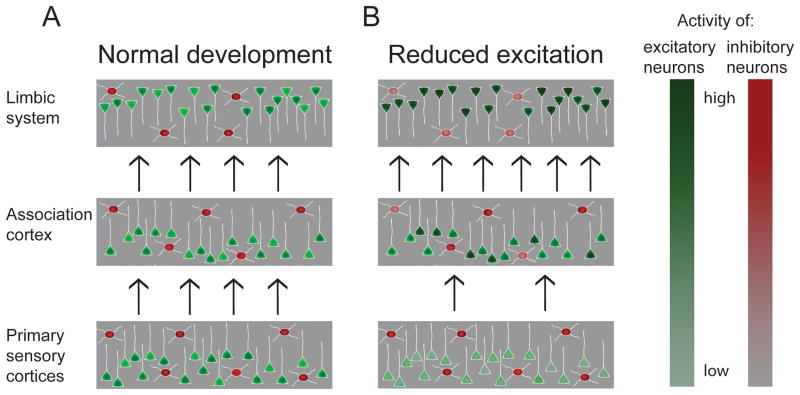
Figure 3. Faithful signal propagation in multilayered cortical networks may require higher order layers to compensate for altered activity in lower layers.
Cortical networks can be schematized as interconnected layers of neurons. Activity in the “input layer” of primary sensory cortices is driven by sensory inputs, while activity in higher order association and limbic regions depends to a greater degree on activity in preceding layers. (A) During normal development excitation and inhibition are balanced to preserve appropriate activity levels across synaptically connected brain regions with the activity of the cells in each layer adjusted to the amount of input this layer receives. (B) If the balance is perturbed so that, for example, input layers have reduced activity (indicated by normal red inhibitory but reduced green excitatory activity), homeostatic mechanisms compensate for the defect and upregulate the excitability of circuits in higher order Association and Limbic regions (indicated by a darker shade of green and lighter shade of red in some neurons) in an attempt to maintain normal levels of propagating activity. However, if not perfectly balanced, this can lead to overactivity in higher order regions coexisting with reduced activity in lower order regions. Networks in higher order regions with enhanced excitation and reduced inhibition may be brittle and prone to develop epileptiform activity.
Lastly, in a multilayered network, like that linking peripheral sensory structures to the thalamus to primary sensory cortices and then to multiple levels of higher order cortices, the faithful propagation of signals from one layer to the next requires that excitation, inhibition and intrinsic excitability in each layer be rather precisely balanced so that the overall gain of signal propagation is close to one. If this “gain” is significantly below one, propagation is likely to fail prior to reaching the higher order regions; if the gain is significantly above one, the signal is likely to saturate and may lead to hyper-synchrony and epilepsy (Figure 3; see also Turrigiano and Nelson, 2004). This scenario becomes even more likely when the homeostatic machinery is compromised by the mutation that caused the imbalance in the first place (Toro et al., 2010; Wondolowski and Dickman, 2013). It has long been known that different forebrain regions have differing propensities to generate seizures, with the highest propensity occurring in the hippocampus and other limbic structures (McCormick and Contreras, 2001). This may reflect differences in circuitry, but may also reflect the relative balance between Hebbian plasticity mechanisms that enhance circuit excitability and homeostatic plasticity mechanisms that dampen circuit excitability. We hypothesize that as developmental disorders progress, homeostatic mechanisms are insufficient to restore normal activity in early cortical areas and are actually maladaptive in higher order limbic regions like the hippocampus, where reduced excitatory drive leads to trophic down-regulation of inhibitory circuits that normally prevent epileptic activity from developing (Figure 3). Developing a more complete understanding of the signaling pathways and effector molecules important for various forms of homeostatic plasticity may allow this hypothesis to be tested. In addition, such an understanding may provide strategies for selectively enhancing or restoring homeostatic plasticity where it is beneficial and/or inhibiting maladaptive forms of plasticity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ben-Ari Y. Epilepsies and neuronal plasticity: for better or for worse? Dialogues Clin Neurosci. 2008;10:17–27. doi: 10.31887/DCNS.2008.10.1/ybenari. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaka Y, Jugloff DG, Zhang L, Eubanks JH, Fitzsimonds RM. Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol Dis. 2006;21:217–227. doi: 10.1016/j.nbd.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Ascano M, Jr, Mukherjee N, Bandaru P, Miller JB, Nusbaum JD, Corcoran DL, Langlois C, Munschauer M, Dewell S, Hafner M, et al. FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature. 2012;492:382–386. doi: 10.1038/nature11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–68. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros CS, Calabrese B, Chamero P, Roberts AJ, Korzus E, Lloyd K, Stowers L, Mayford M, Halpain S, Müller U. Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. PNAS. 2009;106:4507–4512. doi: 10.1073/pnas.0900355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup HS, Takasaki KT, Saulnier JL, Denefrio CL, Sabatini BL. Loss of Tsc1 in vivo impairs hippocampal mGluR-LTD and increases excitatory synaptic function. J Neurosci. 2011;31:8862–8869. doi: 10.1523/JNEUROSCI.1617-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup HS, Johnson CA, Denefrio CL, Saulnier JL, Kornacker K, Sabatini BL. Excitatory/Inhibitory Synaptic Imbalance Leads to Hippocampal Hyperexcitability in Mouse Models of Tuberous Sclerosis. Neuron. 2013;78:510–522. doi: 10.1016/j.neuron.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends in Neurosciences. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Belichenko PV, Kleschevnikov AM, Masliah E, Wu C, Takimoto-Kimura R, Salehi A, Mobley WC. Excitatory-inhibitory relationship in the fascia dentata in the Ts65Dn mouse model of Down syndrome. J Comp Neurol. 2009;512:453–466. doi: 10.1002/cne.21895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béna F, Bruno DL, Eriksson M, van Ravenswaaij-Arts C, Stark Z, Dijkhuizen T, Gerkes E, Gimelli S, Ganesamoorthy D, Thuresson AC, et al. Molecular and clinical characterization of 25 individuals with exonic deletions of NRXN1 and comprehensive review of the literature. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:388–403. doi: 10.1002/ajmg.b.32148. [DOI] [PubMed] [Google Scholar]
