Abstract
Purpose
To determine the feasibility, safety and early effectiveness of percutaneous image-guided ablation as second-line treatment for symptomatic soft tissue vascular anomalies.
Materials and Methods
An IRB-approved retrospective review was undertaken of all patients who underwent percutaneous image-guided ablation as second-line therapy for treatment of symptomatic soft tissue vascular anomalies (VA) during the period from 1/1/2008 to 5/20/2014. US/CT- or MRI-guided and monitored cryoablation or MRI-guided and monitored laser ablation were performed. Clinical follow-up began at one month post-ablation.
Results
Eight patients with nine torso or lower extremity VA were treated with US/CT (N=4) or MRI-guided (N=2) cryoablation or MRI-guided laser ablation (N=5) for moderate to severe pain (N=7) or diffuse bleeding secondary to hemangioma-thrombocytopenia syndrome (N=1). The median maximal diameter was 9.0cm (6.5 to 11.1 cm) and 2.5cm (2.3 to 5.3 cm) for VA undergoing cryoablation and laser ablation, respectively. Seven VA were ablated in one session, one VA initially treated with MRI-guided cryoablation for severe pain was re-treated with MRI-guided laser ablation due to persistent moderate pain and one VA was treated in a planned two-stage session due to large VA size. At an average follow-up of 19.8 months (range 2 to 62 months), 7 of 7 patients with painful VA reported symptomatic pain relief. There was no recurrence of bleeding at five years post ablation in the patient with hemangioma-thrombocytopenia syndrome. There were two minor complications and no major complications.
Conclusion
Image-guided percutaneous ablation is a feasible, safe and effective second-line treatment option for symptomatic vascular anomalies.
Keywords: Vascular anomaly, vascular malformation, vasoproliferative neoplasm, cryoablation, laser ablation, MRI, CT
INTRODUCTION
Peripheral soft-tissue vascular anomalies (VA) are classified as either vasoproliferative neoplasms (VPN) including hemangiomas and hemangioendotheliomas or vascular malformations (VM) including slow-flow (capillary, venous, lymphatic), fast-flow (arterial, arteriovenous) or combined-flow malformations [1]. VA may cause pain, swelling, hematologic abnormalities or high output heart failure secondary to arteriovenous shunting depending on vascular anomaly size, location, histopathology and flow characteristics [1]. For example, Kasabach-Merritt phenomenon (KMP), also known as hemangioma thrombocytopenia syndrome, is a rare thrombocytopenic consumptive coagulopathy that can occur in patients with enlarging hemangioendotheliomas and can result in bleeding, hemolytic anemia, disseminated intravascular coagulation (DIC) and even death [2].
Historically, surgical resection, image-guided percutaneous sclerotherapy or transarterial embolization have been the standard treatments for symptomatic peripheral soft-tissue vascular anomalies depending on flow characteristics [3]. However, patients who fail standard therapy are left with no clear alternatives. Treatment failure may be secondary to small VA size, solid components or lack of conspicuity on ultrasound and fluoroscopy. More recently, there have been a few case reports and a case series of four patients who underwent successful treatment of localized, symptomatic soft-tissue venous malformations with ultrasound (US) or computed tomography (CT)-guided percutaneous radiofrequency ablation or cryoablation [4–7].
Unfortunately, vascular anomaly conspicuity may be poor with US or CT imaging that could limit these modalities for image guidance and monitoring, particularly for slow-flow VM in the absence of contrast or for smaller lesions. Magnetic resonance imaging (MRI) is effective for both diagnostic characterization of vascular anomalies and also provides excellent spatial resolution and frequently increased signal intensity of the VA with T2-weighted imaging for monitoring of treatment response [8]. As such, MRI-guided and monitored ablation, using select laser ablation and cryoablation systems, provide feasible alternatives for treatment of symptomatic vascular anomalies refractory to standard treatment in addition to CT-guided ablation [9; 10].
We report the feasibility, safety and early effectiveness of percutaneous US/CT-guided cryoablation, MRI-guided cryoablation and MRI-guided laser ablation for treatment of symptomatic, peripheral soft tissue, slow-flow vascular anomalies in patients who had failed surgical resection or percutaneous Sotradecol or ethanol sclerotherapy or who were deferred sclerotherapy due to poor visualization on ultrasound secondary to small VA size.
MATERIALS AND METHODS
With approval of the Institutional Review Board, we conducted a retrospective review using the comprehensive electronic medical record system of all patients who underwent percutaneous image-guided ablation for treatment of symptomatic vascular anomalies (VA) during the period from January 1, 2008 to May 20, 2014. Patients who did not give approval for the use of their medical record for research purposes were excluded. Demographic, clinical and imaging data were collected.
Preprocedural Workup
All patients underwent a pre-ablation diagnostic 1.5T MRI (GE, Milwaukee, WI) to assess the VA size, location and flow characteristics.
