Abstract
Purpose
The Minnesota Green Tea Trial (MGTT) was a randomized, placebo-controlled, double-blinded trial investigating the effect of daily green tea extract consumption for 12 months on biomarkers of breast cancer risk.
Methods
Participants were healthy postmenopausal women at high risk of breast cancer due to dense breast tissue with differing catechol-O-methyltransferase (COMT) genotypes. The intervention was a green tea catechin extract containing 843.0 ± 44.0 mg/day epigallocatechin gallate or placebo capsules for one year. Annual digital screening mammograms were obtained at baseline and month 12, and fasting blood and 24-hour urine samples were provided at baseline, months 6, and 12. Primary endpoints included changes in percent mammographic density, circulating endogenous sex hormones and insulin-like growth factor axis proteins; secondary endpoints were changes in urinary estrogens and estrogen metabolites and circulating F2-isoprostanes, a biomarker of oxidative stress.
Results
The MGTT screened more than 100,000 mammograms and randomized 1075 participants based on treatment (green tea extract vs. placebo), stratified by COMT genotype activity (high COMT vs. low/intermediate COMT genotype activity). 937 women successfully completed the study and 138 dropped out (overall dropout rate= 12.8%).
Conclusions
In this paper we report the rationale, design, recruitment, participant characteristics, and methods for biomarker and statistical analyses.
Keywords: Breast cancer, Green tea, Mammographic density, Postmenopausal women, Sex hormones
Breast cancer is the second leading cause of cancer-related death among women in the United States [1]. Diet is a modifiable factor considered to play an important role in the prevention of several types of cancer, including breast cancer [2,3]. Among dietary factors suggested to affect breast cancer risk, green tea has been the subject of a great deal of research within the last two decades.
There is convincing evidence from in vitro and animal studies that green tea has chemopreventive effects, although epidemiological studies are inconsistent [4–7]. The chemoprotective effects of green tea are primarily attributed to bioactive polyphenolic compounds known as catechins, wherein epigallocatechin gallate (EGCG) is the most abundant and active [8]. Some of the purported mechanisms by which green tea is believed to influence breast cancer risk include changes in well-recognized breast cancer biomarkers such as mammographic density [9], circulating sex hormone or urinary estrogen metabolites [10,11], and the insulin-like growth factor (IGF) system [12]. Tea catechins also possess potent antioxidant activities [13] and the role of oxidative stress in carcinogenesis has been established [14]; however, human clinical trial findings are mixed and further research is needed to clarify this [15,16].
The polymorphic COMT enzyme is involved in both estrogen and catechin metabolism. A G to A polymorphism at codon 108/158 of COMT (SNP rs4680) causes a valine to methionine substitution in enzyme, resulting in a 3- to 4-fold decrease in enzymatic activity in individuals possessing homozygous variant alleles A/A relative to homozygous wild-type alleles G/G, and intermediate levels of COMT activity in individuals with heterozygous variant alleles A/G [17,18]. Variability in the COMT enzyme influences catechin excretion and conversion of catechol estrogens to methoxyestrogens [19,20]. In addition, Wu et al [21] found that the inverse association between green tea intake and breast cancer risk is greater in women with COMT A/A alleles than those with G/G alleles. These findings suggest that those with the low-activity COMT enzyme may metabolize tea catechins more slowly, causing greater exposure to catechins and greater benefits from green tea intake.
The MGTT was a randomized, double blind, placebo-controlled trial designed to determine the effects of 12-month daily green tea catechin supplementation on biomarkers of breast cancer risk in 937 high-risk postmenopausal women. Breast cancer biomarkers evaluated include mammographic density, endogenous sex hormones and their metabolites, IGF axis proteins, and F2-isoprostanes, recognized biomarkers of oxidative stress. This paper describes key aspects of the trial including its rationale, design, methods, response rate and the demographic characteristics of the participants.
METHODS
Objectives
The primary objectives of this trial were to investigate the effects of consumption of green tea extract (GTE) containing 800 mg EGCG daily for one year on (i) mammographic density (ii) circulating estrone, estradiol, testosterone, androstenedione, and sex hormone binding globulin (SHBG) (iii) circulating insulin-like growth factor-1 (IGF-1) and IGF binding protein 3 (IGFBP-3) among healthy postmenopausal women at high risk of breast cancer due to dense breast tissue. We hypothesized that consumption of GTE would reduce mammographic density and circulating concentrations of IGF-1, estrone, estradiol, testosterone, and androstenedione, and increase blood levels of IGFBP-3 and SHBG, in directions associated with reduced breast cancer risk.
Secondary endpoints included urinary estrogen metabolites and circulating F2-isoprostanes. The MGTT also aimed to determine whether (i) the effect of GTE supplementation on the primary outcomes differs by COMT genotype and (ii) COMT genotypes alter tea catechin metabolism and urinary excretion. We hypothesized that the low (A/A) and intermediate (A/G) activity COMT genotypes would show the greatest response to catechin consumption and would have lower concentrations of urinary methylated catechins and methoxy estrogens, and higher circulating levels of unmethylated catechins.
This study was approved by the Institutional Review Boards (IRB) of the University of Minnesota, Park Nicollet Institute, the University of Southern California, and the University of Pittsburgh.
Study participants and recruitment
Recruitment for the MGTT took place in the Minneapolis-St. Paul metropolitan area, from August 2009 through April 2013. The eligibility and exclusion criteria of the MGTT, based on scientific and ethical considerations, are listed in Table 1.
Table 1.
Inclusion and Exclusion Criteria of the MGTT, Minnesota, 2009–2014
| Inclusion criteria |
| 50–70 years old |
| Generally healthy postmenopausal womena |
| Heterogeneously (51–75% glandular) or extremely (>75% glandular) dense breastsb |
| Planning to reside in or near Minnesota for study duration |
| Willing to give written informed consent |
| Exclusion criteria |
| Regular green tea intake (i.e. more than one cup per week) |
| Hepatitis B or C viral infection indicated by the presence of hepatitis B surface antigen or antibodies to hepatitis C virus, respectively |
| Elevated liver enzymes level > 1.5 times the upper limit of normal |
| Current or prior (within last 6 months) use of hormone therapy, including systemic postmenopausal hormone therapy (such as oral pills, patch, or gel), chemopreventive agents such as selective estrogen receptor modulators (tamoxifen, raloxifene) or aromatase inhibitors |
| Previous diagnosis of breast cancer, proliferative breast disease, or ovarian cancer |
| History of any other malignancy in the past 5 years (apart from non-melanoma skin cancer) |
| Presence of breast implants |
| Currently taking methotrexate or etanercept (Enbrel) |
| BMI <18.5 or >40 kg/m2 |
| Ongoing enrollment in a weight loss or weight gain program |
| Weight change > 10 lbs during the previous year |
| Alcohol intake >7 drinks per week |
| Current smoker of cigarettes or other tobacco products |
Abbreviation: MGTT, Minnesota Green Tea Trial.
Determined by no menstrual period for at least one year or serum follicle stimulating hormone > 23 mIU/ml (as the lower limit of normal for postmenopausal women set by the Quest Diagnostics, IL).