- Bhakar AL, Dölen G, Bear MF. The pathophysiology of fragile X (and what it teaches us about synapses) Annu Rev Neurosci. 2012;35:417–443. doi: 10.1146/annurev-neuro-060909-153138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman MP, Djukic B, Nelson SB, Turrigiano GG. A critical and cell-autonomous role for MeCP2 in synaptic scaling up. J Neurosci. 2012;32:13529–13536. doi: 10.1523/JNEUROSCI.3077-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume WT. The progression of epilepsy. Epilepsia. 2006;47(Suppl 1):71–78. doi: 10.1111/j.1528-1167.2006.00665.x. [DOI] [PubMed] [Google Scholar]
- Bolliger MF, Pei J, Maxeiner S, Boucard AA, Grishin NV, Südhof TC. Unusually rapid evolution of Neuroligin-4 in mice. PNAS. 2008;105:6421–6426. doi: 10.1073/pnas.0801383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton PF, Carcani-Rathwell I, Hutton J, Goode S, Howlin P, Rutter M. Epilepsy in autism: features and correlates. Br J Psychiatry. 2011;198:289–294. doi: 10.1192/bjp.bp.109.076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, Kajiwara Y, Yang M, Katz AM, Scattoni ML, et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan FX, Albeck DS, Paylor R. Fmr1 knockout mice are impaired in a leverpress escape/avoidance task. Genes Brain Behav. 2006;5:467–471. doi: 10.1111/j.1601-183X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- Brunklaus A, Ellis R, Reavey E, Forbes GH, Zuberi SM. Prognostic, clinical and demographic features in SCN1A mutation-positive Dravet syndrome. Brain. 2012;135:2329–2336. doi: 10.1093/brain/aws151. [DOI] [PubMed] [Google Scholar]
- Budreck EC, Scheiffele P. Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. Eur J Neurosci. 2007;26:1738–1748. doi: 10.1111/j.1460-9568.2007.05842.x. [DOI] [PubMed] [Google Scholar]
- Buonanno A. The neuregulin signaling pathway and schizophrenia: from genes to synapses and neural circuits. Brain Res Bull. 2010;83:122–131. doi: 10.1016/j.brainresbull.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau I, Shepherd GM, Svoboda K. Circuit and plasticity defects in the developing somatosensory cortex of FMR1 knock-out mice. J Neurosci. 2008;28:5178–5188. doi: 10.1523/JNEUROSCI.1076-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Rioult-Pedotti MS, Migani P, Yu CJ, Tiwari R, Parang K, Spaller MR, Goebel DJ, Marshall J. Impairment of TrkB-PSD-95 Signaling in Angelman Syndrome. PLoS Biol. 2013;11:e1001478. doi: 10.1371/journal.pbio.1001478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson ML. Hypothesis: is infantile autism a hypoglutamatergic disorder? Relevance of glutamate - serotonin interactions for pharmacotherapy. J Neural Transm. 1998;105:525–535. doi: 10.1007/s007020050076. [DOI] [PubMed] [Google Scholar]
- Casanova JR, Nishimura M, Le J, Lam TT, Swann JW. Rapid hippocampal network adaptation to recurring synchronous activity--a role for calcineurin. Eur J Neurosci. 2013;38:3115–3127. doi: 10.1111/ejn.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellot G, Cherubini E. GABAergic Signaling as Therapeutic Target for Autism Spectrum Disorders. Front Pediatr. 2014;2 doi: 10.3389/fped.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The Disease Progression of Mecp2 Mutant Mice Is Affected by the Level of BDNF Expression. Neuron. 2006;49:341–348. doi: 10.1016/j.neuron.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Channell MM, Phillips BA, Loveall SJ, Conners FA, Bussanich PM, Klinger LG. Patterns of autism spectrum symptomatology in individuals with Down syndrome without comorbid autism spectrum disorder. J Neurodev Disord. 2015;7 doi: 10.1186/1866-1955-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HT, Zoghbi HY, Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron. 2007;56:58–65. doi: 10.1016/j.neuron.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah CS, Yu FH, Westenbroek RE, Kalume FK, Oakley JC, Potter GB, Rubenstein JL, Catterall WA. Specific deletion of NaV1.1 sodium channels in inhibitory interneurons causes seizures and premature death in a mouse model of Dravet syndrome. Proc Natl Acad Sci U S A. 2012;109:14646–14651. doi: 10.1073/pnas.1211591109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheval H, Guy J, Merusi C, De Sousa D, Selfridge J, Bird A. Postnatal inactivation reveals enhanced requirement for MeCP2 at distinct age windows. Hum Mol Genet. 2012;21:3806–3814. doi: 10.1093/hmg/dds208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B, Gollan L, Scheiffele P. Alternative Splicing Controls Selective Trans-Synaptic Interactions of the Neuroligin-Neurexin Complex. Neuron. 2006;51:171–178. doi: 10.1016/j.neuron.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Coutellier L, Beraki S, Ardestani PM, Saw NL, Shamloo M. Npas4: A Neuronal Transcription Factor with a Key Role in Social and Cognitive Functions Relevant to Developmental Disorders. PLoS ONE. 2012;7:e46604. doi: 10.1371/journal.pone.0046604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino PB. Evolving neurobiology of tuberous sclerosis complex. Acta Neuropathol. 2013;125:317–332. doi: 10.1007/s00401-013-1085-x. [DOI] [PubMed] [Google Scholar]
- Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. The Lancet. 2008;372:657–668. doi: 10.1016/S0140-6736(08)61279-9. [DOI] [PubMed] [Google Scholar]