US/CT or MRI-guided cryoablation and MRI-guided laser ablation
All ablations were performed under general anesthesia with patient positioning to facilitate needle placement. Multiple cryoprobes or laser fibers were used to create overlapping ablation zones to encompass the VA while minimizing ablation of adjacent non-target tissue. Perc-17R and Perc-24 (Endocare, Austin, TX; CT only) and ice-seed, ice-sphere and ice-rod (Galil Medical, Arden Hills, MN) cryoprobes were used for cryoablation. For CT-guided cryoablation, both US (GE logiq E9, Waukesha, WI) and CT (Siemens, Erlangen, Germany) were used for needle placement guidance. For MRI-guided cryoablation (Galil Medical, Arden Hills, MN) or laser ablation (Visualase, Houston, TX), real time 1.5T MR imaging was performed using a balanced steady-state free precession (bSSFP) gradient echo sequence (Siemens, MAGNETOM Espree, Erlangen, Germany) for needle placement guidance. For cryoablation, two-freeze thaw cycles were performed under intermittent CT or MRI monitoring and freezing portion of the procedure was stopped when the lesion was encompassed by the ice ball. Laser ablation was performed with proton-resonance frequency MR thermometry monitoring every seven seconds until the lesion was encompassed by the calculated thermal damage map and there was loss of the abnormal T2 signal within the lesion. Because these were not cancerous lesions, the goal was to encompass the VA with the ablative zone while minimizing injury to the adjacent non-VA tissue. As such, an ablative margin of predetermined distance was not developed around the VA. All patients were admitted for inpatient observation.
Post-Ablation Follow-up
Clinical follow-up began at 1-month post-ablation to assess symptom improvement and follow-up MR imaging was performed at one to six months depending on level of symptom improvement. Minor and major complications were defined based on the Society of Interventional Radiology (SIR) Classification System for Complications by Outcome [11; 12].
Data Management and Statistical Methods
Statistical analyses were performed by using Prism 5.0 (GraphPad Software, Inc., La Jolla, CA). Descriptive statistics were generated.
RESULTS
Patient, soft tissue vascular anomaly and ablation characteristics are summarized in Table 1. Eight patients including five females and three males (ages 10 to 48) with nine vascular anomalies (N=8 intramuscular; N=1 subcutaneous) were treated with US/CT (N=4) or MRI-guided (N=2) cryoablation (Figure 1 and 2A–D, respectively) or MRI-guided laser ablation (N=5; Figure 2E–F and 3) for persistent pain (N=7; Figure 2 and 3) or diffuse bleeding secondary to hemangioma-thrombocytopenia syndrome (N=1; Figure 1). Baseline pain was reported as severe (N=3) or moderate (N=4) with four patients reporting pain that limited physical activity. Prior to percutaneous ablation, five patients were managed with oral opioid analgesics and two patients were managed with oral non-steroidal anti-inflammatory drugs (NSAIDs) for pain control. Seven patients had received at least one prior treatment session with percutaneous Sotradecol or ethanol sclerotherapy (range: 1 to 6 sessions). One patient had previously undergone a failed surgical resection of the VA and another patient was declined sclerotherapy due to poor visualization on ultrasound secondary to small VA size. Seven VA were treated in one session (Figure 3), one VA initially treated with MRI-guided cryoablation was re-treated with MRI-guided laser ablation (Figure 2) and one VA was treated in a planned two-stage CT-guided cryoablation session (Figure 1).