Determined from participant’s annual screening mammogram.
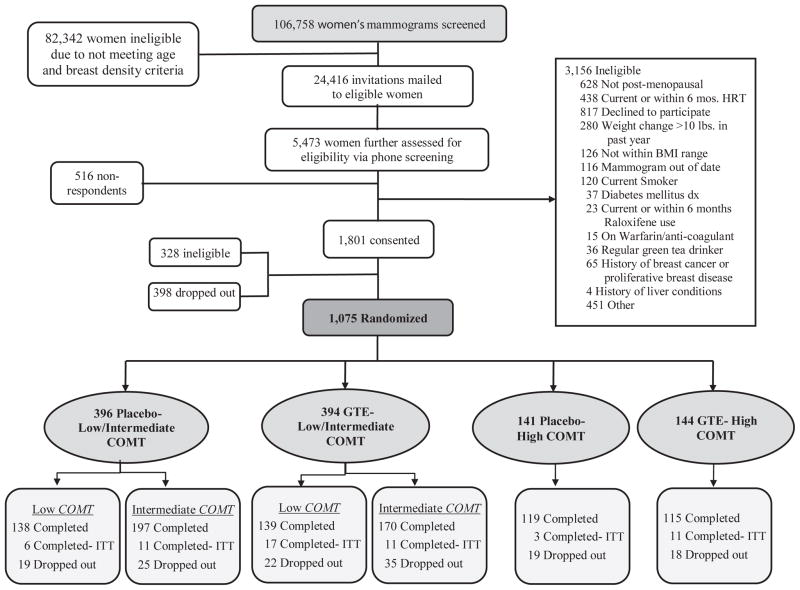
Study staff identified potential participants by reviewing routine screening mammogram reports. Women aged 50 – 70 with either “heterogeneously dense” or extremely dense” breasts, as specified by Breast Imaging and Reporting Data System criteria [22], were eligible for further study screening. Potential participants received a letter describing the intention and basic requirements of the study. If interested, prospective participants contacted the study screening hotline or website to complete a brief screening questionnaire. If they were qualified, research staff scheduled an in-person orientation session. At the end of the orientation, women signed written informed consent. A screening clinic visit was subsequently scheduled to obtain anthropometric measurements, blood pressure, and a blood draw to assess COMT genotype, hepatic function and serological markers of hepatitis B and C virus to avoid potential hepatotoxicity risk in women with compromised liver function. If eligible, participants were randomized into the study and a baseline clinic visit was scheduled. All randomized participants completed the baseline clinic visit within 3.5 months from the date of their baseline mammogram (see Fig. 1).
Fig. 1.
Flow Diagram of Participant Screening, Enrollment, Randomization, and Follow-up of the Minnesota Green Tea Trial, Minnesota, 2009–2014
Abbreviations: BMI, body mass index; COMT, catechol-O-methyltransferase; GTE, green tea extract; HRT, hormone replacement therapy; ITT, intention-to-treat
Randomization
Figure 1 depicts the randomization scheme. Randomization was performed by the Investigational Drug Services (IDS) pharmacy at University of Minnesota Medical Center -Fairview. The IDS pharmacy utilized a computer generated randomization scheme using the permuted block method and randomized participants to GTE or placebo in blocks of 8, stratified by COMT genotype (high activity = G/G or low activity = A/A + A/G). A/A and A/G were combined in the low activity group based on previous studies [21]. Accordingly, participants were randomized and stratified into one of four groups: GTE/low activity COMT; GTE/high activity COMT; placebo/low activity COMT; and placebo/high activity COMT.
Blinding
In this double-blinded study, study staff, participants, laboratory personnel, and all parties involved with assessment of the study endpoints were blinded to treatment assignment. The treatment codes were only available to the IDS pharmacy staff in charge of randomization and a study biostatistician.
Study design, data collection and processing
Table 2 shows the data collection schedule of the trial. The MGTT was a single-center, phase II, randomized, placebo-controlled, double-blind, parallel-arm trial. Participants consumed two GTE capsules or two placebo capsules twice daily for 12 months.
Table 2.
Scheduled Data Collection Part of the Minnesota Green Tea Trial Clinic Visits, Minnesota, 2009–2014
| Screen | Baseline | Month 1 | Month 2 | Month 3 | Month 4 | Month 5 | Month 6 | Month 7 | Month 8 | Month 9 | Month 10 | Month 11 | Month 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 | Visit 6 | Visit 7 | Visit 8 | Visit 9 | Visit 10 | Visit 11 | Visit 12 | Visit 13 | Visit 14 | |
| Mammogram | Xa | Xb | ||||||||||||
| Weight/vital signs | X | X | X | X | X | X | ||||||||
| Height | X | X | ||||||||||||
| Measure waist and hip circumferences | X | X | ||||||||||||
| Distribute supplement | X | X | X | X | ||||||||||
| Pill count for compliance | X | X | X | X | ||||||||||
| Collect 24-hr urine | X | X | X | |||||||||||
| Collect spot urine | X | X | ||||||||||||
| Blood draw | X | X | X | X | X | X | X | X | Xc | Xc | X | Xc | Xc | X |
| Medical history | X | |||||||||||||
| HHQ | X | |||||||||||||
| DHQ | X | X | ||||||||||||
| MENQOL questionnaire | X | X | X | |||||||||||
| Buffy coat collection | X | Xd |
Abbreviations: DHQ, diet history questionnaire; HHQ, health history questionnaire; MENQOL, menopause-specific quality of life questionnaire.
Mammograms were performed up to 3.5 months before the baseline clinic visit.
Mammograms were performed within 5 days of month 12 clinic visit.
Done for 225 (24.0%) of the participants.
Available for 625 (66.7%) of the participants.
At months 0 (i.e., baseline), 6 and 12, fasting blood draws were scheduled for measurement of trial endpoints. The remaining blood draws were non-fasting for the purpose of ALT evaluation. During the first 2 years of the study, participants came to the clinic monthly for ALT monitoring. Because very few women developed elevated ALT, especially after month 6 of the study, clinic visits at months 7, 8, 10, and 11 were omitted for the rest of the study upon receiving food and drug administration (FDA) and IRB approval in September 2011. As a result, clinic visits at those four time points only took place for 24% of the participants who completed the study. In order to retain the participants in the study and keep the dropout rate low, study staff helped participants to schedule their next clinic visit when they were coming for each visit. In addition, participants were contacted via email or phone call one week in advance to remind them of their upcoming clinic visit time.
Biospecimens
Blood
Blood was collected by a trained nurse or phlebotomist via venipuncture.
For fasting blood collections, participants were instructed to refrain from eating and drinking anything other than water for 10 hours prior to blood draw. Blood was drawn into tubes containing sodium heparin for plasma, and serum separator tubes with clot activator and gel for serum. Samples were centrifuged for 10 minutes at 4°C and 3000 rpm. Serum and plasma were separated, aliquoted, and stored at −70°C. For blood samples designated for catechin measurement, 100 μL of ascorbic acid-ethylenediaminetetraacetic acid (EDTA) solution (20% ascorbic acid, 0.1% EDTA, 5.52% sodium phosphate monobasic monohydrate) was added to 1.0 mL plasma aliquots. In addition, fasting blood samples were collected in Cell Preparation Tubes and PAX gene Blood RNA tubes (BD, Franklin Lakes, New Jersey) from nearly 850 participants for future analyses.