- Dani VS, Nelson SB. Intact long-term potentiation but reduced connectivity between neocortical layer 5 pyramidal neurons in a mouse model of Rett syndrome. J Neurosci. 2009;29:11263–11270. doi: 10.1523/JNEUROSCI.1019-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2005;102:12560–12565. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Klann E. The Translation of Translational Control by FMRP: Therapeutic Targets for Fragile X Syndrome. Nat Neurosci. 2013;16:1530–1536. doi: 10.1038/nn.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KYS, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- Dejanovic B, Lal D, Catarino CB, Arjune S, Belaidi AA, Trucks H, Vollmar C, Surges R, Kunz WS, Motameny S, et al. Exonic microdeletions of the gephyrin gene impair GABAergic synaptic inhibition in patients with idiopathic generalized epilepsy. Neurobiology of Disease. 2014;67:88–96. doi: 10.1016/j.nbd.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Derecki NC, Cronk JC, Lu Z, Xu E, Abbott SB, Guyenet PG, Kipnis J. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484:105–109. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djakovic SN, Marquez-Lona EM, Jakawich SK, Wright R, Chu C, Sutton MA, Patrick GN. Phosphorylation of Rpt6 Regulates Synaptic Strength in Hippocampal Neurons. J Neurosci. 2012;32:5126–5131. doi: 10.1523/JNEUROSCI.4427-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolce A, Ben-Zeev B, Naidu S, Kossoff EH. Rett Syndrome and Epilepsy: An Update for Child Neurologists. Pediatric Neurology. 2013;48:337–345. doi: 10.1016/j.pediatrneurol.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Doyle S, Pyndiah S, De Gois S, Erickson JD. Excitation-transcription coupling via calcium/calmodulin-dependent protein kinase/ERK1/2 signaling mediates the coordinate induction of VGLUT2 and Narp triggered by a prolonged increase in glutamatergic synaptic activity. J Biol Chem. 2010;285:14366–14376. doi: 10.1074/jbc.M109.080069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsäter H, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton SB, Makinson CD, Papale LA, Shankar A, Balakrishnan B, Nakazawa K, Escayg A. Preferential inactivation of Scn1a in parvalbumin interneurons increases seizure susceptibility. Neurobiol Dis. 2012;49C:211–220. doi: 10.1016/j.nbd.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert DH, Greenberg ME. Activity-dependent neuronal signalling and autism spectrum disorder. Nature. 2013;493:327–337. doi: 10.1038/nature11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2+/|[minus]| mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English CN, Vigers AJ, Jones KR. Genetic evidence that brain-derived neurotrophic factor mediates competitive interactions between individual cortical neurons. Proc Natl Acad Sci U S A. 2012;109:19456–19461. doi: 10.1073/pnas.1206492109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton MR, Blaiss CA, Powell CM, Südhof TC. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci USA. 2009;106:17998–18003. doi: 10.1073/pnas.0910297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton MR, Tabuchi K, Sharma M, Ko J, Südhof TC. An autism-associated point mutation in the neuroligin cytoplasmic tail selectively impairs AMPA receptor-mediated synaptic transmission in hippocampus. EMBO J. 2011;30:2908–2919. doi: 10.1038/emboj.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DW, Kleinpeter FL, Slane MM, Boomer KB. Adaptive and Maladaptive Correlates of Repetitive Behavior and Restricted Interests in Persons with Down Syndrome and Developmentally-Matched Typical Children: A Two-Year Longitudinal Sequential Design. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez F, Morishita W, Zuniga E, Nguyen J, Blank M, Malenka RC, Garner CC. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci. 2007;10:411–413. doi: 10.1038/nn1860. [DOI] [PubMed] [Google Scholar]
- Fernandez T, Morgan T, Davis N, Klin A, Morris A, Farhi A, Lifton RP, State MW. Disruption of Contactin 4 (CNTN4) Results in Developmental Delay and Other Features of 3p Deletion Syndrome. Am J Hum Genet. 2004;74:1286–1293. doi: 10.1086/421474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, Nicoll RA. Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. J Neurophysiol. 1998;80:3383–3386. doi: 10.1152/jn.1998.80.6.3383. [DOI] [PubMed] [Google Scholar]
- Fu C, Cawthon B, Clinkscales W, Bruce A, Winzenburger P, Ess KC. GABAergic Interneuron Development and Function Is Modulated by the Tsc1 Gene. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- Garg SK, Lioy DT, Cheval H, McGann JC, Bissonnette JM, Murtha MJ, Foust KD, Kaspar BK, Bird A, Mandel G. Systemic Delivery of MeCP2 Rescues Behavioral and Cellular Deficits in Female Mouse Models of Rett Syndrome. J Neurosci. 2013;33:13612–13620. doi: 10.1523/JNEUROSCI.1854-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier J, Spiegelman D, Piton A, Lafrenière RG, Laurent S, St-Onge J, Lapointe L, Hamdan FF, Cossette P, Mottron L, et al. Novel de novo SHANK3 mutation in autistic patients. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:421–424. doi: 10.1002/ajmg.b.30822. [DOI] [PubMed] [Google Scholar]
- Giacometti E, Luikenhuis S, Beard C, Jaenisch R. Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2. PNAS. 2007;104:1931–1936. doi: 10.1073/pnas.0610593104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Bartley AF, Hays SA, Huber KM. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J Neurophysiol. 2008;100:2615–2626. doi: 10.1152/jn.90752.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilby KL, O’Brien TJ. Epilepsy, autism, and neurodevelopment: kindling a shared vulnerability? Epilepsy Behav. 2013;26:370–374. doi: 10.1016/j.yebeh.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Giocomo LM, Hasselmo ME. Neuromodulation by glutamate and acetylcholine can change circuit dynamics by regulating the relative influence of afferent input and excitatory feedback. Mol Neurobiol. 2007;36:184–200. doi: 10.1007/s12035-007-0032-z. [DOI] [PubMed] [Google Scholar]
- Goold CP, Nicoll RA. Single-cell optogenetic excitation drives homeostatic synaptic depression. Neuron. 2010;68:512–528. doi: 10.1016/j.neuron.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorden SMI, van Woerden GM, van der Weerd L, Cheadle JP, Elgersma Y. Cognitive deficits in Tsc1+/− mice in the absence of cerebral lesions and seizures. Ann Neurol. 2007;62:648–655. doi: 10.1002/ana.21317. [DOI] [PubMed] [Google Scholar]
