Abstract
This study delineates the mechanisms by which ultraviolet B (UVB) regulates protein synthesis in human keratinocytes and the importance of translational control in cell survival. Translation initiation is regulated by phosphorylation of eukaryotic initiation factor 2 (eIF2~P), which causes decreased global protein synthesis coincident with enhanced translation of selected stress-related transcripts, such as ATF4. ATF4 is a transcriptional activator of the Integrated Stress Response (ISR), which has cytoprotective functions as well as apoptotic signals through the downstream transcriptional regulator CHOP (GADD153/DDIT3). We determined that UVB irradiation is a potent inducer of eIF2~P in keratinocytes, leading to decreased levels of translation initiation. However, expression of ATF4 or CHOP was not induced by UVB as compared to traditional ISR activators. The rationale for this discordant response is that ATF4 mRNA is reduced by UVB, and despite its ability to be preferentially translated there are diminished levels of available transcript. Forced expression of ATF4 and CHOP protein prior to UVB irradiation significantly enhanced apoptosis, suggesting that this portion of the ISR is deleterious in keratinocytes following UVB. Inhibition of eIF2~P and translational control reduced viability following UVB, which was alleviated by cycloheximide, indicating that translation repression through eIF2~P is central to keratinocyte survival.
INTRODUCTION
Eukaryotic cells have evolved a myriad of mechanisms to protect themselves from environmental stressors such as ultraviolet B (UVB) light. One such mechanism is translational control, which allows stressed cells to conserve resources and rapidly reconfigure gene expression to enhance cytoprotection (Schwanhausser et al., 2011). A central mechanism directing translational control involves phosphorylation of the α subunit of eukaryotic initiation factor 2 (eIF2~P). eIF2~P represses general translation initiation through a reduced ability of eIF2 to combine with GTP and transport the initiator Met-tRNAiMet to ribosomes for the initiation mRNA translation (Baird and Wek, 2012; Wek et al., 2006). Mammalian eIF2 can be phosphorylated by one of four eIF2 kinases, each of which is activated by distinct stress conditions, including UV irradiation (Deng et al., 2002; Donnelly et al., 2013). The convergence of diverse stressors onto this one phosphorylation event has led to this stress response pathway being referred to as the Integrated Stress Response (ISR) (Harding et al., 2003). While UVB-induced protective mechanisms, such as cell cycle checkpoint and mitogen-activated protein kinase pathways, are well characterized (Marrot and Meunier, 2008; Muthusamy and Piva, 2010), the importance of translational control in response to UVB irradiation is less well understood.
Concurrent with global translational repression, canonical eIF2~P enhances the preferential translation of mRNA transcripts such as that encoding activating transcription factor ATF4, a transcriptional activator of ISR genes involved in alleviating stress damage (Harding et al., 2000a; Harding et al., 2003; Vattem and Wek, 2004). Additionally, downstream of ATF4 is the proapoptotic transcription factor CHOP (GADD153/DDIT3), as well as GADD34, which inhibits the ISR by enhancing the dephosphorylation of eIF2~P (Connor et al., 2001; Ma and Hendershot, 2003; McCullough et al., 2001; Novoa et al., 2001). Preferential translation of these transcripts occurs through mechanisms involving upstream open reading frames (uORFs) in the 5′ leaders of the mRNAs (Palam et al., 2011; Vattem and Wek, 2004). It is suggested that chronic stress that induces the eIF2~P/ATF4/CHOP pathway can switch the ISR from its primary survival function to one that facilitates cell death (Marciniak and Ron, 2006; Oslowski and Urano, 2011; Tabas and Ron, 2011; Wek and Anthony, 2009).
In this study we addressed the induction of the ISR mechanism and its biological significance in the response of human keratinocytes to UVB exposure. Our results suggest that a non-canonical induction of the ISR by UVB in human keratinocytes is central for protection provided by translational control.
RESULTS
UVB irradiation induces eIF2 phosphorylation and global repression of translation initiation in human keratinocytes
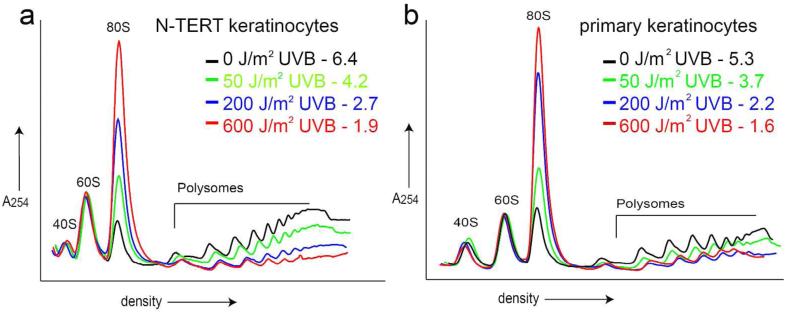
To determine how UVB irradiation affects translational control in human keratinocytes, immortalized (non-transformed) N-TERT keratinocytes and primary human keratinocytes were irradiated with increasing doses of UVB. One, three, and six hours post-irradiation, cell lysates were harvested and subjected to sucrose gradient ultracentrifugation to measure the level of protein synthesis as judged by polysome analyses. UVB irradiation of both N-TERT and primary keratinocytes substantially decreased the amount of cellular mRNAs bound to large polysomes coincident with an increase in mRNAs associated with 80S monosomes, indicating repression of translation initiation in a dose and time-dependent manner (Fig. 1a, b, Fig. S1a, b). Quantitative measurement of translational control was obtained by calculating the ratio of polysomes to monosomes (p/m). For example, N-TERT keratinocytes displayed a p/m ratio of 6.4 when the cells were not subjected to stress, whereas there was a p/m value of 2.7 at 6 hours following treatment with 200 J/m2 UVB (Fig. 1a). This pattern of reduced p/m ratio was observed following increasing doses of UVB in both N-TERT and primary human keratinocytes (Fig. 1a, b). UVB doses as low as 50 J/m2 and as soon as 1 hour post-UVB treatment yielded decreased p/m values, indicating that translational repression occurs following both high and low (non-apoptotic) doses of UVB at times prior to any induction of apoptosis (Fig. S1a, b).