At the screening visit, tubes containing EDTA were extracted for buffy coat, and serum separator tubes were used for hepatic panel evaluation and screening for hepatitis B and C (Quest Diagnostics, Wood Dale, IL). Samples were centrifuged as described above. Buffy coat was collected by removing plasma from whole blood and adding 0.5 mL of 0.9% sodium chloride to each 0.5 mL aliquot, then stored at −70°C.
Urine
Participants collected 24-hour urines at months 0, 6, and 12. Participants collected all urine for 24 hours in a 3-liter plastic container containing 3 g of ascorbic acid. Urine was kept refrigerated until it was brought to the clinic the following day. Urine volume was recorded and aliquots without additive were stored at −20°C. For catechin measurement, 10 μL of ascorbic acid EDTA solution was added to 1.0 mL aliquots before storage at −70°C.
Spot urine samples were collected at the clinic at months 3 and 9 for evaluation of catechins for compliance assessment. Samples were separated into 1.0 mL aliquots, and 10 μL of ascorbic acid-EDTA solution was added before storage at −70°C.
Questionnaires
Health History Questionnaire
At the baseline visit, each participant completed an in-depth health survey including information about demographics, lifestyle factors, medical history, medication use, and full reproductive history.
Diet History Questionnaire I (DHQI)
The DHQI, developed by the National Cancer Institute (NCI)) and validated, includes 124 food items and inquires about the past 12 months of food intake with details regarding portion size and dietary supplement use. Average daily food and nutrient intake were estimated using NCI DietCalc software.
Menopause-Specific Quality of Life Questionnaire (MENQOL)
Quality of life during the previous week, with emphasis on menopausal symptoms, was assessed using the validated, self-administered MENQOL at baseline, months 6 and 12 [23,24].
Anthropometric measurements
Anthropometric measurements including weight, height, and waist and hip circumferences were taken for all participants by trained clinic staff. Weight was measured to the nearest 0.1 kg using a digital scale at screening, baseline and every three months. Standing height was determined to the nearest 0.1 cm at baseline and month 12 with a wall-mounted stadiometer. Waist and hip circumferences were measured using a flexible body tape at baseline and month 12. Waist circumference was measured at the uppermost lateral border of the iliac crest at the narrowest point of the torso, and hip circumference was measured at the widest part of the buttocks. Both measurements were repeated twice and the average was recorded to the nearest 0.1 cm.
Vital signs
At each clinic visit, blood pressure and heart rate were measured using an automated digital vital signs monitor, body temperature was taken orally by a digital thermometer, and respiration rate was estimated at rest by counting the number of breaths per minute.
Intervention
The full composition of the green tea extract is shown in Table 3. Green Tea Extract Catechin Complex (Corban complex GTB; referred to as green tea extract or GTE; Investigational New Drug #103,431) is a decaffeinated green tea extract. Participants were instructed to take two capsules, twice daily with breakfast and dinner, for a daily total of 1315 ± 115.0 mg catechins containing 843.0 ± 44.0 mg EGCG. According to the USDA Database [25], there is 70.2 mg of EGCG in 100 mL of brewed green tea. 843 mg EGCG thus was equivalent to approximately 10 grams of dry tea leaves or five 8-ounce cups of brewed green tea (843 mg/70.2 mg *100mL/237 mL).
Table 3.
Catechin and Caffeine Contents of Corban Complex GTB used in the MGTT, Minnesota, 2009–2014
| Component | Quantity per Capsulea,b (mg) | Dose per Daya (mg) | Quantity per Capsule (%) |
|---|---|---|---|
| Total catechins | 328.8 (28.9) | 1315.3 (115.4) | 80.7 |
| - Epigallocatechin (EGC) | 26.7 (29.7) | 106.8 (118.8) | 6.6 |
| - Catechin | 3.8 (2.1) | 15.2 (8.4) | 0.9 |
| - Epicatechin (EC) | 26.8 (5.9) | 107.2 (23.4) | 6.6 |
| - Epigallocatechin Gallate (EGCG) | 210.7 (11.0) | 842.8 (44.1) | 51.7 |
| - Gallocatechin Gallate (GCG) | 8.4 (1.8) | 33.6 (7.3) | 2.1 |
| - Epicatechin Gallate (ECG) | 50.6 (18.5) | 202.4 (74.0) | 12.4 |
| - Catechin Gallate (CG) | 1.1 (0.5) | 4.2 (1.9) | 0.3 |
| - Gallocatechin (GC) | 1.3 (1.4) | 5.1 (5.6) | 0.3 |
|
| |||
| Caffeine | 3.9 | 15.8 | 1.0 |
Abbreviations: GTB, green tea blend; MGTT, Minnesota Green Tea Trial.
Values are presented as means (SD) from eight batches. Catechin analyses were conducted by Covance Laboratories (Madison, WI).
Each capsule’s entity fill weight equals to 407.3 mg.
Placebo capsules were identical in appearance to the GTE and contained maltodextrin (50%), cellulose (49.5%), and magnesium stearate (0.5%). Both GTE and placebo were supplied by Corban Laboratories/Eniva Nutraceutics (Plymouth, MN), and stored at ambient temperature and moisture conditions.
Eight batches of GTE and placebo were used in this study. Catechins in each batch were analyzed by high-performance liquid chromatography in the laboratory of CS Yang at Rutgers University. Although the originally intended dose was 800 mg, the average EGCG daily dose was 843 mg EGCG.
The IDS pharmacy dispensed and clinic staff distributed capsules to participants at baseline and months 3, 6 and 9. Participants were asked to store pills in a cool and dry place and to return all unused capsules in the original bottles at each clinical visit.
Compliance assessment
To assess compliance, clinic staff counted the number of returned capsules and calculated compliance as the number of capsules actually consumed divided by the number of capsules the participant should have consumed. A second measure of compliance was urinary levels of epigallocatechin (EGC) and epicatechin (EC), measured at baseline, months 3, 6, 9 and 12 in a randomly selected 10% of participants (n = 90)
Endpoint measurement methods
Mammographic density
Digital mammograms at baseline and month 12 were assessed for % mammographic density (%MD) by an experienced researcher on scanned images using a validated, highly reproducible computer-assisted, quantitative method, Madena, developed at the University of Southern California [26,27]. Baseline and month 12 mammograms from a given participant were read in the same batch as a set but the reader was blinded to the timing of the mammogram and treatment.
Circulating sex steroids and SHBG
Estradiol, estrone, androstenedione, and testosterone were analyzed in fasting serum at the Quest Diagnostics Nichols Institute (San Juan Capistrano, CA). All samples for a given participant were analyzed in the same batch by validated liquid chromatography/tandem mass spectrometry (LC/MS/MS) following extraction and separation procedures [28].