Table 1.
Patient, vascular anomaly and ablation characteristics
| Indication | VA Classification |
Prior Treatments | Size (cm) | Location | Guidance- Ablation Type |
Ablation sessions |
Number Probes |
Hospital Days |
Complications | Follow-up (months) |
Symptom Resolution |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 48/F | Consumptive1 coagulopathy | Hemangio-endothelioma | Sclerotherapy (1) | 3.0 × 10.0 × 7.0 | R subscapularis m. | US/CT-Cryoablation | 22 | 5/3 | 3/2 | None | 62 | Complete |
| Consumptive1 coagulopathy | Hemangio-endothelioma | None | 6.5 × 4.2 × 2.8 | R vastus intermedius m. | US/CT-Cryoablation | 1 | 3 | 1 | None | 56 | Complete | |
| 43/F | Severe Pain | Slow Flow VM | Surgery (1) Sclerotherapy (1) |
1.5 × 9.5 × 11.1 | L anterior chest/abdominal wall | US/CT-Cryoablation | 1 | 10 | 1 | None | 6 | Complete |
| 29/M | Moderate Pain | Slow Flow VM | Sclerotherapy (6) | 9.0 × 7.0 × 3.5 | Intermuscular between R infraspinatus m. and deltoid m. | MRI-Cryoablation | 1 | 8 | 2 | None | 5 | Complete |
| 10/M | Severe Pain | Slow Flow VM | Sclerotherapy (2) | 8.0 × 1.0 × 1.6 | R extensor hallucis longus m. and extensor digitorum longus m. | MRI-Cryoablation | 1 | 5 | 1 | Numbness dorsal aspect of 1st toe | 5 | Partial3 |
| Moderate Pain | Slow Flow VM | Sclerotherapy (2) MRI-Cryoablation |
8.0 × 1.0 × 1.6 | R extensor hallucis longus m. and extensor digitorum longus m. | MRI-Laser Ablation | 1 | 2 | 1 | None | 2 | Near Complete4 | |
| 42/F | Severe Pain | Slow Flow VM | Sclerotherapy (1) | 2.3 × 1.1 × 1.2 | Intermuscular between R trapezius m. and rhomboid m. | MRI-Laser Ablation | 1 | 2 | 1 | None | 30 | Complete |
| 44/M | Moderate Pain | Slow Flow VM | Sclerotherapy (1) | 1.5 × 1.3 × 5.3 | Soft tissue deep to R rhomboid m. and medial to subscapularis m. | MRI-Laser Ablation | 1 | 2 | 0 | None | 24 | Complete |
| 13/F | Moderate Pain | Slow Flow VM | Sclerotherapy (1) | 1.2 × 2.1 × 2.5 | R vastus medialis m. | MRI-Laser Ablation | 1 | 2 | 1 | Small Hematoma | 4 | Complete |
| 21/F | Moderate Pain | Slow Flow VM | Sclerotherapy declined5 | 1.7 × 1.1 × 2.5 | L gastrocnemius m. | MRI-Laser Ablation | 1 | 2 | 1 | None | 4 | Complete |
Diffuse bleeding secondary to hemangioma-thrombocytopenia syndrome (Kasabach-Merritt syndrome)
Planned two-stage cryoablation
Re-treatment with MRI-guided laser ablation
No residual pain at rest and occasional mild pain with activity only
VM poorly visualized on ultrasound
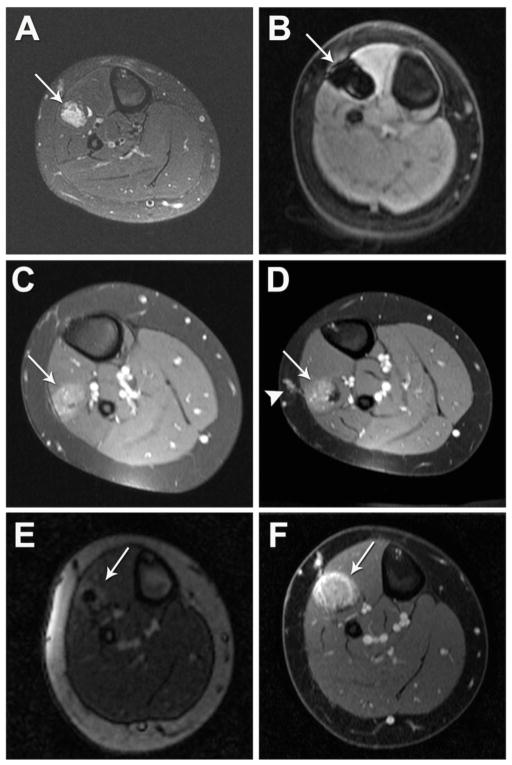
Figure 1. Planned two-stage US/CT-guided cryoablation of a hemangioendothelioma of the right subscapularis muscle in a patient with Kasabach-Merritt Syndrome.
(A) Pre-ablation gadolinium-enhanced axial T1-weight spoiled gradient echo (SPGR) MRI and (B) 3D CTA reconstruction of the vascular anomaly (white arrow). (C) Intra-procedural non-contrast enhanced axial CT during ablation session number one demonstrates zone of hypoattenuation corresponding to the ice-ball (white arrow). (D) Two-month post-ablation gadolinium-enhanced coronal T1-weighted spoiled gradient echo (SPGR) MRI demonstrates residual enhancement of the superior portion of the vascular anomaly (white arrow). (E) Intra-procedural non-contrast enhanced axial CT during ablation session number two demonstrates zone of hypoattenuation corresponding to the ice-ball (white arrow). (F) Six-month post-ablation gadolinium enhanced T1-weighted spoiled gradient echo (SPGR) MRI demonstrates significant reduction in size and no further irregular enhancement of the vascular anomaly (white arrow).
