Abstract
Parasite host range can be influenced by physiological, behavioral, and ecological factors. Combining data sets on host–parasite associations with phylogenetic information of the hosts and the parasites involved can generate evolutionary hypotheses about the selective forces shaping host range. Here, we analyzed associations between the nest-parasitic flies in the genus Philornis and their host birds on Trinidad. Four of ten Philornis species were only reared from one species of bird. Of the parasite species with more than one host bird species, P. falsificus was the least specific and P. deceptivus the most specific attacking only Passeriformes. Philornis flies in Trinidad thus include both specialists and generalists, with varying degrees of specificity within the generalists. We used three quantities to more formally compare the host range of Philornis flies: the number of bird species attacked by each species of Philornis, a phylogenetically informed host specificity index (Poulin and Mouillot's STD), and a branch length-based STD. We then assessed the phylogenetic signal of these measures of host range for 29 bird species. None of these measures showed significant phylogenetic signal, suggesting that clades of Philornis did not differ significantly in their ability to exploit hosts. We also calculated two quantities of parasite species load for the birds – the parasite species richness, and a variant of the STD index based on nodes rather than on taxonomic levels – and assessed the signal of these measures on the bird phylogeny. We did not find significant phylogenetic signal for the parasite species load or the node-based STD index. Finally, we calculated the parasite associations for all bird pairs using the Jaccard index and regressed these similarity values against the number of nodes in the phylogeny separating bird pairs. This analysis showed that Philornis on Trinidad tend to feed on closely related bird species more often than expected by chance.
Keywords: Bird–parasite interactions, community similarity, host specificity, Jaccard index, Philornis, Trinidad
Introduction
One of the most fundamental characteristics of a parasite is the spectrum of host species that it can exploit (Adamson and Caira 1994; Poulin 2007). Interactions between birds and their arthropod parasites illustrate the broad range of host specificity that is possible, ranging from parasites with very broad host ranges such as the hen flea, Ceratophyllus gallinae, which attacks at least 72 host species (Tripet and Richner 1997), to some feather lice species that only attack one species or strain of host (Clayton 1991; Johnson et al. 2002). Bird species can also vary greatly in the number of parasite lineages that they support (e.g., Lacorte et al. 2013), suggesting differences in the host resistance profile or parasite pressure. Here, we take a phylogenetic approach in analyzing host range, parasite species load, and community similarity in associations between a genus of parasitic flies and their bird hosts in Trinidad.
One way of quantifying the host range of a parasite is to simply count the number of hosts it can exploit. However, such estimation lacks important information about the phylogenetic relationship among the host species. Poulin and Mouillot (2003) developed a host specificity index, STD, that measures the standardized taxonomic distinctiveness of all host species used by a parasite. STD allows for the differentiation of parasite species that attack the same numbers of host species at different levels of phylogenetic organization. For example, a parasite species attacking two host species in the same genus has a lower STD value than a parasite species attacking two host species in different genera.
The number of parasite species supported by single host species (the ‘parasite species load’) is another important aspect of host–parasite interactions and can reflect processes such as host susceptibility and parasite competition (Godfray 1994). Here again a phylogenetic approach can be illuminating. In particular, determining the signal of parasite species load on the phylogeny of a group of hosts could be used to pose hypotheses concerning the evolution of susceptibility or resistance in host clades, and of niche partitioning among parasite species (Poulin 2007).
Finally, integrating information on the community structure of parasites and their hosts with information on host phylogenies has the potential to improve our understanding of the patterns and determinants of host range. Recently, indices of species diversity have been applied to calculate how similar the parasite faunas of host species are (Novotny et al. 2002; Weiblen et al. 2006; Vinarski et al. 2007; Davies and Pedersen 2008; Poulin 2010), driving a new interest in the understanding of community similarity of host species, also known as faunal similarity. The Jaccard index is widely used to assess community similarity across geography and phylogeny, and for the case of parasites, a number of studies have shown that the similarity in species composition decreases exponentially with phylogenetic and geographic distance among the host species (Poulin 2003, 2010; Fellis and Esch 2005; Krasnov et al. 2005; Oliva and González 2005; Poulin et al. 2011). At the phylogenetic level, this negative relationship is expected because closely related hosts tend to have similar physiologies and resistance, susceptibility, or tolerance profiles (e.g., Desneux et al. 2012). Therefore, closely related host species are expected to harbor more similar parasite faunas than are distantly related ones (Poulin 2010).