SHBG was quantified in fasting serum samples by commercial ELISA kits (R&D Systems, Inc., Minneapolis, MN) in the laboratory of Mindy Kurzer at the University of Minnesota. All assays were performed on samples collected at baseline and month 12, and both samples from a given participant were run in duplicate in the same batch.
Insulin-like growth factor axis proteins
Fasting plasma concentrations of IGF-1 and IGFBP-3 were quantified in baseline and month 12 samples using commercially available enzyme-linked immunosorbent assay kits (R&D Systems, Inc., Minneapolis, MN) in the laboratory of Mindy Kurzer at the University of Minnesota. Both samples from a given participant were measured in duplicate in the same batch.
For reproductive hormones, IGF-1, and IGFBP3, samples at month 6 were also analyzed on a subset of 374 participants (187 in GTE and 187 in placebo group). Since there were no differences between the months 6 and 12 levels, analyses were done for months 0 and 12 only for the remainder of the participants.
Urinary estrogen metabolites
Urinary estrone (E1), estradiol (E2), and their metabolites were quantified in 24-hour urine samples collected at baseline and month 12 from all participants, in the laboratory of Mindy Kurzer at the University of Minnesota. The metabolites measured were 2-hydroxy E1, 2-hydroxy E2, 4-hydroxy E1, 4-hydroxy E2, 2-methoxy E1, 2-methoxy E2, 4-methoxy E1, 4-methoxy E2, estriol, and 16α-hydroxy E1 using a modification of the validated method developed by Xu [29]. Baseline and month 12 samples from each participant were analyzed in duplicate in the same batch.
F2-isoprostanes
Free F2-isoprostanes were measured in EDTA plasma in the University of Minnesota Molecular Epidemiology and Biomarkers Research Laboratory by a validated gas chromatography-mass spectrometry method as described by Morrow [30–32] and Gross [33].
Catechins
Catechins and their metabolites were quantified in plasma and urine samples collected from participants in the GTE group at month 12. Urinary EGC, methylated-EGC, EC, and catechin ring-fission microbial metabolites 5-(3′, 4′, 5′-trihydroxy-phenyl)-γ-valerolactone (M4) and 5-(3′, 4′-dihydroxy-phenyl)-γ-valerolactone (M6), and plasma EGCG, EGC, epicatechin gallate, and EC were measured using validated methods [34,35]. Urinary creatinine was analyzed in these samples via a modified method as described previously [36].
To measure compliance, urinary levels of EGC and EC were measured at baseline, months 3, 6, 9 and 12 in a randomly selected 10% of participants. In addition, for a subsample of 180 participants urinary and plasma catechins were quantified from samples collected at baseline, months 6 and 12.
All catechin analyses were completed in the laboratory of CS Yang at Rutgers University.
COMT, SULT, and UGT genotyping
DNA was extracted from buffy coat samples using the Qiagen DNAeasy Blood and Tissue Kit method (Qiagen Inc., Gaithersburg, MD) according to the manufacturer’s protocol. A TaqMan assay was developed for determining the COMT A/G polymorphism using a TaqMan PCR Core Reagent kit (Applied Biosystems, Foster City, CA). Forty-six known tag SNPs involved in glucuronidation and sulfation pathways were chosen for exploratory analyses of SULT1A1, SULT1E1, UGT1A1, UGT1A4, UGT1A6, UGT1A8, and UGT2B7 genes. SNP analysis was performed on Sequenom iPlex platform (Sequenom, San Diego, CA) by the University of Minnesota Genomics Center.
Data and safety monitoring
An external data and safety monitoring board (DSMB) monitored the integrity of the trial, data collection, study progress, and adverse events during the study. A biostatistician performed all requested analyses for the DSMB and provided them with the randomization code. Aside from the DSMB, all serious adverse events were reported to the NIH, FDA, the supplement manufacturer, and the IRB of the University of Minnesota within the required time frame specified by each organization. Additionally, a trained clinical monitor from the University of Minnesota periodically audited trial compliance with the protocol approved by the IRB, and ensured that the study was conducted and reported in accordance with FDA Good Clinical Practice.
Sample size estimate and statistical analysis
Sample size was estimated based on the study primary endpoint, change in %MD. With the originally planned sample size of 800 (400 in GTE and 400 in placebo), the MGTT had 81% statistical power to detect 3.4% reduction in the %MD between the GTE vs. placebo groups. Additionally, we had 87% and 100% power to detect 13% change each in circulating estrone and IGF-1 levels, respectively. The calculations assumed a two-sided significance level of 5% (α =0.05). Since the MGTT completed the study for 937 rather than 800 participants, power was enhanced to 86% and 91% to detect desired changes specified above in the %MD and circulating estrone level, respectively.
The intention-to-treat (ITT) principle was used for the analysis of the study results. Participants who withdrew or were suspended from the study due to experiencing ALT elevation were invited to remain in the study and follow all study procedures except taking the study supplements. Comparisons shown in this paper include all those who completed the study (completers). Comparisons between GTE and placebo participants for baseline demographic, dietary intake, and withdrawal reasons were based on Student t-tests for continuous variables and chi-square or Fisher’s exact tests for categorical variables. For compliance assessment, data were analyzed on a log-scale and were reported as geometric means and 95% confidence intervals. Data were analyzed using SAS software, version 9.3 (SAS Institute Inc., Cary, North Carolina). Statistical tests were two-sided, and the value of P < 0.05 was considered statistically significant.
RESULTS
Enrollment
Figure 1 shows the flow of potential participants through the recruitment and study. Mammograms taken for diagnosis of breast cancer or proliferative breast disease, composed almost entirely of fat or scattered fibroglandular tissue were not considered eligible. Participants who responded to the letter of invitation were further screened via telephone (response rate: 24.2%). 1075 women were randomly assigned to either GTE (n = 538) or placebo (n = 537) and stratified by COMT genotype to one of four treatment/genotype groups. A total of 937 women completed the study, 59 of which (6.7% of completers) went off study product during the study period but chose to remain in the study, in accordance with the ITT model. In addition, 138 participants (12.8%) dropped out of the study.
Baseline characteristics and dietary intake
Table 4 summarizes participants’ baseline characteristics for those who completed the study. The majority of participants were white, non-Hispanic, never-smokers, past users of oral contraceptives, and had some college level education. There were no significant differences in baseline characteristics between the two groups except that individuals in the GTE group reported higher intake of vitamin supplements compared with those in the placebo group. The COMT G/G, G/A, and A/A genotypes were distributed as follows: 26.5%, 41.8%, and 31.7%, respectively, close to their distributions in Caucasian women reported previously[37]. The baseline characteristics were not significantly different among high, intermediate, and low genotype participants except for race for which there were more non-white participants in the high and intermediate COMT genotype groups than the low COMT genotype group (P = 0.03). Table 5 also shows the baseline characteristics of all participants who were randomized into the study. Results are mostly similar to the Table 4 with the exception that randomized placebo participants were significantly taller than women in the GTE group.
Table 4.