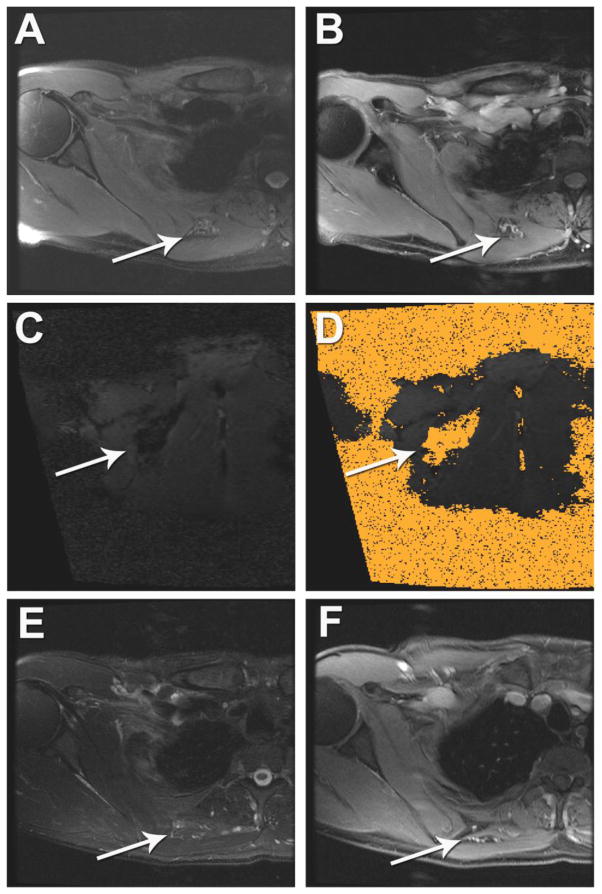
Figure 2. MRI-guided cryoablation and laser ablation of a slow-flow vascular malformation of the right extensor hallucis longus and extensor digitorum longus muscles.
(A) Pre-ablation T2-weighted MRI demonstrates abnormal T2 signal within the vascular anomaly (white arrow). (B) Intra-procedural T1-weighted MRI using a fast 3D gradient-echo sequence during cryoablation shows signal dropout within the ice ball (white arrow). (C) Immediate post-ablation gadolinium-enhanced axial T1-weighted spoiled gradient echo (SPGR) MRI demonstrates minimal enhancement of the vascular anomaly. Due to incomplete resolution of pain at three months post cryoablation, (D) follow-up gadolinium-enhanced axial T1-weighted SPGR MRI demonstrates enhancing draining veins within the center of vascular anomaly (arrow) with persistent drainage to the superficial venous system (arrowhead). (E, F) Patient was re-treated with MRI-guided laser ablation to ablate the central draining outflow veins from the center of the vascular anomaly. (E) Real-time MRI using a using a balanced steady-state free precession (bSSFP) gradient echo sequence was utilized for needle placement and demonstrates needle/laser fiber localization within the center of the vascular anomaly (arrow). (F) Immediate post-ablation gadolinium-enhanced axial T1-weighted SPGR MRI demonstrates loss of enhancement and ablation of the draining outflow veins within the center of the vascular anomaly and a peripheral hyperemic rim (arrow).
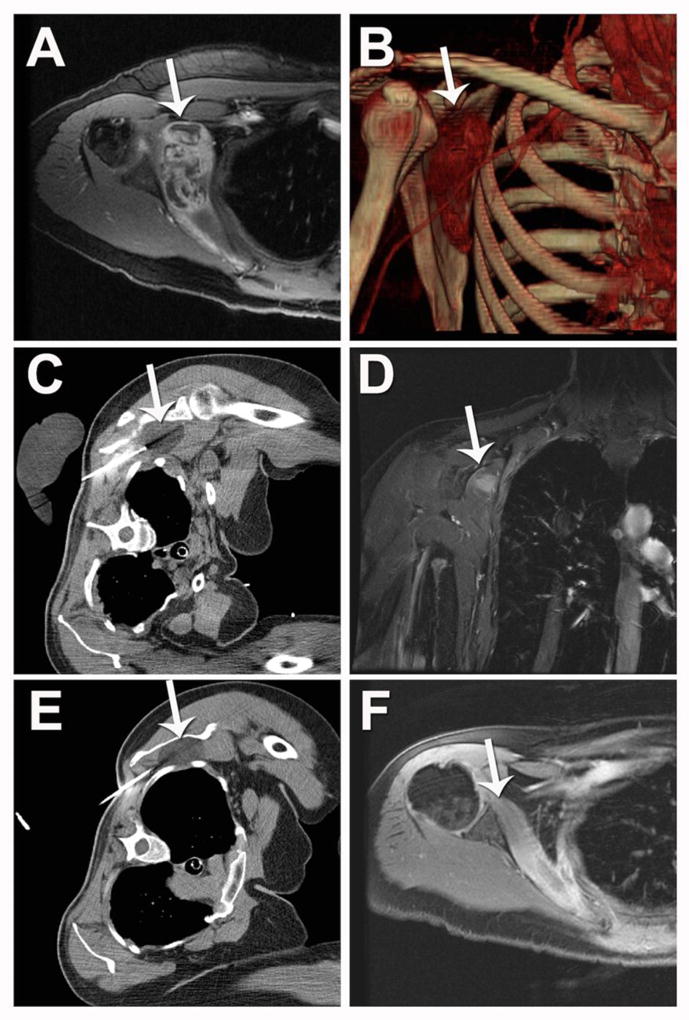
Figure 3. MRI-guided laser ablation of a slow-flow vascular malformation in the soft tissue between the right rhomboid and subscapularis muscles.