Host range, parasite species load, and community similarity are important descriptors of host–parasite interactions. Few studies have used a phylogenetic approach to analyze all three of these processes in a community of bird parasites and their hosts. In this article, we follow such an approach using Philornis parasites on the island of Trinidad.
Materials and Methods
Study system
We studied the associations between the avian-parasitic flies in the genus Philornis (Diptera: Muscidae) and their host birds. In particular, we analyzed data compiled by Dodge and Aitken (1968) over 6 years of fieldwork (1956–1961) for ten Philornis species parasitizing 29 bird species belonging to fourteen families on the island of Trinidad. While conducting arthropod-borne virus research on this island, Dr. Aitken collected large quantities of Philornis material. He reared the adult flies from larvae or pupae taken from the bird nests. The taxonomic classification of the 29 species of birds and the type of nest they use can be found in Table S1.
The distribution of the genus Philornis is Neotropical being found from Argentina and Chile northwards to Texas and Florida, USA (Skidmore 1985), with fifty species currently recognized (Couri et al. 2007). Philornis species depend on birds to complete their life cycle as the larval stage is parasitic. According to the habits of their larvae, they are divided into three feeding guilds. The majority of Philornis species with known larval habits (currently 28 species) are subcutaneous blood feeders, two species are coprophagous with free-living larvae in the nest, and two species have free-living, semi-hematophagous larvae (Teixeira 1999). A list of the ten Philornis species that occur on Trinidad, their larval habits, and taxonomic synonymies is presented in Table1.
Table 1.
The ten species of Philornis occurring in Trinidad, with synonymies, larval habits, number of host birds exploited, the STD index, and the branch length-based STD
| Philornis species | Synonymies | Larval habit | Number of host birds | S TD | Branch length-based STD |
|---|---|---|---|---|---|
| P. aitkeni | Free-living coprophagous | 1 | 1 | 0 | |
| P. falsificus | P. falsifica | Free-living semi-hematophagous | 3 | 4.0 | 163.5 |
| P. downsi | Free-living semi-hematophagous | 16 | 3.2 | 109.4 | |
| P. niger | P. nigra | Subcutaneous | 1 | 1 | 0 |
| P. deceptivus | P. deceptiva | Subcutaneous | 6 | 2.7 | 95.7 |
| P. trinitensis | Subcutaneous | 8 | 2.9 | 93.6 | |
| P. glaucinis | Subcutaneous | 1 | 1 | 0 | |
| P. querulus | P. querula | Subcutaneous | 1 | 1 | 0 |
| P. angustifrons | Subcutaneous | 10 | 3.6 | 141.5 | |
| P. sanguinis | Subcutaneous | 3 | 3.7 | 154.4 |
Host bird and parasite phylogenies
We generated a phylogeny of the 29 bird species reported as hosts of Philornis spp. in Trinidad using the Bayesian pseudo-posterior distribution of time-calibrated bird phylogenies from Jetz et al. (2012) available at the Web site http://birdtree.org. From 5000 trees sampled, we generated a maximum clade credibility (MCC) tree, using the software TreeAnnotator included in BEAST v.1.8.2 (Drummond et al. 2012). This tree was further modified with updated information on Thraupidae (tanagers) from Barker et al. (2015). This bird phylogeny was used in all analyses (Figs.1, 2).
Figure 1.
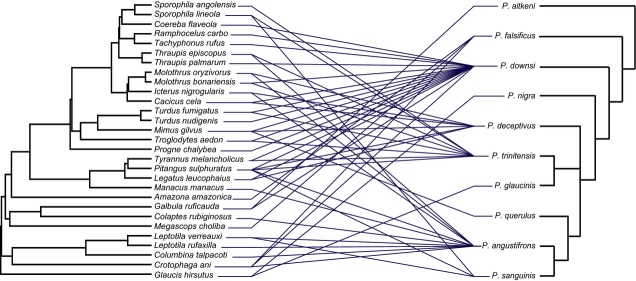
Tanglegram showing the associations between ten Philornis species (on the right) and 29 bird host species (on the left) on the island of Trinidad. Thin lines indicate host–parasite associations.
Figure 2.