Figure 1.
UVB irradiation decreases global translation initiation in human keratinocytes.
Keratinocytes were irradiated with the indicated dose of UVB and harvested at 6 hours post-irradiation. Lysates were prepared from N-TERT (a) or primary human keratinocytes (b) and subjected to ultracentrifugation in a 10-50% sucrose gradient. Polysome profiles were generated, and absorbance was measured at 254 nm. Peaks are indicated as 40S and 60S ribosomal subunits, 80S monosomes, or polysome fractions. Polysome to monosome (p/m) ratios are indicated for each dose. Ratios are calculated by measuring the area under each curve for each dose, and dividing the polysome by monosome (80S) areas.
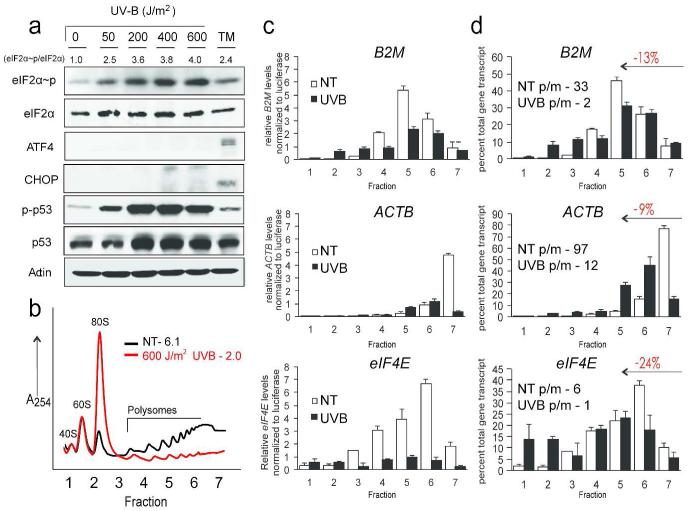
Translational control can be mediated via eIF2~P in response to many environmental stressors. To investigate the levels of eIF2~P in response to UVB irradiation, we treated cultured N-TERTs with increasing doses of UVB. Six hours post-irradiation, cells were harvested and subjected to immunoblot analyses using an antibody that specifically recognizes the α subunit of eIF2 phosphorylated at serine-51. Increasing doses of UVB resulted in enhanced phosphorylation of eIF2 in a dose-dependent manner (Fig. 2a). _Levels of eIF2~P normalized to total levels of eIF2α protein are shown above the immunoblot panel, indicating a maximal response of a 4-fold increase in eIF2~P following 600 J/m2 UVB. eIF2~P occurred as early as 1 hour post-UVB and continued to increase with time, indicating that the trigger for activation of this pathway is something recognized or produced over time (Fig. S1c). Levels of eIF2~P induced by UVB exposure were similar to that seen with tunicamycin (TM), a potent inducer of ER stress and a known trigger of eIF2~P. As a control for the keratinocyte UVB response, we showed that increasing doses of UVB led to increased phosphorylation of p53 at serine-15.
Figure 2.
UVB irradiation induces eIF2~P and translational control of individual transcripts in the absence of ATF4 and CHOP protein expression.
N-TERT keratinocytes were irradiated with the indicated doses of UVB. (a) After 6 hours lysates were prepared and indicated proteins were measured by immunoblot analyses. As controls, cells were subjected to ER stress elicited by 2 μM tunicamycin (TM). Levels of eIF2~P normalized to eIF2 total are indicated below each dose. (b) After 6 hours lysates were subjected to polysome profiling. Seven fractions (indicated on the x-axis) were collected and total RNA isolated from each fraction. (c) Levels of the indicated gene transcripts from fractions collected in (b) were measured by qRT-PCR. (d) mRNA levels are presented as a percent of total gene transcript to illustrate a shift towards lower polysomes, which is quantified and indicated in red. Polysome to monosome (p/m) ratios are calculated by dividing the sum of fractions 5-7 (≥ 4 ribosomes per transcript) by 1-3 (≤2 ribosome per transcript).