- Greer PL, Hanayama R, Bloodgood BL, Mardinly AR, Lipton DM, Flavell SW, Kim TK, Griffith EC, Waldon Z, Maehr R, et al. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140:704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilmatre A, Huguet G, Delorme R, Bourgeron T. The emerging role of SHANK genes in neuropsychiatric disorders. Dev Neurobiol. 2014;74:113–122. doi: 10.1002/dneu.22128. [DOI] [PubMed] [Google Scholar]
- Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Cheval H, Selfridge J, Bird A. The role of MeCP2 in the brain. Annu Rev Cell Dev Biol. 2011;27:631–652. doi: 10.1146/annurev-cellbio-092910-154121. [DOI] [PubMed] [Google Scholar]
- Han K, Holder JL, Schaaf CP, Lu H, Chen H, Kang H, Tang J, Wu Z, Hao S, Cheung SW, et al. SHANK3 overexpression causes manic-like behaviour with unique pharmacogenetic properties. Nature. 2013;503:72–77. doi: 10.1038/nature12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, Potter GB, Rubenstein JL, Scheuer T, de la Iglesia HO, Catterall WA. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–390. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JE, Madison DV. Presynaptic FMR1 genotype influences the degree of synaptic connectivity in a mosaic mouse model of fragile X syndrome. J Neurosci. 2007;27:4014–4018. doi: 10.1523/JNEUROSCI.4717-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman KN, Pal SK, Burrone J, Murthy VN. Activity-dependent regulation of inhibitory synaptic transmission in hippocampal neurons. Nat Neurosci. 2006;9:642–649. doi: 10.1038/nn1677. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Lee JR. Do seizures beget seizures? Prog. Brain Res. 2002;135:215–219. doi: 10.1016/s0079-6123(02)35021-0. [DOI] [PubMed] [Google Scholar]
- Heard TT, Ramgopal S, Picker J, Lincoln SA, Rotenberg A, Kothare SV. EEG abnormalities and seizures in genetically diagnosed Fragile X syndrome. Int J Dev Neurosci. 2014;38:155–160. doi: 10.1016/j.ijdevneu.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Hell JW. CaMKII: Claiming Center Stage in Postsynaptic Function and Organization. Neuron. 2014;81:249–265. doi: 10.1016/j.neuron.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60:610–624. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon M, Soykan T, Falkenburger B, Hammer M, Patrizi A, Schmidt KF, Sassoè-Pognetto M, Löwel S, Moser T, Taschenberger H, et al. Neuroligin-4 is localized to glycinergic postsynapses and regulates inhibition in the retina. PNAS. 2011;108:3053–3058. doi: 10.1073/pnas.1006946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House DRC, Elstrott J, Koh E, Chung J, Feldman DE. Parallel regulation of feedforward inhibition and excitation during whisker map plasticity. Neuron. 2011;72:819–831. doi: 10.1016/j.neuron.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houweling AR, Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Homeostatic synaptic plasticity can explain post-traumatic epileptogenesis in chronically isolated neocortex. Cereb Cortex. 2005;15:834–845. doi: 10.1093/cercor/bhh184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Ma Y, Agmon A. Submillisecond Firing Synchrony between Different Subtypes of Cortical Interneurons Connected Chemically But Not Electrically. J Neurosci. 2011;31:3351–3361. doi: 10.1523/JNEUROSCI.4881-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Ogiwara I, Yamada K, Miyamoto H, Hensch TK, Osawa M, Yamakawa K. Mouse with Na(v)1.1 haploinsufficiency, a model for Dravet syndrome, exhibits lowered sociability and learning impairment. Neurobiol Dis. 2012;49C:29–40. doi: 10.1016/j.nbd.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Jackman C, Horn ND, Molleston JP, Sokol DK. Gene associated with seizures, autism, and hepatomegaly in an Amish girl. Pediatr Neurol. 2009;40:310–313. doi: 10.1016/j.pediatrneurol.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ. Gephyrin Regulates the Cell Surface Dynamics of Synaptic GABAA Receptors. J Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YH, Ehlers MD. Modeling autism by SHANK gene mutations in mice. Neuron. 2013;78:8–27. doi: 10.1016/j.neuron.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Ash RT, Baker SA, Suter B, Ferguson A, Park J, Rudy J, Torsky SP, Chao H-T, Zoghbi HY, et al. Dendritic arborization and spine dynamics are abnormal in the mouse model of MECP2 duplication syndrome. J Neurosci. 2013;33:19518–19533. doi: 10.1523/JNEUROSCI.1745-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Zhang Z, Zhang C, Wang X, Sakata K, Lu B, Sun Q-Q. A key mechanism underlying sensory experience-dependent maturation of neocortical GABAergic circuits in vivo. Proc Natl Acad Sci USA. 2011;108:12131–12136. doi: 10.1073/pnas.1105296108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. GABAergic neurons and their role in cortical plasticity in primates. Cereb Cortex. 1993;3:361–372. doi: 10.1093/cercor/3.5.361-a. [DOI] [PubMed] [Google Scholar]
- Kaphzan H, Hernandez P, Jung JI, Cowansage KK, Deinhardt K, Chao MV, Abel T, Klann E. Reversal of Impaired Hippocampal Long-Term Potentiation and Contextual Fear Memory Deficits in Angelman Syndrome Model Mice by ErbB Inhibitors. Biological Psychiatry. 2012;72:182–190. doi: 10.1016/j.biopsych.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayannis T, Au E, Patel JC, Kruglikov I, Markx S, Delorme R, Héron D, Salomon D, Glessner J, Restituito S, et al. Cntnap4 differentially contributes to GABAergic and dopaminergic synaptic transmission. Nature. 2014;511:236–240. doi: 10.1038/nature13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasnauskiene J, Ciuladaite Z, Preiksaitiene E, Utkus A, Peciulyte A, Kučinskas V. A new single gene deletion on 2q34: ERBB4 is associated with intellectual disability. Am J Med Genet A. 2013;161A:1487–1490. doi: 10.1002/ajmg.a.35911. [DOI] [PubMed] [Google Scholar]