Baseline Demographic and Clinical Characteristics of Randomized Completer Participants of the MGTT; ITT Model, Minnesota, 2009–2014 (n= 937)
| GTE (n= 463) | Placebo (n= 474) | P valuea | |
|---|---|---|---|
| Age at baseline, y | 60.0 (4.9) | 59.7 (5.0) | 0.26 |
| Race | 0.44 | ||
| - White | 453 (97.8%) | 458 (96.6%) | |
| - Asian | 3 (0.7%) | 7 (1.5%) | |
| - Black | 2 (0.4%) | 5 (1.1%) | |
| - Others | 4 (0.9%) | 2 (0.4%) | |
| - Unknown | 1 (0.2%) | 2 (0.4%) | |
| Ethnicity | 0.90 | ||
| - Hispanic | 5 (1.1%) | 4 (0.8%) | |
| - Non-Hispanic | 453 (97.8%) | 464 (97.9%) | |
| - Unknown | 5 (1.1%) | 6 (1.3%) | |
| COMT genotype | 0.32 | ||
| - High | 126 (27.2%) | 122 (25.7%) | |
| - Intermediate | 181 (39.1%) | 208 (43.9%) | |
| - Low | 156 (33.7%) | 144 (30.4%) | |
| Level of education | 0.14 | ||
| - High school or less | 27 (5.8%) | 31 (6.5%) | |
| - Some college | 313 (67.6%) | 287 (60.6%) | |
| - Postgraduate or professional degree | 121 (26.1%) | 152 (32.1%) | |
| - Unknown | 2 (0.4%) | 4 (0.8%) | |
| Family history of breast cancerb | 0.22 | ||
| - No | 332 (71.7%) | 355 (74.9%) | |
| - Yes | 120 (25.9%) | 114 (24.1%) | |
| - Unknown | 11 (2.4%) | 5 (1.1%) | |
| Age at menarche, yc | 13.0 (1.4) | 12.9 (1.4) | 0.78 |
| Parity Status | 0.27 | ||
| - Nulliparous | 108 (23.3%) | 110 (23.2%) | |
| - Parous | 354 (76.5%) | 359 (75.7%) | |
| - Unknown | 1 (0.2%) | 5 (1.1%) | |
| Age at first live birth, yd,e | 27.9 (5.4) | 28.6 (5.4) | 0.07 |
| Breastfeeding, total monthsd,f | 13.3 (17.4) | 13.5 (16.2) | 0.87 |
| Type of menopause | 0.18 | ||
| - Natural | 397 (85.8%) | 409 (86.3%) | |
| - Surgical: bilateral oophorectomy | 35 (7.6%) | 45 (9.5%) | |
| - Surgical: ovarian status unknown | 27 (5.8%) | 15 (3.2%) | |
| - Unknown | 4 (0.9%) | 5 (1.1%) | |
| Age at menopause, yg | 49.0 (5.7) | 49.3 (5.2) | 0.47 |
| Years since menopauseh | 11.1 (7.6) | 10.3 (7.2) | 0.13 |
| Past use of oral contraceptives | 0.85 | ||
| - No | 65 (14.0%) | 70 (14.8%) | |
| - Yes | 395 (85.3%) | 402 (84.8%) | |
| - Unknown | 3 (0.7%) | 2 (0.4%) | |
| Past use of hormone therapy | 0.95 | ||
| - No | 270 (58.3%) | 278 (58.7%) | |
| - Yes | 189 (40.8%) | 191 (40.3%) | |
| - Unknown | 4 (0.9%) | 5 (1.1%) | |
| Height, cm | 163.6 (6.3) | 164.3 (6.1) | 0.10 |
| Weight, kg | 67.3 (10.7) | 67.4 (10.3) | 0.94 |
| BMI, kg/m2 | 25.2 (3.7) | 25.0 (3.7) | 0.52 |
| Waist-to-hip ratio | 0.84i (0.1) | 0.83 (0.1) | 0.21 |
| Use of vitamin supplements | 0.04 | ||
| - No | 42 (9.1%) | 66 (13.9%) | |
| - Yes | 420 (90.7%) | 408 (86.1%) | |
| - Unknown | 1 (0.2%) | 0 (0.0%) | |
| Smoking status | 0.84 | ||
| - Never | 316 (68.3%) | 324 (68.4%) | |
| - Former | 145 (31.3%) | 149 (31.4%) | |
| - Unknown | 2 (0.4%) | 1 (0.2%) | |
| Alcohol drinks/week (drinkers only)j | 3.4 (3.0) | 3.4 (3.0) | 0.81 |
| Self-reported physical activity, MET-h/weekk | 45.7 (54.0) | 51.0 (106.9) | 0.34 |
Abbreviations: BMI, body mass index; COMT, catechol-O-methyltransferase; GTE, green tea extract; ITT, intention-to-treat; MET, metabolic equivalent; MGTT, Minnesota Green Tea Trial.
NOTE: Data presented as the means (SD) for continuous variables and as frequencies (%) for categorical variables.
P value for difference between the GTE-completers with the Placebo-completers.
Only in first degree relatives.
GTE (n): completers= 457; Placebo (n): completers= 460.
Among parous women only.
GTE (n): completers= 354; Placebo (n): completers= 359.
GTE (n): completers= 348; Placebo (n): completers= 353.
GTE (n): completers= 442; Placebo (n): completers= 454.
GTE (n): completers= 440; Placebo (n): completers= 454.
n= 461.
GTE (n): completers= 368; Placebo (n): completers= 396.
GTE (n): completers= 463; Placebo (n): completers= 473.
Table 5.