To address the effects of global repression of translation initiation at the individual gene transcript level, we performed qPCR on individual sucrose fractions collected during polysome analyses (Fig. 2b) of keratinocytes irradiated with 0 or 600 J/m2 UVB using probes specific to beta-2 microglobulin (B2M), β-actin (ACTB), and eukaryotic initiation factor 4E (EIF4E), which are transcripts not involved in the ISR. The levels of the indicated mRNAs in each fraction were normalized to a firefly luciferase RNA control that was added to each fraction as described previously (Baird et al., 2014; Palam et al., 2011). Total levels of each transcript decreased in UVB-irradiated fractions compared to non-irradiated controls (Fig. 2c). Furthermore the percent of each mRNA, independent of changes in total transcript levels, shifted from large polysomes (4 or greater ribosomes bound per transcript) towards smaller polysomes (2 or less ribosomes per transcript) in UVB irradiated keratinocytes compared to non-irradiated controls (Fig. 2d). These results support the idea that the translation of mRNAs genome-wide is reduced in response to UVB irradiation in human keratinocytes, with a gradient of repression among different gene transcripts.
UVB irradiation induces robust eIF2~P in the absence of preferentially translated downstream effectors
Activation of the ISR via eIF2~P is accompanied by upregulation of ATF4 and CHOP. However, despite a robust induction of eIF2~P following both high and low doses of UVB irradiation, the amounts of ATF4 and CHOP protein detected were minimal following any dose of UVB (Fig. 2a). In contrast, there were increased levels of ATF4 and CHOP proteins in response to ER stress. Therefore it appears that the ISR is being activated in a non-canonical fashion, as ATF4 and CHOP are known downstream targets of eIF2~P in response to many stressors not limited to ER stress.
Previous work showed that ATF4 is preferentially translated during ER stress via mechanisms involving upstream open reading frames (uORFs) in the 5′ leader of its mRNA (Vattem and Wek, 2004). We investigated whether ATF4 is preferentially translated as a result of UVB irradiation, even though we observed no UVB-dependent induction of ATF4 protein. To address this question, relative levels of ATF4 mRNA were measured by qPCR in each fraction collected by sucrose gradient ultracentrifugation from N-TERT keratinocytes irradiated with 0 or 600 J/m2 doses of UVB (Fig. 2b). Total levels of ATF4 transcript were decreased in UVB-irradiated sucrose fractions compared to non-irradiated controls (Fig. 3a). Interestingly, despite apparently reduced total ATF4 transcript levels, the percent of ATF4 mRNA among gradient fractions shifted 50% towards higher polysomes in UVB irradiated keratinocytes compared to non-irradiated controls (Fig. 3b). This finding suggests that if ATF4 mRNA is available following UVB stress, the transcript can be preferentially translated in response to eIF2~P. To further test whether ATF4 can undergo preferential translation following UVB irradiation, we transfected N-TERT keratinocytes with a plasmid encoding the 5′ leader of ATF4 mRNA inserted between a constitutive TK promoter and a luciferase coding sequence (Vattem and Wek, 2004). Therefore, any transcriptional regulation is removed and translation can be regulated through uORFs in the 5′ leader. Luciferase activity increased significantly in cells treated with TM as well as UVB, indicating that preferential translation of ATF4 can occur in response to both treatments (Fig. 3c).
Figure 3.
UVB irradiation causes both preferential translation and transcriptional repression of ATF4. (a) Total RNA was isolated from sucrose gradient fractions collected in (2b), and the levels ATF4 mRNA were measured by qRT-PCR. (b) Each of the indicated mRNA levels are presented as a percent of total gene transcript. (c) N-TERTs were co-transfected with pTK-ATF4-luc and control Renilla luciferase plasmids. Luciferase activity is represented as relative light units (RLU). Total levels of ATF4 (d) and CHOP (e) mRNAs following treatment with 600 J/m2 of UVB or TM were measured by qRT-PCR at the indicated time points. (f) N-TERTs were exposed to 0 or 600 J/m2 UVB, and following 1 hour cells were treated with 20 μM actinomycin D for an additional 1, 2, or 4 hours. ATF4 mRNA was measured by qRT-PCR. Values are presented as averages +/− standard deviation of three separate experiments (*, p<0.05 **, p<0.01).
Given the diminished induction of ATF4 protein expression observed in response to UVB irradiation (Fig. 2a), we measured ATF4 and CHOP mRNA expression and at one, three, and six hours post-irradiation via qPCR. Whereas treatment with TM led to an increase in both ATF4 and CHOP mRNA over time, UVB caused a significant lowering of both transcripts following a UVB dose of 600 J/m2 (Fig. 3d, e). This significant decrease in ATF4 and CHOP mRNA levels was also seen following lower doses of UVB irradiation (Fig. S1d, e). It is possible that the decrease in ATF4 following UVB could be a result of a UVB-induced increase in ATF4 mRNA decay. To investigate this idea, we treated N-TERTs with 0 or 600 J/m2 UVB irradiation for 1 hour, followed by an RNA polymerase II inhibitor, 20 μM actinomycin D, for an additional 1, 2, or 4 hours. ATF4 mRNA levels were then measured by qPCR. The half-life of ATF4 mRNA was ~4 hours in both control and irradiated keratinocytes (Fig. 3f), indicating that the decrease in ATF4 in response to UVB is not a result of increased mRNA decay. These results suggest that while ATF4 can be preferentially translated during UVB-irradiation in human keratinocytes, lowered steady-state ATF4 mRNA levels resulting from decreased ATF4 transcription occur in response to UVB and prevent appreciable induction of ATF4 protein.
