TO THE EDITOR
Syringocystadenoma papilliferum (SCAP) is a benign cutaneous hamartomatous tumor (Mammino and Vidmar, 1991) which rarely undergoes transformation to malignant syringocystadenocarcinoma papilliferum (SCACP) (Satter et al., 2014). SCAP are thought to be sweat gland tumors, with evidence suggesting that they may arise from a multipotent progenitor (Yamamoto et al., 2002).
Notably, SCAP rarely spontaneously arise within nevus sebaceus (NS) (OMIM 162900) lesions, caused by somatic mutations in HRAS and KRAS. Additional mutations necessary for SCAP development in NS have yet to be determined (Groesser et al., 2012). Following a report that NS lesions result from PTCH deletion, PTCH deletion was also reported in some SCAP lesions (Boni et al., 2001) though subsequent studies found that NS results nearly exclusively from somatic RAS mutation (Groesser et al., 2012).
SCAP can develop spontaneously as a solitary lesion, or can appear in a linear pattern at birth following Blaschko’s lines. Such linear patterns are rare and have not been reported to progress to malignancy. It is unclear if this is due to the small number of lesions observed, or to distinct molecular pathogenesis. Predicting that a somatic mutation would cause linear SCAP, we interrogated pathogenesis via paired whole exome sequencing (WES) of affected tissue and blood in one case.
This study was approved by the Yale Human Investigation Committee, and complies with the Declaration of Helsinki guidelines. Subjects provided written informed consent, except in the case of archival tissue samples, which were provided anonymized.
Our index case is a 12 year-old, otherwise healthy, girl with no other medical problems and no family history of any unusual nevi. At birth, an erythematous Blaschko-linear plaque composed of individual papules was noted. The lesion remained stable throughout childhood. (Figure 1, A). Histopathology revealed a cystic epithelial invagination containing papillae lined by columnar epithelium (Figure 1, B and C) and underlying dermis with abundant plasma cells.
Figure 1.
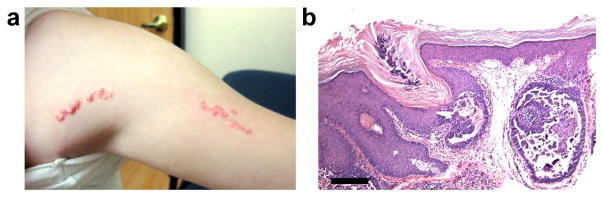
Clinical and histological features of linear SCAP. (A) Linear pink hyperkeratotic papules in a 12-year-old girl have been present since birth. (B) Histopathology demonstrates a cystic epithelial lesion containing papillary projections lined by columnar epithelium and stromal plasma cell infiltration. Scale bar = 500 um.
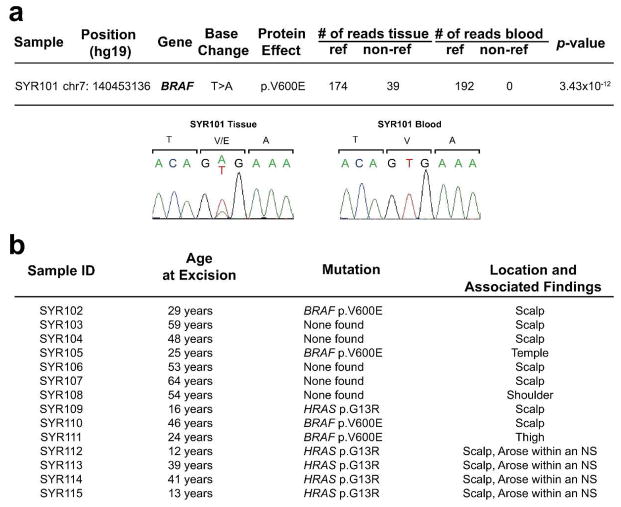
WES was performed with mean coverage depth of 78x in blood and 104x in tissue (Supplementary Table 1). Comparison of single nucleotide variants (SNVs) and small insertions and deletions between tissue and blood revealed a single damaging somatic SNV: BRAF c.T1799A, p.V600E (Figure 2); 22% of reads were mutant in tissue (39/174) and blood had no mutant reads (0/192). Sanger sequencing confirmed this finding. Somatic loss-of-heterozygosity (LOH) and copy number variations were not identified (Supplementary Figure 2). The finding of a single somatic mutation without LOH provides evidence that BRAF V600E mutation is sufficient to cause SCAP. In analysis of ten solitary, sporadic SCAPs from unrelated subjects, we found BRAF V600E somatic mutations in four, using DNA from laser-capture microdissected normal epidermis as a control. To exclude other potential pathogenic BRAF mutations, we also screened exons 6, 8, 11, 12, 13 and 16 in which mutations have been previously found in cancer and cardiofaciocutaneous syndrome (Davies et al., 2002; Niihori et al., 2006), finding none.