Baseline Demographic and Clinical Characteristics of all Randomized Participants; ITT Model, Minnesota, 2009–2014 (n= 1075)
| GTE (n= 538) | Placebo (n= 537) | p valuea | |
|---|---|---|---|
| Age at baseline, y | 59.9 (5.0) | 59.6 (5.1) | 0.35 |
| Race | 0.44 | ||
| - White | 515 (95.7) | 505 (94.0) | |
| - Asian | 3 (0.6) | 7 (1.3) | |
| - Black | 4 (0.7) | 7 (1.3) | |
| - Others | 4 (0.7) | 2 (0.4) | |
| - Unknown | 12 (2.2) | 16 (3.0) | |
| Ethnicity | 0.76 | ||
| - Hispanic | 5 (0.9) | 6 (1.1) | |
| - Non-Hispanic | 516 (95.9) | 510 (95.0) | |
| - Unknown | 17 (3.2) | 21 (3.9) | |
| COMT genotype | 0.51 | ||
| - High | 144 (26.8) | 141 (26.3) | |
| - Intermediate | 216 (40.2) | 233 (43.4) | |
| - Low | 178 (33.1) | 163 (30.3) | |
| Level of education | 0.19 | ||
| - High school or less | 33 (6.1) | 39 (7.3) | |
| - Some college | 348 (64.7) | 313 (58.3) | |
| - Postgraduate or professional degree | 143 (26.6) | 166 (30.9) | |
| - Unknown | 14 (2.6) | 19 (3.5) | |
| Family history of breast cancerb | 0.71 | ||
| - No | 384 (71.4) | 394 (73.4) | |
| - Yes | 130 (24.2) | 123 (22.9) | |
| - Unknown | 24 (4.5) | 20 (3.7) | |
| Age at menarche, yc | 13.0 (1.4) | 13.0 (1.4) | 0.97 |
| Parity Status | 0.46 | ||
| - Nulliparous | 118 (21.9) | 125 (23.3) | |
| - Parous | 407 (75.7) | 393 (73.2) | |
| - Unknown | 13 (2.4) | 19 (3.5) | |
| Age at first live birth, yd,e | 27.8 (5.4) | 28.4 (5.5) | 0.16 |
| Breastfeeding, total monthsd,f | 13.0 (16.7) | 12.9 (15.8) | 0.96 |
| Type of menopause | 0.11 | ||
| - Natural | 448 (83.3) | 445 (82.9) | |
| - Surgical: bilateral oophorectomy | 41 (7.6) | 54 (10.1) | |
| - Surgical: ovarian status unknown | 32 (6.0) | 18 (3.4) | |
| - Unknown | 17 (3.2) | 20 (3.7) | |
| Age at menopause, yg | 49.0 (5.8) | 49.2 (5.3) | 0.53 |
| Years since menopauseh | 10.9 (7.8) | 10.3 (7.2) | 0.20 |
| Past use of oral contraceptives | 0.70 | ||
| - No | 68 (12.6) | 77 (14.3) | |
| - Yes | 455 (84.6) | 444 (82.7) | |
| - Unknown | 15 (2.8) | 16 (3.0) | |
| Past use of hormone therapy | 0.87 | ||
| - No | 306 (56.9) | 302 (56.2) | |
| - Yes | 216 (40.2) | 216 (40.2) | |
| - Unknown | 16 (3.0) | 19 (3.5) | |
| Height, cmi | 163.5 (6.3) | 164.3 (6.2) | 0.04 |
| Weight, kgi | 67.4 (10.6) | 67.9 (10.5) | 0.41 |
| BMI, kg/m2 i | 25.2 (3.7) | 25.2 (3.9) | 0.91 |
| Waist-to-hip ratioj | 0.84 (0.1) | 0.83 (0.1) | 0.26 |
| Use of vitamin supplements | 0.03 | ||
| - No | 48 (8.9) | 76 (14.2) | |
| - Yes | 477 (88.7) | 447 (83.2) | |
| - Unknown | 13 (2.4) | 14 (2.6) | |
| Smoking status | 0.98 | ||
| - Never | 357 (66.4) | 356 (66.3) | |
| - Former | 167 (31.0) | 166 (30.9) | |
| - Unknown | 14 (2.6) | 15 (2.8) | |
| Alcohol drinks/week (drinkers only)k | 3.3 (3.0) | 3.4 (3.0) | 0.77 |
| Self-reported physical activity, MET-h/weekl | 45.3 (53.1) | 51.4 (104.0) | 0.23 |
Abbreviations: ITT, intention-to-treat; GTE, green tea extract; BMI, body mass index; COMT, catechol-O-methyltransferase; MET, metabolic equivalent.
NOTE: Data presented as the means (SD) for continuous variables and as frequencies (%) for categorical variables.
p value for difference between the randomized GTE with the randomized Placebo participants.
Only in first degree relatives.
GTE (n)= 519; Placebo (n)= 509.
Among parous women only.
GTE (n)= 407; Placebo (n)= 393.
GTE (n)= 401; Placebo (n)= 386.
GTE (n)= 500; Placebo (n)= 501.
GTE (n)= 498; Placebo (n)= 501.
GTE (n)= 526; Placebo (n)= 523.
GTE (n)= 524; Placebo (n)= 523.
Only among alcohol drinkers; GTE (n)= 418; Placebo (n)= 434.
The intakes of major food groups, macro- and micro-nutrients, as well as total calories did not differ between the GTE and placebo groups (Supplement Table 1). Participants with high COMT genotype consumed significantly more monounsaturated fat than those with intermediate and low COMT genotypes at baseline (P =0.048).
Compliance
On average, participants in both treatment and placebo groups took 96.5% of prescribed capsules. As expected, urinary levels of catechins including EGC and EC were similar between two groups at baseline, but were significantly higher in the GTE participants at all time points thereafter. Participants in the GTE group experienced, on average, a 10.6-fold increase in urinary levels of EGC and 16.5-fold increase of EC concentrations compared with placebo, with a significant effect of time for both metabolites (Supplement Table 2). Significant positive correlations were noted between the pill counts and urinary levels of EGC and EC among participants in treatment group during the first 3 months of the intervention (Spearman correlation coefficient (r) = 0.33, P = 0.03 for EGC; and r = 0.35, P = 0.02 for EC).
Dropouts
The overall dropout rate of this study was 12.8% (13.9% for GTE and 11.7% for placebo), and characteristics did not differ between GTE and placebo dropouts except for higher weight in placebo-dropouts than GTE-dropouts (Supplement Table 3). Dietary intake did not differ between GTE and placebo dropouts, with the exception of higher weekly intake of soy in the placebo-dropouts compared to the GTE-dropouts (P = 0.02) (Supplement Table 4).
The most common reasons for dropout are shown in Supplement Table 5. The majority of dropouts (94.2%) withdrew from the study within the first 6 months of the intervention. Withdrawals due to adverse events were significantly more frequent in GTE than placebo group.
DISCUSSION
To the best of our knowledge, the MGTT is the largest and longest double-blind, placebo-controlled, randomized intervention study that specifically evaluated the effects of oral GTE containing defined quantities of EGCG on established biomarkers of breast cancer risk, and the only trial in postmenopausal women at high risk of breast cancer with differing COMT genotypes. To date, only two relatively small human intervention trials have examined the effects of green tea intake for 2–6 months on biomarkers of breast cancer risk in either healthy postmenopausal women (n= 103)[38] or breast cancer survivors (n= 40)[39].
The rationale for the GTE dose was based on safety and efficacy described in earlier pharmacokinetic studies and the upper end of green tea consumption in Asian populations [38–41]. Observed adverse events in the MGTT were mild and have been reported separately [42].
The large sample size, 12 month intervention period, and randomized, double-blind, placebo controlled design, excellent compliance, and objective measures of urinary catechin levels are strengths of this study. We also took advantage of a unique nutrigenetic approach to determine individual differences in metabolism of tea catechins based on COMT genotype as well as the state-of-the-art methodologies to measure circulating and urinary levels of estrogens and their metabolites. Furthermore, the study supplements were repeatedly checked to assure catechin stability. Finally, we were successful in retaining the participants in the study, as demonstrated by a dropout rate of 12.8%.