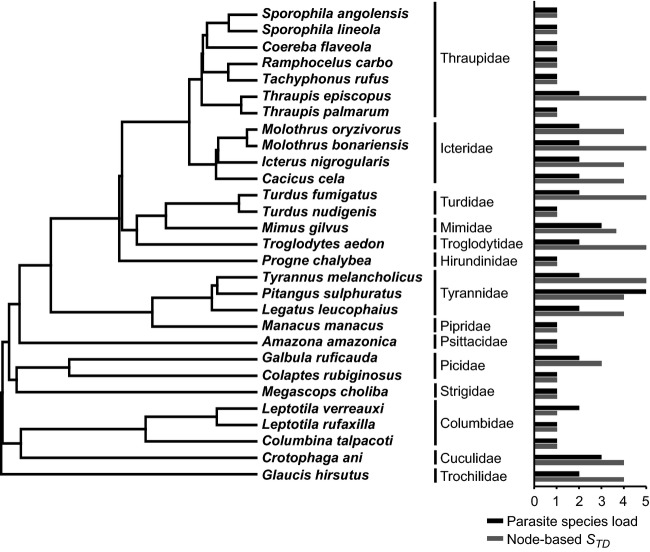
The phylogenetic signal of the birds' parasite species load and node-based STD. Philornis species were reared from bird species at random in relation to the bird phylogeny.
No molecular phylogeny is currently available for the genus Philornis. The only available Philornis phylogeny is based on morphological characters (Couri et al. 2007). We pruned this phylogeny to include only the ten Trinidadian Philornis species and used the software TreeMap v3.0b (Charleston and Robertson 2002) to show the associations between the Philornis and the host bird phylogenies (Fig.1).
Host specificity, parasite species load, and community structure analyses
We recorded the number of bird species exploited by each Philornis species and estimated the host specificity index, STD, for parasite species with more than one host species. To calculate the index, it is necessary to place each pair of host species of a particular parasite within a Linnaean hierarchy (e.g., class, order, family, genus, and species). The average taxonomic distinctness is the number of steps that must be taken to reach a common ancestor to two host species, averaged across all possible pairs of host species as outlined by Poulin and Mouillot (2003). STD is inversely proportional to specificity; a high index value means that, on average, the hosts of a parasite species are not closely related. We calculated STD using the software TaxoBiodiv2 (Poulin and Mouillot 2005). In addition, we modified the STD index by using the branch length values from the bird phylogeny to estimate a ‘branch length-based STD index’ as an additional host specificity measure. In this test, the average branch length between all pairs of hosts of a given parasite species is computed. We then tested for the phylogenetic signal of host range for the raw number of species exploited, the STD index, and the branch length-based STD index by running the Analysis of Traits (AOT) module in the software Phylocom v.4.2 (Webb et al. 2008). Phylocom uses randomization of traits values across the tips of the phylogeny to determine whether patterns of significant clustering of traits can be detected across the phylogeny.
We also assessed the phylogenetic signal of parasite species load using AOT to determine whether the number of Philornis species attacking each host species was significantly clustered on the bird phylogeny. As implemented above for host range, we analyzed both the raw number of species as an indicator of parasite load and a phylogenetically informed measure of parasite species load. Because all parasite species in our study belong in the same genus, we modified the STD index by counting the number of nodes taken to reach a common ancestor between two parasites in the Philornis phylogeny, computed across all possible pairs of parasite species attacking each bird species. We called this modified STD index ‘the node-based STD’. We then performed the AOT in Phylocom to test for the phylogenetic signal of parasite species load for the raw number of parasite each bird harbored (species richness parasite species load) and the node-based STD index (phylogenetically informed parasite species load).
Lastly, we examined the relationship of the parasite community similarity to the phylogenetic distance between the hosts. We calculated community similarity in the parasite faunas for all possible pairs of host birds using the Jaccard index (Jaccard 1912). This index corresponds to the number of parasite species shared by two host species divided by the total number of parasite species occurring in the two host species; it ranges from 0 (no shared parasite) to 1 (the two bird species have exactly the same parasites) (Poulin 2010). We computed the Jaccard index using the software EstimateS v9.10 (Colwell 2013) and used branch length values to calculate the phylogenetic distance between each of the 406 bird species pairs in this study. Following standard practice, similarity (Jaccard index) and phylogenetic distance measures were log + 1-transformed prior to performing a linear regression between the two variables (Poulin 2010).
