To the Editor
Great progress has been made treating patients with disseminated BRAF-mutant melanoma with BRAF inhibitors (Hauschild et al., 2012). Despite this, responses tend to be short-lived and the majority of patients fail therapy. We recently demonstrated that most of the proteins involved in escape from BRAF inhibitor therapy were clients of the heat shock protein (HSP)-90 family of chaperones (Paraiso et al., 2012; Smyth et al., 2014). In cell culture models, the HSP90 inhibitor XL888 overcame acquired BRAF inhibitor resistance, an effect associated with decreased expression of receptor tyrosine kinases (RTKs) PDGFR-β and IGF1R and inhibition of signaling through the mitogen activated protein kinase (MAPK) and PI3K/AKT signaling pathways (Paraiso et al., 2012). In vivo, XL888 treatment decreased growth of BRAF inhibitor-resistant melanoma xenografts (Paraiso et al., 2012). When used as frontline therapy, the combination of another HSP90 inhibitor (AT13387) with vemurafenib prevented escape from BRAF inhibition in melanoma xenograft models (Smyth et al., 2014). On the basis of these data we recently opened a phase I dose-escalation study of XL888 in combination with vemurafenib (960mg PO, BID) in patients with unresectable BRAF-mutant melanoma (NCT01657591).
An initial analysis demonstrated the combination to be well tolerated, with early indications of efficacy. Similar to the experience with single-agent BRAF inhibitor therapy, patients developed hyperproliferative/neoplastic cutaneous lesions, with some notable differences in incidence. These secondary tumors, which arise as a result of the paradoxical activation of the MAPK pathway in keratinocytes, can be at least partly abrogated by the BRAF/MEK inhibitor combination (Flaherty et al., 2012). Activating HRAS mutations are found in up to 71% of these lesions, which can take the form of benign tumors (“BRAF inhibitor-associated verrucous keratosis”), verruca vulgaris (VV), keratoacanthoma-like squamous cell carcinoma (KA-SCC) and typical SCC (Su et al., 2012). Under physiological conditions, MAPK signaling follows the activation of Ras, with the GTP-bound form of Ras inducing RAF to form dimers, which in turn leads to phosphorylation and activation of MEK and ERK (Roring et al., 2012). In cells with upstream Ras activity, the formation of RAF dimers also plays a role in paradoxical MAPK signaling (Gibney et al., 2013; Halaban et al., 2010; Poulikakos et al., 2010). Mechanistically this proceeds through the binding of the BRAF inhibitor to one RAF protomer, which induces the binding and activation of its partner RAF molecule, and an increase in MAPK signaling (Gibney et al., 2013; Poulikakos et al., 2010). Recently, next generation BRAF inhibitors have been developed that limit the paradoxical activation of ERK signaling (Le et al., 2013), but as yet their incidence of associated secondary neoplasms in humans has not been quantified.
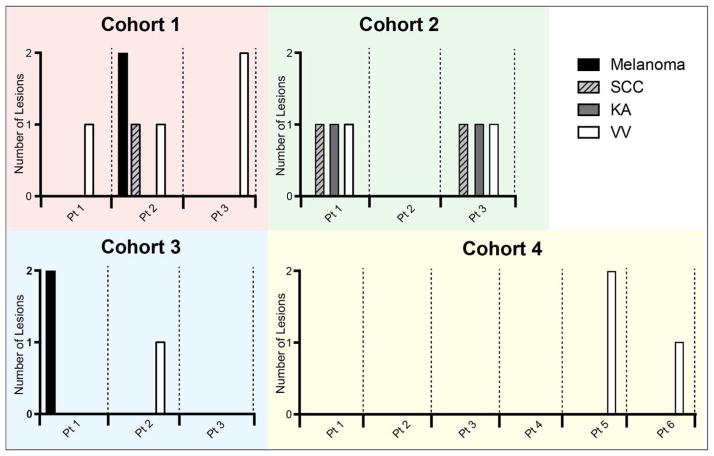
We report herein the incidence of biopsy-proven skin lesions occurring in the first 24 weeks of therapy when vemurafenib was combined with XL888 in increasing doses (Figure 1; Supplemental Figure 1; Supplemental Table 1). Lesions suspicious for malignancy were biopsied in routine dermatologic follow up. All tissue studies took place as part of an IRB-approved protocol after patients gave written, informed consent for tumor biopsies to be performed and used for research purposes. The incidence and number of skin lesions per patient decreased as the dose of XL888 increased in each of the 4 cohorts. In cohort 1 (35mg PO BIW), 3/3 patients (100%) developed a total of 6 lesions (2 melanoma, 1 SCC, 3 VV). In cohort 2 (45mg PO BIW): 2/3 (66%) patients developed 6 lesions (2 KA, 2 SCC, 2 VV). In Cohort 3 (90 mg PO BIW), 2/3 (66%) patients had 3 total lesions (2 melanoma, 1 VV). In cohort 4 (135 mg PO BIW) 2/6 (33%) developed 3 VV (Figure 1). All secondary melanomas were negative by immunohistochemistry for mutant BRAF (VE-1 antibody, Ventana) and were NRAS-wild-type by sequencing (not shown). The differences in incidence of SCC/KA between cohorts 1–3 and cohort 4 were not statistically significant, likely a reflection of the small sample size. The finding that no patients in cohort 4 developed SCCs/KAs compares favorably to the 26% incidence of SCC development previously reported in patients on single agent vemurafenib therapy (Sosman et al., 2012).