(A, B) Pre-ablation (A) axial T2-weighted and (B) gadolinium-enhanced axial T1-weight spoiled gradient echo (SPGR) MRI demonstrates heterogeneous abnormal T2 signal (A, white arrow) and contrast enhancement (B, white arrow) within the small vascular anomaly. (C, D) Intra-procedural coronal oblique (C) phase imaging demonstrates real-time tissue heating using proton resonance frequency MR thermometry and (D) thermal damage map calculated from this using the Arrhenius equation to estimate the ablation zone. (E, F) One-year post-ablation (E) axial T2-weighted MRI and (F) gadolinium-enhanced axial T1-weight spoiled gradient echo (SPGR) MRI demonstrates no significant T2 signal (E, white arrow) and no enhancement of the vascular anomaly (F, white arrow).
Prior to treatment, the VA volume [median, range] was 158.2 cm3 (12.8 to 220.6 cm3) for those undergoing cryoablation and 5.5 cm3 (3.0 to 10.3 cm3) for those undergoing laser ablation. The median (range) of the maximal diameter was 9.0 cm (6.5 to 11.1 cm) for VA undergoing cryoablation and 2.5 cm (2.3 to 5.3 cm) for VA undergoing laser ablation.
The median number of cryoprobes used was 5 (range 3 to 10). Two laser fibers were used per laser ablation session. The median cryoablation time for the freeze-thaw-freeze cycle was 11 minutes (6 to 15 minutes) for freeze one, 7.5 minutes (5 to 23 minute) for the active thaw and 9.5 minutes (8 to 12 minutes) for freeze two. The median laser ablation time was 5 minutes (2.25 to 8 minutes) with a median wattage of 22.5W (7.5 to 30 W).
For the MRI-guided ablations, technical success, defined as complete coverage of the VA by the ice ball (Figure 2) or calculated thermal damage map (Figure 3) and loss of the abnormal T2 signal on non-contrast T2-weighted MRI or loss of enhancement on gadolinium-enhanced MRI (Figure 2), was achieved in 7 of 7 ablations (100%). For CT-guided cryoablation, technical success, defined as complete coverage of the VA with the ice ball or loss of enhancement on post-ablation contrast enhanced CT or MRI, was achieved after a planned two-stage ablation for one VA (Figure 1) and after single ablations for the other two VA. The number of hospital days (median, range) was 1.5 days (1 to 3 days) for cryoablation and 1 day (0 to 1 day) for laser ablation.
Peri-ablation Complications
Minor complications (SIR classifications A–B) included a small intramuscular hematoma after laser ablation, which did not require further intervention and numbness of the dorsal aspect of first toe after cryoablation, which improved without further intervention. In the latter case, the neurovascular bundle containing the deep peroneal nerve was approximately four to five millimeters medial from the margin of the ice ball. There were no major complications (SIR classifications C–E).
Follow-up
The average follow-up was 19.8 months (range 2 to 62 months). There was no recurrence of bleeding at five years post ablation in the patient with hemangioma-thrombocytopenia syndrome. Six of seven patients with initial moderate or severe pain reported complete symptomatic pain relief with decreased need for oral analgesics beginning as early as one-month post ablation. The remaining patient with initial severe pain limiting physical activity had residual moderate pain at three months post MRI-guided cryoablation and was subsequently re-treated with MRI-guided laser ablation (Figure 2). Laser ablation was chosen for re-treatment due to the need to ablate a relatively small volume of tissue comprising the central draining veins to induce vascular thrombosis within the VA (Figure 2E). The patient reported immediate marked decrease in pain twenty-four hours after the second ablation. At two-month follow-up the patient reported no residual pain at rest and only occasional mild pain with activity. Importantly, the patient was able to resume regular physical activity that was previously limited by pain. There were no late minor or major complications.
DISCUSSION
In the present series of eight patients with nine slow-flow VA, percutaneous US/CT and MRI-guided cryoablation and laser ablation were feasible, safe and provided symptomatic relief for the majority of patients at short-term follow-up. Importantly, there were only two early minor complications—small intramuscular hematoma and mild toe numbness—that resolved without further intervention and no late minor or major complications. Of note, six of seven patients referred for persistent moderate or severe pain secondary to slow-flow VA achieved complete symptomatic relief with one ablation session while one patient with initial severe pain limiting physical activity required two ablation sessions to achieve adequate pain relief.
In recent years, there have been a few case reports and a case series of four patients who underwent successful treatment of localized, symptomatic soft-tissue VA with ultrasound (US) or computed tomography (CT)-guided percutaneous radiofrequency ablation or cryoablation [4–7]. The results from the present study confirmed the findings from prior reports in a larger series of patients and demonstrated that in addition to CT-guided ablation, MRI-guided and monitored laser ablation and cryoablation is a feasible, safe and effective treatment option for patients with symptomatic VA refractory to standard treatment. To the best of our knowledge this is the first report of MRI-guided and monitored percutaneous ablation for treatment of symptomatic VA. Moreover, while the prior reports have focused on treatment of painful vascular malformations, the present study demonstrated in a single patient that percutaneous ablation is a feasible and effective option for debulking vasoproliferative neoplasms such as hemangioendotheliomas that cause bleeding secondary to a consumptive coagulopathy.