This study has some limitations as well. We used one dose of tea catechin extract and its impact on breast cancer risk factors may not be extrapolated to other forms of catechin administration or other doses of GTE. We had limited success in recruiting minority populations, and baseline measurement of study biomarkers was based on single sample collection. A further limitation was that we did not capture information on frequency, amount, or duration of green tea drinking prior to the study, thus we cannot rule out potential effect of chronic past green tea intake. Finally, we cannot completely rule out the possibility of a collinearity effect of catechins from other dietary sources such as black tea, dark chocolate, apples, or wine; however, it should be noted that the quantity of catechins provided in the GTE is much higher than that usually found in other catechin-containing foods. For example, provided amount of EGCG from the GTE treatment was more than hundred times of amount usually found in 100 g of normal dietary sources of raw Fuji apples, hazelnuts, or pecans; similarly catechin content of the GTE was more than 5-time than 100 g of dark chocolate, which is one of the best dietary sources of catechin [25,43].
In summary, the MGTT enrolled 1075 participants and completed the trial for 937 women during 2009–2014. The MGTT is the largest long-term study investigating the effects of green tea catechins on well-known biomarkers of breast cancer among postmenopausal women at high risk of breast cancer. This study aims to elucidate the mechanisms by which green tea may reduce breast cancer risk, potentially identifying subgroups of women who may benefit from green tea intake, and lead to improved dietary recommendations for breast cancer prevention.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health/National Cancer Institute (Grant R01 CA127236); the Department of Defense/U.S. Army Medical Research and Materiel Command (Award Number W81XWH-11-1-0013); National Cancer Institute (Award Number T32CA132670); and the Minnesota Agricultural Experiment Station (Project #MIN-18-103). Also, research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (Award Number UL1TR000114) and Cancer Center Support Grant National Institutes of Health/National Cancer Institute (P30 CA077598).
The authors would like to acknowledge the study participants, the data collection team and study administrative staff at the Human Nutrition Research Clinic, Department of Food Science and Nutrition, University of Minnesota; the Oncology Research Department of the Park Nicollet Institute (St. Louis Park, MN), and the Investigational Drug Services pharmacy of the University of Minnesota Medical Center, Fairview. We also thank Corban Laboratories/Eniva Nutraceutics (Plymouth, MN) for providing the study supplement.
Abbreviations
- %MD
% mammographic density
- ALT
alanine aminotransferase
- BMI
body mass index
- CI
confidence interval
- COMT
catechol-O-methyltransferase
- DHA
docosahexaenoic acid
- DHQ1
diet history questionnaire I
- DSMB
data and safety monitoring board
- E1
estrone
- E2
estradiol
- EC
epicatechin
- EDTA
ethylenediaminetetraacetic acid
- EGC
epigallocatechin
- EGCG
epigallocatechin gallate
- EPA
eicosapentaenoic acid
- FDA
food and drug administration
- GTE
green tea extract
- IDS
investigational drug services
- IGF
insulin-like growth factor
- IGFBP-3
IGF binding protein 3
- IRB
institutional review boards
- ITT
intention-to-treat
- LC/MS/MS
liquid chromatography/tandem mass spectrometry
- M4
5-(3′, 4′, 5′-trihydroxy-phenyl)-γ-valerolactone
- M6
5-(3′, 4′-dihydroxy-phenyl)-γ-valerolactone
- MENQOL
menopause-specific quality of life questionnaire
- MET
metabolic equivalent
- MGTT
minnesota green tea trial
- NCI
national cancer institute
- SHBG
sex hormone binding globulin
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest are disclosed.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This trial was registered at clinicaltrials.gov as NCT00917735 on June 8, 2009.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, Gapstur S, Patel AV, Andrews K, Gansler T, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62:30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 3.Thomson CA. Diet and breast cancer: understanding risks and benefits. Nutr Clin Pract. 2012;27:636–650. doi: 10.1177/0884533612454302. [DOI] [PubMed] [Google Scholar]
- 4.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang ZY, Huang MT, Lou YR, Xie JG, Reuhl KR, Newmark HL, Ho CT, Yang CS, Conney AH. Inhibitory effects of black tea, green tea, decaffeinated black tea, and decaffeinated green tea on ultraviolet B light-induced skin carcinogenesis in 7,12-dimethylbenz[a]anthracene-initiated SKH-1 mice. Cancer Res. 1994;54:3428–3435. [PubMed] [Google Scholar]
- 6.Sartippour MR, Shao ZM, Heber D, Beatty P, Zhang L, Liu C, Ellis L, Liu W, Go VL, Brooks MN. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J Nutr. 2002;132:2307–2311. doi: 10.1093/jn/132.8.2307. [DOI] [PubMed] [Google Scholar]
- 7.Ogunleye AA, Xue F, Michels KB. Green tea consumption and breast cancer risk or recurrence: a meta-analysis. Breast Cancer Res Treat. 2010;119:477–484. doi: 10.1007/s10549-009-0415-0. [DOI] [PubMed] [Google Scholar]
- 8.Yang CS, Wang H, Li GX, Yang Z, Guan F, Jin H. Cancer prevention by tea: Evidence from laboratory studies. Pharmacol Res. 2011;64:113–122. doi: 10.1016/j.phrs.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Wu AH, Ursin G, Koh WP, Wang R, Yuan JM, Khoo KS, Yu MC. Green tea, soy, and mammographic density in Singapore Chinese women. Cancer Epidemiol Biomarkers Prev. 2008;17:3358–3365. doi: 10.1158/1055-9965.EPI-08-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu AH, Arakawa K, Stanczyk FZ, Van Den Berg D, Koh WP, Yu MC. Tea and circulating estrogen levels in postmenopausal Chinese women in Singapore. Carcinogenesis. 2005;26:976–980. doi: 10.1093/carcin/bgi028. [DOI] [PubMed] [Google Scholar]
- 11.Fuhrman BJ, Pfeiffer RM, Wu AH, Xu X, Keefer LK, Veenstra TD, Ziegler RG. Green tea intake is associated with urinary estrogen profiles in Japanese-American women. Nutr J. 2013;12:25. doi: 10.1186/1475-2891-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, He Z, Ermakova S, Zheng D, Tang F, Cho YY, Zhu F, Ma WY, Sham Y, Rogozin EA, et al. Direct inhibition of insulin-like growth factor-I receptor kinase activity by (−)-epigallocatechin-3-gallate regulates cell transformation. Cancer Epidemiol Biomarkers Prev. 2007;16:598–605. doi: 10.1158/1055-9965.EPI-06-0892. [DOI] [PubMed] [Google Scholar]
- 13.Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- 14.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 15.Donovan JL, DeVane CL, Chavin KD, Oates JC, Njoku C, Patrick KS, Fiorini RN, Markowitz JS. Oral administration of a decaffeinated green tea (Camellia sinensis) extract did not alter urinary 8-epi-prostaglandin F(2 alpha), a biomarker for in-vivo lipid peroxidation. J Pharm Pharmacol. 2005;57:1365–1369. doi: 10.1211/jpp.57.10.0017. [DOI] [PubMed] [Google Scholar]