Figure 1. Incidence of secondary skin lesions by dose cohort of XL888.
Chart shows the incidence of hyperproliferative/neoplastic skin lesions (melanoma, SCC: squamous cell carcinoma, KA: keratoacanthoma, and VV: verucca vulgaris, stratified by XL888 dose cohort (cohort 1: 30mg; cohort 2: 45mg; cohort 3: 90mg; cohort 4: 135mg). Data shows number of lesions of each type for each individual patient. Cohort had 7 total events (3/3 patients), cohort 2 had 6 total events (2/3 patients), cohort 3 had 3 total events (2/3 patients) and cohort 4 had 3 total events (2/6 patients).
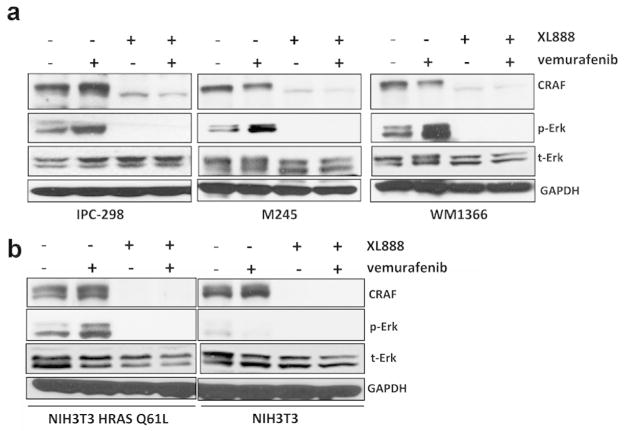
We next determined whether XL888 prevented the growth of vemurafenib-induced skin lesions through the suppression of paradoxical MAPK activation. Previous studies have shown that BRAF inhibitors activate phospho-ERK signaling in NRAS-mutant melanoma cell lines as well as in SCC cell lines that are driven through mutant HRAS (Kaplan et al., 2010; Su et al., 2012). The administration of a BRAF inhibitor has been shown to shorten the latency of SCC induction in a two-step carcinogenesis model, an effect reversible through MEK inhibition (Su et al., 2012). In our studies, treatment of 3 NRAS mutant melanoma cell lines with vemurafenib (1 μM) increased levels of phospho-ERK, an effect that was suppressed following the addition of XL888 (300nM) (Figure 2A). These effects were concentration-dependent, with levels of phospho-ERK being inhibited at concentrations of XL888 >100nM (Supplemental Figure 2A). The ability of BRAF inhibitors to activate MAPK signaling is dependent upon CRAF transactivation (Poulikakos et al., 2011; Wan et al., 2004). As CRAF is an HSP90 client protein, we next asked whether XL888 decreased the expression of CRAF. As expected, the decrease in paradoxical ERK activation observed following combined vemurafenib+XL888 treatment was also associated with decreased CRAF expression in three NRAS-mutant melanoma cell lines (Figure 2A). Similarly, XL888 also suppressed vemurafenib-driven MAPK signaling in NIH3T3 cells transformed by HRAS Q61L (Supplemental Figure 2B), an effect associated with decreased CRAF expression (Figure 2B). Taken together, these data suggest that XL888 prevented the emergence of vemurafenib-associated skin lesions through the inhibition of both CRAF transactivation and paradoxical MAPK signaling. Our clinical findings agree with recent mouse model data showing that topical HSP90 inhibition inhibits UVR-induced SCC development (Singh et al., 2015).