- Katz DM, Berger-Sweeney JE, Eubanks JH, Justice MJ, Neul JL, Pozzo-Miller L, Blue ME, Christian D, Crawley JN, Giustetto M, et al. Preclinical research in Rett syndrome: setting the foundation for translational success. Dis Model Mech. 2012;5:733–745. doi: 10.1242/dmm.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature. 2014;505:318–326. doi: 10.1038/nature12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilman V, van Rossum MCW, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Ziff EB. Calcineurin mediates synaptic scaling via synaptic trafficking of Ca2+-permeable AMPA receptors. PLoS Biol. 2014;12:e1001900. doi: 10.1371/journal.pbio.1001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Venkataraju KU, Pradhan K, Mende C, Taranda J, Turaga SC, Arganda-Carreras I, Ng L, Hawrylycz MJ, Rockland KS, et al. Mapping Social Behavior-Induced Brain Activation at Cellular Resolution in the Mouse. Cell Reports. 2015;10:292–305. doi: 10.1016/j.celrep.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleschevnikov AM, Belichenko PV, Villar AJ, Epstein CJ, Malenka RC, Mobley WC. Hippocampal long-term potentiation suppressed by increased inhibition in the Ts65Dn mouse, a genetic model of Down syndrome. J Neurosci. 2004;24:8153–8160. doi: 10.1523/JNEUROSCI.1766-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Brandstätter JH, Laube B, Stahl S, Müller U, Betz H. Loss of Postsynaptic GABAA Receptor Clustering in Gephyrin-Deficient Mice. J Neurosci. 1999;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krey JF, Dolmetsch RE. Molecular mechanisms of autism: a possible role for Ca2+ signaling. Current Opinion in Neurobiology. 2007;17:112–119. doi: 10.1016/j.conb.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Kron M, Howell CJ, Adams IT, Ransbottom M, Christian D, Ogier M, Katz DM. Brain activity mapping in Mecp2 mutant mice reveals functional deficits in forebrain circuits, including key nodes in the default mode network, that are reversed with ketamine treatment. J Neurosci. 2012;32:13860–13872. doi: 10.1523/JNEUROSCI.2159-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon O-B, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 Reverses Long-Term Potentiation at CA1 Hippocampal Synapses. J Neurosci. 2005;25:9378–9383. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laan LAEM, Vein AA. Angelman syndrome: is there a characteristic EEG? Brain Dev. 2005;27:80–87. doi: 10.1016/j.braindev.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Lasarge CL, Danzer SC. Mechanisms regulating neuronal excitability and seizure development following mTOR pathway hyperactivation. Front Mol Neurosci. 2014;7:18. doi: 10.3389/fnmol.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard M-P, Raynaud M, Ronce N, Lemonnier E, Calvas P, et al. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Rex CS, Kramár E, Chen LY, Pandyarajan V, Lynch G, Gall CM. Brain-Derived Neurotrophic Factor Rescues Synaptic Plasticity in a Mouse Model of Fragile X Syndrome. J Neurosci. 2007;27:10685–10694. doi: 10.1523/JNEUROSCI.2624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leekam SR, Prior MR, Uljarevic M. Restricted and repetitive behaviors in autism spectrum disorders: A review of research in the last decade. Psychological Bulletin. 2011;137:562–593. doi: 10.1037/a0023341. [DOI] [PubMed] [Google Scholar]
- Lesca G, Rudolf G, Labalme A, Hirsch E, Arzimanoglou A, Genton P, Motte J, de Saint Martin A, Valenti MP, Boulay C, et al. Epileptic encephalopathies of the Landau-Kleffner and continuous spike and waves during slow-wave sleep types: genomic dissection makes the link with autism. Epilepsia. 2012;53:1526–1538. doi: 10.1111/j.1528-1167.2012.03559.x. [DOI] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SBE, Meyer EMM, Tovote P, Courtin J, Herry C, Lüthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KX, Lu YM, Xu ZH, Zhang J, Zhu JM, Zhang JM, Cao SX, Chen XJ, Chen Z, Luo JH, et al. Neuregulin 1 regulates excitability of fast-spiking neurons through Kv1.1 and acts in epilepsy. Nat Neurosci. 2012;15:267–273. doi: 10.1038/nn.3006. [DOI] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionel AC, Vaags AK, Sato D, Gazzellone MJ, Mitchell EB, Chen HY, Costain G, Walker S, Egger G, Thiruvahindrapuram B, et al. Rare exonic deletions implicate the synaptic organizer Gephyrin (GPHN) in risk for autism, schizophrenia and seizures. Hum Mol Genet. 2013;22:2055–2066. doi: 10.1093/hmg/ddt056. [DOI] [PubMed] [Google Scholar]
- Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, Hirrlinger PG, Kirchhoff F, Bissonnette JM, Ballas N, et al. A role for glia in the progression of Rett’s syndrome. Nature. 2011;475:497–500. doi: 10.1038/nature10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R, Hare EB, Hagerman RJ. Modulation of the GABAergic pathway for the treatment of fragile X syndrome. Neuropsychiatr Dis Treat. 2014;10:1769–1779. doi: 10.2147/NDT.S42919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozovaya N, Gataullina S, Tsintsadze T, Tsintsadze V, Pallesi-Pocachard E, Minlebaev M, Goriounova NA, Buhler E, Watrin F, Shityakov S, et al. Selective suppression of excessive GluN2C expression rescues early epilepsy in a tuberous sclerosis murine model. Nat Commun. 2014;5 doi: 10.1038/ncomms5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux AL, Osborne JP. The influence of etiology upon ictal semiology, treatment decisions and long-term outcomes in infantile spasms and West syndrome. Epilepsy Research. 2006;70(Supplement):77–86. doi: 10.1016/j.eplepsyres.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Mabb AM, Judson MC, Zylka MJ, Philpot BD. Angelman syndrome: insights into genomic imprinting and neurodevelopmental phenotypes. Trends Neurosci. 