Repression of downstream ISR effectors provides protection from UVB-induced apoptosis
We hypothesized that the discordant ISR triggered by UVB in which ATF4 and CHOP are repressed, rather than induced, during robust eIF2~P provides a survival advantage in human keratinocytes. To test this idea, we utilized a derivative of the drug salubrinal, sal-003 (sal), a potent inhibitor of eIF2 dephosphorylation (Boyce et al., 2005). Cells treated with sal-003 demonstrate enhanced eIF2~P and forced expression of ATF4 and CHOP in the absence of exogenous cell stress. N-TERT keratinocytes were pretreated with sal-003 for 6 hours prior to UVB irradiation. Sal-003 pretreatment increased ATF4 and CHOP protein levels in both untreated and irradiated keratinocytes in contrast to cells treated with UVB alone which showed increased eIF2~P but no ATF4 or CHOP protein (Fig. 4a, 2a). Combined treatment of sal-003 and UVB also caused a significant increase in apoptosis as measured by caspase-3 specific activity at 6 hours post-irradiation when compared to UVB irradiation alone (Fig. 4b). Similar results were observed in primary human keratinocytes (Fig. S2a). Enhanced apoptosis associated with sal-003 pretreatment is seen as early as 3 hours post-UVB, at which point there is no significant induction of caspase-3 activity by UVB alone (Fig. S2b). The negative effects of sal-003 in combination with 600 J/m2 dose UVB was lost at 8 hours (Fig. S2a), indicating that sal-003 is most likely accelerating the onset of apoptosis at higher doses of UVB. Sal-003 alone did not cause a significant increase in caspase-3 activity and did not absorb light in the UVB spectrum. N-TERT keratinocytes collected at three and six hours post-UVB showed a 0 and 4% increase, respectively, in Annexin V-positive cells compared to untreated controls, whereas cells pretreated with sal-003 showed a 38 and 25% increase, respectively. Annexin V staining revealed that the increases in caspase-3 activity with sal-003 pretreatment seen in Fig. 4b and Fig. S2a is a result of an increased population of apoptotic cells, rather than simply an increase in enzyme activity. Taken together, these results indicate that combined treatment of sal-003 and UVB, which induced the expression of ATF4 and CHOP, is deleterious to cell survival.
Figure 4.
Expression of ISR downstream effectors sensitizes cells to UVB-induced apoptosis (a) N-TERT keratinocytes were pretreated with 10 μM sal-003 (Sal) for 6 hours prior to irradiation with the indicated doses of UVB. Alternatively, cells were subjected only to Sal, UVB, or no treatment. Cells were harvested 3 hours post-irradiation and the indicated proteins were measured by immunoblot analyses. As controls, cells were subjected to ER stress elicited by 2 μM tunicamycin (TM). (b) Lysates were assayed for apoptosis 6 hours post-UVB by measuring the induction of caspase-3 specific activity. Data are presented as averages +/− standard deviation of three separate experiments. Asterisks indicate a significant difference between groups treated with UVB alone versus a combined treatment of UVB and Sal (*, p<0.01).
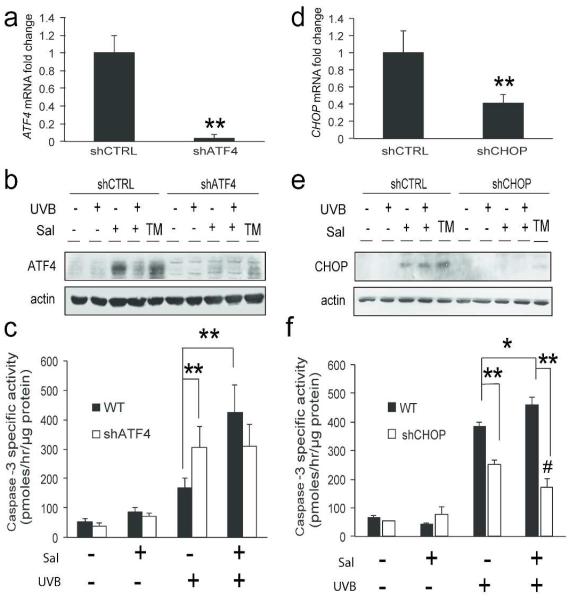
It is possible that the negative effects of combining sal-003 and UVB are a result of something unrelated to the ISR. Therefore to investigate the relative importance of sal-003 induced ATF4 expression, we used short hairpin RNA (shRNA) and a lentiviral delivery system to stably knock down expression of ATF4 in N-TERT keratinocytes. By knocking down ATF4, we prevented its ability to be induced by treatment with sal-003. Knock down of ATF4 resulted in about 80% reduction in basal ATF4 mRNA (Fig. 5a) and substantial reduction of induced ATF4 protein by TM, sal-003, and by combined treatment of sal-003 and UVB (Fig. 5b). The caspase-3 activity of shATF4 keratinocytes was not significantly different between cells treated with UVB alone and cells pretreated with sal-003 prior to UVB irradiation (Fig. 5c). Therefore, the knock down of ATF4 ablated the pro-apoptotic effects of combining sal-003 with UVB. These results suggest that the increase in UVB-induced cell death with sal-003 pretreatment is due to induced expression of ATF4. The N-TERT cells knocked down for ATF4 did experience a modest, albeit significant, increase in caspase-3 activity when treated with UVB alone, which may be a consequence of ATF4 triggering the expression of genes having anti-oxidation functions (Harding et al., 2003).