Results
Host specificity
Four of the ten Philornis species were reared from a single host species: P. aitkeni, P. niger, P. glaucinis, and P. querulus, and the remaining six species were reared from more than one host bird species (Fig.1). Of these, P. falsificus and P. sanguinis parasitized three bird species each, P. deceptivus six, P. trinitensis eight, P. angustifrons ten, and P. downsi sixteen (Table1). Philornis falsificus, with the highest STD value, parasitizes three bird species in three different orders; therefore, this is the least specialized parasite species when host phylogeny is taken into account, whereas P. deceptivus exploits six bird species, all in the Passeriformes, being the most host specific of the Philornis species that has been reared from more than one host species (Table1).
We found no evidence that host specificity is clustered on the Philornis phylogeny (phylogenetic signal analyses in Phylocom: P = 0.88 for the raw number of hosts, P = 0.68 for STD, P = 0.65 for branch length-based STD) or evenly distributed on the phylogeny (P = 0.11 for the raw number of hosts, P = 0.31 for STD, P = 0.35 for branch length-based STD).
Parasite species load
The number of parasite species harbored per bird species also varied, with most birds hosting one or two Philornis species but one bird (the great kiskadee, Pitangus sulphuratus) serving as host to five Philornis species (Fig.1). The analysis of phylogenetic signal of parasite species load on the host phylogeny showed no significant effect for parasite species richness or the node-based STD (Fig.2; P = 0.29 for the parasite species load, P = 0.57 for the node-based STD).
Community structure
We found a significant decrease in the community similarity of host species attacked by Philornis species (measured by the Jaccard index) as a function of increasing phylogenetic distance between the host birds (Fig.3). The significant linear regression (r2 = 0.05, P < 0.0001) of log community similarity against log phylogenetic distance suggests that Philornis species on Trinidad tend to feed on closely related birds more often than on distantly related bird species.
Figure 3.
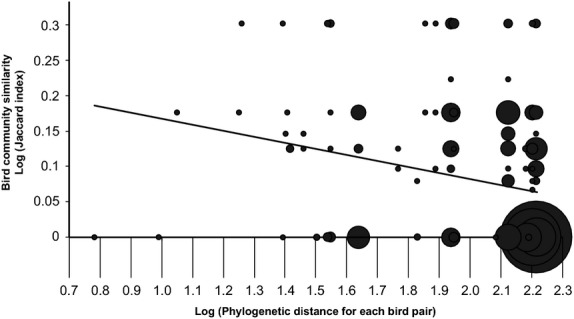
Parasite community similarity as a function of the phylogenetic distance between host birds, in log–log space. Phylogenetic distance is expressed as the total branch length separating each bird pair. The area of the circles is proportional to the number of observations.
Discussion
The Philornis fauna in Trinidad contains both specialists and generalists, with varying degrees of specificity within the generalists, and this general pattern agrees with an analysis of Philornis spp. found outside of Trinidad (Löwenberg-Neto 2008). Four Philornis species were only reared from one bird species in Trinidad. These are P. aitkeni, P. niger, P. querulus, and P. glaucinis. The number of bird species attacked by the remaining six Philornis species ranged from three (P. falsificus and P. sanguinis) to sixteen (P. downsi). The host associations of Philornis spp. in Trinidad included seven bird orders, as compared to ten known throughout its distributional range (Teixeira 1999; Dudaniec and Kleindorfer 2006).
Comparing three measures of host specificity – species richness, the STD index, and the branch length-based STD – showed the usefulness of including phylogenetic information to assess host specificity (Poulin and Mouillot 2003; Poulin et al. 2011). In particular, the two Philornis species that were reared from only three host species had the highest STD values, indicating a broad phylogenetic host range. This is because these three host species belonged to three different bird orders (in the case of P. falsificus) or two host orders with divergent taxa within the common orders (in the case of P. sanguinis) (see Table1 and Fig.1). The four other Philornis species that were reared from between six and sixteen host species attacked birds from within a narrower phylogenetic breadth of hosts. The most specialized of these, P. deceptivus, was reared from six species of birds, all within the order Passeriformes.
The larval feeding habit of Philornis species may have an influence on the host range, but we have insufficient data to evaluate this formally. Coprophagy, which is considered the ancestral feeding habit for Philornis (Couri et al. 2007; but see Dodge 1971), is represented by a single species in our data set (P. aitkeni) which was reared from a single bird species (the rufous-tailed jacamar, Galbula ruficauda). There is no other information range-wide on host associations of this species, but it is interesting that the only other coprophagous Philornis species, P. rufoscutellaris, is also only known from G. ruficauda (Teixeira 1999). Thus, coprophagy may be linked to specialization in Philornis. Of the truly parasitic species, both of the free-living semi-hematophagous species (P. downsi and P. falsificus) exhibited a broad host range (when measured by the number of host species or the STD) while the subcutaneous species had widely varying host ranges (Table1). Free-living semi-hematophagy is considered an evolutionary transition toward subcutaneous feeding (Couri et al. 2007), and the broad patterns exhibited by Philornis in Trinidad are consistent with the hypothesis that this transition is associated with host-range restriction in some cases.