2011;34:293–303. doi: 10.1016/j.tins.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MC, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH, Muotri AR. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527–539. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Goaillard J-M. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- Margolis SS, Salogiannis J, Lipton DM, Mandel-Brehm C, Wills ZP, Mardinly AR, Hu L, Greer PL, Bikoff JB, Ho HY, et al. EphB-mediated degradation of the RhoA GEF Ephexin5 relieves a developmental brake on excitatory synapse formation. Cell. 2010;143:442–455. doi: 10.1016/j.cell.2010.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh E, Fulp C, Gomez E, Nasrallah I, Minarcik J, Sudi J, Christian SL, Mancini G, Labosky P, Dobyns W, et al. Targeted loss of Arx results in a developmental epilepsy mouse model and recapitulates the human phenotype in heterozygous females. Brain. 2009;132:1563–1576. doi: 10.1093/brain/awp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annu Rev Physiol. 2001;63:815–846. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- McNamara JO, Scharfman HE. Temporal Lobe Epilepsy and the BDNF Receptor. TrkB. 2012 [PubMed] [Google Scholar]
- Meisler MH, O’Brien JE, Sharkey LM. Sodium channel gene family: epilepsy mutations, gene interactions and modifier effects. J Physiol. 2010;588:1841–1848. doi: 10.1113/jphysiol.2010.188482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Money KM, Stanwood GD. Developmental origins of brain disorders: roles for dopamine. Front Cell Neurosci. 2013;7:260. doi: 10.3389/fncel.2013.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P, Levenson JM, Battaglia F, Atkinson R, Teague R, Antalffy B, Armstrong D, Arancio O, Sweatt JD, Zoghbi HY. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci. 2006;26:319–327. doi: 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113:e472–e486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- Musumeci SA, Bosco P, Calabrese G, Bakker C, De Sarro GB, Elia M, Ferri R, Oostra BA. Audiogenic seizures susceptibility in transgenic mice with fragile X syndrome. Epilepsia. 2000;41:19–23. doi: 10.1111/j.1528-1157.2000.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Na ES, Nelson ED, Adachi M, Autry AE, Mahgoub MA, Kavalali ET, Monteggia LM. A mouse model for MeCP2 duplication syndrome: MeCP2 overexpression impairs learning and memory and synaptic transmission. J Neurosci. 2012;32:3109–3117. doi: 10.1523/JNEUROSCI.6000-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ED, Kavalali ET, Monteggia LM. MeCP2-dependent transcriptional repression regulates excitatory neurotransmission. Curr Biol. 2006;16:710–716. doi: 10.1016/j.cub.2006.02.062. [DOI] [PubMed] [Google Scholar]
- Neul JL, Kaufmann WE, Glaze DG, Christodoulou J, Clarke AJ, Bahi-Buisson N, Leonard H, Bailey MES, Schanen NC, Zappella M, et al. Rett Syndrome: Revised Diagnostic Criteria and Nomenclature. Ann Neurol. 2010;68:944–950. doi: 10.1002/ana.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noutel J, Hong YK, Leu B, Kang E, Chen C. Experience-dependent retinogeniculate synapse remodeling is abnormal in MeCP2-deficient mice. Neuron. 2011;70:35–42. doi: 10.1016/j.neuron.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numis AL, Major P, Montenegro MA, Muzykewicz DA, Pulsifer MB, Thiele EA. Identification of risk factors for autism spectrum disorders in tuberous sclerosis complex. Neurology. 2011;76:981–987. doi: 10.1212/WNL.0b013e3182104347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley JC, Kalume F, Catterall WA. Insights into pathophysiology and therapy from a mouse model of Dravet syndrome. Epilepsia. 2011;52(Suppl 2):59–61. doi: 10.1111/j.1528-1167.2011.03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, Takeuchi T, Itohara S, Yanagawa Y, Obata K, et al. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 2007;27:5903–5914. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiwara I, Iwasato T, Miyamoto H, Iwata R, Yamagata T, Mazaki E, Yanagawa Y, Tamamaki N, Hensch TK, Itohara S, et al. Nav1.1 haploinsufficiency in excitatory neurons ameliorates seizure-associated sudden death in a mouse model of Dravet syndrome. Hum Mol Genet. 2013;22:4784–4804. doi: 10.1093/hmg/ddt331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okaty BW, Miller MN, Sugino K, Hempel CM, Nelson SB. Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J Neurosci. 2009;29:7040–7052. doi: 10.1523/JNEUROSCI.0105-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivetti PR, Noebels JL. Interneuron, Interrupted: Molecular Pathogenesis of ARX Mutations and X-linked Infantile Spasms. Curr Opin Neurobiol. 2012;22:859–865. doi: 10.1016/j.conb.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluszkiewicz SM, Olmos-Serrano JL, Corbin JG, Huntsman MM. Impaired inhibitory control of cortical synchronization in fragile X syndrome. Journal of Neurophysiology. 2011;106:2264–2272. doi: 10.1152/jn.00421.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AB, Hays SA, Bureau I, Huber KM, Gibson JR. A target cell-specific role for presynaptic Fmr1 in regulating glutamate release onto neocortical fast-spiking inhibitory neurons. J Neurosci. 2013;33:2593–2604. doi: 10.1523/JNEUROSCI.2447-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagarikano O, Geschwind DH. What does CNTNAP2 reveal about autism spectrum disorder? Trends Mol Med. 2012;18:156–163. doi: 10.1016/j.molmed.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, Abrahams BS, Herman EI, Winden KC, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YR, Zeng SY, Song HL, Li MY, Yamada MK, Yu X. Postsynaptic spiking homeostatically induces cell-autonomous regulation of inhibitory inputs via retrograde signaling. J Neurosci. 