Figure 5.
Knockdown of ATF4 or CHOP protects cells during combined sal-003 and UVB treatments Total RNA was isolated from shCTRL, shATF4 (a), or shCHOP (d) cells and analyzed for expression of ATF4 or CHOP mRNAs to validate knockdown efficiency. shCTRL, shATF4 (b), or shCHOP (e) cells were pretreated with 10 μM sal-003 for 6 hours prior to irradiation with 600 J/m2 UVB. Lysates were subjected to immunoblot analysis 3 hours post-irradiation. (c) shCTRL and shATF4 cells were assayed for apoptosis by measuring the induction of caspase-3 specific activity 6 hours post-irradiation. (f) shCTRL and shCHOP cells were separately assayed for caspase-3 specific activity. Error bars represent standard deviation, (*, p<0.05 **, p<0.001 #, p<0.05 UVB+vehicle versus UVB+Sal in shCHOP cells).
The ATF4 target gene CHOP is considered to be a potent pro-apoptotic transcription factor whose expression is induced at the transcriptional and translational level during canonical induction of the ISR (Teske et al., 2013). We used shRNA to carry out a similar analysis of keratinocytes knocked down for CHOP expression. Lentiviral delivery of shCHOP resulted in about a 60% reduction in basal CHOP mRNA (Fig. 5d) as well as the loss of induced expression by TM, sal-003, and combined treatment of sal-003 and UVB (Fig. 5e). Depletion of CHOP provided even greater relief of apoptosis in response to combined sal-003 and UVB treatment than was previously seen in ATF4 depleted cells; shCHOP cells treated with sal-003 and UVB actually had significantly less caspase-3 activity than those treated with UVB alone (Fig. 5f). CHOP depleted cells also showed some protection from UVB exposure compared to control cells, which can be attributed to basal levels of the pro-apoptotic CHOP in keratinocyte controls. These results suggest that expression of downstream ISR effector CHOP, whose expression is transcriptionally enhanced by ATF4, is deleterious to keratinocyte survival in response to UVB irradiation.
Translational control elicited by eIF2~P provides resistance to UVB-induced apoptosis
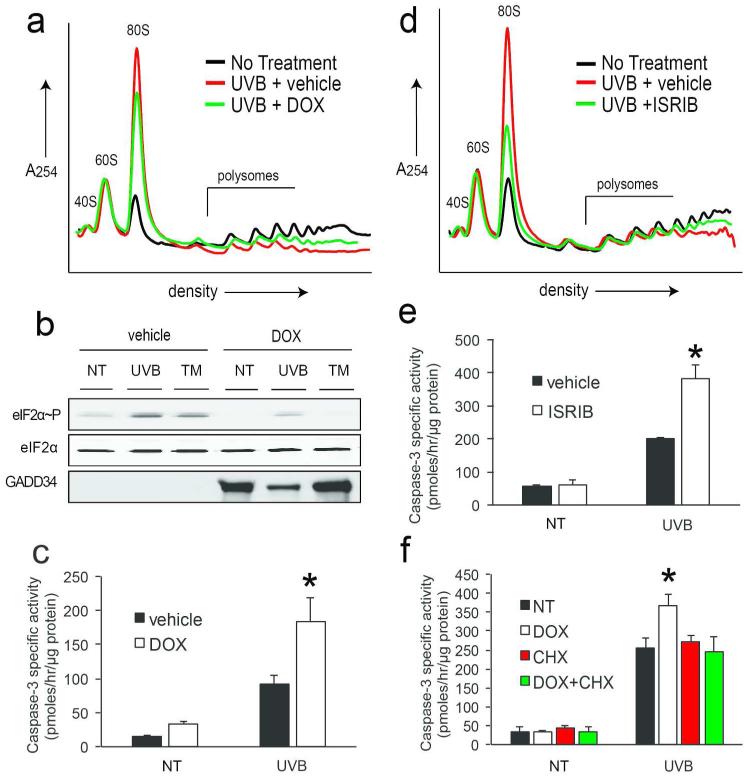
To address the contribution of the ISR to UVB-dependent translation control and keratinocyte survival in response to UVB, we utilized two strategies to inhibit the ISR. We first exploited a lentiviral delivery system to express a doxycycline-inducible GADD34 gene in N-TERT keratinocytes. GADD34 serves as a negative feedback regulator of the ISR by facilitating protein phosphatase 1 dephosphorylation of eIF2~P; therefore, overexpression of GADD34 would effectively block eIF2~P and translational control in response to stress. Treatment of keratinocytes overexpressing GADD34 with doxycycline (DOX) for 24 hours caused a partial restoration of large polysomes coincident with a decrease in monosomes when treated with UVB (Fig. 6a), as well as blocked induction of eIF2~P by both TM and UVB (Fig. 6b). Importantly, induced expression of GADD34 caused an increase in UVB-induced caspase-3 activity (Fig. 6c). Secondly, we used ISRIB, a pharmacological inhibitor of eIF2-dependent translational repression (Sidrauski et al., 2013). Cells were pretreated with ISRIB for 1 hour prior to UVB irradiation and assayed for changes in polysome profiles or caspase-3 activity. ISRIB caused a substantial restoration of large polysomes in keratinocytes treated with UVB, coincident with a decrease in monosomes (Fig. 6d). Pharmacological inhibition of the ISR also significantly increased apoptosis in response to UVB irradiation, indicating that eIF2~P indeed provides protection to keratinocytes in response to both high and low doses of UVB (Fig. 6e). Treatment with ISRIB or DOX alone did not cause any change in polysome profiles and did not absorb light in the UVB spectrum (data not shown).