Despite these trends, we found no significant statistical effect of the Philornis phylogeny on host range, whether it was measured as species richness or the STD index. This is somewhat unexpected as the ability to recognize and successfully exploit hosts is presumed to be under strong selection (Poulin 2007) and thus could lead to lineages with similar host associations. However, various models of speciation involve changes in host specificity. For instance, a generalist lineage may give rise to one or more specialized lineages (Moran 1988) and vice versa (Stireman 2005). Either of these cases would interfere with a phylogenetic signal of host specificity. As we noted above, the most ancestral parasite species in our data set is the specialist P. aitkeni, and its nearest relatives have a very broad host range (Fig.1). This suggests host-range expansion within Trinidad over the timescale that these lineages evolved. A possible case of host-range constriction involves the specialist P. querulus, which was reared only from the tropical mockingbird, Mimus gilvus, while its closest known ancestor P. deceptivus was reared from six hosts, including M. gilvus. Thus, it appears that the evolutionary history of Philornis may involve a mosaic of host-range expansion and contraction resulting in no clear clustering of host range on the phylogeny.
We also did not find a significant effect of the host phylogeny on the parasite species load. Despite this, two bird families did show consistently high values of the node-based STD index (Fig.2): the blackbirds and relatives (Icteridae) and the flycatchers (Tyrannidae). These birds might have a higher level of susceptibility to parasitism by Philornis, or there may be ecological factors (such as abundance) that make them particularly vulnerable.
Finally, we performed a phylogenetically informed analysis of community structure to determine whether Philornis tend to attack birds that are themselves closely related. This is illustrative of a trend to incorporate phylogenetic information into analyses of community structure (Cavender-Bares et al. 2009). We found that Philornis species do indeed tend to parasitize bird species that are more closely related than expected by chance. This is in agreement with the literature on parasite faunas in general (Poulin 2010), but we note that the trend is relatively weak (Fig.3).
A number of difficulties can arise when studying host specificity from published records in the literature (reviewed by Poulin 2007). First, sampling effort can account for much of the variability in the number of known host species. Second, misidentification of parasite or host species can compromise the estimate of host specificity. We note that in the study analyzed here, a substantial amount of time was spent collecting (the sampling period spans 6 years) and the ten Philornis species were presumably correctly identified by the authors, who were themselves the taxonomic authorities on the genus Philornis. However, a more useful index of host specificity would include information on the abundance or prevalence of parasitism (Poulin et al. 2011).
The data on host associations of Philornis species in locations other than Trinidad are rather sparse (Teixeira 1999; Dudaniec and Kleindorfer 2006; Löwenberg-Neto 2008). For some species, however, a comparison between host associations found on Trinidad and in other locations can be made. For example, P. glaucinis has been reared from only one bird species each in Trinidad and Panama (the rufous-breasted hermit, Glaucis hirsutus, and the crimson-backed tanager, Ramphocelus dimidiatus, respectively) (Teixeira 1999; Bermúdez et al. 2010) while in Brazil it is recorded from species belonging to eight bird families, including two species that are also attacked in Trinidad (the shiny cowbird, Molothrus bonariensis, and the tropical screech owl, Megascops choliba) (Teixeira 1999). The intensive sampling in Trinidad suggests true specificity there at least (the case for specificity in Panama is less clear), but as the geographic origin of P. glaucinis is not known, it is not clear whether these differences reflect increasing host specialization in Trinidad or host-range expansion in Brazil. The island of Trinidad is thought to have separated from the South American mainland approximately 1500 years ago (Kenny 2008), so there was likely little physical isolation of the faunas over evolutionary time.