We found that both cryoablation and laser ablation are effective for the treatment of VA, and importantly in a patient cohort that had not responded to conventional therapies. Historically, symptomatic, peripheral soft-tissue VA have been treated with surgery, percutaneous sclerotherapy, embolization or laser photocoagulation depending on size, location and flow-characteristics [13; 14]. A recent systematic review by van der Vleuten et al. examining the effectiveness of surgery, sclerotherapy and laser therapy in patients with slow-flow venous malformations found weighted average success rates of 90% for surgery (N=1 study), 94% for laser photocoagulation (range, 68% to 100%; N=4 studies) and 65% to 90% for sclerotherapy depending on sclerosant (range, 27% to 100%, N=25 studies) [3]. However, the authors noted that the overall methodological quality of the studies was poor and concluded that “the reported success rates suggest that all treatments are effective, but due to the methodological limitations a final conclusion cannot be drawn from the available data” [3]. Another recent systematic review on sclerotherapy for treatment of VA by Gurgacz et al. similarly concluded that given the varied effectiveness of percutaneous sclerotherapy for treatment of vascular malformations, “further evidence is required to delineate which patients will benefit most from percutaneous sclerotherapy to ensure that the advantages of treatment will outweigh the risks” [15]. Additionally, despite empiric use of various medical therapies for treatment of VA, Blatt et al. noted that “the rarity of these tumors has limited development of medical approaches to treatment” [16]. In short, the current evidence suggests that many reported treatment methods are effective but that there is significant variability in the outcomes for patients with symptomatic VA. Although the number of patients that are included in this study is small, cryoablation and laser ablation are safe and effective for treatment of VA and there may be a role for image-guided percutaneous ablation as a first-line treatment of symptomatic soft tissue VA in select patients.
In addition to treating vascular malformations (VMs), percutaneous ablation may be a feasible treatment option for patients with symptomatic vasoproliferative neoplasms (VPN) such as hemangioendotheliomas. In the present series, one patient suffering from diffuse bleeding secondary to hemangioma-thrombocytopenia syndrome (Kasabach-Merritt Phenomenon, KMP) has experienced no recurrence of bleeding five years after undergoing a planned-two stage cryoablation and a single stage cryoablation for two large intramuscular hemangioendotheliomas. Patients with KMP can present with a life-threatening consumptive coagulopathy and treatment of the underlying vascular neoplasm is critical for reversing the coagulopathy and hematologic abnormalities and improving overall patient survival [2]. Various combinations of medical, surgical, embolic and sclerosant therapies have been reported with varying degrees of success for treating vasoproliferative neoplasm such as hemangioendotheliomas [2]. However, Kelly noted that “most patients remain with a large residual tumor after medical or surgical therapies” and that “these quiescent tumors can progress, often resulting in pain, functional impairment, and in rare instances, a late relapse of KMP” [2; 17]. As such, percutaneous ablation may be an alternative treatment option to surgery for debulking symptomatic vasoproliferative neoplasms or serve as an adjunct to medical therapy.
Both laser ablation and cryoablation were feasible and safe. In recent years it has been recognized that a spectrum of injury may occur to peripheral nerves during percutaneous ablation [18; 19]. In the present study, one patient developed temporary numbness of the dorsal aspect of the first toe post cryoablation—likely neurapraxia of the deep peroneal nerve. The margin of the ice ball grew to within four to five millimeters of the neurovascular bundle containing the deep peroneal nerve. As such, a safety margin greater than five millimeters from the edge of the ice ball may be necessary to prevent nerve injury. In such lesions in close proximity to peripheral nerves, adjunctive protective measures and intraprocedural neurologic monitoring should be considered to prevent nerve injury. For example, carbon dioxide (CO2) dissection has been described as a safe and effective technique for displacing nerves for thermal protection during percutaneous ablation [20]. Additionally, temperature or evoked potential monitoring may be used for intraprocedural monitoring of peripheral nerves at higher risk of injury [18; 20–22]. If neurapraxia does occur following cryoablation, it has been reported that steroids may be a useful treatment option [19]. Lastly, there were no instances of frostbite or skin burn in lesions ablated in close proximity to the skin in the present series. However, similar adjunctive measure including CO2 or hydrodissection and temperature monitoring could be used for lesions at high risk for skin injury.
Of note, there was a selection bias of using cryoablation for larger VA (max diameter, 6.0 cm to 11.0 cm) and laser ablation for smaller VA (max diameter, 2.0 cm to 5.0 cm). This is approach to ablation device selection is consistent with the known advantages of cryoablation for generating larger ablation volumes per probe in soft-tissue compared to laser ablation [9; 10]. The present study demonstrated that MRI is feasible for image-guidance and intraprocedural monitoring of both cryoablation (Figure 2) and laser ablation (Figure 2, 3). MRI-guided and monitored laser ablation and cryoablation have previously been shown to be safe and effective for ablation of soft tissue neoplasms [9; 10]. MRI-guidance may be particularly helpful for smaller vascular anomalies, lesions with high inherent T2-signal on MRI and/or low conspicuity on CT without contrast and lesions near critical structures such as peripheral nerves [8].