Figure 6.
eIF2~P dependent translation repression provides resistance to UVB-induced apoptosis GADD34 overexpressing keratinocytes were treated with 1 μg/mL doxycycline (DOX) for 24 hours to induce GADD34 expression prior to irradiation with 600 J/m2 UVB and characterized by (a) polysome profile analysis, (b) immunoblot analysis, or (c) measurements of caspase-3 specific activity. N-TERTs were pretreated with ISRIB for 1 hour and subsequently irradiated with 600 J/m2 UVB and subjected to (d) polysome profile analysis or (e) caspase-3 specific activity. (f) Keratinocytes expressing GADD34 were treated with vehicle, 24 hours of DOX treatment, 30 minutes 20 μg/mL cycloheximide (CHX) treatment, or a combination of 24 hours DOX followed by 30 minutes CHX. Cells were then irradiated with 0 or 600 J/m2 UVB and assayed for induction of caspase-3 activity. Error bars represent standard deviation (*p<0.01).
As described above, eIF2~P causes both a general repression of translation initiation as well as preferential translation of certain mRNAs. Previous studies have shown that the global reduction in protein synthesis elicited by ER stress provides a survival advantage to cells (Han et al., 2013; Harding et al., 2000b). Since ATF4 expression did not provide protection from UVB-induced apoptosis, we hypothesized that an alternative explanation for eIF2~P lies in the importance of global translation repression. To investigate the contribution of general repression of protein synthesis to the survival function of the ISR following UVB irradiation, we co-treated GADD34-overexpressing keratinocytes with both DOX and cycloheximide (CHX), a potent inhibitor of translation elongation, and assayed for apoptosis. Whereas overexpression of GADD34 had a negative impact on ability of keratinocytes to survive UVB-induced stress, co-treatment with CHX rescued this phenotype (Fig 6f). This suggests that reduced survival of eIF2~P-deficient cells following UVB exposure is in part due to their inability to repress protein synthesis levels globally.
DISCUSSION
This study addressed the importance of the ISR in the response of human keratinocytes to UVB irradiation. Translational control through eIF2~P is an important mechanism for enhanced viability of many cells types in response to a multitude of environmental stressors including ER stress and oxidative stress, and in this study we have shown that the ISR is critical for keratinocyte survival following UVB irradiation. Our study has drawn three central conclusions regarding the relative importance of translational control for keratinocyte survival. First, UVB represses global and individual transcript translation initiation in a dose dependent manner (Figs. 1, S1a-c, and 2c, d). This is consistent with previous findings using UVC in mouse embryonic fibroblast and HeLa cells (Dey et al., 2010; Dey et al., 2012; Jiang and Wek, 2005; Powley et al., 2009). Secondly, the ISR response to UVB does not involve appreciable induced expression of ATF4 and its downstream target CHOP in keratinocytes (Figs. 2, 3, and S1d, e). ATF4 mRNA was preferentially translated in response to UVB irradiation (Fig. 3a-c). However, ATF4 and CHOP mRNA levels were significantly reduced (Figs. 3a, d f, and S1d, e), suggesting a lack of gene transcript available for sufficient preferential translation to induce measureable protein expression. As UVB did not affect ATF4 mRNA decay (Fig. 3f), these results suggest that transcription of ATF4 is repressed during the keratinocyte response to UVB. Repression of ATF4 and CHOP appears to be an important feature of the cytoprotective function of the ISR during UVB stress, as forced ATF4 and CHOP expression during UVB by pretreatment with sal-003 had detrimental effects on the ability of keratinocytes to evade UVB-induced apoptosis (Figs. 4, 5, and S2). These findings suggest that expression of CHOP, which can be potently pro-apoptotic during different chronic stresses, leads to sensitivity of keratinocytes to UVB irradiation.
The third conclusion of this study is that the repression of general protein synthesis contributes to protection of keratinocytes from UVB-induced apoptosis. Blocking eIF2-dependent translational repression increased cell death in response to UVB (Fig. 6c, e), and treatment with the protein synthesis inhibitor cycloheximide substantially alleviated the harmful effects of ISR deficiency and protected against UVB-induced cells death (Fig. 6f). These results suggest that the ISR attenuation of global translation provides important advantages to keratinocytes exposed to UVB. Reduced protein synthesis would decrease energy and nutrient expenditure, allowing UVB-stressed cells to focus on quality control of existing proteins that can provide protection to UVB damage, such as those related to DNA repair and cell cycle control. Repression of translation may also contribute to lowered levels of key proteins that are short-lived and that function to enhance apoptosis. Furthermore, part of the deleterious functions of ATF4 and CHOP expression during UVB may involve their transcriptional activation of GADD34, which facilitates feedback dephosphorylation of eIF2~P. ATF4 and CHOP can also promote expression of genes involved in protein synthesis, which can lead to premature resumption of high levels translation that can facilitate cell death during stresses that afflict the ER (Han et al., 2013; Marciniak and Ron, 2006; Marciniak et al., 2004). Therefore, premature resumption of translation accompanying lowered eIF2~P may render UVB-stressed cells susceptible to complications of folding of existing damaged proteins and an enhanced influx of nascent polypeptides.