Another interesting case is Philornis downsi, which is known from a number of localities in mainland South America, and has recently invaded the Galápagos Islands. This species occurs in Argentina (Silvestri et al. 2011), Brazil (Couri 1984, 1999), and mainland Ecuador (Bulgarella et al. 2015). After its accidental introduction into the Galápagos Islands, it has been reported parasitizing 18 species of birds in five families (Fessl and Tebbich 2002; O'Connor et al. 2010; Causton et al. 2013), including 14 endemic species that represent a clear expansion of host range for this fly. In Trinidad, P. downsi was the Philornis species that parasitized the highest number of host birds (16); in Ecuador, P. downsi also appears to be the dominant species of Philornis (Bulgarella et al. 2015) and has been reared from more bird species than other Philornis spp. (M. Bulgarella, M. A. Quiroga, G. A. Brito Vera, and G. E. Heimpel, unpublished). We suggest that the broad host range of this species in its native range may have contributed to its invasiveness in the Galápagos Islands.
Conclusion
Information on host–parasite associations can suggest evolutionary patterns such as host-range expansion or contraction or the co-evolution of host and parasite lineages. Hypotheses such as these can be best addressed when phylogenies are available for the hosts and/or the parasites. Here we have used this approach to explore host associations between birds and their Philornis parasites on the island of Trinidad. No clear trends were uncovered with respect to a phylogenetic pattern of host range or parasite species load, but we found that individual Philornis species were likely to attack bird species that were relatively closely related.
Acknowledgments
We thank Charlotte E. Causton, Martín A. Quiroga, Georgianna May, Ruth Shaw, Meredith Steck, Shan Kothari, and two anonymous reviewers for providing valuable comments on an earlier version of the manuscript; F. Keith Barker for help with the bird phylogeny; and Milan Plećaš and J.J. Weis for useful discussion. We also thank Dave Chadee and Ray Martínez for introducing us to Philornis in Trinidad. This work was supported by a fellowship from the University of Minnesota Institute on the Environment, funding from the International Community Foundation (with a grant awarded by The Leona M. and Harry B. Helmsley Charitable Trust), and an Award from the National Geographic Society. This article was funded by the University of Minnesota's Open Access Publishing Fund. The fund is jointly supported by the University Libraries and the Office of the Vice President of Research.
Conflict of Interest
None declared.
Supporting Information
Table S1. Twenty-nine bird species host to ten Philornis species in the Island of Trinidad.
References
- Adamson ML. Caira JN. Evolutionary factors influencing the nature of parasite specificity. Parasitology. 1994;109:S85–S95. doi: 10.1017/s0031182000085103. [DOI] [PubMed] [Google Scholar]
- Barker FK, Burns KJ, Klicka J, Lanyon SM. Lovette IJ. New insights into New World biogeography: an integrated view from the phylogeny of blackbirds, cardinals, sparrows, tanagers, warblers, and allies. Auk. 2015;132:333–348. [Google Scholar]
- Bermúdez SE, Buenaventura E, Couri M, Miranda RJ. Herrera JM. Mixed myiasis by Philornis glaucinis (Diptera: Muscidae), Sarcodexia lambens (Diptera: Sarcophagidae) and Lucilia eximia (Diptera: Calliphoridae) in Ramphocelus dimidiatus (Aves: Thraupidae) chicks in Panama. Bol. Soc. Entomol. Aragon. 2010;47:445–446. [Google Scholar]
- Bulgarella M, Quiroga MA, Brito Vera GA, Dregni JS, Cunninghame F, Mosquera Muñoz DA, et al. Philornis downsi, an avian nest parasite invasive to the Galápagos Islands, in mainland Ecuador. Ann. Entomol. Soc. Am. 2015;108:242–250. [Google Scholar]
- Causton CE, Cunninghame F. Tapia W. Galapagos report 2011–2012. 2013. Management of the avian parasite Philornis downsi in the Galapagos Islands: a collaborative and strategic action plan; pp. 167–173. , and.. Puerto Ayora, Galápagos, Ecuador. [Google Scholar]