- 16.Qian G, Xue K, Tang L, Wang F, Song X, Chyu MC, Pence BC, Shen CL, Wang JS. Mitigation of oxidative damage by green tea polyphenols and Tai Chi exercise in postmenopausal women with osteopenia. PLoS One. 2012;7:e48090. doi: 10.1371/journal.pone.0048090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Dawling S, Roodi N, Mernaugh RL, Wang X, Parl FF. Catechol-O-methyltransferase (COMT)-mediated metabolism of catechol estrogens: comparison of wild-type and variant COMT isoforms. Cancer Res. 2001;61:6716–6722. [PubMed] [Google Scholar]
- 19.Inoue-Choi M, Yuan JM, Yang CS, Van Den Berg DJ, Lee MJ, Gao YT, Yu MC. Genetic Association Between the COMT Genotype and Urinary Levels of Tea Polyphenols and Their Metabolites among Daily Green Tea Drinkers. Int J Mol Epidemiol Genet. 2010;1:114–123. [PMC free article] [PubMed] [Google Scholar]
- 20.Guldberg HC, Marsden CA. Catechol-O-methyl transferase: pharmacological aspects and physiological role. Pharmacol Rev. 1975;27:135–206. [PubMed] [Google Scholar]
- 21.Wu AH, Tseng CC, Van Den Berg D, Yu MC. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer Res. 2003;63:7526–7529. [PubMed] [Google Scholar]
- 22.ACR. Breast Imaging Reporting and Data System Atlas (BI-RADS® Atlas)-Mammography. 4. Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 23.Hilditch JR, Lewis J, Peter A, van Maris B, Ross A, Franssen E, Guyatt GH, Norton PG, Dunn E. A menopause-specific quality of life questionnaire: development and psychometric properties. Maturitas. 1996;24:161–175. doi: 10.1016/s0378-5122(96)82006-8. [DOI] [PubMed] [Google Scholar]
- 24.Lewis JE, Hilditch JR, Wong CJ. Further psychometric property development of the Menopause-Specific Quality of Life questionnaire and development of a modified version, MENQOL-Intervention questionnaire. Maturitas. 2005;50:209–221. doi: 10.1016/j.maturitas.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Bhagwat S, Haytowitz DB, Holden JM. U.S. Department of Agriculture, Agricultural Research Service, editor. 2014:Data Laboratory Home Page. Dec 13, 2014. USDA Database for the Flavonoid Content of Selected Foods, Release 3.1. [Google Scholar]
- 26.Ursin G, Astrahan MA, Salane M, Parisky YR, Pearce JG, Daniels JR, Pike MC, Spicer DV. The detection of changes in mammographic densities. Cancer Epidemiol Biomarkers Prev. 1998;7:43–47. [PubMed] [Google Scholar]
- 27.Ursin G, Ma H, Wu AH, Bernstein L, Salane M, Parisky YR, Astrahan M, Siozon CC, Pike MC. Mammographic density and breast cancer in three ethnic groups. Cancer Epidemiol Biomarkers Prev. 2003;12:332–338. [PubMed] [Google Scholar]
- 28.Salameh WA, Redor-Goldman MM, Clarke NJ, Reitz RE, Caulfield MP. Validation of a total testosterone assay using high-turbulence liquid chromatography tandem mass spectrometry: total and free testosterone reference ranges. Steroids. 2010;75:169–175. doi: 10.1016/j.steroids.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Xu X, Veenstra TD, Fox SD, Roman JM, Issaq HJ, Falk R, Saavedra JE, Keefer LK, Ziegler RG. Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Anal Chem. 2005;77:6646–6654. doi: 10.1021/ac050697c. [DOI] [PubMed] [Google Scholar]
- 30.Morrow JD, Chen Y, Brame CJ, Yang J, Sanchez SC, Xu J, Zackert WE, Awad JA, Roberts LJ. The isoprostanes: unique prostaglandin-like products of free-radical-initiated lipid peroxidation. Drug Metab Rev. 1999;31:117–139. doi: 10.1081/dmr-100101910. [DOI] [PubMed] [Google Scholar]
- 31.Morrow JD. The isoprostanes: their quantification as an index of oxidant stress status in vivo. Drug Metab Rev. 2000;32:377–385. doi: 10.1081/dmr-100102340. [DOI] [PubMed] [Google Scholar]
- 32.Morrow JD, Roberts LJ. Mass spectrometric quantification of F2-isoprostanes as indicators of oxidant stress. Methods Mol Biol. 2002;186:57–66. doi: 10.1385/1-59259-173-6:57. [DOI] [PubMed] [Google Scholar]
- 33.Gross M, Steffes M, Jacobs DR, Yu X, Lewis L, Lewis CE, Loria CM. Plasma F2-isoprostanes and coronary artery calcification: the CARDIA Study. Clin Chem. 2005;51:125–131. doi: 10.1373/clinchem.2004.037630. [DOI] [PubMed] [Google Scholar]
- 34.Lee MJ, Prabhu S, Meng X, Li C, Yang CS. An improved method for the determination of green and black tea polyphenols in biomatrices by high-performance liquid chromatography with coulometric array detection. Anal Biochem. 2000;279:164–169. doi: 10.1006/abio.2000.4487. [DOI] [PubMed] [Google Scholar]
- 35.Li C, Lee MJ, Sheng S, Meng X, Prabhu S, Winnik B, Huang B, Chung JY, Yan S, Ho CT, et al. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chem Res Toxicol. 2000;13:177–184. doi: 10.1021/tx9901837. [DOI] [PubMed] [Google Scholar]
- 36.Slot C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17:381–387. doi: 10.3109/00365516509077065. [DOI] [PubMed] [Google Scholar]
- 37.Hong CC, Thompson HJ, Jiang C, Hammond GL, Tritchler D, Yaffe M, Boyd NF. Val158Met Polymorphism in catechol-O-methyltransferase gene associated with risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:838–847. [PubMed] [Google Scholar]
- 38.Wu AH, Spicer D, Stanczyk FZ, Tseng CC, Yang CS, Pike MC. Effect of 2-month controlled green tea intervention on lipoprotein cholesterol, glucose, and hormone levels in healthy postmenopausal women. Cancer Prev Res (Phila) 2012;5:393–402. doi: 10.1158/1940-6207.CAPR-11-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crew KD, Brown P, Greenlee H, Bevers TB, Arun B, Hudis C, McArthur HL, Chang J, Rimawi M, Vornik L, et al. Phase IB randomized, double-blinded, placebo-controlled, dose escalation study of polyphenon E in women with hormone receptor-negative breast cancer. Cancer Prev Res (Phila) 2012;5:1144–1154. doi: 10.1158/1940-6207.CAPR-12-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chow HH, Cai Y, Alberts DS, Hakim I, Dorr R, Shahi F, Crowell JA, Yang CS, Hara Y. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev. 2001;10:53–58. [PubMed] [Google Scholar]
- 41.Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, Dorr RT, Hara Y, Alberts DS. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–3319. [PubMed] [Google Scholar]
- 42.Dostal AM, Samavat H, Bedell S, Torkelson C, Wang R, Swenson K, Le C, Wu AH, Ursin G, Yuan JM, et al. The safety of green tea extract supplementation in postmenopausal women at risk for breast cancer: results of the Minnesota Green Tea Trial. Food Chem Toxicol. 2015;83:26–35. doi: 10.1016/j.fct.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neveu V, Perez-Jiménez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford) 2010;2010:bap024. doi: 10.1093/database/bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.