Collectively, these studies emphasize the importance of tightly regulated, non-canonical translational control pathways activated in keratinocytes following UVB. The importance of the ISR in the normal keratinocyte response to UVB as described above illustrates the possibility for therapeutic strategies targeting translational control in human skin.
MATERIALS AND METHODS
Cell culture
Normal human keratinocytes were isolated from neonatal foreskin tissue as described previously (Kuhn et al., 1999). All protocols using human tissue have been reviewed and approved by the Indiana University School of Medicine IRB and Biosafety Committees. Patient consent for experiments was not required because human tissue excised during routine surgery is considered to be discarded material. UVB irradiation of N-TERT and normal human keratinocytes was performed using two Philips FS20T12 UVB broadband light sources as described previously (Lewis et al., 2010). Cultured cells were treated with 2 μM tunicamycin (Sigma), 20 μMM actinomycin D (Sigma), 400 nM ISRIB (Xcess Biosciences, San Diego, CA), 1 μg/mL doxycycline (Sigma), or 20 μg/mL cycloheximide (Sigma) as indicated. Generation of N-TERT cell lines expressing the indicated shRNA or specific genes are discussed in the Supplemental Data.
Polysome Profiling
Polysome analysis used 10 to 50% sucrose gradients containing 20 μM Tris-HCl (pH 7.5), 100 mM NaCl, 5 mM MgCl2, and 50 μg/ml cycloheximide as described previously (Palam et al., 2011; Teske et al., 2011).
Immunoblot analysis
Immunoblots were performed as previously described (Teske et al., 2013). Antibodies used are listed in the Supplemental Data.
Measurement of mRNA levels by qPCR
RNA was isolated from cultured keratinocytes using TRIzol reagent (Invitrogen, Life Technologies). Single-strand cDNA synthesis was conducted using the TaqMan reverse transcriptase kit (Applied Biosystems, Life Technologies). mRNA levels were measured by quantitative PCR using transcript-specific Taqman probes or the SYBR Green (Applied Biosystems, Life Technologies) method for changes in polysome fraction distribution on a Realplex2 Master Cycler (Eppendorf, Hamburg, Germany). To measure the levels of target mRNAs, transcripts were normalized to either 18S rRNA or luciferase control RNA (Promega) for changes in polysome fraction distribution. Primer sequences are listed in the Supplemental Data.
Luciferase Assay
pTK-ATF4-luc and control Renilla luciferase plasmids were co-transfected into N-TERT keratinocytes in triplicate using polyethylenimine (PEI) for 16 hours. Transfected cells were treated with 600 J/m2 UVB or 2μM TM and collected after 6 hours. Firefly luciferase activity was measuring using a Promega Luciferase Assay System as previously described (Vattem and Wek, 2004). Values are a measure of a ratio of firefly versus Renilla luciferase units.
Caspase-3 Assay
Caspase-3 activity was measured using a synthetic fluorogenic substrate (DEVD-AMC, Alexis Biochemicals, San Diego, CA) as previously described (Lewis et al., 2010).
Annexin V Staining
Cells were co-stained with Annexin V (BD Pharminogen, Franklin Lakes, NJ) and propidium iodide (PI) (Life Biosciences). Cells were sorted by flow cytometry; Annexin V positive:PI negative stained cells were considered apoptotic.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Drs. Jeffrey Travers and John Turchi for their helpful suggestions. Furthermore, we thank David Southern and Sheree Wek for their technical support of these studies. We thank Dr. Reddy Palam for his construction of the GADD34-overexpression lentiviral plasmid and Dr. Matt Turner for assistance with flow cytometry. Support for this work was supplied by the National Institute of Environmental Health Sciences (ES020866 to DFS), the National Institute on Aging (AG048946 to DFS), and the National Institute of General Medical Sciences (GM049164 to RCW).
Abbreviations
- UVB
ultraviolet B
- ISR
integrated stress response
- eIF2
eukaryotic initiation factor 2
- eIF2~P
phosphorylated eukaryotic initiation factor 2
- ATF4
activating transcription factor 4
- CHOP
C/EBP homologous protein
- GADD34
growth arrest and DNA damage protein 34
- ISRIB
ISR inhibitor
- sal
salubrinal
- ER
endoplasmic reticulum
- TM
tunicamycin
- J/m2
joules per meter squared
- eIF4E
eukaryotic initiation factor 4E
- B2M
beta-2 microglobulin
- ACTB
β-actin
- DOX
doxycycline
- CHX
cycloheximide
Footnotes
Detailed protocols for the Methods and Materials and additional figures can be found in the Supplementary Materials.