Figure 2.
WES demonstrates BRAF V600E somatic mutation in SCAP. (A) WES was performed paired samples and SNVs and insertions or deletions (indels) were filtered to identify protein damaging variants not found in control exomes. Remaining SNVs were then ranked by fisher score for tissue specificity. Only BRAF V600E surpassed genome wide significance for tissue specificity (2.4 × 10−6), and was confirmed by Sanger sequencing. No other mutations demonstrated a p-value less than 1 × 10−4. There were 39 non-reference reads and 147 reference reads in tissue at this site, demonstrating the presence of wild-type admixture. No non-reference reads were found in blood. Sanger sequencing confirmed that SYR101 has a tissue-specific BRAF V600E mutation. (B) 4 out of 10 sporadic SCAP demonstrated V600E mutations identified via Sanger sequencing. No other damaging mutations were found in exons 6, 8, 11, 12, 13, 15 or 16 in any of the samples. None of the 4 SCAP arising from within an NS that were screened demonstrated a V600E mutation.
SCAP develops in approximately 5% of NS lesions (Groesser et al., 2012). To determine if such NS-associated SCAP lesions are also driven by BRAF mutation, we isolated DNA from four SCAPs that arose within HRAS G13R mutation-positive NS lesions. Sequencing revealed no BRAF mutations in these lesions which were histologically indistinguishable from BRAF V600E-positive lesions (Supplementary Table 2).
Recently, using a mutation-targeted assay to interrogate genes in the mitogen-activated protein kinase and phosphatidylinositol-3′-OH kinase signaling pathways, Shen et al found BRAF V600E in 12/23 screened sporadic SCAP and activating HRAS and KRAS mutations in 7/23 sporadic SCAP (Shen et al., 2015). Consistent with our findings, BRAF mutations were not found in SCAP arising within NS. Notably, 5/6 RAS-positive lesions in the Shen study arose on the head or neck. We also identified a single archival sporadic SCAP sample, which arose on the scalp of a 16-year-old, in which we found a HRAS G13R mutation. There was insufficient tissue to determine if this lesion arose within an NS due to RAS mutation. There is precedent for focal neoplasia within RAS mutant tissue arising via copy number amplification of HRAS alleles as in spitz nevi arising within nevus spilus (Sarin et al., 2013) and papillomas in HRAS G12V mice (Chen et al., 2009). This is one possible explanation for distinct phenotypes of NS and SCAP despite bearing identical somatic RAS mutation.
BRAF is a serine kinase, which plays a crucial role in the RAS-RAF-MEK-ERK signaling pathway, and mutations including V600E have been found in about 50% of melanomas, and in colon, lung and ovarian cancers (Davies et al., 2002). Oncogenic BRAF mutations discovered to date are restricted to exons 11 and 15 (Davies et al., 2002), which encode the P-loop and the activation domains (Wan et al., 2004) that typically interact to inactivate the enzyme. The constitutively active BRAF V600E mutation lies within the activation loop, disrupting this interaction (Wan et al., 2004). Constitutional expression of BRAF V600E causes early embryonic lethality in mice (Dhomen et al., 2010), and keratin-14 driven expression leads to perinatal lethality with craniofacial defects (Krishnaswami et al., 2014), epidermal thickening and loss of keratinocyte differentiation markers.