- Cavender-Bares J, Kozak KH, Fine PVA. Kembel SW. The merging of community ecology and phylogenetic biology. Ecol. Lett. 2009;12:693–715. doi: 10.1111/j.1461-0248.2009.01314.x. [DOI] [PubMed] [Google Scholar]
- Charleston MA. Robertson DL. Preferential host switching by primate lentiviruses can account for phylogenetic similarity with the primate phylogeny. Syst. Biol. 2002;51:528–535. doi: 10.1080/10635150290069940. [DOI] [PubMed] [Google Scholar]
- Clayton DH. Coevolution of avian grooming and ectoparasite avoidance. In: Loye JE, Zuk M, editors. Bird-parasite interactions. Oxford: Oxford Univ. Press; 1991. pp. 258–289. [Google Scholar]
- Colwell RK. 2013. EstimateS: Statistical estimation of species richness and shared species from samples, version 9. Available at http://purl.oclc.org/estimates. (accessed 8 May 2015)
- Couri MS. Notes and descriptions of Philornis flies (Diptera, Muscidae, Cyrtoneurininae) Rev. Bras. Entomol. 1984;2:473–490. [Google Scholar]
- Couri MS. Myiasis caused by obligatory parasites. Ia. Philornis Meinert (Muscidae) In: Guimaraes JH, Papavero N, editors. Myiasis in Man and Animals in the Neotropical Region. São Paulo, Brazil: Editora Pleiade; 1999. pp. 44–70. [Google Scholar]
- Couri MS, de Carvalho CJB. Löwenberg-Neto P. Phylogeny of Philornis Meinert species (Diptera: Muscidae) Zootaxa. 2007;1530:19–26. [Google Scholar]
- Davies TJ. Pedersen AB. Phylogeny and geography predict pathogen community similarity in wild primates and humans. Proc. R. Soc. Lond. B. 2008;275:1695–1701. doi: 10.1098/rspb.2008.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desneux N, Blahnik R, Delebecque CJ. Heimpel GE. Host phylogeny and specialisation in parasitoids. Ecol. Lett. 2012;15:453–460. doi: 10.1111/j.1461-0248.2012.01754.x. [DOI] [PubMed] [Google Scholar]
- Dodge HR. Revisional studies of flies of the genus Philornis (Diptera: Muscidae) Studia Entomol. 1971;14:458–459. [Google Scholar]
- Dodge HR. Aitken THG. Philornis flies from Trinidad (Diptera: Muscidae) J. Kansas Entomol. Soc. 1968;41:134–154. [Google Scholar]
- Drummond AJ, Suchard MA, Xie D. Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudaniec RY. Kleindorfer S. Effects of the parasitic flies of the genus Philornis (Diptera: Muscidae) on birds. Emu. 2006;106:13–20. [Google Scholar]
- Fellis KJ. Esch GW. Autogenic-allogenic status affects interpond community similarity and species-area relationship of macroparasites in the bluegill sunfish, Lepomis macrochirus, from a series of freshwater ponds in the Piedmont area of North Carolina. J. Parasitol. 2005;91:764–767. doi: 10.1645/GE-451R.1. [DOI] [PubMed] [Google Scholar]
- Fessl B. Tebbich S. Philornis downsi– a recently discovered parasite on the Galápagos archipelago – a threat for Darwin's finches? The Ibis. 2002;144:445–451. [Google Scholar]
- Godfray HCJ. Parasitoids: Behavioral and Evolutionary Ecology. Princeton, NJ: Princeton Univ. Press; 1994. [Google Scholar]
- Jaccard P. The distribution of the flora in the alpine zone. New Phytol. 1912;11:37–50. [Google Scholar]
- Jetz W, Thomas GH, Joy JB, Hartmann K. Mooers AO. The global diversity of birds in space and time. Nature. 2012;491:444–448. doi: 10.1038/nature11631. [DOI] [PubMed] [Google Scholar]
- Johnson KP, Williams BL, Drown DM, Adams RJ. Clayton DH. The population genetics of host specificity: genetic differentiation in dove lice (Insecta: Phthiraptera) Mol. Ecol. 2002;11:25–38. doi: 10.1046/j.0962-1083.2001.01412.x. [DOI] [PubMed] [Google Scholar]
- Kenny J. The Biological Diversity of Trinidad and Tobago. Port of Spain, Trinidad and Tobago: Prospect Press; 2008. [Google Scholar]
- Krasnov BR, Shenbrot GI, Mouillot D, Khokhlova IS. Poulin R. Spatial variation in species diversity and composition of flea assemblages in small mammalian hosts: geographical distance or faunal similarity? J. Biogeog. 2005;32:633–644. [Google Scholar]