Figure 2. XL888 suppresses paradoxical MAPK signaling in NRAS-mutant melanoma and HRAS-transformed NIH3T3 cells.
a: XL888 prevents the paradoxical MAPK signaling in 3 NRAS mutant melanoma cell lines. IPC-298, M245 and WM1366 cell lines were treated with vemurafenib (1 μM, 72 hrs) in the absence and presence of XL888 (300 nM). Western blots show total CRAF, phospho-ERK and total ERK. b: XL888 (300 nM) suppressed vemurafenib-driven (1 μM, 72 hrs) paradoxical MAPK signaling in NIH3T3 cells transduced with HRAS Q61L.
Our conclusion is that the HSP90 inhibitor XL888 suppresses paradoxical MAPK activation in NRAS and HRAS-driven in vitro models and is associated with fewer hyperproliferative cutaneous lesions in melanoma patients receiving the combination of vemurafenib and XL888. These data suggest that the reduced incidence of cutaneous adverse events could be used as a biomarker of HSP90 inhibition in this setting.
Supplementary Material
Acknowledgments
Grant support: Supported by SPORE grant 1P50CA168536-01A1
Footnotes
Conflicts of interest
G. Gibney has served as a consultant for BMS, Genentech and Novartis. V. Sondak has served as a consultant for Merck, OncoSec, MabVax, Polynoma and Genentech. J. Weber has received honoraria from Genentech and GSK. All other authors declare no conflict of interest.
References
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. New Engl J Med. 2012;367:1694–703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney GT, Messina JL, Fedorenko IV, et al. Paradoxical oncogenesis--the long-term effects of BRAF inhibition in melanoma. Nat Rev Clin Oncol. 2013;10:390–9. doi: 10.1038/nrclinonc.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaban R, Zhang W, Bacchiocchi A, et al. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 2010;23:190–200. doi: 10.1111/j.1755-148X.2010.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–65. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- Kaplan FM, Shao Y, Mayberry MM, et al. Hyperactivation of MEK-ERK1/2 signaling and resistance to apoptosis induced by the ongenic B-RAF inhibitor, PLX4720, in mutant N-Ras melanoma cell lines. Oncogene. 2010;30:366–71. doi: 10.1038/onc.2010.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le K, Blomain E, Aplin AE. Selective RAF inhibitor impairs ERK1/2 phosphorylation and growth in mutant NRAS, vemurafenib-resistance melanoma cells. Pigment Cell Melanoma Res. 2013;26:509–17. doi: 10.1111/pcmr.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraiso KH, Haarberg HE, Wood E, et al. The HSP90 inhibitor XL888 overcomes BRAF inhibitor resistance mediated through diverse mechanisms. Clinical cancer research. 2012;18:2502–14. doi: 10.1158/1078-0432.CCR-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos PI, Persaud Y, Janakiraman M, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–U144. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–30. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roring M, Herr R, Fiala GJ, et al. Distinct requirement for an intact dimer interface in wild-type, V600E and kinase-dead B-Raf signalling. EMBO Journal. 2012;31:2629–47. doi: 10.1038/emboj.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Singh A, Sand JM, et al. Topically Applied Hsp90 Inhibitor 17AAG Inhibits UVR-Induced Cutaneous Squamous Cell Carcinomas. J Invest Dermatol. 2015;135:1098–107. doi: 10.1038/jid.2014.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth T, Paraiso KH, Hearn K, et al. Inhibition of HSP90 by AT13387 Delays the Emergence of Resistance to BRAF Inhibitors and Overcomes Resistance to Dual BRAF and MEK Inhibition in Melanoma Models. Mol Cancer Ther. 2014;13:2793–804. doi: 10.1158/1535-7163.MCT-14-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. New Engl J Med. 2012;366:707–14. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su F, Viros A, Milagre C, et al. RAS Mutations in Cutaneous Squamous-Cell Carcinomas in Patients Treated with BRAF Inhibitors. New Engl J Med. 2012;366:207–15. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.