RAS-RAF-MEK-ERK dysfunction has also been observed in RASopathies with skin and other organ abnormalities. Weakly-activating BRAF mutations cause cardiofaciocutaneous syndrome (CFC) (OMIM 115150), featuring craniofacial abnormalities, intellectual disability and cardiomyopathy (Niihori et al., 2006).
CFC can demonstrate hyperkeratotic skin lesions on extensor surfaces of the limbs and on the scalp and nipples, or generalized ichthyotic scale (Niihori et al., 2006). The marked epithelial hyperplasia of SCAP may be due to stronger BRAF activation by the V600E mutation than by more weakly-activating mutations in CFC, though further experimental investigation is warranted.
Despite SCAP’s limited malignant potential, it may be clinically important to consider the possibility of transformation to carcinoma and risk of internal malignancy in patients presenting with large mosaic SCAP lesions at birth. Mosaic disorders have been shown to extend beyond the epidermis to affect other tissues including melanocytes, bone, and neural tissue (Lim et al., 2013).
Since BRAF mutations are found in cancer, therapeutics targeting mutant BRAF have been developed. Vemurafenib, originally developed to treat melanoma, targets cells with a V600E mutation (Bollag et al., 2010). This or similar therapeutics may provide benefit for patients with BRAF V600E-positive SCAP lesions that are intractable to resection as well as for patients with SCACP.
Materials and Methods
Human Subjects
This study was approved by the Yale Human Investigation Committee, and complies with the Declaration of Helsinki guidelines. Subjects provided written informed consent, except in the case of archival tissue samples, which were provided anonymized.
DNA Extraction
For linear SCAP, DNA was directly extracted from a punch biopsy of and excised lesion. Fat and underlying dermis were trimmed to leave clinically homogeneous lesional tissue.
For archival SCAP specimens, 2–3 1mm cores were taken from the center of lesional tissue based upon a hematoxylin-eosin stained slide from an adjacent section. DNA from formalin-fixed paraffin-embedded (FFPE) archival tissue samples was extracted using an FFPE extraction kit (QIAGEN, Valencia, CA). DNA was extracted from fresh tissue and blood via standard methods.
Whole Exome Sequencing
DNA was sheared, and captured using EZexome V2 capture probes (Roche). Paired-end sequencing was performed on an Illumina HiSeq2000. Raw reads were aligned to the hg19 reference genome using BWA-mem [1]. PCR duplicates were excluded and reads were trimmed to fit the targeted regions. Variants (SNVs and indels) were called using SAMtools [2], and common variants (dbSNP 137) were excluded. A Perl script was used to identify mutations with increased non-reference reads in tissue versus blood, and manually filtered for novel, coding mutations with ≥4 non-reference reads in tissue and ≤3 non-reference reads in blood. Mutations were manually inspected using the Integrative Genomics Viewer to ensure that reads were not mismapped [3].
Copy Number Variation and Loss-of-Heterozygosity
Copy number variation and loss-of-heterozygosity events were evaluated using CoNIFER [4] (267 control exomes, SVD 20).
Sanger Sequencing
Kapa 2G polymerase was used for PCR. The following primers were used for amplification and sequencing:
BRAF_exon11_F: TTCTGTTTGGCTTGACTTGAC
BRAF_exon11_R: GACTTGTCACAATGTCACCAC
BRAF_exon15_F: TCATAATGCTTGCTCTGATAGGA
BRAF_exon15_R: GGCCAAAAATTTAATCAGTGGA
HRAS_exon2_F: CTCCTTGGCAGGTGGGGCAG
HRAS_exon2_R: AGCCCTATCCTGGCTGTGTCCTG
KRAS_exon2_F: TGAGTTTGTATTAAAAGGTACTGGTGGAG
KRAS_exon2_R: AACTTGAAACCCAAGGTACATTTCAG
Supplementary Material
Acknowledgments
We would like to thank Young Lim, Evelyn Lily and Lynn Boyden for review of the manuscript and Nouf Hijazi for technical assistance. This work was supported by the Yale Center for Mendelian Genomics (NIH U54 HG006504). JLL is supported by the Medical Scientist Training Program (NIH NIGMS GM007205) and is a recipient of a clinical research mentorship award from Doris Duke Charitable Foundation.