- Lacorte GA, Félix GMF, Pinheiro RRB, Chaves AV, Almeida-Neto G, Neves FS, et al. Exploring the diversity and distribution of Neotropical avian malaria parasites – A molecular survey from southeast Brazil. PLoS ONE. 2013;8:e57770. doi: 10.1371/journal.pone.0057770. . doi: 10.1371/journal.pone.0057770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwenberg-Neto P. The structure of the parasite-host interactions between Philornis (Diptera: Muscidae) and neotropical birds. J. Trop. Ecol. 2008;24:575–580. [Google Scholar]
- Moran NA. The evolution of host-plant alteration in aphids: evidence for specialization as a dead end. Am. Nat. 1988;132:681–706. [Google Scholar]
- Novotny V, Basset Y, Miller SE, Weiblen GD, Bremer B, Cizek L, et al. Low host specificity of herbivorous insects in a tropical forest. Nature. 2002;416:841–844. doi: 10.1038/416841a. [DOI] [PubMed] [Google Scholar]
- O'Connor JA, Sulloway FJ, Robertson J. Kleindorfer S. Philornis downsi parasitism is the primary cause of nestling mortality in the critically endangered Darwin's medium tree finch (Camarhynchus pauper. Biodivers. Conserv. 2010;19:853–866. [Google Scholar]
- Oliva ME. González MT. The decay of similarity over geographical distance in parasite communities of marine fishes. J. Biogeog. 2005;32:1327–1332. [Google Scholar]
- Poulin R. The decay of similarity with geographical distance in parasite communities of vertebrate hosts. J. Biogeog. 2003;30:1609–1615. [Google Scholar]
- Poulin R. Evolutionary Ecology of Parasites. 2nd ed. Princeton, NJ: Princeton Univ. Press; 2007. [Google Scholar]
- Poulin R. Decay of similarity with host phylogenetic fdistance in parasite faunas. Parasitology. 2010;137:733–741. doi: 10.1017/S0031182009991491. [DOI] [PubMed] [Google Scholar]
- Poulin R. Mouillot D. Parasite specialization from a phylogenetic perspective: a new index of host specificity. Parasitology. 2003;126:473–480. doi: 10.1017/s0031182003002993. [DOI] [PubMed] [Google Scholar]
- Poulin R. Mouillot D. Combining phylogenetic and ecological information into a new index of host specificity. J. Parasitol. 2005;91:511–514. doi: 10.1645/GE-398R. [DOI] [PubMed] [Google Scholar]
- Poulin R, Krasnov BR. Mouillot D. Host specificity in phylogenetic and geographic space. Trends Parasitol. 2011;27:355–361. doi: 10.1016/j.pt.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Silvestri L, Antoniazzi LR, Couri MS, Monje LD. Beldomenico PM. First record of the avian ectoparasite Philornis downsi Dodge and Aitken, 1968 (Diptera: Muscidae) in Argentina. Syst. Parasitol. 2011;80:137–140. doi: 10.1007/s11230-011-9314-y. [DOI] [PubMed] [Google Scholar]
- Skidmore P. Genus Philornis Meinert (Neomusca Malloch) the biology of the Muscidae of the world. The Hague, The Netherlands: Dr. W. Junk Publishers; 1985. [Google Scholar]
- Stireman JO. The evolution of generalization? Parasitoid flies and the perils of inferring host range evolution from phylogenies. J. Evol. Biol. 2005;18:325–336. doi: 10.1111/j.1420-9101.2004.00850.x. [DOI] [PubMed] [Google Scholar]
- Teixeira DM. Myiasis caused by obligatory parasites. Ib. General observations on the biology of species of the genus Philornis Meinert, 1890 (Diptera, Muscidae) In: Guimaraes JH, Papavero N, editors. Myiasis in Man and Animals in the Neotropical Region. São Paulo, Brazil: Editora Pleiade; 1999. pp. 71–96. [Google Scholar]
- Tripet F. Richner H. The coevolutionary potential of a ‘generalist’ parasite, the hen flea Ceratophyllus gallinae. Parasitology. 1997;115:419–427. doi: 10.1017/s0031182097001467. [DOI] [PubMed] [Google Scholar]
- Vinarski MV, Korallo NP, Krasnov BR, Shenbrot GI. Poulin R. Decay of similarity of gamasid mite assemblages parasitic on Palaearctic small mammals: geographic distance, host-species composition or environment. J. Biogeog. 2007;34:1691–1700. [Google Scholar]
- Webb CO, Ackerly DD. Kembel SW. Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics. 2008;24:2098–2100. doi: 10.1093/bioinformatics/btn358. [DOI] [PubMed] [Google Scholar]
- Weiblen GD, Webb CO, Novotny V, Basset Y. Miller SE. Phylogenetic dispersion of host use in a tropical insect herbivore community. Ecology. 2006;87:S62–S75. doi: 10.1890/0012-9658(2006)87[62:pdohui]2.0.co;2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Twenty-nine bird species host to ten Philornis species in the Island of Trinidad.