2010;30:16220–16231. doi: 10.1523/JNEUROSCI.3085-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petazzi P, Akizu N, García A, Estarás C, Martínez de Paz A, Rodríguez-Paredes M, Martínez-Balbás MA, Huertas D, Esteller M. An increase in MECP2 dosage impairs neural tube formation. Neurobiology of Disease. 2014;67:49–56. doi: 10.1016/j.nbd.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16:1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z, Sylwestrak EL, Lieberman DN, Zhang Y, Liu XY, Ghosh A. The Rett syndrome protein MeCP2 regulates synaptic scaling. J Neurosci. 2012;32:989–994. doi: 10.1523/JNEUROSCI.0175-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabaneda LG, Robles-Lanuza E, Nieto-González JL, Scholl FG. Neurexin Dysfunction in Adult Neurons Results in Autistic-like Behavior in Mice. Cell Reports. 2014;8:338–346. doi: 10.1016/j.celrep.2014.06.022. [DOI] [PubMed] [Google Scholar]
- Ramamoorthi K, Fropf R, Belfort GM, Fitzmaurice HL, McKinney RM, Neve RL, Otto T, Lin Y. Npas4 regulates a transcriptional program in CA3 required for contextual memory formation. Science. 2011;334:1669–1675. doi: 10.1126/science.1208049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramocki MB, Zoghbi HY. Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature. 2008;455:912–918. doi: 10.1038/nature07457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico B, Marín O. Neuregulin signaling, cortical circuitry development and schizophrenia. Curr Opin Genet Dev. 2011;21:262–270. doi: 10.1016/j.gde.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Roohi J, Montagna C, Tegay DH, Palmer LE, DeVincent C, Pomeroy JC, Christian SL, Nowak N, Hatchwell E. Disruption of contactin 4 in three subjects with autism spectrum disorder. J Med Genet. 2009;46:176–182. doi: 10.1136/jmg.2008.057505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Ercument Cicek A, Kou Y, Liu L, Fromer M, Walker S, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014 doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M, Westenbroek RE, Yu FH, Jones CJ, Scheuer T, Catterall WA. Genetic background modulates impaired excitability of inhibitory neurons in a mouse model of Dravet syndrome. Neurobiol Dis. 2015;73:106–117. doi: 10.1016/j.nbd.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata K, Woo NH, Martinowich K, Greene JS, Schloesser RJ, Shen L, Lu B. Critical role of promoter IV-driven BDNF transcription in GABAergic transmission and synaptic plasticity in the prefrontal cortex. Proc Natl Acad Sci USA. 2009;106:5942–5947. doi: 10.1073/pnas.0811431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Neul JL. Complexities of Rett Syndrome and MeCP2. J Neurosci. 2011;31:7951–7959. doi: 10.1523/JNEUROSCI.0169-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Mandel-Brehm C, Chao HT, Ward CS, Fyffe-Maricich SL, Ren J, Hyland K, Thaller C, Maricich SM, Humphreys P, et al. Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities. Proc Natl Acad Sci U S A. 2009;106:21966–21971. doi: 10.1073/pnas.0912257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro MR, Bray SM, Warren ST. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu Rev Pathol. 2012;7:219–245. doi: 10.1146/annurev-pathol-011811-132457. [DOI] [PubMed] [Google Scholar]
- Sarasua SM, Boccuto L, Sharp JL, Dwivedi A, Chen C-F, Rollins JD, Rogers RC, Phelan K, DuPont BR. Clinical and genomic evaluation of 201 patients with Phelan-McDermid syndrome. Hum Genet. 2014;133:847–859. doi: 10.1007/s00439-014-1423-7. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Wenzel HJ. Are developmental dysplastic lesions epileptogenic? Epilepsia. 2012;53(Suppl 1):35–44. doi: 10.1111/j.1528-1167.2012.03473.x. [DOI] [PubMed] [Google Scholar]
- Shoubridge C, Fullston T, Gécz J. ARX spectrum disorders: making inroads into the molecular pathology. Hum Mutat. 2010;31:889–900. doi: 10.1002/humu.21288. [DOI] [PubMed] [Google Scholar]
- Soden ME, Chen L. Fragile X protein FMRP is required for homeostatic plasticity and regulation of synaptic strength by retinoic acid. J Neurosci. 2010;30:16910–16921. doi: 10.1523/JNEUROSCI.3660-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Südhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci USA. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel I, Mardinly AR, Gabel HW, Bazinet JE, Couch CH, Tzeng CP, Harmin DA, Greenberg ME. Npas4 Regulates Excitatory-Inhibitory Balance within Neural Circuits through Cell-Type-Specific Gene Programs. Cell. 2014;157:1216–1229. doi: 10.1016/j.cell.2014.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss KA, Puffenberger EG, Huentelman MJ, Gottlieb S, Dobrin SE, Parod JM, Stephan DA, Morton DH. Recessive Symptomatic Focal Epilepsy and Mutant Contactin-Associated Protein-like 2. New England Journal of Medicine. 2006;354:1370–1377. doi: 10.1056/NEJMoa052773. [DOI] [PubMed] [Google Scholar]
- Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai C, Abe Y, Westenbroek RE, Scheuer T, Catterall WA. Impaired excitability of somatostatin- and parvalbumin-expressing cortical interneurons in a mouse model of Dravet syndrome. Proc Natl Acad Sci USA. 2014;111:E3139–E3148. doi: 10.1073/pnas.1411131111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan GH, Liu YY, Hu XL, Yin DM, Mei L, Xiong ZQ. Neuregulin 1 represses limbic epileptogenesis through ErbB4 in parvalbumin-expressing interneurons. Nat Neurosci. 2012;15:258–266. doi: 10.1038/nn.3005. [DOI] [PubMed] [Google Scholar]
- Tanaka M, DeLorey TM, Delgado-Escueta A, Olsen RW. GABRB3, Epilepsy, and Neurodevelopment. 2012 [PubMed] [Google Scholar]
- Thalhammer A, Cingolani LA. Cell adhesion and homeostatic synaptic plasticity. Neuropharmacology. 2014;78:23–30. doi: 10.1016/j.neuropharm.2013.03.015. [DOI] [PubMed] [Google Scholar]