Abbreviations
- SCAP
syringocystadenoma papliliferum
- SCACP
syryingocystadenocarcinoma Papilliferum
- KEN
keratinocytic epidermal nevus
- NS
nevus sebaceus
- CFC
cardiofascialcutaneous syndrome
- SNV
single nucleotide variation
- LOH
loss of heterozygosity
Footnotes
Work was done in New Haven, Connecticut
The authors state no conflict of interest.
References
- Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni R, Xin H, Hohl D, et al. Syringocystadenoma papilliferum: a study of potential tumor suppressor genes. The American Journal of dermatopathology. 2001;23:87–9. doi: 10.1097/00000372-200104000-00001. [DOI] [PubMed] [Google Scholar]
- Chen X, Mitsutake N, LaPerle K, et al. Endogenous expression of Hras (G12V) induces developmental defects and neoplasms with copy number imbalances of the oncogene. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7979–84. doi: 10.1073/pnas.0900343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Dhomen N, Da Rocha Dias S, Hayward R, et al. Inducible expression of (V600E) Braf using tyrosinase-driven Cre recombinase results in embryonic lethality. Pigment cell & melanoma research. 2010;23:112–20. doi: 10.1111/j.1755-148X.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- Groesser L, Herschberger E, Ruetten A, et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nature genetics. 2012;44:783–7. doi: 10.1038/ng.2316. [DOI] [PubMed] [Google Scholar]
- Krishnaswami SR, Kumar S, Ordoukhanian P, et al. Fate and Plasticity of the Epidermis in Response to Congenital Activation of BRAF. The Journal of investigative dermatology. 2014 doi: 10.1038/jid.2014.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YH, Ovejero D, Sugarman JS, et al. Multi-lineage somatic activating mutations in HRAS and NRAS cause mosaic cutaneous and skeletal lesions, elevated FGF23, and hypophosphatemia. Human molecular genetics. 2013 doi: 10.1093/hmg/ddt429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammino JJ, Vidmar DA. Syringocystadenoma papilliferum. International journal of dermatology. 1991;30:763–6. doi: 10.1111/j.1365-4362.1991.tb04780.x. [DOI] [PubMed] [Google Scholar]
- Niihori T, Aoki Y, Narumi Y, et al. Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nature genetics. 2006;38:294–6. doi: 10.1038/ng1749. [DOI] [PubMed] [Google Scholar]
- Sarin KY, Sun BK, Bangs CD, et al. Activating HRAS mutation in agminated Spitz nevi arising in a nevus spilus. JAMA dermatology. 2013;149:1077–81. doi: 10.1001/jamadermatol.2013.4745. [DOI] [PubMed] [Google Scholar]
- Satter E, Grady D, Schlocker CT. Syringocystadenocarcinoma papilliferum with locoregional metastases. Dermatology online journal. 2014;20:22335. [PubMed] [Google Scholar]
- Shen AS, Peterhof E, Kind P, et al. Activating mutations in the RAS/mitogen-activated protein kinase signaling pathway in sporadic trichoblastoma and syringocystadenoma papilliferum. Human pathology. 2015;46:272–6. doi: 10.1016/j.humpath.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto O, Doi Y, Hamada T, et al. An immunohistochemical and ultrastructural study of syringocystadenoma papilliferum. The British journal of dermatology. 2002;147:936–45. doi: 10.1046/j.1365-2133.2002.05027.x. [DOI] [PubMed] [Google Scholar]
Supplemental References
- 1.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thorvaldsdottir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 2013;14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krumm N, Sudmant PH, Ko A, O’Roak BJ, Malig M, et al. Copy number variation detection and genotyping from exome sequence data. Genome Res. 2012;22:1525–1532. doi: 10.1101/gr.138115.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.