There are limitations to this study. This was a retrospective review of a small cohort of patients with relatively short-term follow-up for the majority of patients (<1 year, N=5). Although there was longer-term follow-up for three of the patients, additional follow-up will be needed for the remainder of the cohort to assess the duration of symptomatic relief post ablation. Patient pain response to treatment was subjective although all patients treated for moderate to severe pain reported symptomatic pain relief following one (N=6) or two ablations (N=1) with decreased need for oral analgesics and/or increased ability to perform physical activity no longer limited by pain. Next, multiple imaging modalities were used for ablation guidance including US, CT and MRI as well as both cryoablation and laser ablation. Although both cryoablation and laser ablation were effective treatments, it is not known if one of these treatment methods is better suited for treatment of VA. Although MRI-guidance may provide certain advantages for ablation of VA, dedicated interventional MRI suites or MRI-compatible ablation equipment may not be readily available at many institutions. Larger prospective studies or multicenter registries of both US/CT and MRI-guided thermal ablation and cryoablation are needed to help further define the role of percutaneous ablation in the treatment of symptomatic vascular anomalies. Lastly, general anesthesia was used for all ablations in the present study and is employed for nearly all ablations at our institution. We acknowledge that general anesthesia may increase procedural time and cost and that avoiding nerve injury through direct patient feedback is not possible. As such, the aforementioned adjunctive neuroprotective and monitoring measures may be particularly important with general anesthesia. Monitored sedation and analgesia may be feasible for certain laser ablation cases but may be more challenging in the MRI environment. This study demonstrates that image-guided percutaneous thermal ablation and cryoablation are feasible, safe and effective second-line therapeutic modalities for treatment of symptomatic vascular anomalies in patients refractory to standard treatment. Both US/CT and MRI are feasible for image-guidance and intraprocedural ablation monitoring for VA treatment.
Acknowledgments
Funding Information
This publication was supported by Center for Clinical and Translational Science (CCaTS) Grant Number TL1 TR000137 from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Scott M. Thompson thanks the Mayo Clinic MSTP for fostering an outstanding environment for physician-scientist training.
Footnotes
CONFLICT OF INTEREST STATEMENT
Matthew R. Callstrom has received research grants from Thermedical Inc., General Electric Company, Siemens AG and Galil Medical Ltd. Scott Thompson, Michael A. McKusick and David A. Woodrum report no conflicts of interest related to the subject of this manuscript.
STATEMENT OF INFORMED CONSENT
A waiver of informed consent was granted by the local institutional review board in accordance with 45 CFR 46.116(d). For this type of retrospective study formal consent is not required. All individual participants consented to use of their medical record for research purposes in accordance with Minnesota state law.
STATEMENT OF HUMAN RIGHTS
For this type of retrospective study formal consent is not required.
Contributor Information
Scott M. Thompson, Email: Thompson.scott@mayo.edu, Mayo Graduate School, Mayo Medical School and the Mayo Clinic Medical Scientist Training Program, Mayo Clinic, 200 First Street SW Rochester, MN, 55905; USA
Matthew R. Callstrom, Email: callstrom.matthew@mayo.edu, Department of Radiology, Mayo Clinic, 200 First Street SW Rochester, MN, 55905; USA
Michael A. McKusick, Email: mckusick.michael@mayo.edu, Department of Radiology, Mayo Clinic, 200 First Street SW Rochester, MN, 55905; USA
David A. Woodrum, Email: woodrum.david@mayo.edu, Department of Radiology, Mayo Clinic, 200 First Street SW Rochester, MN, 55905; USA
References
- 1.Lowe LH, Marchant TC, Rivard DC, Scherbel AJ. Vascular malformations: classification and terminology the radiologist needs to know. Semin Roentgenol. 2012;47(2):106–117. doi: 10.1053/j.ro.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Kelly M. Kasabach-Merritt phenomenon. Pediatr Clin North Am. 2010;57(5):1085–1089. doi: 10.1016/j.pcl.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 3.van der Vleuten CJ, Kater A, Wijnen MH, Schultze Kool LJ, Rovers MM. Effectiveness of sclerotherapy, surgery, and laser therapy in patients with venous malformations: a systematic review. Cardiovasc Intervent Radiol. 2014;37(4):977–989. doi: 10.1007/s00270-013-0764-2. [DOI] [PubMed] [Google Scholar]