- The Dutch-Belgian Fragile X Consorthium. Bakker CE, Verheij C, Willemsen R, van der Helm R, Oerlemans F, Vermey M, Bygrave A, Hoogeveen A, Oostra BA, et al. Fmr1 knockout mice: A model to study fragile X mental retardation. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- Thibert RL, Larson AM, Hsieh DT, Raby AR, Thiele EA. Neurologic manifestations of Angelman syndrome. Pediatr Neurol. 2013;48:271–279. doi: 10.1016/j.pediatrneurol.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Ting JT, Peca J, Feng G. Functional consequences of mutations in postsynaptic scaffolding proteins and relevance to psychiatric disorders. Annu Rev Neurosci. 2012;35:49–71. doi: 10.1146/annurev-neuro-062111-150442. [DOI] [PubMed] [Google Scholar]
- Toro R, Konyukh M, Delorme R, Leblond C, Chaste P, Fauchereau F, Coleman M, Leboyer M, Gillberg C, Bourgeron T. Key role for gene dosage and synaptic homeostasis in autism spectrum disorders. Trends Genet. 2010;26:363–372. doi: 10.1016/j.tig.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Too Many Cooks? Intrinsic and Synaptic Homeostatic Mechanisms in Cortical Circuit Refinement. Annual Review of Neuroscience. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Tyagarajan SK, Fritschy J-M. Gephyrin: a master regulator of neuronal function? Nat. Rev Neurosci. 2014;15:141–156. doi: 10.1038/nrn3670. [DOI] [PubMed] [Google Scholar]
- Ullrich B, Ushkaryov YA, Südhof TC. Cartography of neurexins: More than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron. 1995;14:497–507. doi: 10.1016/0896-6273(95)90306-2. [DOI] [PubMed] [Google Scholar]
- Vaags AK, Lionel AC, Sato D, Goodenberger M, Stein QP, Curran S, Ogilvie C, Ahn JW, Drmic I, Senman L, et al. Rare deletions at the neurexin 3 locus in autism spectrum disorder. Am J Hum Genet. 2012;90:133–141. doi: 10.1016/j.ajhg.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente KD, Koiffmann CP, Fridman C, Varella M, Kok F, Andrade JQ, Grossmann RM, Marques-Dias MJ. Epilepsy in patients with angelman syndrome caused by deletion of the chromosome 15q11-13. Arch Neurol. 2006;63:122–128. doi: 10.1001/archneur.63.1.122. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Südhof TC, Brose N. Neuroligins Determine Synapse Maturation and Function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Viemari J-C, Roux J-C, Tryba AK, Saywell V, Burnet H, Peña F, Zanella S, Bévengut M, Barthelemy-Requin M, Herzing LBK, et al. Mecp2 Deficiency Disrupts Norepinephrine and Respiratory Systems in Mice. J Neurosci. 2005;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace ML, Burette AC, Weinberg RJ, Philpot BD. Maternal loss of Ube3a produces an excitatory/inhibitory imbalance through neuron type-specific synaptic defects. Neuron. 2012;74:793–800. doi: 10.1016/j.neuron.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Thompson SM. Maladaptive Homeostatic Plasticity in a Rodent Model of Central Pain Syndrome: Thalamic Hyperexcitability after Spinothalamic Tract Lesions. J Neurosci. 2008;28:11959–11969. doi: 10.1523/JNEUROSCI.3296-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, McCoy PA, Rodriguiz RM, Pan Y, Je HS, Roberts AC, Kim CJ, Berrios J, Colvin JS, Bousquet-Moore D, et al. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet. 2011;20:3093–3108. doi: 10.1093/hmg/ddr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Huber KM. Protein translation in synaptic plasticity: mGluR-LTD, Fragile X. Current Opinion in Neurobiology. 2009;19:319–326. doi: 10.1016/j.conb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wondolowski J, Dickman D. Emerging links between homeostatic synaptic plasticity and neurological disease. Front Cell Neurosci. 2013;7:223. doi: 10.3389/fncel.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Crino PB. Tuberous sclerosis and epilepsy: Role of astrocytes. Glia. 2012;60:1244–1250. doi: 10.1002/glia.22326. [DOI] [PubMed] [Google Scholar]
- Woo NH, Lu B. Regulation of cortical interneurons by neurotrophins: from development to cognitive disorders. Neuroscientist. 2006;12:43–56. doi: 10.1177/1073858405284360. [DOI] [PubMed] [Google Scholar]
- Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, Neiswender H, Dong XP, Wu J, Gassmann M, et al. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Xu H, Jeong HY, Tremblay R, Rudy B. Neocortical Somatostatin-expressing GABAergic Interneurons Disinhibit the Thalamorecipient Layer 4. Neuron. 2013;77:155–167. doi: 10.1016/j.neuron.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Atallah BV, Scanziani M. Equalizing excitation-inhibition ratios across visual cortical neurons. Nature. 2014;511:596–600. doi: 10.1038/nature13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J-M, Zhang J, Chen X-J, Geng H-Y, Ye M, Spitzer NC, Luo J-H, Duan S-M, Li X-M. Development of GABA Circuitry of Fast-Spiking Basket Interneurons in the Medial Prefrontal Cortex of erbb4-Mutant Mice. J Neurosci. 2013;33:19724–19733. doi: 10.1523/JNEUROSCI.1584-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Hou D, Jiang W, Zhang C. Intercellular protein–protein interactions at synapses. Protein Cell. 2014;5:420–444. doi: 10.1007/s13238-014-0054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Peterson M, Beyer B, Frankel WN, Zhang Z. Loss of MeCP2 From Forebrain Excitatory Neurons Leads to Cortical Hyperexcitation and Seizures. J Neurosci. 2014;34:2754–2763. doi: 10.1523/JNEUROSCI.4900-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Li H, Chang Q. MeCP2 phosphorylation is required for modulating synaptic scaling through mGluR5. J Neurosci. 2012;32:12841–12847. doi: 10.1523/JNEUROSCI.2784-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Hong EJ, Cohen S, Zhao W, Ho HH, Schmidt L, Chen WG, Lin Y, Savner E, Griffith EC, et al. Brain-Specific Phosphorylation of MeCP2 Regulates Activity-Dependent Bdnf Transcription, Dendritic Growth, and Spine Maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]