CONFLICT OF INTEREST
The authors report no conflicts of interest with any of the data presented in this study.
REFERENCES
- Baird TD, Palam LR, Fusakio ME, et al. Selective mRNA translation during eIF2 phosphorylation induces expression of IBTKalpha. Mol Biol Cell. 2014;25:1686–97. doi: 10.1091/mbc.E14-02-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird TD, Wek RC. Eukaryotic initiation factor 2 phosphorylation and translational control in metabolism. Advances in nutrition (Bethesda, Md) 2012;3:307–21. doi: 10.3945/an.112.002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M, Bryant KF, Jousse C, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–9. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- Connor JH, Weiser DC, Li S, et al. Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol Cell Biol. 2001;21:6841–50. doi: 10.1128/MCB.21.20.6841-6850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Harding HP, Raught B, et al. Activation of GCN2 in UV-irradiated cells inhibits translation. Curr Biol. 2002;12:1279–86. doi: 10.1016/s0960-9822(02)01037-0. [DOI] [PubMed] [Google Scholar]
- Dey S, Baird TD, Zhou D, et al. Both transcriptional regulation and translational control of ATF4 are central to the integrated stress response. J Biol Chem. 2010;285:33165–74. doi: 10.1074/jbc.M110.167213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S, Savant S, Teske BF, et al. Transcriptional repression of ATF4 gene by CCAAT/enhancer-binding protein beta (C/EBPbeta) differentially regulates integrated stress response. J Biol Chem. 2012;287:21936–49. doi: 10.1074/jbc.M112.351783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly N, Gorman AM, Gupta S, et al. The eIF2alpha kinases: their structures and functions. Cell Mol Life Sci. 2013;70:3493–511. doi: 10.1007/s00018-012-1252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Back SH, Hur J, et al. ER-stress induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–90. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000a;6:1099–108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, et al. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000b;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–33. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Jiang HY, Wek RC. GCN2 phosphorylation of eIF2alpha activates NF-kappaB in response to UV irradiation. Biochem J. 2005;385:371–80. doi: 10.1042/BJ20041164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn C, Hurwitz SA, Kumar MG, et al. Activation of the insulin-like growth factor-1 receptor promotes the survival of human keratinocytes following ultraviolet B irradiation. Int J Cancer. 1999;80:431–8. doi: 10.1002/(sici)1097-0215(19990129)80:3<431::aid-ijc16>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Travers JB, Somani AK, et al. The IGF-1/IGF-1R signaling axis in the skin: a new role for the dermis in aging-associated skin cancer. Oncogene. 2010;29:1475–85. doi: 10.1038/onc.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM. Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J Biol Chem. 2003;278:34864–73. doi: 10.1074/jbc.M301107200. [DOI] [PubMed] [Google Scholar]
- Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–49. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- Marciniak SJ, Yun CY, Oyadomari S, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–77. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrot L, Meunier JR. Skin DNA photodamage and its biological consequences. J Am Acad Dermatol. 2008;58:S139–48. doi: 10.1016/j.jaad.2007.12.007. [DOI] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, et al. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–59. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthusamy V, Piva TJ. The UV response of the skin: a review of the MAPK, NFkappaB and TNFalpha signal transduction pathways. Arch Dermatol Res. 2010;302:5–17. doi: 10.1007/s00403-009-0994-y. [DOI] [PubMed] [Google Scholar]
- Novoa I, Zeng H, Harding HP, et al. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–22. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslowski CM, Urano F. The binary switch that controls the life and death decisions of ER stressed beta cells. Curr Opin Cell Biol. 2011;23:207–15. doi: 10.1016/j.ceb.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palam LR, Baird TD, Wek RC. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol Chem. 2011;286:10939–49. doi: 10.1074/jbc.M110.216093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powley IR, Kondrashov A, Young LA, et al. Translational reprogramming following UVB irradiation is mediated by DNA-PKcs and allows selective recruitment to the polysomes of mRNAs encoding DNA repair enzymes. Genes Dev. 2009;23:1207–20. doi: 10.1101/gad.516509. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Schwanhausser B, Busse D, Li N, et al. Global quantification of mammalian gene expression control. Nature. 2011;473:337–42. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- Sidrauski C, Acosta-Alvear D, Khoutorsky A, et al. Pharmacological brake-release of mRNA translation enhances cognitive memory. eLife. 2013;2:e00498. doi: 10.7554/eLife.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–90. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teske BF, Baird TD, Wek RC. Methods for analyzing eIF2 kinases and translational control in the unfolded protein response. Methods Enzymol. 2011;490:333–56. doi: 10.1016/B978-0-12-385114-7.00019-2. [DOI] [PubMed] [Google Scholar]
- Teske BF, Fusakio ME, Zhou D, et al. CHOP induces activating transcription factor 5 (ATF5) to trigger apoptosis in response to perturbations in protein homeostasis. Mol Biol Cell. 2013;24:2477–90. doi: 10.1091/mbc.E13-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A. 2004;101:11269–74. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek RC, Anthony TG. Beta testing the antioxidant function of eIF2alpha phosphorylation in diabetes prevention. Cell metabolism. 2009;10:1–2. doi: 10.1016/j.cmet.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.