- 4.Kim AH, Ko HK, Won JY, Lee do Y. Percutaneous radiofrequency ablation: a novel treatment of facial venous malformation. J Vasc Surg. 2009;50(2):424–427. doi: 10.1016/j.jvs.2009.03.047. [DOI] [PubMed] [Google Scholar]
- 5.Childs DD, Emory CL. Successful treatment of intramuscular venous malformation with image-guided radiofrequency ablation. J Vasc Interv Radiol. 2012;23(10):1391–1393. doi: 10.1016/j.jvir.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Cornelis F, Neuville A, Labreze C, et al. Percutaneous cryotherapy of vascular malformation: initial experience. Cardiovasc Intervent Radiol. 2013;36(3):853–856. doi: 10.1007/s00270-012-0434-9. [DOI] [PubMed] [Google Scholar]
- 7.Cornelis F, Havez M, Labreze C, et al. Percutaneous cryoablation of symptomatic localized venous malformations: preliminary short-term results. J Vasc Interv Radiol. 2013;24(6):823–827. doi: 10.1016/j.jvir.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Flors L, Leiva-Salinas C, Maged IM, et al. MR imaging of soft-tissue vascular malformations: diagnosis, classification, and therapy follow-up. Radiographics. 2011;31(5):1321–1340. doi: 10.1148/rg.315105213. discussion 1340–1321. [DOI] [PubMed] [Google Scholar]
- 9.Woodrum DA, Kawashima A, Karnes RJ, et al. Magnetic resonance imaging-guided cryoablation of recurrent prostate cancer after radical prostatectomy: initial single institution experience. Urology. 2013;82(4):870–875. doi: 10.1016/j.urology.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Woodrum DA, Mynderse LA, Gorny KR, Amrami KK, McNichols RJ, Callstrom MR. 3.0T MR-guided laser ablation of a prostate cancer recurrence in the postsurgical prostate bed. J Vasc Interv Radiol. 2011;22(7):929–934. doi: 10.1016/j.jvir.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 11.Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–202. doi: 10.1097/01.rvi.0000094584.83406.3e. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed M, Solbiati L, Brace CL, et al. Image-guided Tumor Ablation: Standardization of Terminology and Reporting Criteria-A 10-Year Update. Radiology. 2014;273(1):241–260. doi: 10.1148/radiol.14132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gloviczki P, Duncan A, Kalra M, et al. Vascular malformations: an update. Perspect Vasc Surg Endovasc Ther. 2009;21(2):133–148. doi: 10.1177/1531003509343019. [DOI] [PubMed] [Google Scholar]
- 14.Alomari A, Dubois J. Interventional management of vascular malformations. Tech Vasc Interv Radiol. 2011;14(1):22–31. doi: 10.1053/j.tvir.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Gurgacz S, Zamora L, Scott NA. Percutaneous sclerotherapy for vascular malformations: a systematic review. Ann Vasc Surg. 2014;28(5):1335–1349. doi: 10.1016/j.avsg.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Blatt J, McLean TW, Castellino SM, Burkhart CN. A review of contemporary options for medical management of hemangiomas, other vascular tumors, and vascular malformations. Pharmacol Ther. 2013;139(3):327–333. doi: 10.1016/j.pharmthera.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Enjolras O, Mulliken JB, Wassef M, et al. Residual lesions after Kasabach-Merritt phenomenon in 41 patients. J Am Acad Dermatol. 2000;42(2 Pt 1):225–235. doi: 10.1016/s0190-9622(00)90130-0. [DOI] [PubMed] [Google Scholar]
- 18.Kurup AN, Morris JM, Schmit GD, et al. Neuroanatomic considerations in percutaneous tumor ablation. Radiographics. 2013;33(4):1195–1215. doi: 10.1148/rg.334125141. [DOI] [PubMed] [Google Scholar]
- 19.Philip A, Gupta S, Ahrar K, Tam AL. A spectrum of nerve injury after thermal ablation: a report of four cases and review of the literature. Cardiovasc Intervent Radiol. 2013;36(5):1427–1435. doi: 10.1007/s00270-012-0491-0. [DOI] [PubMed] [Google Scholar]
- 20.Buy X, Tok CH, Szwarc D, Bierry G, Gangi A. Thermal protection during percutaneous thermal ablation procedures: interest of carbon dioxide dissection and temperature monitoring. Cardiovasc Intervent Radiol. 2009;32(3):529–534. doi: 10.1007/s00270-009-9524-8. [DOI] [PubMed] [Google Scholar]
- 21.Tsoumakidou G, Garnon J, Ramamurthy N, Buy X, Gangi A. Interest of electrostimulation of peripheral motor nerves during percutaneous thermal ablation. Cardiovasc Intervent Radiol. 2013;36(6):1624–1628. doi: 10.1007/s00270-013-0641-z. [DOI] [PubMed] [Google Scholar]
- 22.Kurup AN, Morris JM, Boon AJ, et al. Motor Evoked Potential Monitoring during Cryoablation of Musculoskeletal Tumors. J Vasc Interv Radiol. 2014;25(11):1657–1664. doi: 10.1016/j.jvir.2014.08.006. [DOI] [PubMed] [Google Scholar]